Submitted:
06 January 2023
Posted:
10 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Literature search
2.1.1. Databases and key terms searched
2.1.2. Inclusion criteria
2.1.3. Search process and study selection
2.1.4. Data Extraction and Analysis
3. Results
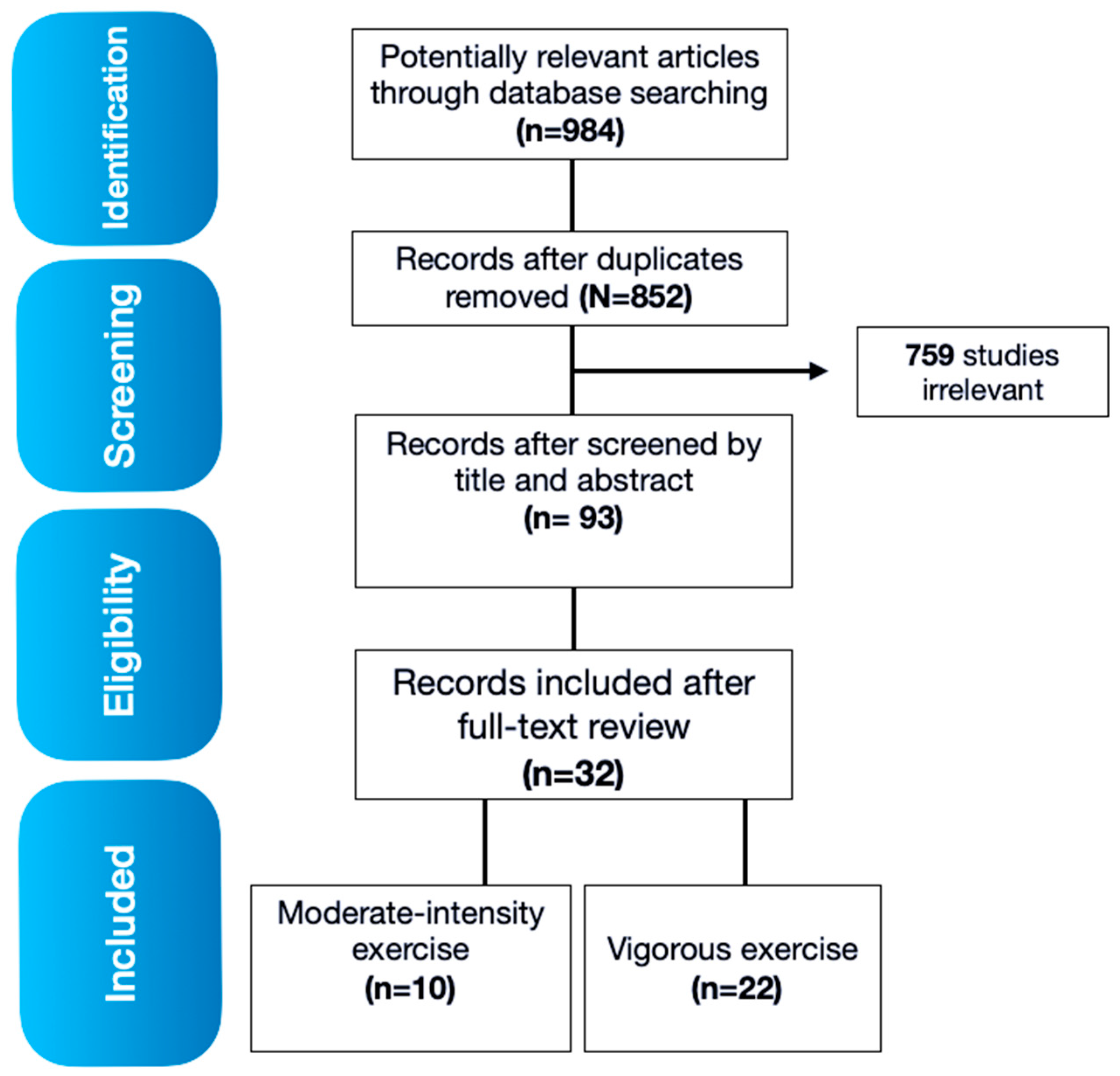
3.1. Search results
3.2. Effects of moderate physical activity intensity on immune responses in female and male subjects
| Author, year, country [Reference #] |
Study goal | Exercise protocol [Exercise type] |
N subjects [Sex] |
Key findings |
|---|---|---|---|---|
| Nieman, 1990, USA [29] |
Randomized controlled trial to investigate the relationship between improvement in cardiorespiratory fitness, changes in natural killer (NK) cell number and activity, and acute upper respiratory tract infection (URTI) symptomatology. | A randomly controlled 15-wk exercise training (ET) study (five 45-min sessions/wk, brisk walking at 60% heart rate reserve). Conducted using 2 (exercise and non-exercise groups) × 3 (baseline, 6-, and 15-wk testing sessions). [brisk walking] |
36 mildly obese sedentary women (aged 34.4 +/- 1.1 years) | The ET group was found to have significantly fewer URTI symptom days/incident than the nonexercised group. Moderate ET was associated with elevated NK cell activity after six but not 15 weeks, and reduced URTI symptomatology in comparison to a randomized, sedentary control group. |
| Nieman, 1993, USA [30] |
Randomized controlled trial to investigate the relationship between cardiorespiratory exercise, immune function, and URTI in elderly women. | Sedentary women randomized to a 12-week moderate intensity (30- to 40-min/day x 5 days/week) walking program or stretching (45 min/day x 5 days/week) in fall season. [brisk walking or stretching] |
32 sedentary women (aged 73.4 +/-1.2 years); 12 highly conditioned women (aged 72.5 +/- 1.8 years) | URTI incidence 8% in highly conditioned, 21% in walkers, and 50% in controls. |
| Nieman, 1998, USA [31] |
Randomized controlled trial to investigate the effect of ET and/or moderate energy restriction on innate and adaptive immunity, NK cell activity, and mitogen-stimulated lymphocyte proliferation. | 12-week (five 45-min walking sessions/wk at 60-75% maximum heart rate) and/or moderate energy restriction (4.19-5.44 MJ or 1,200-1,300 kcal x d(-1)) randomized to one of four groups: control, exercise, diet, exercise + diet. [brisk walking] |
N = 91 obese women, aged 45.6 +/- 1.1 yr., body mass index 33.1 +/- 0.6 kg x m(-2) | Days with symptoms of URTI were reduced in subjects in the exercise group, relative to subjects in the non-exercise groups. Energy restriction and weight loss increased mitogen-stimulated lymphocyte proliferation but had no effect on NK cell activity. |
| Chubak, 2006, USA [32] |
Randomized controlled trial to investigate the effect of a moderate-intensity, year-long exercise program on the risk of colds and other URTI in postmenopausal women. | Subjects randomized to 12 months of moderate intensity exercise (45 min/day x 5 days/week) or stretching control (45 min/day x 1 day/week) [brisk walking or stretching] |
115 overweight and obese, sedentary, postmenopausal women (aged 60.7 +/- 6.9 years) | URTI incidence was 30% in exercise vs. 48% in controls. |
| Barrett, 2012, USA [34] |
Randomized controlled trial to investigate potential preventive effects of meditation or exercise on incidence, duration, and severity of acute respiratory infection (ARI) illness | Randomized to 1 of 3 study groups: 8-week training in mindfulness meditation, matched 8-week training in moderate-intensity sustained exercise, or observational control. [brisk walking or jogging] |
149 (82% female, 94% white, mean age 59.3 ± 6.6 years) | Both global severity and total days of illness (duration) displayed lower trends the exercise group. |
| Barrett, 2018, USA [35] |
Randomized controlled trial to investigate preventive effects of meditation and exercise on ARI illness. | Randomized to 1) 8-week behavioral training in mindfulness-based stress reduction (MBSR); 2) matched 8-week training in moderate intensity sustained exercise; or 3) observational waitlist control. [brisk walking or jogging] |
390 male and female (76% female) (mean age 49.6 +/- 11.6 years) | There were 112 ARI episodes and 1045 days of ARI illness, compared to 120 episodes and 1010 illness days in the moderate intensity sustained exercise group, and 134 episodes with 1210 days of ARI illness for controls. |
| Fondell, 2011, Sweeden [36] |
Epidemiologic study to investigate differences in URTI risk between physically inactive and moderately active adults. Population-based prospective cohort study with baseline questionnaire on physical activity; illness symptoms assessed every 3 weeks for a follow-up period of 4 months. | Three 24-h physical activity recalls per evaluation were obtained and averaged to quantify total moderate-vigorous activity (> or =3.0 MET) [Several] |
1509 (725 male, 784 female) aged 20-60 yr. | High levels of physical activity were associated with an 18% reduced risk of self-reporting URTI compared with low levels of physical activity. |
| Matthews, 2002, USA [33] |
Epidemiologic study (observational) to investigate differences in URTI risk between physically inactive and moderately active adults. | Reported URTI events at 90-d intervals over 12-month of follow-up (5 evaluations) were measured. Three 24hr physical activity recalls per evaluation were obtained and averaged to quantify total moderate-vigorous activity (> or = 3.0 metabolic equivalent, MET) [Several] |
