Introduction
Lung cancer is the leading cause of cancer death worldwide. In 2020, 2,206,771 new cases have occurred and caused 1,796,144 deaths [
1]. Also in 2020, 4,509 cases and 3,548 deaths were registered in Catalonia (7.7 million inhabitants), Spain [
2]. Among patients with non-small cell lung cancer (NSCLC), the 5-year survival rate is 26% for all stages and below 3% for stage 4 disease. Unfortunately, less than 20% of lung cancers are detected in localised tumor stages when it is most treatable [3-5]. In 1999, the Early Lung Cancer Action Programme (ELCAP) study showed that screening with low-dose computed tomography (LDCT) improved early lung cancer detection [
6]. In 2011, the U.S. National Lung Screening Trial (NLST) reported a 20% reduction in lung cancer-specific mortality using LDCT compared with chest radiography (CXR) screening in a high-risk population [
7]. In 2017, the implementation of LDCT screening throughout Europe was recommended by an European Union (EU) position statement [
8]. In 2020, the Dutch-Belgian lung-cancer screening trial (NELSON study) in high-risk male participants showed a cumulative rate ratio for death of 0.76 at 10 years in the LDCT screening group as compared with no screening [
9]. At the beginning of 2022, the European Parliament resolution recognised the evidence that proves the positive effect of targeted lung cancer screening on mortality [
10], and a recent resolution from the European Commission Directorate-General for Health and Food Safety (COM 2022/474) has included lung cancer screening as a recommendation for the first time [
11]. Once the clinical benefits of the LDCT screening on the high-risk population have been demonstrated, there are currently multiple initiatives focused on improving its cost-effectiveness, implementation in populations at risk and smoking cessation interventions [
12].
The introduction of immunotherapy and targeted therapies in the management of advanced stages of lung cancer have shown considerable impact on overall survival (from 11.0% to 17.8% for the period between 2011 and 2014) in the subset of responders [
13,
14]. These important changes are achieved at a high economical cost but could be offset by identifying early-stage tumours and treating them with the current standards of surgery or stereotactic body radiotherapy (SBRT). Lung cancer screening could avoid the expensive and less effective current treatments in use for advanced stage disease [
15].
Cost-effectiveness analyses of lung cancer screening using LDCT is sensitive to different key model parameters (e.g. incidence, sensitivity and specificity of the tests, selection criteria, definition of high-risk population, utility values, etc.) showing inconclusive data and some uncertainty [16-18]. In the Pan-Canadian Early Detection Lung Cancer Study, the average cost to screen individuals with a high risk for developing lung cancer using LDCT and the average initial cost of curative intent treatment were found to be lower than the average per-person cost of treating advanced stage lung cancer [
19]. Late treatment rarely results in a cure. In Catalonia, a cost-effectiveness analysis of different smoking cessation approaches concluded that the most cost-effective strategy would be to implement intensive smoking cessation interventions at ages 35-45, combined with LDCT screening every three years between the ages of 55 and 65 [
20]. Another study in the same region showed that surgical treatment for early stage lung cancer is cheaper and offered better outcome than advanced stage medical treatment and that the intervention would save money between 3-6 years after its launch [
21]. Other studies have shown similar results [
22].
