Submitted:
17 January 2023
Posted:
17 January 2023
You are already at the latest version
Abstract
Keywords:
Environmental Significance
Introduction
Radiocarbon Summary
Revelle Isotopic Anomaly
CO2 Finite Reserve Model
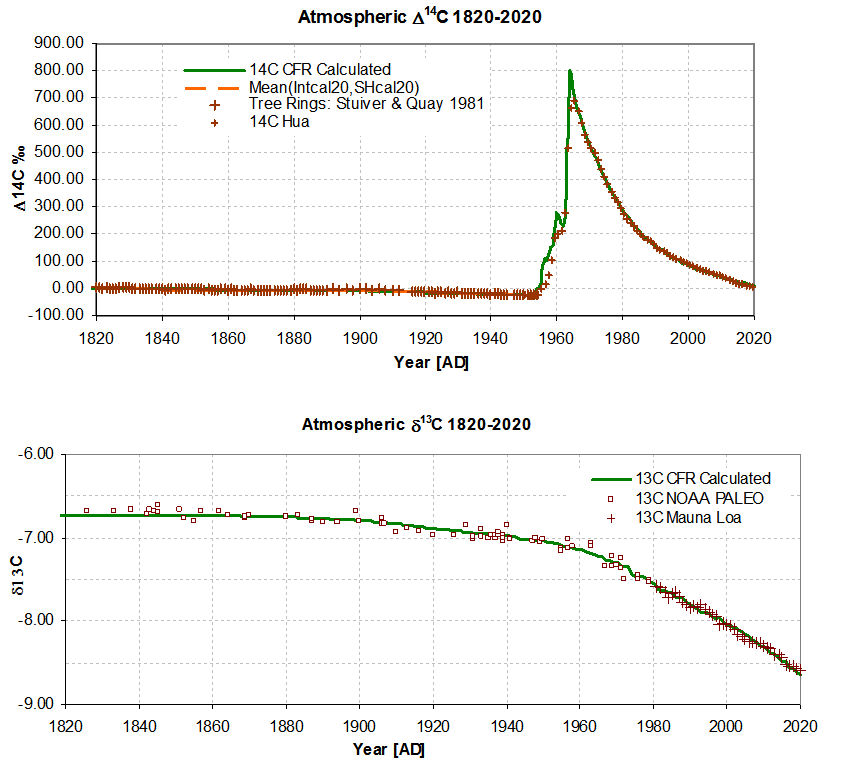
