1. The plant immune system
Plants are exposed to diverse types of stresses, both biotic and abiotic, and need to adjust their metabolism to respond rapidly to the changes that appear in their natural conditions. Plant cells harbor an array of receptors allowing the identification of self and non-self (He et al., 2018; Gust et al., 2017). Intracellular and cell-surface receptors work in combination to solve the identity of the threat and to activate immunity (Ngou et al., 2021)
Recognition of non-self-molecules belonging to a specific type of insect or microbe that are termed Pathogen/ Microbe/ Herbivore Associated Molecular Patterns (PAMPs/ MAMPs/ HAMPs, respectively) (Yu et al., 2017). These molecular signatures belong to bacteria, fungi, oomycetes, or arthropods and are often peptides, fatty acids, or oligosaccharides. PAMPs are exogenous danger signals that are recognized by cell surface receptors so-called pattern recognition receptors (PRRs). Following the binding of their substrates, these receptors trigger the first layer of plant innate immunity known as pattern-triggered immunity (PTI). PTI is a general response that contributes to defense against a broad range of attackers. PTI responses are characterized by a cascade of signaling events that trigger the production of reactive oxygen species (ROS), increase in intracellular calcium (Ca2+), activation of Mitogen-activated and/or calcium-dependent protein kinases (MPKs and CDPKs) and transcriptional changes (Hou et al., 2019). Some well-studied MAMPs are the bacterial peptide flg22 or the elf18 from the EF-Tu (Felix et al., 1999; Kunze et al., 2004), as well as lipopolysaccharides and chitin, components of the cell wall of bacteria and fungi respectively (Couto and Zipfel, 2016). On the other hand, herbivore oral secretions are an example of HAMPs (Mithöfer and Boland, 2008).
In addition to PAMPs recognition, pest or pathogen invasion also triggers the production and release of host-derived molecules that are also perceived by specific PRRs to trigger PTI. Pathogens and insects produce lytic enzymes to degrade plant tissues and access host cells leading to the release of degradation products. These molecules are commonly known as Damage-Associated Molecular Patterns (DAMPs; Heil, 2009; Albert et al., 2013). Recently, a more accurate classification for endogenous danger signals was proposed: a) those host molecules that are passively released after cell damage and disruption are primary endogenous danger signals and include the “classical” DAMPs (Gust et al., 2017) like cell wall fragments such as oligogalacturonides or cellulose fragments (Hou et al., 2019); and b) peptides that are produced actively by cells under biotic attack are secondary endogenous danger signals termed phytocytokines (Gust et al.,2017). Production of phytocytokines often involves processing from a larger precursor that leads to the release of the mature peptide which is perceived by neighboring cells to spread the danger alarm. Thus, unlike classical DAMPs, peptides may be present at the site of infection even if there is no cell disruption and they can also be released in adjacent intact cells (Ma et al., 2013).
2. Phytocytokines in basal immunity
Accumulating studies reveal the importance of small, secreted peptides in cell-to-cell signaling to coordinate cellular function including defense response in plants. Phytocytokines are small peptides secreted after damage perception that induce the amplification of immune responses in damaged and undamaged cells (Gust et al., 2017). Tomato Systemin was the first signaling peptide found in plants (Pearce and Ryan 1991). Later many peptides with a defense signaling function were identified in different plant species, such as PEPs (Plant Elicitor Peptides) from Arabidopsis, maize, and soybean (Huffaker et al., 2006; Huffaker et al., 2011; Yamaguchi et al., 2011). Recently there is an emerging number of studies reporting the discovery of new peptides involved in plant defense against a variety of biotic stressors in different plant species (
Table 1; Hou et al., 2014; Chen et al., 2014; Gully et al., 2019; Huang et al., 2021).
Defense peptides are usually short in their amino acid chain. There are reported biologically active peptides ranging from 5aa in length such as phytosulfokine (Zhang et al., 2018) to 67aa from the stable antimicrobial peptides biosynthesized by citrus in response to Huanglongbing disease (Huang et al., 2021), and they can be active at concentrations as low as femtomolar (Roy et al., 2018). Regarding these mentioned features and their ubiquitous participation in plant physiological events and cell-to-cell communication, they have been considered by many authors as peptidic hormones (Matsubayashi and Sakagami, 2006; Roy et al., 2018) hence suitable candidates to be used as induced resistance (IR) elicitors.
The release of small defense peptides often involves the processing of a larger precursor propeptide which differs in structure, indicating different processing mechanisms (Yamaguchi and Huffaker, 2011). According to their precursor structure, there are peptides derived from precursors having an N-terminal secretion signal, derived from precursors not having an N-terminal secretion signal, and derived from proteins that have a different biological function (Yamaguchi and Huffaker, 2011; Albert., 2013). Systemin precursor, ProSystemin, or the precursors of Arabidopsis PEPs, PROPEPS, are examples of proteins not having an N-terminal secretion signal (Pearce et., 1991; Huffaker et al., 2006). Recently, some research studies have shed light on the mechanism by which these two peptides are being processed in plants. ProSystemin is hydrolyzed by subtilisins that release an inactive Systemin peptide that is further processed by a leucine aminopeptidase that removes the N terminal aa activating the functional peptide (Beloshistov et al., 2017). On the other hand, the Pep1 precursor, PROPEP1, is processed by Calcium-dependent metacaspases which directly release the mature peptide (Hander et al., 2019). Alternatively, Hydroxyproline-rich Systemins (HypSys) peptides derive from a precursor with an N-terminal secretion signal (Pearce et al., 2001; Pearce and Ryan 2003), and GmSubPep from soybean derives from a protein with a distinct primary function (Pearce et al., 2010). However, the processing mechanisms occurring to release HypSys and GmSubPep are poorly understood. Besides proteolytic processing, some peptides require posttranslational modification to be biologically active and to interact with their receptor (Matsubayashi, 2014). Posttranslational modifications include tyrosine sulfation, proline hydroxylation, and hydroxyproline arabinosylation (Matsubayashi, 2014; Stührwohldt & Schaller, 2019). Phytosulfokine (PSK) was the first identified peptide with posttranslational modifications, having sulfation at the two tyrosine residues (Matsubayashi, 1996). Later HypSys peptides were identified in tobacco and tomato as having proline hydroxylation (Pearce et al., 2001; Pearce and Ryan, 2003).
Once the mature peptide is released it triggers a cascade of signaling events and defense responses upon its perception by a membrane receptor. Peptides’ perception, signal transduction, and triggered defense responses are reviewed in the following sections.
2.1. Peptides perception and signal transduction
A fast and efficient perception of plant surroundings is indispensable for plant survival. Similarly, to classical DAMPs or PAMPs, phytocytokines perception by membrane receptors of damaged and adjacent cells is crucial to ensure danger alarm spread leading the amplification of immune signaling in undamaged tissues and resistance to pests and pathogens.
As other danger signals, plant defense peptides are perceived by membrane receptors that are usually receptor-like kinases (RLKs) with an extracellular domain that binds the peptide ligand, a transmembrane domain, and an intracellular kinase domain that ensures the initiation of an intracellular signaling cascade (Li et al., 2020). These cell surface receptors often form complexes with coreceptors that enable the activation of downstream signaling upon ligand perception (He et al., 2018). The receptor-like Kinase BRI1-associated receptor Kinase (BAK1) functions as a coreceptor of multiple PRRs including those perceiving phytocytokines (
Table 1). In addition, some receptor-like cytoplasmatic kinases such as Botrytis-induced kinase (BIK1) interact with PRRs complexes to initiate the signal transduction upon complex activation in response to danger signals (Lu et al., 2010; Liu et al., 2013a). Remarkably, although BAK1 associates with multiple PRRs upon danger perception enabling signal transduction, it has been shown that pathogens induce BAK1 depletion hijacking PTI responses (Macho and Zypfel, 2015). When this happens, it was demonstrated that the PEPR pathway ensures basal resistance inducing cell death and salicylate-related defenses (Yamada et al., 2016). This suggests that phytocytokines-triggered immune responses can also occur independently of common PTI signaling actors.
An increasing number of peptide-receptors pairs have been discovered in the last few years (
Table 1). Arabidopsis Plant elicitor peptide 1 (Pep1) is perceived either by PEP RECEPTOR 1 (PEPR1) or 2 (PEPR2) whereas Arabidopsis Pathogen induced peptide 1 (PIP1) is perceived by RLK7 (Yamaguchi et al., 2006; Hou et al., 2014). Very recently RLK7 was identified also as a receptor of C-TERMINAL ENCODED PEPTIDE 4 (CEP4; Rzemieniewski et al., 2022). Both PEPR1 and RLK7 form a complex with BAK1, although early signaling triggered by PIP1 is only partially dependent on BAK1 (Liu et al., 2013; Hou et al., 2014). Similarly, PEPR1 can directly phosphorylate BIK1, without relying on BAK1 (Liu et al., 2013). However, PIP1 signal transduction was demonstrated to be BIK1-independent (Hou et al., 2014). Recently, MIK2 was demonstrated to be the SCOOP12 receptor in Arabidopsis. Interestingly, MIK2 does not only perceive SCOOPs but also sense microbial proteins from fungi and bacteria (Hou et al., 2021). MIK2 also associates with BAK1 and its close homolog SERK4 and relies on BIK1 and PBL1 for the downstream signaling events (Hou et al., 2021).
On the other hand, tomato Systemin is perceived by both LRR-RK receptors SYR1 and SYR2, that bind Systemin with high and low affinity respectively although some more research is needed to confirm the binding mechanism (Wang et al., 2018). The PEPR tomato ortholog PORK1 is also necessary to trigger Systemin-induced signaling since plants with silenced PORK1 but intact SYRs lack some Systemin responses (Xu et al., 2018). However, it has not been demonstrated if PORK1 binds directly to Systemin or may function as a coreceptor of SYRs similar to the Arabidopsis receptor protein complexes mentioned above. Interestingly some peptides can be perceived by more than one receptor generating a different signal according to the peptide-receptor pair.
Although many peptide-receptor complex pairs have been elucidated in the past few years, there are still many phytocytokines in which perception is still elusive. For instance, how maize ZmPeps and Zip1, soybean Peps, tomato CAPE1, or MaSAMP are perceived is still unknown (
Table 1). Further research is needed to address this issue and improve our knowledge of phytocytokines perceptions and signal transduction. Techniques and methods to find new peptide ligand-receptor pairs are extensively reviewed elsewhere (Roy et al., 2018; Olsson et al., 2019)
2.2. Peptides perception and signal transduction
The binding of phytocytokines to their receptor triggers a cascade of defense signaling that leads to an amplification of the plant immune system to mount a defense response against invading attackers (
Figure 1A). Defense peptides share common intracellular signaling elements with other self and non-self-defense elicitors (
Table 1). Although there is specific recognition of peptides by PRRs, triggered defense responses and intracellular signaling often overlap as it happens in response to PAMPs (Yu et al., 2017). Resistance inducers and priming agents also trigger typical PTI defense responses and primed plants have potentiated defense in response to a challenge (Mauch-Mani et al., 2017). The next sections present the most common defense responses that are triggered by plant defense peptides and their natural role against biotic stresses.