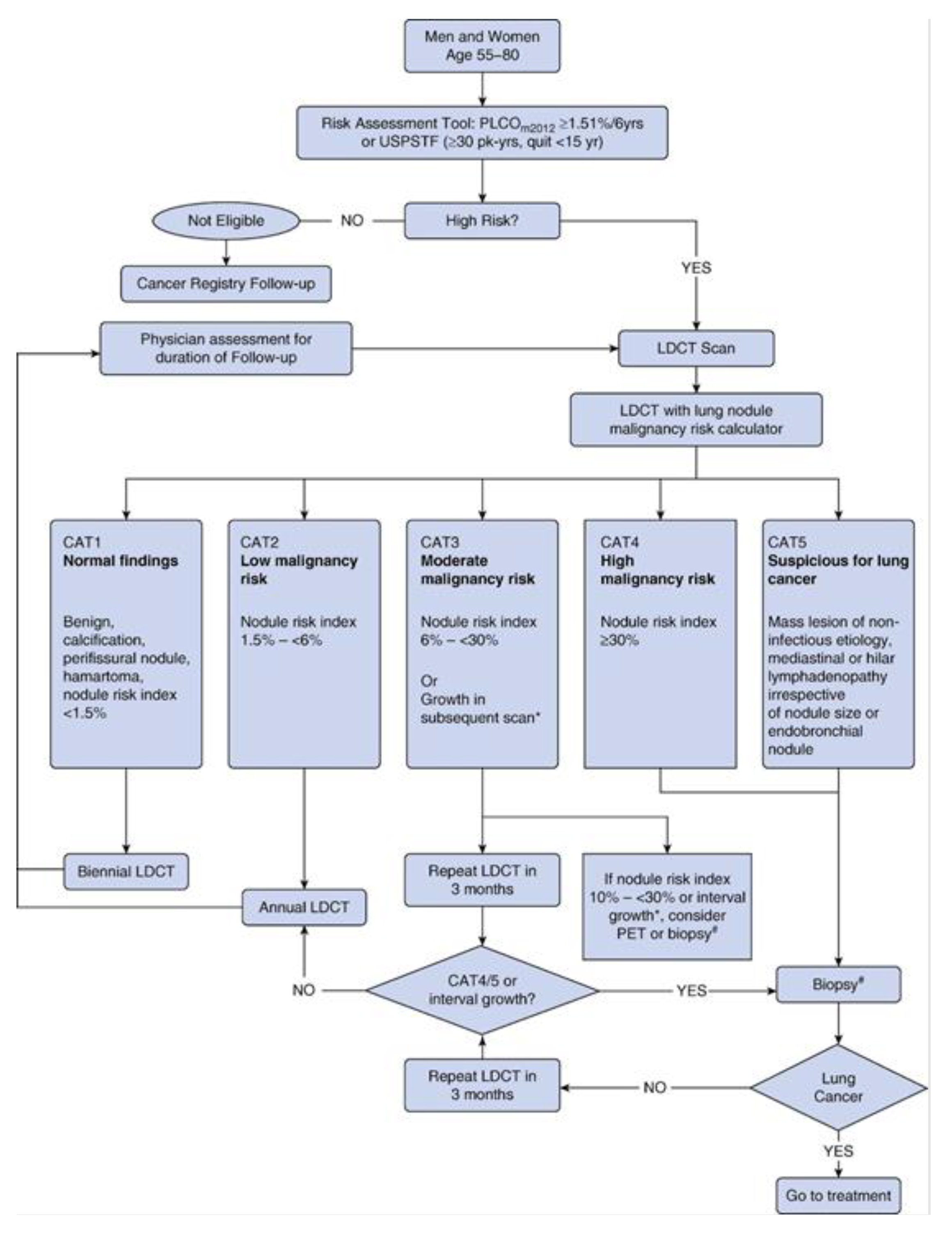
In December 2020, an experimental lung cancer LDCT screening (LCS) programme was implemented in the Northern Metropolitan Area of Barcelona through the participation of Germans Trias i Pujol University Hospital (HUGTP) in the international consortium of the International Lung Screen Trial (ILST), in which two patient selection strategies (PLCOm2012 vs. USPSTF 2013) and two protocols to classify the detected pulmonary nodules (PanCan vs. LUNG-RADS) were compared [
23]. In this context, the aim of the present study was to perform a direct cost analysis comparing the reduction of overall lung cancer treatment expenses arising from stage shift due to screening, with the costs associated with usual care (no screening) in patients diagnosed with NSCLC in the framework of the public national health system in Catalonia (Spain).
Methods
Study Design and Objective
A cost analysis study was designed from the perspective of the Catalan Health Service (CatSalut), the public health system that offers universal, free healthcare in Catalonia.
