Submitted:
27 December 2022
Posted:
07 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Characterization of Col11a1 Expression during Chondrogenesis in ATDC5 Cells.
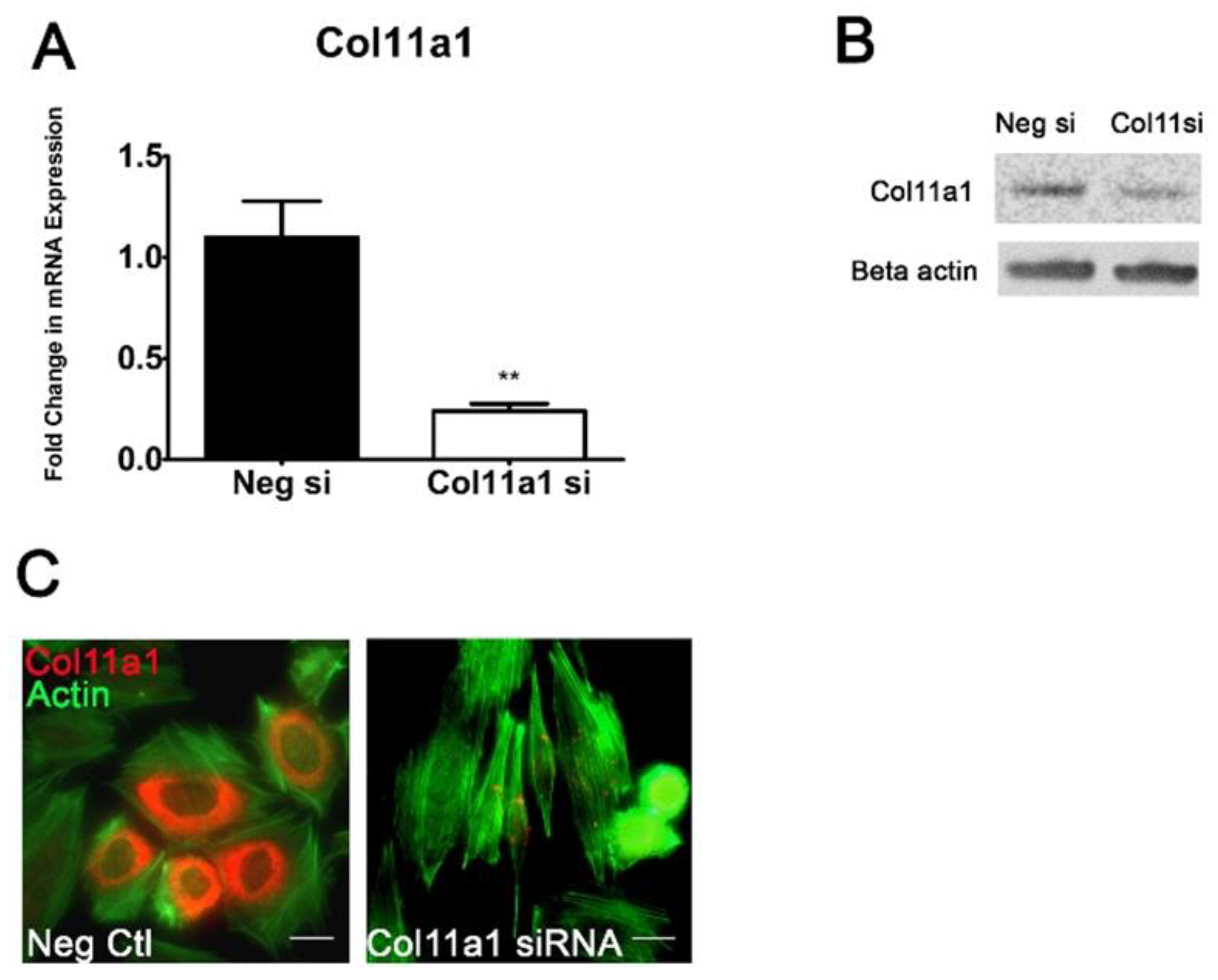
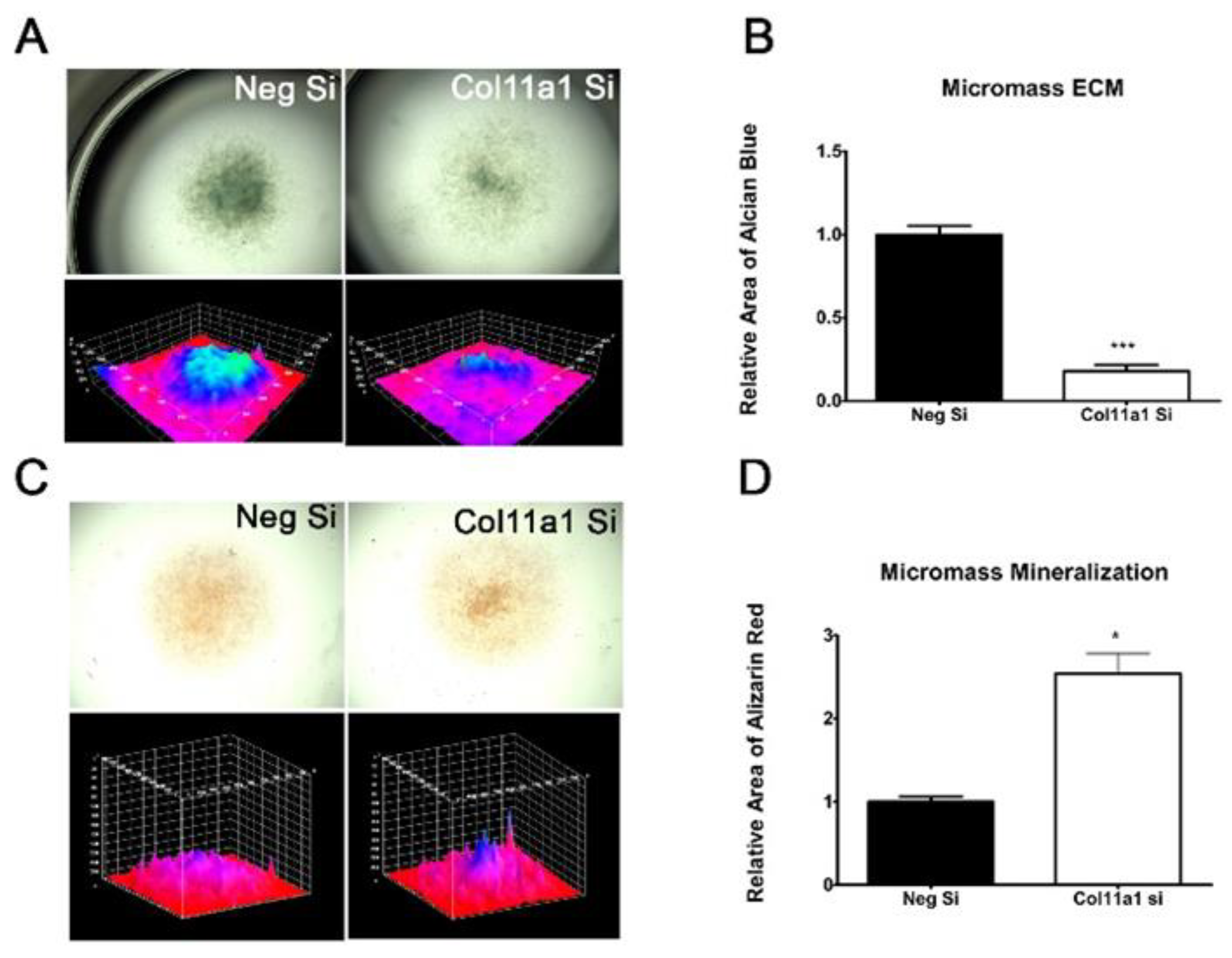
