Submitted:
31 December 2022
Posted:
03 January 2023
You are already at the latest version
Abstract
Keywords:
MSC: 92C05, 92C15, 92C40, 92C45, 80Axx, 82Cxx, 82B35, 82C26
1. Introduction
2. Physical, Chemical, and Optical Properties of Archean Vesicles
2.1. Properties of Fatty Acid Vesicles at the Origin of Life
2.2. Photochemical Dissipative Structuring of Fatty Acids
2.3. Vesicle formation
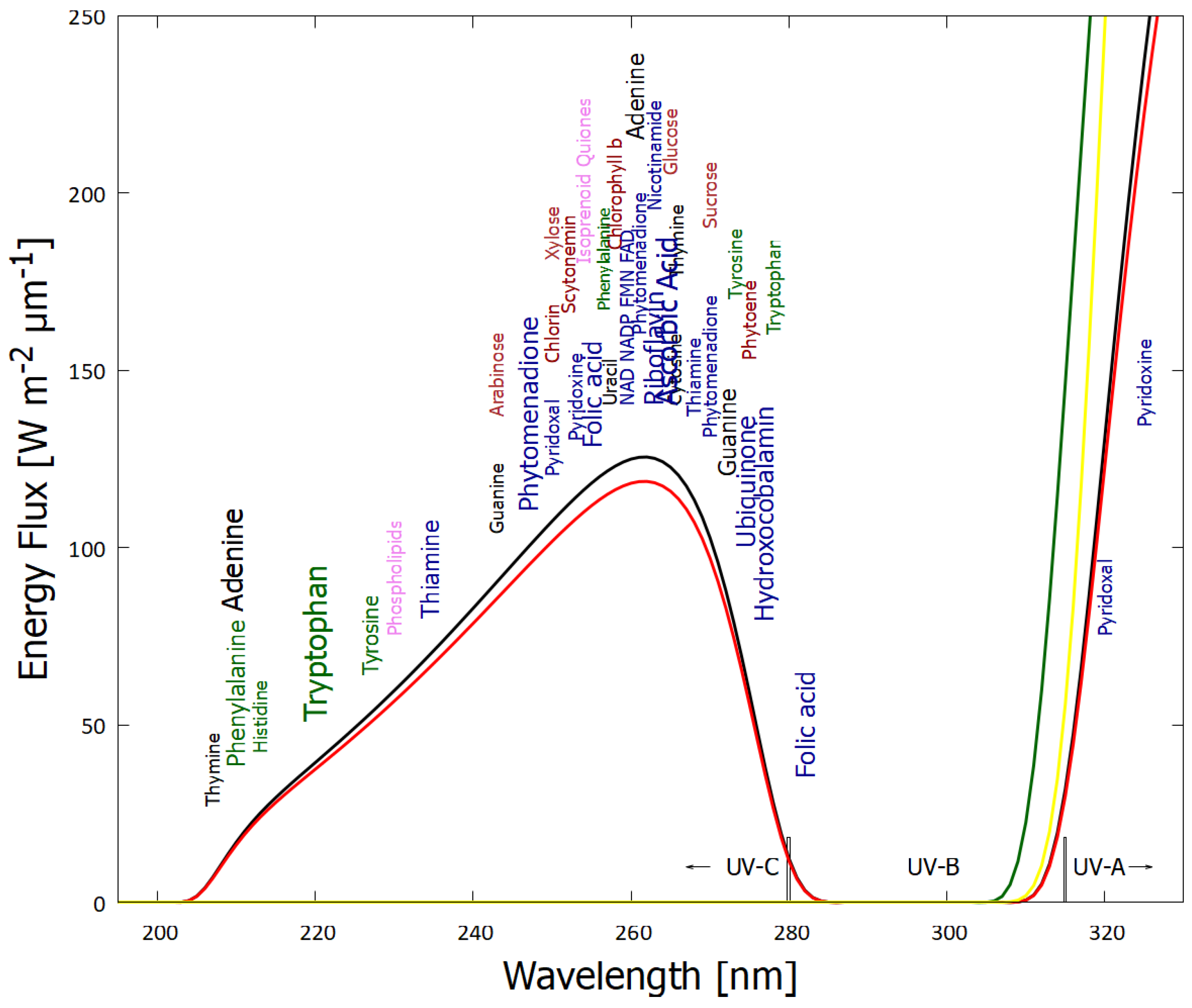
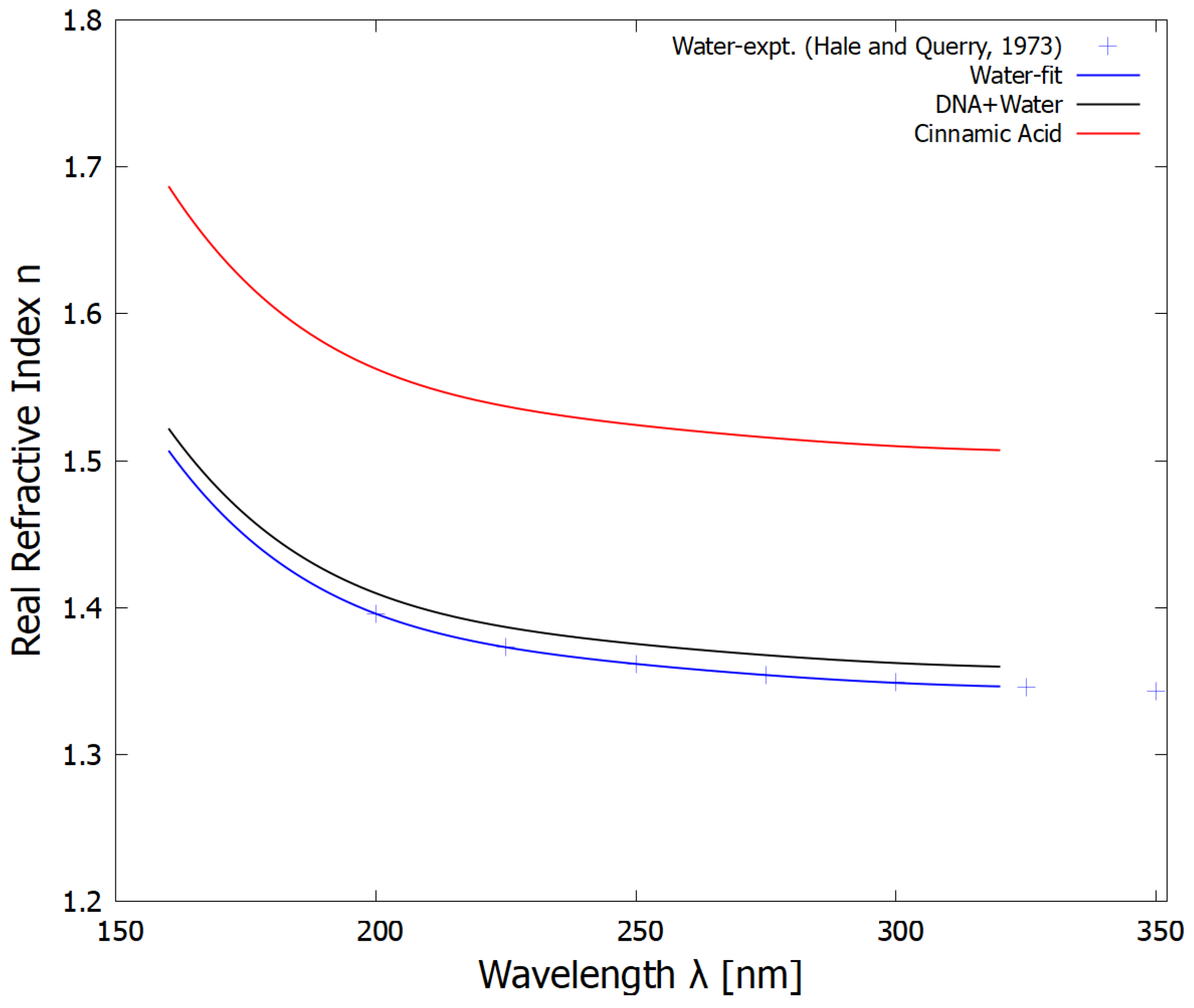
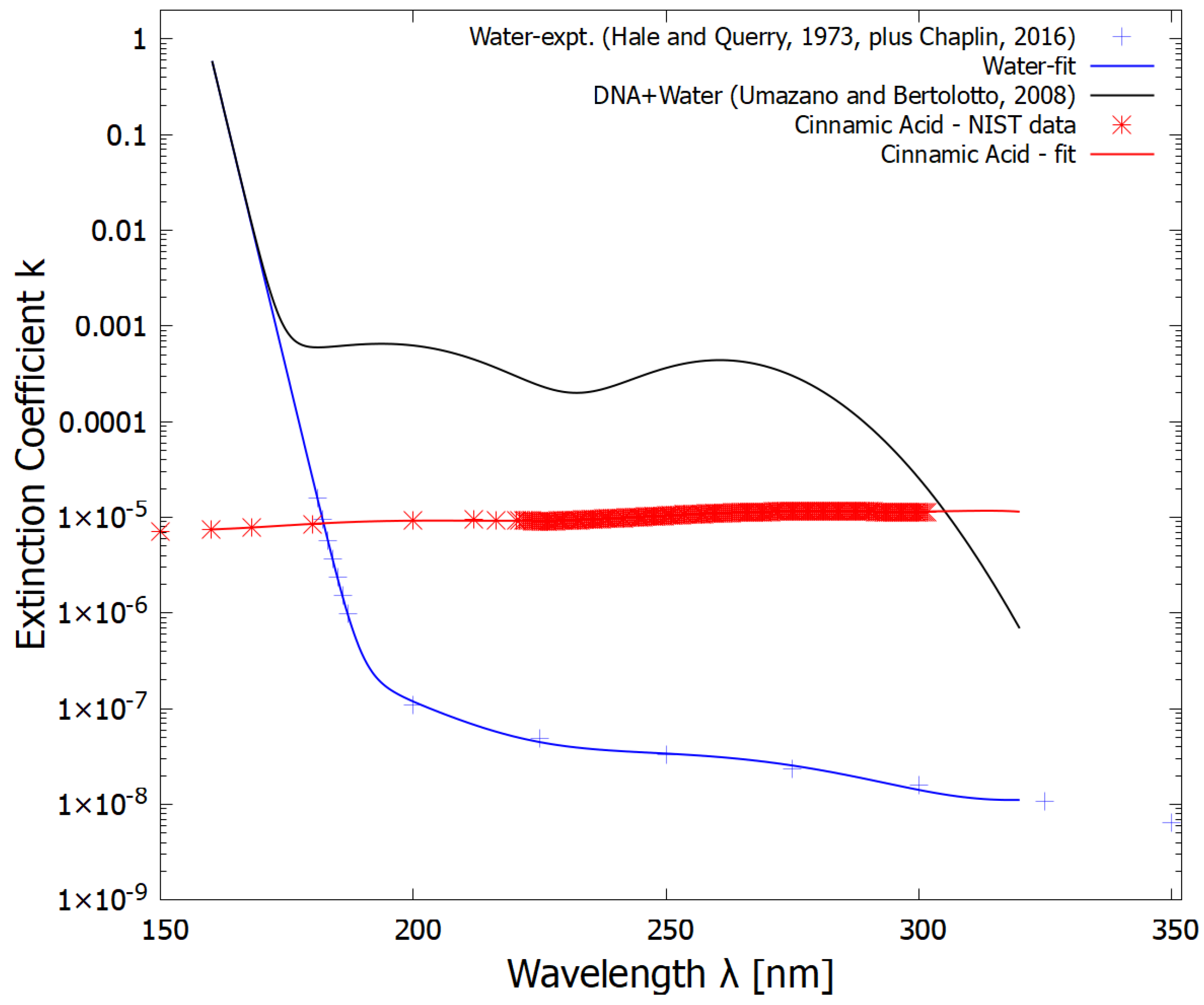
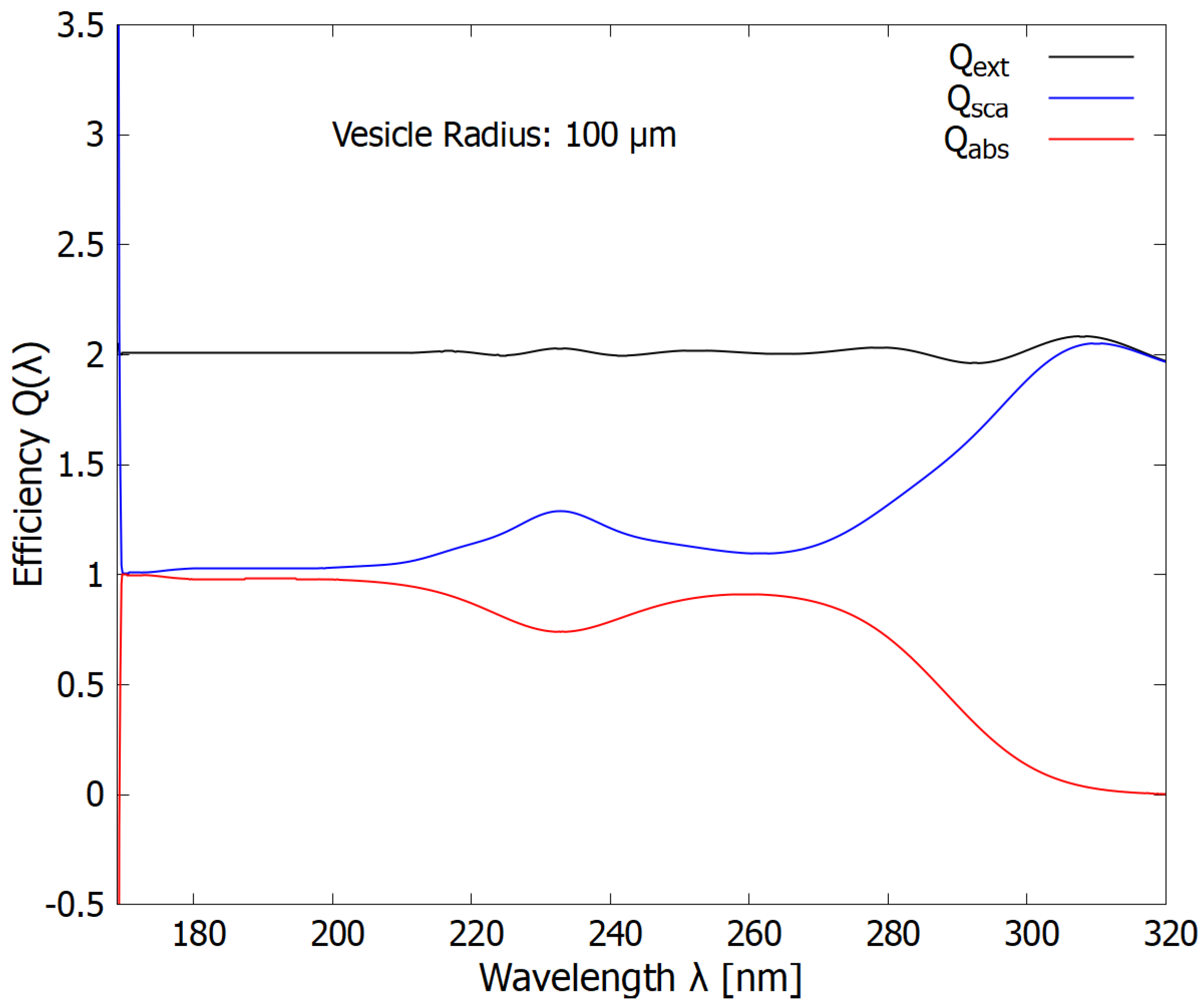
