1. Introduction
Diabetic foot infections (DFI), including osteomyelitis (DFO), are associated with high rates of treatment failure, even when treated with prolonged antibiotic therapy, adequate surgical debridement, and appropriate wound care [
1]. There are multiple reasons for the poor outcomes, including limb ischemia, inadequate pressure off-loading of the foot, and a lack of patient adherence to the prescribed treatment [
1]. In contrast to what many clinicians believe, the specific causative DFI pathogen is generally not a major determinant for the outcome of therapy [
2,
3,
4,
5,
6,
7,
8], unless it is resistant to multiple antibiotic agents [
4]. Indeed, in almost all published reports regarding DFIs, clinical or microbiological outcomes are no worse for patients infected with “difficult” pathogens such as methicillin-resistant
Staphylococcus aureus [
2],
Pseudomonas aeruginosa [
3,
5], or obligately anaerobic bacteria than with other pathogens [
6,
7,
8]. Even in randomized, controlled trials of treatment of DFI, the causative pathogen(s) is a negligible factor in treatment failure, compared to other parameters [
9,
10].
Unlike pathogenic bacteria, such as
S. aureus,
P. aeruginosa,
Enterobacteriaceae, enterococci, or streptococci, skin commensals isolated from swab cultures are not usually considered true pathogens, even when grown repeatedly from specimens [
11,
12,
13,
14]. According to widespread clinical experience and a very few retrospective studies, skin commensals [
15] (mostly coagulase-negative staphylococci [
16,
17,
18], micrococci [
15,
16], cornynebacteria [
15,
16,
19], cutibacteria [
15,
16,
20,
21]) demonstrate lower clinical virulence than other bacterial genera upon the manifestation of infection. However, there are few published data to inform whether skin commensals are clinically associated with a better outcome after therapy for DFI. We investigate this gap in the literature.
2. Results
2.1. Study Population and Infections
All authors of this paper worked at Geneva University Hospitals during the composition of he scientific database of the Clinical Pathway for DFI. Using that pathway database, we identified 1,018 DFI episodes (median age 81 years, 73% males, 610 [60%] with peripheral arterial disease). Among these, skin commensals were the sole isolates from wound cultures (without any pathogenic pathogens detected) in 54 cases (5%), and in of 23 of these 1018 (2% of all cases) the patient was diagnosed as having DFO. The proportion of DFI episodes caused entirely by pathogenic pathogens was 63% (641/1,018). Among these patients whose cultures grew at least one pathogenic pathogen (the control group), the most common isolates were Staphylococcus aureus (389 cases [38%]) and Pseudomonas aeruginosa (61, 6%) cases, but cultures yielded 30 other pathogenic pathogens (e.g., β-hemolytic streptococci or Enterobacteriaceae).
Overall, we detected 68 different microbiological constellations. The five most frequent, monobacterial, predominant, and pathogenic species were Staphylococcus aureus (38%), streptococci (6%), enterococci (5%), and Gram-negative microorganisms (18%), of which Pseudomonas aeruginosa to 6%, Skin commensals were retrieved as (co)-pathogens, together with pathogenic bacteria, in 161 DFI cases (16%). Blood cultures grew organisms that we believed represented clinically plausible bacteremia in 80 episodes (8%). The median serum C-reactive protein (CRP) level among all enrolled subjects on admission was 81 mg/L. Among the 392 [39%] episodes of DFO with a positive bone culture, the diagnosis of chronic osteitis was confirmed by histology in 275 (70%), while the rest by clinical and imaging findings.
