1. Introduction
Renal cell carcinoma (RCC) accounts for around 3% of all cancer and 80-85% of primary renal neoplasms [1]. The estimated average 5-year survival rates for patients with RCC are 96% for those presenting with stage II, 64% for stage III, and 23% for stage IV [2].
The 20-40% of localized RCC develop metastases late, with 5-year survival below 10% [3] and the most common sites of recurrences are lung, regional lymph nodes and bone [4]. Lung metastases from RCC are usually small (0.5 to 2 cm diameter) well-defined round or ovoid nodules, solitary or multiple (the “cannonball” metastases), and asymptomatic [5].
Fluorodeoxyglucose ([18F]FDG) is the predominant PET tracer broadly used in oncology. Its uptake depends on the cancer’s ability to use the glucose transporter GLUT1, that is usually upregulated in cancerous cells, or other overexpressed enzymes such as lactate dehydrogenase (LDH) [6].
RCC is not typically evaluated with FDG due to its physiologic renal excretion that might limit its diagnostic accuracy [7]. As a result, FDG is not routinely recommended as an imaging tool in this setting by clinical guidelines such as AUA, ESMO and EAU [3,4,7]. However, [18F]FDG-PET/CT can be proposed in those presenting a high likelihood of metastatic disease, including lung metastases, when molecular imaging is suggested in addition to CT.
Therefore, the aim of this study was to evaluate the diagnostic accuracy of [18F]FDG-PET/CT to correctly identify RCC lung metastases in a cohort of patients with diagnostic suspicion of lung nodules and using histopathological analysis of the surgical specimen as standard of truth.
2. Materials and Methods
2.1. Objectives
The primary endpoint of this study was to assess the diagnostic accuracy of [18F]FDG-PET/CT in assessing the presence of lung metastases from RCC, in terms of positive predictive value (defined as the proportion of true positive subjects in the total group with positive result).
The secondary endpoint of this study was to evaluate the correlation between [18F]FDG-PET/CT semi-quantitative parameters (Standardized Uptake Value [SUVmax], Metabolic Tumor Volume [MTV] and Total Lesion Glycolysis [TLG]) and the tumor histology (RCC metastases vs. other malignancies).
2.2. Study Design and Patients Selection
This is a retrospective, single-center, single-arm, open-label study in RCC patients treated and followed-up at our institution. Clinical records of patients from February 2004 to October 2020 (study cut-off date) were evaluated.
Inclusion criteria were: 1) biopsy or histologically proven RCC; 2) suspicion of lung metastasis (at least one lung nodule observed in contrast enhanced CT (ceCT); 3) [18F]FDG-PET/CT as baseline procedure, prior to lung surgery; 4) lung surgery with subsequent histopathological analysis of surgical specimens; 5) availability of all clinical, pathology and imaging data (no lost follow-up). Exclusion criteria were: 1) low-quality PET images (e.g., wrong input of PET parameters, glycemia > 200 mg/dL, para-vein injection, 2D scanners); 2) proven metastatic disease other than lung. The study was approved by the local ethical committee and institutional scientific review board (IEO Trial-ID: 3490).
2.3. [18F]FDG-PET/CT
[18F]FDG-PET/CT was performed with a standard procedure according to the European Association of Nuclear Medicine (EANM) procedural guidelines [ i ]. PET images were acquired on PET/CT scanner (GE DMI-DDR, GE Healthcare, Milwaukee, WI, USA), 60 minutes after the intravenous injection of the radiopharmaceutical. An activity of 3MBq/Kg was injected (median 262 MBq; range 171-388 MBq). SUV/body-weighted (SUV/bw) was defined as the ratio of activity per unit volume of a region of interest (ROI) to the activity per unit whole body volume (SUV= activity concentration/[injected dose/body weight]), calculated on attenuation-corrected images.
2.4. Image Analysis
First, [18F]FDG-PET/CT images were reviewed by three nuclear medicine physicians (LLT, MC, LSAF) and interpreted with consensus (rule 2:1) as positive or negative according to a qualitative evaluation. Subsequently, each [18F]FDG-PET/CT was re-examined and the positivity of the [18F]FDG-PET/CT was determined on the basis of an arbitrary cut-off of SUVmax ≥ 2 determined from data in the literature.
