Submitted:
31 December 2022
Posted:
04 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
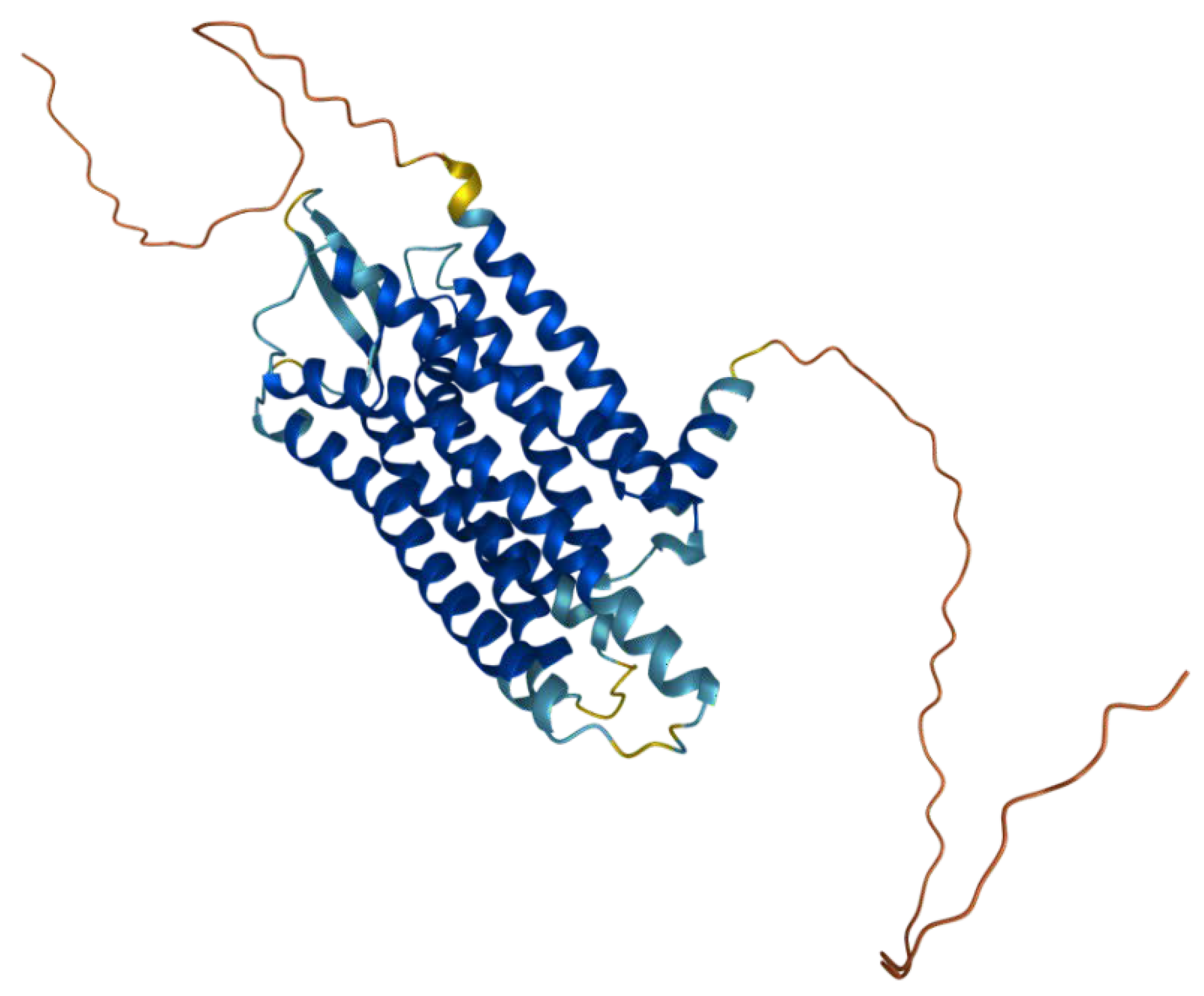
2. Gastrin-releasing peptide receptor - overview
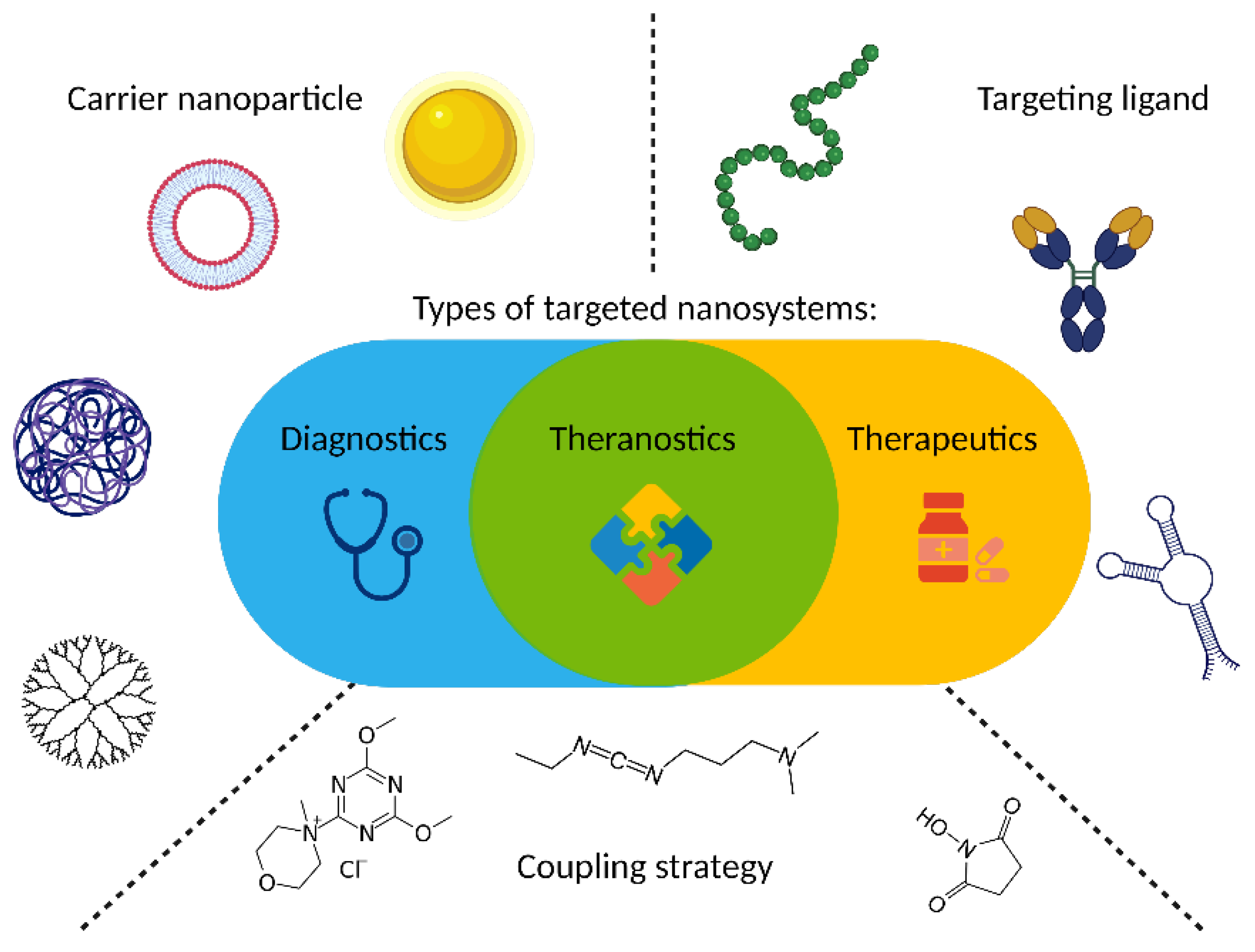
3. GRPR targeting and nanosystems
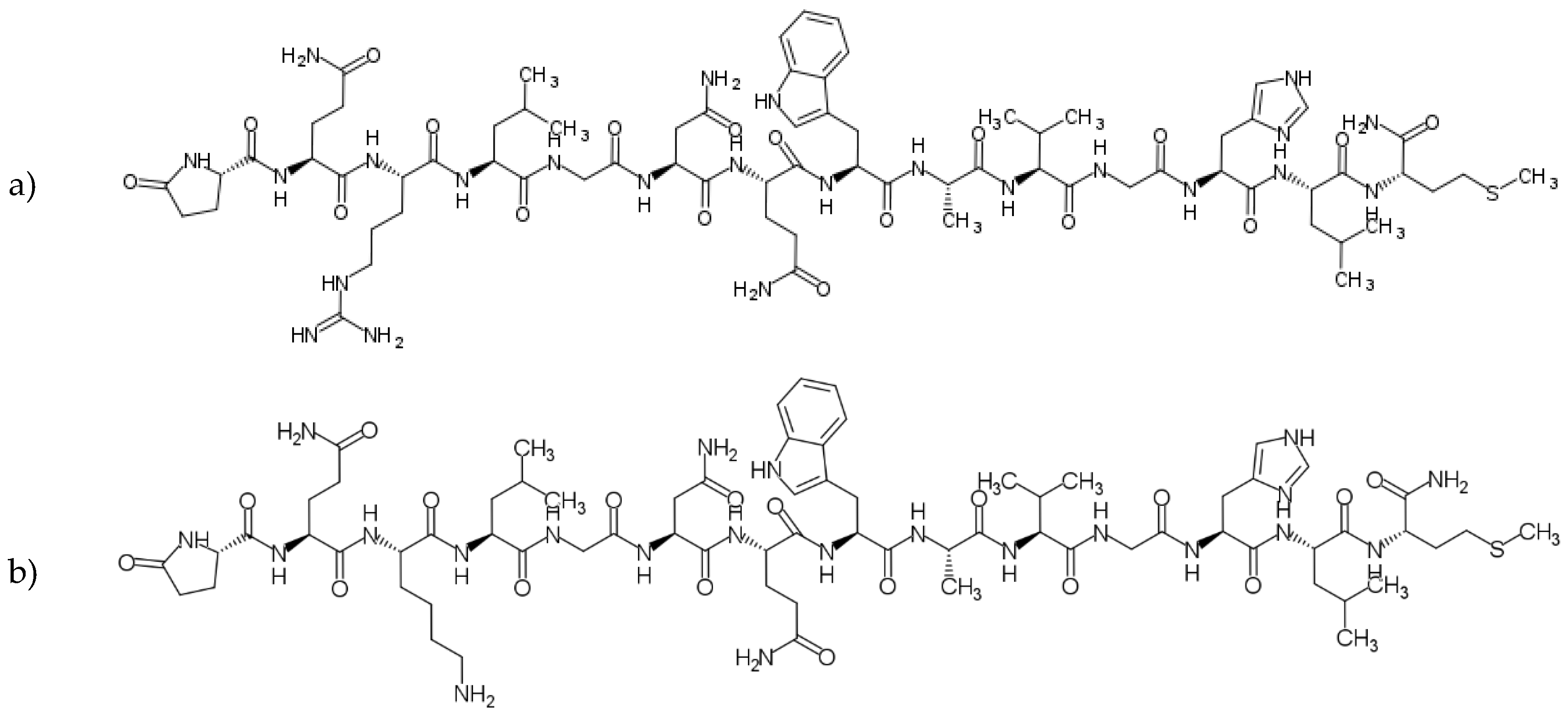
3.1. Targeting ligands
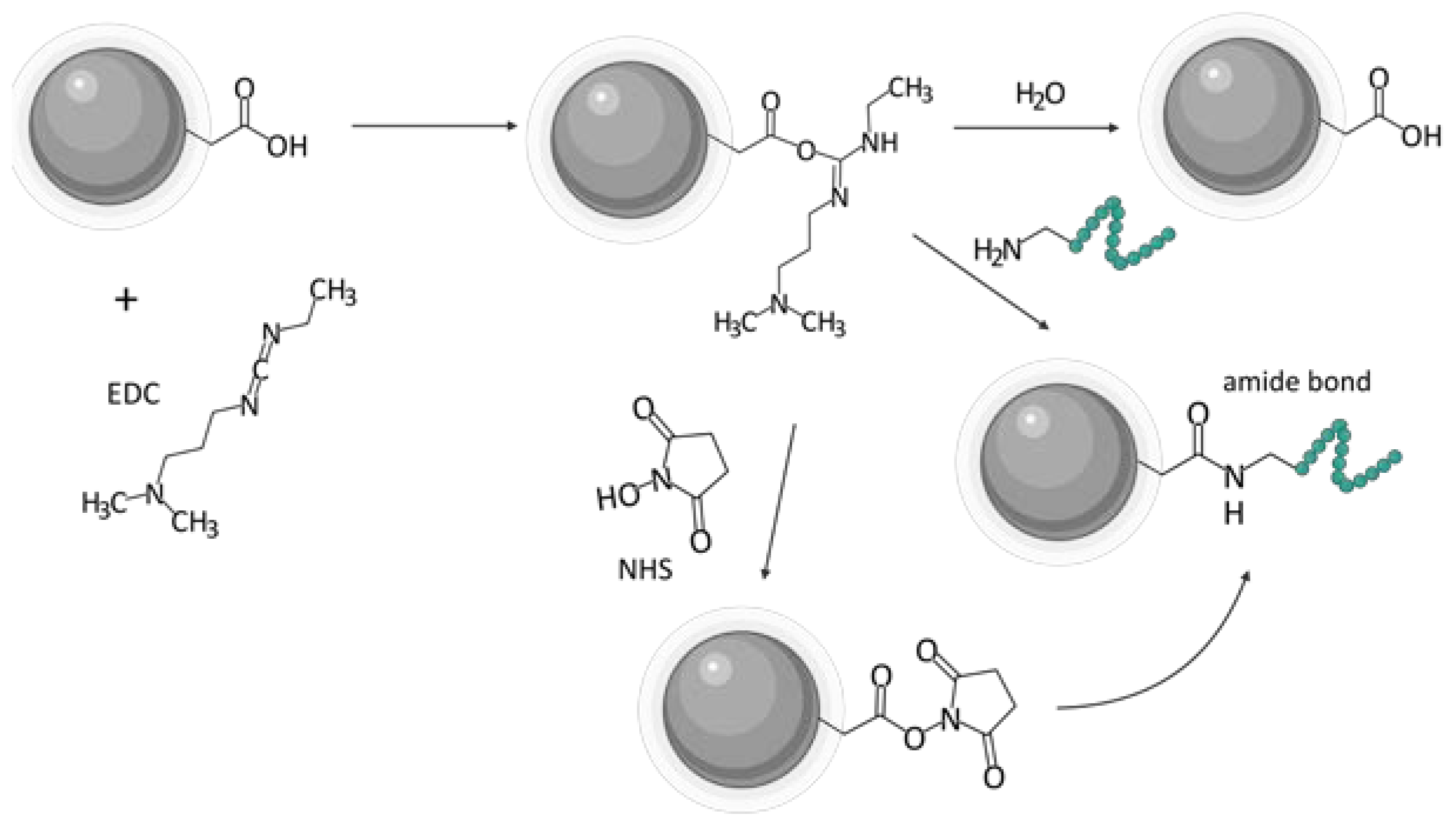
3.2. Ligand incorporation
4. Targeted delivery of biologically active compounds
4.1. Micelles, liposomes, and other self-assembled structures
4.2. Gold nanomaterials
4.3. PLGA nanoparticles
4.4. Other materials and structures
5. Molecular imaging
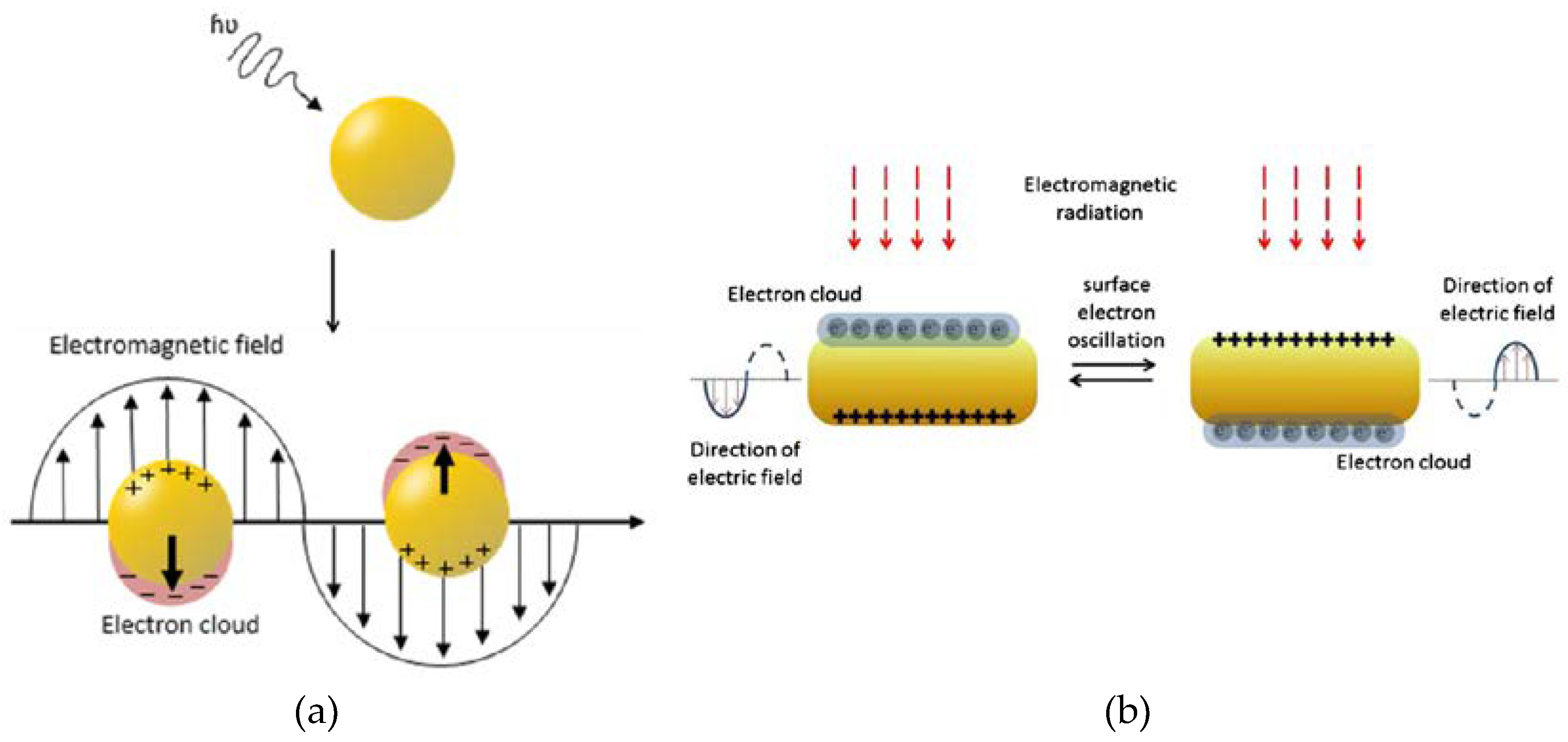
5.1. Gold nanomaterials
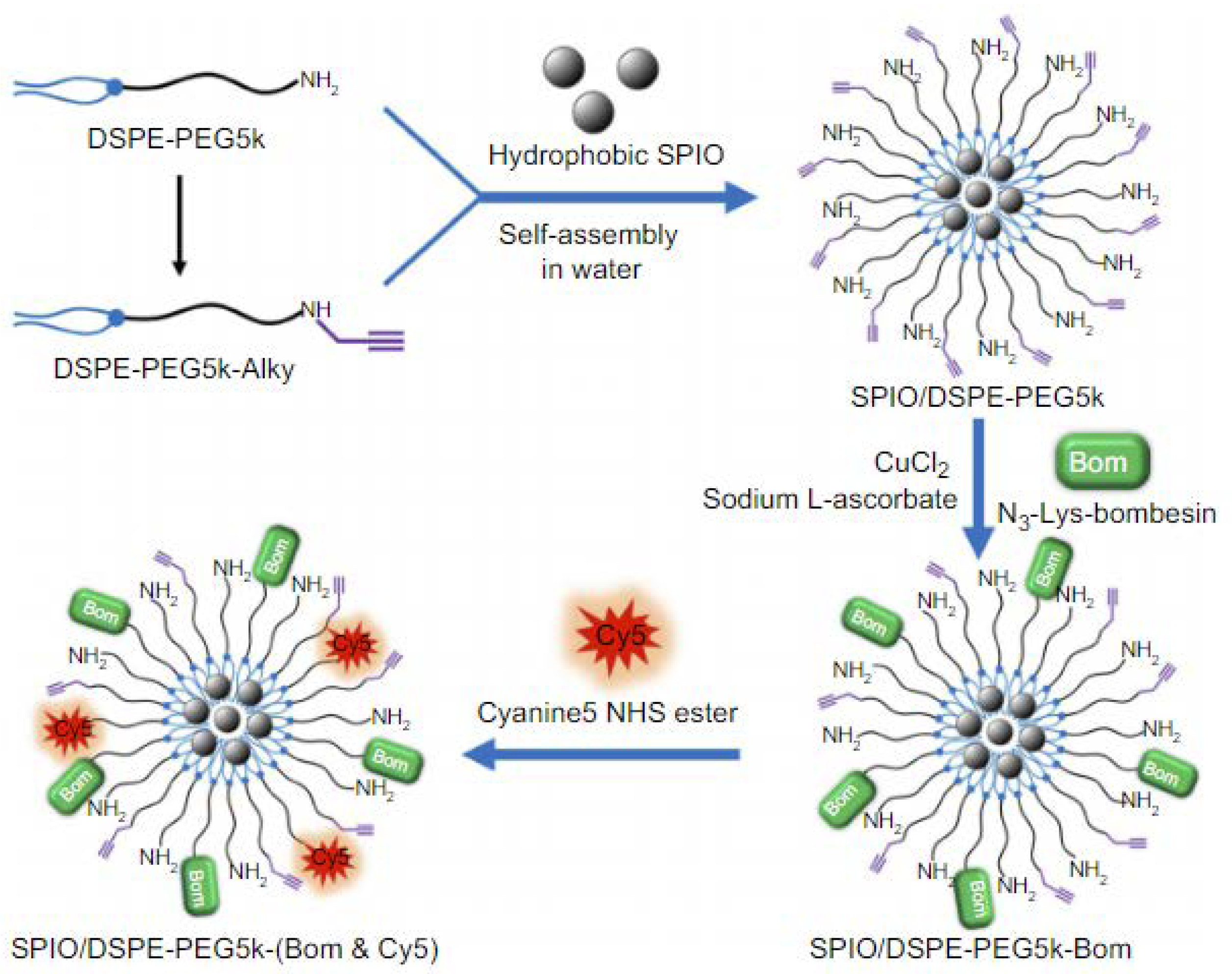
5.2. Iron oxide nanoparticles
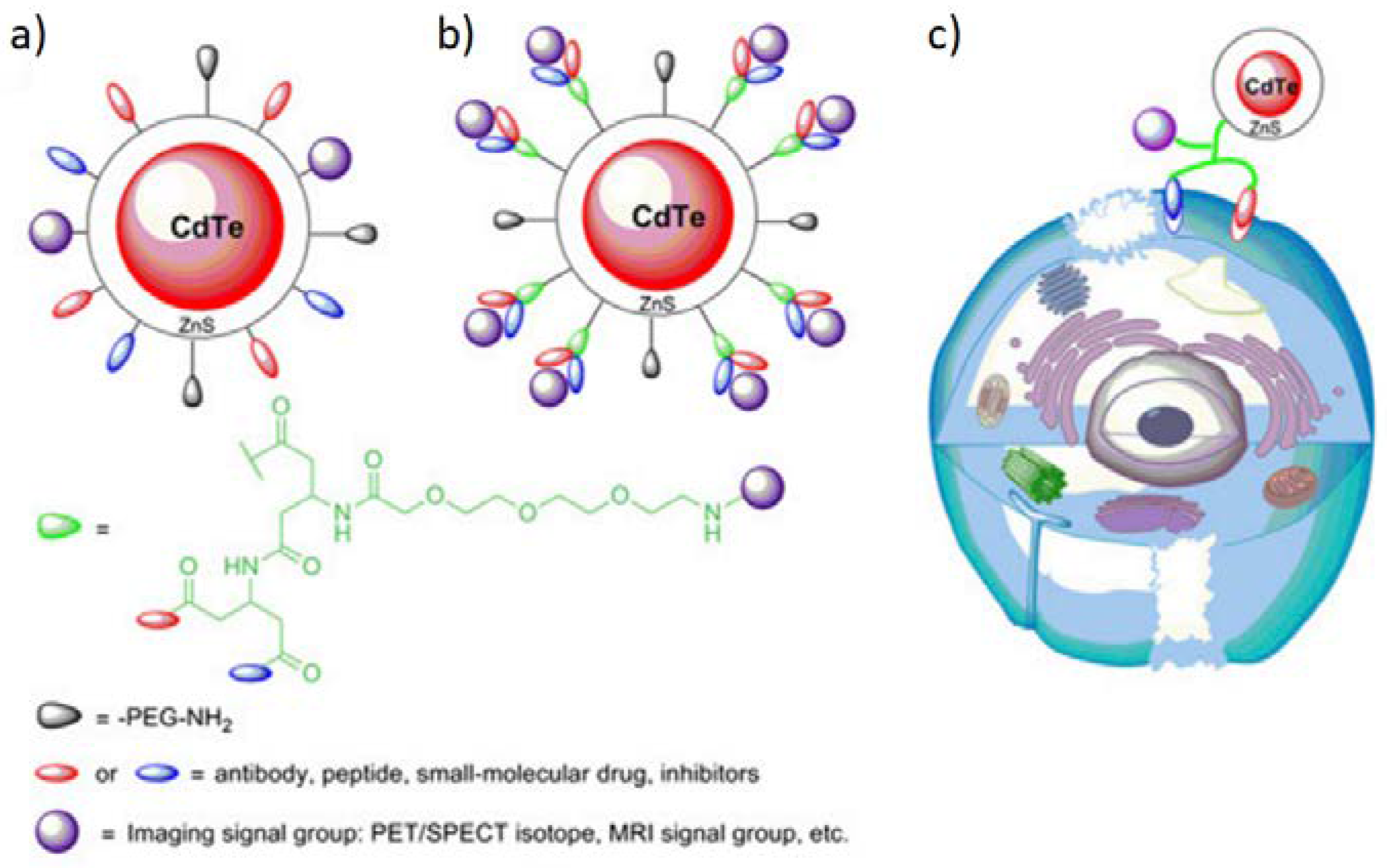
5.3. Quantum Dots
5.4. Other materials
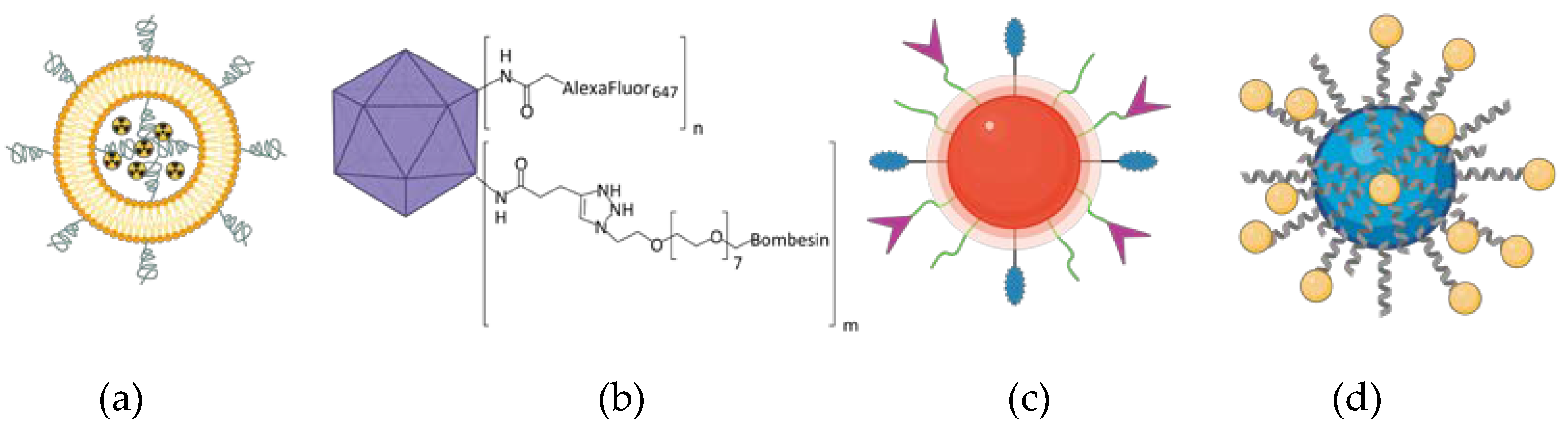
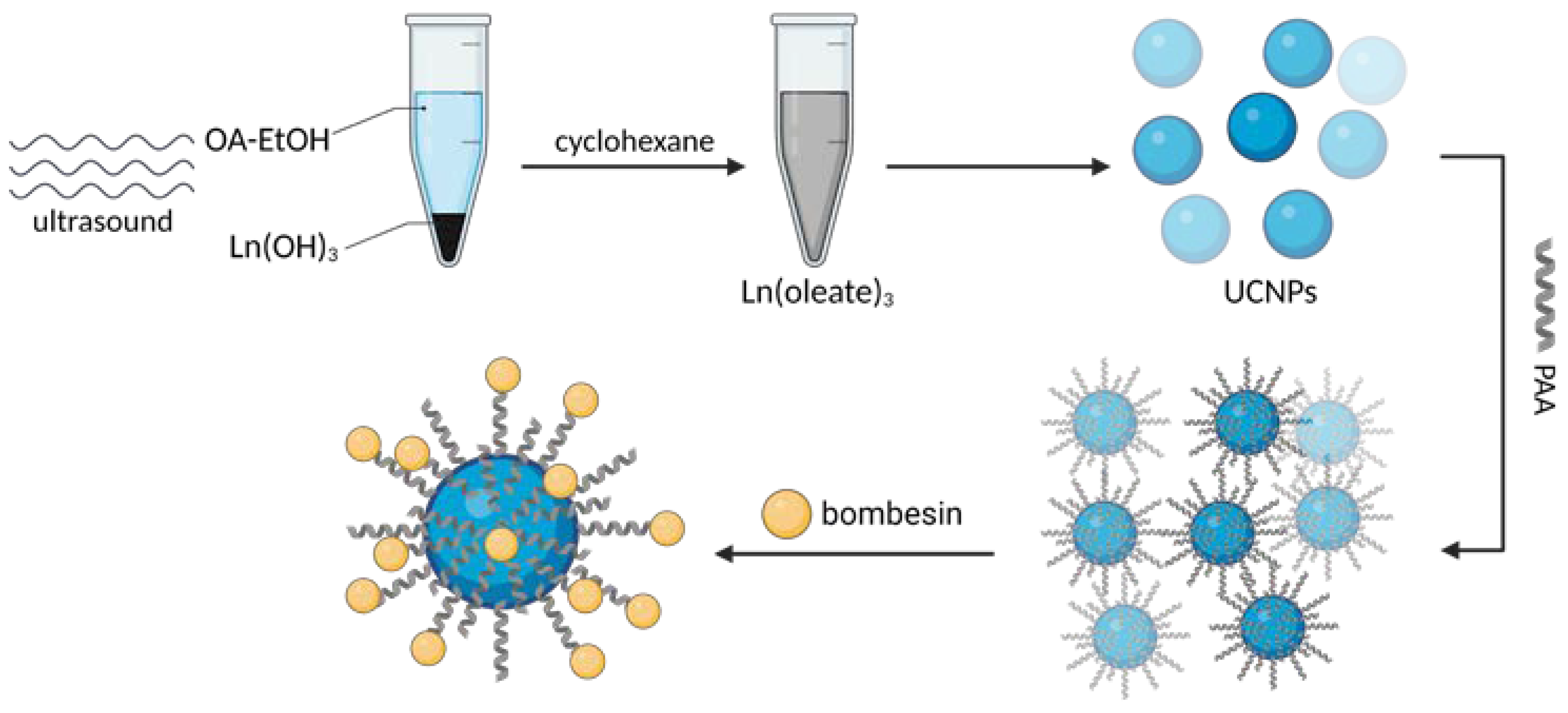
- Besides the beforementioned groups of particles, scientists explore a variety of other nanosystems to target GRPR in bioimaging applications. Liposomes, graphene derivatives, and biobased structures are among the nanomaterials which are gaining more and more attention from the research community (Figure 17). What is important is that some of those are dedicated not only to managing breast and prostate malignancies but also, for example, head and neck cancer. It clearly shows how broad the spectrum of material science applications in nanomedicine can be.