2.2. Therapy and Outcomes
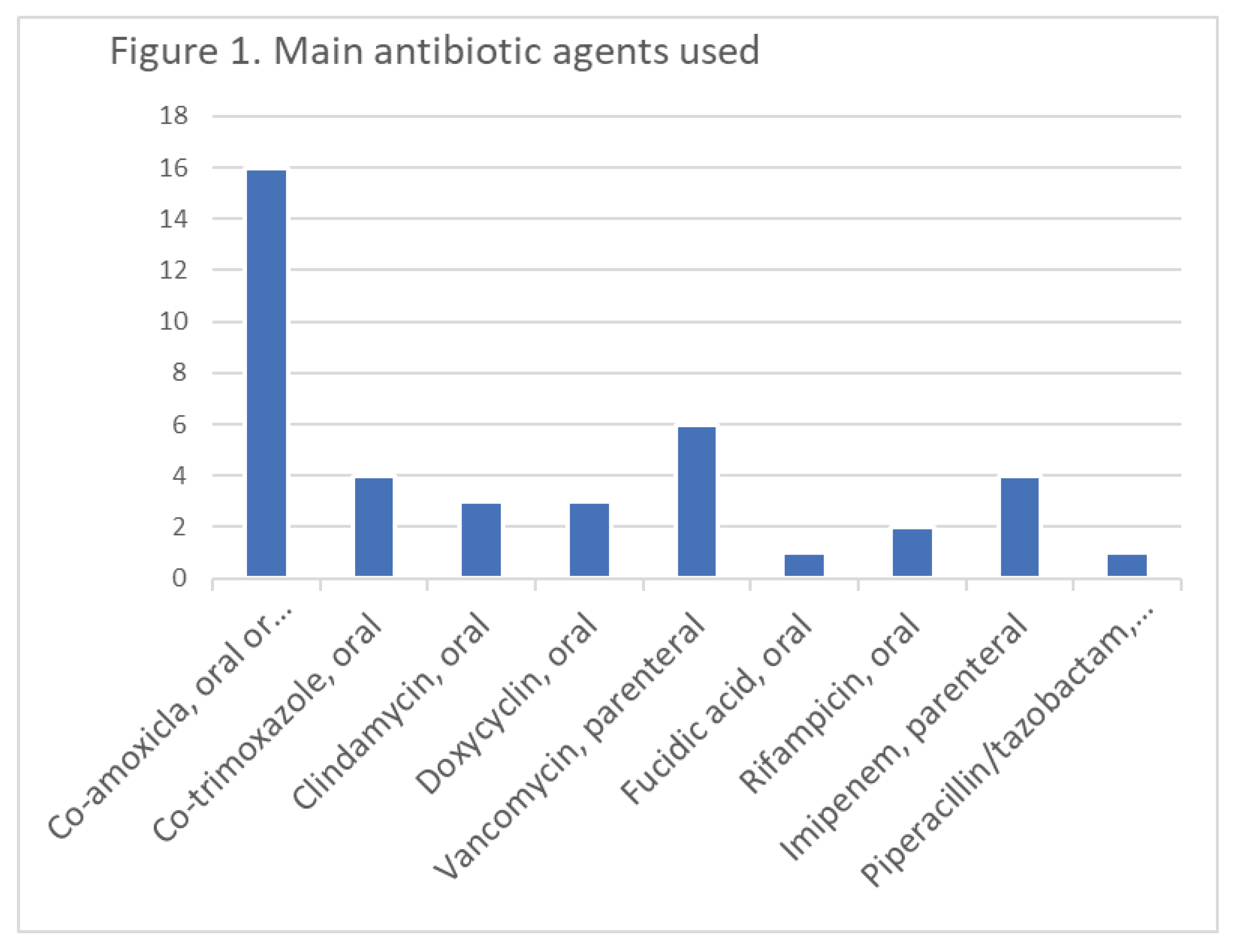
After treatment (including at least one surgical debridement in all, and partial amputation in 596 [58%], antibiotic therapy [45 different regimens, (
Figure 1) with a median duration of 20 days, of which 5 days were administered parenterally], hyperbaric oxygen therapy (98 cases [10%]), 251 (25%) of the episodes met our definition of clinical failure. Of these, 119 cases (12%) met our definition of microbiological recurrence. The follow-up duration for these episodes was a minimum of six months, and a median of 3.3 years. The six main antibiotic agents used for skin commensals were co-amoxiclav (40%; practically for all susceptible commensals), vancomycin (15%), co-trimoxazole (10%), clindamycin 8%, doxycyclin 8%, fusidic acid with rifampicin (8%). Hence, 34% were treated with co-trimoxazole, or doxycycline, or rifampicin plus fusidic acid because that was the best choice based on the antibiogram. Linezolid and daptomycin were very rarely used, and only for a short period.
