Submitted:
31 December 2022
Posted:
03 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Auxins
2.1. Auxin biosynthesis
2.2. Auxin transport
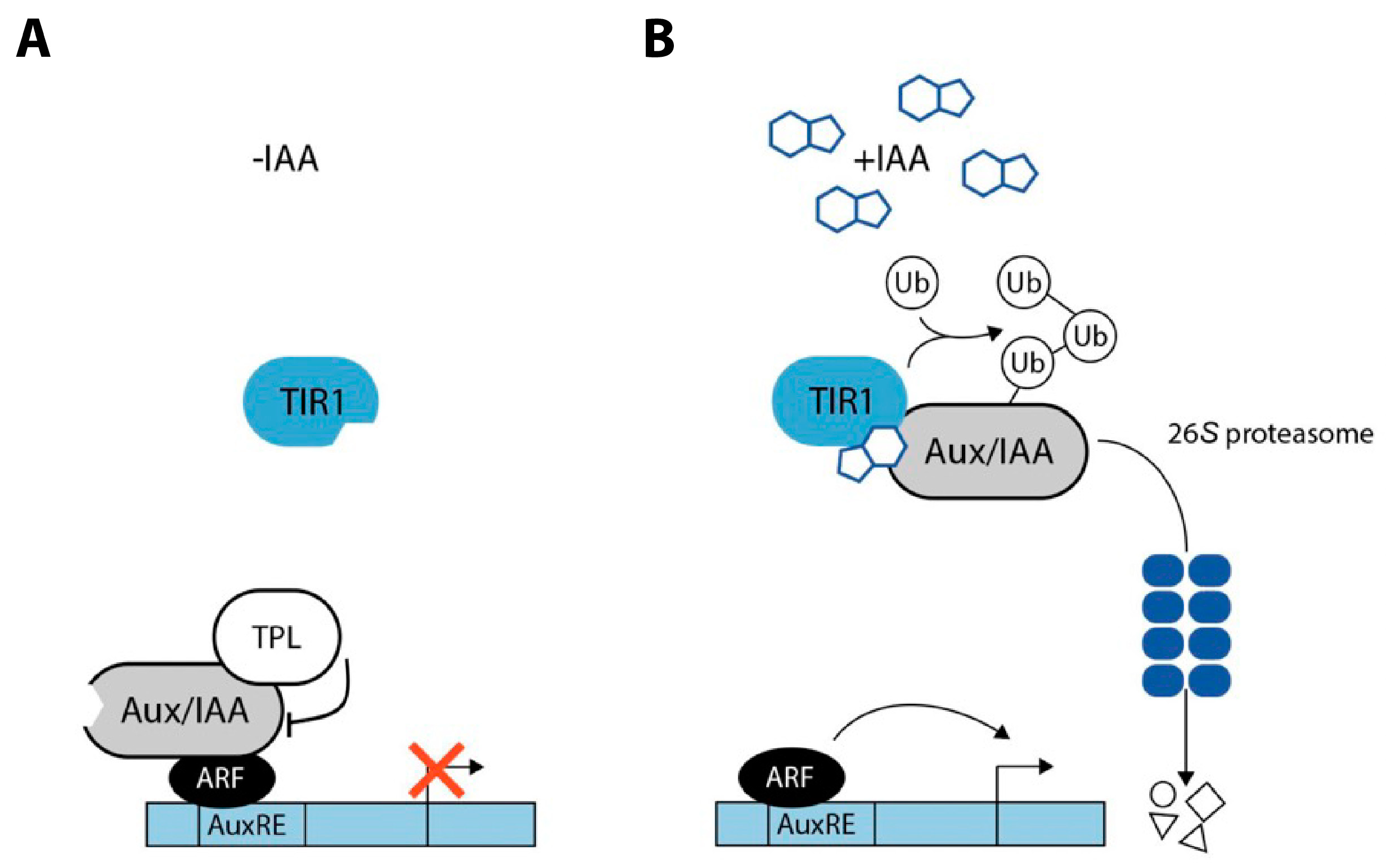
2.3. Auxin signaling
3. Abscisic acid
3.1. ABA biosynthesis
3.2. ABA transport
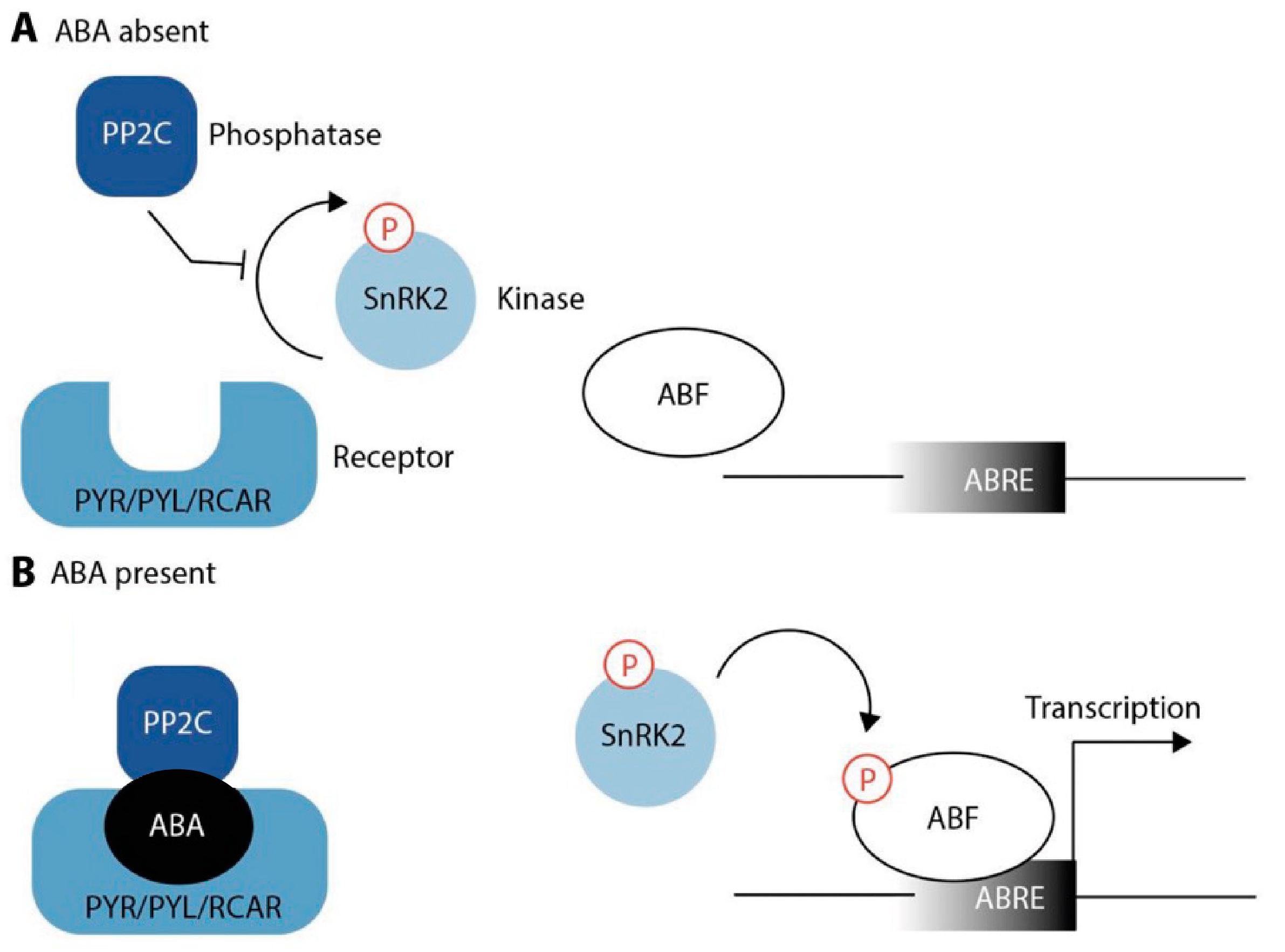
3.3. ABA signaling
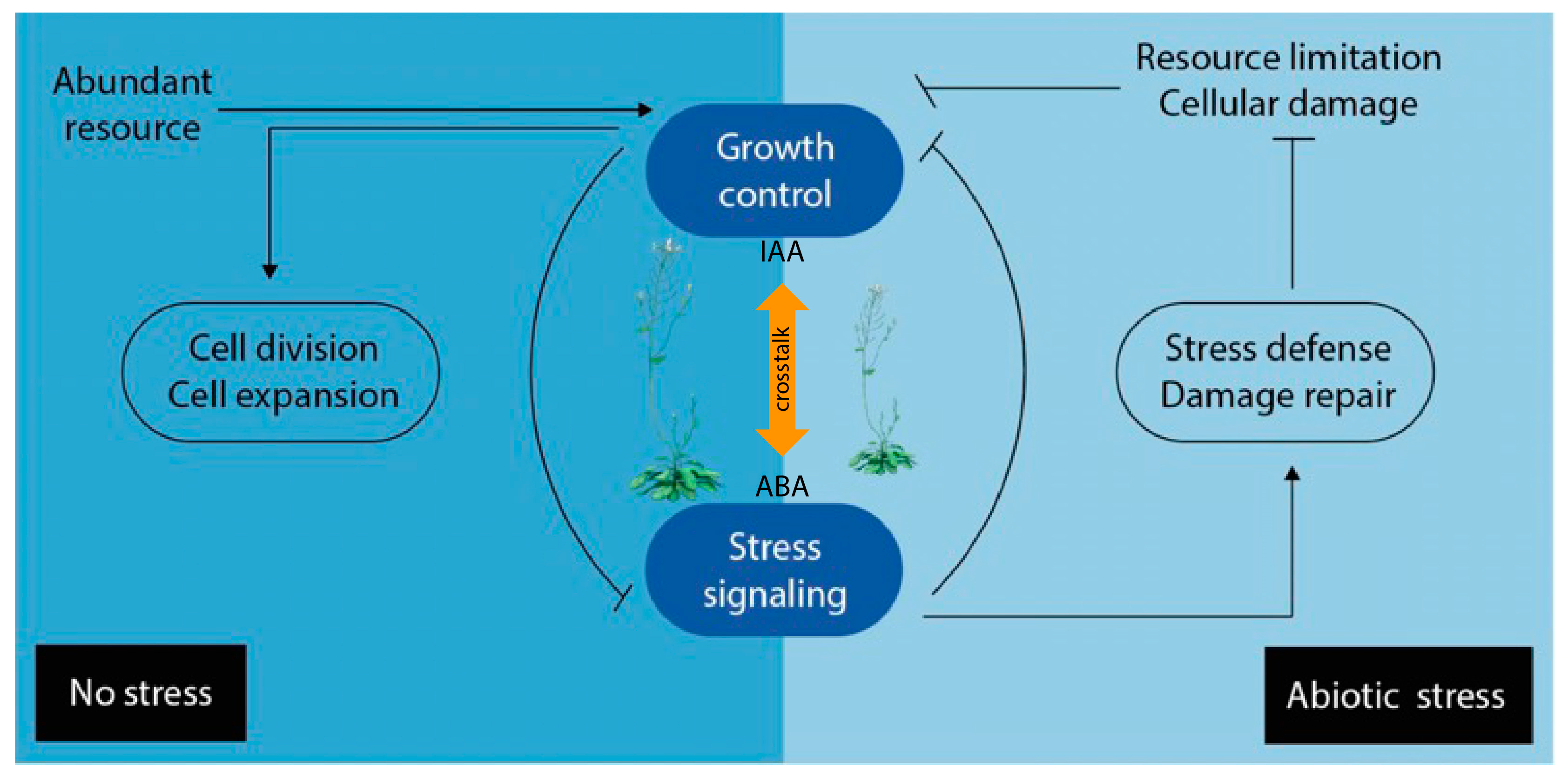
4. Plant hormone crosstalk
4.1. Crosstalk under abiotic stress conditions
4.2. Further examples for auxin–ABA crosstalk
5. Open challenges in auxin–ABA crosstalk
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mazdiyasni, O.; Aghakouchak, A. Substantial Increase in Concurrent Droughts and Heatwaves in the United States. Proc. Natl. Acad. Sci. USA 2015, 112, 11484–11489. [Google Scholar] [CrossRef] [PubMed]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; Huang, M.; Leitzell, K.; Lonnoy, E.; Matthews, J.B.R.; Maycock, T.K.; Waterfield, T.; Yelekçi, O.; Yu, R.; Zhou, B. (Eds.) IPCC Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. Ros Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Shinozaki, K. Research on Plant Abiotic Stress Responses in the Post-Genome Era: Past, Present and Future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Claeys, H.; Inzé, D. The Agony of Choice: How Plants Balance Growth and Survival under Water-Limiting Conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Azapagic, A.; Shokri, N. Global Predictions of Primary Soil Salinization under Changing Climate in the 21st Century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Mustafa, Y.; İre, P.; Aslinur, Ç.; Yasin, Ö.; Ramazan, B. Plant Responses to Salt Stress. In Plant Breeding, edited by Y. Abdurakhmonov Ibrokhim, 93920. Rijeka: IntechOpen, 2020.
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Affairs, U.N.-D.O.E.a.S. World Population Prospects 2017 - Volume I: Comprehensive Tables, 2021.
