1. Tobacco
Lung cancer is the leading cause of cancer-related mortality worldwide. (Gao et al. 2016). Smoking is the leading risk factor for lung cancer and accounts for 80% of lung cancer in males and 50% in females. While the risk is likely to increase in a dose-dependent relationship, genetic predisposition may play a role in tobacco-related cancers, as evidenced by the familial occurrenceof some tobacco-related cancers (Kotnis et al., 2012). Tobacco usehas been directly linked to at least 19 cancers. Some of these cancers include; lung, larynx, and head and neck, (Petros et al., 2012).
Tobacco smoke causes oxidative stress via reactive oxygen species that affect many cell types, including fibroblasts (The primary cell type of tumour stroma) and adjacent epithelial cells. It results in cancerous properties such as cell growth, adaptation, and survival. Tobacco smoke also negatively affects the innate (DCs, macrophages, NK cells) and adaptive (T-cells, B-cells) immune system, weakening the immune response pathologically (Cao et al., 2021). While many carcinogens in tobacco, such as benzopyrene diol epoxide, are directly associated with lung cancer (Rajalakshmi et al., 2015), smoking contributes to cancer and remains fully understood.
The activation of NF-κB is complex and involves stimuli that activate the inhibitor of the κB (IκB), IKappaB Kinase (IKK) complex. This complex contains IKK1, IKK2, and NEMO gene complexes. Once activated, the IKK complex phosphorylates IκB, which leads to its degradation. It leads to free NF-κB dimers that can translocate from the cytoplasm to the nucleus and facilitate gene transcription. Many stimuli lead to the activation of NF-κB. These include cytokines such as TNF-a and IL-1. It also includes epidermic growth factors (EGF), bacterial and viral components (lipopolysaccharide), radiation, reactive oxygen species, and DNA damage from intracellular oncogenic stress. (Xia et al., 2014)
It has undoubtedly been reported as the main modifiable factor responsible for the development of lung cancer worldwide: Ding et al., 2021 and Shen et al., 2021 linked cigarette smoking to lung cancer occurrence. Ding et al. 2021 noted an increased likelihood of lung cancer in smokers versus non-smokers. Additionally, Bernatsky et al., 2018 further define this link, remarking, regarding Systemic Lupus erythematosus (SLE) patients who developed lung cancer, that most of the reported lung cancer cases in SLE were ever-smokers. Furthermore, Ding et al., 2021 identified a dose-response relationship between many cigarettes and lung cancer. Kaliaand Kwong, 2019 also mention that smoking and exposureto second-hand smoke increase the risk for skin cancers. More specifically, Arafa et al., 2020 suggest that chronic and regular smokers are at a higher risk of developing squamous cell carcinoma but at a decreased risk of developing Basal Cell carcinoma.
Most studies have reported positive associations between paternal smoking during pregnancy and childhood brain tumour risk. However, only one study was statistically significant (Preston-Martin et al., 1982). The authors proposed a hypothesis that brain tumours were related to in utero exposure to N-nitroso compounds in young people as it was well-known that
N-nitroso was the most striking among carcinogens of the brain system known in experimental animals. However it was investigated investigated the impact of parental smoking on the development of brain tumours. Smoking during pregnancy posed an increased relative risk for brain tumours in offspring, with increased risk associated with parental exposure to polycyclic aromatic hydrocarbons (Zumel-Marne et al., 2019).
A study compared with non-smokers showed a 51% increase in the risk of hepatocellular carcinoma (HCC) in ongoing smokers and a 12% increase in the risk of developing HCC in prior smokers (Massarweh & El-Serag, 2017). In another study, population-based smoking increased the odds of intrahepatic cholangiocarcinoma (ICC) by 80% (Massarweh & El-Serag, 2017). Lastly, according to Baecker et al., 2018 tobacco smoking is attributed to a13%risk for all liver cancer cases worldwide.
HIV and Tobacco
HIV may cause lung carcinoma owing to T-lymphocyte depletion. There is evidence that chronic HIV infection can contribute to the development of lung cancer through HIV-specific mechanisms. HIV is associated with a greater risk of being diagnosed with COPD, which may be due to the higher smoking rates in this population and inappropriate immune responses resulting from CD8+ T cell over-activity within the lungs, all of which contribute to more significant amounts of inflammation. Recurrent infections can be compound. These factors contribute to an increased risk of COPD (Sigel et al., 2017).
