Introduction
Generally in order to elude immunorejection in preclinical model various methods have being used, such as transgenic pig expressing the human complemented inhibitor, hCD59 in rat transacted spinal cord which shows axonal regeneration (Imaizumi T. et al., 2000). In our study we use the daily injection of 100 ml of diluted cyclosporine. Whether the OEC transplanted AS rats can survive ipsilateral forefaw reaching after stoping cyclosporine for up to 6 months is controversial issue which was investigated specifically at this study. As this study shows the animals continue recovering after trasnplantion of OECs despite the halt of cyclosporine.
Material and methods
In our study animal were used in accordance with the UK Home Office regulations for the care and use of laboratory animal, the Uk Animals (Scientific Prosecures) Act 1989, with the ethical approval of the University College London, Institute of Neurology. The study was supported by the British Neurological Research Trust and the international Spinal Research Trust.
In order to study whether there is any immunorejection against OECs xenotransplant at grafted tissue, we divided 15 adult female rats (180-210 g body weight) of a locally inbred Albino Swiss Strain (AS) in 5 groups. OCEs labelled by green fluorescent protein (GFP). The operation was performed. Animal was perfused and tissue was prepared. Animals were divided in 5 groups with the survival time of 4 days, 1week, 2 weeks, 3 weeks, 4 weeks, 5 weeks for group 1 ( n=2), group 2 (n=2), group 3 (n=4), group 4 (n=4) and group5 (n=3) respectively. To study the immunorejection of the grafted tissue at all groups the sections were stained by Hematoxylin & Eosin (H&E). To look at the GFP labeled OECs cells, sections were counterstained by Sytox Orange (Invitrogen, Uk) and viewed by confocal microscope. According to Keyvan-Fouladi N., et