2.2.1. Increment of cytosolic Ca2+
An increase of cytosolic Ca2+ is one of the earlier responses triggered by some phytocytokines as well as by other PAMPs and DAMPs during PTI, occurring within a few minutes, or even seconds after perception, upstream of subsequent immune responses (Yu et al., 2017). Systemin perception triggers an increase in the intracellular calcium in mesophyll cells (Moyen et al., 1998). Similarly, Pep1, Pep3, SCOOPs, and CEP4 treatment produce an augmentation of cytosolic calcium in Arabidopsis (Ma et al., 2012; Ma et al., 2013; Rhodes et al., 2021; Rzemieniewski et al., 2022). Remarkably, it was recently reported that the release of Pep1 from PROPEP1 processing is catalyzed by Ca2+-dependent metacaspases (Hander et al., 2019; Shen et al., 2019). On the other hand, PSK in tomatoes not only induces a Ca2+ increase but Ca2+-dependent auxin responses for the protection against Botrytis cinerea (Zhang et al., 2018). These findings suggest the importance of cytosolic Ca2+ in phytocytokines-triggered defense signaling.
2.2.2. Effect on ion channels and extracellular pH
Opening of ion channels and extracellular alkalinization is a hallmark response occurring after peptide treatment (
Figure 1). Media alkalinization occurs also very rapidly (1min) upon Flg22 or Elf18 treatment (Jeworutzki et al., 2010). Rapid alkalinization factors (RALFs) peptides owe their name to their ability to alkalinize the extracellular media when applied to a cell suspension culture (Pearce et al., 2001). Similarly, tobacco and tomato HypSys, as well as peptides from soybean (GmSubPep, GmPep914, and GmPep890), also induce extracellular alkalinization when supplied to suspension-cultured cells (
Table 1; Pearce et al., 2001; Pearce and Ryan 2003; Pearce et al 2010; Yamaguchi et al., 2011). In addition, the opening of ion channels by the modulation of plasma membrane H+ATPase activity is a Systemin-triggered early event (Schaller et al., 1999).
2.2.3. Production of reactive oxygen species (ROS) and activation of mitogen-activated protein kinases (MAPK)
ROS production is another cellular responses upon pathogen recognition and it mediates other defense responses in the plant (Torres et al., 2006; Fichman and Mittler 2020). PAMPs, defense elicitors, and many phytocytokines perceptions produce an increase in the oxidative burst (
Table 1). In Arabidopsis, exogenous foliar application of PEP1, as well as PIP1, causes the production of H
2O
2 (Huffaker et al., 2006; Hou et al., 2014). Pep3 induces both H
2O
2 and NO production which are essential for a functional Pep3-triggered immunity against
PstDC3000 since it is compromised in
rbohD/F and
noa1 mutants (Ma et al., 2013). Similarly, SCOOP12 induces ROS as well as Phosphatidic acid (PA) in Arabidopsis, suggested to be involved in ROS production, MAPK activation, and defense gene induction (Testerink et al., 2011; Gully et al., 2019). In addition, in Arabidopsis GRIM REAPER peptide (GRI) was shown to regulate ROS-dependent cell death (Wrzaczek et al., 2009; 2014). In tomato, both CAPE1 and Systemin treatment trigger H
2O
2 formation (Chen et al., 2014; Wang et al., 2018) whereas in potato plants HypSys also elicits H
2O
2 generation (Bhattacharya et al., 2013). Conversely, RALF23 is a negative regulator of PAMP-induced ROS (Stregmann et al., 2017).
Activation of protein kinase cascades is a hallmark of PTI responses. MAPKs cascades are essential signaling elements to ensure defense signaling activation downstream pattern recognition receptor complexes (Yu et al 2017). PIP1, SCOOPs, CEP4, Systemin, and Hypsys peptides induce MAPK activation in their respective species of origin (Hou et al 2014; Lee et al., 2020; Rzemieniewski et al., 2022; Stratmann 1997; Pearce et al 2001). Additionally, Systemin primes MPK3 and MPK6 phosphorylation upon Plectosphaerella cucumerina infection in Arabidopsis thaliana (Pastor-Fernández et al., 2022). Parallelly, Calcium-dependent protein kinases (CDPKs), which are Ca2+ sensor protein kinases, are also activated upon several danger signals perception and trigger downstream defense responses (Boudsocq and Sheen, 2013). However, very little is known about their implication in peptide-activated defenses. In this regard, Pep3 induction of MPK3 and WRKY33 and Pep-triggered immunity against PstDC3000 is CDPK-dependent since it is impaired in the cpk mutants or when a kinase inhibitor is applied (Ma et al., 2013).
2.2.4. Expression of defense-related genes and protease inhibitors
Most phytocytokines induce the expression of a variety of defense-related genes in different plant species (
Table 1). Although there are peptide-specific transcriptomic fingerprints, transcriptional changes triggered by defense peptides often overlap. In Arabidopsis treatment with Peps induced the expression of plant defensin
PDF1.2,
MPK3, and
WRKY33 transcription factor (Huffaker et al., 2006; Ma et al., 2013). PIP1 treatment induces immune-related Flg22-INDUCED RECEPTOR KINASE1 (FRK1), the transcription factors
WRKY30,
WRKY33, and
WRKY53 gene expression as well as expression of pathogen-related PR1 in protoplasts, and the transcription factor
MYB51 in roots (Hou et al., 2014). As PIP1, SCOOPs also induced
FRK1,
WRKY30/33 gene expression as well as
CYP81F2, involved in glucosinolate metabolism and resistance to fungi (Gully et al., 2019; Hou et al., 2021). Similarly, it was recently observed that CEP4 also triggered the expression of the PTI marker gene
FRK1 in Arabidopsis (Rzemieniewski et al., 2022). Systemin treatment induces the expression of defense-related genes, especially genes involved in the synthesis of JA, such as
AOS and JA marker genes
PI-I and
PI-II (Coppola et al., 2019). Like Systemin, the HypSys peptides activate the expression of octadecanoid pathway genes as well as essential pathogen- and herbivore-related genes (Bhattacharya et al., 2013). CAPE1 activates the expression of pathogen-related genes
PR1b,
BETA-1,
3-GLUCANASE (PR2),
CYS PROTEASE (PR7), a chitinase,
ETHYLENE RESPONSE FACTOR5 (ERF5) and
AvrPto-DEPENDENT Pto-INTERACTING PROTEIN3 (Adi3) among others (Chen et al., 2014). In soybean GmSubPep, GmPep914 and GmPep890 peptides induce
CYP93A1 gene expression, involving synthesis of a phytoalexin, a chitinase and chalcone synthase gene expression (Pearce et al., 2010; Yamaguchi et al., 2011). In Maize ZmPep1 induces some defense genes activation encoding for defense proteins which includes endochitinase A, PR-4, PRms, and SerPIN, and a gene involved in the biosynthesis of the phytoalexin benzoxazinoid (Huffaker et al., 2011). On the other hand, ZmPep3 increases the expression of indole biosynthetic genes together with genes encoding proteins associated with herbivory defense and biosynthetic enzymes for the production of volatile terpenes and benzoxazinoids (Huffaker et al 2013). Also in maize, Zip1 induces the expression of SA and JA marker genes and other defense-related genes such as WRKY transcription factors (Ziemann et al., 2018). A new class of peptides named “stable microbial peptides” (SAMPs) were identified in some tolerant citrus hybrids to Huanglongbing (HLB) disease caused by the bacterial pathogen
Candidatus liberibacter asiaticus. This peptide induce the expression of several defense-related genes, including pathogen-related
PR1 and
PR2 as well as phenylalanine ammonia-lyase 1 (
PAL), involved in SA and phenylpropanoid biosynthesis through a pathogenesis-related gene1 (
NPR1) and suppressor of G2 allele of skp1 (
SGT1) -dependent manner (Huang et al., 2021).
On the other hand, some released peptides participate in positive feedback inducing the expression of their precursors. This is the case of AtPep1, which activates the expression of PROPEP1 (Huffaker et al., 2006). PEPR activation also mediates PROPEP2/PROPEP3 activation (Yamaguchi & Huffaker, 2011). SCOOP12 as well as Pep1 triggers the expression of PROSCOOPS (Gully et al., 2019). Similarly, CAPE1 induces the expression of its precursor protein PR1b (Chen et al 2014) and Systemin induces the expression of ProSystemin (Coppola et al., 2019). An additional example shows that Zip1 maize peptide induces the activity of the proteases that process its precursor PROZIP1 (Ziemann et al., 2018). These findings suggest that a likely biological function of the positive feedback loop is to amplify defense responses improving resistance efficiency.
A very common response triggered by defense peptides in tomato and other solanaceous species is the induction of Protease inhibitors (PIs). PIs inhibit insect digestive enzymes being key elements in plant defense against herbivory (Green and Ryan, 1972). In fact, Systemin was identified when looking for signals that induced PIs accumulation in tomato. Later, it was reported that Systemin is also present in potato, pepper, and nightshade where it also induces the accumulation of PIs. Similarly, HypSys found in tobacco, tomato, and potato can also trigger PIs against insects (Bhattacharya et al., 2013). On the other hand, CAPE1 a tomato peptide embedded in PR1b was found to induce the expression of PIs (Chen et al., 2014). In addition, induction of PIs biosynthetic genes was also observed in maize due to ZmPep3 treatment (Huffaker et al., 2013).
Table 1.
Main features of phytocytokines in biotic stress.
Table 1.