2. Alcohol
Alcohol consumption is a risk factor for cancers of the upper aerodigestive tract. It includes the oral cavity, pharynx, hypopharynx, oesophagus and other gastrointestinal tract organs, liver, pancreas, colon, and rectum. Contrary to this, moderate alcohol intake (less than 30g daily) may protect against kidney cancer (Mentella et al., 2019). Consumption of more than 30g increases the risk of liver cirrhosis, while intake of more significant than 60g of alcohol daily has a linear increased risk of developing hepatocellular carcinoma (HCC). The risk of HCC is increased 3- to 10-fold in alcohol abuse. Ethanol has been classified as a Group 1 carcinogen by the International Agency for Research on Cancer (Matsushita and Takaki. 2019). Ethanol and its metabolite, acetaldehyde, are carcinogenic. While the exact aetiology of alcohol consumption and cancer formation is not fully understood (Xu & Luo, 2017). Ethanol is digested via acetaldehyde dehydrogenase into acetaldehyde. It leads to free radicals, which bind onto DNA proteins, destroying folate and resulting in hyperproliferation. Free radicals may also be formed via alcohol-induced oxidative stress (activating cytochrome P450 2E1, CYP2E1) as well as lipid peroxidation (Rumgay et al., 2021; Gianni et al., 2014). Increased oestrogen and metabolism leading to decreased folate and retinoids due to alcohol use may play a role in cancer development (Ratna and Mandrekar. 2017).
Moreover, Ding et al., 2021 and Ko et al., 2020 also suggest an association between smoking behaviour and alcohol intake behaviour, thus accounting for lung cancer in smokers with heavy alcohol intake. Ko et al., 2020 indicate that establishments where alcohol is consumed, can pose as presumed environments for second-hand smoke exposure, which can be related to lung cancer development. However, Ding et al., 2021, deny alcohol intake as a direct risk factor in non-smokers but note that increased alcohol consumption is linked to a higher BMI, potentially increasing the risk of lung squamous cell carcinoma. Ko et al., 2020, which looked at risk factors for lung cancer in non-smokers, perceived alcohol as carcinogenic.
The next most important risk factor for gastric cancer is alcohol consumption by increasing nitrosamine intake and creating a mechanism that causes chronic inflammation (Poorolajal et al., 2020). Alcohol has many adverse effects on the liver because of the amount and rate at which one drink can cause fat deposition. This deposition can cause fatty liver disease, alcoholic hepatitis, and cirrhosis, previously reported as risk factors. Approximately 13% to 23% of HCC cases are due to alcohol-related illnesses, with a higher risk in males, whites, blacks, and Hispanics (Massarweh & El-Serag, 2017). The relationship between ICC and alcohol consumption has yet to be studied at the current moment. A meta-analysis of 19 studies conducted by the World Cancer Research Fund reported a substantial increased risk per 10 g alcohol intake per day (Yang et al., 2019). Another study showed that over 150,000 cases of HCC were attributed to alcohol consumption, which accounted for 26% of the worldwide total (Baecker et al., 2018).
3. Diet as a Risk Factor for Cancer
A Mediterranean diet, a traditional diet in Mediterranean countries, is characterised primarily by high consumption of vegetables and olive oil and moderate protein consumption. Moreover, thought to confer health benefits that can reduce the risk of many cancers including oesophageal carcinoma, colorectal, uterine, kidney, liver, thyroid and many others cancers, which we discussed in a further chapter (Mentella et al., 2019).
Ultra-processed foods have undergone multiple biological and chemical processes (for example, the addition of food preservatives) to become palatable and affordable (Fiolet et al., 2018). The use of food additives or cooking can introduce many carcinogens such as nitrates, nitrosamines, pesticides, and dioxins, that are then consumed; nitrates occur naturally in soil and water but are frequently used as food preservatives in processed meats (Chazelas et al., 2021). The packaging of food is also associated with cancer. Plastic food containers contain carcinogenic compounds, such as bisphenol, that can be incorporated into food products and may increase cancer risk (Muncke, 2021).