al., 2003 “Slides were overstained in hematoxylin first to ensure saturation of chemical binding sites and excess staining is then removed by controlled leaching in an alcoholic acid solution (differentiation)″. Leaching of the stain is arrested to a basic environment (20 sec) in Ammonia solution) whereby the stain becomes blue and is permanently fixed to cell structure. Finally sectioned were counterstained by eosin and mounted by DPX after dehydration. The result shows surviving transplant cells only at group 1 and 2 where the animal survival time was 1 week and 2 weeks respectively. No GFP labelled OEC could be observed at group 2, 3, and 5 where the survival time is over 2 weeks. Therefore the results prove that the animals need to be under immunosuppressant reagent after xeno-transplantation by mouse OECs in order to avoid immune rejection by leaving the animals under trial over 2 weeks time.
Result
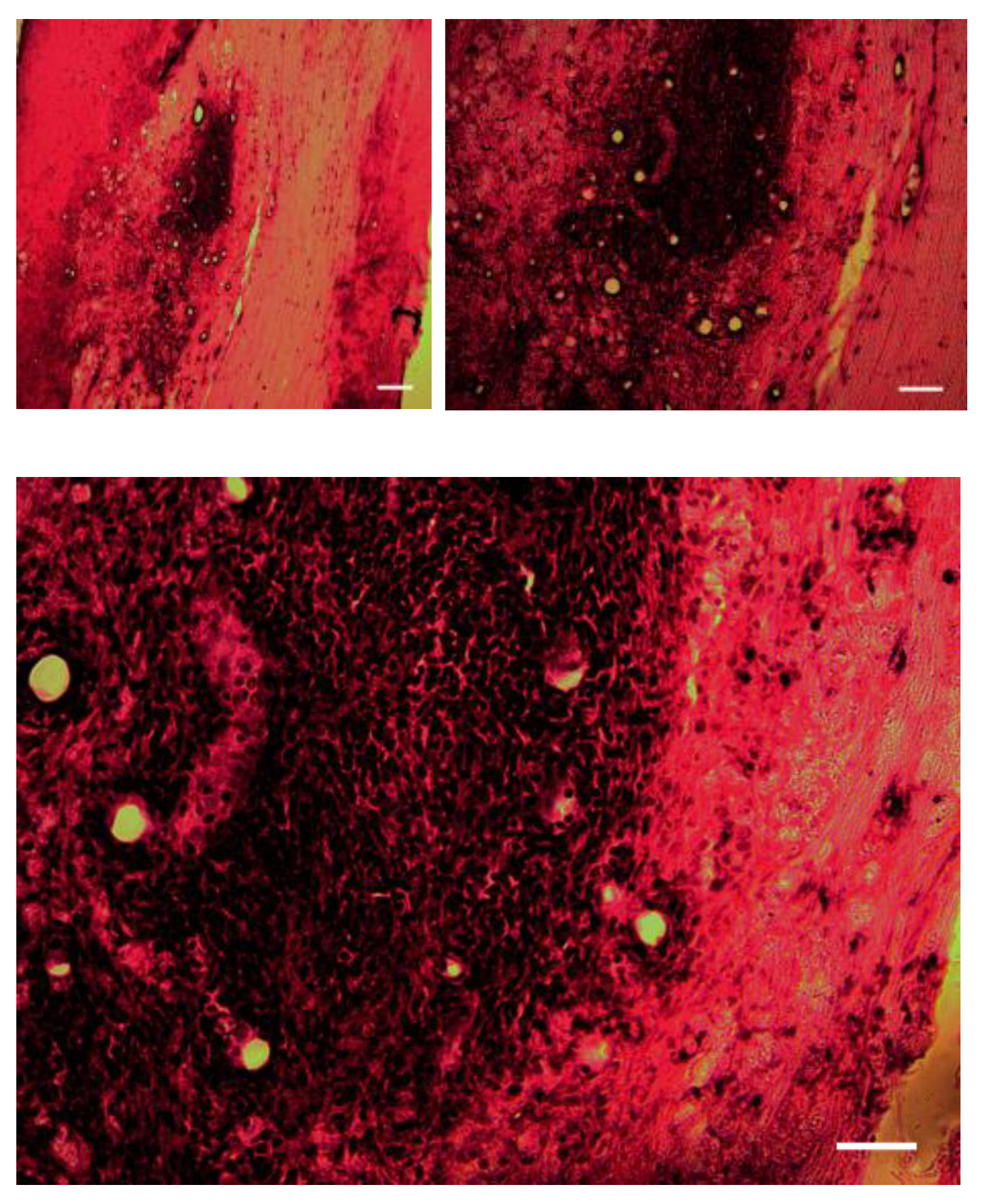
The histology images show that the animal after 10 days starts immunorjecting the xenotransplanted mice OECs in grafted tissue. Among five groups of animals the one who had survival time of 4 and 1 week did not show any immunorejection and the GFP labeled OEC cells can be visualised and there is not inflammation sign. However the histology images of groups 3, 4 and 5 animals with the survival time of 10-14 days, 3, 4 and 5 weeks show immune reaction to xenogeraft transplant of OEC degenerated cells and the central necrosis and lymphocyte infiltration in lesion area (
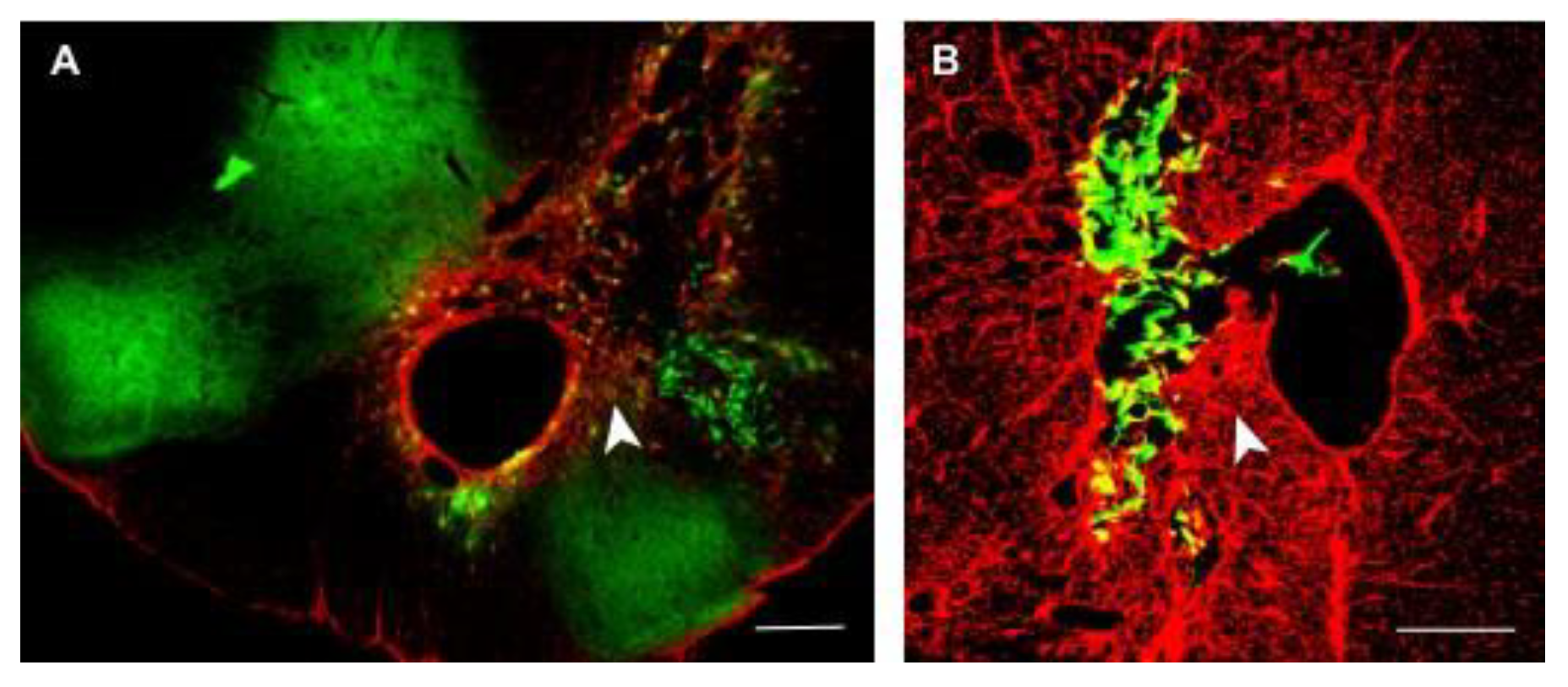
Figure 1). Moreover, the images of postoperative xenotransplanted OECs under daily subcutaneous injection of 100 ml of a dosage of 6 mg/kg/day avoid immunorejection (
Figure 2) which shows the misplace of GFP labeled transplanted OECs with the survival time of 4 weeks and there is no sign of inflammation.
