1. Introduction
Gastrointestinal (GI) disorders and anxiety are widespread and growing clinical concerns worldwide [
1,
2]. The clinical characteristics of GI disorders are complex that often known to be associated with anxiety-related symptoms [
3,
4,
5]. The etiology and overlapping pathomechanisms responsible for GI disorders and anxiety remain largely unknown. Histamine is an important neuromodulator, recognized to be
involved in homeostasis, allergy, immune
functions, and neuroplasticity [
6,
7]
. In the GI tract, histamine is released by the enterochromaffin-like cells of the gastric mucosa which binds to H2 receptors of oxyntic cells and stimulates the secretion of HCl [
8]. Besides, mast cells and basophils of the immune system are the key sources of histamine in circulation [
9]. In the brain, histaminergic neurons and microglia produce histamine which acts as a neurotransmitter responsible for various neurophysiological functions [
10]. While the physiological level of histamine is important for the functions of the GI tract, an elevated level of histamine appears to induce over-secretion of gastric HCl leading to peptic ulcer [
11,
12]. Notably, basal level of histamine in the brain have been identified to play key roles in circadian rhythm, mood, and cognitive functions, its elevated level leads to pathogenic activation of microglial cells resulting in neuroinflammation noticed in mood disorders and various neurocognitive impairments [
6,
13,
14]. Therefore, antihistamine medications that are used to mitigate gastritis, allergy, and inflammation provide considerable relief against many neurological illnesses, psychotic episodes, and mood disorders including anxiety [
15,
16,
17]. However, the effects of antihistamines on the outcome of anxiety-related behavioral symptoms in GI disorders have been less explored. Recently, the hippocampus is a crucial portion of the limbic system in the brain which plays an important role in pattern separation, memory, and mood [
18,
19,
20,
21,
22]. Mounting evidence suggests that the Cornu Ammonis (CA)1 region of the hippocampus plays a vital role in fear conditioning, while abnormalities in the hippocampal CA3 region are involved in anxiety [
23,
24,
25]. Considering the facts, an increased level of histamine in the circulation or the brain could be proposed to alter the neuroimmune mechanism and neuroplasticity in the CA3 region of the hippocampus which is accountable for anxiety-related symptoms [
25].
Many antihistamine drugs act as antagonists of H1 receptors and are used against allergic reaction receptors, while ranitidine is a potent blocker of H2 receptors that has been widely used to treat various GI disorders including peptic ulcers [
26,
27]. The anti-gastric ulcerative effect of ranitidine is well established but the effect of ranitidine on brain function and behavioral outcomes remains largely undetermined. A growing body of experimental data supports that ranitidine acts as an effective anti-depressant [
15,
28]. A new line of scientific evidence indicates that the blockade of H2 receptors by ranitidine is highly beneficial against many inflammatory disorders [
29,
30,
31]. However, the reports on the possible effect of ranitidine treatment on the regulation of neuroimmune function and anxiety are highly limited. Considering the aforementioned facts, it can be posited a hypothesis that ranitidine might contribute in reducing neuroinflammation at the level of microglia activation in the brain. Unexpectedly, few reports highlight the caution that ranitidine treatment induces some unprecedented adverse effects in the brain [
32].
Thus, this study has been intended to investigate the effect of ranitidine against anxiety-related behaviors in a cysteamine HCl-induced mouse model of gastrointestinal disorders in association with the difference in the number of densities of pyramidal neurons and microglia in the CA3 region of the hippocampus of the brain.
2. Materials and Methodology
2.1. Experimental Animals
Three to four months old BALB/c mice were procured from the Biogen Laboratory, Bangalore, India and housed in the animal house facility of Bharathidasan University and maintained at air-conditioned room temperature of 20-22°C with a 12-h light/dark cycle. Mice were freely allowed to access standard animal feed and water. A total of 24 experimental mice were randomly divided into four groups namely, 1) control group (N=6), 2) cysteamine HCl group (N=6), 3) ranitidine group (N=6), 4) cysteamine HCl + ranitidine group (N=6). While group 1 mice received normal tap water, mice in group 2 and group 4 received an intraperitoneal injection of cysteamine HCl (Sigma Aldrich, USA) (60mg/per kilogram (Kg) body weight (BW) for three alternate days to induce GI disorder. Group 3 and 4 animals were orally administered ranitidine (Cadila Pharmaceuticals Limited, India) (30mg/ per Kg, BW) in drinking water for 14 consecutive days. After the treatment, the experimental mice have undergone behavioral experiments such as the open field test (OFT), light and dark box test (LDBT), and elevated plus maze (EPM) test. The behavioral room was equipped with a proper light setting and a video camera was placed above the center of the behavioral apparatuses. The camera was connected to a semi-automated computer system equipped with the SMART 3.0 module (Pan lab, Harvard apparatus, Spain) a video tracking module through which digital tracking of all the animal behavioral experiments was made. After the behavior experiments, the animals were sacrificed, perfused tissues were processed as earlier described Kandasamy and colleagues [
19,
33]
According to the guidelines of the Indian government, the experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) Bharathidasan University, with registration no. 418/GO/Re/S/01/CPCSEA as protocol no. BDU/IAEC/P10/2019 dated on 30.11.2019.
2.2. Open field test
The OFT was conducted to assess the locomotive and anxiety-like behaviors in the experimental mice. A standard wooden arena (120 cm × 120 cm) consisting of 16 grids sized 30 cm each was used for the OFT. The arena was digitally subdivided into the outer zone (red color) and inner zone (dark green) using SMART 3.0 a video tracking module. Each animal was gently released into the center of the OFT and allowed to freely explore the entire arena without any interruption. Three trials were conducted for three consecutive days with a duration of 5 minutes each. The total number of grids crossed, time spent in the outer zone and inner zone, and locomotive path were observed and measured for each animal using SMART 3.0 a video tracking module. After OFT, the animal was placed in its home cage. At the end of each trial, the open field arena was cleaned with 70% ethanol [
5,
19,
20].
2.3. Light and dark box test
LDB test was used to determine the photic-related preference and unconditioned avoidance or preference responses in the experimental mice. A standard black wooden rectangular box consisting of closed dark, and open light compartments connected via a slit was used for the LDBT. Using SMART 3.0, digitally two zones were inserted to designate the dark and light compartments. While the area outlined with blue color represents the untrackable covered dark area, and the red color denotes the trackable open zone exposed to light. Each mouse was placed in the middle of the apparatus and allowed to explore both dark and bright areas without any disturbance. Three trials were conducted for three consecutive days with a duration of 5 minutes per trial. After the experiments, the mouse was gently returned to the home cage. The entire LTB was wiped with 70% ethanol and dried. The total time spent in the light, and dark zone, by experimental animals were estimated from trajectories recorded using SMART 3.0 software [
5,
20].
2.4. Elevated plus maze
The EPM was used to analyze the anxiety in the experimental mice. The EPM apparatus consists of 4 walking arms connected in the middle zone. The two arms were protected by 30 cm high sidewalls and the two open arms were kept unprotected. The whole EPM setup was 90 cm elevated from the ground level using a stable central stand. Using the SMART 3.0 video tracking module, all four arms were digitally designated in different colors. While two closed arms were marked blue and brown, and two open arms were marked red and violet respectively. Each animal was gently placed in the middle zone and allowed for 5 minutes to explore all four arms. Three trials were carried out for 3 consecutive days. The time spent by the animal in open and closed arms was calculated using the SMART 3.0. After the completion of each trial, the animal was placed back into the home cage. The arms and central area of EPM were cleaned with 70% ethanol [
5,
20].