Consumption of red meat is also associated with an increased risk of cancer (Wie et al., 2014). A ketogenic diet creates an unfavourable environment for cancer cells by limiting tumour growth and protecting healthy cells from chemotherapy and radiation (Weber et al., 2020). Lian et al., 2017 fish intake reduces the risk of brain cancers since they exhibit neuroprotective mechanisms when consumed. As well, adequate vegetables and antioxidants (such as vitamins C and A) provided with a diet could have a protective effect. In contrast, other factors have shown no correlation with the glioma incidence, according to Bielecka & Markiewicz-Zukowska. 2020.
A study conducted by Yamamuraet al., 2013 examined the relationship between dietary factors and adult de novo acute myeloid leukaemia. It revealed a notable increase in risk in individuals who consumed significant amounts of red meat often instead of those who mainly consumed dark green vegetables, seafood and nuts. Furthermore, Ko et al., 2020 also describe an increased risk for lung cancer in persons ≥ 70 who consume a meat-based diet, while Shen et al., 2021 found no connection between meat intake and lung cancer. It may be attributed to red meat, which leads to increased production of nitrosamines, phenols, and hydroquinones. At the same time, fruits and vegetables contain compounds that may have anticarcinogenic properties like lycopene, flavonoids and folic acid, as indicated by the same authors. Many studies have linked high salt intake to an increased risk of stomach cancer. According to these studies, the OR for salt intake is a critical risk factor (Poorolajal et al., 2020). Inadequate fresh fruits and vegetable intake is another risk factor for cancer, whereas a higher food intake of fruits and vegetables decreases this risk (Peltzer & Phaswana-Mafuya. 2012).
The fact that 90–95% of cancers are due to environment and lifestyle provides significant opportunities for preventing cancer even if there is a genetic predisposition. Diet, obesity and metabolic syndrome account for 30-35% of cancer incidence, emphasising that there can be a significant reduction in cancer-related mortality by modifying theselifestylefactors. Morethan 25 000 phytochemicals have been identified as protectors against cancer. Include lycopene, catechins, capsaicin (Alok et al., 2019).
Carotenoids are found in many fruits and vegetables, and lycopene has shown anti-cancerproperties in vitro and Vivo. Theproposed mechanism involves ROS scavenging up-regulation of detoxification systems, interference with cell proliferation, induction of gap-junctional communication, inhibition of cell-cycle progression, and modulation of signal transduction pathway (Ranjan et al., 2019).
Quercetin is a flavonoid found in many fruits and vegetables. It has antioxidant, anti-inflammatory, and antiproliferative properties. It is known to block NF-κB activation, which may prevent cancer formation. Sulforaphane is acompound foundin vegetables, especially broccoli. In vitro and in vivo studies have found chemopreventive effects. There are many mechanisms in cell signalling that Sulforaphane inhibits, which like most other phytochemicals, includes blocking NF-κB activation (Dandawate et al., 2016).
Hua et al., 2020 and Belfiore et al., 2018 reported that insulin and IGF-1 are stimulated, they lead to signalling via the PI3K and Mitogen-Activated Protein Kinase (MAPK) transduction pathways, resulting in cellular growth, proliferation, differentiation, metabolism, and apoptosis. Aberrant signalling from the Insulin and IGF-1 axis may lead to malignant cell transformation and progression. It is further supported by the overexpression of the mitogenic insulin receptor isoform A (IR-A), and in addition, the insulin-like growth factor receptor (IGF-1R) in cancer cells. Nimptsch and Pischon. (2016) proposed that activation of NF-kB may link obesity and cancer.
Adipokines are cytokines secreted by adipose tissue that affect satiety, metabolism, signalling pathways, and inflammation (Hursting et al., 2012). Leptin is an adipokine responsible for energy intake, homeostasis, and immune response. Leptin is a tumorigenic adipokine. It activates multiple signalling transduction pathways, including Janus kinase/signal transducers and activators of transcription (JAK/STAT), MAPK, and PI3K pathways (Hopkins et al., 2016).
Leptin can bind to its receptor (ObR), leading to a leptin/ObR axis involved in hallmark cancer features such as cellular survival, metabolism, angiogenesis, and metastasis. Leptin can also interact with other pathways such as sex hormones, such as oestrogen, and induceinflammation via cytokine production, for example, IL-6, which can lead to further stimulation of cellular signal transduction pathways (Barone and Giordano. 2021).