Following the stop of cyclosporine after 4 weeks postsurgery the animals were tested for the paw reaching tasks for 6 months, the results indicated that despise the halt of cyclosporine the animals preserved paw reaching for over 6 months survival time.
Discussion
The histology analysis (
Figure 1) show that the animals after two weeks start Immunorejecting the xenotransplanted mice OECs in grafted tissue. Among five groups of animals group 1 and 2 with the one who had survival time of 4-7 days did not show any immunorejection and the GFP labeled OEC cells can be visualised and there is not any inflammation sign. However the histology images of groups 3, 4 and 5 animals with the survival time of 10-14 days 3,4 and 5 weeks show immune reaction to xenogarft transplant of OEC degenerated cells and the central necrosis and lymphocyte infiltration in lesion area. Moreover, the images of postoperative xenotransplanted OECs under daily subcutaneous injection of 100 ml of a dosage of 6 mg/kg/day avoid immunorejection (
Figure 2) which shows the misplace of GFP labeled transplanted OECs with the survival time of 4 weeks and there is no sign of inflammation. In general in preclinical model different approaches has being used to avoid immunorejection , such as genetically engineered pig expressing the human complemented inhibitor, hCD59 in rat transacted spinal cord which shows axonal regeneration (Imaizumi T. et al., 2000). Our study uses the daily injection of 100 ml of diluted cyclosporine. However, the study done by (E. Karaoz S, et al., 2012) shows the greater survival of groin flaps and reduces in calcineur in inhibitor drug toxicity by combination therapy of triptolide and cyclosporine A than recipients treated with cyclosporine A only. Therefore, in our conclusion, the mice xenograft model of 10 AS rats delayed OEC transplant shows the regeneration of damaged cortiocospinal tract and ipsilateral DFR function return, and also the result shows that the daily injection of 100ml diluted cyclosporine avoids immune attack and reduces inflammatory cells infiltrate in delayed CST ipsilateral rat model and also our study proved that stop of cyclosporine injection would not prevent DFR function in over 6 months AS rats and the regenerated fibers can still be noticed.
Conflicts of Interest
Corresponding Author Declaration Form Manuscript title Mouse xenograft model of Corticospinal Tract by Delayed Transplantation of Olfactory Ensheating Cells in Adult Rats Corresponding author: Maryam Naghynajadfard Additional authors in the order provided in the manuscript: Maryam Naghynajadfard The corresponding author must provide statements of authorship, originality, conflicts of interest, and research funding on behalf of all authors of the manuscript. Corresponding author, on behalf of all authors, is equired to report the following information with each submission: 1. All third- party financial support for the work in the submitted manuscript. 2. All financial relationships with any entities that could be viewed as relevant to the general area of the submitted manuscript. 3. All sources of revenue with relevance to the submitted work who made payments to you, or to your institution on your behalf, in the 36 months prior to submission. 4. Any other interactions with the sponsor of outside of the submitted work should also be reported. 5. Any relevant patents or copyrights (planned, pending, or issued). 6. Any other relationships or affiliations that may be perceived by readers to have influenced, or give the appearance of potentially influencing, what you wrote in the submitted work. As a general guideline, it is usually better to disclose a relationship than not. This information will be acknowledged at publication in a Transparency Document. Additional information on the ICMJE recommendations can be found at:
http://www.icmje.org. The corresponding author must complete the Corresponding Author declaration form on behalf of all authors of the manuscript. The corresponding author signed this statement on behalf of all co-authors to indicate that the above information is true, correct and complete. Signature: Naghynajadfard Print name: Maryam Naghynajadfard Date: 22/12/2022.
References
- E. Karaoz S, Kabatas G, Duruksu et al. Reduction of lesion in injured rat spinal cord and partial functional recovery of motility after bone marrow derived mesenchymal stem cell transplantation. Turkish Neurosurgery 2012, 2, 207–217. [CrossRef]
- Keyvan-Fouladi N., Raisman G., Li Y. Funtional Repair of the Corticospinal Tract by Delayed Transplantation of Olfactrory Ensheating Cells in Adult Rats. 2003, 23, 9428–9434. [CrossRef]
- T. Imaizumi, K.L. Lankford, J.D. Kocsis. Transplantation of olfactory ensheathing cells or Schwann cells restores rapid and secure conduction across the transected spinal cord. Brain Res. 2000, 854, 70–78. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).