Main features of phytocytokines in biotic stress.
| Phytocytokine |
Species of origin |
Signal
transduction |
Induced defense responses and signaling |
References |
| Peps |
Arabidopsis |
PEPR1 and PEPR2 |
media alkalinization, H2O2
PDF1.2 and PROPEPs expression |
Huffaker et al., 2006 |
| BAK1 |
Ca2+, ET, callose |
Ma et al., 2013 |
| BIK1/PBL1 |
Ca2+, H2O2 NO
MPK3 and WRKY33 expression |
Bartels and Boller, 2015 |
| ZmPep1 |
Maize |
|
JA, ET, defense gene expression, defense metabolites accumulation |
Huffaker et al., 2011 |
| ZmPep3 |
Maize |
|
JA, ET, defense gene expression, volatiles emission, phytoalexin |
Huffaker et al., 2013 |
| PIP1 |
Arabidopsis |
RLK7 |
ROS,
FRK1, WRKY30, WRKY33, WRKY53, MYB51 and PR1 expression |
Hou et al., 2014 |
| partially BAK1- dependent |
MAPK, Callose, Stomatal closure |
| SCOOP12 |
Arabidopsis |
MIK2-BAK1/SERK4 |
ROS, callose |
Gully et al., 2019 |
Phosphatidic acid (PA)
FRK1 expression |
Rhodes et al., 2021 |
| SCOOPs |
|
BIK1/PBL1 |
Ca2+, ROS, MAPK
Ethylene, defense gene expression |
Hou et al., 2021 |
| PNP-A |
Arabidopsis |
PNP-R2 |
antagonizes SA responses, stomatal closure |
Lee et al., 2020 |
| RALF23 |
Arabidopsis |
FER-BAK1 |
Ca2+, Media alkalinization |
Stregmann et al 2017 |
| Antagonizes PAMP-induced ROS |
| IDL6 |
Arabidopsis |
HAE and HSL2 |
Poligalacturonase gene ADPG2 |
Wang et al., 2017 |
| GRI |
Arabidopsis |
PRK5 |
ROS-dependent Cell death, hormones |
Wrzaczek et al., 2009 and 2014 |
| CEP4 |
Arabidopsis |
CEPR1/2 and RLK7 |
Ca2+, MAPK
Ethylene, FRK1 expression |
Rzemieniewski et al., 2022 |
| Systemin |
Tomato |
SYR1 |
Opening of ion channels, Ca2+, MAPKs
JA, defense genes |
Pearce et al., 1991 |
| SYR2 |
CDPKs, ROS
Protease inhibitors |
Zhang et al., 2020 |
| PORK1 |
CAT and APX activity
Volatiles emission |
Molisso et al., 2021 |
| PotSys1 and 2 |
Potato |
SYR1 and SYR2 |
Proteinase inhibitors |
Constabel el et., 1998 |
| PepSys |
Pepper |
| NishSys |
Nightshade |
| HypSys1, 2 and 3 |
Tomato |
|
Media alkalinization
JA, PI-I, and PI-II |
Pearce and Ryan, 2003 |
| Potato |
H2O2
PIs, JA, defense-related genes, antioxidant defensive enzymes |
Bhattacharya et al., 2013 |
| TobHypSys 1 and 2 |
tobacco |
|
Media alkalinization, MAPK
Proteinase inhibitors |
Pearce et al., 2001 |
| CAPE1 |
Tomato |
|
H2O2
SA, defense gene expression |
Chen et al., 2014 |
| PSK |
Arabidopsis |
PSRKs |
Ca2+
IAA and Auxin-dependent responses |
Shen and Diener, 2013 |
| Tomato |
Zhang et al., 2018 |
| PSY1 |
Arabidopsis |
PSY1R |
|
Shen and Diener, 2013 |
| SubPep |
Soybean |
|
Media alkalinization
Chitinase1b, CYP93A1, chalcone synthase and PDR12 gene expression |
Pearce et al., 2010 |
| Pep914 |
Soybean |
|
Media alkalinization
CYP93A1, Chib1-1, and chalcone synthase gene expression |
Yamaguchi et al., 2011 |
| Pep890 |
| Zip1 |
Maize |
|
SA, SA, and JA marker genes, defense-related genes |
Ziemann et al., 2018 |
| SAMP |
Citrus |
|
Defense gene expression |
Huang et al., 2021 |
2.2.5. Metabolic changes after phytocytokine perception
Phytohormones are well known for their implication in plant defense and their production in plants under attack is a conserved response across species. SA, ET, JA, and ABA are the main hormones regulating many resistance responses associated with basal immunity as well as gene-for-gene and systemic resistance. Among the literature, there are some examples of phytohormonal production upon defense peptide perception. In Arabidopsis, Pep1, SCOOPs, and CEP4 induce the accumulation of ET in Arabidopsis (Gully et al., 2019; Rzemieniewski et al., 2022). In maize both ZmPep1 and ZmPep3 induce JA and ET (Huffaker et al., 2011; 2013) whereas Zip1 was observed to induce both JA and SA marker gene expression and strongly induce SA accumulation (Ziemann et al., 2018). In Solanaceous species, Systemin induces the release of linolenic acid that leads to the production of JA and JA-Ile as well as the biosynthesis of ET (Sun et al., 2011; Wang et al., 2018), and HypSys from tomato and potato were reported to activate the octadecanoid pathway and the production of JA (Pearce and Ryan, 2003; Bhattacharya et al., 2013). On the contrary, CAPE1 significantly induces SA accumulation in tomato (Chen et al., 2014). In addition, other peptides seem to be involved in hormonal regulation upon different stresses. In fact, Arabidopsis PLANT NATRIURETIC PEPTIDE A, PNP-A, was shown to antagonize SA-mediated responses (Lee et al., 2020). Similarly, GRIM REAPER peptide was shown to be involved in hormonal regulation since SA and JA accumulation upon stress induced by O3 exposure was strongly reduced in the gri knock-out plants (Wrzaczek et al., 2009). On the other hand, PSK induces IAA and auxin-dependent responses in tomato plants against Botrytis cinerea infection (Zhang et al., 2018). Besides the classical hormones, in tomato, the constitutive expression of ProSystemin which leads to a higher accumulation of Systemin, enhances the production of secondary metabolites including lignans and tyrosine derivatives (Chen et al 2006; Pastor et al., 2018).
Plants under pathogenic or insect attack trigger a metabolic rearrangement to coordinate the biosynthesis of defense compounds with antimicrobial effects, such as phytoalexins. Very few reports have studied the production of defense metabolites downstream of defense peptides perception. In maize, it was found that ZmPep1 as well as ZmPep3 treatment induce the accumulation of benzoxazinoid phytoalexin, involved in lepidopteran defense (Huffaker et al 2011; Huffaker et al 2013).
2.2.6. Callose accumulation
Among the inducible downstream defense responses, we found a few reports of peptides inducing callose accumulation. Callose is a β-1,3 glucan polymer that accumulates in the plant cell wall in response to pathogen infection to strengthen the plant cell wall and restrict their entry (Luna et al., 2011; Ellinger and Voigt, 2014). Augmented callose formation is an important feature of β-aminobutiric acid (BABA)-induced resistance against pathogenic fungi that leads to plant protection (Conrath et al., 2006). Regarding peptide-triggered responses, Pep1, PIP1 and SCOOP12 were reported to induce the accumulation of callose in Arabidopsis plants although to a much lesser extent than flagellin or chitin (Hou et al 2014; Gully et al 2019).
2.2.7. Stomatal movement
Stomatal closure is also among the inducible defenses triggered by plants under attack since stomata are sites of bacterial pathogen entry in the plant (Melotto et al 2006). In this regard, PIP1 was found to induce stomatal closure in Arabidopsis (Hou et al., 2014). On the other hand, PNP-A was also reported to regulate stomatal closure upon biotic stress since pnp-A mutant displayed reduced stomatal closure and higher SA-related responses to bacterial infection, while a PNP-A overexpressing line closed its stomata more efficiently and lower SA-responses (Lee et al., 2020). Interestingly, the pnp-A displayed higher resistance against Pseudomonas syringae pv tomato DC3000 while the overexpression of PNP-A showed higher susceptibility, agreeing with the negative relation with SA-responsive defenses, but not with the ability to regulate the stomatal closure. In this study the authors infiltrated the bacteria, thus overpassing the defense mediated by the stomata closure. Indeed, Ficarra et al (2018) had the opposite phenotype, when using surface inoculation of the bacteria, then, the bacterium had to deal first with stomatal immunity.
2.2.8. Indirect defenses
Finally, sometimes plants can induce indirect defenses upon stimuli perception that includes the release of volatile organic compounds (VOCs) to attract pest natural enemies. Additionally, the released VOCs also primes distal parts of the plant or alert neighbor plants of upcoming stress. In maize, ZmPep3 treatment triggered an enhanced emission of volatiles which included terpenes and shikimate pathway-derived compounds that made plants more attractive to lepidopteran herbivore parasitoids (Huffaker et al., 2013). In tomato, Systemin induces the emission of volatiles that, on the one hand, attract pest natural enemies and, on the other hand, alert neighboring plants priming their defenses (Corrado et al., 2007; Coppola et al., 2017).
2.3. Role of phytocytokines in the defense response against pests and pathogens
Several studies have demonstrated that changing endogenous levels of some phytocytokines by overexpressing or silencing the precursor peptide produces changes in the natural resistance of plants against different attackers confirming their key role in plant defense (
Table 2).
Constitutive overexpression of the Pep1 precursor PROPEP1 confers resistance to the root pathogen Pythium irregulare in Arabidopsis (Huffaker et al., 2006). Similarly, overexpression of prePIP1 and prePIP2 in Arabidopsis induces resistance against P. syringae Pst DC3000 and Foc 699 (Hou et al., 2014). In the same line, overexpression lines of the CEP4 precursor displayed enhanced resistance against Pseudomonas syringae pv. tomato (Pto), whereas lost-of-function mutants showed susceptibility against the same pathogen (Rzemieniewski et al., 2022). In tomato, Prosystemin overexpressing plants are more resistant to several attackers including aphids, larvae, and necrotrophic fungi (Coppola et al., 2015) as well as plant viruses (Bubici et al., 2017). HypSys overexpression in tobacco leads to enhanced resistance to Helicoverpa armigera larvae (Ren and Lu, 2006). On the contrary, plants expressing antisense ProSystemin were more susceptible to Manduca sexta larvae (Orozco-Cádenas et al., 1993). A knockout mutant of SCOOP12 precursor showed higher susceptibility to Erwinia amylovora but enhanced resistance to Alternaria brassicicola (Gully et al 2019). Seemingly, loss of PSK signaling reduces resistance against necrotrophic fungi (Mosher et al 2013), whereas at the same time increases resistance to biotrophic bacteria (Igarashi et al., 2012) and fungi (Shen and Diener, 2013). Another example is the GRI peptide that triggers an increase in cell death and raises the resistance to virulent bacteria (Wrzaczek et al., 2009).
A contrasting effect on resistance to biotrophic and necrotrophic pathogens is observed among phytocytokines. This indicates specific roles of plant phytocytokines in resistance according to the attacker lifestyle and might be correlated with the specific hormonal regulation upon phytocytokine perception. Thus, it makes sense that peptides involved in defense against herbivores may also defend against necrotrophs since both defense responses usually involve JA regulation. For instance, Systemin is effective against several types of herbivores, such as caterpillars and aphids, as well as against necrotrophic fungi such as B. cinerea (Coppola et al., 2015), whereas although not tested it’s likely not involved in defense against hemibiotrophic such as P. syringae. Similarly, in Arabidopsis PNP-A was shown to antagonize SA-mediated and SA-primed defenses, thus overexpression of PNP-A resulted in compromised resistance to Pst DC3000 (Lee et al., 2020).