For further analyses, we compared the 54 DFIs solely caused by skin commensals to the 641 DFIs caused solely by pathogenic pathogens (
Table 1). As noted, we censored episodes with a mixture between both types of isolates [
16] and found no difference in the incidence of clinical failure between the skin commensals and pathogenic pathogens (17% vs 24 %, respectively;
p = 0.23) or microbiological recurrence (11% vs 17 %, respectively;
p = 0.23). Clinically, the study groups only significantly differed in the CRP values at admission (median of 25 mg/L vs. 105 mg/L, respectively;
p < 0.01). The number of surgical debridement, proportion of DFO cases, occurrence of bacteremia, and the duration of antibiotic therapy (including the parenteral part) were not significantly different between the groups. With further stratifications upon soft tissue DFI and DFO, we found no significant differences in both strata. Treating only skin pathogens among cases with only soft tissue infections revealed a similar of clinical failure rate as for the pathogenic pathogens (7/24; 29% vs 94/296; 32%,
p = 0.85). The same was true for the clinical failure rate for cases with DFO (2/21 vs 59/192;
p = 0.10).
Using multivariate adjustment with the outcome "clinical failure" (
Table 2), growth of skin commensals on wound culture was not determinant of clinical failure (odds ratio 0.4, 95% confidence interval 0.1-3.8), but the presence of ipsilateral lower extremity ischemia was (OR 3.0, 95% CI 1.1–8.5). These findings were similar in a multivariate analysis for "microbiological recurrence" (
Table 2). The Receiver-Operating-Curve (ROC) value was 0.83, representing a good accuracy of our multivariate model.
3. Discussion
In this single-center study, we did not detect any association of the clinical or microbiological outcomes of DFI with the presence of skin commensals compared with pyrogenic pathogens. Furthermore, we found that the number of surgical debridement, the incidence of bacteremia, the percentage of patients with DFO, and the length of antibiotic therapy were quite similar for the two microbiological groups. These results suggest there was not a major comparison bias in management related to the two microbiological groups. The clinical “virulence” of both pathogen groups was similar. Hence, if both patient groups with skin commensals and “pathogens” are equally treated with sensitive antibiotics based on respective antibiograms, there are no clinical differences.
The only two differences of note between the groups was a significantly lower C-reactive protein level at admission in those with skin commensals and the association of lower extremity ischemia with a higher rate of clinical failure [
22], but not microbiological recurrence [
23]. Only the choice of the antibiotic agent was different. Secondly, contrary to pathogenic DFI pathogens, for which (oral) co-amoxiclav is the hallmark in the Swiss medical culture [
24], we mostly used non-beta-lactam and non-quinolone antibiotic agents; with similar clinical efficacy as oral beta-lactam agents. We conclude that while skin commensals may induce a lesser degree of inflammation (CRP elevation), they do not appear to be less virulent than the classical bacteria in patients treated for DFI. Thus, there does not appear to be a reason to select less aggressive surgical or antibiotic therapy for DFIs caused by these bacteria.
Besides its retrospective nature, a relatively small sample size of only some 60 DFI episodes in the skin commensals’ group, and the large case-mix inherent to the adult DFI population, our study has other several limitations. First, we somewhat arbitrarily created two microbiological groups, one with only skin commensals and the other with only pathogenic pathogens, while in reality two-thirds of skin commensals are co-pathogens with other pathogenic bacteria. However, for formal comparative statistics, we had to exclude mixed-group cases in order to perform a true statistical comparison of sharply distinguished groups of interest. Similarly, our skin commensal classification was composed of many species (e.g. micrococci,
S. epidermidis [
16,
18] and
S. lugdunensis [
17])
, each of which might have a different level of clinical virulence or ability to cause persistent infection. Even with our large number of DFI episodes, it is not impossible to adjust for the effect of a single species in the frequently polymicrobial infections in our study population [
16].