- Moreover, upconversion luminescence (UCL), MRI and CT examinations disclosed that the implementation of the newly synthesized construct greatly enhanced the tumor in vivo visualization in the Nude mice animal model. Interestingly, UCL turned out to be of great importance as the signal could have been observed only 3 minutes post-injection and was still detectable after 4 hours. For magnetic resonance and computed tomography, the signal amelioration was discerned after 4 hours of the administration of UCNPs-BBN, the former method being slightly more efficient as there was a 25% enhancement of MR signal (for CT: 20% signal intensity improvement).
6. Multifunctional particles for theranostic interventions
7. Summary and conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roser, M.; Ritchie, H. Cancer. Available online: https://ourworldindata.org/cancer (accessed on 14.12.2022).
- Arachchige, M.C.M.; Reshetnyak, Y.K.; Andreev, O.A. Advanced targeted nanomedicine. J. Biotechnol. 2015, 202, 88–97. [Google Scholar] [CrossRef]
- Pooja, D.; Gunukula, A.; Gupta, N.; Adams, D.J.; Kulhari, H. Bombesin receptors as potential targets for anticancer drug delivery and imaging. Int. J. Biochem. Cell Biol. 2019, 114, 105567. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, A.R.; Ocampo-García, B.; Pasanphan, W.; Sakr, T.M.; Melendez-Alafort, L.; Grasselli, M.; Lugao, A.B.; Yousefnia, H.; Dispenza, C.; Janib, S.M.; et al. IAEA Contribution to Nanosized Targeted Radiopharmaceuticals for Drug Delivery. Pharmaceutics 2022, 14(5), 1060. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.T. Gastrin-Releasing Peptide. In Encyclopedia of Gastroenterology; Johnson, L.R., Alpers, D.H., Barrett., K.E., Carethers., J.M., Feldman, M., Gores, G.J., Grand, R.J., Kagnoff, M.F., Liddle, R.A., Lu, S., Madara, J.L., II, C.M.M., Margulis, A.R., Williams, J.A., Wilmore, D.W., Wood, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 179–184. [Google Scholar]
- Jensen, R.T.; Battey, J.F.; Spindel, E.R.; Benya, R. V. International union of pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Akeson, M.; Sainz, E.; Mantey, S.A.; Jensen, R.T.; Battey, J.F. Identification of four amino acids in the gastrin-releasing peptide receptor that are required for high affinity agonist binding. J. Biol. Chem. 1997, 272, 17405–17409. [Google Scholar] [CrossRef] [PubMed]
- Tokita, K.; Katsuno, T.; Hocart, S.J.; Coy, D.H.; Llinares, M.; Martinez, J.; Jensen, R.T. Molecular basis for selectivity of high affinity peptide antagonists for the gastrin-releasing peptide receptor. J. Biol. Chem. 2001, 276, 36652–36663. [Google Scholar] [CrossRef] [PubMed]
- UniProt: P30550 GRPR_HUMAN. Available online: https://www.uniprot.org/uniprot/P30550 (accessed on 14.12.2022).