2.5. Perfusion of animals and brain tissue processing
The experimental mice were deeply anesthetized and transcardially perfused with 0.9% sterile saline followed by 4% paraformaldehyde (PFA) (Himedia, India). The brains were dissected from the skull and submerged in 4% PFA for 24 hours. Then the brains were transferred into 30% sucrose (SRL, India) and maintained at 4℃. After a week, the brain was placed on the tissue holder of a sliding microtome (Weswox, India), a solution of optimal cutting temperature compound (OCT) (Sigma Aldrich, USA) was added and the brain was frozen using dry ice. The brain was cut into 30 µm serial sagittal sections and systematically collected in sterile tubes 12 containing a mixer of cryoprotectant solution made up of glycerol (Merck, USA), ethylene glycol (Himedia, India), and phosphate buffer in a 1:1:2 ratio. The sections were stored at -20°C. The brain sections from 1 out of 12 tubes (360 µm apart) were used for immunohistochemical and histological assessments [
19,
33].
2.6. Nissl staining
The cryosections were mounted on
(3-Aminopropyl) triethoxysilane (APTES) coated slides (Borosil, India), and air-dried overnight. 100 mg of cresyl violet acetate (SRL, India) was dissolved in 100 ml of distilled water together with 250 µl of glacial acetic acid (SRL, India) using a magnetic stirrer at 60 °C. After cooling down to room temperate, the cresyl violet solution was then filtered using Whatman filter paper. First, the slides containing brain sections were dipped into alcohol: chloroform (1:1) solution for 3 minutes, and the sections were rehydrated through 100%, 95% of ethanol, and double distilled water for 3 minutes. And then, the sections were stained with cresyl violet solution for 30 minutes. And further, the sections were rinsed with double distilled water and 100%, and 95% of ethanol for 3 minutes, followed by xylene (Merck, USA). The sections were airdried and mounted with Dibutylphthalate polystyrene xylene (DPX) (Merk, Germany). The pyramidal cells were counted in the hippocampus as described by Yesudas A et al [
19].
2.7. Immunohistochemistry of microglia
The brain sections were washed thrice with 1x Tris-buffered saline (TBS) for 10 minutes, then the sections were placed in sodium citrate buffer at 65℃ for 90 minutes for antigen retrieval. After incubation, the sections were washed thrice with TBS for 10 minutes. Then, the sections were blocked with 3 % bovine serum albumin (BSA) for an hour before being treated with the primary antibody. The brain sections were transferred to a solution containing rabbit α ionized calcium-binding adapter molecule (Iba)-1 antibody, (Cell Signalling Technology, USA, 1:250) and incubated at 4℃ for 48 hours. After incubation with the primary antibody, the sections were washed with TBS for 10 minutes. Further, brain sections were incubated with goat α rabbit daylight 594 secondary antibody (Novus Biologicals, USA, 1:500) was added and kept at 4℃ for 24 hours. The next day, the sections were washed twice with TBS for 10 minutes. The sections were incubated with 4′,6-diamidino-2-phe-nylindole (DAPI) (Himedia, India) (0.1 mg/ml) for 5 minutes, and washed again with TBS for 10 minutes. After that, the sections were mounted with ProLongTM Glass antifade mountant (Thermo Fisher Scientific, USA) and dried overnight. The slides were blind coded and the brain sections were analyzed and photographed using a epifluorescence microscope (DM750, Leica Microsystems, Germany). The total number of Iba-1 immune-positive cells were counted in 5 different non-overlapping photomicrographs in the CA3 of the hippocampus using the image J plugin with the cell counter. Besides, 50 cells in the brains of each animal were assessed for the morphology of Iba-1 positive cells based on the ramified, hypertrophic and ameboid characteristic to obtain percentage of different types of microglia cells in the CA3 of the hippocampus.
2.8. Statistical Analyses
The values have been represented as mean ± standard deviation (SD). Results from behaviuoral, immunohistochemical and histological assessments were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. Graph Pad Prism was used to assess all the statistical analyses. The level of statistical significance was considered at P < 0.05 unless otherwise indicated.
3. Results
3.1. Ranitidine treatment increased the locomotory exploration-based anxiety-related behaviours of mice in the cysteamine HCl-treated group
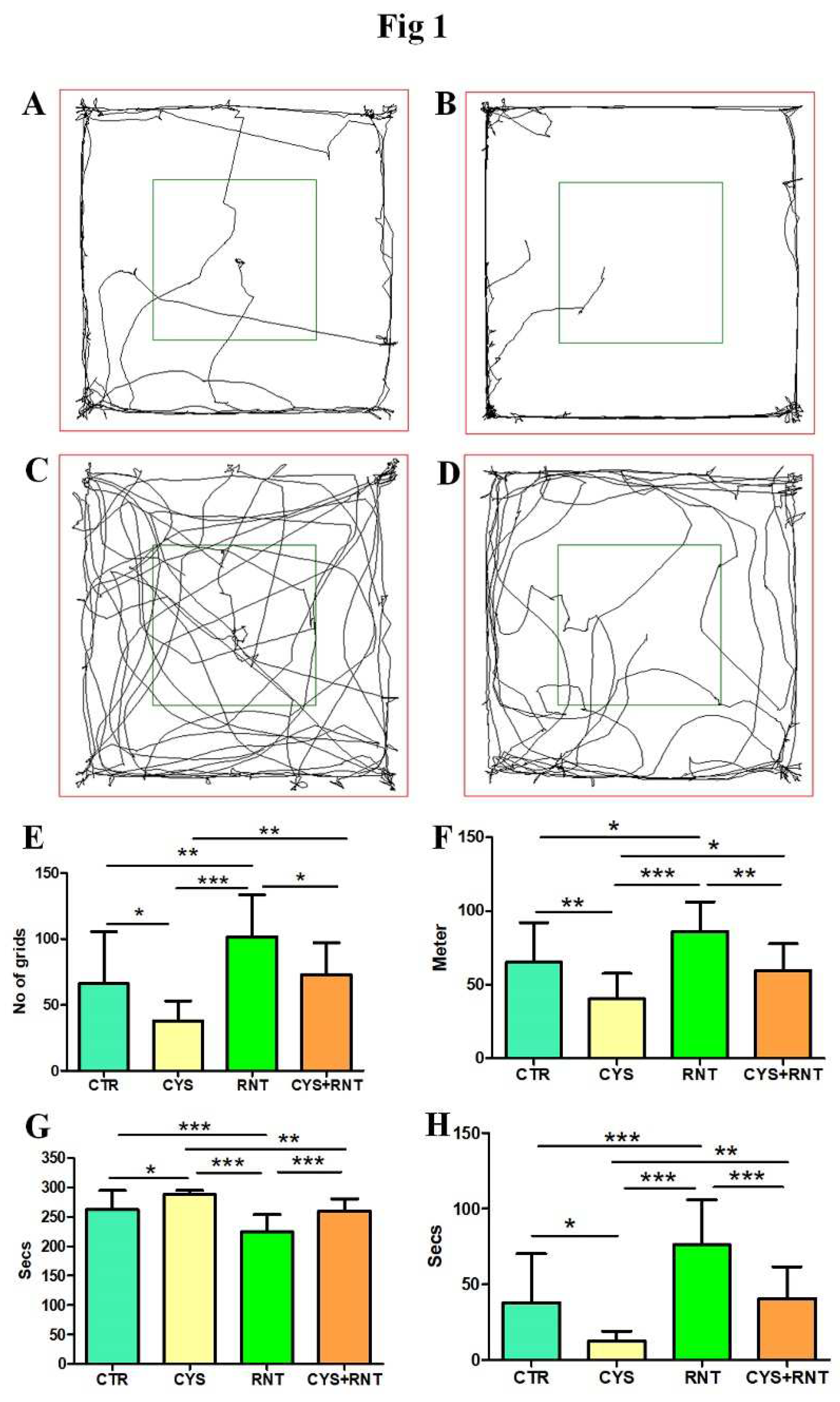
In the assessment of locomotion-based exploratory behavior in OFT, the total number of grids crossed by the cysteamine HCl-treated mice was found to be less when compared to the control, ranitidine alone, and cysteamine HCl + ranitidine-treated group. Ranitidine-treated animals have crossed significantly more grids than mice in control and cysteamine HCl + ranitidine-treated groups (CTR = 66±40; CYS = 37 ± 15; RNT =101 ± 32; CYS + RNT = 73 ± 24). Also, the total distance traveled by the cysteamine HCl-treated animals was reduced than that of mice in the control, ranitidine alone and cysteamine HCl + ranitidine treated groups. Cysteamine HCl + ranitidine treated group showed a significantly decreased distance traveled than the ranitidine treated group (CTR = 65±27; CYS = 40±17; RNT =86 ± 20; CYS + RNT = 60 ± 18) (
Figure 1)
.