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global Food Demand and the Sustainable Intensification of Agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic Strategies for Improving Crop Yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Weiler, E.W. Sensory Principles of Higher Plants. Angew. Chemie Int. Ed. 2003, 42, 392–411. [Google Scholar] [CrossRef]
- Miyakawa, T.; Tanokura, M. Structural Basis for the Regulation of Phytohormone Receptors. Biosci. Biotechnol. Biochem. 2017, 81, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Hormonal Impact on Photosynthesis and Photoprotection in Plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant Hormones Are Versatile Chemical Regulators of Plant Growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hentrich, M.; Pollmann, S. Auxin-Oxylipin Crosstalk: Relationship of Antagonists. J. Integr. Plant Biol. 2011, 53, 429–445. [Google Scholar] [CrossRef]
- Pozo, M.J.; López-Ráez, J.A.; Azcón-Aguilar, C.; García-Garrido, J.M. Phytohormones as Integrators of Environmental Signals in the Regulation of Mycorrhizal Symbioses. New Phytol. 2015, 205, 1431–1436. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, M.; Böttcher, C.; Düchting, P.; Cheng, Y.; Zhao, Y.; Berkowitz, O.; Masle, J.; Medina, J.; Pollmann, S. The Jasmonic Acid Signaling Pathway Is Linked to Auxin Homeostasis through the Modulation of YUCCA8 and YUCCA9 Gene Expression. Plant J. 2013, 74, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alonso, M.M.; Sánchez-Parra, B.; Ortiz-García, P.; Santamaria, M.E.; Diaz, I.; Pollmann, S. Jasmonic Acid-Dependent Myc Transcription Factors Bind to a Tandem G-Box Motif in the YUCCA8 and YUCCA9 Promoters to Regulate Biotic Stress Responses. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Perrot-Rechenmann, C.; Napier, R.M. Auxins. Vitam. Horm. 2005, 72, 203–233. [Google Scholar] [PubMed]
- Davies, P.J. Plant Hormones. Biosynthesis, Signal Transduction, Action! 3 ed. Dordrecht, Boston, London: Springer Netherlands, 2010.
- Went, F.W. Auxin, the Plant Growth-Hormone. Bot. Rev. 1935, 1, 162–182. [Google Scholar] [CrossRef]
- Went, F.W.; Thimann, K.V. Phytohormones. New York: Macmillan Company, 1937.
- Won, C.; Shen, X.; Mashiguchi, K.; Zheng, Z.; Dai, X.; Cheng, Y.; Kasahara, H.; Kamiya, Y.; Chory, J.; Zhao, Y. Conversion of Tryptophan to Indole-3-Acetic Acid by Tryptophan Aminotransferases of Arabidopsis and Yuccas in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18518–18523. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.; Benavente, L.M.; Xie, D.Y.; Dolezal, K.; Schlereth, A.; Jürgens, G.; Alonso, J.M. Taa1-Mediated Auxin Biosynthesis Is Essential for Hormone Crosstalk and Plant Development. Cell 2008, 133, 177–191. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Yun, J.; Robles, L.M.; Novak, O.; He, W.; Guo, H.; Ljung, K.; Alonso, J.M. The Arabidopsis Yucca1 Flavin Monooxygenase Functions in the Indole-3-Pyruvic Acid Branch of Auxin Biosynthesis. Plant Cell 2011, 23, 3961–3973. [Google Scholar] [CrossRef] [PubMed]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H.; Mcsteen, P.; Zhao, Y.; Hayashi, K.; Kamiya, Y.; Kasahara, H. The Main Auxin Biosynthesis Pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef] [PubMed]
- Tivendale, N.D.; Ross, J.J.; Cohen, J.D. The Shifting Paradigms of Auxin Biosynthesis. Trends Plant Sci. 2014, 19, 44–51. [Google Scholar] [CrossRef]
- Wright, A.D.; Sampson, M.B.; Neuffer, M.G.; Michalczuk, L.; Slovin, J.P.; Cohen, J.D. Indole-3-Acetic Acid Biosynthesis in the Mutant Maize Orange Pericarp, a Tryptophan Auxotroph. Science 1991, 254, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Normanly, J.; Cohen, J.D.; Fink, G.R. Arabidopsis Thaliana Auxotrophs Reveal a Tryptophan-Independent Biosynthetic Pathway for Indole-3-Acetic Acid. Proc. Natl. Acad. Sci. USA 1993, 90, 10355–10359. [Google Scholar] [CrossRef]
- Müller, A.; Weiler, E.W. Indolic Constituents and Indole-3-Acetic Acid Biosynthesis in the Wild-Type and a Tryptophan Auxotroph Mutant of Arabidopsis thaliana. Planta 2000, 211, 855–63. [Google Scholar] [CrossRef]
- Wang, B.; Chu, J.; Yu, T.; Xu, Q.; Sun, X.; Yuan, J.; Xiong, G.; Wang, G.; Wang, Y.; Li, J. Tryptophan-Independent Auxin Biosynthesis Contributes to Early Embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 4821–48226. [Google Scholar] [CrossRef]
- Tao, Y.; Ferrer, J.L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J.; Cheng, Y.; Lim, J.; Zhao, Y.; Ballare, C.L.; Sandberg, G.; Noel, J.P.; Chory, J. Rapid Synthesis of Auxin Via a New Tryptophan-Dependent Pathway Is Required for Shade Avoidance in Plants. Cell 2008, 133, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A Role for Flavin Monooxygenase-Like Enzymes in Auxin Biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, S.; Müller, A.; Weiler, E.W. Many Roads Lead to "Auxin": Of Nitrilases, Synthases, and Amidases. Plant Biol. (Stuttg) 2006, 8, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Nemoto, K. The Pathway of Auxin Biosynthesis in Plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H. Current Aspects of Auxin Biosynthesis in Plants. Biosci. Biotechnol. Biochem. 2016, 80, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.D.; Hansen, C.H.; Wittstock, U.; Halkier, B.A. Cytochrome P450 Cyp79b2 from Arabidopsis Catalyzes the Conversion of Tryptophan to Indole-3-Acetaldoxime, a Precursor of Indole Glucosinolates and Indole-3-Acetic Acid. J. Biol. Chem. 2000, 275, 33712–33717. [Google Scholar] [CrossRef] [PubMed]
- Hull, A.K.; Vij, R.; Celenza, J.L. Arabidopsis Cytochrome P450s That Catalyze the First Step of Tryptophan-Dependent Indole-3-Acetic Acid Biosynthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2379–2384. [Google Scholar] [CrossRef]
- Pérez, V.C.; Dai, R.; Bai, B.; Tomiczek, B.; Askey, B.C.; Zhang, Y.; Rubin, G.M.; Ding, Y.; Grenning, A.; Block, A.K.; Kim, J. Aldoximes Are Precursors of Auxins in Arabidopsis and Maize. New Phytol. 2021, 231, 1449–1461. [Google Scholar] [CrossRef]
- Irmisch, S.; Zeltner, P.; Handrick, V.; Gershenzon, J.; Kollner, T.G. The Maize Cytochrome P450 Cyp79a61 Produces Phenylacetaldoxime and Indole-3-Acetaldoxime in Heterologous Systems and Might Contribute to Plant Defense and Auxin Formation. BMC Plant Biol. 2015, 15, 128. [Google Scholar] [CrossRef]