547 healthy adults (49% female) aged 20-70 yr. | Male and female subjects reported mean and (SD) of 1.2 (1.4) and 1.2 (1.2) URTI events per year, respectively. This effect was stronger in males although at similar expenditure levels, risk was reduced by about 20% in both sexes. |
| Zhou, 2018, China [37] |
Epidemiologic study (cross-sectional) to investigate associations between the frequency of leisure-time exercise, cigarette smoking status, and frequency of the common cold in a cold area. Participants retrospectively reported frequency of illness and physical activity over the past year | [Any type of moderate-intensity exercise] | 1413 male and female adults (aged 38.9 +/- 9.04 years) 44.4% of them were male |
A high frequency of leisure-time exercise (≥3 days/week) was associated with a 26% reduced risk of having at least one episode of the common cold compared with a low frequency group (< 4 days/month). |
| Nieman, 2011, USA [38] |
Epidemiologic study to investigate URTI symptoms and severity in a heterogeneous group of community adults with various physical activity and fitness levels. | Assessment over 12 weeks during the winter and fall seasons while monitoring URTI symptoms and severity using the Wisconsin Upper Respiratory Symptom Survey and reported frequency of aerobic activity. [Aerobic activity (mild)] |
1002 adults (ages 18-85 years, 60% female, 40% male) | The number of days with URTI during the 12-week period was significantly reduced by 43% in subjects reporting ≥ 5 days/week aerobic exercise compared to those who were largely sedentary (≤ 1 day/week), and by 46% when comparing subjects in the high versus low fitness. URTI severity and symptomatology were also reduced in the physical fitness group. |
3.3. Effects of vigorous physical activity intensity on immune responses in females and males
| Author, year, country [Reference #] |
Study goal | Exercise protocol [Exercise type] |
N subjects [Sex] |
Key findings |
|---|---|---|---|---|
| Peters, 1983, South Africa [39] |
Prospective study of the incidence of symptoms of upper respiratory tract infections (URTI) in 150 randomly selected runners taking part of the 1982 Two Oceans Marathon in Cape Town, and compared incidence in individually matched controls who did not run. | Participants reported 2-week recall of illness symptoms after 56km race [Ultramarathon] |
41 ultramarathon runners and 124 controls (aged 18-65 years) [No sex reported] |
Illness incidence was 2-times higher in runners (after race) vs. controls. |
| Nieman, 1993, USA [45] |
Epidemiologic study of Los Angeles Marathon applicants to investigate the relationship between self-reported infectious episodes and training. | Participants were asked to report illness symptoms 2 months before and 1 week after March 42.2-km race. [Marathon] |
2311 marathon runners (aged 36.9 + 0.2 years) [male: 1941 and female: 370] |
Runners experienced increased odds for infectious episodes during heavy training or following a marathon race. |
| Raysmith, 2016, multiple countries [43] |
Retrospective cohort to investigate the impact of training modification on achieving performance goals and illness. | Participants reported illness symptoms during 6 months across five international competition seasons. [Track and Field] |
33 international track and field athletes. [No sex reported] |
Illness incidence was 23%; one-half of illnesses occurred 2 months before competition. |
| Heath, 1991, USA [46] |
Prospective cohort study. To investigate illness patterns in a cohort of 530 male and female runners who completed a monthly log for 12 months. | Participants reported training log and illness symptoms every month for 1 year. [Running] |
530 runners (average age 39.4 years) [male: 447 and female: 83] |
Participants running > 485 miles/year (780 km/year) displayed an increased risk of illness. The average number of events per person per year was 1.2, and slightly higher in females than males (1.2 for males vs. 1.3 for females). |
| König, 2000, Germany [40] |
Epidemiological study to investigate the association between incidence of illness and exercise, stress and sleep deprivation. | Participants retrospectively reported illness episodes over the past 12 months. [Athletics] |
852 German athletes (aged 23.6 + 9.5 years) [No sex reported] |
Illness incidence was 2 times higher in participants conducting endurance sports. |
| Alonso, 2012, Korea [50] |
Epidemiological study to investigate the incidence and characteristics of newly incurred injuries and illnesses the 13th International Association of Athletics Federations World Championships in Athletics 2011 in Daegu, Korea. | Medical staff reported illness symptoms during competition event (<4 weeks) [Athletics] |
1,851 registered athletes (1,063 males and 964 females). | No differences in illness and time-loss illness incidence were reported between male and female athletes. |
| Timpka, 2017, China [59] |
Cohort study to investigate pre-participation predictors of injury and illness during the 15th International Association of Athletics Federations World Athletics Championships in Beijing (22–30 August 2015). | Athletes answered pre-participation health questionnaire including individual pre-participation information (personal characteristics and health status during the month preceding the championship). [Athletics] |