The objective of the study was to compare the anticipated costs of diagnosis, treatment (surgery, radiotherapy and drugs) and follow-up associated with two approaches to the NSCLC diagnosis and care: the lung cancer LDCT screening according to the ILST protocol [
23] versus the diagnosis in the usual care (
Figure 1). Usual care treatment was defined as the application of clinical practice guidelines developed by the Spanish Society of Medical Oncology for the treatment of NSCLC [
24]. In both alternatives, all lifetime costs (up to healing or death) were taken into account. The costs, expressed in euros (2021), were calculated based on the standard retail prices of CatSalut, the health resources contractor of the Catalan Ministry of Health [
25].
Estimated costs
The anticipated costs of the LCS programme were calculated as the sum of the prices of radiological tests, medical visits, endoscopic or
computed tomography-guided biopsies, laboratory tests, lung function tests and positron emission tomography (PET-CT), which were expected to be performed over a duration of 5 years according to the ILST protocol [
23], considering 300 participants and assuming the following patients’ expected distribution: CAT1 (normal findings) 65.7%, CAT2 (low malignancy risk) 17.5%, CAT3 (moderate malignancy risk) 13.1%, and CAT4/5 (high malignancy risk/suspicious for lung cancer) 3.6%
26. The costs associated with smoking cessation treatment (9 visits per patient) were also included, assuming that it will be necessary for 50% of participants, based on the patients enrolled at the LCS programme during 2021. The costs of nicotine replacement treatment are not included in this study because they are not funded by CatSalut.
Diagnostic procedures including bronchoscopy, staging CT, PET-CT, brain nuclear magnetic resonance and follow-up visits were recorded as per local practice guidelines (usual care). Treatment-related costs were calculated based on the main treatment schedules' retail prices, administered for each stage of the disease and according to the institutional activity registry, which includes surgery, chemotherapy, radiotherapy, immunotherapy and targeted therapy. Total costs were estimated adjusted by overall survival based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual (TNM) [
27]. Although the public health system covers the whole treatment, the model did not include complications and second-line treatments; other, minor costs were not considered.
The stages distribution for usual care (stage I 14%, stage II 6%, stage III 12%, stage IV 68%) corresponded to data observed in 388 patients diagnosed with NSCLC at the Catalan Institute of Oncology, an institution that includes a network of public hospitals that in 2022 covered 40% of the population of Catalonia. The stages distribution contemplated for the screening alternative was estimated from the weighted average of the detections in the NELSON [
9] and NLST [
7] studies, eliminating the missing values (stage I 63%, stage II 8%, stage III 17%, stage IV 12%).
The total anticipated costs derived from the diagnosis, treatment and follow-up of the different lung cancer stages identified in each alternative were compared in order to assess the economic impact of early detection of the disease, using a 5-years average
cancer detection rate (CDR) of 1.6%. As we present the first-round data, the average CDR corresponding to the 5 years programme was taken from the Lahey programme, which has a baseline CT CDR of 2%, identical to our site [
28].
Results
Screening programme
In the LCS programme, total costs estimated for 300 patients expected to be managed in our centre amounted to €409,917. As shown in
Table 1, the most substantial anticipated expenditure corresponded to LDCT tests (23.2%) followed by PET-CT (19.4%) and smoking cessation visits (16.7%), whereas biopsies (2.4%) and laboratory tests (0.4%) accounted for a minor part of anticipated costs. The average cost per participant for the 5-year duration of the LCS programme was €1,349.72. Differences in cost of screening due to variations in the management of the lung nodule as established in the ILST protocol for each of the CAT (based on PanCan lung nodule risk-based protocol) categories are shown in
Table 2. The cost per participant increased from €1,054 for CAT1 to €1,832 for CAT4/5 (a 74% increase).