- Buezo, J.; Esteban, R.; Cornejo, A.; López-Gómez, P.; Marino, D.; Chamizo-Ampudia, A.; Gil, M.J.; Martínez-Merino, V.; Moran, J.F. IAOx Induces the Sur Phenotype and Differential Signalling from IAA under Different Types of Nitrogen Nutrition in Medicago Truncatula Roots. Plant Sci. 2019, 287, 110176. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Hishiyama, S.; Jikumaru, Y.; Hanada, A.; Nishimura, T.; Koshiba, T.; Zhao, Y.; Kamiya, Y.; Kasahara, H. Biochemical Analyses of Indole-3-Acetaldoxime-Dependent Auxin Biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 5430–5435. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.M.; Böttcher, C.; Glawischnig, E. Dissection of the Network of Indolic Defence Compounds in Arabidopsis thaliana by Multiple Mutant Analysis. Phytochemistry 2019, 161, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J. Auxin and Plant-Microbe Interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, S.; Neu, D.; Weiler, E.W. Molecular Cloning and Characterization of an Amidase from Arabidopsis Thaliana Capable of Converting Indole-3-Acetamide into the Plant Growth Hormone, Indole-3-Acetic Acid. Phytochemistry 2003, 62, 293–300. [Google Scholar] [CrossRef]
- Sánchez-Parra, B.; Frerigmann, H.; Pérez-Alonso, M.M.; Carrasco-Loba, V.; Jost, R.; Hentrich, M.; Pollmann, S. Characterization of Four Bifunctional Plant IAM/PAM-Amidohydrolases Capable of Contributing to Auxin Biosynthesis. Plants (Basel) 2014, 3, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Hara, M.; Suzuki, M.; Seki, H.; Muranaka, T.; Mano, Y. The Ntami1 Gene Functions in Cell Division of Tobacco by-2 Cells in the Presence of Indole-3-Acetamide. FEBS Lett. 2009, 583, 487–492. [Google Scholar] [CrossRef]
- Keereetaweep, J.; Blancaflor, E.B.; Hornung, E.; Feussner, I.; Chapman, K.D. Ethanolamide Oxylipins of Linolenic Acid Can Negatively Regulate Arabidopsis Seedling Development. Plant Cell 2013, 25, 3824–3840. [Google Scholar] [CrossRef]
- Aziz, M.; Chapman, K.D. Fatty Acid Amide Hydrolases: An Expanded Capacity for Chemical Communication? Trends Plant Sci. 2020, 25, 236–249. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.M.; Ortiz-García, P.; Moya-Cuevas, J.; Lehmann, T.; Sánchez-Parra, B.; Björk, R.G.; Karim, S.; Amirjani, M.R.; Aronsson, H.; Wilkinson, M.D.; Pollmann, S. Endogenous Indole-3-Acetamide Levels Contribute to the Crosstalk between Auxin and Abscisic Acid, and Trigger Plant Stress Responses in Arabidopsis thaliana. J. Exp. Bot. 2021, 72, 459–475. [Google Scholar] [CrossRef]
- Gao, Y.; Dai, X.; Aoi, Y.; Takebayashi, Y.; Yang, L.; Guo, X.; Zeng, Q.; Yu, H.; Kasahara, H.; Zhao, Y. Two Homologous Indole-3-Acetamide (IAM) Hydrolase Genes Are Required for the Auxin Effects of Iam in Arabidopsis. J. Genet. Genom. 2020, 47, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Petrásek, J.; Friml, J.I. Auxin Transport Routes in Plant Development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, Action, and Interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Michniewicz, M.; Brewer, P.B.; Friml, J. Polar Auxin Transport and Asymmetric Auxin Distribution. The Arabidopsis Book 2007, 5, e0108–e08. [Google Scholar] [PubMed]
- Adamowski, M.; Friml, J. Pin-Dependent Auxin Transport: Action, Regulation, and Evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of Auxin in Regulating Arabidopsis Flower Development. Planta 2006, 223, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Guyomarc'h, S.; Mandel, T.; Reinhardt, D.; Kuhlemeier, C.; Prusinkiewicz, P. A Plausible Model of Phyllotaxis. Proc. Natl. Acad. Sci. USA 2006, 103, 1301–1306. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Auxin in Root Development. Cold Spring Harb. Perspect. Biol. 2022, 14. [Google Scholar] [CrossRef]
- Goyal, A.; Szarzynska, B.; Fankhauser, C. Phototropism: At the Crossroads of Light-Signaling Pathways. Trends Plant Sci. 2013, 18, 393–401. [Google Scholar] [CrossRef]
- Geilfus, C.-M. The Ph of the Apoplast: Dynamic Factor with Functional Impact under Stress. Mol. Plant 2017, 10, 1371–1386. [Google Scholar] [CrossRef]
- Swarup, R.; Bhosale, R. Developmental Roles of Aux1/Lax Auxin Influx Carriers in Plants. Front. Plant Sci. 2019, 10, 1306. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Luo, J. The Pin-Formed Auxin Efflux Carriers in Plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef]
- Geisler, M.; Aryal, B.; Di Donato, M.; Hao, P. A Critical View on Abc Transporters and Their Interacting Partners in Auxin Transport. Plant Cell Physiol. 2017, 58, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-Box Protein Tir1 Is an Auxin Receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Wang, X.J.; Hagen, G.; Guilfoyle, T.J. Aux/Iaa Proteins Are Active Repressors, and Their Stability and Activity Are Modulated by Auxin. Plant Cell 2001, 13, 2809–2822. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.W. Auxin Response Factors. Plant Cell Environ. 2016, 39, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Quint, M.; Gray, W.M. Auxin Signaling. Curr. Opin. Plant Biol. 2006, 9, 448–453. [Google Scholar] [CrossRef]
- Leyser, O. Auxin Signaling. Plant Physiol. 2017, 176, 465–479. [Google Scholar] [CrossRef]
- Hertel, R.; Thomson, K.S.; Russo, V.E.A. In-Vitro Auxin Binding to Particulate Cell Fractions from Corn Coleoptiles. Planta 1972, 107, 325–340. [Google Scholar] [CrossRef]
- Friml, J.; Gallei, M.; Gelová, Z.; Johnson, A.; Mazur, E.; Monzer, A.; Rodriguez, L.; Roosjen, M.; Verstraeten, I.; Živanović, B.D.; Zou, M.; Fiedler, L.; Giannini, C.; Grones, P.; Hrtyan, M.; Kaufmann, W.A.; Kuhn, A.; Narasimhan, M.; Randuch, M.; Rýdza, N.; Takahashi, K.; Tan, S.; Teplova, A.; Kinoshita, T.; Weijers, D.; Rakusová, H. ABP1–TMK Auxin Perception for Global Phosphorylation and Auxin Canalization. Nature 2022, 609, 575–581. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.-K. Regulation of Abscisic Acid Biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Milborrow, B.V. The Pathway of Biosynthesis of Abscisic Acid in Vascular Plants: A Review of the Present State of Knowledge of ABA Biosynthesis. J. Exp. Bot. 2001, 52, 1145–1164. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H.; Takei, K.; Ueda, N.; Hishiyama, S.; Yamaya, T.; Kamiya, Y.; Yamaguchi, S.; Sakakibara, H. Distinct Isoprenoid Origins of Cis- and Trans-Zeatin Biosyntheses in Arabidopsis. J. Biol. Chem. 2004, 279, 14049–14054. [Google Scholar] [CrossRef] [PubMed]