2.4. Optical Properties of fatty acids vesicles
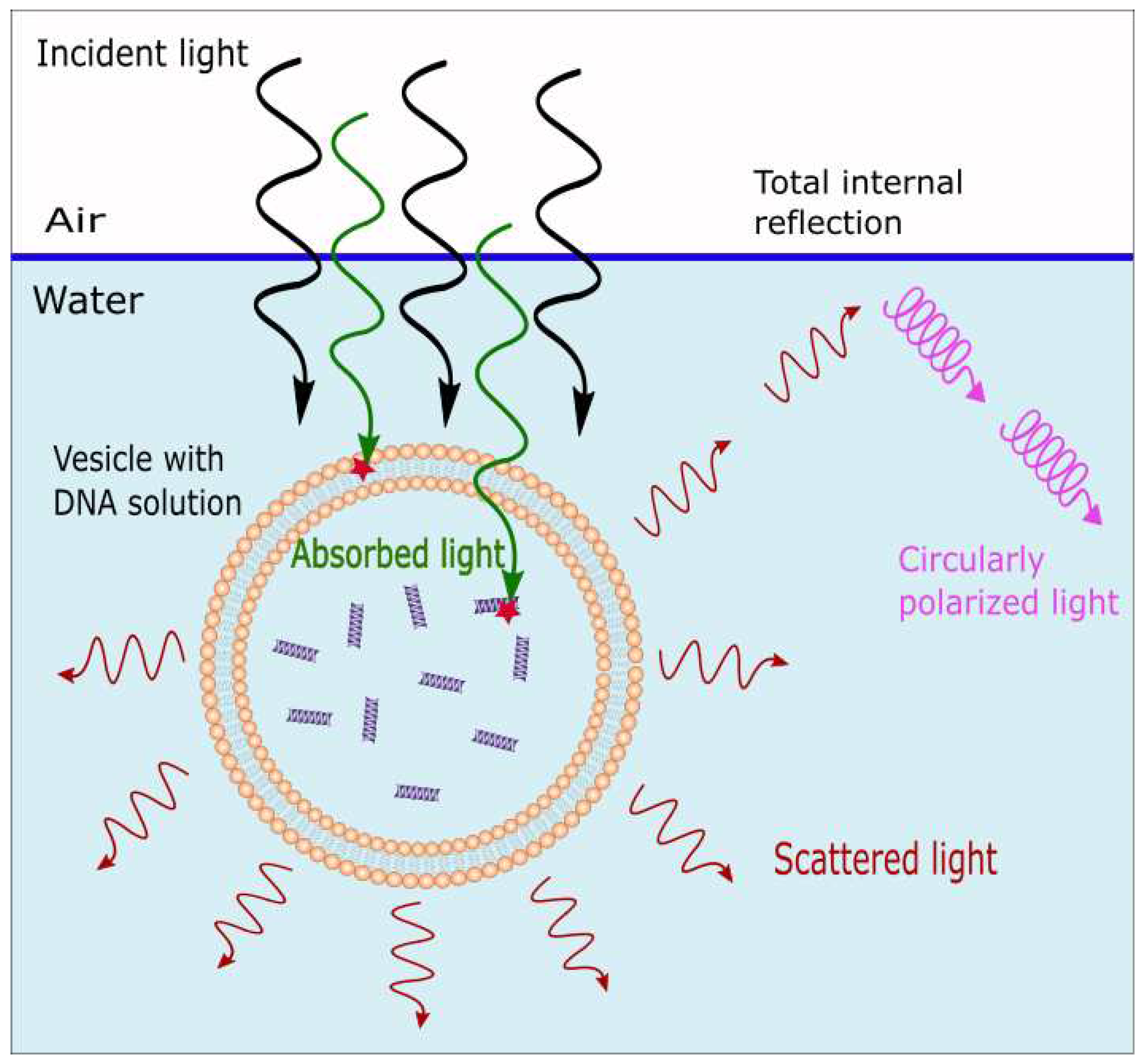
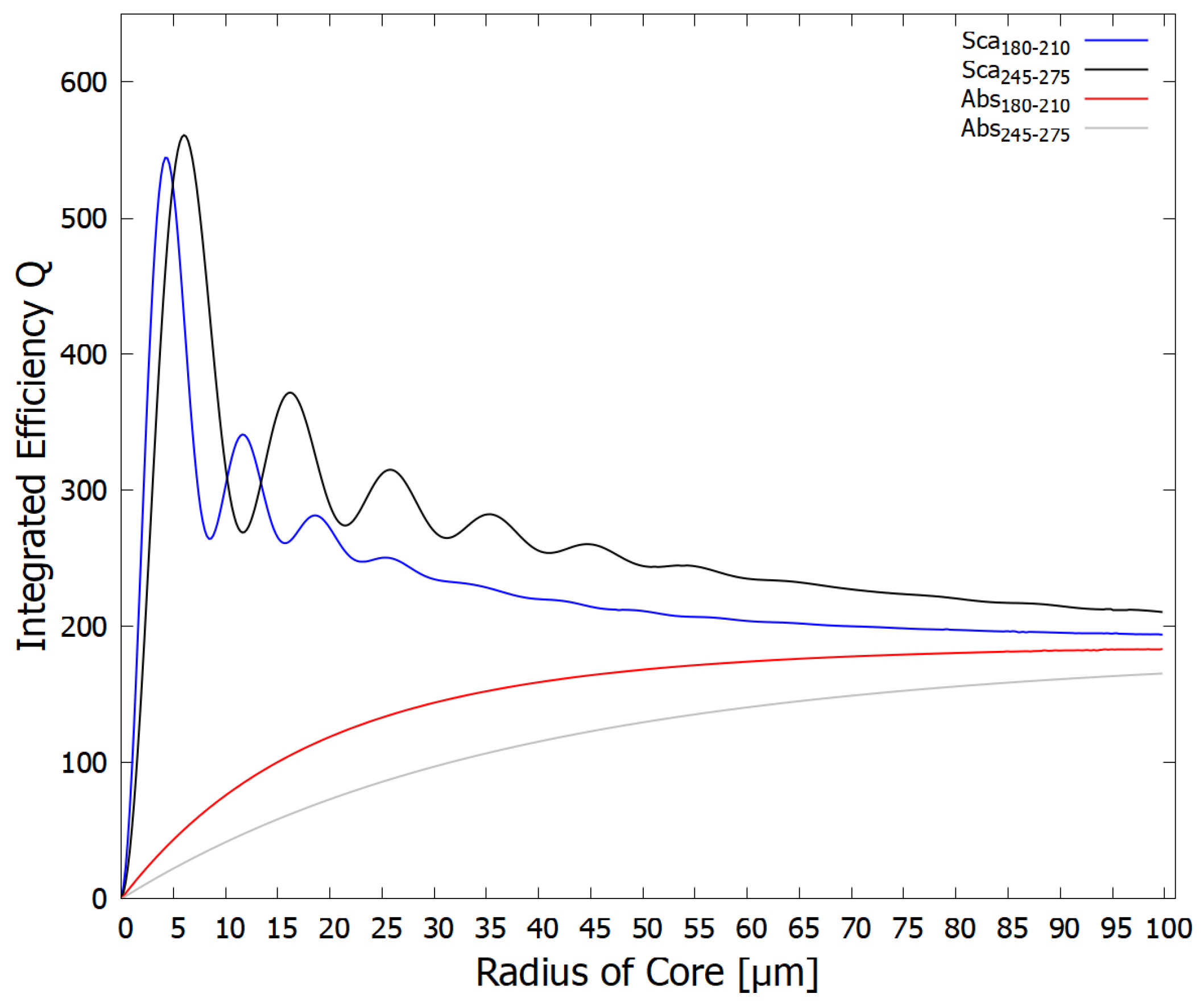
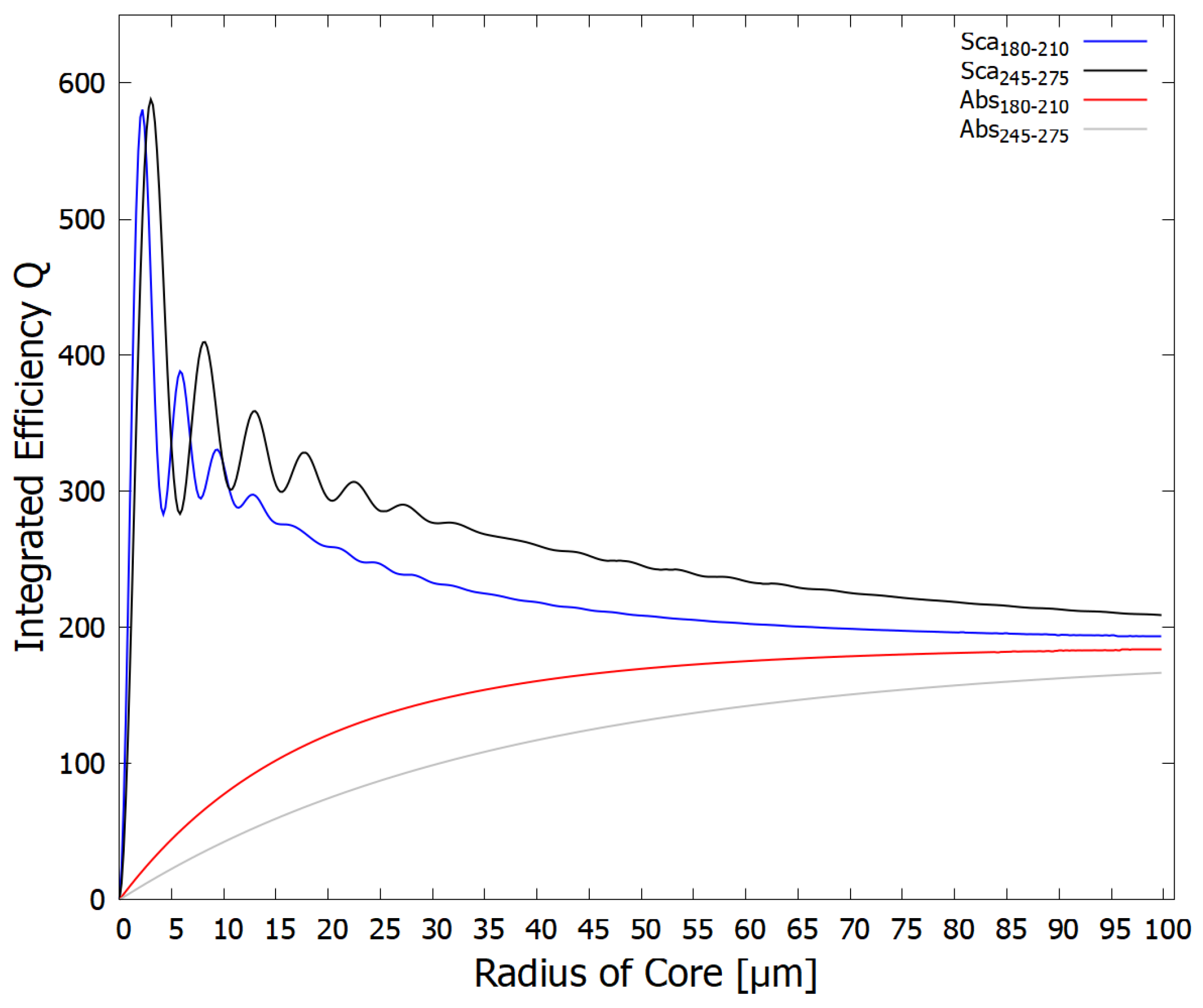
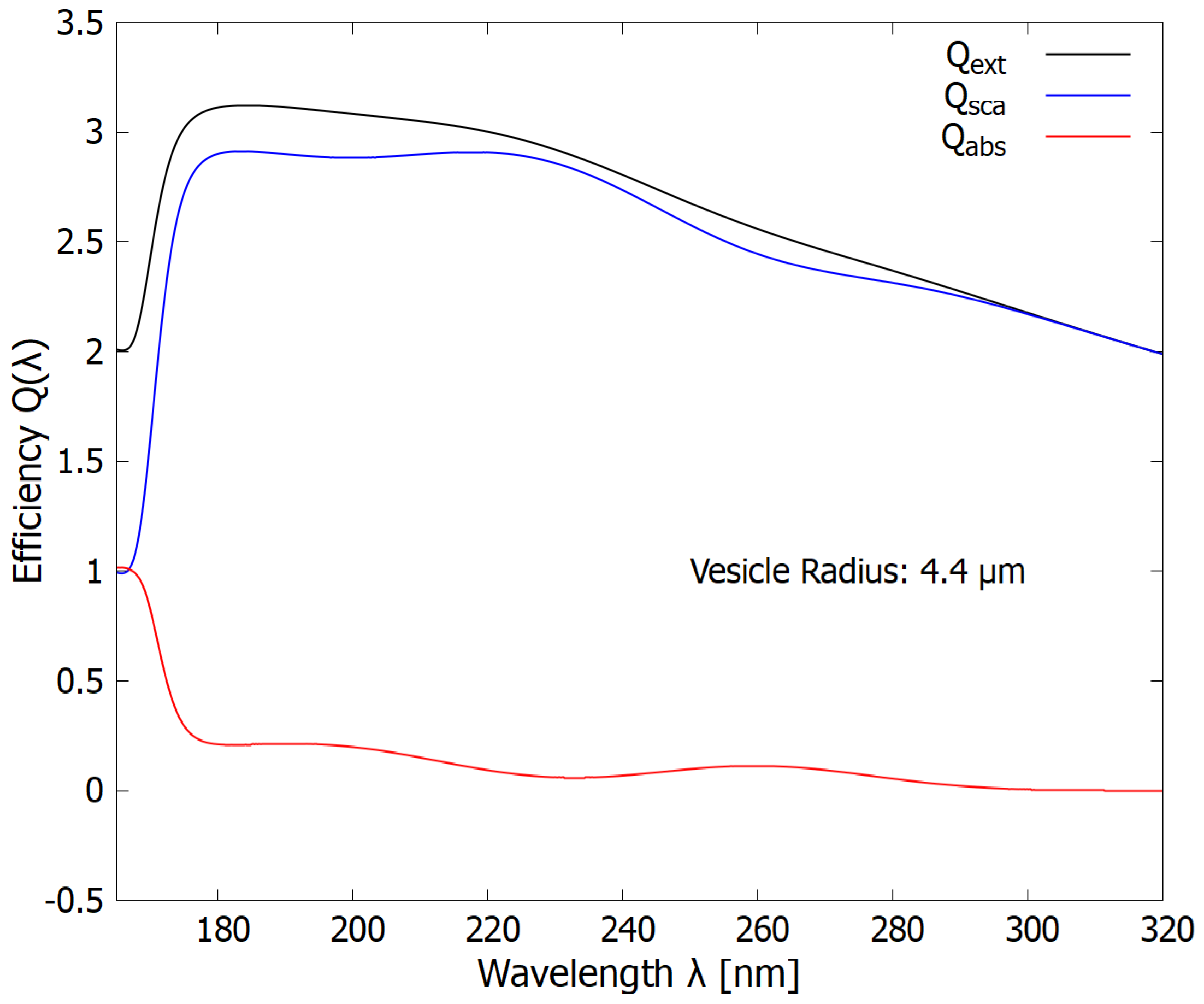
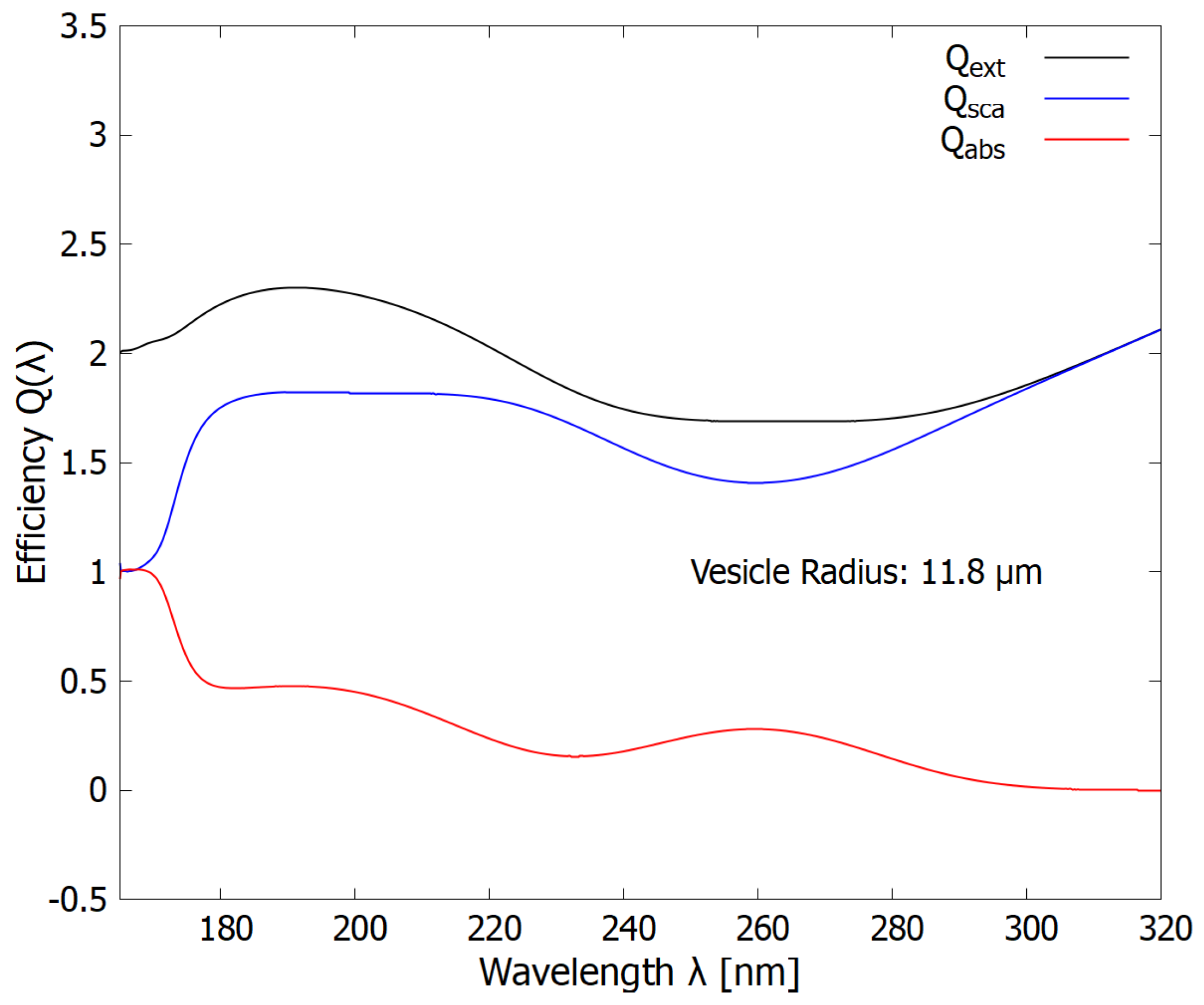
2.4.1. Scattering
2.4.2. Backscattering, Optical Dichroism, and Homochirality
3. Discussion and Conclusions
Funding
Abbreviations
| CO2 | carbon dioxide |
| DNA | deoxyribonucleic acid |
| HCN | hydrogen cyanide |
| H2S | hydrogen sulfide |
| RNA | ribonucleic acid |
| UV-A | light in the region 360-400 nm |
| UV-B | light in the region 285-360 nm |