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, 1–16. [Google Scholar] [CrossRef]
- Cescato, R.; Maina, T.; Nock, B.; Nikolopoulou, A.; Charalambidis, D.; Piccand, V.; Reubi, J.C. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J. Nucl. Med. 2008, 49, 318–326. [Google Scholar] [CrossRef]
- Bombesin acetate salt hydrate. Available online: https://www.sigmaaldrich.com/PL/pl/product/sigma/b4272 (accessed on 14.12.2022).
- Kulhari, H.; Kulhari, D.P.; Singh, M.K.; Sistla, R. Colloidal stability and physicochemical characterization of bombesin conjugated biodegradable nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 443, 459–466. [Google Scholar] [CrossRef]
- Kulhari, H.; Pooja, D.; Shrivastava, S.; V.G.M, N.; Sistla, R. Peptide conjugated polymeric nanoparticles as a carrier for targeted delivery of docetaxel. Colloids Surfaces B Biointerfaces 2014, 117, 166–173. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Pooja, D.; Kulhari, H.; Gudem, S.; Ravuri, H.G.; Bhargava, S.; Ramakrishna, S. Bombesin conjugated solid lipid nanoparticles for improved delivery of epigallocatechin gallate for breast cancer treatment. Chem. Phys. Lipids 2019, 224, 104770. [Google Scholar] [CrossRef]
- Cai, H.; Xie, F.; Mulgaonkar, A.; Chen, L.; Sun, X.; Hsieh, J.T.; Peng, F.; Tian, R.; Li, L.; Wu, C.; et al. Bombesin functionalized 64Cu-copper sulfide nanoparticles for targeted imaging of orthotopic prostate cancer. Nanomedicine 2018, 13, 1695–1705. [Google Scholar] [CrossRef]
- Du, J.; Li, L. Which one performs better for targeted lung cancer combination therapy: pre- or post-bombesin-decorated nanostructured lipid carriers? Drug Deliv. 2016, 23, 1799–1809. [Google Scholar] [CrossRef]
- Ocampo-García, B.; Ferro-Flores, G.; Morales-Avila, E.; Ramírez, F.D.M. Kit for preparation of multimeric receptor-specific 99mTc- radiopharmaceuticals based on gold nanoparticles. Nucl. Med. Commun. 2011, 32, 1095–1104. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, A.N.; Ferro-Flores, G.; Ocampo-García, B.E.; Morales-Avila, E.; Ramírez, F.D.M.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; Medina, L.A.; Rojas-Calderón, E.L.; Camacho-López, M.A. Lys3-bombesin conjugated to 99mTc-labelled gold nanoparticles for in vivo gastrin releasing peptide-receptor imaging. J. Biomed. Nanotechnol. 2010, 6, 375–384. [Google Scholar] [CrossRef]
- Bleul, R.; Thiermann, R.; Marten, G.U.; House, M.J.; Pierre, T.G.S.; Häfeli, U.O.; Maskos, M. Continuously manufactured magnetic polymersomes-a versatile tool (not only) for targeted cancer therapy. Nanoscale 2013, 5, 11385–11393. [Google Scholar] [CrossRef]
- [Lys3]-Bombesin. Available online: https://www.sigmaaldrich.com/PL/pl/product/sigma/b1647 (accessed on 14.12.2022).
- Suresh, D.; Zambre, A.; Chanda, N.; Hoffman, T.J.; Smith, C.J.; Robertson, J.D.; Kannan, R. Bombesin peptide conjugated gold nanocages internalize via clathrin mediated endocytosis. Bioconjug. Chem. 2014, 25, 1565–1579. [Google Scholar] [CrossRef]
- Silva, F.; Paulo, A.; Pallier, A.; Même, S.; Tóth, É.; Gano, L.; Marques, F.; Geraldes, C.F.G.C.; Castro, M.M.C.A.; Cardoso, A.M.; et al. Dual imaging gold nanoplatforms for targeted radiotheranostics. Materials (Basel). 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Silva, F.; Gano, L.; Cabral Campello, M.P.; Marques, R.; Prudêncio, I.; Zambre, A.; Upendran, A.; Paulo, A.; Kannan, R. In vitro / in vivo “peeling” of multilayered aminocarboxylate gold nanoparticles evidenced by a kinetically stable 99mTc-label. Dalt. Trans. 2017, 46, 14572–14583. [Google Scholar] [CrossRef] [PubMed]
- Chilug, L.E.; Niculae, D.; Leonte, R.A.; Nan, A.; Turcu, R.; Mustaciosu, C.; Serban, R.M.; Lavric, V.; Manda, G. Preclinical evaluation of NHS-activated gold nanoparticles functionalized with bombesin or neurotensin-like peptides for targeting colon and prostate tumours. Molecules 2020, 25, 3363. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Galli, F.; Mansi, R.; Del Pozzo, L.; Aurilio, M.; Morisco, A.; Ringhieri, P.; Signore, A.; Morelli, G.; Aloj, L. Pre-clinical evaluation of eight DOTA coupled gastrin-releasing peptide receptor (GRP-R) ligands for in vivo targeting of receptor-expressing tumors. EJNMMI Res. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Matusiak, M.; Rurarz, B.P.; Kadłubowski, S.; Wolszczak, M.; Karczmarczyk, U.; Maurin, M.; Kolesińska, B.; Ulański, P. Synthesis and properties of targeted radioisotope carriers based on poly(acrylic acid) nanogels. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- Santos-Cuevas, C.L.; Ferro-Flores, G.; Arteaga de Murphy, C.; Ramírez, F. de M.; Luna-Gutiérrez, M.A.; Pedraza-López, M.; García-Becerra, R.; Ordaz-Rosado, D. Design, preparation, in vitro and in vivo evaluation of 99mTc-N2S2-Tat(49-57)-bombesin: A target-specific hybrid radiopharmaceutical. Int. J. Pharm. 2009, 375, 75–83. [Google Scholar] [CrossRef]
- Hu, K.; Wang, H.; Tang, G.; Huang, T.; Tang, X.; Liang, X.; Yao, S.; Nie, D. In vivo cancer dual-targeting and dual-modality imaging with functionalized quantum dots. J. Nucl. Med. 2015, 56, 1278–1284. [Google Scholar] [CrossRef]
- Trujillo-Benítez, D.; Ferro-Flores, G.; Morales-Avila, E.; Jiménez-Mancilla, N.; Ancira-Cortez, A.; Ocampo-García, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutiérrez, M.; Azorín-Vega, E. Synthesis and biochemical evaluation of samarium-153 oxide nanoparticles functionalized with iPSMA-bombesin heterodimeric peptide. J. Biomed. Nanotechnol. 2020, 16, 689–701. [Google Scholar] [CrossRef]