- Addicott, F.T.; Lyon, J.L.; Ohkuma, K.; Thiessen, W.E.; Carns, H.R.; Smith, O.E.; Cornforth, J.W.; Milborrow, B.V.; Ryback, G.; Wareing, P.F. Abscisic Acid: A New Name for Abscisin II (Dormin). Science 1968, 159, 1493. [Google Scholar] [CrossRef]
- Bennet-Clark, T.A.; Kefford, N.P. Chromatography of the Growth Substances in Plant Extracts. Nature 1953, 171, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the Basal Role of ABA – Roles Outside of Stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of Basal Aba in Plant Growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef]
- Dörffling, K. The Discovery of Abscisic Acid: A Retrospect. J. Plant Growth Regul. 2015, 34, 795–808. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Ann. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Müller, M. Foes or Friends: Aba and Ethylene Interaction under Abiotic Stress. Plants (Basel) 2021, 10, 448. [Google Scholar] [CrossRef]
- Barrero, J.M.; Piqueras, P.; González-Guzmán, M.; Serrano, R.; Rodríguez, P.L.; Ponce, M.R.; Micol, J.L. A Mutational Analysis of the ABA1 Gene of Arabidopsis thaliana Highlights the Involvement of ABA in Vegetative Development. J. Exp. Bot. 2005, 56, 2071–2083. [Google Scholar] [CrossRef]
- Perreau, F.; Frey, A.; Effroy-Cuzzi, D.; Savane, P.; Berger, A.; Gissot, L.; Marion-Poll, A. Abscisic Acid-Deficient4 Has an Essential Function in Both Cis-Violaxanthin and Cis-Neoxanthin Synthesis. Plant Physiol. 2020, 184, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- North, H.M.; Almeida, A.D.; Boutin, J.-P.; Frey, A.; To, A.; Botran, L.; Sotta, B.; Marion-Poll, A. The Arabidopsis ABA-Deficient Mutant aba4 Demonstrates That the Major Route for Stress-Induced ABA Accumulation Is Via Neoxanthin Isomers. Plant J. 2007, 50, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Tan, B.C.; Gage, D.A.; Zeevaart, J.a.D.; Mccarty, D.R. Specific Oxidative Cleavage of Carotenoids by Vp14 of Maize. Science 1997, 276, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of Drought Tolerance by Gene Manipulation of 9-Cis-Epoxycarotenoid Dioxygenase, a Key Enzyme in Abscisic Acid Biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-H.; Endo, A.; Zhou, L.; Penney, J.; Chen, H.-C.; Arroyo, A.; Leon, P.; Nambara, E.; Asami, T.; Seo, M.; Koshiba, T.; Sheen, J. A Unique Short-Chain Dehydrogenase/Reductase in Arabidopsis Glucose Signaling and Abscisic Acid Biosynthesis and Functions. Plant Cell 2002, 14, 2723–2743. [Google Scholar] [CrossRef]
- González-Guzmán, M.; Abia, D.; Salinas, J.; Serrano, R.; Rodríguez, P.L. Two New Alleles of the Abscisic Aldehyde Oxidase 3 Gene Reveal Its Role in Abscisic Acid Biosynthesis in Seeds. Plant Physiol. 2004, 135, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Bittner, F.; Oreb, M.; Mendel, R.R. Aba3 Is a Molybdenum Cofactor Sulfurase Required for Activation of Aldehyde Oxidase and Xanthine Dehydrogenase in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 40381–40384. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Scazzocchio, C.; Fluhr, R. The Absence of Molybdenum Cofactor Sulfuration Is the Primary Cause of the Flacca Phenotype in Tomato Plants. Plant J. 2002, 31, 305–317. [Google Scholar] [CrossRef]
- Léon-Kloosterziel, K.M.; Gil, M.A.; Ruijs, G.J.; Jacobsen, S.E.; Olszewski, N.E.; Schwartz, S.H.; Zeevaart, J.a.D.; Koornneef, M. Isolation and Characterization of Abscisic Acid-Deficient Arabidopsis Mutants at Two New Loci. Plant J. 1996, 10, 655–661. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R.; Lips, S.H. Aldehyde Oxidase and Xanthine Dehydrogenase in a Flacca Tomato Mutant with Deficient Abscisic Acid and Wilty Phenotype. Plant Physiol. 1999, 120, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, J.; He, J.; Chen, Q.; Li, X.; Yang, Y. Molecular Mechanism for the Regulation of Aba Homeostasis During Plant Development and Stress Responses. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Dahuja, A.; Kumar, R.R.; Sakhare, A.; Watts, A.; Singh, B.; Goswami, S.; Sachdev, A.; Praveen, S. Role of Atp-Binding Cassette Transporters in Maintaining Plant Homeostasis under Abiotic and Biotic Stresses. Physiol. Planta. 2021, 171, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Miyaji, T.; Yabuuchi, H.; Shimizu, H.; Sugimoto, E.; Kamiya, A.; Moriyama, Y.; Shinozaki, K. Abc Transporter Atabcg25 Is Involved in Abscisic Acid Transport and Responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2361–2366. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.-U.; Lee, M.; Kim, Y.-Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. Pdr-Type Abc Transporter Mediates Cellular Uptake of the Phytohormone Abscisic Acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kilambi, H.V.; Liu, J.; Bar, H.; Lazary, S.; Egbaria, A.; Ripper, D.; Charrier, L.; Belew, Z.M.; Wulff, N.; Damodaran, S.; Nour-Eldin, H.H.; Aharoni, A.; Ragni, L.; Strader, L.; Sade, N.; Weinstain, R.; Geisler, M.; Shani, E. Aba Homeostasis and Long-Distance Translocation Are Redundantly Regulated by Abcg Aba Importers. Sci. Adv. 2021, 7, eabf6069. [Google Scholar] [CrossRef]
- Kanno, Y.; Hanada, A.; Chiba, Y.; Ichikawa, T.; Nakazawa, M.; Matsui, M.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of an Abscisic Acid Transporter by Functional Screening Using the Receptor Complex as a Sensor. Proc. Natl. Acad. Sci. USA 2012, 109, 9653–9658. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.; Pan, Y.; Yu, Y.; Luan, S.; Li, L. A Dtx/Mate-Type Transporter Facilitates Abscisic Acid Efflux and Modulates Aba Sensitivity and Drought Tolerance in Arabidopsis. Mol. Plant 2014, 7, 1522–1532. [Google Scholar] [CrossRef]
- Boursiac, Y.; Léran, S.; Corratgé-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA Transport and Transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Lozano-Juste, J.; Albert, A. Pyr/Pyl/Rcar Aba Receptors. In Advances in Botanical Research, edited by Mitsunori Seo and Annie Marion-Poll, 51-82: Academic Press, 2019.