307 athletes (Female 135 and male 172) | No sex difference found in athletes reporting an illness symptom. |
| Alonso, 2010, Germany [51] |
Epidemiological study to investigate the frequency and characteristics of sports injuries and illnesses incurred during the 12th International Association of Athletics Federations World Championships in Athletics 2009 in Berlin, Germany. | Medical staff reported illness symptoms during competition event (<4 weeks) [Athletics] |
2,378 athletes (1,301 males and 1,077 females) | A total of 382 injury and illness report forms were returned. More illnesses were reported for female than for male athletes. |
| Gleeson, 2013, UK [44] |
Prospective cohort to investigate the effect of training load on URTI incidence in men and women engaged in endurance-based physical activity during the winter. | Participants followed for 4 months in winter; reported weekly illness symptoms and training. Exercised 3-6 h/week (low), 7-10 h/week (medium) or ≥ 11 h/week (high). [Endurance training] |
75 endurance trained university students (aged 18-35 years) [male and female number not reported] |
The high and medium intensity groups reported higher rates of URTI episodes than the low intensity group. |
| Rama, 2013, Portugal [41] |
Prospective cohort study to investigate the occurrence of episodes of upper respiratory symptoms (URS), over a winter swimming season. | Participants followed for 7 months in winter; reported daily illness symptoms. [Swimming] |
19 elite swimmers vs. 11 nonathlete controls (aged 17.6 + 1.0 years) [No sex reported] |
67% of URS episodes occurred during high volume training in swimmers vs. no illness in control at same time points. |
| Hellard, 2015, France [42] |
Prospective cohort study to investigate the relationship between sport training and the risk of common illnesses: URTI and pulmonary infections, muscular affections, and all-type pathologies in highly trained swimmers. | Participants followed for 4 years; monitored weekly for illness. [Swimming] |
28 elite swimmers (aged 1630 years) [No sex reported] |
Illness increased 1.08 times for every 10% increase in resistance training and 1.10 times for every 10% increase in high-load training. |
| Prien, 2017, Russia [60] |
Comparative study to investigate the frequency and characteristics of injuries/illnesses in the 4 weeks prior to, and during the Fédération Internationale de Natation (FINA) World Championships in 2015. | Athletes answered a retrospective questionnaire, and medical staff reported injuries/illnesses prospectively. [Swimming] |
2413 athletes who competed at the FINA World Championships 2015 in one of the six aquatic disciplines. Both female (n=1262) and male athletes (n=1151) participated; age ranged from 10-40 years with an average of 22.1 years (SD=4.5) | Around a quarter (26.1%) of athletes reported health complaints in the 4 weeks prior to the championships. |
| Spence, 2007, Australia [47] |
Prospective cohort to investigate the incidence, pathogenic etiology, and symptomatology of acute URTI during a 5-month training and competition period. | Participants followed for 5 months in summer/autumn; reported daily illness symptoms. [Triathlon and cycling] |
17 elite male and female triathletes and cyclists (age 18–34 yr.), 30 male and female recreationally competitive triathletes and cyclists (age 19–34 yr.), and 18 male and female untrained sedentary controls who did less than 60 min walk (age 19–29 yr.) | Elite athletes had higher rates of illness than recreationally competitive athletes and sedentary controls. |
| Svendsen, 2015, multitple countries [48] |
Prospective cohort study to investigate whether participating in a cross-country skiing stage race (Tour de Ski) affects subsequent illness incidence, training, and race performance. | Participants followed for 8 years; reported illness symptoms daily for 10 days after the Tour de Ski race. [Cross-country skiing] |
42 male and female elite cross-country skiers (aged 24 + 4 years) | Illness incidence was 3 times higher in skiers who raced the Tour de Ski vs. non-competing skiers. |
| Drew, 2018, Brazil [49] |
Retrospective Cohort. To investigate the prevalence of illness symptoms, poor sleep quality, poor mental health symptoms, low energy availability and stress-recovery state in an Olympic cohort late in the 3 months prior to the Summer Olympic Games. | 3 months before competition, participants reported illness symptoms during a 1-month time period. [Summer Olympic sports] |
132 elite athletes preparing for the Olympics (male, n=47, age 25.8±4.1 years; female, n=85, age 24.3±3.9 years) | Illness symptoms were found in 100% athletes. Risk factors were female sex, low energy availability, and a combination of anxiety and stress-recovery states. |
| Mountjoy, 2015, Spain [52] |
Epidemiological study to investigate injuries among athletes of aquatic disciplines in the 4 weeks prior to and during the 2013 FINA World Championships. | Medical staff reported illness symptoms during competition event (<4 weeks) [Water sports] |
1,110 (500 males and 610 females) Mean age: 22.5 (4.35) |
Significantly more females than males reported physical complaint prior to the Championships. |
| Mountjoy, 2010, Italy [53] |