Table 2.
Effect of overexpression of phytocytokines or their precursors.
Table 2.
Effect of overexpression of phytocytokines or their precursors.
| Plan species of origin |
Peptide /precursor |
Recipient plant/organism |
Effect |
References |
| Arabidopsis |
PROPEP1 |
Arabidopsis |
Resistance to Pythium irregulare and Pseudomonas syringae
|
Huffaker et al., 2006 |
| Arabidopsis |
PrePIP1 |
Arabidopsis |
Resistance to foc 699
|
Hou et al., 2014 |
| Arabidopsis |
SCOOP |
Arabidopsis |
Resistance to Alternaria brassicicola
|
Gully et al., 2019 |
| Susceptibility against E. amylovora
|
Rhodes et al., 2021 |
| Arabidopsis |
RALF23 |
Arabidopsis |
Susceptibility to Pto DC3000 COR and P. cucumerina
|
Stregmann et al 2017 |
| Arabidopsis |
IDL6 |
Arabidopsis |
Susceptibility to P. syringae Pst DC3000 |
Wang et al., 2017 |
| Arabidopsis |
GRI |
Arabidopsis |
Susceptibility to P. syringae Pst DC3000
|
Wrzaczek et al., 2009 and 2014 |
| Arabidopsis |
CEP4 |
Arabidopsis |
Resistance to P. syringae Pto |
Rzemieniewski et al., 2022 |
| Tomato |
ProSystemin |
Tomato |
Resistance to herbivore |
Coppola et al., 2015 |
| Resistance to aphids |
Coppola et al., 2015 |
| Resistance to B. cinerea and A. alternata
|
Coppola et al., 2015 |
| Reduced susceptibility to Cucumber mosaic virus
|
Bubici et al., 2017 |
| Tomato |
ProSystemin |
Arabidopsis |
Resistance to B. cinerea
|
Zhang et al., 2017 |
| Tomato |
PSK |
Arabidopsis |
Susceptibility to Fusarium oxysporum
|
Shen and Diener, 2013 |
| Arabidopsis |
PSK |
Tomato |
Botrytis cinerea |
Zhang et al., 2018 |
| Maize |
Zip1 |
Ustilago maydis |
Resistance against Ustilago maydis
|
Ziemann et al., 2018 |
| Tobacco |
HypSys |
Tobacco |
Resistance to Helicovera armigera
|
Ren and Lu, 2006 |
3. Phytocytokines/peptides in plant-induced resistance and priming
In addition to direct responses to a challenge, plants also evolved the ability to activate stronger defense by inducing resistance mechanisms (IR) at local and distal plant tissues through the so-called systemic induced resistance (ISR; de Kessel et al 2021). The state of IR can be achieved by exposing plants to biological organisms but also by treating plants with proteins, xenobiotics, natural extracts, DNA, volatile organic compounds (VOCs), physical damage, or chemicals (Conrath et al 2006; Pastor et al 2013; Mauch-Mani et al., 2017; Thevenet et al., 2017; de Kesel et al., 2021). Plants under the IR state show augmented defense responses and better performance upon different challenges (Pieterse et al., 2012; Walters et al., 2013; Mauch-Mani et al., 2017; Kessel et al., 2021). Moreover, plants expressing IR trigger specific short term defense mechanisms (Pastor et al., 2013) and activate chromatin remodeling providing a longer term “plant memory” (Luna et al., 2012). When the plant's perception of an IR stimulus does not trigger major changes in the plant metabolism directly but rather shows an augmented response only when the challenge appears it is known as “defense priming” (Conrath et al., 2006; Martínez-Medina et al., 2016; Mauch-Mani et al., 2017). Primed plants exhibit a faster and stronger defense response that leads to enhanced disease protection against a broad range of pathogens and associated with low fitness cost (van Hulten et al., 2006; Pastor et al 2014; Martínez-Medina et al., 2016). Recently there has been a consensus that the IR phenotype is a sum of both direct and primed defense activation (De Kesel et al., 2021). Priming during SAR and ISR is expressed in distal tissues upon the perception of the secondary challenge (Mauch-Mani et al 2017). Sometimes the same stimuli can trigger either direct induced resistance or primed defenses depending on the concentration (Conrath et al., 2006). This indicates the importance of establishing an optimal dose threshold for achieving beneficial effects when using a resistance elicitor.
The biological function of small secreted peptides in plant basal immunity is extensively studied. In the above-mentioned sections, we describe endogenous phytocytokines triggering a huge range of defense responses and signaling cascades upon cell damage by pests and pathogens to amplify the defense response (
Figure 1A). Similar responses are observed when their precursor is overexpressed. But what is the outcome of their exogenous application against an upcoming attack? Because of their ability to activate the plant immune system and induce defensive responses at very low concentrations, they can be considered suitable candidates as defense elicitors (
Figure 1 B).
In natural environments, phytocytokines are released after plant perception of a biotic challenge during the activation of the first layer of immune responses, PTI. Then phytocytokines bind to their receptors to amplify and strengthen the already activated defenses and spread the danger alarm to adjacent cells (
Figure 1A). Hence, as common defense strategies, cellular responses are activated when both the phytocytokine and the challenge of PAMP are present. On the contrary, when peptides are used as defense elicitors, the plant perceives the phytocytokine prior the challenge. The plant recognizes them as danger signals and activates moderate defense signaling, thus when a future biotic challenge occurs the plant poses an enhanced defensive response displaying peptide-IR (
Figure 1B). However, the effect of exogenously applied peptides in the plant's defensive responses may differ from that triggered naturally when the endogenous peptide is released after the challenge.
Figure 1.
The natural function of defense peptides vs Peptide -Induced Resistance. (A) Cellular responses against a pathogenic fungi infection. 1, Pathogen penetration in the cell is perceived by plant membrane receptors. 2, Intracellular signaling and defense responses are produced, including ROS production, the opening of ion and Ca2+ channels, and MAPK cascade activation that lead to downstream transcriptional reprogramming and defense compounds production. In parallel, peptide precursors are synthesized and phytocytokines are released. 3, mature peptides are released to the apoplast where they are perceived by damaged and adjacent cells. 4, phytocytokines trigger the amplification of defense responses. 5, the battery of defensive elements impairs pathogen success. (B) Mechanisms of Peptide-IR. 1, cell membrane receptors perceive the exogenously applied phytocytokine. 2, Immune responses are activated as in (A).3, an invading fungal pathogen is perceived. 4, the plant is already prepared to counteract the infection, displaying a faster and stronger defense response that leads to enhanced resistance.
Figure 1.
The natural function of defense peptides vs Peptide -Induced Resistance. (A) Cellular responses against a pathogenic fungi infection. 1, Pathogen penetration in the cell is perceived by plant membrane receptors. 2, Intracellular signaling and defense responses are produced, including ROS production, the opening of ion and Ca2+ channels, and MAPK cascade activation that lead to downstream transcriptional reprogramming and defense compounds production. In parallel, peptide precursors are synthesized and phytocytokines are released. 3, mature peptides are released to the apoplast where they are perceived by damaged and adjacent cells. 4, phytocytokines trigger the amplification of defense responses. 5, the battery of defensive elements impairs pathogen success. (B) Mechanisms of Peptide-IR. 1, cell membrane receptors perceive the exogenously applied phytocytokine. 2, Immune responses are activated as in (A).3, an invading fungal pathogen is perceived. 4, the plant is already prepared to counteract the infection, displaying a faster and stronger defense response that leads to enhanced resistance.
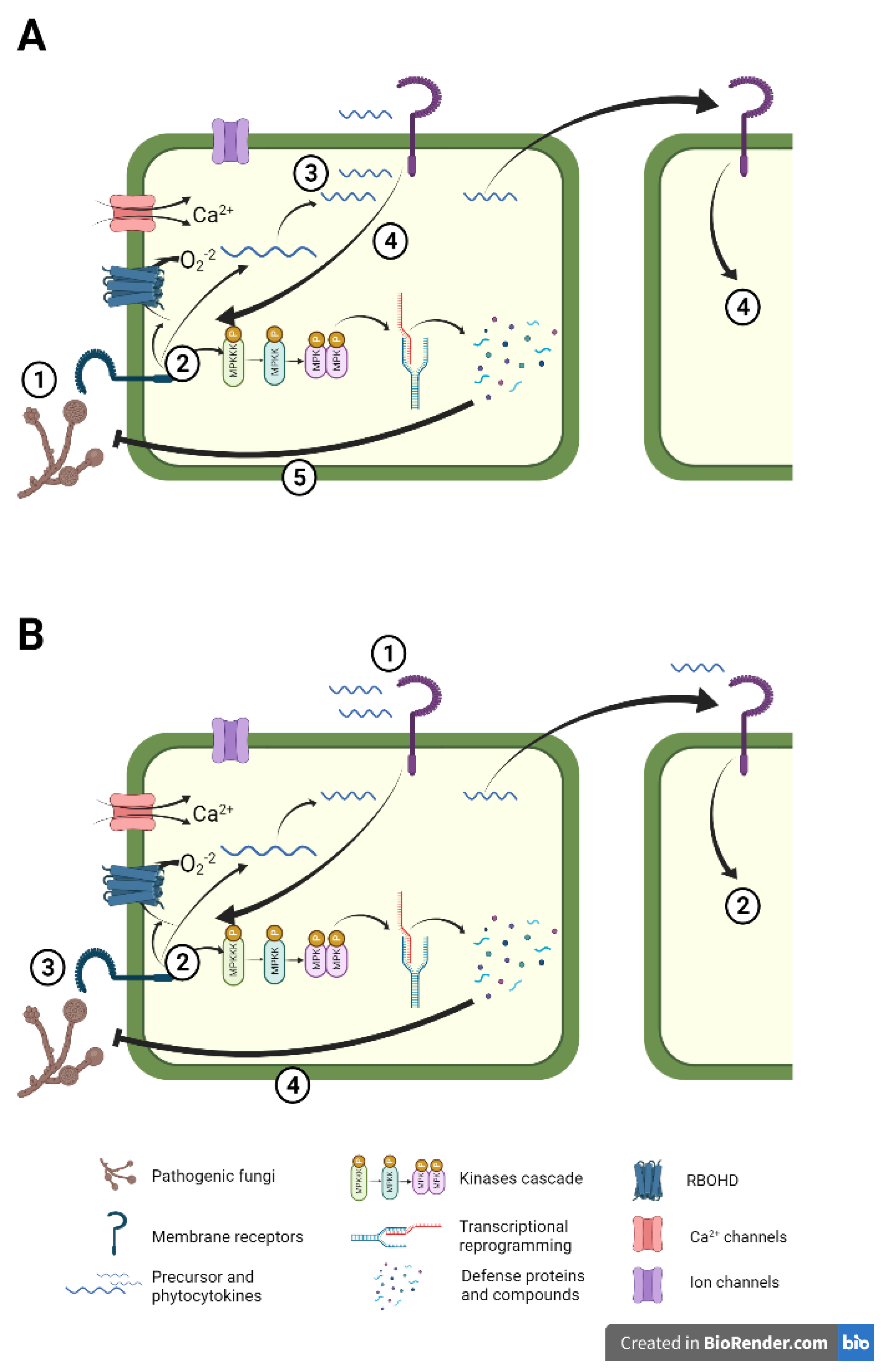
3.1. Peptide-Induced resistance against pests and pathogens
Although the natural function of phytocytokines is currently under study, their potential for induced resistance when applied exogenously needs further research. Nevertheless, there is some promising evidence of their benefits in plant effect (
Table 3).