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of Pp2c Phosphatase Activity Function as Abscisic Acid Sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.F.; Alfred, S.E.; Bonetta, D.; Finkelstein, R.; Provart, N.J.; Desveaux, D.; Rodriguez, P.L.; Mccourt, P.; Zhu, J.-K.; Schroeder, J.I.; Volkman, B.F.; Cutler, S.R. Abscisic Acid Inhibits Type 2c Protein Phosphatases Via the Pyr/Pyl Family of Start Proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Geiger, D.; Scherzer, S.; Mumm, P.; Stange, A.; Marten, I.; Bauer, H.; Ache, P.; Matschi, S.; Liese, A.; Al-Rasheid, K.a.S.; Romeis, T.; Hedrich, R. Activity of Guard Cell Anion Channel SLAC1 Is Controlled by Drought-Stress Signaling Kinase-Phosphatase Pair. Proc. Natl. Acad. Sci. USA 2009, 106, 21425–21430. [Google Scholar] [CrossRef] [PubMed]
- Brandt, B.; Brodsky, D.E.; Xue, S.; Negi, J.; Iba, K.; Kangasjärvi, J.; Ghassemian, M.; Stephan, A.B.; Hu, H.; Schroeder, J.I. Reconstitution of Abscisic Acid Activation of SLAC1 Anion Channel by CPK6 and OST1 Kinases and Branched ABI1 PP2C Phosphatase Action. Proc. Natl. Acad. Sci. USA 2012, 109, 10593–10598. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling Mechanisms in Abscisic Acid-Mediated Stomatal Closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef]
- Ali, A.; Pardo, J.M.; Yun, D.-J. Desensitization of ABA-Signaling: The Swing from Activation to Degradation. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Konopka-Postupolska, D.; Dobrowolska, G. Aba Perception Is Modulated by Membrane Receptor-Like Kinases. J. Exp. Bot. 2020, 71, 1210–1214. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.-Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.-K. In Vitro Reconstitution of an Abscisic Acid Signalling Pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Leopold, A.C.; Noodén, L.D. Hormonal Regulatory Systems in Plants. In Hormonal Regulation of Development Ii: The Functions of Hormones from the Level of the Cell to the Whole Plant, edited by Tom K. Scott, 4-22. Berlin, Heidelberg: Springer Berlin Heidelberg, 1984.
- Chandler, J.W. Auxin as Compère in Plant Hormone Crosstalk. Planta 2009, 231, 1–12. [Google Scholar] [CrossRef]
- Parwez, R.; Aftab, T.; Gill, S.S.; Naeem, M. Abscisic Acid Signaling and Crosstalk with Phytohormones in Regulation of Environmental Stress Responses. Environ. Exp. Bot. 2022, 104885. [Google Scholar] [CrossRef]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk Complexities between Auxin, Cytokinin, and Ethylene in Arabidopsis Root Development: From Experiments to Systems Modeling, and Back Again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Prasad, S.M.; Munné-Bosch, S.; Müller, M. Editorial: Phytohormones and the Regulation of Stress Tolerance in Plants: Current Status and Future Directions. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Gazzarrini, S.; Mccourt, P. Cross-Talk in Plant Hormone Signalling: What Arabidopsis Mutants Are Telling Us. Ann. Bot. 2003, 91, 605–12. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The Dilemma of Plants: To Grow or Defend. Quart. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [PubMed]
- Emenecker, R.J.; Strader, L.C. Auxin-Abscisic Acid Interactions in Plant Growth and Development. Biomolecules 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, N.; Fu, J.; Wang, S.; Li, X.; Xiao, J.; Xiong, L. A GH3 Family Member, OsGH3-2, Modulates Auxin and Abscisic Acid Levels and Differentially Affects Drought and Cold Tolerance in Rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Xiong, L. Endogenous Auxin and Jasmonic Acid Levels Are Differentially Modulated by Abiotic Stresses in Rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-Acetic Acid Improves Drought Tolerance of White Clover Via Activating Auxin, Abscisic Acid and Jasmonic Acid Related Genes and Inhibiting Senescence Genes. BMC Plant Biol. 2020, 20, 150. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Li, M.; Lamin-Samu, A.T.; Yang, D.; Yu, X.; Izhar, M.; Jan, I.; Ali, M.; Lu, G. The Arabidopsis Small Auxin up Rna32 Protein Regulates Aba-Mediated Responses to Drought Stress. Front. Plant Sci. 2021, 12, 625493. [Google Scholar] [CrossRef]
- Kale, L.; Nakurte, I.; Jalakas, P.; Kunga-Jegere, L.; Brosché, M.; Rostoks, N. Arabidopsis Mutant Dnd2 Exhibits Increased Auxin and Abscisic Acid Content and Reduced Stomatal Conductance. Plant Physiol. Biochem. 2019, 140, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-García, P.; Pérez-Alonso, M.M.; González Ortega-Villaizan, A.; Sánchez-Parra, B.; Ludwig-Müller, J.; Wilkinson, M.D.; Pollmann, S. The Indole-3-Acetamide-Induced Arabidopsis Transcription Factor MYB74 Decreases Plant Growth and Contributes to the Control of Osmotic Stress Responses. Front. Plant Sci. 2022, 13, 928386. [Google Scholar] [CrossRef] [PubMed]
- Sirko, A.; Wawrzyńska, A.; Brzywczy, J.; Sieńko, M. Control of ABA Signaling and Crosstalk with Other Hormones by the Selective Degradation of Pathway Components. Int. J. Mol. Sci. 2021, 22, 4638. [Google Scholar] [CrossRef] [PubMed]
- Hugouvieux, V.; Kwak, J.M.; Schroeder, J.I. An Mrna Cap Binding Protein, Abh1, Modulates Early Abscisic Acid Signal Transduction in Arabidopsis. Cell 2001, 106, 477–487. [Google Scholar] [CrossRef]
- Gibson, S.I. Plant Sugar-Response Pathways. Part of a Complex Regulatory Web. Plant Physiol. 2000, 124, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shang, L.; Wang, X.; Xing, Y.; Xu, W.; Zhang, Y.; Wang, T.; Li, H.; Zhang, J.; Ye, Z. Mapk11 Regulates Seed Germination and Aba Signaling in Tomato by Phosphorylating Snrks. J. Exp. Bot. 2020, 72, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated Role of ABA in Seed Maturation, Dormancy, and Germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Essemine, J.; Pang, X.; Chen, H.; Jin, J.; Cai, W. Abscisic Acid Regulates the Root Growth Trajectory by Reducing Auxin Transporter Pin2 Protein Levels in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 632676. [Google Scholar] [CrossRef]
- Wang, L.; Hua, D.; He, J.; Duan, Y.; Chen, Z.; Hong, X.; Gong, Z. Auxin Response Factor2 (Arf2) and Its Regulated Homeodomain Gene Hb33 Mediate Abscisic Acid Response in Arabidopsis. PLOS Genet. 2011, 7, e1002172. [Google Scholar] [CrossRef]