- Jimenez-Mancilla, N.; Ferro-Flores, G.; Ocampo-Garcia, B.; Luna-Gutierrez, M.; De Maria Ramirez, F.; Pedraza-Lopez, M.; Torres-Garcia, E. Multifunctional targeted radiotherapy system for induced tumours expressing gastrin-releasing peptide receptors. Curr. Nanosci. 2012, 8, 193–201. [Google Scholar] [CrossRef]
- Jiménez-Mancilla, N.; Ferro-Flores, G.; Santos-Cuevas, C.; Ocampo-García, B.; Luna-Gutiérrez, M.; Azorín-Vega, E.; Isaac-Olivé, K.; Camacho-López, M.; Torres-García, E. Multifunctional targeted therapy system based on 99mTc/ 177Lu-labeled gold nanoparticles-Tat(49-57)-Lys3-bombesin internalized in nuclei of prostate cancer cells. J. Label. Compd. Radiopharm. 2013, 56, 663–671. [Google Scholar] [CrossRef]
- Lahooti, A.; Shanehsazzadeh, S.; Laurent, S. Preliminary studies of 68Ga-NODA-USPION-BBN as a dual-modality contrast agent for use in positron emission tomography/magnetic resonance imaging. Nanotechnology 2020, 31, 015102. [Google Scholar] [CrossRef]
- Accardo, A.; Mansi, R.; Salzano, G.; Morisco, A.; Aurilio, M.; Parisi, A.; Maione, F.; Cicala, C.; Ziaco, B.; Tesauro, D.; et al. Bombesin peptide antagonist for target-selective delivery of liposomal doxorubicin on cancer cells. J. Drug Target. 2013, 21, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Ringhieri, P.; Iannitti, R.; Nardon, C.; Palumbo, R.; Fregona, D.; Morelli, G.; Accardo, A. Target selective micelles for bombesin receptors incorporating Au(III)-dithiocarbamato complexes. Int. J. Pharm. 2014, 473, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Mannucci, S.; Nicolato, E.; Vurro, F.; Diaferia, C.; Bontempi, P.; Marzola, P.; Morelli, G. Easy formulation of liposomal doxorubicin modified with a bombesin peptide analogue for selective targeting of GRP receptors overexpressed by cancer cells. Drug Deliv. Transl. Res. 2019, 9, 215–226. [Google Scholar] [CrossRef]
- Li, R.; Gao, R.; Wang, Y.; Liu, Z.; Xu, H.; Duan, A.; Zhang, F.; Ma, L. Gastrin releasing peptide receptor targeted nano-graphene oxide for near-infrared fluorescence imaging of oral squamous cell carcinoma. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Tagliavini, V.; Honisch, C.; Serratì, S.; Azzariti, A.; Bonchio, M.; Ruzza, P.; Carraro, M. Enhancing the biological activity of polyoxometalate-peptide nano-fibrils by spacer design. RSC Adv. 2021, 11, 4952–4957. [Google Scholar] [CrossRef]
- Heidari, Z.; Salouti, M.; Sariri, R. Breast cancer photothermal therapy based on gold nanorods targeted by covalently-coupled bombesin peptide. Nanotechnology 2015, 26, 195101. [Google Scholar] [CrossRef]
- Heidari, Z.; Sariri, R.; Salouti, M. Gold nanorods-bombesin conjugate as a potential targeted imaging agent for detection of breast cancer. J. Photochem. Photobiol. B Biol. 2014, 130, 40–46. [Google Scholar] [CrossRef]
- Salouti, M.; Saghatchi, F. BBN conjugated GNPs: a new targeting contrast agent for imaging of breast cancer in radiology. IET Nanobiotechnology 2017, 11, 604–611. [Google Scholar] [CrossRef]
- Jafari, A.; Salouti, M.; Shayesteh, S.F.; Heidari, Z.; Rajabi, A.B.; Boustani, K.; Nahardani, A. Synthesis and characterization of Bombesin-superparamagnetic iron oxide nanoparticles as a targeted contrast agent for imaging of breast cancer using MRI. Nanotechnology 2015, 26, 075101. [Google Scholar] [CrossRef]
- Martin, A.L.; Hickey, J.L.; Ablack, A.L.; Lewis, J.D.; Luyt, L.G.; Gillies, E.R. Synthesis of bombesin-functionalized iron oxide nanoparticles and their specific uptake in prostate cancer cells. J. Nanoparticle Res. 2010, 12, 1599–1608. [Google Scholar] [CrossRef]
- Steinmetz, N.F.; Ablack, A.L.; Hickey, J.L.; Ablack, J.; Manocha, B.; Mymryk, J.S.; Luyt, L.G.; Lewis, J.D. Intravital imaging of human prostate cancer using viral nanoparticles targeted to gastrin-releasing peptide receptors. Small 2011, 7, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.H.; Wang, J.; Yang, C.X.; Dong, L.X.; Kong, D.; Yan, X.P. Ultrasonic assisted preparation of lanthanide-oleate complexes for the synthesis of multifunctional monodisperse upconversion nanoparticles for multimodal imaging. Nanoscale 2014, 6, 8037–8044. [Google Scholar] [CrossRef] [PubMed]
- Mansour, N.; Paquette, M.; Ait-Mohand, S.; Dumulon-Perreault, V.; Guérin, B. Evaluation of a novel GRPR antagonist for prostate cancer PET imaging: [64Cu]-DOTHA2-PEG-RM26. Nucl. Med. Biol. 2018, 56, 31–38. [Google Scholar] [CrossRef]
- Tangthong, T.; Piroonpan, T.; Thipe, V.C.; Khoobchandani, M.; Katti, K.; Katti, K. V.; Pasanphan, W. Bombesin peptide conjugated water-soluble chitosan gallate—a new nanopharmaceutical architecture for the rapid one-pot synthesis of prostate tumor targeted gold nanoparticles. Int. J. Nanomedicine 2021, 16, 6957–6981. [Google Scholar] [CrossRef]
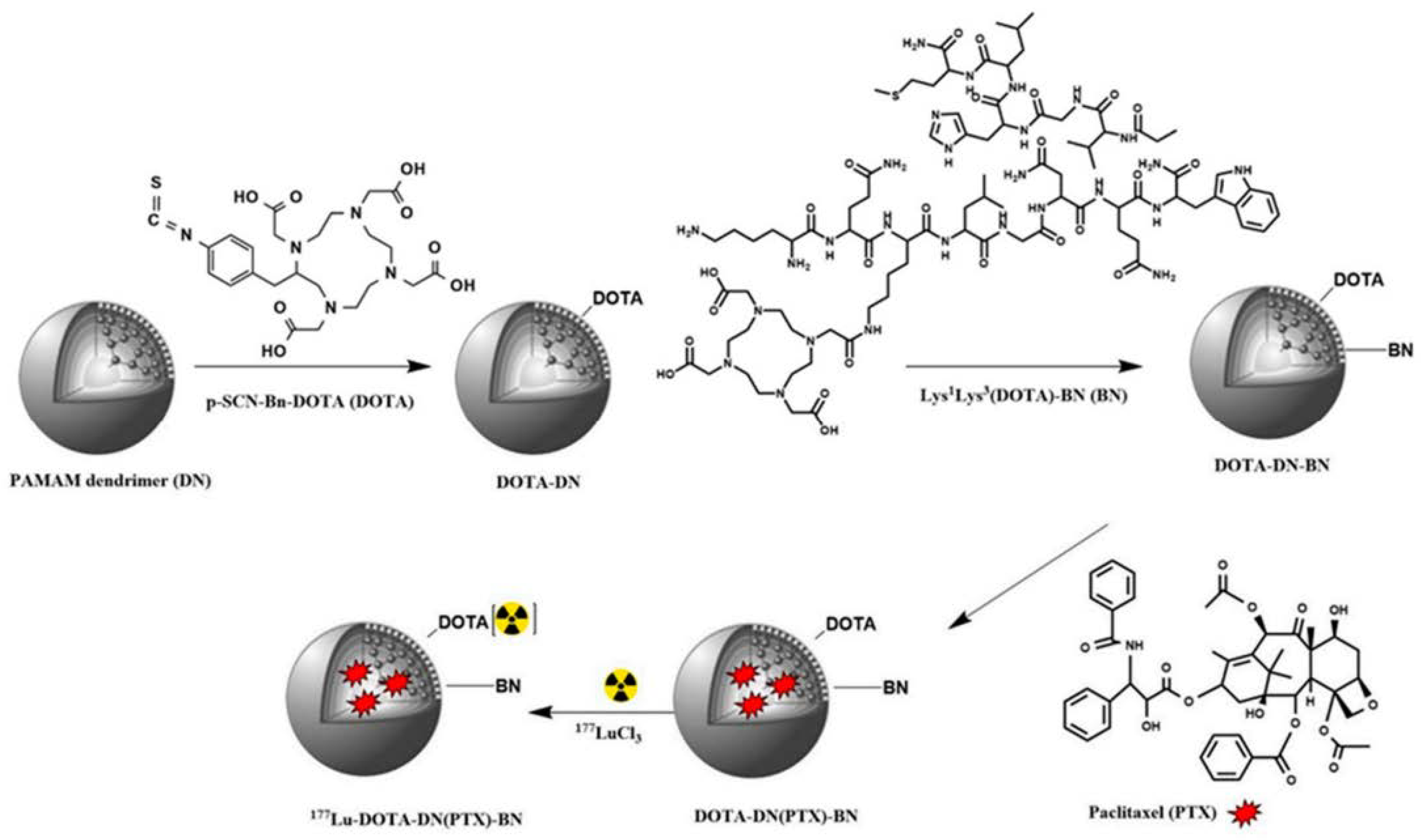
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramírez, F.D.M.; Ocampo-García, B.; Santos-Cuevas, C.; Aranda-Lara, L.; Azorín-Vega, E.; Morales-Avila, E.; Isaac-Olivé, K. 177Lu-dendrimer conjugated to folate and bombesin with gold nanoparticles in the dendritic cavity: a potential theranostic radiopharmaceutical. J. Nanomater. 2016, 2016, ID 1039258. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, M.; Ma, D.; Shen, J.; Fang, F. Engineering of 177Lu-labeled gold encapsulated into dendrimeric nanomaterials for the treatment of lung cancer. J. Biomater. Sci. Polym. Ed. 2022 2022, 33, 197–211. [Google Scholar] [CrossRef]