Exogenous treatment of Pep3, PIP1, or SCOOP12 leads to Arabidopsis resistance to Pst DC 3000 (Ma et al., 2013; Hou et al., 2014; Gully et al 2019). In maize, the ZmPep1 treatment confers resistance to necrotrophic fungal pathogens Cochliobolis heterostrophus and Colletotrichum graminicola (Huffaker et al., 2011) whereas ZmPep3 treatment produced a reduction in Spodoptera exigua larval growth (Huffaker et al., 2013). In tomato, CAPE1 application induces resistance to both the herbivore Spodoptera litura and the biotrophic pathogen P. syringae DC3000 (Chen et al., 2014). Similarly, exogenously applied phytosulfokine (PSK), as well as Systemin, enhances resistance to the necrotrophic fungus B. cinerea in tomato (Zhang et al., 2018; Coppola et al., 2019). In addition, Systemin treatment also impairs the larval growth of Spodoptera litoralis (Coppola et al., 2019). SAMPs produced by citrus resistant to Candidatus liberibacter asiaticus induce systemic resistance against this bacterium when sprayed to the leaves of HLB-sensitive cultivars (Huang et al., 2021). Hence it seems clear that peptides induce resistance against herbivores and necrotrophs.
Seemingly to endogenous peptides, exogenous treatments trigger specific defense pathways inducing resistance against pathogens with the same lifestyle. In this line of evidence, when alternative pathogens with opposite lifestyles infect the plant, a pretreatment with peptides may trigger susceptibility. This is the case for Zip1 and PNP-A treatments that trigger susceptibility against B. cinerea and Pst DC3000 respectively (Ziemann et al 2018; Lee et al 2020).
3.2. Cross-species perception and peptide.induced resistance
Interestingly, a few studies have reported heterologous peptide sensing and signaling in taxonomically distant plant species. Although a report claims that tobacco cells do not respond to exogenous Systemin treatment (Scheer et al., 2003), a later study showed that tobacco calli and suspension cells responded to Systemin by both MAPK activation and weak-medium alkalinization (Malinowski et al., 2009). In addition, constitutive expression of the tomato ProSystemin gene in tobacco considerably affected the plant metabolism by inducing the synthesis of host proteins, several of which are involved in protection against pathogens, suggesting the ability of tobacco to reproduce Systemin signaling (Rocco et al., 2008). More surprisingly, Zhang et al. (2017), reported that tomato Systemin was sensed by Arabidopsis plants, leading to an inhibition of seedling root growth and the expression of the plant defensin PDF1.2. On the other hand, tobacco cells transformed with the Arabidopsis Pep1 receptor PEPR1 responded to nanomolar concentrations of Pep1, producing a strong alkalinization of the cell culture medium, suggesting again a capacity of tobacco to activate signaling upon a heterologous peptide treatment (Yamaguchi et al., 2006). Later, Huffaker and coworkers (2013) found ZmPep orthologs in rice (OsPep2) and sorghum (SbPep1) and tested their ability to induce volatile emissions in maize plants. They found that both peptides elicited a full spectrum of herbivore-associated volatiles at the same level as those induced by maize Peps. This suggests that Peps from rice and sorghum species might be able to induce resistance in maize similarly to ZmPeps.
However, evidence of peptide-induced resistance in heterologous species is very scarce (
Table 3). Heterologous peptides, including Sytemins from Solanaceae and AFPs from radish, confer resistance to the necrotroph Plectosphaerella cucumerina in Arabidopsis (Pastor-Fernández et al., 2020). In addition, very recently it was demonstrated that Systemin is also able to induce resistance against necrotrophic fungi in the taxonomically distant species Vitis vinifera, as well as in Solanum melongea, which is taxonomically closer but still does not produce the peptide (Molisso et al., 2021). The functionality of peptide treatments in crosspieces-IR is emerging as a very interesting tool to be used as general agents of biocontrol and thus deserves further research.
Table 3.
Effects of exogenous peptide applications in resistance against pest and pathogens
Table 3.
Effects of exogenous peptide applications in resistance against pest and pathogens
| Plant species of origin |
Peptide |
Recipient plant |
effect |
References |
| Arabidopsis |
Pep3 |
Arabidopsis |
Resistance to Pst DC 3000
|
Ma et al., 2013 |
| Arabidopsis |
PIP1 |
Arabidopsis |
Resistance to Pst DC 3000
|
Hou et al., 2014 |
| Arabidopsis |
SCOOP12 |
Arabidopsis |
Resistance to Pst DC 3000
|
Gully et al., 2019 |
| Maize |
ZmPep1 |
Maize |
Resistance to Cochliobolis heterostrophus and C. graminicola
|
Huffaker et al., 2011 |
| Maize |
ZmPep3 |
Maize |
Resistance to Spodoptera exigua
|
Huffaker et al., 2013 |
| Tomato |
CAPE1 |
Tomato |
Resistance to Spodoptera litura
|
Chen et al., 2014 |
| Resistance to Pst DC 3000
|
| Tomato |
PSK |
Tomato |
Resistance to B. cinerea
|
Zhang et al., 2018 |
| Tomato |
Systemin |
Tomato |
Resistance to Spodoptera litoralis
|
Coppola et al., 2019 |
| |
|
|
Resistance to B. cinerea
|
|
| Arabidopsis |
PNP-A |
Arabidopsis |
Susceptibility to P. syringae
|
Lee et al., 2020 |
| Maize |
Zip1 |
Maize |
Susceptibility to B. cinerea
|
Ziemann et al., 2018 |
| Tomato |
Systemin |
Arabidopsis |
Resistance to P. cucumerina
|
Pastor-Fernández et al., 2020 |
| Potato |
PotSysII |
| Pepper |
PepSys |
| Nightshade |
Nishsys |
| Tomato |
HypSys |
| Radish |
AFP's |
| Arabidopsis |
Pep1 |
Arabidopsis |
Resistance to P. cucumerina
|
Pastor-Fernández et al., 2020 |
| Tomato |
Systemin |
Eggplant |
Resistance to B. cinerea
|
Molisso et al., 2021 |
| Vitis vinifera |
| Citrus |
SAMP |
Citrus |
Resistance to Candidatus liberibacter asiaticus
|
Huang et al., 2021 |
4. Cooperative functioning of peptides in innate immunity and induced resistance
As previously mentioned, defense peptides function as amplifiers of the “warning alarm”. The increasing number of identified peptides functioning as phytocytokines within the same plant species such as Arabidopsis, maize, or tomato suggests a possible interaction between them to coordinate the immune response. Interesting studies indicate a complex network of interconnected peptides in the plant response to stress and defense by performing an in silico analysis of the predicted peptide interactome (Marmiroli and Maestri 2014).
There is evidence of peptide cooperation to amplify the defense response PIP-RLK7 and PEP1-PEPR1 cooperate amplifying the immune response triggered by the PAMP flagellin in Arabidopsis (Hou et al., 2014). Similarly, SCOOP12 and Pep1 induce the expression of several of the SCOOP precursors genes, PROSCOOPs, (Gully et al., 2019), suggesting that Pep1 is cooperating with SCOOPs to amplify its feedback loop. In tomato, Systemin and HypSys function together in the regulation of the long-distance wound signaling response in tomato through the upregulation of the octadecanoid pathway and the synthesis of jasmonates (Narvaéz-Vázquez et al., 2007). Finally, CAPE1 is among the signals induced upon wounding plus MeJA treatment together with Systemin, both with a similar expression pattern, which means that both peptides regulate the response to the same stress (Chen et al., 2014). All these findings suggest synergistic effects between specific peptides, raising the question of the possible coapplication of different peptides, or peptides plus other danger signals as an interesting strategy to potentiate Peptide-IR.
In this regard, evidence of the interdependence of PAMP and DAMP signaling has been already reported in the literature. Functional Pep/PEPR1 system is required for complete FLS2 immune signaling, including flg22-induced Ca2+ increase, H2O2 production, defense gene activation as well as to flg22/FLS2 induced hampering of pathogen growth, whereas loss of FLS2 similarly impaired PEPR1 signaling (Ma et al., 2012). Later, Ma and coworkers (2013) also observed that maximal H2O2 and NO production in response to Pep3 required the presence of both PEPR1 and FLS2 receptors, suggesting again a cooperation between flg22 and Peps signaling.
In terms of IR, a beneficial effect of the coapplication of PAMPs and DAMPs has been described, although it is poorly explored. Klauser et al., (2013) observed that Pep1 triggered oxidative burst when applied to leaf discs and was enhanced by a subsequent application of either Flg22 or chitin. Recently, Pastor et al., (2022) demostrated that the simultaneous perception of Flg22 (non-self) and DAMPs (self) produce an amplification of PTI, as well as the production of phytocytokines. Similarly, an integration between HAMP and DAMP signaling was evidenced since the application of a rice Pep3 together with insect oral secretions produced an amplification of a great variety of defense responses in rice plants, such as activation of MAPK and production of defense hormones and metabolites (Shinya et al 2018).
The previous observations demonstrate that the co-application of danger signals of different natures could be a useful tool to enhance the IR defensive response, and mediated bt phytocytokines.
5. Cost of peptide-induced resistance
Activation of plant defenses is commonly associated with fitness costs due to the allocation of energy and resources toward the production of defensive compounds (Hulten et al., 2006). As mentioned above, a beneficial trait of defense priming is the low fitness cost produced by the stimulus (Martínez-medina et al., 2016). Since phytocytokines are danger signals triggering direct and early defense responses, their perception is expected to have a substantial cost in plant growth and development, although, so far, very few studies have measured the possible fitness costs of using peptides as defense inducers.