Epidemiological study to investigate the frequency and characteristics of injuries and illnesses occurring during the 13th FINA World Championships 2009 | Medical staff reported illness symptoms during competition event (<4 weeks) [Water sports] |
2,592 athletes (1,293 females and 1,299 males) | Female athletes had a higher risk of injury than male athletes. |
| Engebretsen, 2010, Canada [58] |
Epidemiological study to investigate the frequencies and characteristics of injuries and illnesses during the XXI Winter Olympic Games in Vancouver (2010) | Medical staff reported illness symptoms during the competition event (<4 weeks) [Winter Olympic sports] |
2,567 athletes (1,045 females and 1,522 males) | There was a significantly higher proportion of illness in female athletes compared to male athletes. |
| Soligard, 2015, Russia [55] |
Epidemiological study to investigate injuries and illnesses that occurred during the XXII Olympic Winter Games, held in Sochi in 2014 | Medical staff reported illness symptoms during competition event (<4 weeks) [Winter Olympic sports] |
2,780 athletes (1,121 females and 1,659 males) | Female athletes were at significantly higher risk of contracting an illness than male athletes. |
| Palmer-Green, 2015, Russia [56] |
Observational prospective cohort study to investigate the prevalence, severity, nature, and causes of athlete injuries and illnesses in the Great Britain Olympic Team during the Sochi 2014 Winter Olympic Games | Medical staff reported illness symptoms during competition event (<4 weeks) [Winter Olympic sports] |
56 Athletes (33 males and 23 females) members of the Great Britain Olympic Team (13 sports represented). |
There were more illnesses sustained by female athletes compared to male athletes. |
| Engebretsen, 2013, UK [57] |
Epidemiological study to investigate injuries and illnesses that occurred during the Games of the XXX Olympiad, held in London in 2012 | Medical staff reported illness symptoms during the competition event (<4 weeks) [Summer Olympic sports] |
10,568 athletes (4,676 females and 5,892 males) | Overall, female athletes suffered higher rates of illnesses than males. |
| Soligard, 2017, Brazil [54] |
Epidemiological study to investigate the recorded daily incidence of athlete injuries and illnesses through the reporting of all National Olympic Committee medical teams, and in the polyclinic and medical venues in Rio de Janeiro 2016. | Medical staff reported illness symptoms during competition event (<4 weeks) [Summer Olympic sports] |
11,274 athletes (5,089 female, 6,185 male) participating in the Games of the XXXI Olympiad, hosted in Rio de Janeiro. | Women were at significantly higher risk of contracting an illness than men. |
3.4. Effects of exercise in immune cell function
| Author, year [Reference #] |
Study goal | Key findings |
|---|---|---|
| Kraemer, 1996 [14] |
Examine the impact of heavy-resistance exercise-induced elevations of plasma cortisol on circulating leukocyte counts. | Significant acute increases in total leukocyte counts (but no differential counts) in response to heavy-resistance exercise that does not significantly elevate plasma cortisol. |
| Potteiger, 2001 [15] |
Examine white blood cell counts (WBC), immunoglobulin (IgA, IgG, IgM) levels, and T-cell proliferation following acute resistance training. | WBCs were significantly elevated in subjects 1.5 and 3 hours post exercise compared with pre- and immediately post-exercise. T-cell proliferation was significantly decreased at 3 hours post-exercise |
| Dohi, 2001 [16] |
Examine the influence of physical strength on lymphocyte proliferation after an acute bout of heavy resistance exercise | Squat exercise was associated with a decrease in lymphocyte responsiveness in the high strength but not in the low strength |
| Miles, 2003 [17] |
Examine whether the immune response to resistance exercise was associated with changes in workload or anaerobic exercise intensity | Exercise induced increases in NK, CD4+, CD8+ and B lymphocyte concentrations. |
| Ramel, 2003 [18] |
Examine the acute effects of submaximal resistance exercise on immunological and hormonal parameters | Total leukocytes, neutrophils, lymphocytes, and monocytes increased during exercise. T-helper cells returned to resting values after exercise, NK and T-suppressor cells decreased below resting values. The CD4/CD8 ratio decreased during exercise but increased during recovery. Resistance-trained participants tended to have lower T-helper cell counts before, during and immediately after exercise and a lower CD4/CD8 ratio during recovery than the non-resistance-trained. |
| Simonson, 2004 [19] |
Examine the effects of a single bout of resistance exercise on immune cell numbers. | Resistance exercise induced leukocytosis was due to an increase in circulating LY (natural killer cells increased most, CD4+/CD8+ ratio unchanged) and monocytes (MO). The transient, inconsequential immune cell population responses to resistance exercise are similar to those during aerobic activity |
| Natale, 2003 [20] |
Examine the effects of three different exercise protocols on blood leukocyte count during and following exercise | The peak aerobic and prolonged submaximal exercise induced similar alterations in cell counts. Both resistance and peak aerobic exercise resulted in a significantly longer-lasting decrease in the CD4+/CD8+ ratio than the submaximal exercise bout. |