With reference to preference-based anxiety behaviors in the OFT, experimental mice in the cysteamine HCl treated group spent more time in the outer zone when compared to mice in the control, ranitidine alone, and cysteamine HCl + ranitidine treated groups (CTR = 262±32; CYS = 288±67; RNT =224 ± 30; CYS + RNT = 259 ± 21). As a result, the time explored in the inner zone by cysteamine HCl-treated animals were considerably reduced when compared to mice in the control, ranitidine alone, and cysteamine HCl + ranitidine treated groups (
Figure 1)
. The experimental mice in the ranitidine alone treated group explored markedly more time in the inner zone compared to mice in the control, ranitidine alone, and cysteamine HCl + ranitidine treated groups. Moreover, the time spent in the inner zone by cysteamine HCl + ranitidine treated mice were also significantly increased than mice treated with cysteamine HCl group (CTR = 38±32; CYS = 12±7; RNT =76± 30; CYS + RNT = 40 ± 21) (
Figure 1). Overall, the results obtained from the OFT indicate that ranitidine treatment abolishes the cysteamine HCl-induced abnormalities in locomotive exploratory and anxiety-related behavioural deficits in the experimental animals.
3.2. Ranitidine treatment reduced preference-based and unconditioned anxiety-like behavior in cysteamine HCl-treated mice in the LDBT
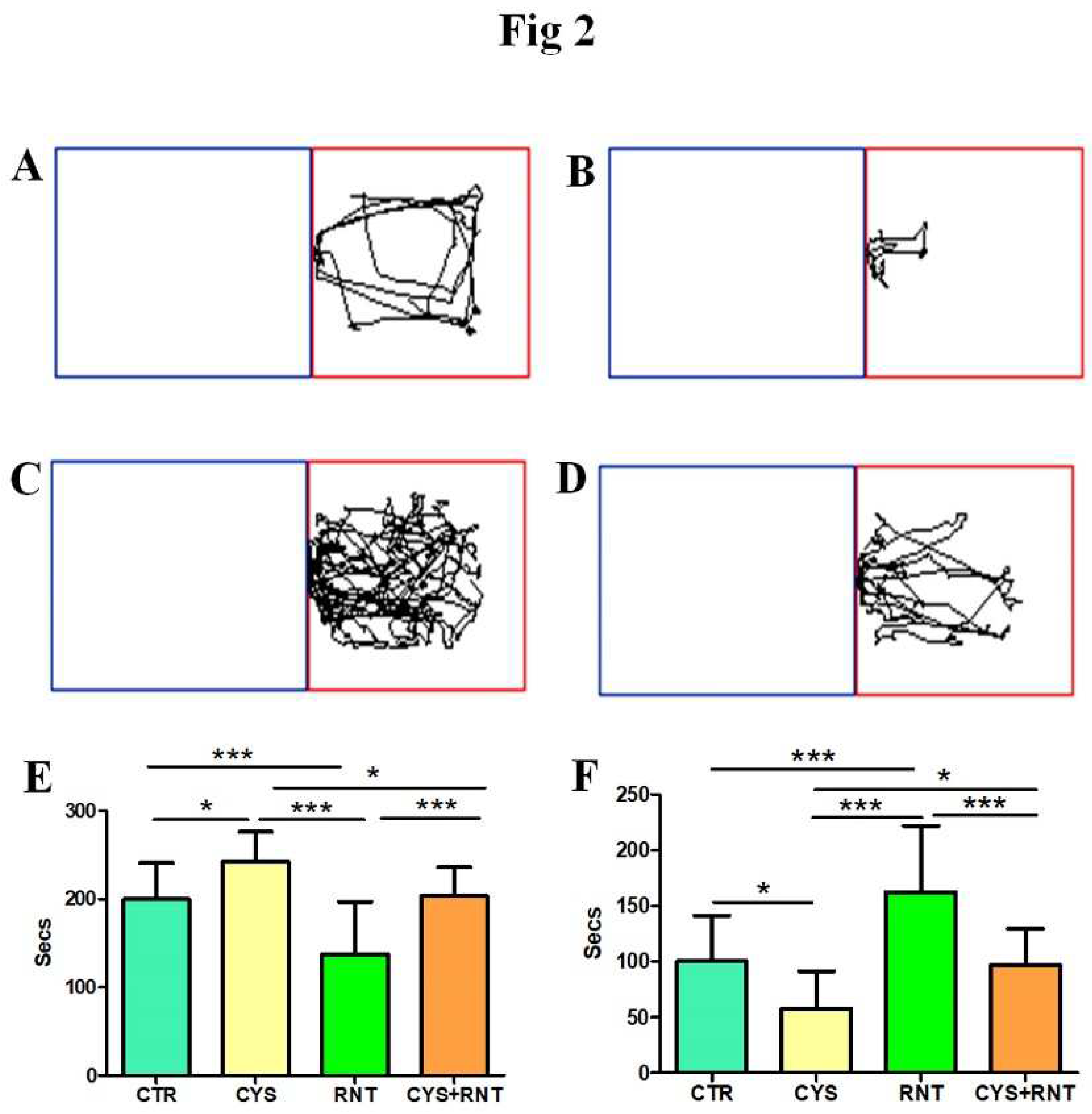
During the LDBT, the experimental mice in the cysteamine HCl-treated group stayed more time in the dark compartment when compared to other groups (CTR = 200±41; CYS = 242±34; RNT =137± 59; CYS + RNT = 203 ± 33). Hence, the time spent in the light compartment by mice in the cysteamine HCl group was significantly reduced when compared to the control, ranitidine alone, and cysteamine HCl+ranitidine treated groups. The time spent in the dark compartment by the ranitidine alone treated mice was considerably reduced as they explored more time in the light compartment than the control, cysteamine alone, and cysteamine HCl + ranitidine-treated groups. Notably, the time spent in the dark compartment by the ranitidine-treated group was also considerably reduced as they explored more time in the light compartment than the control, cysteamine HCl, and cysteamine HCl + ranitidine-treated groups (CTR = 100±41; CYS = 57±38; RNT =162± 59; CYS + RNT = 96 ± 33). The result obtained from LDBT suggests that ranitidine treatment decreases cysteamine HCl-induced anxiety-like symptoms in experimental animals (
Figure 2)
.