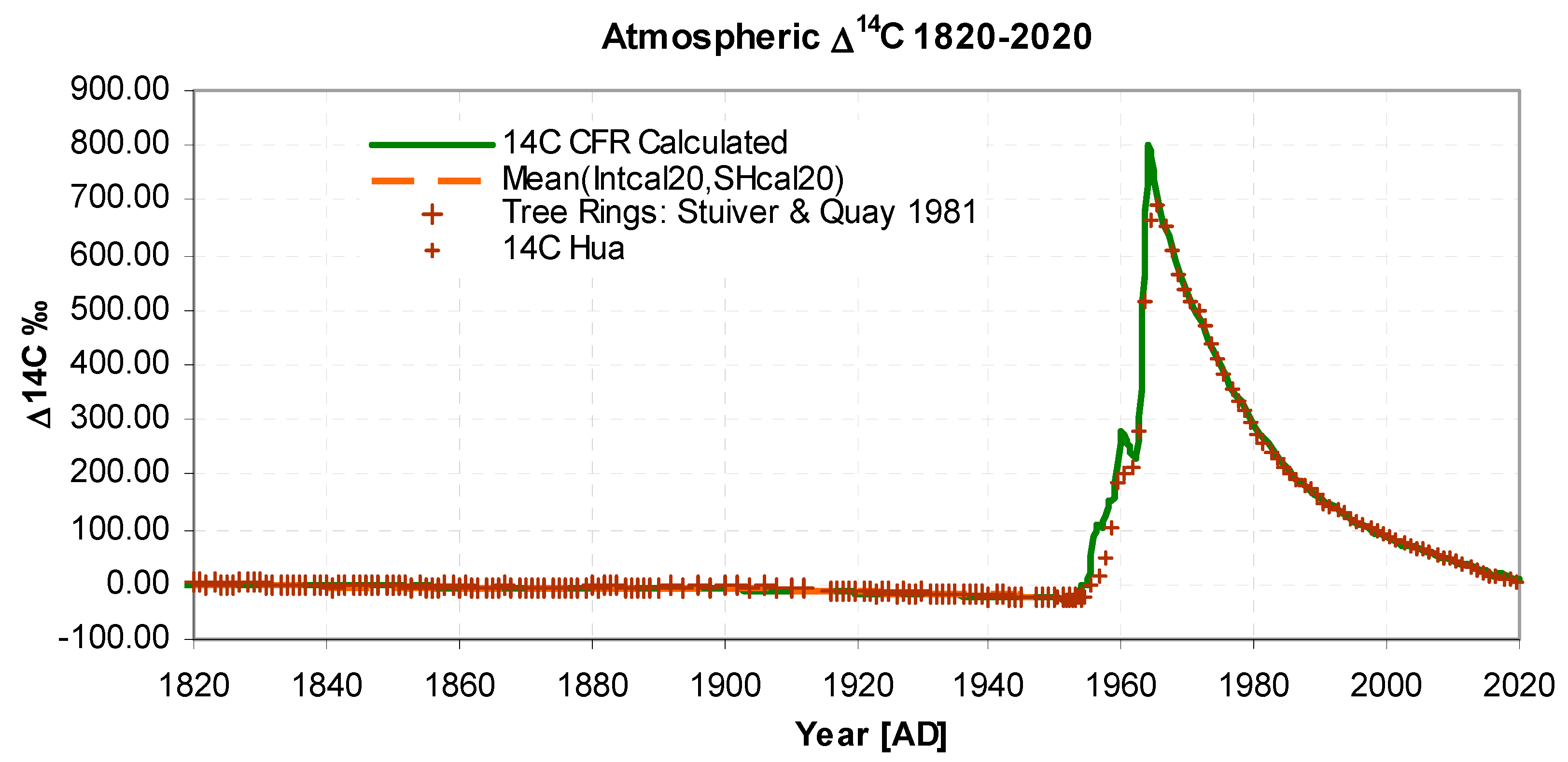
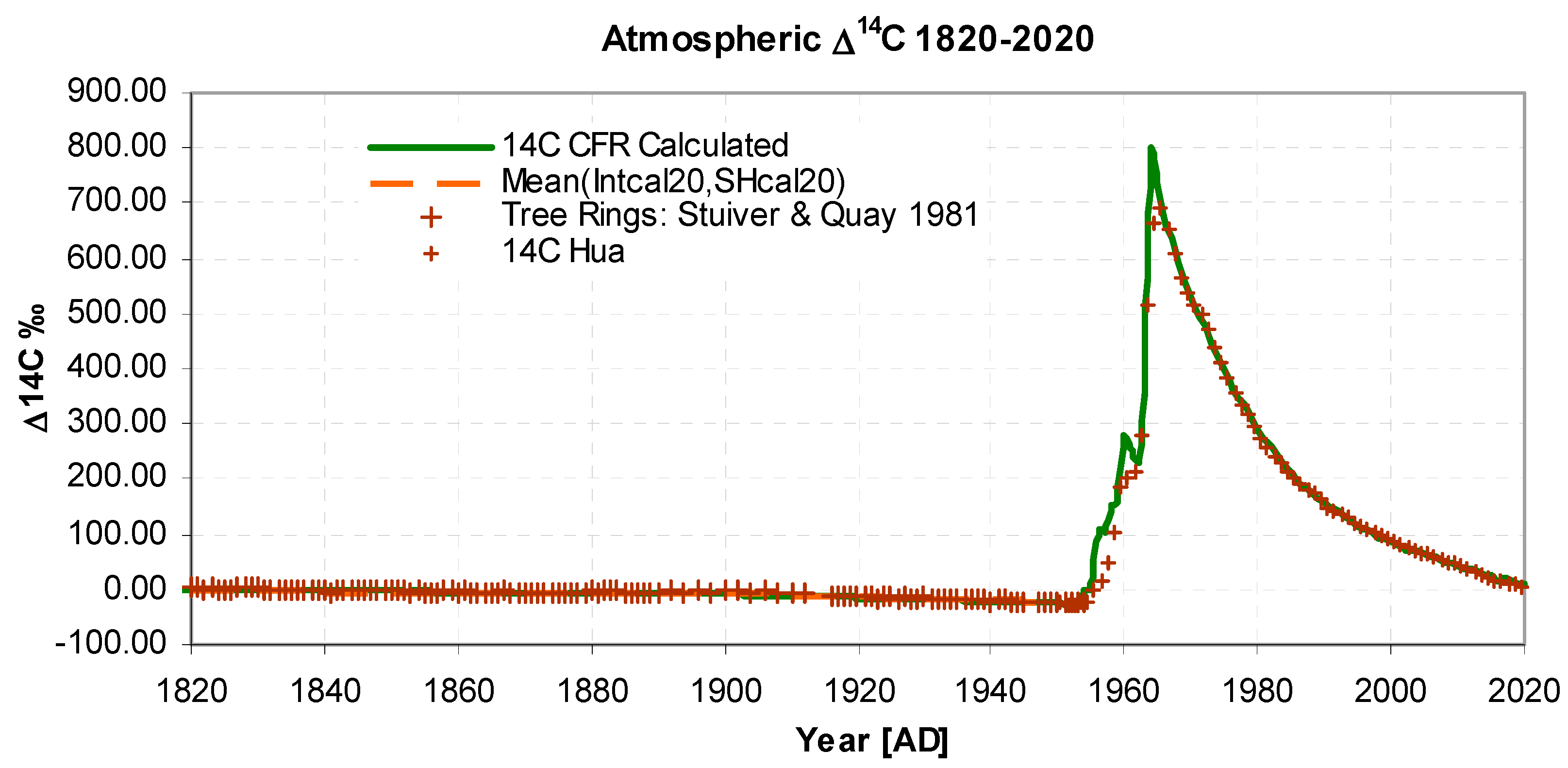
- There is a continuous CO2 outflow AOUT, from the atmosphere to a global carbon mixing reservoir, the flow being proportional to the CO2 atmospheric level, ACO2, as listed (Data Ref 1,2). The constant of proportionality is the inverse turnover time, T (IPCC 2013 Glossary); it determines the initial rate of fall of the 14C bomb pulse, (Figures 2 and 3), and is a solution parameter.