Second, our analysis may lack other important variables such as the ulcer or infection healing time. Ulcer healing is heavily influenced by off-loading, patient’s compliance and professional wound debridement, and probably only to a minor extent by antibiotic therapy. The role of pathogens in ulcer colonizing, diabetic foot microbiome, and ulcer healing is a matter of debate in human ulcers. The role of microbial bioburden in ulcer outcomes and complications remains ambiguous, including the significance of microbial load and diversity and the role of specific microorganisms, including known wound pathogens and microorganisms considered as skin commensals or environmental contaminants. In experimental studies, the cultured wound isolates of
S. aureus elicited differential phenotypes in mouse models that corresponded with patient outcomes, while wound “bystanders” such as
Corynebacterium striatum and
Alcaligenes faecalis typically considered commensals or contaminants also significantly impacted wound severity and healing [
25].
Furthermore, as we relied on classical, clinical culture techniques, we might have missed unidentified species within the microbiome [
15,
26]. These might have been detected by molecular methods such as "shotgun" and other DNA-enhancing techniques [
12,
15]. There is a growing literature assessing the effects of these “hidden” bacteria (based on standard cultures) within the microbiome or the biofilm. For example, some research groups advocate that these hidden commensals may interact with other bacteria, perhaps even promoting wound healing by inhibiting the virulent
S. aureus [
27] that are so often found in diabetic foot wounds [
28,
29]. Undertaking such a study would require expensive and limited academic laboratory facilities, making it beyond our routine clinical evaluation.
Lastly, some clinicians might argue that the presence of skin commensals on wound culture is more a sign of specimen contamination than of true infection, or organism selection by prior antibiotic therapy. We do not think this is so, as our diagnostic criteria are based on the IWGDF guidelines [
13] and on a high proportion of histologically-confirmed DFO episodes. Moreover, on the clinical side, we managed patients with these skin commensals the same as those with every other pathogen, and still saw no difference. If these bacteria play a less virulent role, we think we should have found at least some hints in favor of an altered outcome when studying 1,018 episodes in the same Clinical Pathway.
4. Conclusion
In one of the largest single-center case-control studies in the field of DFi and DFo, our retrospective results suggest that skin commensals isolated from DFIs, or of DFOs, are neither clinically virulent, nor more microbiologically persistent, than other bacteria. They can be treated also by oral antibiotic agents. Clinicians should therefore perhaps consider these bacteria as potential pathogens when selecting an antibiotic Regimen. Similarly, there is probably no need to advocate a different antibiotic treatment (e.g. shorter or longer treatments) when compared to the therapy of pathogenic bacteria. Further clinical confirmatory stories are needed.
5. Methods
At the Geneva University Hospitals, we have established a database (embedded in a hospital-wide Clinical Pathway for DFI [
1]) for managing DFI. We examined all DFI episodes identified from April 24
, 2013 to July 31, 2016 for which microbiological samples were collected. Furthermore, our Clinical Pathway prospectively assessed all DFI and DFO that we encountered in the entire hospital. The pathway involved hospitalized patients and those in the outpatient settings. All physicians and surgeons were asked to report all DFI patients. Moreover, in the context of the Clinical Pathway implementation, a Research Nurse specialized in DFI regularly screened all hospitalization wards for diabetic patients with and without foot problems, and identified potential DFI candidates.
We identified all pathogens from these specimens using internationally recommendedculture methods [
2,
3,
4]. Wound cultures were only accepted from depth samples (including pus) of the wound after the start of debridement, and/or intraoperatively. In the Clinical Pathway orienting on the IWGDF guidance, we avoided superficial microbiological swab sampling.[
13]
.We defined DFI based on the International Working Group on the Diabetic Foot (IWGDF) criteria [
13] and a “clinical failure” as: (1) the persistence or recurrence of any clinical indication for revision surgery; (2) the development of a recurrent infection (same site, same causative pathogen[s]; (3) or the occurrence of a new infection in the same foot [
9]. We defined “microbiological recurrence” as a “clinical failure” predominantly caused by the same pathogens as in the index episode. We recorded the three most frequent pathogens per episode, and censored any other quantitatively fewer common microorganisms. We developed our Clinical Pathway for DFI as a quality program, for which the patients were not required to provide individual consent. However, many of them concomitantly participated in at least one of the many randomized DFI trials we conducted [
9,
10,
30,
31] that required signed consent forms.