| UV-C | light in the region 100-285 nm (only the region 180-285 nm is relevant here since |
| shorter wavelengths are well shielded by atmospheric CO2) | |
| UVTAR | Ultraviolet and Temperature Assisted Replication |
References
- Fan, Y.; Fang, Y.; Ma, L. The self-crosslinked ufasome of conjugated linoleic acid: Investigation of morphology, bilayer membrane and stability. Colloids and Surfaces B: Biointerfaces 2014, 123, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D. The Role of Lipid Membranes in Life’s Origin. Life 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Yi, R.; Fahrenbach, A.C.; Wang, A.; Jia, T.Z. A Physicochemical Consideration of Prebiotic Microenvironments for Self-Assembly and Prebiotic Chemistry. Life 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Gözen, I.; Köksal, E.S.; Põldsalu, I.; Xue, L.; Spustova, K.; Pedrueza-Villalmanzo, E.; Ryskulov, R.; Meng, F.; Jesorka, A. Protocells: Milestones and Recent Advances. Small 2022, 18, 2106624, [https://onlinelibrary.wiley.com/doi/pdf/10.1002/smll.202106624]. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K. The Dissipative Photochemical Origin of Life: UVC Abiogenesis of Adenine. Entropy 2021, 23. [https://www.mdpi.com/1099-4300/23/2/217]. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Michaelian, K. Dissipative Photochemical Abiogenesis of the Purines. Entropy 2022, 24, 1027. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Fiore, M. Investigating Prebiotic Protocells for a Comprehensive Understanding of the Origins of Life: A Prebiotic Systems Chemistry Perspective. Life 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K. Thermodynamic origin of life. ArXiv 2009, [arXiv:physics.gen-ph/0907.0042].
- Michaelian, K. Thermodynamic dissipation theory for the origin of life. Earth Syst. Dynam. 2011, 224, 37–51, [https://esd.copernicus.org/articles/2/37/2011/esd-2-37-2011.html]. [Google Scholar] [CrossRef]
- Michaelian, K.; Simeonov, A. Fundamental molecules of life are pigments which arose and co-evolved as a response to the thermodynamic imperative of dissipating the prevailing solar spectrum. Biogeosciences 2015, 12, 4913–4937, [https://bg.copernicus.org/articles/12/4913/2015/]. [Google Scholar] [CrossRef]
- Michaelian, K. Thermodynamic Dissipation Theory of the Origina and Evolution of Life: Salient characteristics of RNA and DNA and other fundamental molecules suggest an origin of life driven by UV-C light; Self-published. Printed by CreateSpace. Mexico City. ISBN:9781541317482., 2016.
- Michaelian, K. Microscopic Dissipative Structuring and Proliferation at the Origin of Life. Heliyon 2017, 3, e00424, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5647473/]. [Google Scholar] [CrossRef]
- Glansdorff, P.; Prigogine, I. Thermodynamic Theory of Structure, Stability and Fluctuations; Wiley - Interscience., 1971.