Corrado and coworkers (2011) reported that overexpression of the Systemin precursor ProSystemin resulted in constitutive activation of inducible defenses that was costly in tomato, affecting plant growth, development, and reproduction. Note that the cost of a constitutive overexpressing line may differ substantially from that of a punctual treatment. On the contrary, a very recent study reported that PS+ plants did not show fitness reduction, in fact, they increased their growth and productivity (Luna-Martínez et al, 2021) suggesting a role of ProSystemin in the defense-growth balance. Indeed, further research is needed to address this issue.
6. Conclusions and future perspective
Phytocytokines are a type of small molecule that is present in plants when stress appears. A long way is still to be done, to decipher the role of these molecules in cell-to-cell communication or their induction of downstream cell events. Being small molecules, the processing can be really complex as already related along the review, and it is of great interest to clarify each step in the production of the peptide and its role in defense against fungi, bacteria, viruses, nematodes, and herbivores. Studies at both levels, molecular and applied, are necessary to fully explore the action of the phytocytokines, including their precursors. The plant application or co-application of these small molecules will also allow us to explore the possibilities of adaptive immunity against biotic stress, and crop protection using natural compounds that exert an effect on plants. In this regard, also may be interesting to follow the entire biological cycle of the plant, to see when is the best moment for the application and which is the impact on yield production.
Author Contributions
Conceptualization, V.P. and J.P.F.; writing—original draft preparation, J.P.F., and P.S.B.; writing—review and editing, V.F and V.P.; visualization, M.C.; literature search, M.C., J.P.F; supervision, V.P. and P.S.B.; funding acquisition, V.F., P.S.B., V.P.; figures and tables J.P.F, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: GV/2018/115 and CIACO/2021/092 projects from Generalitat Valenciana; RTI2018-094350-B-C33 and PID2021-124813OB-C32 from the Spanish Government; also, the project of INTOMED-PRIMA, from the PRIMA Foundation.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albert, M. (2013). Peptides as triggers of plant defence. In Journal of Experimental Botany (Vol. 64, Issue 17, pp. 5269–5279). Oxford Academic. [CrossRef]
- Bartels S, Boller T. (2015). Quo vadis, Pep? Plant elicitor peptides at the crossroads of immunity, stress, and development.
J Exp Bot. 2015 Aug;66(17):5183-93. Epub 2015 Apr 23. PMID: 25911744. [CrossRef]
- Beloshistov, R.E. , Dreizler, K., Galiullina, R.A., Alexander, I., Tuzhikov, A.I., Serebryakova, M.V., et al., (2017). Phytaspase-mediated precursor processing and maturation of the wound hormone systemin. New Phytologist. 218(3):1167-1178. [CrossRef]
- Bhattacharya R, Koramutla MK, Negi M, Pearce G, Ryan CA. (2013). Hydroxyproline-rich glycopeptide signals in potato
elicit signalling associated with defense against insects and pathogens. Plant Sci. 2013 Jun;207:88-97. Epub 2013 Mar 15. PMID: 23602103. [CrossRef]
- Boudsocq M, Sheen J. (2012). CDPKs in immune and stress signaling. Trends Plant Sci. 2013 Jan;18(1):30-40. . Epub 2012 Sep 10. PMID: 22974587; PMCID: PMC3534830. [CrossRef]
- Bubici, G. , Carluccio, A. V., Stavolone, L., & Cillo, F. (2017). Prosystemin overexpression induces transcriptional modifications of defense-related and receptor-like kinase genes and reduces the susceptibility to Cucumber mosaic virus and its satellite RNAs in transgenic tomato plants. PLOS ONE, 12(2), e0171902. [CrossRef]
- Chen H, Jones AD, Howe GA. (2006). Constitutive activation of the jasmonate signaling pathway enhances the production
of secondary metabolites in tomato. FEBS Lett. 2006 May 15;580(11):2540-6. Epub 2006 Apr 7. PMID: 16647069. [CrossRef]
- Chen YL, Lee CY, Cheng KT, Chang WH, Huang RN, Nam HG, Chen YR (2014). Quantitative peptidomics study reveals
that a wound-induced peptide from PR-1 regulates immune signaling in tomato. Plant Cell. 2014
Oct;26(10):4135-48. Epub 2014 Oct 31. PMID: 25361956; PMCID: PMC4247587. [CrossRef]
- Conrath U, Beckers GJ, Flors V, García-Agustín P, Jakab G, Mauch F, Newman MA, Pieterse CM, Poinssot B, Pozo MJ, Pugin A, Schaffrath U, Ton J, Wendehenne D, Zimmerli L, Mauch-Mani B. (2006). Priming: getting ready for battle. Mol Plant Microbe Interact. 2006 Oct;19(10):1062-71. PMID: 17022170. [CrossRef]
- Constabel, C.P. , Yip, L. and Ryan, C.A. (1998). Prosystemin from potato, black nightshade, and bell pepper: primary structure and biological activity of predicted systemin polypeptides. Plant Molecular Biology 36: 55–62. [CrossRef]
- Coppola M, Cascone P, Madonna V, Di Lelio I, Esposito F, Avitabile C, Romanelli A, Guerrieri E, Vitiello A, Pennacchio F, Rao R, Corrado G. (2017). Plant-to-plant communication triggered by systemin primes anti-herbivore resistance in tomato. Sci Rep. 2017 Nov 14;7(1):15522. . PMID: 29138416; PMCID: PMC5686165. [CrossRef]
- Coppola, M., Corrado, G., Coppola, V., Cascone, P., Martinelli, R., Digilio, M. C., Pennacchio, F., & Rao, R. (2015). Prosystemin Overexpression in Tomato Enhances Resistance to Different Biotic Stresses by Activating Genes of Multiple Signaling Pathways. Plant Molecular Biology Reporter, 33(5), 1270–1285. [CrossRef]
- Coppola, M., Di Lelio, I., Romanelli, A., Gualtieri, L., Molisso, D., Ruocco, M., Avitabile, C., Natale, R., Cascone, P., Guerrieri, E., Pennacchio, P., and Rao, R. (2019). Tomato Plants Treated with Systemin Peptide Show Enhanced Levels of Direct and Indirect Defense Associated with Increased Expression of Defense-Related Genes. Plants 2019, 8, 395. [CrossRef]
- Corrado, G. , Agrelli, D., Rocco, M., Basile, B., Marra, M., & Rao, R. (2011). Systemin-inducible defence against pests is costly in tomato. Biologia plantarum, 55(2), 305-311. [CrossRef]
- Corrado, G. , Sasso, R., Pasquariello, M., Iodice, L., Carretta, A., Cascone, P., Ariati, L., Digilio, M. C., Guerrieri, E., & Rao, R. (2007). Systemin regulates both systemic and volatile signaling in tomato plants. Journal of Chemical Ecology, 33(4), 669–681. [CrossRef]
- Couto, D. , & Zipfel, C. (2016). Regulation of pattern recognition receptor signalling in plants. In Nature Reviews Immunology (Vol. 16, Issue 9, pp. 537–552). Nature Publishing Group. [CrossRef]
- De Kesel J, Conrath U, Flors V, Luna E, Mageroy MH, Mauch-Mani B, Pastor V, Pozo MJ, Pieterse CMJ, Ton J, Kyndt T (2021). The Induced Resistance Lexicon: Do's and Don'ts. Trends Plant Sci. 2021 Jul;26(7):685-691. Epub 2021 Jan 30. PMID: 33531282. [CrossRef]
- Ellinger D, Voigt CA. (2014). Callose biosynthesis in Arabidopsis with a focus on pathogen response: what we have learned within the last decade. Ann Bot. 2014 Oct;114(6):1349-58. Epub 2014 Jul 1. PMID: 24984713; PMCID: PMC4195556. [CrossRef]
- Felix G, Duran JD, Volko S, Boller T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999 May;18(3):265-76. PMID: 10377992. [CrossRef]
- Ficarra, F.A. , Grandellis, C., Garavaglia, B.S., Gottig, N., andOttado, J. (2018). Bacterial and plant natriuretic peptides improveplant defence responses against pathogens. Mol. Plant Pathol. 19:801–811. [CrossRef]
- Fichman Y, Mittler R. (2020). Rapid systemic signaling during abiotic and biotic stresses: is the ROS wave master of all trades? Plant J. 2020 Jun;102(5):887-896. Epub 2020 Feb 18. PMID: 31943489. [CrossRef]
- Green TR, Ryan CA. (1972). Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776-7. PMID: 17836138. [CrossRef]
- Gully, K., Pelletier, S., Guillou, M.-C., Ferrand, M., Aligon, S., Pokotylo, I., Perrin, A., Vergne, E., Fagard, M., Ruelland, E., Grappin, P., Bucher, E., Renou, J.-P., & Aubourg, S. (2019). The SCOOP12 peptide regulates defense response and root elongation in Arabidopsis thaliana. Journal of Experimental Botany, 70(4), 1349–1365. [CrossRef]
- Gust, A. A. , Pruitt, R., & Nürnberger, T. (2017). Sensing Danger: Key to Activating Plant Immunity. In Trends in Plant Science (Vol. 22, Issue 9, pp. 779–791). Elsevier Ltd. [CrossRef]
- Hander T, Fernández-Fernández ÁD, Kumpf RP, Willems P, Schatowitz H, Rombaut D, Staes A, Nolf J, Pottie R, Yao P, Gonçalves A, Pavie B, Boller T, Gevaert K, Van Breusegem F, Bartels S, Stael S. (2019). Damage on plants activates Ca2+-dependent metacaspases for release of immunomodulatory peptides. Science. 2019 Mar 22;363(6433):eaar7486. PMID: 30898901. [CrossRef]
- He Y, Zhou J, Shan L, Meng X (2018). Plant cell surface receptor-mediated signaling - a common theme amid diversity. J Cell Sci. 2018 Jan 29;131(2):jcs209353. PMID: 29378836; PMCID: PMC6518216. [CrossRef]
- Heil, M. (2009). Damaged-self recognition in plant herbivore defence. Trends Plant Sci. 14 356–363. [CrossRef]
- 28. Hou S, Liu D, Huang S, Luo D, Liu Z, Xiang Q, Wang P, Mu R, Han Z, Chen S. (2021) The Arabidopsis MIK2 receptor elicits immunity by sensing a conserved signature from phytocytokines and microbes. Nat Commun 12(1), 5494. [CrossRef]