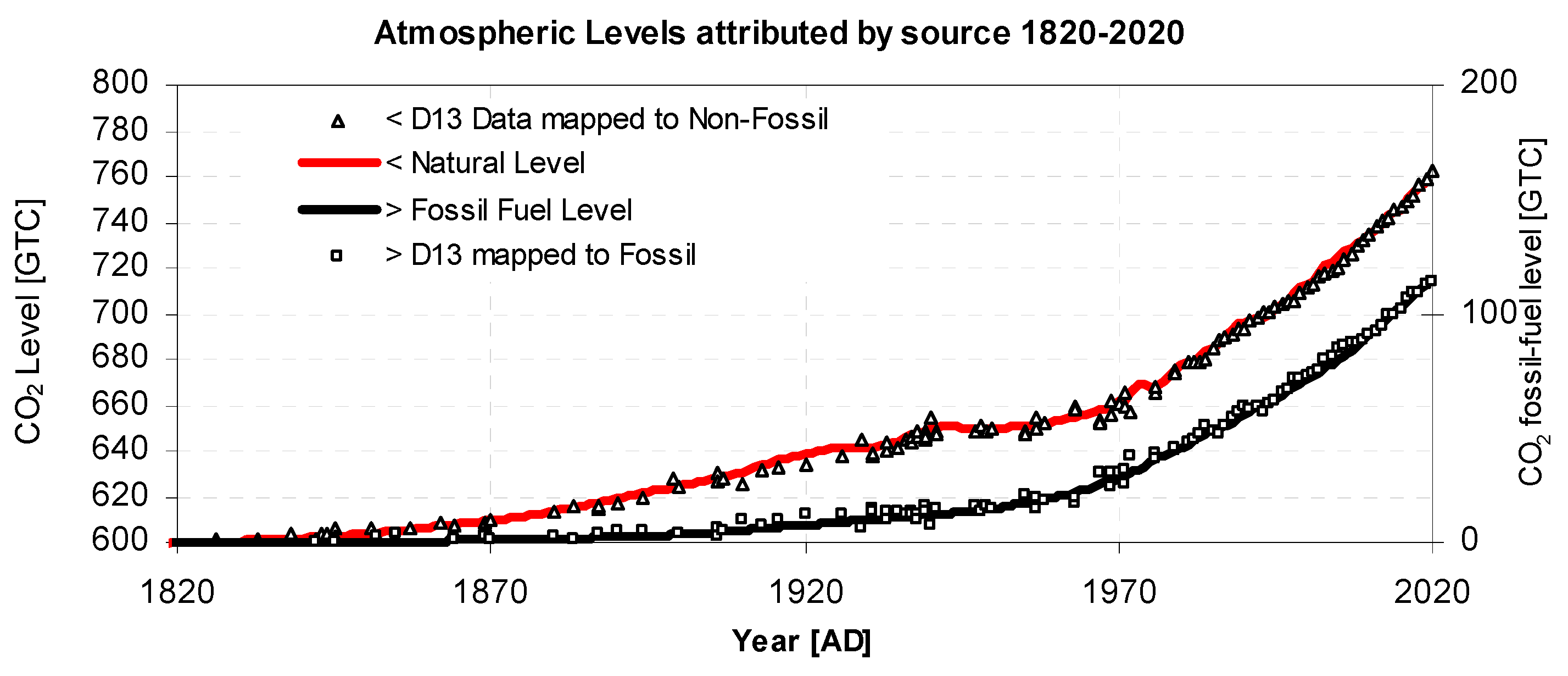
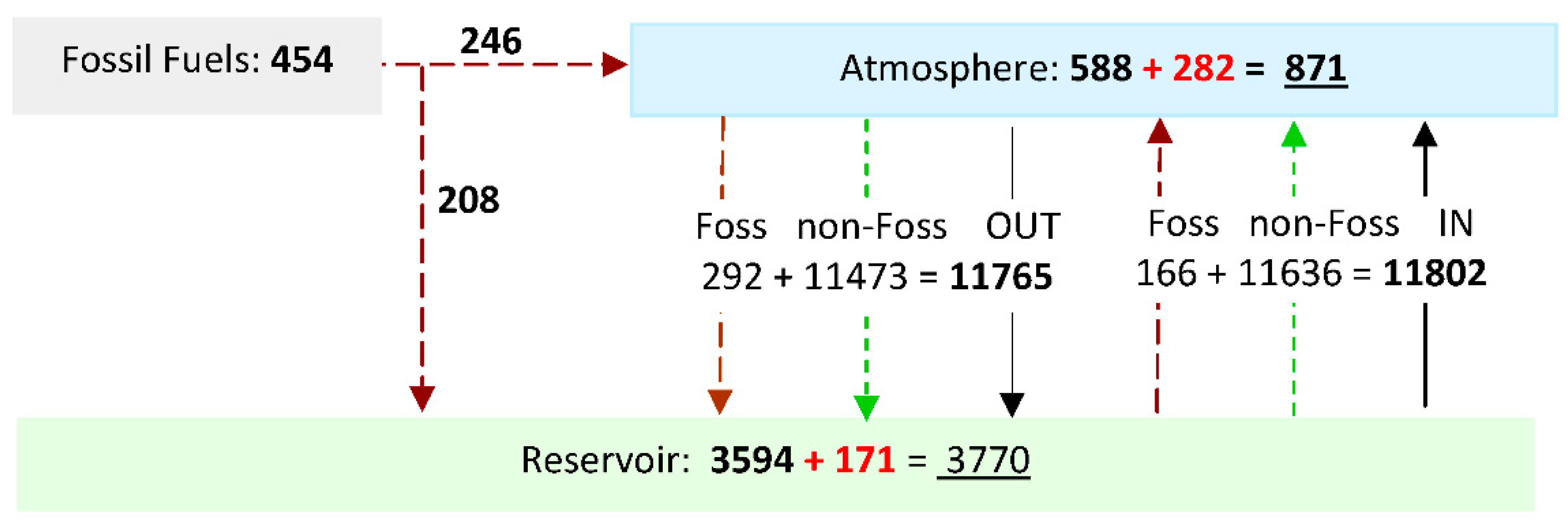
- Carbon is returned to the atmosphere from the reservoir via an inflow of CO2. The amount returned, AIN, is calculated by "balancing the budget" of outflow with the known atmospheric growth of CO2, Δ(ACO2) and fossil fuel emissions input AFF, as shown in Figure 1. CO2 inflow from a reservoir in which atmospheric CO2 has previously accumulated hinders the fall in value of 14C. Hence it predominantly determines the shape of the tail of the 14C bomb pulse, the rate of fall of δ13C, and recent δ13C levels, see Figure 3, Figure 4 and Figure 5. Note that some inflows are roughly independent of atmospheric CO2 level (e.g., fire / respiration) while others may be dependant upon pressure difference (e.g., oceanic flux). Both types of inflow are computed within this balanced budget method. The relative reservoir size, RCO2, is a solution parameter.
- Total fossil fuel emissions inflows (CO2FF) are derived from known listings (Data Ref 3). A portion of the inflow, as described by an Airborne Factor, AF, is directly mixed into the atmosphere, while the remaining portion (1-AF) is absorbed directly by the reservoir. This does not imply the absorption is instantaneous, because each cycle is annual. AF is a solution parameter. It predominantly determines the shape of the tail of the 14C bomb pulse, the rate of fall of δ13C, and recent δ13C levels Figure 3, Figure 4 and Figure 5.