- Cancé, C.; Martin-Arevalillo, R.; Boubekeur, K.; Dumas, R. Auxin Response Factors Are Keys to the Many Auxin Doors. New Phytol. 2022, 235, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, S.; Wu, H.; Wang, H. Protein Levels of Several Arabidopsis Auxin Response Factors Are Regulated by Multiple Factors and Aba Promotes Arf6 Protein Ubiquitination. Int. J. Mol. Sci. 2020, 21, 9437. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, M.J.; Bentsink, L.; Soppe, W.J.J. Molecular Networks Regulating Arabidopsis Seed Maturation, after-Ripening, Dormancy and Germination. New Phytol. 2008, 179, 33–54. [Google Scholar] [CrossRef]
- Kozaki, A.; Aoyanagi, T. Molecular Aspects of Seed Development Controlled by Gibberellins and Abscisic Acids. Int. J. Mol. Sci. 2022, 23, 1876. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin Controls Seed Dormancy through Stimulation of Abscisic Acid Signaling by Inducing ARF-Mediated ABI3 Activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Nonogaki, H.; Carrington, J.C. Repression of Auxin Response Factor10 by Microrna160 Is Critical for Seed Germination and Post-Germination Stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Wang, F.; Zheng, Q.; Niza, V.M.a.G.E.; Downie, A.B.; Perry, S.E. Direct and Indirect Targets of the Arabidopsis Seed Transcription Factor Abscisic Acid Insensitive3. Plant J. 2020, 103, 1679–1694. [Google Scholar] [CrossRef]
- Hussain, S.; Kim, S.H.; Bahk, S.; Ali, A.; Nguyen, X.C.; Yun, D.-J.; Chung, W.S. The Auxin Signaling Repressor Iaa8 Promotes Seed Germination through Down-Regulation of Abi3 Transcription in Arabidopsis. Front. Plant Sci. 2020, 11, 11111. [Google Scholar] [CrossRef]
- Wang, J.; Yan, D.-W.; Yuan, T.-T.; Gao, X.; Lu, Y.-T. A Gain-of-Function Mutation in IAA8 Alters Arabidopsis Floral Organ Development by Change of Jasmonic Acid Level. Plant Mol. Biol. 2013, 82, 71–83. [Google Scholar] [CrossRef]
- Brocard, I.S.M.; Lynch, T.J.; Finkelstein, R.R. Regulation and Role of the Arabidopsis Abscisic Acid-Insensitive 5 Gene in Abscisic Acid, Sugar, and Stress Response. Plant Physiol. 2002, 129, 1533–1543. [Google Scholar] [CrossRef]
- Li, L.; Zhu, T.; Song, Y.; Feng, L.; Farag, E.a.H.; Ren, M. Abscisic Acid Insensitive5 Interacts with Ribosomal S6 Kinase2 to Mediate Aba Responses During Seedling Growth in Arabidopsis. Front. Plant Sci. 2021, 11, 598654. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Li, Z.; Hsu, C.-C.; Liu, X.; Fu, L.; Hou, Y.-J.; Du, Y.; Xie, S.; Zhang, C.; Gao, J.; Cao, M.; Huang, X.; Zhu, Y.; Tang, K.; Wang, X.; Tao, W.A.; Xiong, Y.; Zhu, J.-K. Reciprocal Regulation of the TOR Kinase and ABA Receptor Balances Plant Growth and Stress Response. Mol. Cell 2018, 69, 100-12.e6. [Google Scholar] [CrossRef] [PubMed]
- Schepetilnikov, M.; Makarian, J.; Srour, O.; Geldreich, A.; Yang, Z.; Chicher, J.; Hammann, P.; Ryabova, L.A. Gtpase Rop2 Binds and Promotes Activation of Target of Rapamycin, TOR, in Response to Auxin. EMBO J. 2017, 36, 886–903. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-T.; Xu, H.-H.; Zhang, K.-X.; Guo, T.-T.; Lu, Y.-T. Glucose Inhibits Root Meristem Growth Via Aba Insensitive 5, Which Represses PIN1 Accumulation and Auxin Activity in Arabidopsis. Plant Cel Environ. 2014, 37, 1338–1350. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, Y.; Luo, L.; Tian, W.; Gong, Q.; Liu, X. Abscisic Acid Employs NRP-Dependent PIN2 Vacuolar Degradation to Suppress Auxin-Mediated Primary Root Elongation in Arabidopsis. New Phytol. 2022, 233, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, R.-J.; Han, T.-T.; Cai, W.; Fu, Z.-W.; Lu, Y.-T. Salt Stress Reduces Root Meristem Size by Nitric Oxide-Mediated Modulation of Auxin Accumulation and Signaling in Arabidopsis. Plant Physiol. 2015, 168, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Albertos, P.; Romero-Puertas, M.C.; Tatematsu, K.; Mateos, I.; Sánchez-Vicente, I.; Nambara, E.; Lorenzo, O. S-Nitrosylation Triggers Abi5 Degradation to Promote Seed Germination and Seedling Growth. Nat. Commun. 2015, 6, 8669. [Google Scholar] [CrossRef]
- Manrique-Gil, I.; Sánchez-Vicente, I.; Torres-Quezada, I.; Lorenzo, O. Nitric Oxide Function During Oxygen Deprivation in Physiological and Stress Processes. J. Exp. Bot. 2020, 72, 904–916. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Li, Z.; Qiao, J.; Quan, R.; Wang, J.; Huang, R.; Qin, H. Salt and ABA Response ERF1 Improves Seed Germination and Salt Tolerance by Repressing Aba Signaling in Rice. Plant Physiol. 2022, 189, 1110–1127. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, H.; Li, L.; Zhai, Q.; Qi, L.; Zhou, W.; Liu, X.; Li, H.; Zheng, W.; Sun, J.; Li, C. The Arabidopsis Mediator Subunit Med25 Differentially Regulates Jasmonate and Abscisic Acid Signaling through Interacting with the MYC2 and ABI5 Transcription Factors. Plant Cell 2012, 24, 2898–2916. [Google Scholar] [CrossRef]
- Ito, J.; Fukaki, H.; Onoda, M.; Li, L.; Li, C.; Tasaka, M.; Furutani, M. Auxin-Dependent Compositional Change in Mediator in ARF7- and ARF19-Mediated Transcription. Proc. Natl. Acad. Sci. USA 2016, 113, 6562–6567. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chong, L.; Wu, F.; Hsu, C.-C.; Li, C.; Zhu, J.-K.; Zhu, Y. Mediator Tail Module Subunits Med16 and Med25 Differentially Regulate Abscisic Acid Signaling in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Maymon, T.; Eisner, N.; Bar-Zvi, D. The Abcisic Acid Insensitive (ABI) 4 Transcription Factor Is Stabilized by Stress, Aba and Phosphorylation. Plants 2022, 11, 2179. [Google Scholar] [CrossRef] [PubMed]
- Matilla, A.J. Auxin: Hormonal Signal Required for Seed Development and Dormancy. Plants (Basel) 2020, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Shkolnik-Inbar, D.; Bar-Zvi, D. Abi4 Mediates Abscisic Acid and Cytokinin Inhibition of Lateral Root Formation by Reducing Polar Auxin Transport in Arabidopsis. Plant Cell 2010, 22, 3560–3573. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, U.; Luo, X.; Zhou, W.; Shu, K. Multifaceted Signaling Networks Mediated By abscisic Acid Insensitive 4. Plant Commun. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Nakayama, N.; Smith, Richard s. ; Mandel, T.; Robinson, S.; Kimura, S.; Boudaoud, A.; Kuhlemeier, C. Mechanical Regulation of Auxin-Mediated Growth. Curr. Biol. 2012, 22, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic Acid Regulates Root Growth under Osmotic Stress Conditions Via an Interacting Hormonal Network with Cytokinin, Ethylene and Auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.J.; Yan, J.Y.; Li, C.X.; Li, G.X.; Wu, Y.R.; Zheng, S.J. Transcription Factor WRKY46 Modulates the Development of Arabidopsis Lateral Roots in Osmotic/Salt Stress Conditions Via Regulation of ABA Signaling and Auxin Homeostasis. Plant J. 2015, 84, 56–69. [Google Scholar] [CrossRef]