- Hou S, Liu D, Huang S, Luo D, Liu Z, Xiang Q, Wang P, Mu R, Han Z, Chen S. (2021) The Arabidopsis MIK2 receptor elicits immunity by sensing a conserved signature from phytocytokines and microbes. Nat Commun 12(1), 5494. 1. [CrossRef]
- Hou, S. , Liu, Z., Shen, H., & Wu, D. (2019). Damage-associated molecular pattern-triggered immunity in plants. In Frontiers in Plant Science (Vol. 10, p. 646). Frontiers Media S.A. [CrossRef]
- Hou, S. , Wang, X., Chen, D., Yang, X., Wang, M., Turrà, D., Pietro, A. Di, & Zhang, W. (2014). The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7. PLOS Pathogens, 10(9), e1004331. [CrossRef]
- Huang CY, Araujo K, Sánchez JN, Kund G, Trumble J, Roper C, Godfrey KE, Jin H. A (2021). stable antimicrobial peptide with dual functions of treating and preventing citrus Huanglongbing. Proc Natl Acad Sci U S A. 2021 Feb 9;118(6):e2019628118. PMID: 33526689; PMCID: PMC8017978. [CrossRef]
- Huffaker A, Dafoe NJ, Schmelz EA. (2011). ZmPep1, an ortholog of Arabidopsis elicitor peptide 1, regulates maize innate immunity and enhances disease resistance. Plant Physiol. 2011 Mar;155(3):1325-38. Epub 2011 Jan 4. PMID: 21205619; PMCID: PMC3046589. [CrossRef]
- Huffaker A, Pearce G, Veyrat N, Erb M, Turlings TC, Sartor R, Shen Z, Briggs SP, Vaughan MM, Alborn HT, Teal PE, Schmelz EA. (2013). Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense. Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5707-12. Epub 2013 Mar 18. PMID: 23509266; PMCID: PMC3619339. [CrossRef]
- Huffaker, A., Pearce, G., and Ryan, C.A. (2006). An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. U.S.A. 103, 10098–10103. [CrossRef]
- Igarashi D, Tsuda K, Katagiri F. (2012). The peptide growth factor, phytosulfokine, attenuates pattern-triggered immunity. Plant J. 2012 Jul;71(2):194-204. Epub 2012 May 14. PMID: 22353039. [CrossRef]
- Jeworutzki E, Roelfsema MR, Anschütz U, Krol E, Elzenga JT, Felix G, Boller T, Hedrich R, Becker D.(2010). Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca-associated opening of plasma membrane anion channels. Plant J. 2010 May;62(3):367-78. Epub 2010 Jan 25. PMID: 20113440. [CrossRef]
- Klauser D, Flury P, Boller T, Bartels S. (2013). Several MAMPs, including chitin fragments, enhance AtPep-triggered oxidative burst independently of wounding. Plant Signal Behav. 2013 Sep;8(9):e25346. Epub 2013 Jun 19. PMID: 23803750; PMCID: PMC4002628. [CrossRef]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. (2004). The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell. 2004 Dec;16(12):3496-507. Epub 2004 Nov 17. PMID: 15548740; PMCID: PMC535888. [CrossRef]
- Lee, S.-M. , Kim, S.-K., Lee, N., Ahn, C.-Y. and Ryu, C.-M. (2020). d-Lactic acid secreted by Chlorella fusca primes pattern-triggered immunity against Pseudomonas syringae in Arabidopsis. Plant J, 102: 761-778. [CrossRef]
- Li Q, Wang C, Mou Z (2020). Perception of Damaged Self in Plants. Plant Physiol. 2020 Apr;182(4):1545-1565. Epub 2020 Jan 6. PMID: 31907298; PMCID: PMC7140957. [CrossRef]
- Liu Z, Wu Y, Yang F, Zhang Y, Chen S, Xie Q, Tian X, Zhou JM. (2013a). BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proceedings of the National Academy of Sciences of the United States of America 110, 6205–6210. [CrossRef]
- Lu, D. , Wu, S., Gao, X., Zhang, Y., Shan, L., & He, P. (2010). A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proceedings of the National Academy of Sciences of the United States of America, 107(1), 496–501. [CrossRef]
- Luna E, Bruce TJ, Roberts MR, Flors V, Ton J (2012). Next-generation systemic acquired resistance. Plant Physiol. 2012 Feb;158(2):844-53. Epub 2011 Dec 5. PMID: 22147520; PMCID: PMC3271772. [CrossRef]
- Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J (2011). Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact. 2011 Feb;24(2):183-93. PMID: 20955078. [CrossRef]
- Luna-Martínez, M.; Martínez-Gallardo, N.; Casarrubias-Castillo, K.; Monti, S.M.; Coppola, M.; Rao, R.; Délano-Frier, J.P. (2021). Development and Yield Traits Indicate That the Constitutive Wound Response Phenotype of Prosystemin Overexpressing Tomato Plants Entails No Fitness Penalty. Agronomy 2021, 11, 1148. [CrossRef]
- Ma Y, Walker RK, Zhao Y, Berkowitz GA. (2012). Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc Natl Acad Sci U S A. 2012 Nov 27;109(48):19852-7. Epub 2012 Nov 12. PMID: 23150556; PMCID: PMC3511762. [CrossRef]
- Ma Y, Zhao Y, Walker RK, Berkowitz GA. (2013). Molecular steps in the immune signaling pathway evoked by plant elicitor peptides: Ca2+-dependent protein kinases, nitric oxide, and reactive oxygen species are downstream from the early Ca2+ signal. Plant Physiol. 2013 Nov;163(3):1459-71. Epub 2013 Sep 9. PMID: 24019427; PMCID: PMC3813664. [CrossRef]
- Macho AP, Zipfel C. (2014). Plant PRRs and the activation of innate immune signaling. Mol Cell. 2014 Apr 24;54(2):263-72. PMID: 24766890 . [CrossRef]
- Malinowski, R. , Higgins, R., Luo, Y., Piper, L., Nazir, A., Bajwa, V. S., Clouse, S. D., Thompson, P. R., & Stratmann, J. W. (2009). The tomato brassinosteroid receptor BRI1 increases binding of systemin to tobacco plasma membranes, but is not involved in systemin signaling. Plant Molecular Biology 2009 70:5, 70(5), 603–616. [CrossRef]
- Marmiroli N, Maestri E. (2014). Plant peptides in defense and signaling. Peptides. Jun;56:30-44. Epub 2014 Mar 26. PMID: 24681437.
- Martínez-Medina, A. , Flors, V., Heil, M., Mauch-Mani, B., Pieterse, C. M. J. J., Pozo, M. J., Ton, J., van Dam, N. M., & Conrath, U. (2016). Recognizing plant defense priming. Trends in Plant Science, 21(10), 818–822. [CrossRef]
- Matsubayashi Y, Sakagami Y. (1996). Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7623-7. PMID: 8755525; PMCID: PMC38796. [CrossRef]
- Matsubayashi Y, Sakagami Y. (2006). Peptide hormones in plants. Annu Rev Plant Biol. 2006;57:649-74. PMID: 16669777. [CrossRef]
- Matsubayashi, Y. (2014). Posttranslationally modified small-peptide signals in plants. Annu Rev Plant Biol. 2014;65:385-413. PMID: 24779997. [CrossRef]
- Mauch-Mani, B. , Baccelli, I., Luna, E. and Flors, V. (2017). Defense Priming: An Adaptive Part of Induced Resistance. Anuu. Rev. Plant Biol. 68:485-512. [CrossRef]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell. 2006 Sep 8;126(5):969-80. PMID: 16959575. [CrossRef]
- Mithofer, A. , & Boland, W. (2008). Recognition of herbivory-associated molecular patterns. Plant Physiology, 146(3), 825-831. [CrossRef]
- Molisso D, Coppola M, Aprile AM, Avitabile C, Natale R, Romanelli A, Chiaiese P, Rao R. (2021). Colonization of Solanum melongena and Vitis vinifera Plants by Botrytis cinerea Is Strongly Reduced by the Exogenous Application of Tomato Systemin. J Fungi (Basel). (2021) Dec 29;7(1):15. PMID: 33383908; PMCID: PMC7824362. [CrossRef]
- Mosher S, Seybold H, Rodriguez P, Stahl M, Davies KA, Dayaratne S, Morillo SA, Wierzba M, Favery B, Keller H, Tax FE, Kemmerling B. (2013). The tyrosine-sulfated peptide receptors PSKR1 and PSY1R modify the immunity of Arabidopsis to biotrophic and necrotrophic pathogens in an antagonistic manner. Plant J. 2013 Feb;73(3):469-82. Epub 2012 Nov 26. PMID: 23062058. [CrossRef]
- Moyen C, Hammond-Kosack KE, Jones J, Knight MR, Johannes E. (1998). Systemin triggers an increase in cytoplasmic calcium in tomato mesophyll cells: Ca2+ mobilization from intra- and extracellular compartments. Plant Cell Environ. 21:1101–11. [CrossRef]
- Narváez-Vásquez J, Orozco-Cárdenas ML, Ryan CA.(2007). Systemic wound signaling in tomato leaves is cooperatively regulated by systemin and hydroxyproline-rich glycopeptide signals. Plant Mol Biol. 2007 Dec;65(6):711-8. Epub 2007 Sep 25. PMID: 17899396 . [CrossRef]
- Ngou BPM, Ahn HK, Ding P, Jones JDG. Mutual potentiation of plant immunity by cell-surface and intracellular receptors (2021). Nature. 2021 Apr;592(7852):110-115. Epub 2021 Mar 10. [CrossRef]
- Olsson V, Joos L, Zhu S, Gevaert K, Butenko MA, De Smet I. (2019). Look Closely, the Beautiful May Be Small: Precursor-Derived Peptides in Plants. Annu Rev Plant Biol. 2019 Apr 29;70:153-186. Epub 2018 Dec 10. PMID: 30525926. [CrossRef]
- Orozco-Cardenas M, McGurl B, Ryan CA. (1993). Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc. Natl. Acad. Sci. USA 90:8273–76. [CrossRef]
- Pastor V, Balmer A, Gamir J, Flors V, Mauch-Mani B. (2014). Preparing to fight back: generation and storage of priming compounds. Front Plant Sci. 2014 Jun 24;5:295. PMID: 25009546; PMCID: PMC4068018. [CrossRef]
- Pastor V, Cervero R, Gamir J. (2022). The simultaneous perception of self- and non-self-danger signals potentiates plant innate immunity responses. Planta. 2022 Jun 13;256(1):10. PMID: 35697869; PMCID: PMC9192368. [CrossRef]
- Pastor, V. , Luna, E., Ton, J., Cerezo, M., García-Agustín, P. and Flors, V. (2013). Fine Tuning of Reactive Oxygen Species Homeostasis Regulates Primed Immune Responses in Arabidopsis. MPMI Vol. 26, No. 11, pp. 1334–1344. [CrossRef]
- Pastor, V. , Sánchez-Bel, P., Gamir, J., Pozo, M.J. and Flors, V. (2018). Accurate and easy method for Systemin quantification and examining metabolic changes under different endogenous levels. Plant Methods 14:33. [CrossRef]