3.3. Ranitidine treatment reduced phobia-related anxiety-like behavior in cysteamine HCl-treated mice in the EPM
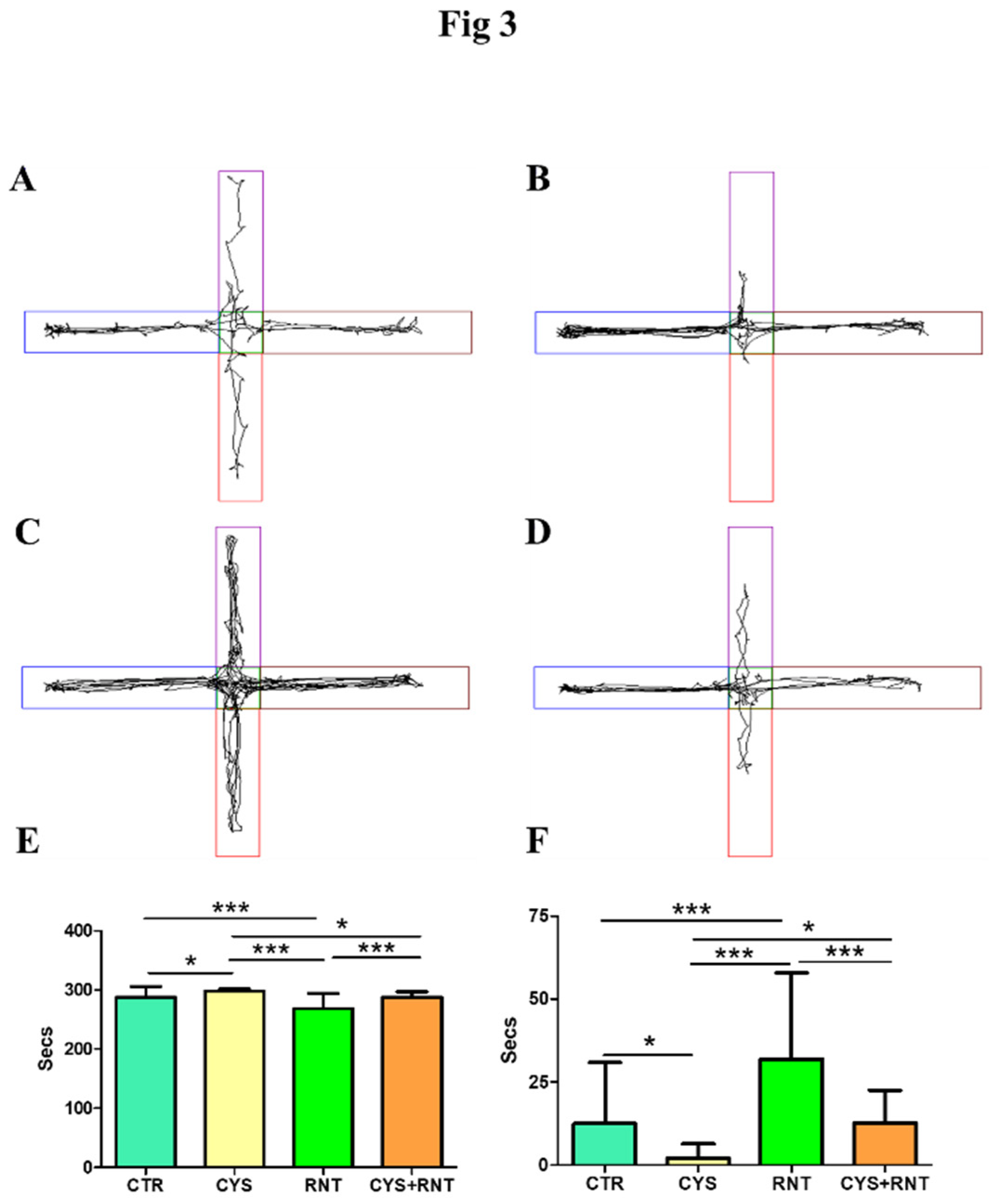
Upon exposure to EPM, the experimental mice in the cysteamine HCl-treated group were found to stay more time in the protected closed arms of EPM, while their activities to explore the open arms were drastically decreased when compared to the control, ranitidine alone, and cysteamine HCl + ranitidine-treated groups (CTR = 288±18; CYS =298±4; RNT =268± 26; CYS + RNT = 287 ± 10). In contrast, the experimental mice treated with ranitidine alone showed high tendency to explore the open arms than the control, cysteamine HCl and cysteamine HCl + ranitidine-treated groups. Moreover, the time spent in the open arms by cysteamine HCl + ranitidine-treated groups mice was also considerably increased than the mice treated with cysteamine HCl (CTR = 12±18; CYS = 2±4; RNT =32± 26; CYS + RNT = 13 ± 10). Hence, the results obtained from EPM corroborate that the ranitidine treatment mitigates cysteamine HCl-mediated anxiety in the experimental animals (
Figure 3).
3.4. Ranitidine treatment increased the neuronal density in the CA3 region of the hippocampus in cysteamine HCl-treated mice.