- Inflow of 14CO2 from known listed atmospheric atomic weapon detonations B14, (Data Ref 5) are assumed to be linearly related to the bomb yield. The conversion factor Yb (14C [in 1820 background units] per megaton) is a solution parameter. It has the main effect of scaling the bomb pulse portion of the graph after 1960, Figure 3.
- Isotopic 13C and 14C concentrations are calculated using Dalton’s mixing laws, see Appendix B. Fractionation is considered negligible at the reservoir-atmosphere boundary so fractionation factors are implicitly unity. The isotopic equilibrium is identical for 13C ,14C and 12C hence there is no Revelle exception. In accounting for isotopic concentration, it is not necessary to explicitly embed Stuiver's attenuation factor, Suess dilution or a general Revelle factor (see Radiocarbon above), because they are implicitly represented. The initial values i.e. δ13Cinit, Δ14Cinit , determine the initial level of the curves in Figure 3 and Figure 4 and for δ13CFF determine the curve slopes in Figure 4 and Figure 5. δ13Cinit, Δ14Cinit and fossil fuel δ13Cff content are solution parameters.


Results
6. Discussion
7. Conclusions
Acknowledgments
Appendix A. Notes on Tans 1993 and Tans 2022
Appendix B. Notes on Isotopic Mixtures and Radiocarbon Levels
Appendix C. Implementation
Appendix D. Attenuation Factor of 14C and 13C
References
- Archer, D et al. 2009. Atmospheric lifetime of fossil fuel carbon dioxide. Annu. Rev. Earth Planet. Sci., 2009. 37:117–134.