Treatment costs of the NSCLC patient
There were relevant differences in the total cost of diagnosis, treatment and follow-up depending on the TNM stage in which the disease is detected, with 12 times higher costs for stage IV than those associated with stage I (
Table 3).
Stage shift and cost reduction
The differential costs arising from stage shift are detailed in
Table 4. The average expected cost of diagnosis, treatment, and follow-up for lung cancer detected in LCS programme was €32,431 as compared with €91,959 in the usual care. In the LCS programme strategy, costs were mostly associated with stages I, II, and III (positive differential costs) because of a higher number of cases detected in the initial stages. However, the high cost associated with stage IV and the high proportion of these stages in the usual care programme offset the expenditure on initial stage treatments in the screening programme approach. Consequently, the differential cost per participant diagnosed with lung cancer was estimated at €59,528.
For a 5-year average CDR of 1.6%, the expected cost reduction per LCS for each participant was €952. Taking the expected cost reduction into account, the screening programme in discounted cost was €397 (€1,349-€952), or €119.180 for 300 participants. The decrease in costs of diagnosis, treatment and follow-up resulting from stage shift offset 70.6% of the costs of the LCS programme. Assuming a 1% cancer detection rate, decrease in costs of diagnosis, treatment and follow-up resulting from stage shift offsets 44% of the costs of the screening programme. With a 2.2% cancer detection rate, 97% of the total costs would be offset (
Table 5). In all the cases studied, the cost reduction resulting from the stage shift due to the screening programme offset a substantial part of the screening programme costs.
Discussion
The stage at lung cancer diagnosis is a major determinant of lung cancer prognosis. A late diagnosis is the main contributing factor to the high frequency of individuals with advanced disease at presentation who are unlikely to benefit from curative treatment and accounts for the high lung cancer-specific mortality rates. In addition to the high mortality, the costs of non-curative treatment options, including new therapeutic targets, are very high and continue to rise. LCS programmes are therefore essential to find lung cancers earlier and diagnose and treat more patients at early stages.
This study highlights the importance of the participant selection criteria and recruitment effectiveness in the potential savings of a LCS programme. If only a prevalence of 1% is found, then the savings covers less than half of the direct costs of screening, while if a 2.2% cancer detection rate is achieved, then savings almost cover costs (
Table 5). There is only one prospective study in which the effectiveness of two approaches to selection of individuals to invite to participate in a lung cancer screening programme was compared [
29]. The two approaches were the USPSTF2013 categorical age and smoking criteria, and the PLCOm2012 risk prediction model. The PLCOm2012 appeared to be more efficient than the USPSTF2013, with a cancer sensitivity improvement of 15.8% (95% confidence interval [CI] 10.7–22.1%; absolute odds ratio 4.00, 95% CI 1.89-9.44;
p < 0.0001) [
29]. Another approach to achieve a better recruitment is screening low socioeconomic status population. Results of the UK Lung Cancer Screening trial [
30], in which LDCT screening was evaluated in a large population sample of people aged 50-70 years, showed that higher socioeconomic status correlated positively with response, but inversely with risk. The proportion of individuals with high-risk of developing lung cancer (5% or greater over next 5 years – LLP risk prediction model) decreased with higher economic status, ranging from 17.7% in the most deprived quintile to 8.0% in the least-deprived quintile (
p < 0.001). A cost-effective screening programme should include a significant proportion of women as the benefit has proved to be greater. In the NELSON study women achieved a 59% reduction in lung cancer-specific mortality as compared to 24% for men at 8 years of follow-up (although this difference decreases after 10 years) and 27% reduction for women in the NLST as compared to 8% for men [
10,
31].
This study also differs from previous ones as the cost of smoking cessation is included. The total cost of smoking cessation visits represents a 16.7% of the total cost of the LCS programme. Including smoking cessation interventions within LCS programmes have additional benefits of improving tobacco-related health outcomes, such as chronic obstructive pulmonary disease, heart disease and other malignancies. This impact has not been quantified yet, but it is expected that it will contribute to cost-effectiveness of the LCS programme [
20].