- Hajiramezanali, M.; Atyabi, F.; Mosayebnia, M.; Akhlaghi, M.; Geramifar, P.; Jalilian, A.R.; Mazidi, S.M.; Yousefnia, H.; Shahhosseini, S.; Beiki, D. (68)Ga-radiolabeled bombesin-conjugated to trimethyl chitosan-coated superparamagnetic nanoparticles for molecular imaging: preparation, characterization and biological evaluation. Int. J. Nanomedicine 2019, 14, 2591–2605. [Google Scholar] [CrossRef]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramírez, F. de M.; Ocampo-García, B.; Santos-Cuevas, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Luna-Gutiérrez, M.; Isaac-Olivé, K. Fluorescent, plasmonic, and radiotherapeutic properties of the 177Lu–dendrimer-AuNP–folate–bombesin nanoprobe located inside cancer cells. Mol. Imaging 2017, 16, 153601211770476. [Google Scholar] [CrossRef] [PubMed]
- Gibbens-Bandala, B.; Morales-Avila, E.; Ferro-Flores, G.; Santos-Cuevas, C.; Meléndez-Alafort, L.; Trujillo-Nolasco, M.; Ocampo-García, B. 177Lu-Bombesin-PLGA (paclitaxel): A targeted controlled-release nanomedicine for bimodal therapy of breast cancer. Mater. Sci. Eng. C 2019, 105, 110043. [Google Scholar] [CrossRef]
- Gibbens-Bandala, B.; Morales-Avila, E.; Ferro-Flores, G.; Santos-Cuevas, C.; Luna-Gutiérrez, M.; Ramírez-Nava, G.; Ocampo-García, B. Synthesis and evaluation of 177Lu-DOTA-DN(PTX)-BN for selective and concomitant radio and drug-therapeutic effect on breast cancer cells. Polymers (Basel). 2019, 11, 1572. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Xu, R.; Wu, X.; Gillespie, D.; Jensen, R.; Lu, Z.R. Targeted systemic delivery of a therapeutic siRNA with a multifunctional carrier controls tumor proliferation in mice. Mol. Pharm. 2009, 6, 738–746. [Google Scholar] [CrossRef]
- Akbar, M.J.; Ferreira, P.C.L.; Giorgetti, M.; Stokes, L.; Morris, C.J. Bombesin receptor-targeted liposomes for enhanced delivery to lung cancer cells. Beilstein J. Nanotechnol. 2019, 10, 2553–2562. [Google Scholar] [CrossRef]
- Li, L.; Wu, C.; Pan, L.; Li, X.; Kuang, A.; Cai, H.; Tian, R. Bombesin-functionalized superparamagnetic iron oxide nanoparticles for dual-modality MR/NIRFI in mouse models of breast cancer. Int. J. Nanomedicine 2019, Volume 14, 6721–6732. [Google Scholar] [CrossRef]
- Hussein, W.M.; Cheong, Y.S.; Liu, C.; Liu, G.; Begum, A.A.; Attallah, M.A.; Moyle, P.M.; Torchilin, V.P.; Smith, R.; Toth, I. Peptide-based targeted polymeric nanoparticles for siRNA delivery. Nanotechnology 2019, 30, 415604. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.S.; Lu, Y.-J.; Chen, H.-A.; Chuang, C.-C.; Chen, J.-P. Magnetic and GRPR-targeted reduced graphene oxide/doxorubicin nanocomposite for dual-targeted chemo-photothermal cancer therapy. Mater. Sci. Eng. C 2021, 128, 112311. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Wang, K.; Wang, Y.; Yang, F.; Wang, H. Breast cancer targeted chemotherapy based on doxorubicin-loaded bombesin peptide modified nanocarriers. Drug Deliv. 2016, 23, 2697–2702. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Mansi, R.; Morisco, A.; Mangiapia, G.; Paduano, L.; Tesauro, D.; Radulescu, A.; Aurilio, M.; Aloj, L.; Arra, C.; et al. Peptide modified nanocarriers for selective targeting of bombesin receptors. Mol. Biosyst. 2010, 6, 878–887. [Google Scholar] [CrossRef]
- Kanazawa, T.; Taki, H.; Okada, H. Nose-to-brain drug delivery system with ligand/cell-penetrating peptide-modified polymeric nano-micelles for intracerebral gliomas. Eur. J. Pharm. Biopharm. 2020, 152, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Neef, T.; Mittelholzer, C.; Garcia Garayoa, E.; Bläuenstein, P.; Schibli, R.; Aebi, U.; Burkhard, P. The biodistribution of self-assembling protein nanoparticles shows they are promising vaccine platforms. J. Nanobiotechnology 2013, 11, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Garg, S.; Eldi, P.; Zhou, F.H. huan; Johnson, I.R.D.; Brooks, D.A.; Lam, F.; Rychkov, G.; Hayball, J.; Albrecht, H. Targeting prostate cancer cells with genetically engineered polypeptide-based micelles displaying gastrin-releasing peptide. Int. J. Pharm. 2016, 513, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Achilli, E.; Flores, C.Y.; Temprana, C.F.; Alonso, S. del V.; Radrizzani, M.; Grasselli, M. Enhanced gold nanoparticle-tumor cell recognition by albumin multilayer coating. OpenNano 2021, 1–12. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.S.; Min, K.H.; Kim, Y.-H.; Chen, X.; Kim, J.; Kim, J.S.; Min, K.H.; Kim, Y.-H.; Chen, X. Bombesin-tethered reactive oxygen species (ROS)-responsive nanoparticles for monomethyl auristatin F (MMAF) delivery. Bioeng. 2021, 8, 43. [Google Scholar] [CrossRef]
- Montet, X.; Weissleder, R.; Josephson, L. Imaging pancreatic cancer with a peptide-nanoparticle conjugate targeted to normal pancreas. Bioconjug. Chem. 2006, 17, 905–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Jeong, H.J.; Cheong, S.J.; Kim, E.M.; Kim, D.W.; Lim, S.T.; Sohn, M.H. Prostate cancer-targeted imaging using magnetofluorescent polymeric nanoparticles functionalized with bombesin. Pharm. Res. 2010, 27, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Chanda, N.; Kattumuri, V.; Shukla, R.; Zambre, A.; Katti, K.; Upendran, A.; Kulkarni, R.R.; Kan, P.; Fent, G.M.; Casteel, S.W.; et al. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 8760–8765. [Google Scholar] [CrossRef] [PubMed]