- Pastor-Fernández J, Gamir J, Pastor V, Sanchez-Bel P, Sanmartín N, Cerezo M, Flors V. (2020). Arabidopsis Plants Sense Non-self Peptides to Promote Resistance Against Plectosphaerella cucumerina. Frontiers in Plant Science 11, 529. [CrossRef]
- Pastor-Fernández J, Sánchez-Bel P, Gamir J, Pastor V, Sanmartín N, Cerezo M, Andrés-Moreno S, Flors V. (2022) Tomato Systemin induces resistance against Plectosphaerella cucumerina in Arabidopsis through the induction of phenolic compounds and priming of tryptophan derivatives. Plant Sci. 2022 Aug;321:111321. Epub 2022 May 14. PMID: 35696921. [CrossRef]
- Pearce, G., and Ryan, C. A. (2003). Systemic signaling in tomato plants for defense against herbivores. Isolation and characterization of three novel defense signalling glycopeptide hormones coded in a single precursor gene. J. Biol. Chem. 278, 30044–30050. [CrossRef]
- Pearce, G., Moura, D. S., Stratmann, J., and Ryan, C. A. (2001). Production of multiple plant hormones from a single polyprotein precursor. Nature 411, 817–820. [CrossRef]
- Pearce, G. , Strydom, D., Johnson, S., & Ryan, C. A. (1991). A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science, 253(5022), 895–898. [CrossRef]
- Pearce, G. , Yamaguchi, Y., Barona, G., and Ryan, C. A. (2010). A subtilisin-like protein from soybean contains an embedded, cryptic signal that activates defense-related genes. Proc. Natl. Acad. Sci. U.S.A. 107, 14921–14925. [CrossRef]
- Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC. (2012). Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489-521. Epub 2012 May 3
PMID: 22559264. [CrossRef]
- Ren F. and Lu Y. (2006). Overexpression of tobacco hydroxyproline-rich glycopeptide systemin precursor A gene in
transgenic tobacco enhances resistance against Helicoverpa armigera larvae. Plant Science Volume 171, Issue 2, August
2006, Pages 286-292. [CrossRef]
- Rhodes, J. , Yang, H., Moussu, S., Boutrot, F., Santiago, J., & Zipfel, C. (2021). Perception of a divergent family of phytocytokines by the Arabidopsis receptor kinase MIK2. Nature Communications 2021 12:1, 12(1), 1–10. [CrossRef]
- Rocco, M. , Corrado, G., Arena, S., D’Ambrosio, C., Tortiglione, C., Sellaroli, S., Marra, M., Rao, R., Scaloni, A.l. (2008). The expression of tomato prosystemin gene in tobacco plants highly affects host proteomic repertoire. Journal of Proteomics 71: 176–185. [CrossRef]
- Roy, S., Lundquist, P. Michael Udvardi, M. and Wolf-Rüdiger Scheible W. (2018). Small and Mighty: Peptide hormones
in plant biology, The Plant Cell, Volume 30, Issue 8, August 2018, tpc.118.tt0718. [CrossRef]
- Rzemieniewski, J. , Leicher, H., Lee, H., Broyart, C., Nayem, S., Wiese, C., Maroschek, J., Camgoez, Z., Lalun, V., Djordjevic, M., Vlot, C., Hückelhoven, R., Santiago, J., Stegmann, M. (2022). Phytocytokine signaling integrates cell surface immunity and nitrogen limitation. [CrossRef]
- Schaller, A. (1999). Oligopeptide signalling and the action of systemin. Plant Mol Biol. 1999 Jul;40(5):763-9. PMID: 10487211. [CrossRef]
- Scheer, J.M. , Pearce, G., Ryan, C.A. (2003). Generation of systemin signaling in tobacco by transformation with the tomato systemin receptor kinase gene. Proceedings of the National Academy of Sciences, USA 100: 10114–10117. [CrossRef]
- Shen Y, Diener AC. (2013). Arabidopsis thaliana resistance to fusarium oxysporum 2 implicates tyrosine-sulfated peptide signaling in susceptibility and resistance to root infection. PLoS Genet. 2013 May;9(5):e1003525. Epub 2013 May 23. PMID: 23717215; PMCID: PMC3662643. [CrossRef]
- Shen, W. , Liu, J., Li JF. (2019) Type-II Metacaspases Mediate the Processing of Plant Elicitor Peptides in Arabidopsis. Molecular Plant 12, 1524–1533. [CrossRef]
- Shinya T, Yasuda S, Hyodo K, Tani R, Hojo Y, Fujiwara Y, Hiruma K, Ishizaki T, Fujita Y, Saijo Y, Galis. (2018). I. Integration of danger peptide signals with herbivore-associated molecular pattern signaling amplifies anti-herbivore defense responses in rice. Plant J. 2018 May;94(4):626-637. Epub 2018 Apr 1. PMID: 29513388. [CrossRef]
- Stegmann M, Monaghan J, Smakowska-Luzan E, Rovenich H, Lehner A, Holton N, Belkhadir Y, Zipfel C. (2017). The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science. 2017 Jan 20;355(6322):287-289. PMID: 28104890. [CrossRef]
- Stratmann JW, Ryan CA. (1997). Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc. Natl. Acad. Sci. USA 94:11085–89. [CrossRef]
- Stührwohldt, N. , Schaller A. (2019). Regulation of plant peptide hormones and growth factors by post-translational modification. Plant Biology 21, 49-63. [CrossRef]
- Sun JQ, Jiang HL, Li CY. (2011). Systemin/Jasmonate-mediated systemic defense signaling in tomato. Mol Plant. 2011 Jul;4(4):607-15. Epub 2011 Feb 28. PMID: 21357647. [CrossRef]
- Testerink C, Munnik T (2011). Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J Exp Bot. 2011 Apr;62(7):2349-61. Epub 2011 Mar 23. PMID: 21430291. [CrossRef]
- Thevenet D, Pastor V, Baccelli I, Balmer A, Vallat A, Neier R, Glauser G, Mauch-Mani B. (2017). The priming molecule β-aminobutyric acid is naturally present in plants and is induced by stress. New Phytol. 2017 Jan;213(2):552-559. Epub 2016 Oct 26. PMID: 27782340. [CrossRef]
- Torres MA, Jones JD, Dangl JL. (2006). Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006 Jun;141(2):373-8. PMID: 16760490; PMCID: PMC1475467. Wang, X., Hou, S., Wu, Q., Lin, M., Acharya, B. R., Wu, D., et al. (2017). IDL6-HAE/HSL2 impacts pectin degradation and resistance to Pseudomonas syringae pv tomato DC3000 in Arabidopsis leaves. Plant J. 89, 250–263. 10.1111/tpj.13380. [CrossRef]
- van Hulten M, Pelser M, van Loon LC, Pieterse CM, Ton J. (2006). Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci U S A. 2006 Apr 4;103(14):5602-7. Epub 2006 Mar 24. PMID: 16565218; PMCID: PMC1459400. [CrossRef]
- Walters DR, Ratsep J, Havis ND. (2013). Controlling crop diseases using induced resistance: challenges for the future. J Exp Bot. 2013 Mar;64(5):1263-80. Epub 2013 Feb 5. PMID: 23386685. [CrossRef]
- Wang, L., Einig, E., Almeida-Trapp, M., Albert, M., Fliegmann, J., Mithöfer, A., Kalbacher, H., & Felix, G. (2018). The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nature Plants, 4(3), 152–156. [CrossRef]
- Wrzaczek M, Vainonen JP, Stael S, Tsiatsiani L, Help-Rinta-Rahko H, Gauthier A, Kaufholdt D, Bollhöner B, Lamminmäki A, Staes A, Gevaert K, Tuominen H, Van Breusegem F, Helariutta Y, Kangasjärvi J. (2014). GRIM REAPER peptide binds to receptor kinase PRK5 to trigger cell death in Arabidopsis. EMBO J. 2015 Jan 2;34(1):55-66. Epub 2014 Nov 14. PMID: 25398910; PMCID: PMC4291480. [CrossRef]
- Wrzaczek, M., Brosche, M., Kollist, H., and Kangasjarvi, J. (2009). Arabidopsis GRI is involved in the regulation of cell death induced by extracellular ROS. Proc. Natl. Acad. Sci. U.S.A. 106, 5412–5417. [CrossRef]
- Xu, S. , Liao, C. J., Jaiswal, N., Lee, S., Yun, D. J., Lee, S. Y., Garvey, M., Kaplan, I., & Mengiste, T. (2018). Tomato PEPR1 ORTHOLOG RECEPTOR-LIKE KINASE1 regulates responses to systemin, necrotrophic fungi, and insect herbivory. Plant Cell, 30(9), 2214–2229. [CrossRef]
- Yamada K, Yamashita-Yamada M, Hirase T, Fujiwara T, Tsuda K, Hiruma K, Saijo Y. (2016). Danger peptide receptor signaling in plants ensures basal immunity upon pathogen-induced depletion of BAK1. EMBO J. 2016 Jan 4;35(1):46-61. Epub 2015 Nov 16. PMID: 26574534; PMCID: PMC4718002. [CrossRef]
- Yamaguchi, Y. , Barona, G., Ryan, C. A., and Pearce, G. (2011). GmPep914, an eight-amino acid peptide isolated from soybean leaves, activates defense-related genes. Plant Physiol. 156, 932–942. [CrossRef]
- Yamaguchi, Y. , Pearce, G., & Ryan, C. A. (2006). The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proceedings of the National Academy of Sciences of the United States of America, 103(26), 10104–10109. [CrossRef]
- Yu, X. , Feng, B., He, P., & Shan, L. (2017). From Chaos to Harmony: Responses and Signaling upon Microbial Pattern Recognition. Annual Review of Phytopathology, 55(1), 109–137. [CrossRef]
- Zhang, H. , Hu, Z., Lei, C., Zheng, C., Wang, J., Shao, S., et al. (2018). A plant phytosulfokine peptide initiates auxin-dependent immunity through cytosolic Ca(2+) signaling in tomato. Plant Cell 30, 652–667. [CrossRef]
- Zhang, H. , Yu, P., Zhao, J., Jiang, H., Wang, H. et al. (2017). Expression of tomato prosystemin gene in Arabidopsis reveals systemic translocation of its mRNA and confers necrotrophic fungal resistance. New Phytologist. [CrossRef]
- Zhang, H. , Zhang, H. y Lin, J. (2020), Respuestas de defensa sistémica a larga distancia mediadas por Systemin. New Phytol, 226: 1573-1582. [CrossRef]
- Ziemann S, van der Linde K, Lahrmann U, Acar B, Kaschani F, Colby T, Kaiser M, Ding Y, Schmelz E, Huffaker A, Holton N, Zipfel C, Doehlemann G. (2018). An apoplastic peptide activates salicylic acid signalling in maize. Nat Plants. 2018 Mar;4(3):172-180. Epub 2018 Feb 26. PMID: 29483684. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).