5.1. Statistical Analyses
For this study, we divided the isolated microorganisms into two groups: those that we regarded, based on the literature and our extensive experience, as only commensals (coagulase-negative staphylococci, micrococci, cutibacteria, cornynebacteria); and pathogenic pathogens composed of bacteria commonly regarded as virulent isolated causing DFI. The primary objective of this study was to define the role of skin commensals in DFI by examining the likelihood of clinical remission of DFI overall, and diabetic foot osteomyelitis (DFO) separately. We compared the skin commensal with pathogenic pathogen groups using the Pearson-χ2 or the Wilcoxon-ranksum-test, as appriopiate. In these comparisons, we only analysed infections caused entirely by skin commensals and those caused entirely due to pathogenic bacteria, excluding for these group comparisons any polymicrobial DFIs with mixed groups (i.e., pathogenic pathogens AND skin commensals). We furthermore adjusted for our large case-mix with two identical, cluster-controlled (clustering on the individual patient) multivariate logistic regression analyses with the separate outcomes “clinical failure” and “microbiological recurrence”. We performed all statistical calculations using STATA™ software (Version 14, College Station, Texas, USA).
Author Contributions
I.U.: Idea: Drafting, Sponsor, Principal Investigator, Funding, Conduct, Analyses, Writing. D.L.: Study Conduct: Inclusion, Database. B.K.: Study Nurse: Conduct, Corrections, Supervision. B.A.L.: Concept: Writing, Corrections. K.G.: Idea: Writing, Database. All authors have agreed to the published version of the manuscript.
Funding
The prior Medical Director of Geneva University Hospitals provided 50,000 Swiss Francs ($50,000 USD) for the “Clinical Pathway of DFI” as a “quality of care” project.
Informed Consent Statement
The project was part of a quality of Care Approach for DFI at Geneva University Hospitals. The necessity of an individual consent was waived by the Direction of the Hospital. The project approved by the Ethics Committee’ of Geneva Canton (Ethical Committee NAC 13-178).
Data Availability Statement
Key data are available in an anonymous form upon reasonable scientific request to the corresponding author. They are not publicly available.
Acknowledgments
We thank to the former Medical Director Prof. Pierre Dayer for his support and to all laboratory and clinical teams of Geneva University Hospitals treating our DFI patients. We are indebted to Mrs. Elodie von Dach for her invaluable help in composing the database.
Transparency Declarations
All authors do not have any financial conflicts of interest and the funder has not played any decision-making role in this research.
References
- Uçkay, I., Gariani, K., Pataky, Z., Lipsky, B.A. Diabetic foot infections: state-of-the-art. Diabetes, Obes, Metab, 2014, 16, 305-316. [CrossRef]
- Zenelaj, B. , Bouvet, C., Lipsky, B.A., Uçkay, I. Do diabetic foot infections with methicillin-resistant Staphylococcus aureus differ from those with other pathogens? Int. J. Low. Extrem. Wounds. 2014, 13, 263–272. [Google Scholar] [CrossRef] [PubMed]
- eghrouchni, K., van Delden, C., Dominguez, D., Benkabouche, M., Bernard, L., Assal, M., Hoffmeyer, P., Uçkay, I. Remission after treatment of osteoarticular infections due to Pseudomonas aeruginosa versus Staphylococcus aureus: a case-controlled study. Int. Orthop. 2012, 36, 1065-1071. [CrossRef]