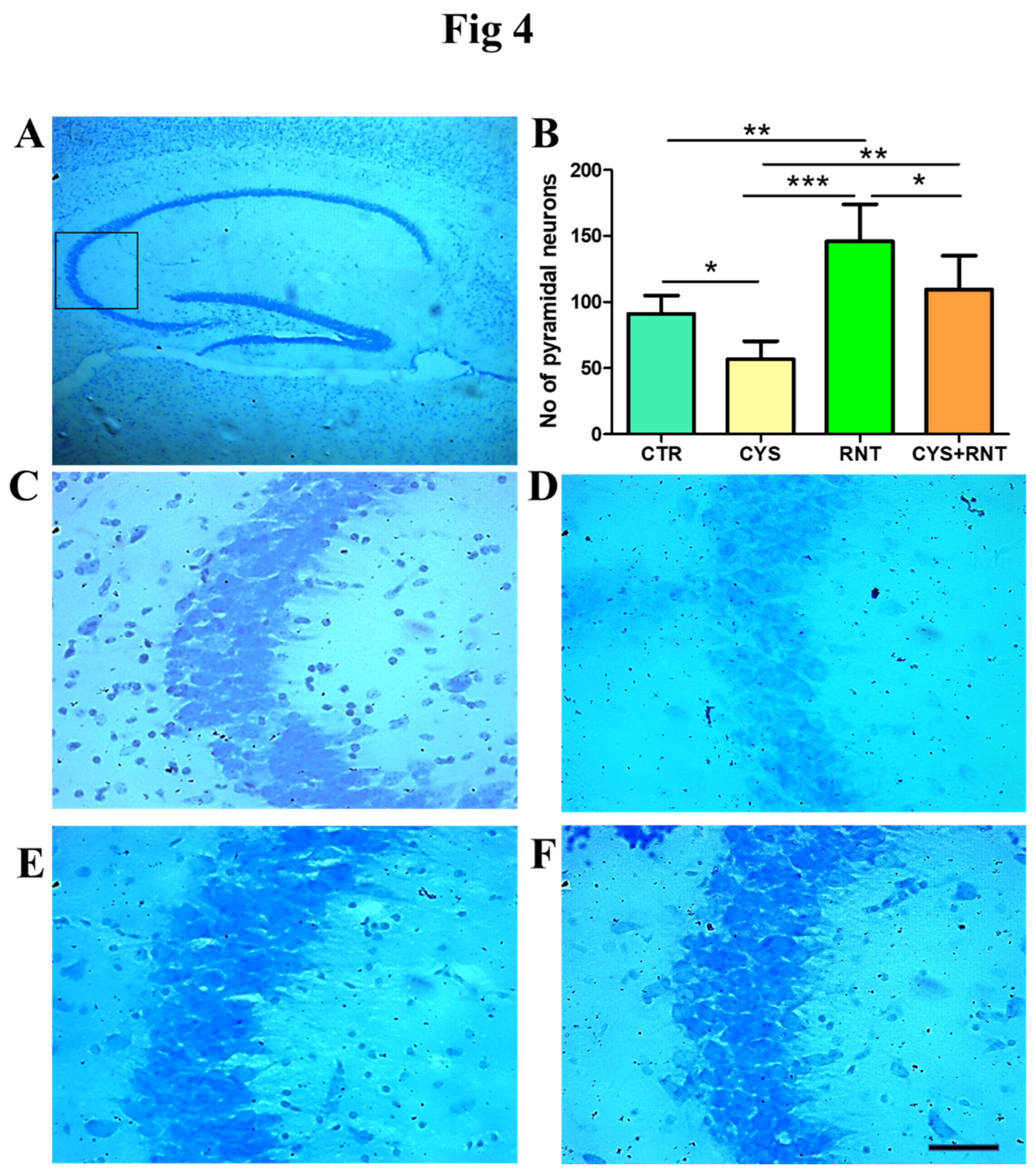
Recent experimental proof suggests that abnormal changes in the pyramidal neurons in CA3 region of the hippocampus contribute to anxiety-related behaviors. Thus, the cresyl violet stained brain sections obtained from experimental animals were examined for morphology and density of Nissl bodies in the CA3 region of the hippocampus. In the cysteamine HCl-treated group, there were omnipresent signs of the loss of cellular integrity and degenerative-like changes in the CA3 region of the hippocampus. The number of pyramidal neurons were significantly reduced in the CA3 region of the hippocampus in the brain of the cysteamine HCl-treated group compared to the control, ranitidine alone, and cysteamine HCl+ranitidine treated groups. Whereas in the ranitidine alone treated group, the number of pyramidal neurons were significantly increased in the CA3 region of the hippocampus of the brain compared to that of the control, cysteamine HCl, and cysteamine HCl+ranitidine treated groups. Notably, mice in the cysteamine HCl+ranitidine-treated groups also exhibited an increased number of pyramidal neurons in the hippocampal CA3 region compared to that of the cysteamine HCl-treated group (CTR = 91±14; CYS = 57±14; RNT =146± 28; CYS + RNT = 109 ± 26). (
Figure 4). Thus, the result suggests that the ranitidine treatment provides neuroprotection against cysteamine HCl-induced neuropathogenic modifications.
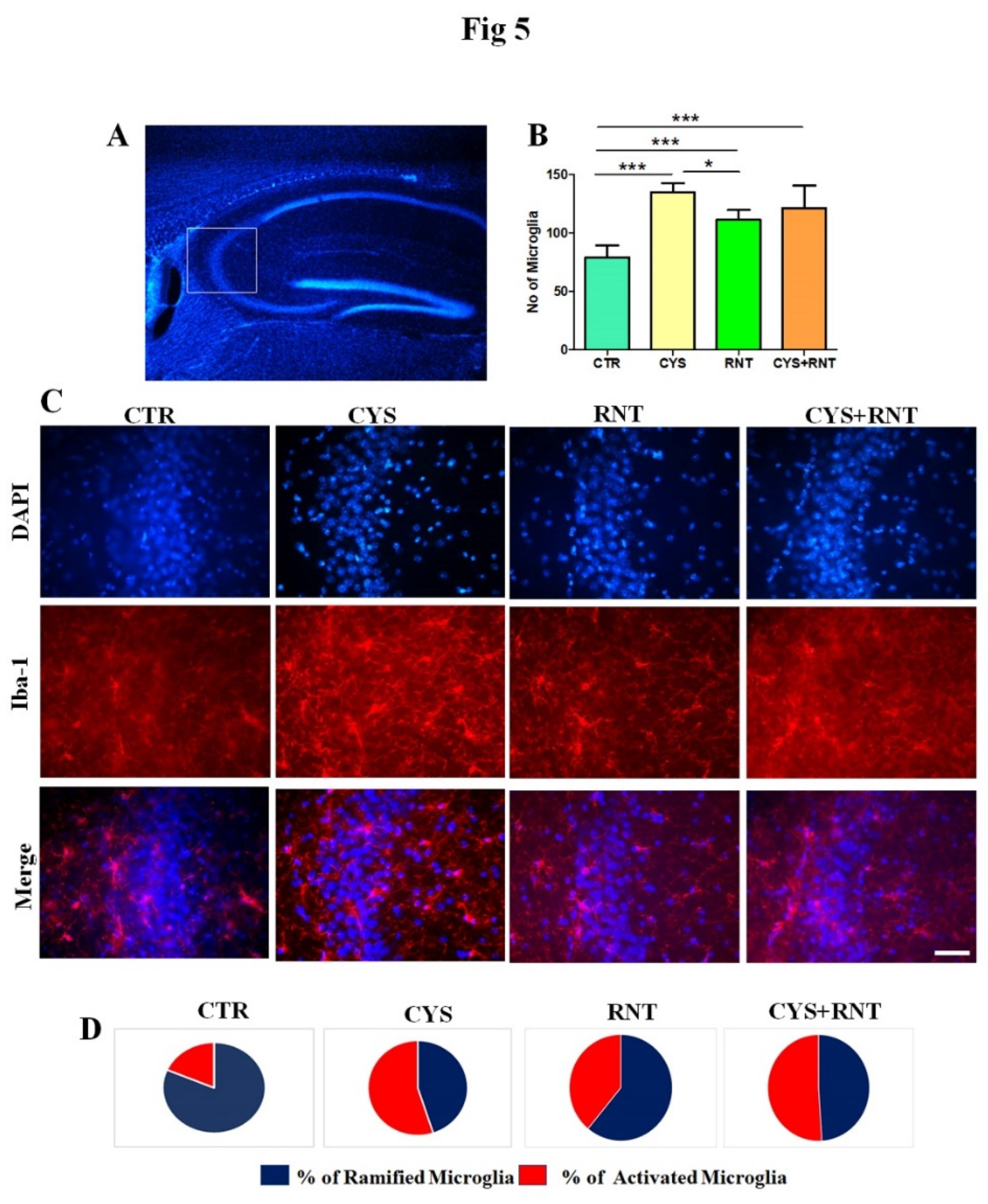
3.5. Ranitidine treatment attenuated cysteamine HCl induced activation of microglia in the hippocampal CA3 region of the brain
As microglial activation in the brain has increasingly been identified as a pathogenic determinant of anxiety and aberrant hippocampal plasticity, this study was extended to assess the changes in Iba 1 positive microglia in the CA3 region of the hippocampus in the brain. Surprisingly, the overall number of microglial cells regardless of morphological changes was found to be increased in the CA3 region of the hippocampus in the brains of the experimental mice in all the treatment group when compared to control However, decreased number of microglial cells was evident in the CA3 region of the hippocampus in the brains of the mice in ranitidine treated groups when compared to cysteamine HCl-treated and cysteamine HCl+ranitidine treated groups (CTR = 79±14; CYS = 126±17; RNT =108± 9; CYS + RNT = 118 ± 29) (
Figure 5).. It led us to assess and compare the morphological differences of microglial with ramified, hypertrophy, and ameboid-like features in the CA3 region of the hippocampus of the brains among experimental groups. While experimental mice in the cysteamine HCl-treated group showed a considerable raise in the proportion of activated microglia with hypertrophic and amoeboid-like characteristics, the percentage of activated microglial cells was found to be reduced in the CA3 region of the hippocampus in the brains of mice in the ranitidine alone treated group than that of cysteamine HCl-treated and cysteamine HCl+ranitidine treated groups Eventually, a non-significant reduction in the percentage of activated microglial cells was also noticed in the CA3 region of the hippocampus in the brains of the cysteamine HCl+ranitidine-treated mice than that of the cysteamine HCl-treated group(% of ramified microglia: CTR = 81.5±8; CYS = 45±7; RNT =61± 6; CYS + RNT = 49± ) and (% of activated microglia: CTR = 18.5.5±8; CYS = 55±7; RNT =39± 6; CYS + RNT = 51±7 ). Considering the aforementioned facts, induced microglial activation upon exposure to cysteamine HCl appears to be attenuated by ranitidine-mediated effects in the brain during anxiety.