- Hosta-Rigau, L.; Olmedo, I.; Arbiol, J.; Cruz, L.J.; Kogan, M.J.; Albericio, F. Multifunctionalized gold nanoparticles with peptides targeted to gastrin-releasing peptide receptor of a tumor cell line. Bioconjug. Chem. 2010, 21, 1070–1078. [Google Scholar] [CrossRef]
- Young, S.H.; Rozengurt, E. Qdot Nanocrystal Conjugates conjugated to bombesin or ANG II label the cognate G protein-coupled receptor in living cells. Am. J. Physiol. - Cell Physiol. 2006, 290, 728–732. [Google Scholar] [CrossRef] [PubMed]
- De Barros, A.L.B.; Mota, L.D.G.; Soares, D.C.F.; Coelho, M.M.A.; Oliveira, M.C.; Cardoso, V.N. Tumor bombesin analog loaded long-circulating and pH-sensitive liposomes as tool for tumor identification. Bioorg. Med. Chem. Lett. 2011, 21, 7373–7375. [Google Scholar] [CrossRef]
- De Barros, A.L.B.; Mota, L.D.G.; Soares, D.C.F.; De Souza, C.M.; Cassali, G.D.; Oliveira, M.C.; Cardoso, V.N. Long-circulating, pH-sensitive liposomes versus long-circulating, non-pH-sensitive liposomes as a delivery system for tumor identification. J. Biomed. Nanotechnol. 2013, 9, 1636–1643. [Google Scholar] [CrossRef]
- De Barros, A.L.B.; Das Graças Mota, L.; Coelho, M.M.A.; Corrêa, N.C.R.; De Góes, A.M.; Oliveira, M.C.; Cardoso, V.N. Bombesin encapsulated in long-circulating pH-sensitive liposomes as a radiotracer for breast tumor identification. J. Biomed. Nanotechnol. 2015, 11, 342–350. [Google Scholar] [CrossRef]
- Zhang, W.; Song, Y.; Eldi, P.; Guo, X.; Hayball, J.D.; Garg, S.; Albrecht, H. Targeting prostate cancer cells with hybrid elastin-like polypeptide/liposome nanoparticles. Int. J. Nanomedicine 2018, 13, 293–305. [Google Scholar] [CrossRef]
- Kulhari, H.; Pooja, D.; Singh, M.K.; Kuncha, M.; Adams, D.J.; Sistla, R. Bombesin-conjugated nanoparticles improve the cytotoxic efficacy of docetaxel against gastrin-releasing but androgen-independent prostate cancer. Nanomedicine 2015, 10, 2847–2859. [Google Scholar] [CrossRef]
- Tangthong, T.; Piroonpan, T.; Thipe, V.C.; Khoobchandani, M.; Katti, K.; Katti, K. V.; Pasanphan, W. Water-soluble chitosan conjugated DOTA-bombesin peptide capped gold nanoparticles as a targeted therapeutic agent for prostate cancer. Nanotechnol. Sci. Appl. 2021, 14, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sheng, G.; Lu, L.; Wang, C.; Zhang, Y.; Feng, L.; Meng, L.; Min, P.; Zhang, L.; Wang, Y.; et al. GRPr-mediated photothermal and thermodynamic dual-therapy for prostate cancer with synergistic anti-apoptosis mechanism. Nanoscale 2021, 13, 4249–4261. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, S.A.; Hempel, N.; Bergkvist, M. Development of nanoscale approaches for ovarian cancer therapeutics and diagnostics. Crit. Rev. Oncog. 2014, 19, 281–316. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured lipid carriers: a groundbreaking approach for transdermal drug delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S. Radionanomedicine. Combined Nuclear and Nanomedicine, 1st ed.; Lee, D.S., Ed.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials (Basel). 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Theodosiou, M.; Toniolo, G.; Abu-Thabit, N.Y. 15 - Stimuli-responsive biopolymer nanocarriers for drug delivery applications. In Woodhead Publishing Series in Biomaterials; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Sawston, Great Britain, 2018; pp. 405–432. [Google Scholar]
- Lee, S.; Shanti, A. Effect of exogenous pH on cell growth of breast cancer cells. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Pretze, M.; Hien, A.; Rädle, M.; Schirrmacher, R.; Wängler, C.; Wängler, B. Gastrin-releasing peptide receptor- and prostate-specific membrane antigen-specific ultrasmall gold nanoparticles for characterization and diagnosis of prostate carcinoma via fluorescence imaging. Bioconjug. Chem. 2018, 29, 1525–1533. [Google Scholar] [CrossRef]
- Pretze, M.; van der Meulen, N.P.; Wängler, C.; Schibli, R.; Wängler, B. Targeted 64Cu-labeled gold nanoparticles for dual imaging with positron emission tomography and optical imaging. J. Label. Compd. Radiopharm. 2019, 62, 471–482. [Google Scholar] [CrossRef]
- Chanda, N.; Shukla, R.; Katti, K. V.; Kannan, R. Gastrin releasing protein receptor specific gold nanorods: breast and prostate tumor avid nanovectors for molecular imaging. Nano Lett. 2009, 9, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Lu, X.; Yan, C.; Liu, X.; Hou, M.; Xia, Q.; Xu, Y.; Liu, R. Gastrin-releasing peptide receptor-targeted gadolinium oxide-based multifunctional nanoparticles for dual magnetic resonance/fluorescent molecular imaging of prostate cancer. Int. J. Nanomedicine 2017, 12, 6787–6797. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in cancer treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Mahan, M.M.; Doiron, A.L. Gold Nanoparticles as X-Ray, CT, and Multimodal imaging contrast agents: formulation, targeting, and methodology. J. Nanomater. 2018, 2018, ID 5837276. [Google Scholar] [CrossRef]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef]