The evidence from this research is consistent with previous studies [
15] showing that, in high-risk populations, the average costs of LCS using LDCT plus the average initial cost of treatment with curative intent are less than the average cost per NSCLC patient with advanced stage in the usual care approach.
This study has some limitations. First, the cost analysis was based on a combination of our own data and estimated distribution of lung cancer obtained in a LCS programme from the weighted average of two main published studies [
7,
9]. Second, although the financial cost of lung cancer is high, the cost range in our study has been restricted to the healthcare system’s perspective, which according to some studies may account for less than 25% of the total costs attributable to the disease [
32]. It does not consider a set of other potentially relevant costs, such as mortality and morbidity losses informal costs [
33], costs related to primary care [
34] or cancer drugs prices [
35]. In addition, the expected positive effects of the smoking cessation programme have not been taken into account. Third, adherence to the LCS programme has been considered to be 100%, while published data varies between 50% and 90% so that our cost savings estimates are likely optimistic [
36]. Fourth, as we only have baseline results, we cannot provide our own average CDR for the 5-year programme. For our model, we have used the average CDR from the Lahey programme [
28] (1.6%) as their baseline CT CDR is equal to ours (2%).
The present findings invite a reflection on the current global health policy management of lung cancer along time. Current policy seems to be focused on short-term costs, to the detriment of the implementation of more mid-long term cost-effective solutions. A part from increasing the smoking prevention and cessation programmes, including the cost of screening into the current budget for lung cancer diagnosis and treatment, will result in a certain cost increase of the budget for an indeterminate period of time. If the implementation of the LCS programme is rapid, the selection criteria used is highly cost-effective, and the recruitment manages to reach the entire at-risk population, but especially the most socioeconomically deprived population, the increased cost of screening will be offset by the reduced treatment costs for early-stage in a relatively short time.
Research on LCS should continue to further optimise workflows so that they become even more cost-effective. In addition to the use of LDCT as a screening tool, other fields of research such as blood biomarkers focused on both screening participant selection and the management of detected lung nodules [
37,
38] or the introduction of radiomics [
39,
40] are being developed. Once these techniques are validated in clinical practice, further cost-effectiveness studies will be needed.
Interpretation
In conclusion, the decrease in direct costs associated with lung cancer treatment due to a stage shift resulting from LCS of high-risk populations compensates for a substantial part of the LCS programme costs. Since the economic impact of a LCS programme depends mostly on the cancer detection rate achieved, crucial aspects include the choice of the most effective selection criteria, the implementation of a robust public health policy to promote smoking cessation, screening the majority of the high-risk population especially the highest risk individuals, and achieving adherence to follow-up annual and biennial screening.
Author Contributions
A.R.G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.R.G contributed to conceptualisation, methodology, supervision, data curation, writing—original draft preparation, and writing—review and editing. S.M.B contributed to conceptualization, conducted data curation, and contributed data resources, writing—original draft preparation, and writing—review and editing. R.M.R. contributed to methodology, writing—original draft preparation, and writing—review and editing. M.S. conducted data curation and contributed data resources. M.M conducted data curation and contributed data resources. P.L.C writing—original draft preparation and writing—review and editing. F.L.S contributed to conceptualization, methodology, supervision, data curation, writing—original draft preparation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by MINECO [Núm. Expedient: RTI2018-095209-B-C21-22]; Barcelona Respitatory Network (BRN)- Fundació Ramon Pla i Armengol; “Clinical project” grant, Fundació Acadèmia Ciències Mèdiques de Catalunya i de Balears; “Talents” grant 2020, Fundació La Pedrera and Hospital Universitari Germans Trias i Pujol.
Financial/Nonfinancial Disclosures
The authors do not have any financial or non-financial disclosures to declare.
Data Sharing Statement
Study data are available from the corresponding author upon request.