- Arnold J.R. & Anderson E.C. 1957. The Distribution of Carbon-14 in Nature. Tellus IX 1957.
- Ballantyne, A. P et al. 2012. Increase in observed net carbon dioxide uptake by land and oceans during the last 50 years. Nature, 488, 70–72.
- Bauska et al. 2014 Carbon isotopes characterize rapid changes in atmospheric carbon dioxide during the last deglaciation. PNAS | March 29, 2016 | vol. 113 | no. 13 | 3465–3470.
- Bengston et al. 2020 Lower oceanic δ13C during the last interglacial period compared to the Holocene. Clim. Past, 17, 507–528. [CrossRef]
- Bindoff, N.L. et al. 2013, Detection and Attribution of Climate Change: from Global to Regional. Climate Change 2013: The Physical Science Basis. WG1 AR5, IPCC, Cambridge University Press.
- Bolin B, Eriksson E. 1959. Changes in the carbon dioxide content of the atmosphere and sea due to fossil fuel combustion. In Rossby Memorial Volume, ed. B Bolin:130-42. New York: Rockefeller Institute Press, Oxford University Press. Number of 130-42 pp.
- Broecker W. & Peng T, 1994. Stratospheric contribution to the global bomb radiocarbon inventory: Model versus observation.
- Bush S.E. et al 2007. Sources of variation in d13C of fossil fuel emissions in Salt Lake City, USA , Applied Geochemistry 22 (2007) 715–723.
- Canadell, J.G et al. 2021: Global Carbon and other Biogeochemical Cycles and Feedbacks. In Climate Change 2021: The Physical Science Basis. WG1 AR6 IPCC, [Masson-Delmotte V et al.]. Cambridge University Press, pp. 673–816. [CrossRef]
- Ciais, P. et al, 2013. Carbon and Other Biogeochemical Cycles. Chapter 6. Climate Change 2013: The Physical Science Basis. [Stocker et. al] WG1 AR5 IPCC 2013.
- Ciais, P. et al, 1995. Partitioning of ocean and land uptakeof CO2 as inferred by d13C measurements from the NOAA Climate Monitoring and Diagnsotics Laboratory Global Air Sampling Network, Journal of Geophysical Research Vol. 100 D3, 5051-5070, March 20, 1995.
- Flato, G.et al., 2013: Evaluation of Climate Models. Climate Change 2013: The Physical Science Basis. WG1 AR5, IPCC. Cambridge University Press, UK and USA.
- Friedlingstein P. et al, 2021. Global Carbon Budget 2021, Earth Syst. Sci. Data, 14,1917-2005, 2022. [CrossRef]
- Gruber, N. et al. 2019. The oceanic sink for anthropogenic CO2 from 1994 to 2007. Science 363, 1193–1199 (2019).
- Harde H. 2017. Scrutinizing the carbon cycle and CO2 residence-time in the atmosphere , Global and Planetary Change 152 (2017) 19–26.
- Harvey Danny LD 2000, Global Warming: The Hard Science, Pearson Education Limited, UK. ISBN 0582-38167-3.
- Hesshaimer, V et al. 1994, Radiocarbon evidence for a smaller oceanic carbon dioxide sink than previously believed. Nature Vol 370 21 July 1994.
- Hua Q. et al. Atmospheric Radiocarbon For The Period 1950–2019, Radiocarbon Vol 0 Nr00 2021. Submitted. Private Communication.
- IPCC 2013: Glossary. Climate Change 2013: The Physical Science Basis. [Stocker et. al] WG1 AR5 IPCC 2013.
- IPCC, 2021: Annex II: Models [Gutiérrez, J M., A.-M. Tréguier (eds.)]. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte V. et al]. Cambridge University Press, 2087–2138. [CrossRef]
- Joos F. 1994. , Imbalance in the Budget, Nature Vol 370 21July 1994.