- Michaelian, K. Homochirality through Photon-Induced Denaturing of RNA/DNA at the Origin of Life. Life 2018, 8. [http://www.mdpi.com/2075-1729/8/2/21]. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K.; Rodriguez, O. Prebiotic fatty acid vesicles through photochemical dissipative structuring. Revista Cubana de Química 2019, 31, 354–370. [Google Scholar]
- Michaelian, K.; Santillan, N. UVC photon-induced denaturing of DNA: A possible dissipative route to Archean enzyme-less replication. Heliyon 2019, 5, e01902, [https://www.heliyon.com/article/e01902]. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K. Non-Equilibrium Thermodynamic Foundations of the Origin of Life. Foundations 2022, 2, 308–337. [Google Scholar] [CrossRef]
- Schirrmeister, B.E.; Sanchez-Baracaldo, P.; Wacey, D. Cyanobacterial evolution during the Precambrian. International Journal of Astrobiology 2016, 15, 187–204. [Google Scholar] [CrossRef]
- Johnson, D. A synthesis of unsaturated very long chain fatty acids. Chemistry and Physics of Lipids 1990, 56, 65–71. [Google Scholar] [CrossRef]
- Pereto, J.; Lopez-Garcia, P.; Moreira, D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem. Sci. 2004, 29, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.; López-García, P.; Moreira, D. The early evolution of lipid membranes and the three domains of life. Nature Reviews, Microbiology 2012, 10, 507–515. [Google Scholar] [CrossRef]
- Knauth, L.P.; Lowe, D.R. High Archean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland group, South Africa. Geol. Soc. Am. Bull. 2003, 115, 566–580. [Google Scholar] [CrossRef]
- Knauth, L.P. Temperature and salinity history of the Precambrian ocean: implications for the course of microbial evolution. Paleogeography, Paleoclimatology, Paleoecology 2005, 219, 53–69. [Google Scholar] [CrossRef]
- Meixnerová, J.; Blum, J.D.; Johnson, M.W.; Stüeken, E.E.; Kipp, M.A.; Anbar, A.D.; Buick, R. Mercury abundance and isotopic composition indicate subaerial volcanism prior to the end-Archean “whiff” of oxygen. Proceedings of the National Academy of Sciences 2021, 118, e2107511118, [https://www.pnas.org/doi/pdf/10.1073/pnas.2107511118]. [Google Scholar] [CrossRef] [PubMed]
- Sagan, C. Ultraviolet Selection Pressure on the Earliest Organisms. J. Theor. Biol. 1973, 39, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Calvin, M. Occurrence of fatty acids and aliphatic hydrocarbons in a 3.4 billion-year-old sediment. Nature 1969, 224, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Van Hoeven, W.; Maxwell, J.; Calvin, M. Fatty acids and hydrocarbons as evidence of life processes in ancient sediments and crude oils. Geochimica et Cosmochimica Acta 1969, 33, 877–881. [Google Scholar] [CrossRef]
- Rossignol, S.; Tinel, L.; Bianco, A.; Passananti, M. Atmospheric photochemistry at a fatty acid–coated air-water interface. Science 2016, 353, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.N.; Kloxin, C.J. Toward an enhanced understanding and implementation of photopolymerization reactions. AIChE J. 2008, 54, 2775–2795. [Google Scholar] [CrossRef]
- Botta, L.; Bizzarri, B.M.; Piccinino, D.; Fornaro, T.; Brucato, J.R.; Saladino, R. Prebiotic synthesis of carboxylic acids, amino acids and nucleic acid bases from formamide under photochemical conditions. Eur. Phys. J. Plus 2017, 132, 317. [Google Scholar] [CrossRef]
- Vicente, A.; Antunes, R.; Almeida, D.; Franco, I.J.A.; Hoffmann, S.V.; Mason, N.J.; Eden, S.; Duflot, D.; Canneaux, S.; Delwiche, J.; Hubin-Franskin, M.J.; Limão-Vieira, P. Photoabsorption measurements and theoretical calculations of the electronic state spectroscopy of propionic, butyric, and valeric acids. Phys. Chem. Chem. Phys. 2009, 11, 5729–5741. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.K.; Chatterjee, S.N. Ultraviolet- and Sunlight-Induced Lipid Peroxidation in Liposomal Membrane. Radiation Research 1980, 83, 290–302. [Google Scholar] [CrossRef]
- Celani, P.; Garavelli, M.; Ottani, S.; Bemardi, F.; Robb, M.A.; Olivucci, M. Molecular “Trigger” for Radiationless Deactivation of Photoexcited Conjugated Hydrocarbons. J. Am. Chem. Soc. 1995, 117, 11584–11585. [Google Scholar] [CrossRef]
- Bassas, M.; Marqués, A.M.; Manresa, A. Study of the crosslinking reaction (natural and UV induced) in polyunsaturated PHA from linseed oil. Biochemical Engineering Journal 2007, 40, 275–283. [Google Scholar] [CrossRef]
- Fan, Y.; Fang, Y.; L. , M.; Jiang, H. Investigation of Micellization and Vesiculation of Conjugated Linoleic Acid by Means of Self-Assembling and Self-Crosslinking. J. Surfact. Deterg. 2015, 18, 179–188. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, 1998. [Google Scholar]
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-nm to 200-μm Wavelength Region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, J.; Reale, M.P.; Rodriguez, M.B. DETERMINACIÓN DEL TENSOR POLARIZABILIDAD ÓPTICA DEL ADN TIPO VARILLA. ANALES AFA 2013, 11. [Google Scholar]
- Liu, P.Y.; Chin, L.K.; Ser, W.; Chen, H.F.; Hsieh, C.M.; Lee, C.H.; Sung, K.B.; Ayi, T.C.; Yap, P.H.; Liedberg, B.; Wang, K.; Bourouina, T.; Leprince-Wang, Y. Cell refractive index for cell biology and disease diagnosis: past, present and future. Lab Chip 2016, 16, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Shaw, M.; Hole, P.; Smith, J.; Tannetta, D.; Redman, C.W.; Sargent, I.L. Measurement of refractive index by nanoparticle tracking analysis reveals heterogeneity in extracellular vesicles. Journal of extracellular vesicles 2014, 3, 25361. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, M. Water Structure and Science 2016. [https://water.lsbu.ac.uk/water].
- Umazano, J.; Bertolotto, J. Optical properties of DNA in aqueous solution. J Biol Phys. 2008, 34, 163–177. [Google Scholar] [CrossRef]
- Wolstencroft, R.D. Terrestrial and Astronomical Sources of Circular Polarisation: A Fresh Look at the Origin of OF Homochirality on Earth. Bioastronomy 2002: Life Among the Stars; Norris, R.; Stootman, F., Eds., 2004, Vol. 213, p. 154.
- Michaelian, K. Thermodynamic stability of ecosystems. Journal of Theoretical Biology 2005, 237, 323–335, [https://www.sciencedirect.com/science/article/pii/S0022519305001839?via%3Dihub]. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