| Ramel, 2004 [21] |
Examine noradrenaline concentrations, neutrophil counts, plasma antioxidants, and lipid oxidation products before and after acute resistance exercise. | Neutrophils, noradrenaline, fat soluble antioxidants, and lipid oxidation products increased after exercise. Neutrophil counts were related to higher concentrations of conjugated dienes. |
| Mayhew, 2005 [22] |
Examine the effect of varying rest intervals on leukocyte levels during heavy resistance exercise | Greater lymphocytosis and monocytosis following exercise. Serum creatine kinase (CK) activity was increased and correlated to lymphocytosis. |
| Peake, 2006 [23] |
Examined changes in markers of muscle damage and systemic inflammation after submaximal and maximal lengthening muscle contractions of the elbow flexors | Total leukocyte and neutrophil numbers, and serum TNFa1 receptor were elevated after both trials. Serum IL-6 was elevated 3h after submaximal contractions. Plasma myoglobin concentration and creatine kinase activity increased after both trials but were not significantly different between trials. |
| Ghanbari-Niaki, 2010 [24] |
Examined effects of circuit resistance exercise (CRE) on peripheral blood lymphocyte (PBL) AgRP mRNA expression and its concentrations in lymphocytes and plasma | CRE increased AgRP mRNA lymphocyte expression at all intensities. A higher and significant increase was found in the resistance exercise group. The CRE-induced lymphocyte AgRP expression was accompanied by elevations in plasma AgRP, glucose and GH levels as well as higher WBCs, lymphocytes and neutrophil counts. Lymphocyte AgRP and GH concentrations were significantly reduced |
| Mooren, 2012 [25] |
Examined the influence of a long-distance exercise on neutrophil apoptosis | After both marathon run and intensive laboratory exercise tests, neutrophil apoptosis was delayed. Neutrophils mitochondrial membrane potential and death receptor/ligand expression were not affected by exercise. Apoptosis delay was accompanied by enhanced intracellular calcium transients and decreased glutathione levels. |
| Ihalainen, 2014 [27] |
Examine the acute immune response (circulating levels of leukocytes, cytokines and adipocytokines) to maximal resistance and hypertrophic resistance exercise bouts. | Leukocytes (WBC) significantly increased immediately after exercise. Monocyte chemoattractant protein-1 (MCP-1) decreased and interleukin-1 receptor antagonist (IL-1ra) increased after exercise. |
| Jamurtas, 2018 [29] |
Examine the effects of HIIT on hematological profile and redox status compared with those following traditional continuous aerobic exercise (CET) | WBC increased after both exercise protocols immediately post-exercise. Both HIIT and CET increased uric acid after exercise. There were no significant changes for TBARS and catalase following either exercise protocol. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larrabee, R.C. Leucocytosis after violent Exercise. J Med Res 1902, 7, 76–82. [Google Scholar] [PubMed]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol Basis Dis 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; Medicine, A.C.o.S. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Lippi, L.; Folli, A.; Turco, A.; Zattoni, L.; Maconi, A.; de Sire, A.; Fusco, N. Integrating molecular biomarkers in breast cancer rehabilitation. What is the current evidence? A systematic review of randomized controlled trials. Front Mol Biosci 2022, 9, 930361. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol 2019, 19, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef]
- Bartlett, D.B.; Fox, O.; McNulty, C.L.; Greenwood, H.L.; Murphy, L.; Sapey, E.; Goodman, M.; Crabtree, N.; Thøgersen-Ntoumani, C.; Fisher, J.P.; et al. Habitual physical activity is associated with the maintenance of neutrophil migratory dynamics in healthy older adults. Brain Behav Immun 2016, 56, 12–20. [Google Scholar] [CrossRef]
- Bartlett, D.B.; Willis, L.H.; Slentz, C.A.; Hoselton, A.; Kelly, L.; Huebner, J.L.; Kraus, V.B.; Moss, J.; Muehlbauer, M.J.; Spielmann, G.; et al. Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: a pilot study. Arthritis Res Ther 2018, 20, 127. [Google Scholar] [CrossRef]
- Kruijsen-Jaarsma, M.; Révész, D.; Bierings, M.B.; Buffart, L.M.; Takken, T. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev 2013, 19, 120–143. [Google Scholar]
- Huff, W.X.; Kwon, J.H.; Henriquez, M.; Fetcko, K.; Dey, M. The Evolving Role of CD8. International journal of molecular sciences 2019, 20. [Google Scholar] [CrossRef]
- Minuzzi, L.G.; Rama, L.; Bishop, N.C.; Rosado, F.; Martinho, A.; Paiva, A.; Teixeira, A.M. Lifelong training improves anti-inflammatory environment and maintains the number of regulatory T cells in masters athletes. Eur J Appl Physiol 2017, 117, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.F.M.; Abaraogu, U.; Bourgois, J.G.; Dall, P.M.; Darnborough, J.; Duncan, E.; Dumortier, J.; Pavón, D.J.; McParland, J.; Roberts, N.J.; et al. Effects of Regular Physical Activity on the Immune System, Vaccination and Risk of Community-Acquired Infectious Disease in the General Population: Systematic Review and Meta-Analysis. Sports Med 2021, 51, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; de Sire, A.; Mezian, K.; Curci, C.; Perrero, L.; Turco, A.; Andaloro, S.; Ammendolia, A.; Fusco, N.; Invernizzi, M. Impact of exercise training on muscle mitochondria modifications in older adults: a systematic review of randomized controlled trials. Aging Clin Exp Res 2022, 34, 1495–1510. [Google Scholar] [CrossRef]