Adiponectin counteracts many of the pro-tumorigenic effects of leptin. By activating adenosine monophosphate-activated protein kinase (AMPK), adiponectin leads to cell cycle arrest. It inhibits the mammalian target of rapamycin (mTOR) activity. It results in adiponectin being an anti-diabetogenic. In addition, it is anti-atherogenic, anti-inflammatory, and anti-cancer adipokine (Hopkins et al., 2016).
Nindrea et al., 2017 found that BMI indicative of overweight/obese (>25 kg/m2) exhibits tremendous odds for breast cancer development second to nulliparity. Obesity not only increases the risk of breast cancer by 1.5 to 2 times among post-menopausal women but also worsens prognosis from increased recurrence and morbidity (Ligibel et al., 2019).
4. Physical Inactivity and Exercise
An estimated 40% of cancers can be prevented through lifestyle modifications (Friedenreich et al., 2021). In American adults, increased BMI>40 is associated with increased death rates in all cancers for males and females compared to patients with a lower BMI< 24.9 (Brown et al., 2012). Exercise leads to multifactorial bodily changes such as changes in body composition, hormonelevels, decreased inflammation, and improved cellularimmunity improving outcomes in patients diagnosed with cancer. Oxidative stress is known to affect DNA and negatively increase cancer risk. Regular physical exercise induces cellular responses that augment an antioxidant response (Idorn & thor Straten., 2017).
While a lack of exercise is linked to obesity and a higher BMI, it is vital to understand the impact of this factor individually. Physical activity reduces breast cancer risk independent of BMI, smoking, and hormone therapy. The WHO recommended that 10 minutes workout markedly reduces risk. Physical activity even delays breast cancer development in the BRCA-genetic mutation carriers. Mechanisms facilitating risk reduction remain elusive but involve desirable falls in oestrogen and inflammation and regulation of metabolic function and body composition. Therefore, physical activity, diet modification, and weight control are key management strategies for these patients (Ligibel et al., 2019).