- Chen, C.; Letnik, I.; Hacham, Y.; Dobrev, P.; Ben-Daniel, B.-H.; Vanková, R.; Amir, R.; Miller, G. Ascorbate Peroxidase 6 Protects Arabidopsis Desiccating and Germinating Seeds from Stress and Mediates Cross Talk between Reactive Oxygen Species, Abscisic Acid, and Auxin. Plant Physiol. 2014, 166, 370–383. [Google Scholar] [CrossRef]
- Chen, C.; Twito, S.; Miller, G. New Cross Talk between ROS, ABA and Auxin Controlling Seed Maturation and Germination Unraveled in Apx6 Deficient Arabidopsis Seeds. Plant Signal. Behav. 2014, 9, e976489. [Google Scholar] [CrossRef]
- Munguía-Rodríguez, A.G.; López-Bucio, J.S.; Ruiz-Herrera, L.F.; Ortiz-Castro, R.; Guevara-García, Á.A.; Marsch-Martínez, N.; Carreón-Abud, Y.; López-Bucio, J.; Martínez-Trujillo, M. Yucca4 Overexpression Modulates Auxin Biosynthesis and Transport and Influences Plant Growth and Development Via Crosstalk with Abscisic Acid in Arabidopsis thaliana. Genet. Mol. Biol. 2020, 43, e20190221. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Qiao, L.; Chen, Y.; Zhang, D.; Jing, X.; Gan, P.; Huang, Y.; Gao, J.; Liu, W.; Shi, C.; Cui, H.; Li, H.; Chen, K. Characterization of Wavy Root 1, an Agravitropism Allele, Reveals the Functions of Ospin2 in Fine Regulation of Auxin Transport and Distribution and in Aba Biosynthesis and Response in Rice (Oryza Sativa L.). Crop J. 2022, 10, 980–992. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Lu, J.; Song, F.; Sun, J.; Wang, C.; Lian, J.; Zhao, L.; Zhao, B. Osiaa20, an Aux/Iaa Protein, Mediates Abiotic Stress Tolerance in Rice through an ABA Pathway. Plant Sci. 2021, 308, 110903. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Niu, H.; Xin, D.; Long, Y.; Wang, G.; Liu, Z.; Li, G.; Zhang, F.; Qi, M.; Ye, Y.; Wang, Z.; Pei, B.; Hu, L.; Yuan, C.; Chen, X. OsIAA18, an Aux/IAA Transcription Factor Gene, Is Involved in Salt and Drought Tolerance in Rice. Front. Plant Sci. 2021, 12, 738660. [Google Scholar] [CrossRef]
- Li, G.; Ye, Y.X.; Ren, X.Q.; Qi, M.Y.; Zhao, H.Y.; Zhou, Q.; Chen, X.H.; Wang, J.; Yuan, C.Y.; Wang, F.B. The Rice Aux/Iaa Transcription Factor Gene Osiaa18 Enhances Salt and Osmotic Tolerance in Arabidopsis. Biol. Planta. 2020, 64, 454–464. [Google Scholar] [CrossRef]
- Li, W.; Dang, C.; Ye, Y.; Wang, Z.; Hu, L.; Zhang, F.; Zhang, Y.; Qian, X.; Shi, J.; Guo, Y.; Zhou, Q.; Wang, T.; Chen, X.; Wang, F. Overexpression of Grapevine VvIAA18 Gene Enhanced Salt Tolerance in Tobacco. Int. J. Mol. Sci. 2020, 21, 1323. [Google Scholar] [CrossRef]
- Shani, E.; Salehin, M.; Zhang, Y.; Sanchez, S.E.; Doherty, C.; Wang, R.; Mangado, C.C.; Song, L.; Tal, I.; Pisanty, O.; Ecker, J.R.; Kay, S.A.; Pruneda-Paz, J.; Estelle, M. Plant Stress Tolerance Requires Auxin-Sensitive Aux/IAA Transcriptional Repressors. Curr. Biol. 2017, 27, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shu, D.; Tan, Z.; Ma, M.; Guo, N.; Gao, S.; Duan, G.; Kuai, B.; Hu, Y.; Li, S.; Cui, D. The Arabidopsis IDD14 Transcription Factor Interacts with bZIP-Type ABFs/AREBs and Cooperatively Regulates Aba-Mediated Drought Tolerance. New Phytol. 2022, 236, 929–942. [Google Scholar] [CrossRef]
- Zhang, T.; Tan, M.; Geng, L.; Li, J.; Xiang, Y.; Zhang, B.; Zhao, Y. New Insight into Comprehensive Analysis of Indeterminate Domain (IDD) Gene Family in Rice. Plant Physiol. Biochem. 2020, 154, 547–556. [Google Scholar] [CrossRef]
- Cui, D.; Zhao, J.; Jing, Y.; Fan, M.; Liu, J.; Wang, Z.; Xin, W.; Hu, Y. The Arabidopsis IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport. PLOS Genet. 2013, 9, e1003759. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, J.; Zhou, J.; Qiao, J.; Li, Y.; Quan, R.; Huang, R. Abscisic Acid Promotes Auxin Biosynthesis to Inhibit Primary Root Elongation in Rice. Plant Physiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.J.; Yan, J.Y.; Li, G.X.; Wu, Z.C.; Zhang, S.Q.; Zheng, S.J. WRKY41 Controls Arabidopsis Seed Dormancy Via Direct Regulation of ABI3 Transcript Levels Not Downstream of Aba. Plant J. 2014, 79, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-T.; Xiang, Z.-X.; Li, W.; Gao, X.; Lu, Y.-T. Osmotic Stress Represses Root Growth by Modulating the Transcriptional Regulation of PIN-Formed3. New Phytol. 2021, 232, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Cackett, L.; Cannistraci, C.V.; Meier, S.; Ferrandi, P.; Pěnčík, A.; Gehring, C.; Novák, O.; Ingle, R.A.; Donaldson, L. Salt-Specific Gene Expression Reveals Elevated Auxin Levels in Arabidopsis thaliana Plants Grown under Saline Conditions. Front. Plant Sci. 2022, 13, 804716. [Google Scholar] [CrossRef] [PubMed]
- Mega, R.; Meguro-Maoka, A.; Endo, A.; Shimosaka, E.; Murayama, S.; Nambara, E.; Seo, M.; Kanno, Y.; Abrams, S.R.; Sato, Y. Sustained Low Abscisic Acid Levels Increase Seedling Vigor under Cold Stress in Rice (Oryza Sativa L.). Sci. Rep. 2015, 5, 13819. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Rahman, A. Cold Stress Response in Arabidopsis thaliana Is Mediated by Gnom ARF-GEF. Plant J. 2019, 97, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Ioio, R.D.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A Genetic Framework for the Control of Cell Division and Differentiation in the Root Meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, K.-X.; Wang, W.-S.; Gong, W.; Liu, W.-C.; Chen, H.-G.; Xu, H.-H.; Lu, Y.-T. Low Temperature Inhibits Root Growth by Reducing Auxin Accumulation Via ARR1/12. Plant Cell Physiol. 2014, 56, 727–736. [Google Scholar] [CrossRef]
- Vissenberg, K.; Claeijs, N.; Balcerowicz, D.; Schoenaers, S. Hormonal Regulation of Root Hair Growth and Responses to the Environment in Arabidopsis. J. Exp. Bot. 2020, 71, 2412–2427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