Semi-quantitative analysis was performed using AW Server Workstation 2.0 (GE Medical Systems, Milwaukee, WI, USA) providing multiplanar reformatted images. The three nuclear medicine physicians identified all the known lung nodules on CT and calculated, on corresponding PET images, the semiquantitative parameters: SUVmax, MTV and TLG, by setting a spherical volume of interest (VOI) over the regions of interest.
2.5. Statistical Analysis
Continuous data were reported as median and interquartile ranges (IQR). Categorical data were reported as counts and percentages.
The accuracy in defining a lesion as a RCC lung metastases, according to both PET/CT lung result and SUVmax, was evaluated using the histopathological result as standard of truth. Sensitivity (SE) and specificity (SP), with their 95% confidence interval (95% CI), were calculated for the two methods. The Wilcoxon rank-sum test was performed to evaluate the distribution of [18F]FDG-PET/CT semi-quantitative body parameters (SUVmax, MTV and TLG) among lesions with and without metastases. A p-value less than 0.05 was considered statistically significant.
All analyses were performed with the statistical software SAS 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patient population
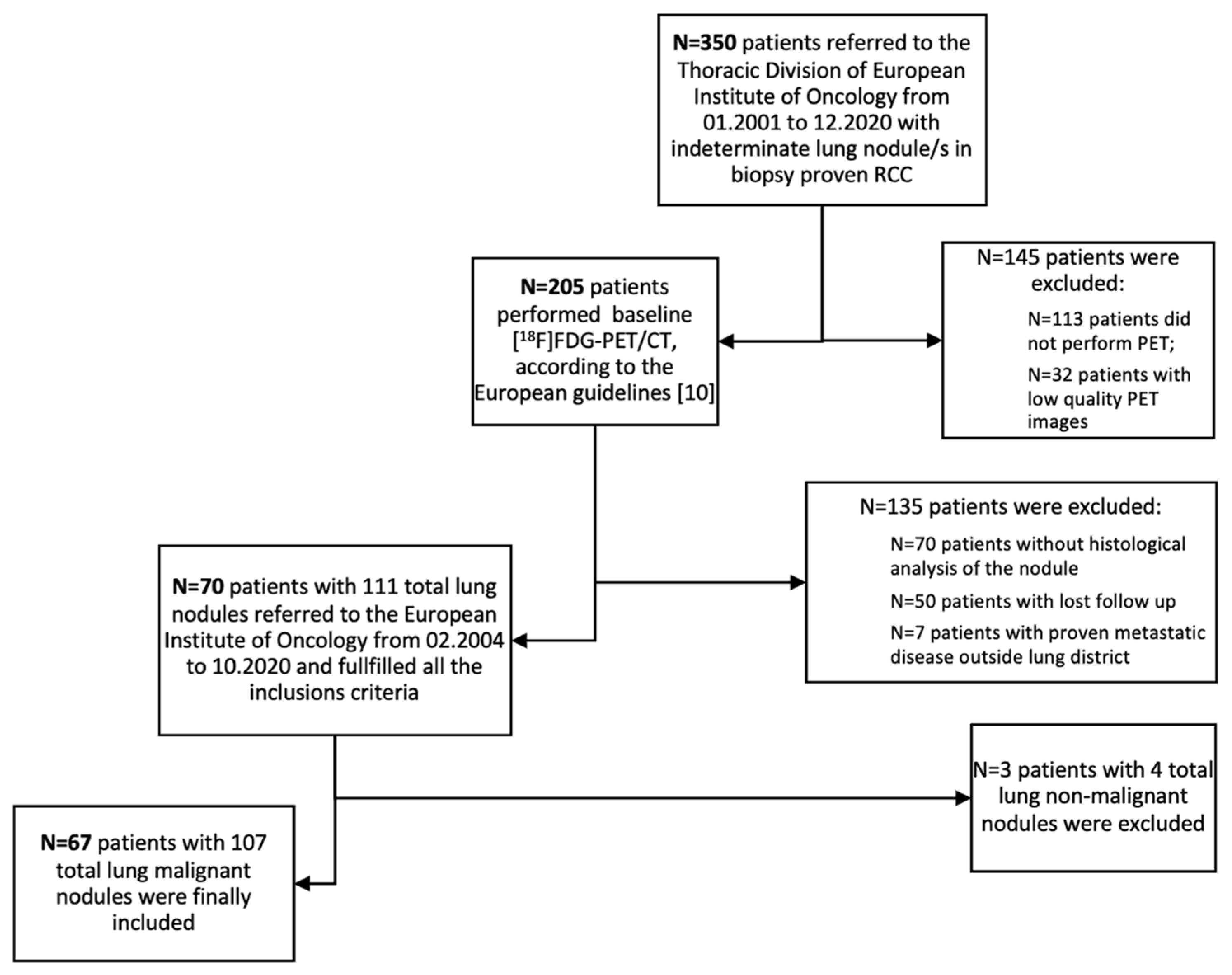
Seventy patients (n=70/350; 20%) with 111 lung nodules fulfilled all the inclusion criteria and were initially included in the analysis. Three patients were subsequently excluded from this population since showed non-malignant nodules at the histopathological analysis. Accordingly, sixty-seven (n=67/70; 96%) patients presenting 107 lung nodules was considered as the patient cohort eligible for primary endpoint analysis (
Figure 1). Population characteristics are summarized in
Table 1. Almost all patients (n=65/67) underwent surgery as primary therapy. Total nephrectomy was performed in most cases (79%), while partial nephrectomy in 6% and simple resection/enucleation in 12%. Two patients did not perform surgery due to clinical decision.