- Jokerst, J. V.; Cole, A.J.; Van De Sompel, D.; Gambhir, S.S. Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via Raman imaging in living mice. ACS Nano 2012, 6, 10366–10377. [Google Scholar] [CrossRef]
- Fuller, M.A.; Köper, I. Biomedical applications of polyelectrolyte coated spherical gold nanoparticles. Nano Converg. 2019, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Gotman, I.; Psakhie, S.G.; Lozhkomoev, A.S.; Gutmanas, E.Y. Iron oxide and gold nanoparticles in cancer therapy. AIP Conf. Proc. 2016, 1760, 20020. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic iron oxide nanoparticles: cytotoxicity, metabolism, and cellular behavior in biomedicine applications. Int. J. Nanomedicine 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- Yen, S.K.; Padmanabhan, P.; Selvan, S.T. Multifunctional iron oxide nanoparticles for diagnostics, therapy and macromolecule delivery. Theranostics 2013, 3, 986–1003. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Yu, C.-Y.; Hsu, C.-W.; Lee, W.-C.; Chen, S.-J.; Chang, C.-H.; Lee, T.-W. Molecular imaging and therapeutic efficacy of 188Re-(DXR)-liposome-BBN in AR42J pancreatic tumor-bearing mice. Oncol. Rep. 2012, 28, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Bleul, R.; Thiermann, R.; Saatchi, K.; Häfeli, U.O.; Maskos, M. Multifunctional nanocarriers for biomedical applications. Colloid. Nanocrystals Biomed. Appl. VIII 2013, 8595, 85951N. [Google Scholar] [CrossRef]

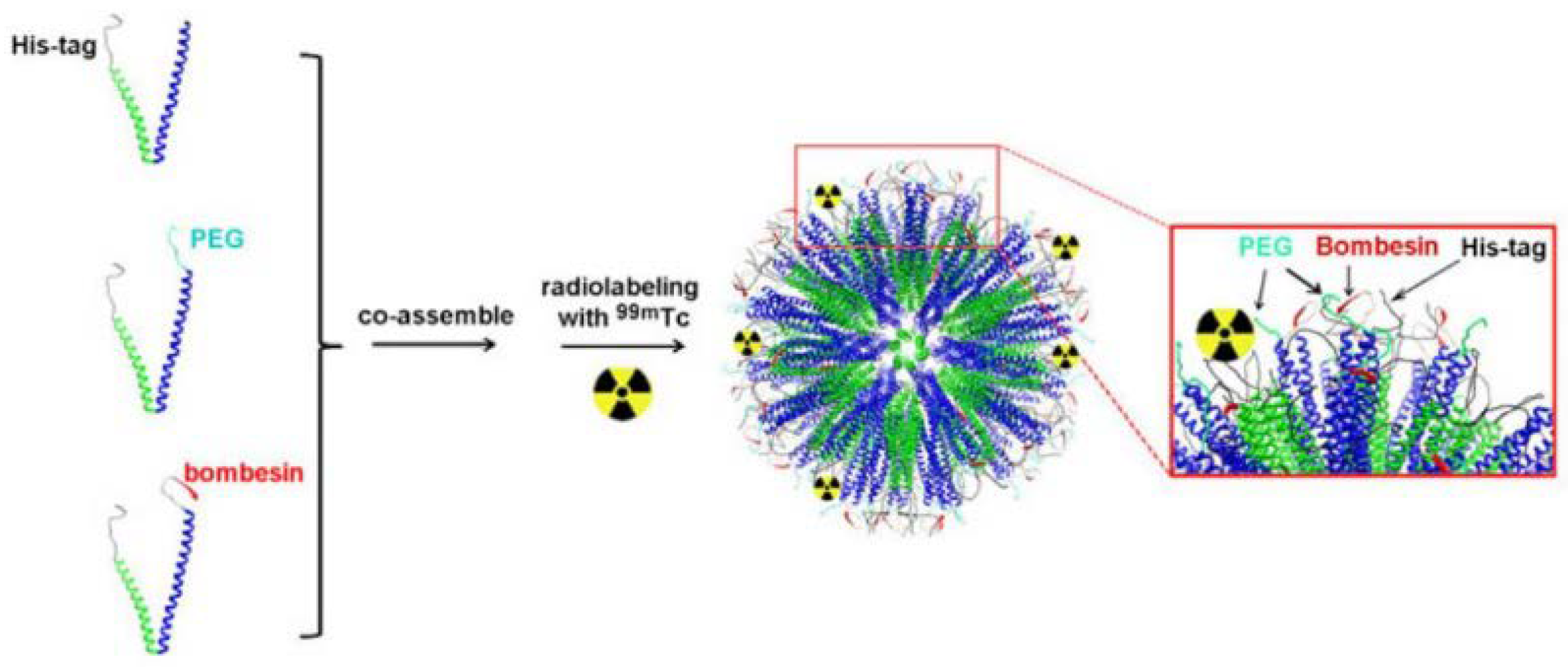
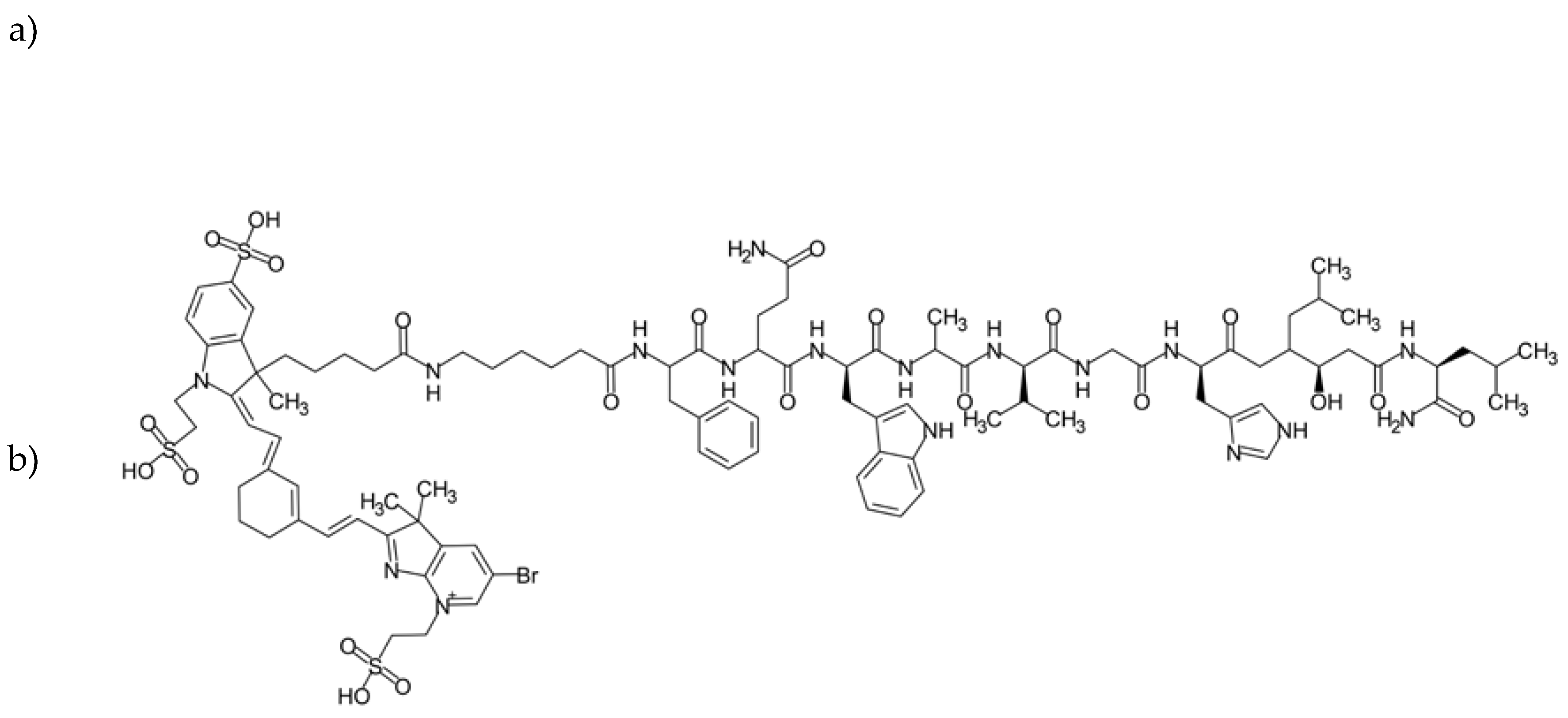
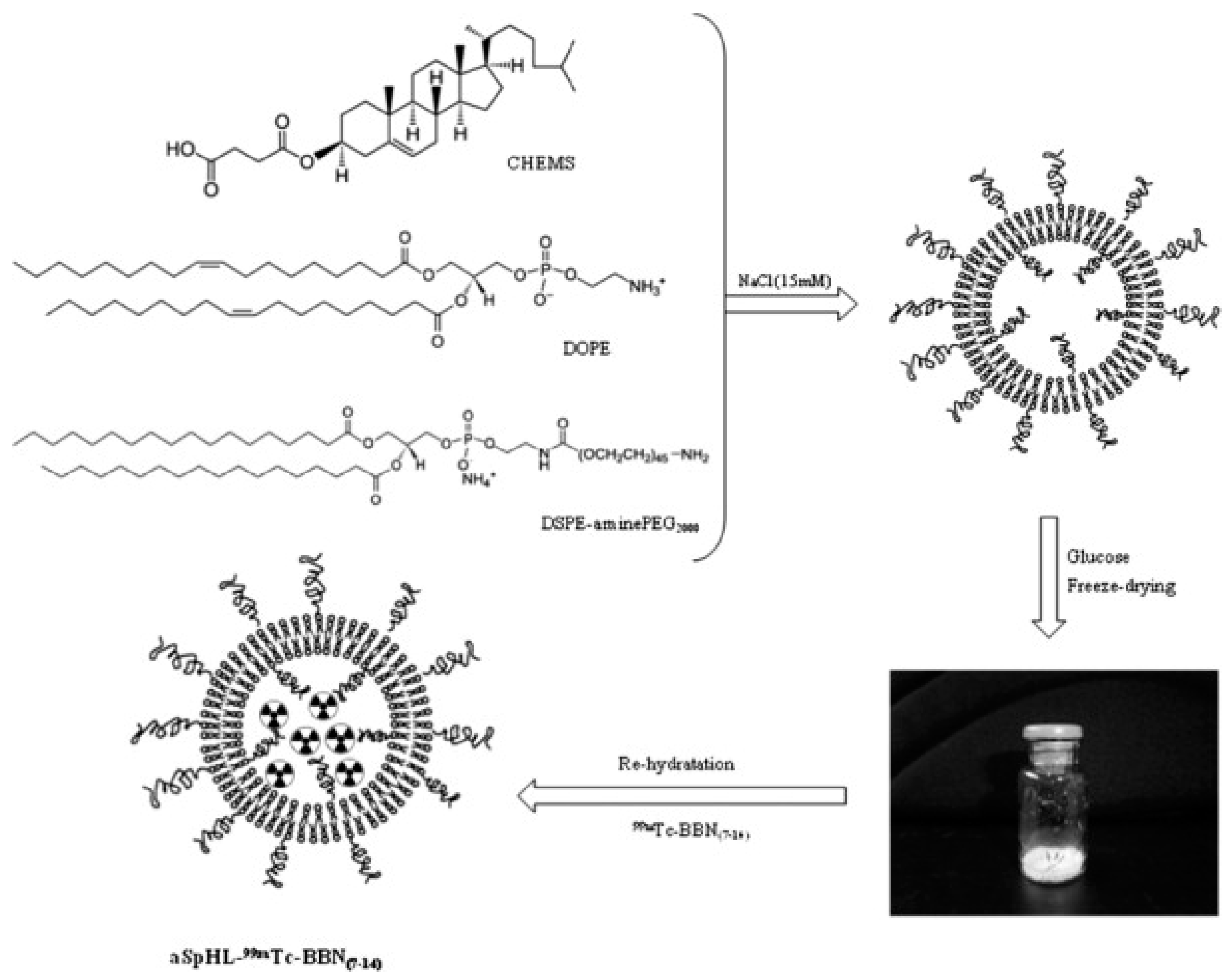
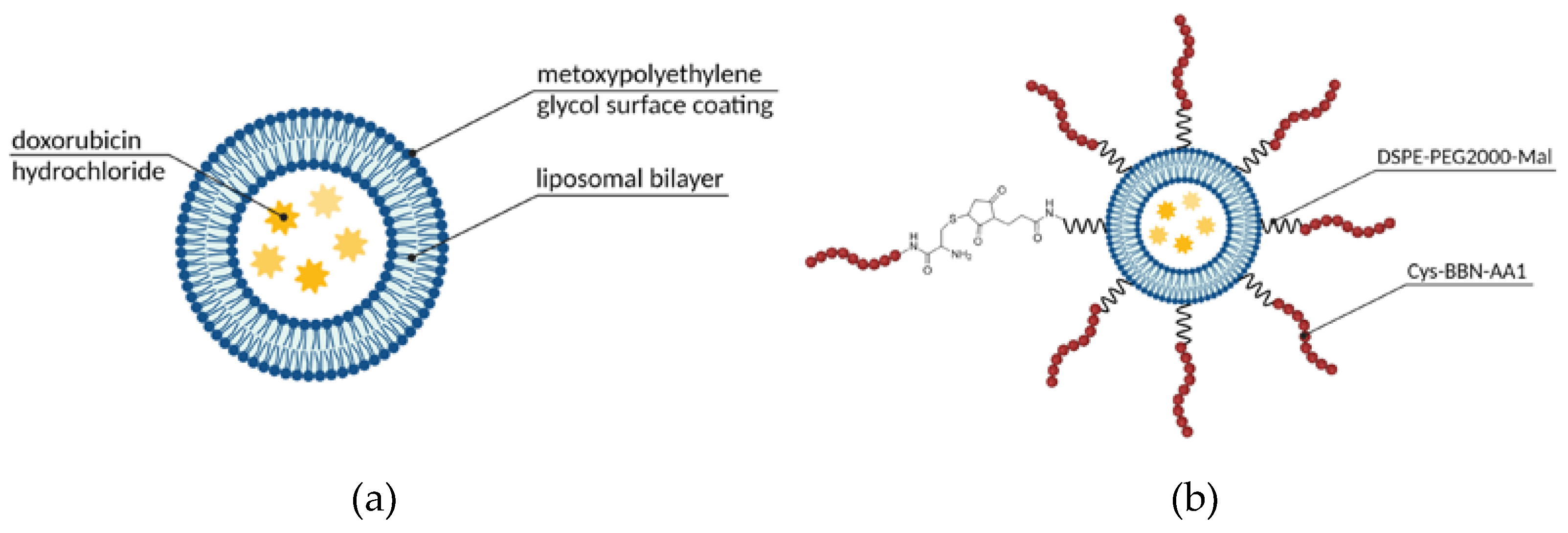
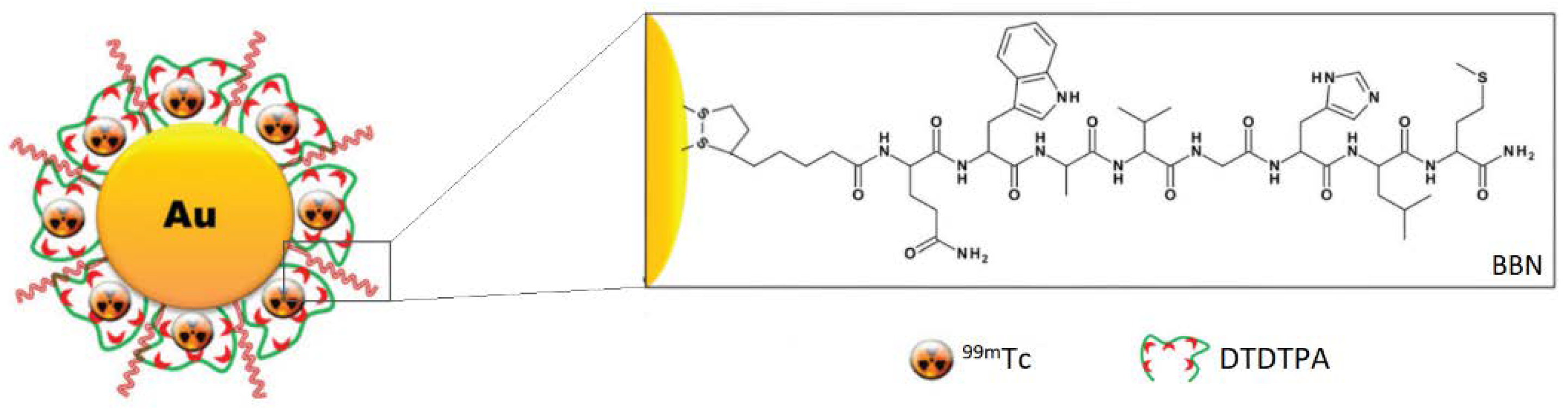
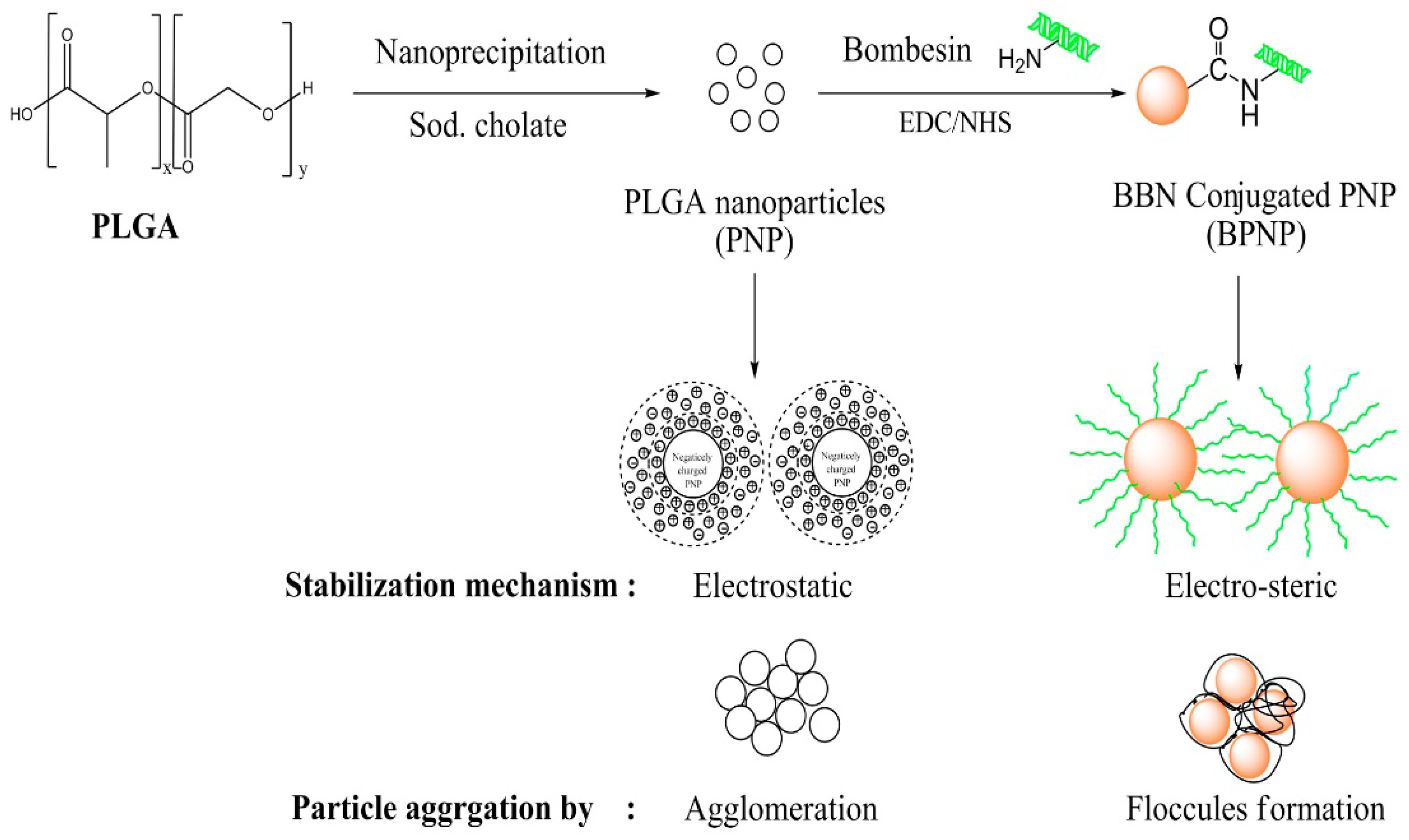
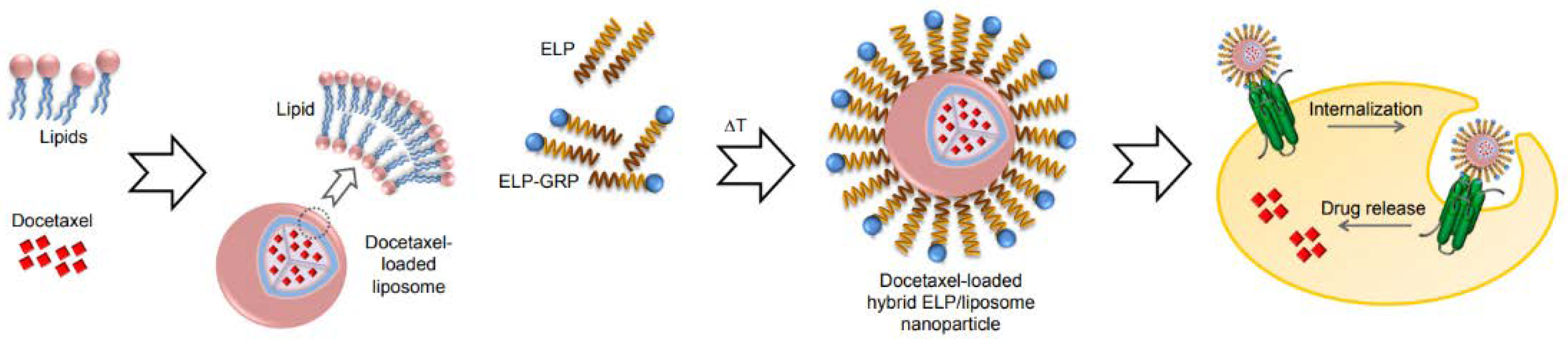
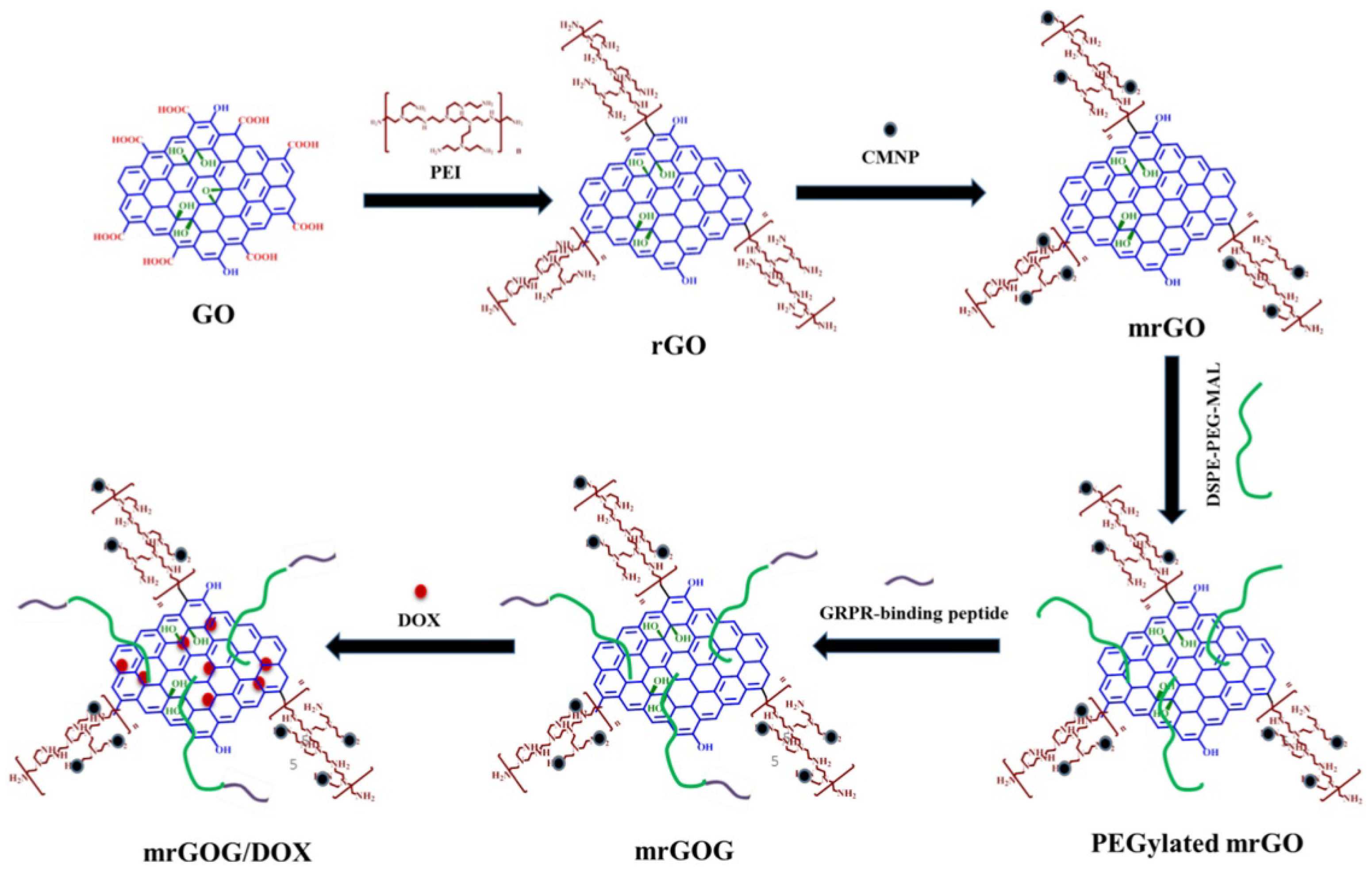

| Nanocarrier | Target condition | Drug/therapeutic means | Reference |
|---|---|---|---|
| phospholipid micelles | prostate cancer | Au(III)-dithiocarbamate complex (AuL12) | [35] |
| polymeric micelles | glioma | Coumarin-6 Camptothecin (CPT) |
[61] |
| prostate cancer | Monomethyl auristatin F (MMAF) | [65] | |
| elastin-like micelles | prostate cancer | - | [63] |
| Docetaxel (DTX) | [74] | ||
| nanostructured lipid carriers |
lung cancer | Doxorubicin (DOX) | [17] |
| solid lipid nanoparticles | breast cancer | Doxorubicin (DOX) | [59] |
| PLGA nanoparticles | breast cancer | Docetaxel (DTX) | [14] |
| prostate cancer | Docetaxel (DTX) | [75] | |
| gold nanoparticles | cervical cancer | RAF peptide analog (Ac-Cys-Ahx-RAF) | [69] |
| prostate cancer | 99mTc | [24] | |
| 99mTc, 177Lu | [31] | ||
| - | [76] | ||
| Gallic acid (GA) | [47] | ||
| prostate cancer, colon cancer |
68Ga | [25] | |
| gold nanorods | breast cancer | Photothermal therapy – use of NIR laser irradiation |
[39] |
| prostate cancer | Heat-labile cytotoxic free radical donor - AIPH | [77] | |
| graphene oxide | glioblastoma | Magnetic targeting, doxorubicin (DOX), NIR laser irradiation | [58] |
| Nanocarrier | Target condition | Imaging modality | Reference |
|---|---|---|---|
| gold nanoparticles | breast cancer | X-ray imaging | [41] |
| prostate cancer | CT imaging | [68] | |
| fluorescence imaging, CT imaging | [86] | ||
| PET imaging, CT imaging | [87] | ||
| microSPECT imaging, CT imaging | [19] | ||
| microSPECT imaging, CT imaging | [18] | ||
| gold nanorods | breast cancer | PA imaging | [40] |
| breast cancer, prostate cancer |
- | [88] | |
| iron oxide nanoparticles | breast cancer | MR imaging, NIRF imaging | [56] |
| MR imaging , PET imaging | [50] | ||
| PET imaging, CT imaging, MR imaging | [33] | ||
| MR imaging | [42] | ||
| pancreatic cancer | MR imaging | [66] | |
| prostate cancer | fluorescence imaging, MR imaging | [43] | |
| [67] | |||
| gadolinium oxide nanoparticles |
prostate cancer | fluorescence imaging, MR imaging | [89] |
| quantum dots | - | fluorescence imaging | [70] |
| prostate cancer | PET imaging, NIRF imaging | [29] | |
| PET imaging, CT imaging | [16] | ||
| liposomes | breast cancer | scintigraphy | [73] |
| Ehrlich tumor | [71] | ||
| upconversion nanoparticles |
prostate cancer | MR imaging, CT imaging, UC luminescence | [45] |
| graphene oxide | oral squamous cell carcinoma | fluorescence imaging | [37] |
| cowpea mosaic virus | prostate cancer | fluorescence imaging | [44] |
| Nanocarrier | Target | Imaging modality | Drug/therapeutic means | Reference |
|---|---|---|---|---|
| gold nanoparticles | prostate cancer | MR imaging (Gd) SPECT imaging (67Ga) |
radiosensitization | [23] |
| hybrid gold nanoparticle - PAMAM dendrimer | breast cancer | optical imaging | 177Lu radiotherapy, plasmonic photothermal therapy | [51] |
| lung cancer | optical imaging | 177Lu radiotherapy, plasmonic photothermal therapy | [49] | |
| hybrid polymeric vesicles - iron oxide nanoparticles | prostate cancer | fluorescence imaging; potential for MRI | camptothecin delivery | [20] |
| liposomes | pancreatic cancer | microSPECT/CT imaging (188Re) | doxorubicin delivery | [99] |
| prostate cancer | gamma imaging (111In) | doxorubicin delivery | [34] | |
| PAMAM dendrimer | breast cancer | microSPECT imaging/CT imaging (177Lu) |
177Lu radiotherapy, paclitaxel delivery |
[53] |
| PLGA nanoparticles | breast cancer | microSPECT imaging/CT imaging (177Lu) |
177Lu radiotherapy, paclitaxel delivery |
[52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