4. Discussion
The present study demonstrates that ranitidine increases locomotion, and exploratory behavior and reduces anxiety in association with increased neuronal density upon deactivation of microglial cells in the CA3 region of the cysteamine HCl-induced mouse model of GI disorder. Recent scientific evidence unequivocally shows that the pathogenicity of GI disorders affects the brain and leads to anxiety-related symptoms. However, the overlapping pathomechanism by which GI disorders and anxiety are connected remains unknown. While the overproduction of histamine in the stomach has been identified as a cause of GI disorder, abnormal levels of histamine can lead to development of anxiety. In the brain, histamine plays a crucial role in the regulation of neurotransmission of ACh, serotonin, GABA, and vasopressin [
6]. Histamine has been identified to be important for the physiology of glial cells, thermoregulation, energy metabolism, and blood-brain barrier permeability [
10]. Moreover, histamine has been known to be involved in the regulation of the HPA and HPG axes [
34,
35] . While aberrant levels of histamine-mediated alteration in the synthesis and downstream signaling of corticosteroid hormones and the reuptake process of serotonin by glial cells have increasingly been recognized in the development of stress and depression, pathomechanism leading to anxiety by elevated histamine remains obscure [
36].
A potential cross-talk between peripheral immune cells and brain resident immune cells during various pathogenic processes appears to be linked to anxiety [
37]. Notably, excess-level of histamine appears to induce anxiogenic effects through the activation of neuroimmune cells in the brain [
38]. In general, the hippocampus of the brain has been established to play role in cognitive functions [
18]. Recent association studies and animal experiments strongly point towards that alteration in the hippocampal volume, distorted pyramidal neurons, and microglial activation in the CA3 region of the hippocampus can contribute to anxiety. Gene expression studies on cultured microglial cell lines revealed that microglia express all histamine receptors thus, surplus circulation of histamine can stimulate microglial activation leading to the subsequent production of triradicals and proinflammatory factors such as TNF-α and IL-6 in the hippocampus [
39]. Considering the fact, neuroinflammation resulting from activated microglia may lead to detrimental effects on the neurons in the brain [
40]. Notably, many antihistamines have been known to provide antidepressant and anxiolytic effects. Among various antihistamine drugs developed, ranitidine has been widely used to mitigate GI disorders as it effectively reduces the gastric section of HCl [
41]. While the anti-inflammatory properties of ranitidine have been increasingly evident, previous research finding provided considerable relief against pathogenicity resulting from traumatic brain injury [
42]. Moreover, ranitidine treatment has been suggested to be effective in treating stress and depression-related symptoms. Though some reports indicated the anxiolytic effect of ranitidine, studies for its neurotherapeutic roles, and possible cellular mechanism in the brain through which it attenuates the clinical symptoms of anxiety remain to be limited. Moreover, the effects of ranitidine against anxiety at the level of microglial activation and density of pyramidal neurons in the hippocampus in the brain of subjects with GI disorders have not been addressed.
In this study, we used OFT, LDBT, and EPM to examine the effects of ranitidine on the outcome of innate and GI disorder related anxiety-like behaviours in control and cysteamine HCl treated animals. Results revealed that cysteamine HCl-treated animal exhibit enhanced anxiety-related behavior. This data validates our previous findings that cysteamine HCl treatment can mediate ulcerative problems in the GI tract and induce oxidative stress and neuroinflammation in the brain [
5]. While the neuroinflammation seen in various GI disorders has been known to induce pathogenic alteration in the hippocampus affecting the morphological features and neuroplasticity of the pyramidal neurons predominately in the CA3 region, subjects with gastric and duodenal ulcers have been shown to exhibit an increased level of histamine [
43,
44]. Besides, the infusion of exogenous histamine into the substantia nigra and striatum of the experimental animals resulted in neurodegeneration in association with the activation of microglia [
45]. Therefore, increased anxiety-related behavior noticed in cysteamine HCl-treated mice could be related to an increased level of histamine-related neuropathogenic changes in the brain. In contrast, ranitidine-treated mice exhibited less anxiety than control as well as cysteamine HCl-treated animals. Despite its well-known anti-ulcerative effect in the GI tract, ranitidine has been known to mediate anti-inflammatory and cytoprotective effects [
29,
46,
47]. In this study, the quantification of nissl stained neurons revealed an increase in the density of pyramidal neurons in the CA3 region of the hippocampus in the brain of ranitidine alone and cysteamine HCl+ranitidine treated groups than that of cysteamine HCl-treated mice. Notably, Malagelada C and coworkers demonstrated that ranitidine treatment provided neuroprotection in cultured rat brain cortical neurons which is largely due to reduced level of Caspase-3 mediated apoptotic signaling [
48]. In another study by Park HJ et al reported that ranitidine provides neuroprotection against rotenone-induced apoptosis as it inhibits the phosphorylation of JNK and P38 in human dopaminergic SH-SY5Y cells [
49]. Thus, enhanced neuronal density noticed in the CA3 region of the ranitidine treated groups can presumably be related to enhanced density of pyramidal neurons due to its possible anti-apoptotic signaling based neuroprotective mechanism in brain.
Recently, the ramified microglia have been known to exhibit neuroprotective functions in the hippocampus and contributes to synaptic plasticity [
50,
51]. Whereas activated microglial cells with hypertrophic and ameboid features are recognized and have been known to induce deleterious effects to neurons of the brain during the neuropathogenic conditions [
52,
53]. Taken together, it can be hypothesized that, ranitidine treatment mitigates cysteamine HCl induced anxiety-related behavior at the level of providing neuroprotection in association with modulation of activated microglial cells in the CA3 region of the hippocampus of the brain. However, molecular mechanism by which ranitidine treatment facilitates anxiolytic effects through neuroprotection in association with microglia remains less clear. Thus, future studies are needed to understand the dysregulation of histaminergic system and mechanisms of ranitidine in health and disease with reference to regulation of neuroplasticity responsible for cognitive, mood and emotions.