ceCT was performed in all patients prior to PET imaging. Twenty-five patients (37%) had more than one lung nodule in ce-CT, while forty-two patients (63%) had a solitary lung nodule. All patients underwent lung surgery with histological analysis of surgical specimens. In particular, 52% of patients (35/67) underwent wedge resection, 43% (29/67) underwent lobectomy and 5% (3/67) had pneumonectomy. Histological analysis of the 107 malignant nodules, revealed lung metastases from RCC in 53% and primary adenocarcinoma in 33%. Other less frequent malignant lesions were squamous carcinoma (6%), and other tumors (8%, such as large cell neuroendocrine carcinoma, large cell undifferentiated carcinoma, lung carcinosarcoma and lymphoma).
All patients included were treated and followed-up according to the best standard-of-care clinical practice at our institution and according to international procedural guidelines. All decisions were taken in a multi-disciplinary tumor board setting.
3.2. PET parameters
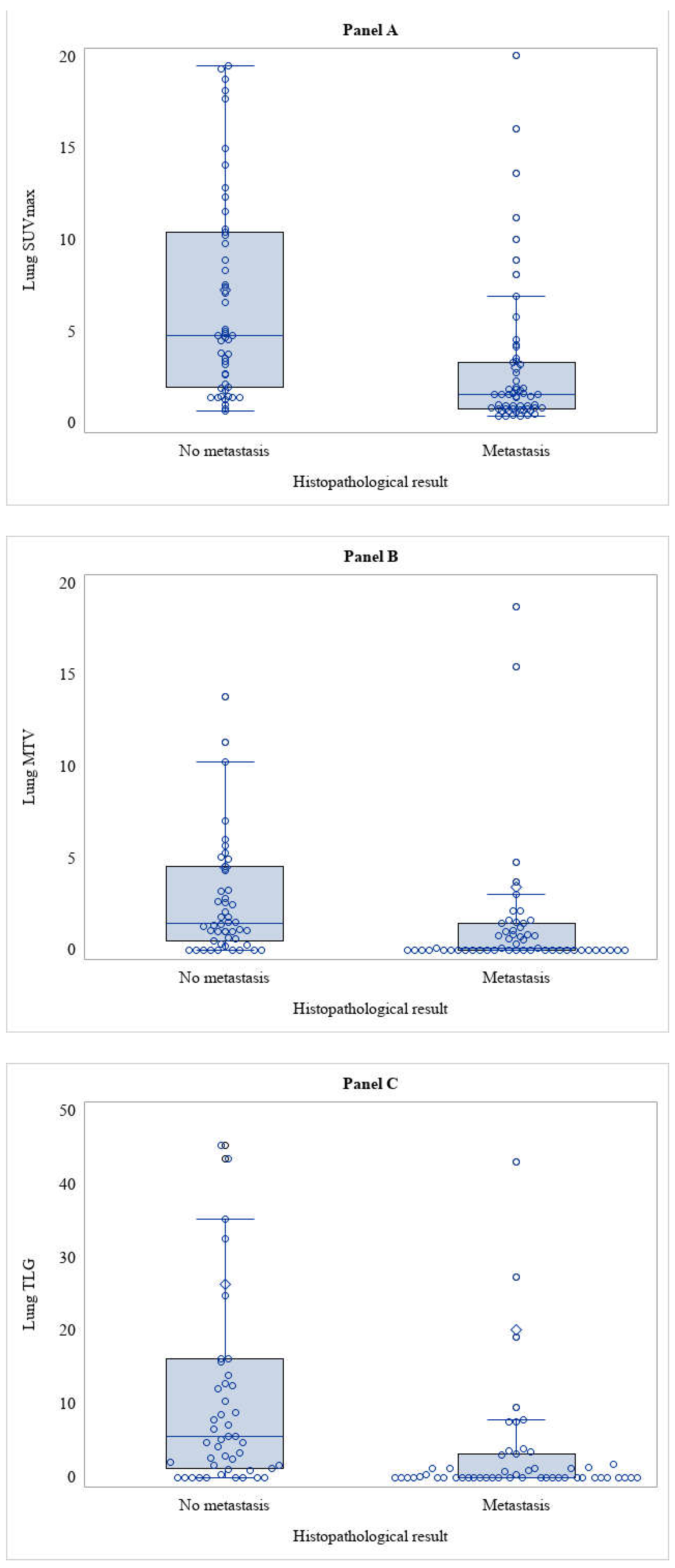
In the primary endpoint analysis, the 107 nodules analysed were sorted into two subgroups based on histological result post-lung surgery: the first subgroup included lung metastases from RCC (53%) while the other subgroup included all other malignant nodules (47%). A first analysis was performed considering the qualitative analysis of [
18F]FDG-PET/CT image, yielding a sensitivity of 49.1% (95% CI: 36.1% - 62.1%) and a specificity of 10.0% (95% CI: 5.3% - 24.3%). Then, images were read applying a semiquantitative analysis with SUVmax. SUVmax was significantly lower in RCC metastasis compared to other lung malignancies (
Table 3 and
Figure 2 Panel A). From this finding, a Receiver Operating Characteristic (ROC) curve was developed to identify the best optimal cut-off of SUVmax that could improve the diagnostic accuracy of [