- Khakroo Abkenar, I.; Rahmani-Nia, F.; Lombardi, G. The Effects of Acute and Chronic Aerobic Activity on the Signaling Pathway of the Inflammasome NLRP3 Complex in Young Men. Medicina (Kaunas) 2019, 55. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Lowder, T.W.; Spielmann, G.; Bigley, A.B.; LaVoy, E.C.; Kunz, H. Exercise and the aging immune system. Ageing Res Rev 2012, 11, 404–420. [Google Scholar] [CrossRef]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 2010, 10, 594–604. [Google Scholar] [CrossRef]
- Lang, T.J. Estrogen as an immunomodulator. Clin Immunol 2004, 113, 224–230. [Google Scholar] [CrossRef]
- Kadel, S.; Kovats, S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front Immunol 2018, 9, 1653. [Google Scholar] [CrossRef]
- Laffont, S.; Blanquart, E.; Guéry, J.C. Sex Differences in Asthma: A Key Role of Androgen-Signaling in Group 2 Innate Lymphoid Cells. Front Immunol 2017, 8, 1069. [Google Scholar] [CrossRef]
- Klein, S.L. The effects of hormones on sex differences in infection: from genes to behavior. Neuroscience and biobehavioral reviews 2000, 24, 627–638. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol 2018, 9, 2279. [Google Scholar] [CrossRef]
- Del Rio, L.; Murcia-Belmonte, A.; Buendía, A.J.; Navarro, J.A.; Ortega, N.; Alvarez, D.; Salinas, J.; Caro, M.R. Effect of Female Sex Hormones on the Immune Response against. Pathogens 2022, 11. [Google Scholar] [CrossRef]
- Umair, M.; Fazazi, M.R.; Rangachari, M. Biological Sex As a Critical Variable in CD4. Antioxid Redox Signal 2022. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, S.E.; Cardet, J.C.; Prakash, Y.S. Sex, Cells, and Asthma. Mayo Clin Proc 2021, 96, 1955–1969. [Google Scholar] [CrossRef]
- Mashhouri, S.; Koleva, P.; Huynh, M.; Okoye, I.; Shahbaz, S.; Elahi, S. Sex Matters: Physiological Abundance of Immuno-Regulatory CD71+ Erythroid Cells Impair Immunity in Females. Front Immunol 2021, 12, 705197. [Google Scholar] [CrossRef] [PubMed]
- Markle, J.G.; Fish, E.N. SeXX matters in immunity. Trends Immunol 2014, 35, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.P.; Chiang, B.L. Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun Rev 2012, 11, A422–429. [Google Scholar] [CrossRef]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Markoff, P.A.; Balk-Lamberton, A.J.; Yang, H.; Chritton, D.B.; Lee, J.W.; Arabatzis, K. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med 1990, 11, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Gusewitch, G.; Warren, B.J.; Dotson, R.C.; Butterworth, D.E.; Nehlsen-Cannarella, S.L. Physical activity and immune function in elderly women. Med Sci Sports Exerc 1993, 25, 823–831. [Google Scholar] [CrossRef]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Henson, D.A.; Koch, A.J.; Butterworth, D.E.; Fagoaga, O.R.; Utter, A. Immune response to exercise training and/or energy restriction in obese women. Med Sci Sports Exerc 1998, 30, 679–686. [Google Scholar] [CrossRef]
- Chubak, J.; McTiernan, A.; Sorensen, B.; Wener, M.H.; Yasui, Y.; Velasquez, M.; Wood, B.; Rajan, K.B.; Wetmore, C.M.; Potter, J.D.; et al. Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med 2006, 119, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Ockene, I.S.; Freedson, P.S.; Rosal, M.C.; Merriam, P.A.; Hebert, J.R. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc 2002, 34, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.; Hayney, M.S.; Muller, D.; Rakel, D.; Ward, A.; Obasi, C.N.; Brown, R.; Zhang, Z.; Zgierska, A.; Gern, J.; et al. Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Ann Fam Med 2012, 10, 337–346. [Google Scholar] [CrossRef]
- Barrett, B.; Hayney, M.S.; Muller, D.; Rakel, D.; Brown, R.; Zgierska, A.E.; Barlow, S.; Hayer, S.; Barnet, J.H.; Torres, E.R.; et al. Meditation or exercise for preventing acute respiratory infection (MEPARI-2): A randomized controlled trial. PLoS One 2018, 13, e0197778. [Google Scholar] [CrossRef] [PubMed]
- Fondell, E.; Lagerros, Y.T.; Sundberg, C.J.; Lekander, M.; Bälter, O.; Rothman, K.J.; Bälter, K. Physical activity, stress, and self-reported upper respiratory tract infection. Med Sci Sports Exerc 2011, 43, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, H.; He, M.; Yue, M.; Gong, P.; Wu, F.; Li, X.; Pang, Y.; Yang, X.; Ma, J.; et al. Smoking, leisure-time exercise and frequency of self-reported common cold among the general population in northeastern China: a cross-sectional study. BMC public health 2018, 18, 294. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Austin, M.D.; Sha, W. Upper respiratory tract infection is reduced in physically fit and active adults. Br J Sports Med 2011, 45, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.; Bateman, E.D. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S Afr Med J 1983, 64, 582–584. [Google Scholar]
- König, D.; Grathwohl, D.; Weinstock, C.; Northoff, H.; Berg, A. Upper respiratory tract infection in athletes: influence of lifestyle, type of sport, training effort, and immunostimulant intake. Exerc Immunol Rev 2000, 6, 102–120. [Google Scholar]