Authors Contributions
Conceptualization, M.K; methodology, M.K, DB.S ; validation, M.K.,M.A and formal analysis, M.K DB.S and JF.VA; investigation, M.K; resources; data curation, M.K, DB.S, JF. VA; writing—original draft preparation, M.K, DB.S; writing—review and editing, JF. VA, M.K,.M.A.; visualization, M.K, DB.S;; supervision, M.K. MA; project administration, M.K; funding acquisition, M.K. All authors have read and agreed to publish the manuscript.
Funding
MK has been supported by University Grants Commission-Faculty Recharge Programme (UGC-FRP), New Delhi, India. M.K. gratefully acknowledges a research grant (SERB-EEQ/2016/000639), an Early Career Research Award (SERB-ECR/2016/000741) from the Science and Engineering Research Board (SERB) under the Department of Science and Technology (DST), Government of India and UGC-SAP and DST-FIST for the infrastructure of the Department of Animal Science and Bharathidasan University. MA would like to thank, ICMR, New Delhi, India (ICMR/Adhoc/BMS/2019-2605-CMB), DST, New Delhi, India (DST/CSRI/2018/343), and MHRD, New Delhi, India for the financial support. M.K and MA acknowledges RUSA 2.0, Biological Sciences, Bharathidasan University for their financial support. DBS is the recipient of RUSA 2.0 project fellowship (Ref. No. BDU/RUSA 2.0/TRP/BS/Date 22/04/2021). Jemi Feiona Vergil Andrews was supported as a project assistant from the project grant SERB EEQ/2016/000639.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of, Institutional Animal Ethics Committee (IAEC) and approved by the Institutional Ethics Committee of Bharathidasan University, Tiruchirappalli Reg. No. 418/GO/Re/S/01/CPCSEA as protocol no. BDU/IAEC/P10/2019 dated on 30.11.2019.
Data Availability Statement
All data needed to evaluate the conclusions are present in the paper.
Conflict of Interest
The author declares no conflict of Interest
References
- Bandelow, B.; Michaelis, S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin Neurosci 2015, 17, 327–335. [Google Scholar] [CrossRef]
- Fikree, A.; Byrne, P. Management of Functional Gastrointestinal Disorders. Clin Med (Lond) 2021, 21, 44–52. [Google Scholar] [CrossRef]
- Shah, E.; Rezaie, A.; Riddle, M.; Pimentel, M. Psychological Disorders in Gastrointestinal Disease: Epiphenomenon, Cause or Consequence? Ann Gastroenterol 2014, 27, 224–230. [Google Scholar]
- Spiegel, B.M.R.; Khanna, D.; Bolus, R.; Agarwal, N.; Khanna, P.; Chang, L. Understanding Gastrointestinal Distress: A Framework for Clinical Practice. Am J Gastroenterol 2011, 106, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Sri Rethinavel, H.; Selvaraj, D.B.; Balakrishnan, S.J.; Vergil Andrews, J.F.; Joseph, J.H.M.; Kandasamy, M. Omeprazole Treatment Manifests Anxiolytic Effects in a Cysteamine Hydrochloride Induced Mouse Model of Gastrointestinal Disorder. Heliyon 2022, 8, e09787. [Google Scholar] [CrossRef]
- Hough, L.B. Histamine Actions in the Central Nervous System. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition 1999.
- Jutel, M.; Akdis, M.; Akdis, C.A. Histamine, Histamine Receptors and Their Role in Immune Pathology. Clin Exp Allergy 2009, 39, 1786–1800. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Jutel, M.; Crameri, R.; O’Mahony, L. Histamine and Gut Mucosal Immune Regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Borriello, F.; Iannone, R.; Marone, G. Histamine Release from Mast Cells and Basophils. Handb Exp Pharmacol 2017, 241, 121–139. [Google Scholar] [CrossRef]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the Nervous System. Physiol Rev 2008, 88, 1183–1241. [Google Scholar] [CrossRef]
- Shamburek, R.D.; Schubert, M.L. Control of Gastric Acid Secretion. Histamine H2-Receptor Antagonists and H+K(+)-ATPase Inhibitors. Gastroenterol Clin North Am 1992, 21, 527–550. [Google Scholar] [CrossRef]
- Anton, A.H.; Woodward, E.R. High Levels of Blood Histamine and Peptic Ulcer. Arch Surg 1966, 92, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Passani, M.B.; Panula, P.; Lin, J.-S. Histamine in the Brain. Front Syst Neurosci 2014, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Passani, M.B.; Giannoni, P.; Bucherelli, C.; Baldi, E.; Blandina, P. Histamine in the Brain: Beyond Sleep and Memory. Biochem Pharmacol 2007, 73, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Robins, A.H.; Lucke, W.; McFadyen, M.L.; Wright, J.P. Effect of the H2-Receptor Antagonist Ranitidine on Depression and Anxiety in Duodenal Ulcer Patients. Postgrad Med J 1984, 60, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, P.G.; Karadag, A.S.; Selvi, Y.; Boysan, M.; Bilgili, S.G.; Aydin, A.; Onder, S. Assessment of the Effects of Antihistamine Drugs on Mood, Sleep Quality, Sleepiness, and Dream Anxiety. Int J Psychiatry Clin Pract 2014, 18, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.; Bukatina, E.; Lermontova, N.; Tkachenko, S.; Afanasiev, A.; Grigoriev, V.; Grigorieva, I.; Ivanov, Y.; Sablin, S.; Zefirov, N. Antihistamine Agent Dimebon as a Novel Neuroprotector and a Cognition Enhancer. Ann N Y Acad Sci 2001, 939, 425–435. [Google Scholar] [CrossRef]
- Anand, K.S.; Dhikav, V. Hippocampus in Health and Disease: An Overview. Ann Indian Acad Neurol 2012, 15, 239–246. [Google Scholar] [CrossRef]
- Yesudhas, A.; Roshan, S.A.; Radhakrishnan, R.K.; Abirami, G.P.; Manickam, N.; Selvaraj, K.; Elumalai, G.; Shanmugaapriya, S.; Anusuyadevi, M.; Kandasamy, M. Intramuscular Injection of BOTOX® Boosts Learning and Memory in Adult Mice in Association with Enriched Circulation of Platelets and Enhanced Density of Pyramidal Neurons in the Hippocampus. Neurochemical Research 2020, 45, 2856–2867. [Google Scholar] [CrossRef]
- Yesudhas, A.; Radhakrishnan, R.K.; Sukesh, A.; Ravichandran, S.; Manickam, N.; Kandasamy, M. BOTOX® Counteracts the Innate Anxiety-Related Behaviours in Correlation with Increased Activities of Key Antioxidant Enzymes in the Hippocampus of Ageing Experimental Mice. Biochemical and Biophysical Research Communications 2021, 569, 54–60. [Google Scholar] [CrossRef]
- Campbell, S.; MacQueen, G. The Role of the Hippocampus in the Pathophysiology of Major Depression. J Psychiatry Neurosci 2004, 29, 417–426. [Google Scholar]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: How the Hippocampus Contributes to Memory, Navigation and Cognition. Nat Neurosci 2017, 20, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Maren, S. Differential Roles for Hippocampal Areas CA1 and CA3 in the Contextual Encoding and Retrieval of Extinguished Fear. Learn Mem 2008, 15, 244–251. [Google Scholar] [CrossRef]