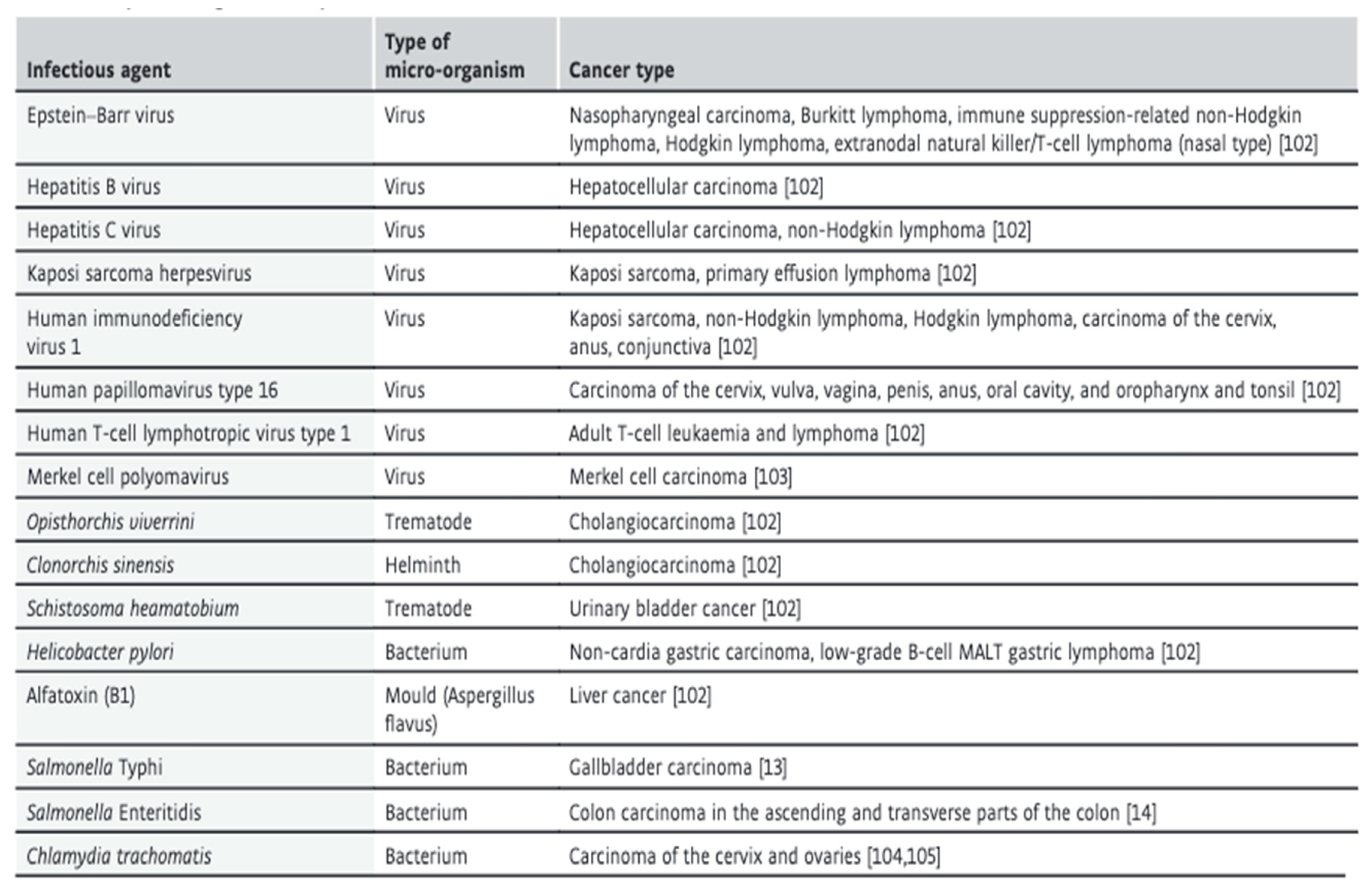
5. Infectious Agents
An estimated 17.8% of neoplasms worldwide are associated with infections. In developing countries, infections-related cancers are as high as 22% and 6% in developed countries.
(Pappas. 2009). Specifically, viruses account for most infection-mediated cancers, while other microorganisms such as parasites, for example,
Opisthorchis viverrini or
Schistosoma haematobium and bacteria, for example,
Helicobacter Pylori (
H. pylori) are also caused of cancer as shown in
Table 1 (van Elsland and Neefjes. 2018).
The aetiology of infection-related cancers is complex and likely involves multiple pathways.
The infectious agent can possess oncogenes or tumour-suppressing genes that can lead to cancer formation. Infectious agents implicated in this pathway include; HPV, EBV, HHV-8 and HTLV-1 (Islami et al., 2018). Infectious agents can also indirectly lead to cancers via chronic inflammation that can produce metabolites harmful to host cells and DNA and alter the normal progression of the cell cycle. It is the proposed mechanism of H. pylori and parasitic-related cancers. Additionally, infectious agents can suppress the hostimmuneresponse, leading to cancer formation. It, however, is likely a result of chronic inflammation, which shows severe overlap in infection-related cancers (Pappas, 2009). HPV accounts for 90% of cervical cancers, while Hepatitis B and C account for 80% of hepatocellular carcinomas (van Elsland and Neefjes. 2018). Human papillomaviruses are associated with mutations that integrate the HPV DNA into the host cells. It results in premalignant and malignant changes in gynaecological tissue. HPV has been named the most important risk factor worldwide for cervical cancer. Thus, HPV vaccination and procedures such as pap smears aimed at early detection of HPV infection may aid in the prevention of these cancers (Viarisio et al., 2017).
We conclude that the facts suggest that a number of individuals present risk factors for cancer, which can be modifiable.