18F]FDG-PET/CT. However, there was no cut-off values whose diagnostic performance could be higher than the arbitrary value of SUVmax ≥ 2, so the main analysis was conducted using qualitative analysis. Applying the SUVmax ≥ 2 arbitrary cut-off to distinguish between RCC lung metastasis and other malignancies, a sensitivity of 33.3% (95% CI: 21.4% - 47.1%) and specificity of 26% (95% CI: 14.6% - 40.3%) were observed (
Table 2).
In all lesions, the semiquantitative volumetric parameters (MTV and TLG) have been also calculated. MTV and TLG were significantly lower in RCC metastasis compared to other lung malignancies (
Table 3 and
Figure 2 Panel B-C). Two ROC curves were developed for MTV and TLG in this case as well, but these also did not show to be statistically significant. Furthermore, while the cut-off value of ≥2 was used for SUVmax (as derived by clinical practice), no cut-off value could be identified for the semi-quantitative volumetric parameters due to the lack of data in the literature to support this choice.
A secondary analysis was then performed considering only lung lesions equal to or less than 10 mm in CT or ceCT (46 out of 107 nodules) and subdividing them into the same two histologic subgroups mentioned above: lung metastases from RCC (n=36/46; 78%) and other lung malignancies (n=10/36; 22%). We then compared these histological subgroups of lung nodules equal or less than 10 mm with lung findings from the original [
18F]FDG-PET/CT report, yielding a sensitivity and a specificity of 30.6% (95% CI: 16.4% - 48.1%) and 20% (95% CI: 2.5% - 55.6%), respectively; and with SUVmax ≥2 as cut-off discriminator obtaining sensitivity 11.1% (95% CI: 3.1% - 26.1%) and specificity 50% (95% CI: 18.7% - 81.3%) (
Table 4).