- Conrad, C.D. What Is the Functional Significance of Chronic Stress-Induced CA3 Dendritic Retraction Within the Hippocampus? Behav Cogn Neurosci Rev 2006, 5, 41–60. [Google Scholar] [CrossRef]
- Engin, E.; Smith, K.S.; Gao, Y.; Nagy, D.; Foster, R.A.; Tsvetkov, E.; Keist, R.; Crestani, F.; Fritschy, J.-M.; Bolshakov, V.Y.; et al. Modulation of Anxiety and Fear via Distinct Intrahippocampal Circuits. eLife 2016, 5, e14120. [Google Scholar] [CrossRef] [PubMed]
- Baroody, F.M.; Naclerio, R.M. Antiallergic Effects of H1-Receptor Antagonists. Allergy 2000, 55 Suppl 64, 17–27. [Google Scholar] [CrossRef]
- Grant, S.M.; Langtry, H.D.; Brogden, R.N. Ranitidine. An Updated Review of Its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Use in Peptic Ulcer Disease and Other Allied Diseases. Drugs 1989, 37, 801–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, Z.; Gao, Y.; Wu, Y.; Li, Z.; Liu, H.; Zhang, C. Bidirectional Crosstalk between Stress-Induced Gastric Ulcer and Depression under Chronic Stress. PLoS One 2012, 7, e51148. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, M.D.; Muley, M.P.; Manekar, M.S. Anti-Inflammatory Property of Ranitidine, a Specific H2-Receptor Antagonist. Indian J Physiol Pharmacol 1986, 30, 205–209. [Google Scholar] [PubMed]
- Lancaster-Smith, M.J.; Jaderberg, M.E.; Jackson, D.A. Ranitidine in the Treatment of Non-Steroidal Anti-Inflammatory Drug Associated Gastric and Duodenal Ulcers. Gut 1991, 32, 252–255. [Google Scholar] [CrossRef]
- Okajima, K.; Harada, N.; Uchiba, M. Ranitidine Reduces Ischemia/Reperfusion-Induced Liver Injury in Rats by Inhibiting Neutrophil Activation. J Pharmacol Exp Ther 2002, 301, 1157–1165. [Google Scholar] [CrossRef]
- Werbel, T.; Cohen, P.R. Ranitidine-Associated Sleep Disturbance: Case Report and Review of H2 Antihistamine-Related Central Nervous System Adverse Effects. Cureus 2018, 10, e2414. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.; Lehner, B.; Kraus, S.; Sander, P.R.; Marschallinger, J.; Rivera, F.J.; Trümbach, D.; Ueberham, U.; Reitsamer, H.A.; Strauss, O.; et al. TGF-Beta Signalling in the Adult Neurogenic Niche Promotes Stem Cell Quiescence as Well as Generation of New Neurons. J Cell Mol Med 2014, 18, 1444–1459. [Google Scholar] [CrossRef] [PubMed]
- Scaccianoce, S.; Lombardo, K.; Nicolai, R.; Affricano, D.; Angelucci, L. Studies on the Involvement of Histamine in the Hypothalamic-Pituitary-Adrenal Axis Activation Induced by Nerve Growth Factor. Life Sci 2000, 67, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Roberts, F.; Calcutt, C.R. Histamine and the Hypothalamus. Neuroscience 1983, 9, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Shu, C.; Xiao, L.; Wang, G. Histamine and Histamine Receptors: Roles in Major Depressive Disorder. Front Psychiatry 2022, 13, 825591. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Vandenbark, A.A.; Offner, H. Cross-Talk of the CNS With Immune Cells and Functions in Health and Disease. Front Neurol 2021, 12, 672455. [Google Scholar] [CrossRef] [PubMed]
- Carthy, E.; Ellender, T. Histamine, Neuroinflammation and Neurodevelopment: A Review. Front Neurosci 2021, 15, 680214. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, W.; Zeng, X.; Hu, G.; Zhang, H.; He, S.; Zhang, S. Histamine Induces Upregulated Expression of Histamine Receptors and Increases Release of Inflammatory Mediators from Microglia. Mol Neurobiol 2014, 49, 1487–1500. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Frontiers in Cellular Neuroscience 2018, 12. [Google Scholar] [CrossRef]
- Farzam, K.; Sabir, S.; O’Rourke, M.C. Antihistamines. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2022. [Google Scholar]
- Michinaga, S.; Sonoda, K.; Inazuki, N.; Ezaki, M.; Awane, H.; Shimizu, K.; Hishinuma, S.; Mizuguchi, H. Selective Histamine H2 Receptor Agonists Alleviate Blood-Brain Barrier Disruption by Promoting the Expression of Vascular Protective Factors Following Traumatic Brain Injury in Mice. Journal of Pharmacological Sciences 2022, 150, 135–145. [Google Scholar] [CrossRef]
- Parsons, M.E. Histamine and the Pathogenesis of Duodenal Ulcer Disease. Gut 1985, 26, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Haith, L.R.; Reynolds, E.S. Pathogenesis of Duodenal Ulceration Produced by Cysteamine or Propionitrile. Digest Dis Sci 1979, 24, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Vizuete, M.L.; Merino, M.; Venero, J.L.; Santiago, M.; Cano, J.; Machado, A. Histamine Infusion Induces a Selective Dopaminergic Neuronal Death along with an Inflammatory Reaction in Rat Substantia Nigra. J Neurochem 2000, 75, 540–552. [Google Scholar] [CrossRef]
- Konturek, S.J.; Radecki, T.; Brzozowski, T.; Piastucki, I.; Dembińska-Kieć, A.; Zmuda, A. Gastric Cytoprotection by Prostaglandins, Ranitidine, and Probanthine in Rats. Role of Endogenous Prostaglandins. Scand J Gastroenterol 1981, 16, 7–12. [Google Scholar] [PubMed]
- Conchillo, A.; Mola, C.; Navarro, C.; Bravo, L.; Bulbena, O. Cytoprotective and Antisecretory Activity of a Ranitidine-Zinc Complex. Prostaglandins Leukot Essent Fatty Acids 1995, 52, 393–397. [Google Scholar] [CrossRef]
- Malagelada, C.; Xifró, X.; Badiola, N.; Sabrià, J.; Rodríguez-Álvarez, J. Histamine H2-Receptor Antagonist Ranitidine Protects Against Neural Death Induced by Oxygen-Glucose Deprivation. Stroke 2004, 35, 2396–2401. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, H.J.; Park, H.-K.; Chung, J.-H. Protective Effect of Histamine H2 Receptor Antagonist Ranitidine against Rotenone-Induced Apoptosis. Neurotoxicology 2009, 30, 1114–1119. [Google Scholar] [CrossRef]
- Fumagalli, M.; Lombardi, M.; Gressens, P.; Verderio, C. How to Reprogram Microglia toward Beneficial Functions. Glia 2018, 66, 2531–2549. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293. [Google Scholar] [CrossRef]
- Dheen, S.T.; Kaur, C.; Ling, E.-A. Microglial Activation and Its Implications in the Brain Diseases. Curr Med Chem 2007, 14, 1189–1197. [Google Scholar] [CrossRef]
- Barnes, N.; Beddall, A.C.; Bird, C.C.; Bradfield, J.W.; Brown, I.L.; Burnett, A.K.; Cartwright, R.A.; Davies, J.D.; Edwards, M.S.; Ellis, I.O. Distribution of Leukemia, Lymphoma, and Allied Disease in Parts of Great Britain: Analysis by Administrative Districts and Simulations of Adjacencies. Leukaemia Research Fund 1984 Data Collection Group. Leukemia 1987, 1, 78–81. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).