- Kutschera, W., (2013) Applications of accelerator mass spectrometry. International Journal of Mass Spectrometry 349, 203-218.
- Levin I. et al. 2010 Observations and modelling of the global distriution and long-term trend of atmospheric 14CO2. Tellus B, 62: 26-46 2010.
- Marcott A S et al., 2014. Centennial-scale changes in the global carbon cycle during the last deglaciation. Nature,Vol 514,30October 2014.
- Paola, C. & Leeder M, Simplicity versus complexity. Nature 469, 38–39(2011).
- Quirk T. 2021 Suggested method using inversion formulae for d13C. Private Communication 2021.
- Revelle, R & Suess H, 1957. Carbon Dioxide Exchange Between Atmosphere and Ocean and the Question of an Increase of Atmospheric CO2 during the Past Decades. Tellus IX(1957).1.
- Rubino, M et al 2013. A revised 1000 year atmospheric δ13C-CO2 record from Law Dome and South Pole, Antarctica. Journal of Geophysical Research: Atmospheres, 118(15), pp.8482-8499. 2013.
- Physics, Division of Nuclear Physics. Sweden. 2011.
- Stenström K. E. 2011. A guide to radiocarbon units and calculations, Lund University, Department of Stuiver, M. & Quay, P.D. 1981. Atmospheric 14C changes resulting from fossil fuel CO2 release and cosmic ray flux variability. Earth and Planetary Science Letters, 53 (1981) 349-362.
- Stuiver M & Polach H A. 1977 Discussion: Reporting of 14C Data, Radiocarbon, Vol19 No 3 1977 P355-363.
- Stuiver, M et al. 1998 "Intcal98 Radiocarbon Age Calibration, 24,000-0 Cal Bp", Radiocarbon, Vol. 40, No. 3, 1998, P.1041-1083.
- Suess, H.E. 1955 Radiocarbon concentration in modern wood. Science 122, 415.
- Svetlik I. 2010 Estimation of Long-Term Trends in the Tropospheric 14CO2 Activity Concentration. Proceedings of the 20th International Radiocarbon Conference, edited by A J T Jull, RADIOCARBON, Vol 52, Nr 2–3, 2010, p 815–822.
- Tans, P., Berry J., Keeling R. 1993. Oceanic 13C/12C Observation: A new window on ocean CO2 uptake. Global Biogeochemical Cycles Vol 7 No2 P353-368) June 1993.
- Tans P. 2022. Reminiscing On The Use And Abuse Of 14c And 13c In Atmospheric Co2. Radiocarbon, Vol 64, Nr 4, 2022, p 747–760. [CrossRef]
- Zeebe, R.E. and Wolf-Gladrow, D.A. 2001. CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Gulf Professional Publishing. http://store.elsevier.com/product.jsp?isbn=9780444509468.