Other Contributions
Editing and editorial assistance in the development of this manuscript were provided by Marta Pulido, MD (freelance medical editor, Spain). Mrs Adela Gonzalez and Mrs Ester Cervera, as nurse navigators of the ILST (Hospital Universitari Germans Trias i Pujol, Spain), have selected and managed the participants. Dra Anna Nuñez (Hospital Universitari Germans Trias i Pujol, Spain) has contributed to filling in the ILST database. Ms Andrea Borondy Kitts MS, MPH (Lung Cancer and Patient Advocate, Consultant, USA, has reviewed the manuscript.
Abbreviations
AJCC = American Joint Committee on Cancer; CatSalut = Catalan healthcare service; CXR = chest radiography; ELCAP = Early Lung Cancer Action Programme; HUGTP = Germans Trias i Pujol University Hospital; NLST = National Lung Screening Trial; NSCLC = non-small cell lung cancer; LDCT = low-dose computed tomography; PET = positron emission tomography; SBRT = stereotactic body radiotherapy; SCLC = small cell lung cancer; LCS = lung cancer screening; CAT = PanCan lung nodule risk based protocol; CDR = cancer detection rate.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Asociación Española contra el Cáncer (AEEC). Impacto del Cáncer en Cataluña 2020. AEEC website. Accessed February 26, 2022. https://observatorio.contraelcancer.es/sites/default/files/informes/cataluna/Catalu%C3%B1a.pdf.
- Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Schabath MB, Cote ML. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015, 10, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999, 354, 99–105. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol. 2017, 18, e754–e766. [Google Scholar] [CrossRef]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. REPORT on strengthening Europe in the fight against cancer – towards a comprehensive and coordinated strategy (2020/2267(INI)). Accessed July 20, 2022. https://www.europarl.europa.eu/doceo/document/A-9-2022-0001_EN.pdf.
- European Parliament. Proposal for a COUNCIL RECOMMENDATION on strengthening prevention through early detection: A new EU approach on cancer screening replacing Council Recommendation 2003/878/EC. Accessed November 09, 2022. (https://eur-lex.europa.eu/procedure/EN/2022_290).
- Han D, Heuvelmans MA, Vliegenthart R, et al. An update on the European Lung Cancer Screening Trials and comparison of lung cancer screening recommendations in Europe. J Thorac Imaging 2019, 34, 65–71. [Google Scholar] [CrossRef]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 Study. J Clin Oncol 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Grover H, King W, Bhattarai N, et al. Systematic review of the cost-effectiveness of screening for lung cancer with low dose computed tomography. Lung Cancer 2022, 170, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Snowsill T, Yang H, Griffin E, et al. Low-dose computed tomography for lung cancer screening in high-risk populations: a systematic review and economic evaluation. Health Technol Assess 2018, 22, 1–276. [Google Scholar] [CrossRef] [PubMed]
- Raymakers AJN, Mayo J, Lam S, et al. Cost-effectiveness analyses of lung cancer screening strategies using low-dose computed tomography: a systematic review. Appl Health Econ Health Policy 2016, 14, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Ngo PJ, Cressman S, Behar-Harpaz S, et al. Applying utility values in cost-effectiveness analyses of lung cancer screening: a review of methods. Lung Cancer 2022, 166, 122–131. [Google Scholar] [CrossRef]
- Cressman S, Peacock SJ, Tammemägi MC, et al. The cost-effectiveness of high-risk lung cancer screening and drivers of programme efficiency. J Thorac Oncol 2017, 12, 1210–1222. [Google Scholar] [CrossRef]
- Diaz M, Garcia M, Vidal C, et al. Health and economic impact at a population level of both primary and secondary preventive lung cancer interventions: A model-based cost-effectiveness analysis. Lung Cancer 2021, 159, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Guzman R, Guirao À, Vela E, et al. Outcomes and cost of lung cancer patients treated surgically or medically in Catalunya: cost-benefit implications for lung cancer screening programmes. Eur J Cancer Prev 2020, 29, 486–492. [Google Scholar] [CrossRef]
- Gómez-Carballo N, Fernández-Soberón S, Rejas-Gutiérrez J. Cost-effectiveness analysis of a lung cancer screening programmeme in Spain. Eur J Cancer Prev 2022, 3, 235–244. [Google Scholar]
- Lim KP, Marshall H, Tammemägi M, et al. Protocol and rationale for the International Lung Screening Trial. Ann Am Thorac Soc 2020, 17, 503–512. [Google Scholar] [CrossRef]
- Majem M, Juan O, Insa A, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol 2019, 21, 3–17. [Google Scholar] [CrossRef] [PubMed]
- ORDER SLT/71/2020, of 2 June, Which Regulates the Billable Cases and Concepts and Approves the Public Prices Corresponding to the Services Provided by the Catalan Institute of Health. Accessed December 29, 2021. http://cido.diba.cat/legislacio/10263520/ordre-slt712020-de-2-de-juny-per-la-qual-es-regulen-els-suposits-i-conceptes-facturables-i-saproven-els-preus-publics-corresponents-als-serveis-que-presta-linstitut-catala-de-la-salut-departament-de-salut.