- Rama, L.; Teixeira, A.M.; Matos, A.; Borges, G.; Henriques, A.; Gleeson, M.; Pedreiro, S.; Filaire, E.; Alves, F.; Paiva, A. Changes in natural killer cell subpopulations over a winter training season in elite swimmers. Eur J Appl Physiol 2013, 113, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Hellard, P.; Avalos, M.; Guimaraes, F.; Toussaint, J.F.; Pyne, D.B. Training-related risk of common illnesses in elite swimmers over a 4-yr period. Med Sci Sports Exerc 2015, 47, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Raysmith, B.P.; Drew, M.K. Performance success or failure is influenced by weeks lost to injury and illness in elite Australian track and field athletes: A 5-year prospective study. J Sci Med Sport 2016, 19, 778–783. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.; Oliveira, M.; Tauler, P. Influence of training load on upper respiratory tract infection incidence and antigen-stimulated cytokine production. Scand J Med Sci Sports 2013, 23, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness 1990, 30, 316–328. [Google Scholar]
- Heath, G.W.; Ford, E.S.; Craven, T.E.; Macera, C.A.; Jackson, K.L.; Pate, R.R. Exercise and the incidence of upper respiratory tract infections. Med Sci Sports Exerc 1991, 23, 152–157. [Google Scholar] [CrossRef]
- Spence, L.; Brown, W.J.; Pyne, D.B.; Nissen, M.D.; Sloots, T.P.; McCormack, J.G.; Locke, A.S.; Fricker, P.A. Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med Sci Sports Exerc 2007, 39, 577–586. [Google Scholar] [CrossRef]
- Svendsen, I.S.; Gleeson, M.; Haugen, T.A.; Tønnessen, E. Effect of an intense period of competition on race performance and self-reported illness in elite cross-country skiers. Scand J Med Sci Sports 2015, 25, 846–853. [Google Scholar] [CrossRef]
- Drew, M.; Vlahovich, N.; Hughes, D.; Appaneal, R.; Burke, L.M.; Lundy, B.; Rogers, M.; Toomey, M.; Watts, D.; Lovell, G.; et al. Prevalence of illness, poor mental health and sleep quality and low energy availability prior to the 2016 Summer Olympic Games. Br J Sports Med 2018, 52, 47–53. [Google Scholar] [CrossRef]
- Alonso, J.M.; Edouard, P.; Fischetto, G.; Adams, B.; Depiesse, F.; Mountjoy, M. Determination of future prevention strategies in elite track and field: analysis of Daegu 2011 IAAF Championships injuries and illnesses surveillance. Br J Sports Med 2012, 46, 505–514. [Google Scholar] [CrossRef]
- Alonso, J.M.; Tscholl, P.M.; Engebretsen, L.; Mountjoy, M.; Dvorak, J.; Junge, A. Occurrence of injuries and illnesses during the 2009 IAAF World Athletics Championships. Br J Sports Med 2010, 44, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Junge, A.; Benjamen, S.; Boyd, K.; Diop, M.; Gerrard, D.; van den Hoogenband, C.R.; Marks, S.; Martinez-Ruiz, E.; Miller, J.; et al. Competing with injuries: injuries prior to and during the 15th FINA World Championships 2013 (aquatics). Br J Sports Med 2015, 49, 37–43. [Google Scholar] [CrossRef]
- Mountjoy, M.; Junge, A.; Alonso, J.M.; Engebretsen, L.; Dragan, I.; Gerrard, D.; Kouidri, M.; Luebs, E.; Shahpar, F.M.; Dvorak, J. Sports injuries and illnesses in the 2009 FINA World Championships (Aquatics). Br J Sports Med 2010, 44, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Soligard, T.; Steffen, K.; Palmer, D.; Alonso, J.M.; Bahr, R.; Lopes, A.D.; Dvorak, J.; Grant, M.E.; Meeuwisse, W.; Mountjoy, M.; et al. Sports injury and illness incidence in the Rio de Janeiro 2016 Olympic Summer Games: A prospective study of 11274 athletes from 207 countries. Br J Sports Med 2017, 51, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Soligard, T.; Steffen, K.; Palmer-Green, D.; Aubry, M.; Grant, M.E.; Meeuwisse, W.; Mountjoy, M.; Budgett, R.; Engebretsen, L. Sports injuries and illnesses in the Sochi 2014 Olympic Winter Games. Br J Sports Med 2015, 49, 441–447. [Google Scholar] [CrossRef]
- Palmer-Green, D.; Elliott, N. Sports injury and illness epidemiology: Great Britain Olympic Team (TeamGB) surveillance during the Sochi 2014 Winter Olympic Games. Br J Sports Med 2015, 49, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Engebretsen, L.; Soligard, T.; Steffen, K.; Alonso, J.M.; Aubry, M.; Budgett, R.; Dvorak, J.; Jegathesan, M.; Meeuwisse, W.H.; Mountjoy, M.; et al. Sports injuries and illnesses during the London Summer Olympic Games 2012. Br J Sports Med 2013, 47, 407–414. [Google Scholar] [CrossRef]
- Engebretsen, L.; Steffen, K.; Alonso, J.M.; Aubry, M.; Dvorak, J.; Junge, A.; Meeuwisse, W.; Mountjoy, M.; Renström, P.; Wilkinson, M. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med 2010, 44, 772–780. [Google Scholar] [CrossRef]
- Timpka, T.; Jacobsson, J.; Bargoria, V.; Périard, J.D.; Racinais, S.; Ronsen, O.; Halje, K.; Andersson, C.; Dahlström, Ö.; Spreco, A.; et al. Preparticipation predictors for championship injury and illness: cohort study at the Beijing 2015 International Association of Athletics Federations World Championships. Br J Sports Med 2017, 51, 271–276. [Google Scholar] [CrossRef]
- Prien, A.; Mountjoy, M.; Miller, J.; Boyd, K.; van den Hoogenband, C.; Gerrard, D.; Cherif, M.Y.; Lu, Y.; Nanousis, K.; Ortiz Liscano, E.I.; et al. Injury and illness in aquatic sport: how high is the risk? A comparison of results from three FINA World Championships. Br J Sports Med 2017, 51, 277–282. [Google Scholar] [CrossRef]
- Ceddia, M.A.; Woods, J.A. Exercise suppresses macrophage antigen presentation. J Appl Physiol (1985) 1999, 87, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Bruunsgaard, H.; Galbo, H.; Halkjaer-Kristensen, J.; Johansen, T.L.; MacLean, D.A.; Pedersen, B.K. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol 1997, 499 ( Pt 3) Pt 3, 833–841. [Google Scholar] [CrossRef]