References
- Alok, Ranjan., Ramachandran, Sharavan., Gupta, Nehal., Kaushik, Itishree., Wright, Stephen., Srivastava, Suyash., Das, Hiranmoy., Srivastava, Sangeeta., Prasad, Sahdeo., and Srivastava, Sanjay. (2019). “Role of Phytochemicals in Cancer Prevention.” International Journal of Molecular Sciences 20 (20). [CrossRef]
- Andrieu, Nadine, Douglas F. Easton, Jenny Chang-Claude, Matti A. Rookus, Richard Brohet, Elisabeth Cardis, Antonis C. Antoniou, et al. (2006). “Effect of Chest X-Rays on the Risk of Breast Cancer Among BRCA1/2 Mutation Carriers in the International BRCA1/2 Carrier Cohort Study: A Report from the EMBRACE, GENEPSO, GEO-HEBON, and IBCCS Collaborators’ Group.” Journal of Clinical Orthodontics: JCO 24 (21): 3361–66. [CrossRef]
- Arafa, A., Mostafa, A., Navarini, A. A., & Dong, J. Y. (2020). The association between smoking and risk of skin cancer: a meta-analysis of cohort studies. Cancer causes & control: CCC, 31(8), 787–794. [CrossRef]
- Baecker, A., Liu, X., La Vecchia, C., & Zhang, Z. F. (2018). Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP), 27(3), 205–212. [CrossRef]
- Barone, Ines, and Cinzia Giordano. (2021). “Leptin and Beyond: Actors in Cancer.” Biomolecules 11 (12). [CrossRef]
- Belfiore, Antonino, Roberta Malaguarnera, Maria Luisa Nicolosi, Rosamaria Lappano, Marco Ragusa, Andrea Morrione, and Veronica Vella. (2018). “A Novel Functional Crosstalk between DDR1 and the IGF Axis and Its Relevance for Breast Cancer.” Cell Adhesion & Migration 12 (4): 305–14. [CrossRef]
- Bernatsky, S., Ramsey-Goldman, R., Petri, M., Urowitz, M. B., Gladman, D. D., Fortin, P. R., Yelin, E. H., Ginzler, E., Hanly, J. G., Peschken, C., Gordon, C., Nived, O., Aranow, C., Bae, S. C., Isenberg, D., Rahman, A., Hansen, J. E., Pierre, Y. S., & Clarke, A. E. (2018). Smoking Is the Most Significant Modifiable Lung Cancer Risk Factor in Systemic Lupus Erythematosus. The Journal of rheumatology, 45(3), 393–396. [CrossRef]
- Bielecka, J., & Markiewicz-Żukowska, R. (2020). TheInfluenceof Nutritional and Lifestyle Factors on Glioma Incidence. Nutrients, 12(6), 1812. [CrossRef]
- Brown, Justin C., Kerri Winters-Stone, Augustine Lee, and Kathryn H. Schmitz. (2012). “Cancer, Physical Activity, and Exercise.” Comprehensive Physiology 2 (4): 2775–2809.
- Cao, Zai-Zai, Yin-Jie Ao, and Shui-Hong Zhou. (2021). “The Role of Cancer Stromal Fibroblasts in Mediating the Effects of Tobacco-Induced Cancer Cell Growth.” Cancer Cell International 21 (1): 707. [CrossRef]
- Chazelas, E., F. Pierre, N. Druesne-Pecollo, S. Gigandet, B. Srour, I. Huybrechts, C. Julia, E. Kesse-Guyot, M. Deschasaux-Tanguy, and M. Touvier. (2021). “Nitrites and Nitrates from Food Additives and Cancer Risk: Results from the NutriNet-Santé Cohort.” European Journal of Public Health 31 (Supplement_3). [CrossRef]
- Dandawate, Prasad R., Dharmalingam Subramaniam, Roy A. Jensen, and Shrikant Anant. (2016). “Targeting Cancer Stem Cells and Signaling Pathways by Phytochemicals: Novel Approach for Breast Cancer Therapy.” Seminars in Cancer Biology 40-41 (October): 192–208. [CrossRef]
- Ding, J., Tu, Z., Chen, H., & Liu, Z. (2021). Identifying modifiable risk factors of lung cancer: Indications from Mendelian randomization. PloS one, 16(10), e0258498. [CrossRef]
- Elsland, Daphne van, and Jacques Neefjes. (2018). “Bacterial Infections and Cancer.” EMBO Reports 19 (11): e46632. [CrossRef]
- Fiolet, Thibault, Bernard Srour, Laury Sellem, Emmanuelle Kesse-Guyot, Benjamin Allès, Caroline Méjean, Mélanie Deschasaux, et al. (2018). “Consumption of Ultra-Processed Foods and Cancer Risk: Results from NutriNet-Santé Prospective Cohort.” BMJ 360 (February): k322.
- Friedenreich, Christine M., Charlotte Ryder-Burbidge, and Jessica McNeil. (2021). “Physical Activity, Obesity and Sedentary Behavior in Cancer Etiology: Epidemiologic Evidence and Biologic Mechanisms.” Molecular Oncology 15 (3): 790–800. [CrossRef]
- Gao, Xu, Yan Zhang, Lutz Philipp Breitling, and Hermann Brenner. (2016). “Tobacco Smoking and Methylation of Genes Related to Lung Cancer Development.” Oncotarget 7 (37): 59017–28.