- Zeng J. et al. 2020. Global terrestrial carbon fluxes of 1999–2019 estimated by upscaling eddy covariance data with a random forest . Scientific Data (2020) 7:313. [CrossRef]
| Parameter | Symbol | Value | SD ± |
| Turnover Time = ACO2 /AOUT | T | 14.9 yr | 1.7 |
| Fossil-fuel Inflow Fraction | AF | 0.54 | 0.11 |
| Nuclear Bomb Yield* | Yb | 1.60 | 0.1 |
| Rel. Reserve Size | RCO2 | 6.1 | 1.4 |
| 14C Pre-industrial | Δ14Cinit | -3.0‰ | 10‰ |
| 13C Pre-industrial | δ13Cinit | -6.7‰ | 0.2‰ |
| 13C fossil fuel | δ13Cff | -20.8‰ | 4‰ |
| Duration | 1750 - | 2020 | 1850 - | 2020 | 1960 - | 2020 | ||
| GTC | % | GTC | % | GTC | % | |||
| CO2FF Supplied | ||||||||
| CO2FF delivered to Atmos. (AF) | 246 | 54 | 245 | 54 | 202 | 45 | ||
| CO2FF delivered to Rsvr. (1-AF) | 208 | 46 | 207 | 46 | 170 | 38 | ||
| CO2FF Total | 454 | 100 | 452 | 100 | 372 | 82 | ||
| CO2FF Destination | ||||||||
| CO2FF present in Atmos. | 120 | 26 | 120 | 26 | 99 | 26 | ||
| CO2FF present in Rsvr. | 333 | 74 | 333 | 74 | 273 | 72 | ||
| CO2FF Total | 454 | 100 | 452 | 100 | 382 | 100 | ||
| Atmospheric Growth | ||||||||
| Atmos. CO2 Growth due to CO2FF | 120 | 43 | 120 | 44 | 99 | 49 | ||
| Atmos. CO2 Growth due to non-Foss* | 162 | 57 | 152 | 56 | 104 | 51 | ||
| Atmospheric Growth Total | 282 | 100 | 272 | 100 | 204 | 100 | ||
| Reservoir Growth | ||||||||
| Reservoir Growth due to CO2FF | 333 | 333 | 273 | |||||
| Reservoir Growth due to non-Foss* | -158 | -143 | -97 | |||||
| Reservoir Growth Total | 171 | 185 | 171 | |||||
| Atmospheric Outflow | ||||||||
| Atmos Outflow CO2FF | 292 | 291 | 245 | |||||
| Atmos Outflow non-Foss | 11473 | 7459 | 2806 | |||||
| Atmospheric Outflow Total | 11765 | 7750 | 3051 | |||||
| Reservoir Outflow | ||||||||
| Reservoir Outflow CO2FF | 166 | 166 | 143 | |||||
| Reservoir Outflow non-Foss | 11636 | 7606 | 2907 | |||||
| Reservoir Outflow Total | 11802 | 7772 | 3050 | |||||
| CO2FF rel. to CO2 atmos. 2020† (%) | 120/876 | 13.7 | 120/876 | 13.7 | 99/876 | 11.3 | ||
| Average Annual Flux from Reservoir | 43.77 | 45.79 | 50.96 | |||||
| Average Annual Flux to Reservoir | 43.63 | 45.67 | 50.99 |
Data References
- Institute for Atmospheric and Climate Science (IAC), CO2 Mean Global AD0 to AD2014 ftp://data.iac.ethz.ch/CMIP6/input4MIPs/UoM/GHGConc/CMIP/yr/atmos/UoM-CMIP-1-1- 0/GHGConc/gr3-GMNHSH/v20160701/ mole_fraction_of_ carbon_dioxide_in_air_input 4MIPs_GHGConcentrations_CMIP_UoM-CMIP-1-1-0_gr3-GMNHSH_0000-2014.csv
- NOOAA GML. Accessed 04-March-2022. https://gml.noaa.gov/ccgg/trends/gl_data.html File: https://gml.noaa.gov/webdata/ccgg/trends/co2/co2_annmean_gl.txt
- Global Carbon Budget: National_Carbon_Emissions_2021v0.4.xlsx Historical Budget, Global Fossil Emissions Visited 04 March 2022.Friedlingstein et al (2021),
- World Data Service for Paleoclimatology, Boulder and NOAA Paleoclimatology Program, National Centers for Environmental Information (NCEI) https://www1.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/law/law2018d13c-co2.txt, https://doi.org/10.25919/5bfe29ff807fb
- UNSCEAR: United Nations Scientific Committee on the Effects of Atomic Radiation 2000 Report To The General Assembly. Volume I: Sources. Annex C: Exposures To The Public From Man-Made Sources Of Radiation 207 Sources And Effects Of Ionizing Radiation. . Table 4. Annual Fission And Fusion Yields.
- Calib: INTCAL20/SGCAL20. Stuiver, M. et al, 2022 CALIB 8.2 [WWW program] at http://calib.org, accessed 2022-3-4 Rev 8.1.0 intcal20.14c, shcal20.14c
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