- Henig, O., Pogue, J.M., Martin, E., Hayat, U., Ja'ara, M., Kilgore, P.E., Cha, R., Dhar, S., Kaye, K.S. The Impact of Multidrug-Resistant Organisms on Outcomes in Patients With Diabetic Foot Infections. Open. Forum. Infect. Dis. 2020, 7, 161. [CrossRef]
- Uçkay, I., Lebowitz, D., Kressmann, B., von Dach, E., Lipsky, B.A., Gariani, K. Pseudomonal Diabetic Foot Infections: Vive la Différence? Mayo. Clin. Proc. Innov. Qual. Outcomes. 2022, 6, 250-256. [CrossRef]
- Charles, P.G., Uçkay, I., Kressmann, B., Emonet, S., Lipsky, B.A. The role of anaerobes in diabetic foot infections. Anaerobe. 2015, 34, 8-13. [CrossRef]
- Percival, S.L. , Malone, M., Mayer, D., Salisbury, A.M., Schultz, G. Role of anaerobes in polymicrobial communities and biofilms complicating diabetic foot ulcers. Int. Wound. J. 2018, 15, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Lebowitz, D. , Kressmann, B., Gjoni, S., Zenelaj, B., Grosgurin, O., Marti, C., Zingg, M., Uçkay, I. Clinical features of anaerobic orthopaedic infections. Infect. Dis. (Lond). 2017, 49, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K., Pham, T.T., Kressmann, B., Jornayvaz, F.R., Gastaldi, G., Stafylakis, D., Philippe, J., Lipsky, B.A., Uçkay, I. Three Weeks Versus Six Weeks of Antibiotic Therapy for Diabetic Foot Osteomyelitis: A Prospective, Randomized, Noninferiority Pilot Trial. Clin. Infect. Dis. 2021, 73, 1539-1545. [CrossRef]
- Pham, T.T., Gariani, K., Richard, J.C., Kressmann, B., Jornayvaz, F.R., Philippe, J., Lipsky, B.A., Uçkay, I. Moderate to Severe Soft Tissue Diabetic Foot Infections: A Randomized, Controlled, Pilot Trial of Post-debridement Antibiotic Treatment for 10 versus 20 days. Ann. Surg. 2022, 276, 233-238. [CrossRef]
- Tae, K. K. , Armstrong, D.G. Microbiology and Antimicrobial Therapy for Diabetic Foot Infections. Infect. Chemother. 2018, 50, 11–20. [Google Scholar] [CrossRef]
- Sadeghpour Heravi, F., Zakrzewski, M., Vickery, K., Armstrong, D.G., Hu, H. Bacterial Diversity of Diabetic Foot Ulcers: Current Status and Future Prospectives. J. Clin. Med. 2019, 8, 1935. [CrossRef]
- Lipsky, B.A., Sennevill,e E., Abbas, Z.A., et al. IWGDF Guideline on the diagnosis and treatment of foot infection in persons with diabetes. www.iwgdfguidelines.org (last accessed 19 December 2022). 19 December.
- Uçkay, I. , Pires, D., Agostinho, A., Guanziroli, N., Öztürk, M., Bartolone, P., Tscholl, P., Betz, M., Pittet, D. Enterococci in orthopaedic infections: Who is at risk getting infected? J. Infect. 2017, 75, 309–314. [Google Scholar] [CrossRef] [PubMed]
- van Asten, S.A. , La Fontaine, J., Peters, E.J.G., Bhavan, K., Kim, P.J., Lavery, L.A. The microbiome of diabetic foot osteomyelitis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Citron, D.M., Goldstein, E.J.C., Merriam, C.V., Lipsky, B.A., Abramson, M.A. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J. Clin. Microbiol. 2007, 45, 2819-2828. J. [CrossRef]
- Mohamad, M. , Uçkay, I., Hannouche, D., Miozzari, H. Particularities of Staphylococcus lugdunensis in orthopaedic infections. Infect. Dis. (Lond). 2018, 0, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I., Harbarth, S., Ferry, T., Lübbeke, A., Emonet, S., Hoffmeyer, P, Pittet, D.. Meticillin resistance in orthopaedic coagulase-negative staphylococcal infections. J. Hosp. Infect. 2011, 79, 248-253. [CrossRef]