4. Discussion
Chest ceCT scan is strongly recommended in patients with RCC (except cT1a) firstly because it is considered it is the most accurate method to identify the presence of lung metastasis; secondly because lung is one of the most common sites of metastasis from RCC. In addition, it is well known that patients who have only lung metastasis from RCC have a better prognosis than those who also have metastasis to other organs (especially bone and liver). Furthermore, the correct identification of lung metastasis is crucial as surgical resection can be performed as soon as the lung nodule is identified [4,11,12]. As already mentioned, the potential role of [18F]FDG-PET/CT in the evaluation of recurrent RCC rather than in staging grew in this context deserves to be explored. [18F]FDG-PET/CT is a one-stop-shop imaging procedure able to give a correct evaluation of the real burden of the disease in many solid malignancies. However, its role in RCC is still debated due to the low FDG-avidity exhibited by this specific tumor histotype. Some recent studies emphasize the quality of [18F]FDG-PET/CT in differentiating solitary pulmonary nodules when chest ceCT results are indeterminate. In this regard, some authors who performed three sequential scans in 43 patients with metastatic RCC found that the most common metabolically active metastatic sites were precisely lungs and lymph nodes [13]. The findings in literature seem promising, as the diagnostic accuracy of [18F]FDG-PET/CT in these studies ranged from 87% to 92%, and many of them also highlighted that [18F]FDG-PET/CT can also show lesions not visible on ceCT [14,15].
However, in our study less promising results have been observed, and very low diagnostic accuracy when comparing the qualitative analysis of [18F]FDG-PET/CT with the effective histology of the nodule. The results were slightly better when considering an arbitrary cut-off of SUVmax ≥. SUVmax, indeed, showed a statistically significant lower value in lung metastasis from RCC than in other lung malignancies, as expected since RCC is a low glucose metabolism disease. Although no cut-off value of SUVmax was found to provide adequate diagnostic accuracy, SUVmax was still higher than the background in RCC lung metastasis, resulting in the greater significance of the volumetric semiquantitative parameters (MTV and TLG), which were shown to be significantly different.
In our analysis, the standard-of-truth was histology and not another diagnostic procedure, such as ceCT or clinical follow-up. This methodology is different compared to other studies published at present in the literature. As is reported in a study that compared the accuracy of [
18F]FDG-PET/CT with chest CT, showing a sensitivity of 75.0% and specificity of 97.1% for the lung metastases compared with 91.1% and 73.1%, respectively, for chest CT [16]. Similar results were indeed obtained from another study that used, as a comparator, follow-up by conventional imaging (CT/MRI), [
18F]FDG-PET-CT, and histopathological confirmation only when possible, achieving a sensitivity of 80.6% with [
18F]FDG PET/CT compared with 100% obtained with ceCT [15]. However, authors reported how that nodules truly positive on CT and missed by [
18F]FDG-PET/CT had a smaller diameter (less than 12 mm, [16,17]). Excluding small nodules, might result in an overestimation of the diagnostic accuracy of [
18F]FDG-PET/CT because lung metastases from RCC present generally small dimensions. Lung nodules greater than 10 mm are well studied with CT due to their morphologic features and changes over time which are frequently sufficient to identify RCC metastasis over other lung malignancies, whereas distinguishing nonspecific lesions from small metastatic nodules with CT is more challenging [18]. Moreover, biopsy or histological characterization is clinically indicated for lung nodules greater than 10 mm, and thus [
18F]FDG-PET/CT positivity or negativity assumes limited significance, even if it has been observed that [
18F]FDG-PET/CT may have an unfavorable prognostic value in the case of a positive scan [18,19]. All the patients in our cohort, including patients with nodules lower than 10 mm underwent surgery. However, as also noted in
Figure 1, many of the patients who had [
18F]FDG-PET/CT did not then had surgery (70/205; 34%): so in a real-world clinical scenario it’s not true that all patients with a history of RCC and CT finding of lung nodule undergo surgery, and this is especially true for small nodules.
On the basis of these considerations (although it may appear unusual), we carried out a second analysis taking into account only smaller nodules (< 10mm), for which a surgical approach is not usually indicated and which are generally excluded from other studies in the literature and showed that, even in this sample of our cohort, a poor diagnostic accuracy is detected which is, by the way, quite similar to data obtained by considering also larger nodules. A diagnostic accuracy that, again, is better when a qualitative analysis of [18F]FDG-PET/CT is performed rather than using the arbitrary cut-off of SUVmax ≥2.
Finally, we thought it would be useful to make a third analysis using both the qualitative analysis of [18F]FDG-PET-CT and the arbitrary cut-off of SUVmax ≥2 to discriminate malignant nodules (107/111) from non-malignant nodules (4/111): an analysis that would show a much better diagnostic accuracy.