- Myers R, Mayo J, Atkar-Khattra S, et al. MA10.01 Prospective Evaluation of the International Lung Screening Trial (ILST) protocol for management of first screening LDCT. J Thorac Oncol 2021, 16, S913–S914. [Google Scholar] [CrossRef]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Regis SM, Borondy-Kitts A, McKee AB, et al. Outcomes of positive and suspicious findings in clinical computed tomography lung cancer screening and the road ahead. Ann Am Thorac Soc 2022, 19, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Tammemägi MC, Ruparel M, Tremblay A, et al. USPSTF2013 versus PLCOm2012 lung cancer screening eligibility criteria (International Lung Screening Trial): interim analysis of a prospective cohort study. Lancet Oncol 2022, 23, 138–148. [Google Scholar] [CrossRef] [PubMed]
- McRonald FE, Yadegarfar G, Baldwin DR, et al. The UK Lung Screen (UKLS): demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev Res (Phila) 2014, 7, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Pinsky PF, Church TR, Izmirlian G, et al. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer 2013, 119, 3976–3983. [Google Scholar] [CrossRef] [PubMed]
- Luengo-Fernandez R, Leal J, Gray A, et al. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol 2013, 14, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Wood R, Taylor-Stokes G. Cost burden associated with advanced non-small cell lung cancer in Europe and influence of disease stage. BMC Cancer 2019, 19, 214. [Google Scholar] [CrossRef]
- van Meerbeeck JP, Franck C. Lung cancer screening in Europe: where are we in 2021? Transl Lung Cancer Res 2021, 10, 2407–2417. [Google Scholar] [CrossRef]
- Vogler S, Vitry A, Babar ZU. Cancer drugs in 16 European countries, Australia, and New Zealand: a cross-country price comparison study. Lancet Oncol. 2016, 17, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Triplette M, Thayer JH, Kross EK, et al. The impact of smoking and screening results on adherence to follow-up in an academic multisite lung cancer screening programme. Ann Am Thorac Soc 2021, 18, 541–544. [Google Scholar]
- Pastorino U, Boeri M, Sestini S, et al. Baseline computed tomography screening and blood microRNA predict lung cancer risk and define adequate intervals in the BioMILD trial. Ann Oncol 2022, 33, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez M, Ajona D, Seijo LM, et al. Molecular biomarkers in early stage lung cancer. Transl Lung Cancer Res 2021, 10, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang Z, Li N, Zheng F, et al. Optimizing the timing of diagnostic testing after positive findings in lung cancer screening: a proof-of-concept radiomics study. J Transl Med 2021, 19, 191. [Google Scholar] [CrossRef]
- Torres G, Baeza S, Sanchez C, et al. An intelligent radiomic approach for lung cancer screening. Appl Sci (Basel) 2022, 12, 1568. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).