- Gianni, Testino., Leone, Silvia., and Borro, Paolo. (2014). “Alcohol and Hepatocellular Carcinoma: A Review and a Point of View.” World Journal of Gastroenterology: WJG 20 (43): 15943–54.
- Hopkins, Benjamin D., Marcus D. Goncalves, and Lewis C. Cantley. (2016). “Obesity and Cancer Mechanisms: Cancer Metabolism.” Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 34 (35): 4277–83.
- Hua, Hui, Qingbin Kong, Jie Yin, Jin Zhang, and Yangfu Jiang. (2020). “Insulin-like Growth Factor Receptor Signaling in Tumorigenesis and Drug Resistance: A Challenge for Cancer Therapy.” Journal of Hematology & Oncology 13 (1): 64. [CrossRef]
- Hursting, Stephen D., John Digiovanni, Andrew J. Dannenberg, Maria Azrad, Derek Leroith, Wendy Demark-Wahnefried, Madhuri Kakarala, Angela Brodie, and Nathan A. Berger. (2012). “Obesity, Energy Balance, and Cancer: New Opportunities for Prevention.” Cancer Prevention Research 5 (11): 1260–72. [CrossRef]
- Idorn, Manja, and Per, thor Straten. (2017). “Exercise and Cancer: From ‘healthy’ to ‘therapeutic’?” Cancer Immunology, Immunotherapy: CII 66 (5): 667–71. [CrossRef]
- Islami, F., Goding Sauer, A., Miller, K. D., Siegel, R. L., Fedewa, S. A., Jacobs, E. J., McCullough, M. L., Patel, A. V., Ma, J., Soerjomataram, I., Flanders, W. D., Brawley, O. W., Gapstur, S. M., & Jemal, A. (2018). Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA: a cancer journal for clinicians, 68(1), 31–54. [CrossRef]
- Ko, Y. H., Kim, S. J., Kim, W. S., Park, C. K., Park, C. K., Suh, Y. G., Eom, J. S., Cho, S., Hur, J. Y., Hwang, S. H., & Myong, J. P. (2020). Risk factors forprimarylung canceramong never-smoking women in South Korea: a retrospective nationwide population-based cohort study. The Korean journal of internal medicine, 35(3), 692–702. [CrossRef]
- Kotnis, Ashwin, Junghyun Namkung, Sadhana Kannan, Nallala Jayakrupakar, Taesung Park, RajivSarin, and RitaMulherkar. (2012).“MultiplePathway-Based GeneticVariations Associated with Tobacco Related Multiple Primary Neoplasms.” PloS One 7 (1): e30013. [CrossRef]
- Lian, W., Wang, R., Xing, B., & Yao, Y. (2017). Fish intake and the risk of brain tumour: a meta-analysis with systematic review. Nutrition journal, 16(1), 1. [CrossRef]
- Ligibel, J. A., Basen-Engquist, K., & Bea, J. W. (2019). Weight Management and Physical Activity for Breast Cancer Prevention and Control. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting, 39, e22–e33. [CrossRef]
- Massarweh, N. N., & El-Serag, H. B. (2017). Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer control: journal of the Moffitt Cancer Center, 24(3), 1073274817729245. [CrossRef]
- Matsushita, Hiroshi, and Akinobu Takaki. (2019). “Alcohol and Hepatocellular Carcinoma.” BMJ Open Gastroenterology 6 (1): e000260. [CrossRef]
- Mentella, Maria Chiara, Franco Scaldaferri, Caterina Ricci, Antonio Gasbarrini, and Giacinto Abele Donato Miggiano. (2019). “Cancer and Mediterranean Diet: A Review.” Nutrients 11 (9). [CrossRef]
- Nimptsch, Katharina, and Tobias Pischon. (2016). “Obesity Biomarkers, Metabolism and Risk of Cancer: An Epidemiological Perspective.” Recent Results in Cancer Research. Fortschritte Der Krebsforschung. Progres Dans Les Recherches Sur Le Cancer 208: 199–217. [CrossRef]
- Pappas, G. (2009). “Infectious Causes of Cancer: An Evolving Educational Saga.” Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases 15 (11): 961–63.