Definitely, one of the limitations of the latter analysis would have been that the non-malignant nodule sample is quite small compared with the malignant one, but this is a limitation caused by the obvious selection bias of this study, as all 70 patients had a history of RCC and thus they were cancer patients; it is also a selection bias due to the fact that all our patients had histological confirmation but it is clear that a patient with a nodule that was frankly benign would not undergo surgery and thus subsequent histological characterization.
One of the biggest strengths of this study, however, remains that it provides a real-world scenario, which is even more accurate when we consider that our institute is a tertiary cancer center, which is therefore indeed a single center but still a reference center.
A final consideration is that the diagnostic accuracy improves by considering the results of the [18F]FDG-PET-CT reports rather than the arbitrary cutoff of SUVmax ≥2: this might indicate how, in such nuanced cases, the nuclear physician’s expertise has greater accuracy than the semi-quantitative PET parameters.
5. Conclusions
In accordance with these data, obtained from a histology-based validated cohort, the use of [18F]FDG-PET/CT to correctly identify lung metastases from RCC should be discouraged, as our analysis showed suboptimal diagnostic accuracy of [18F]FDG-PET/CT in discriminating between lung metastases from RCC and other lung malignancies. It is necessary to point out, however, that metastatic patients in whom [18F]FDG-PET/CT has been shown to have added value, including prognostic value, were excluded in this analysis. So the take-home message is that, when [18F]FDG-PET/CT is used in the restaging setting of metastatic RCC patient, it is necessary to keep in mind that metastases from RCC have a low SUVmax value (however, generally higher than background), and it is important not to rely only on qualitative analysis but it is also necessary to make a semiquantitative analysis using also volumetric parameters (MTV and TLG) in addition to SUVmax, because they can support image interpretation.
Further perspective multi-center studies with a larger study case number are required to establish the diagnostic value of [18F]FDG-PET/CT imaging for RCC patients with lung nodules.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, L.S.A.F, L.L.T. and F.C.; methodology, F.M., L.M. and F.C.; formal analysis, S.F. and V.B.; investigation, L.S.A.F., L.L.T., M.C. and G.B.; resources, D.G., V.B., L.S.; data curation, S.F. and V.B.; writing—original draft preparation, L.S.A.F., L.L.T. and F.C.; writing—review and editing, F.M., L.M., G.Z. and F.C.; visualization, L.S.A.F., S.F. and V.B.; supervision, L.L.T, D.G., L.S. and F.C.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local ethical committee and institutional scientific review board (IEO Trial-ID: 3490).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. Supplementary data are available from the corresponding author, F.C., upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest regarding this study.
References
- Escudier, B., Porta, C., Schmidinger, M., Rioux-Leclercq, N., Bex, A., Khoo, V., Grünwald, V., Gillessen, S., Horwich, A., & ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org (2019). Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of oncology: official journal of the European Society for Medical Oncology, 30(5), 706–720. [CrossRef]
- Motzer, R.J., Hutson, T.E., Tomczak, P., Michaelson, M.D., Bukowski, R.M., Oudard, S., Negrier, S., Szczylik, C., Pili, R., Bjarnason, G.A., Garcia-del-Muro, X., Sosman, J.A., Solska, E., Wilding, G., Thompson, J.A., Kim, S.T., Chen, I., Huang, X., & Figlin, R.A. (2009). Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 27(22), 3584–3590. [CrossRef]
- Van Brussel, J.P., & Mickisch, G.H. (1999). Prognostic factors in renal cell and bladder cancer. BJU international, 83(8), 902–909. [CrossRef]
- Flanigan, R.C., Campbell, S.C., Clark, J.I., & Picken, M.M. (2003). Metastatic renal cell carcinoma. Current treatment options in oncology, 4(5), 385–390. [CrossRef]