- Keller, C.; Steensberg, A.; Pilegaard, H.; Osada, T.; Saltin, B.; Pedersen, B.K.; Neufer, P.D. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. Faseb j 2001, 15, 2748–2750. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Prog Mol Biol Transl Sci 2015, 135, 355–380. [Google Scholar] [CrossRef] [PubMed]
- Bertone-Johnson, E.R.; Tworoger, S.S.; Hankinson, S.E. Recreational physical activity and steroid hormone levels in postmenopausal women. Am J Epidemiol 2009, 170, 1095–1104. [Google Scholar] [CrossRef]
- Jasienska, G.; Ziomkiewicz, A.; Thune, I.; Lipson, S.F.; Ellison, P.T. Habitual physical activity and estradiol levels in women of reproductive age. Eur J Cancer Prev 2006, 15, 439–445. [Google Scholar] [CrossRef]
- Bouman, A.; Heineman, M.J.; Faas, M.M. Sex hormones and the immune response in humans. Hum Reprod Update 2005, 11, 411–423. [Google Scholar] [CrossRef]
- Bird, M.D.; Karavitis, J.; Kovacs, E.J. Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immunol 2008, 252, 57–67. [Google Scholar] [CrossRef]
- Samy, T.S.; Schwacha, M.G.; Cioffi, W.G.; Bland, K.I.; Chaudry, I.H. Androgen and estrogen receptors in splenic T lymphocytes: effects of flutamide and trauma-hemorrhage. Shock 2000, 14, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.A. Novel aspects of estrogen action. J Soc Gynecol Investig 2000, 7, S8–9. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, C.C.; Reingold, S.C.; O'Looney, P.A. A gender gap in autoimmunity. Science 1999, 283, 1277–1278. [Google Scholar] [CrossRef] [PubMed]
- Girón-González, J.A.; Moral, F.J.; Elvira, J.; García-Gil, D.; Guerrero, F.; Gavilán, I.; Escobar, L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol 2000, 143, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Bouman, A.; Schipper, M.; Heineman, M.J.; Faas, M.M. Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol 2004, 52, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol 2019, 116, 135–170. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Diaz, M.; Song, M.; Heller, N. Androgen and Androgen Receptors as Regulators of Monocyte and Macrophage Biology in the Healthy and Diseased Lung. Front Immunol 2020, 11, 1698. [Google Scholar] [CrossRef]
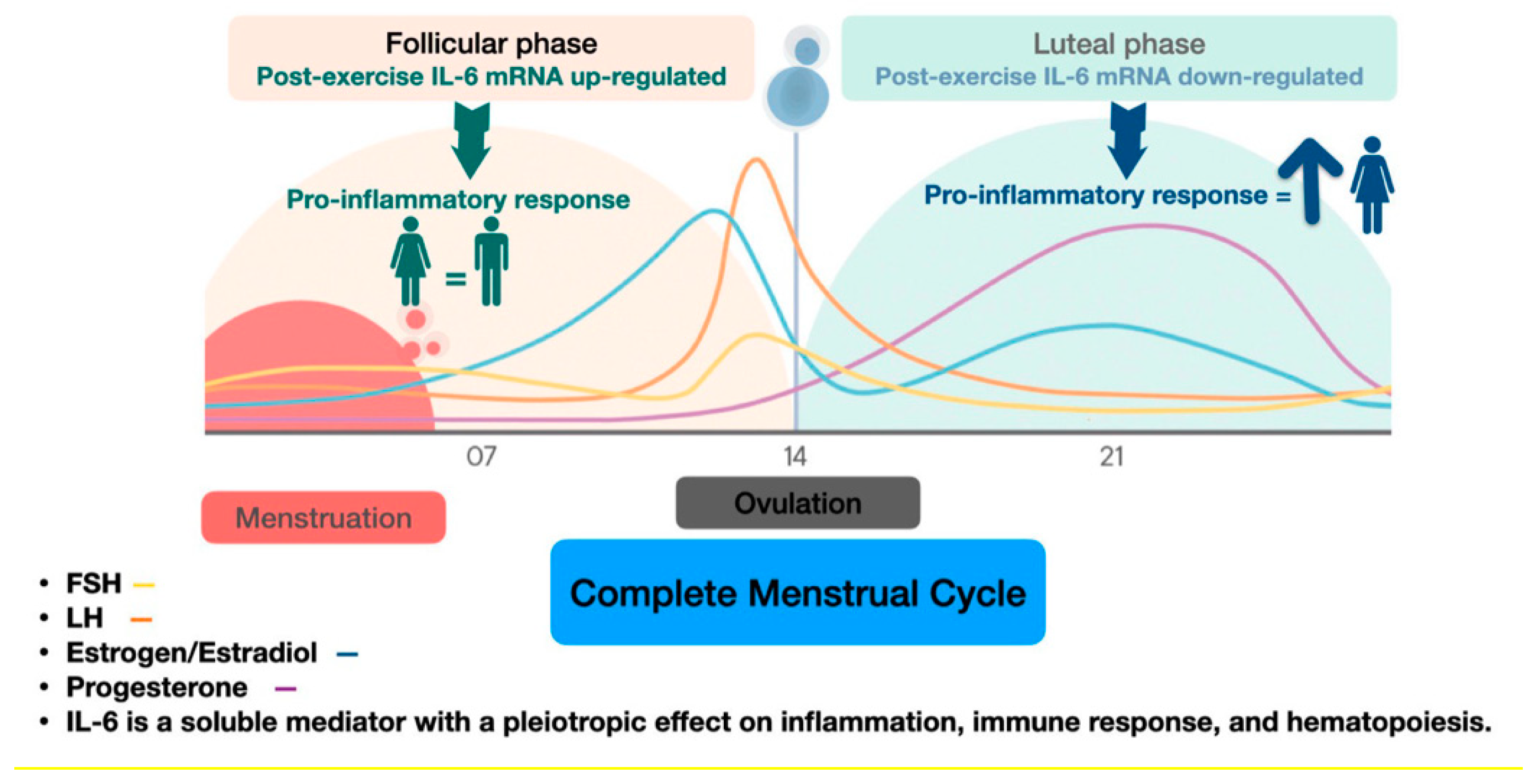
- Northoff, H.; Symons, S.; Zieker, D.; Schaible, E.V.; Schäfer, K.; Thoma, S.; Löffler, M.; Abbasi, A.; Simon, P.; Niess, A.M.; et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev 2008, 14, 86–103. [Google Scholar]
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev 2016, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 2000, 80, 1055–1081. [Google Scholar] [CrossRef]
- Nieman, D.C. Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc 1994, 26, 128–139. [Google Scholar] [CrossRef]
- Gokhale, R.; Chandrashekara, S.; Vasanthakumar, K.C. Cytokine response to strenuous exercise in athletes and non-athletes--an adaptive response. Cytokine 2007, 40, 123–127. [Google Scholar] [CrossRef]
- Stewart, L.K.; Flynn, M.G.; Campbell, W.W.; Craig, B.A.; Robinson, J.P.; McFarlin, B.K.; Timmerman, K.L.; Coen, P.M.; Felker, J.; Talbert, E. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun 2005, 19, 389–397. [Google Scholar] [CrossRef]
- Moyna, N.M.; Acker, G.R.; Fulton, J.R.; Weber, K.; Goss, F.L.; Robertson, R.J.; Tollerud, D.J.; Rabin, B.S. Lymphocyte function and cytokine production during incremental exercise in active and sedentary males and females. Int J Sports Med 1996, 17, 585–591. [Google Scholar] [CrossRef]
- Timmons, B.W.; Hamadeh, M.J.; Devries, M.C.; Tarnopolsky, M.A. Influence of gender, menstrual phase, and oral contraceptive use on immunological changes in response to prolonged cycling. J Appl Physiol (1985) 2005, 99, 979–985. [Google Scholar] [CrossRef]
- Cannon, J.G.; St Pierre, B.A. Gender differences in host defense mechanisms. J Psychiatr Res 1997, 31, 99–113. [Google Scholar] [CrossRef]
- Spitzer, J.A.; Zhang, P. Gender differences in neutrophil function and cytokine-induced neutrophil chemoattractant generation in endotoxic rats. Inflammation 1996, 20, 485–498. [Google Scholar] [CrossRef]
- Tinahones, F.J.; Gómez-Zumaquero, J.M.; Garrido-Sánchez, L.; García-Fuentes, E.; Rojo-Martínez, G.; Esteva, I.; Ruiz de Adana, M.S.; Cardona, F.; Soriguer, F. Influence of age and sex on levels of anti-oxidized LDL antibodies and anti-LDL immune complexes in the general population. J Lipid Res 2005, 46, 452–457. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Smith, L.L.; Utter, A.C.; Vinci, D.M.; Davis, J.M.; Kaminsky, D.E.; Shute, M. Cytokine changes after a marathon race. J Appl Physiol (1985) 2001, 91, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc 1990, 22, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Morris, F.L.; Payne, W.R.; Wark, J.D. Prospective decrease in progesterone concentrations in female lightweight rowers during the competition season compared with the off season: a controlled study examining weight loss and intensive exercise. Br J Sports Med 1999, 33, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.P.; Perlroth, N.E. The effects of intense exercise on the female reproductive system. J Endocrinol 2001, 170, 3–11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