- Petros, William P., Islam R. Younis, James N. Ford, and Scott A. Weed. (2012). “Effects of Tobacco Smoking and Nicotine on Cancer Treatment.” Pharmacotherapy 32 (10): 920–31. [CrossRef]
- Poorolajal, J., Moradi, L., Mohammadi, Y., Cheraghi, Z., & Gohari-Ensaf, F. (2020). Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiology and health, 42, e2020004. [CrossRef]
- Preston-Martin, S., Yu, M. C., Benton, B., & Henderson, B. E. (1982). N-Nitroso compounds and childhood brain tumors: a case-control study. Cancer research, 42(12), 5240–5245.
- Rajalakshmi, T. R., N. Aravindha Babu, K. T. Shanmugam, and K. M. K. Masthan. (2015). “DNA Adducts-Chemical Addons.” Journal of Pharmacy & Bioallied Sciences 7 (Suppl 1): S197–99. [CrossRef]
- Ranjan, A., Ramachandran, S., Gupta, N., Kaushik, I., Wright, S., Srivastava, S., Das, H., Srivastava, S., Prasad, S., & Srivastava, S. K. (2019). Role of Phytochemicals in Cancer Prevention. International journal of molecular sciences, 20(20), 4981. [CrossRef]
- Ratna, Anuradha, and Pranoti Mandrekar. (2017). “Alcohol and Cancer: Mechanisms and Therapies.” Biomolecules 7 (3). [CrossRef]
- Rumgay, Harriet, Neil Murphy, Pietro Ferrari, and Isabelle Soerjomataram. (2021). “Alcohol and Cancer: Epidemiology and Biological Mechanisms.” Nutrients 13 (9). [CrossRef]
- Shen, J., Zhou, H., Liu, J., Zhang, Y., Zhou, T., Yang, Y., Fang, W., Huang, Y., & Zhang, L. (2021). A modifiable risk factors atlas of lung cancer: A Mendelian randomization study. Cancer medicine, 10(13), 4587–4603. [CrossRef]
- Sigel, K., Makinson, A., & Thaler, J. (2017). Lung cancer in persons with HIV. Current opinion in HIV and AIDS, 12(1), 31–38. [CrossRef]
- Viarisio, D., Gissmann, L., & Tommasino, M. (2017). Human papillomaviruses and carcinogenesis: well-established and novel models. Current opinion in virology, 26, 56–62. [CrossRef]
- Weber, Daniela D., Sepideh Aminzadeh-Gohari, Julia Tulipan, Luca Catalano, René G. Feichtinger, and Barbara Kofler. (2020). “Ketogenic Diet in the Treatment of Cancer -Where Do We Stand?” Molecular Metabolism 33 (March): 102–21.
- Wie, G. A., Cho, Y. A., Kang, H. H., Ryu, K. A., Yoo, M. K., Kim, Y. A., Jung, K. W., Kim, J., Lee, J. H., & Joung, H. (2014). Red meat consumption is associated with an increased overall cancer risk: a prospective cohort study in Korea. The British journal of nutrition, 112(2), 238–247. [CrossRef]
- Xia, Yifeng., Shen., and Inder M. Verma. (2014). “NF-κB, an Active Player in Human Cancers.” Cancer Immunology Research 2 (9): 823–30.
- Xu, Mei, and Jia Luo. (2017). “Alcohol and Cancer Stem Cells.” Cancers 9 (11). [CrossRef]
- Yamamura, Y., Oum, R., Gbito, K. Y., Garcia-Manero, G., & Strom, S. S. (2013). Dietary intake of vegetables, fruits, and meats/beans as potential risk factors of acute myeloid leukemia: a Texas case-control study. Nutrition and cancer, 65(8), 1132–1140. [CrossRef]
- Yang, J. D., Hainaut, P., Gores, G. J., Amadou, A., Plymoth, A., & Roberts, L. R. (2019). A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nature reviews. Gastroenterology & hepatology, 16 (10), 589–604. [CrossRef]
- Zhang, Faming, Yanyun Chen, Mark Heiman, and Richard Dimarchi. (2005). “Leptin: Structure, Function and Biology.” Vitamins and Hormones 71: 345–72.
- Zumel-Marne, A., Castano-Vinyals, G., Kundi, M., Alguacil, J., & Cardis, E. (2019). Environmental Factors and the Risk of Brain Tumours in Young People: A Systematic Review. Neuroepidemiology, 53(3-4), 121–141. [CrossRef]
Table 1.
Link between infections and respective cancers.
Table 1.
Link between infections and respective cancers.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).