- Griffin, N., Gore, M.E., & Sohaib, S.A. (2007). Imaging in metastatic renal cell carcinoma. AJR. American journal of roentgenology, 189(2), 360–370. [CrossRef]
- Majhail, N.S., Urbain, J.L., Albani, J.M., Kanvinde, M.H., Rice, T.W., Novick, A.C., Mekhail, T.M., Olencki, T.E., Elson, P., & Bukowski, R.M. (2003). F-18 fluorodeoxyglucose positron emission tomography in the evaluation of distant metastases from renal cell carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 21(21), 3995–4000. [CrossRef]
- Kang, D.E., White, R.L., Jr, Zuger, J.H., Sasser, H.C., & Teigland, C.M. (2004). Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. The Journal of urology, 171(5), 1806–1809. [CrossRef]
- Boellaard, R., Delgado-Bolton, R., Oyen, W.J., Giammarile, F., Tatsch, K., Eschner, W., Verzijlbergen, F.J., Barrington, S.F., Pike, L.C., Weber, W.A., Stroobants, S., Delbeke, D., Donohoe, K.J., Holbrook, S., Graham, M.M., Testanera, G., Hoekstra, O.S., Zijlstra, J., Visser, E., Hoekstra, C.J., … European Association of Nuclear Medicine (EANM) (2015). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. European journal of nuclear medicine and molecular imaging, 42(2), 328–354. [CrossRef]
- McKay, R.R., Kroeger, N., Xie, W., Lee, J.L., Knox, J.J., Bjarnason, G.A., MacKenzie, M.J., Wood, L., Srinivas, S., Vaishampayan, U.N., Rha, S.Y., Pal, S.K., Donskov, F., Tantravahi, S.K., Rini, B.I., Heng, D.Y., & Choueiri, T.K. (2014). Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. European urology, 65(3), 577–584. [CrossRef]
- Flanigan, R.C., Salmon, S.E., Blumenstein, B.A., Bearman, S.I., Roy, V., McGrath, P.C., Caton, J.R., Jr, Munshi, N., & Crawford, E.D. (2001). Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. The New England journal of medicine, 345(23), 1655–1659. [CrossRef]
- Voss, J., Drake, T., Matthews, H., Jenkins, J., Tang, S., Doherty, J., Chan, K., Dawe, H., Thomas, T., Kearley, S., Manners, J., Carter, C., Al-Buheissi, S., & Klatte, T. (2020). Chest computed tomography for staging renal tumours: validation and simplification of a risk prediction model from a large contemporary retrospective cohort. BJU international, 125(4), 561–567. [CrossRef]
- Cozzoli, A., Milano, S., Cancarini, G., Zanotelli, T., & Cosciani Cunico, S. (1995). Surgery of lung metastases in renal cell carcinoma. British journal of urology, 75(4), 445–447. [CrossRef] [PubMed]
- Kayani, I., Avril, N., Bomanji, J., Chowdhury, S., Rockall, A., Sahdev, A., Nathan, P., Wilson, P., Shamash, J., Sharpe, K., Lim, L., Dickson, J., Ell, P., Reynolds, A., & Powles, T. (2011). Sequential FDG-PET/CT as a biomarker of response to Sunitinib in metastatic clear cell renal cancer. Clinical cancer research: an official journal of the American Association for Cancer Research, 17(18), 6021–6028. [CrossRef]
- Gupta, N.C., Maloof, J., & Gunel, E. (1996). Probability of malignancy in solitary pulmonary nodules using fluorine-18-FDG and PET. Journal of nuclear medicine: official publication, Society of Nuclear Medicine, 37(6), 943–948. [PubMed]
- Chang, C.H., Shiau, Y.C., Shen, Y.Y., Kao, A., Lin, C.C., & Lee, C.C. (2003). Differentiating solitary pulmonary metastases in patients with renal cell carcinomas by 18F-fluoro-2-deoxyglucose positron emission tomography--a preliminary report. Urologia internationalis, 71(3), 306–309. [CrossRef]
- Kang, D.E., White, R.L., Jr, Zuger, J.H., Sasser, H.C., & Teigland, C.M. (2004). Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. The Journal of urology, 171(5), 1806–1809. [CrossRef]
- Elahmadawy, M.A., Elazab, M.S.S., Ahmed, S., & Salama, M. (2018). Diagnostic value of F-18 FDG PET/CT for local and distant disease relapse surveillance in surgically treated RCC patients: Can it aid in establishing consensus follow up strategy?. Nuclear medicine review. Central & Eastern Europe, 21(2), 85–91. [CrossRef]
- Gould, M.K., Fletcher, J., Iannettoni, M.D., Lynch, W.R., Midthun, D.E., Naidich, D.P., Ost, D.E., & American College of Chest Physicians (2007). Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest, 132(3 Suppl), 108S–130S. [CrossRef]
- Liu Y. (2016). The Place of FDG PET/CT in Renal Cell Carcinoma: Value and Limitations. Frontiers in oncology, 6, 201. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).