- Kalt, F., Schulthess, B., Sidler, F., Herren, S., Fucentese, S.F., Zingg, P.O., Berli, M., Zinkernagel, A.S., Zbinden, R., Achermann, Y. Corynebacterium Species Rarely Cause Orthopedic Infections. J. Clin. Microbiol. 2018, 56, 1200-1218. [CrossRef]
- Uçkay, I. , Agostinho, A., Landelle, C., Coppens, E., Cunningham, G., Pittet, D. Incidence of Propionibacterium acnes infection in orthopedic and trauma surgery. Antimicrob. Resist. Infect. Control. 2015, 4, 28. [Google Scholar] [CrossRef]
- Coenye, T. , Spittaels, K.J., Achermann, Y. The role of biofilm formation in the pathogenesis and antimicrobial susceptibility of Cutibacterium acnes. Biofilm. 2021, 4, 100063. [Google Scholar] [CrossRef]
- Hinchliffe, R.J., Brownrigg, J.R., Apelqvist, J., Boyko, E.J., Fitridge, R., Mills, J.L., Reekers, J, Shearman, C.P., Zierler, R.E., Schaper, N.C. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes. Metab. Res. Rev. 2016, 32, 37-44. [CrossRef]
- Pham, T.T. , Wetzel, O., Gariani, K., Kressmann, B., Jornayvaz, F.R., Lipsky, B.A., Uçkay, İ. Is routine measurement of the serum C-reactive protein level helpful during antibiotic therapy for diabetic foot infection? Diabetes. Obes. Metab. 2021, 23, 637–641. [Google Scholar] [CrossRef]
- Gariani, K., Lebowitz, D., Kressmann, B., von Dach, E., Sendi, P., Waibel, F., Berli, M., Huber, T., Lipsky, B.A., Uçkay, I. Oral amoxicillin-clavulanate for treating diabetic foot infections. Diabetes. Obes. Metab. 2019, 21, 1483-1486. [CrossRef]
- Kalan, L. R, Meisel, J.S., Loesche, M.A., Horwinski, J., Soaita, I., Chen, X., Uberoi, A., Gardner, S.E., Grice, E.A. Strain- and Species-Level Variation in the Microbiome of Diabetic Wounds Is Associated with Clinical Outcomes and Therapeutic Efficacy. Cell. Host. Microbe. 2019, 2, 641–655. [Google Scholar] [CrossRef]
- Jneida, J., Lavigne, J.P., La Scolaa, B., Cassira, N. The diabetic foot microbiota: A review. Human. Microbiom. J. 2017, 5, 1-6. [CrossRef]
- Lai, Y., Di Nardo, A., Nakatsuji, T., Leichtle, A., Yang, Y., Cogen, A.L., Wu, Z.R., Hooper, L.V., Schmidt, R. R., von Aulock. S., Radek, K.A, Huang, C.M., Ryan, A.F., Gallo, R.L Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 2009, 15, 1377-1382.
- Kadamb Patel, B.K., Patel, K.H., Huang, R.Y., Chuen Neng Lee, C., Moochhala, S.M. The Gut-Skin Microbiota Axis and Its Role in Diabetic Wound Healing - A Review Based on Current Literature. Int. J. Mol. Sci. 2022, 23, 2375. [CrossRef]
- Bouvet, C. , Gjoni, S., Zenelaj, B., Lipsky, B.A., Hakko, E., Uçkay, I. Staphylococcus aureus soft tissue infection may increase the risk of subsequent staphylococcal soft tissue infections. Int. J. Infect. Dis. 2017, 60, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Waibel, F., Berli, M., Catanzaro, S., Sairanen, K., Schöni, M., Böni, T., Burkhard, J., Holy, D., Huber, T., Bertram, M., Läubli, K., Frustaci, D., Rosskopf, A., Botter, S., Uçkay, I. Optimization of the antibiotic management of diabetic foot infections: protocol for two randomized controlled trials. Trials. 2020, 21, 54. [CrossRef]
- Uçkay, I., Kressmann, B., Malacarne, S., Toumanova, A., Jaafar, J., Lew, D., Lipsky, B.A. A randomized, controlled study to investigate the efficacy and safety of a topical gentamicin-collagen sponge in combination with systemic antibiotic therapy in diabetic patients with a moderate or severe foot ulcer infection. BMC. Infect. Dis. 2018, 18, 361. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).