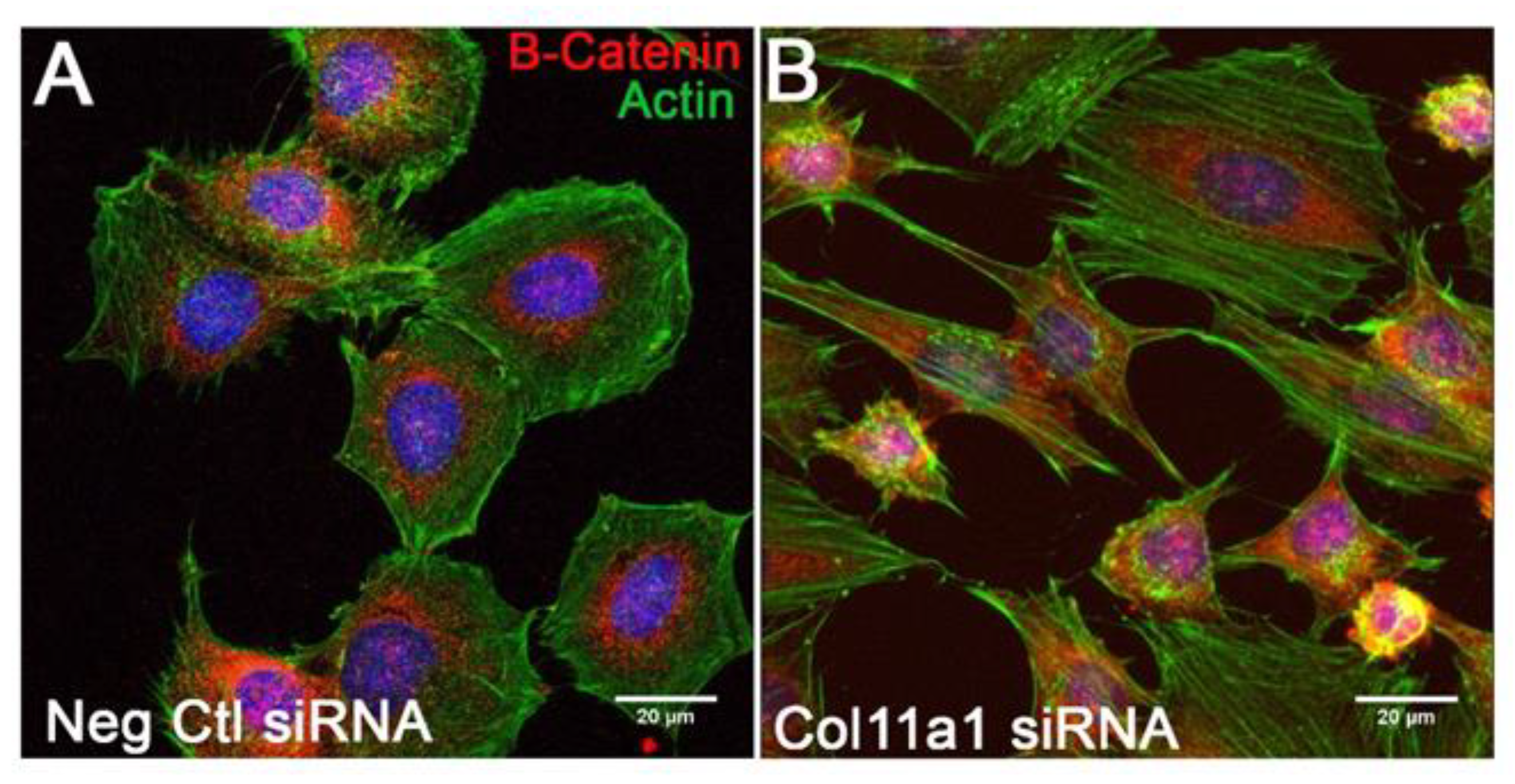
2.2. Col11a1 Knockdown Induces Fibroblast-like Morphology, Inhibits Chondrogenesis, and Increases Mineralization
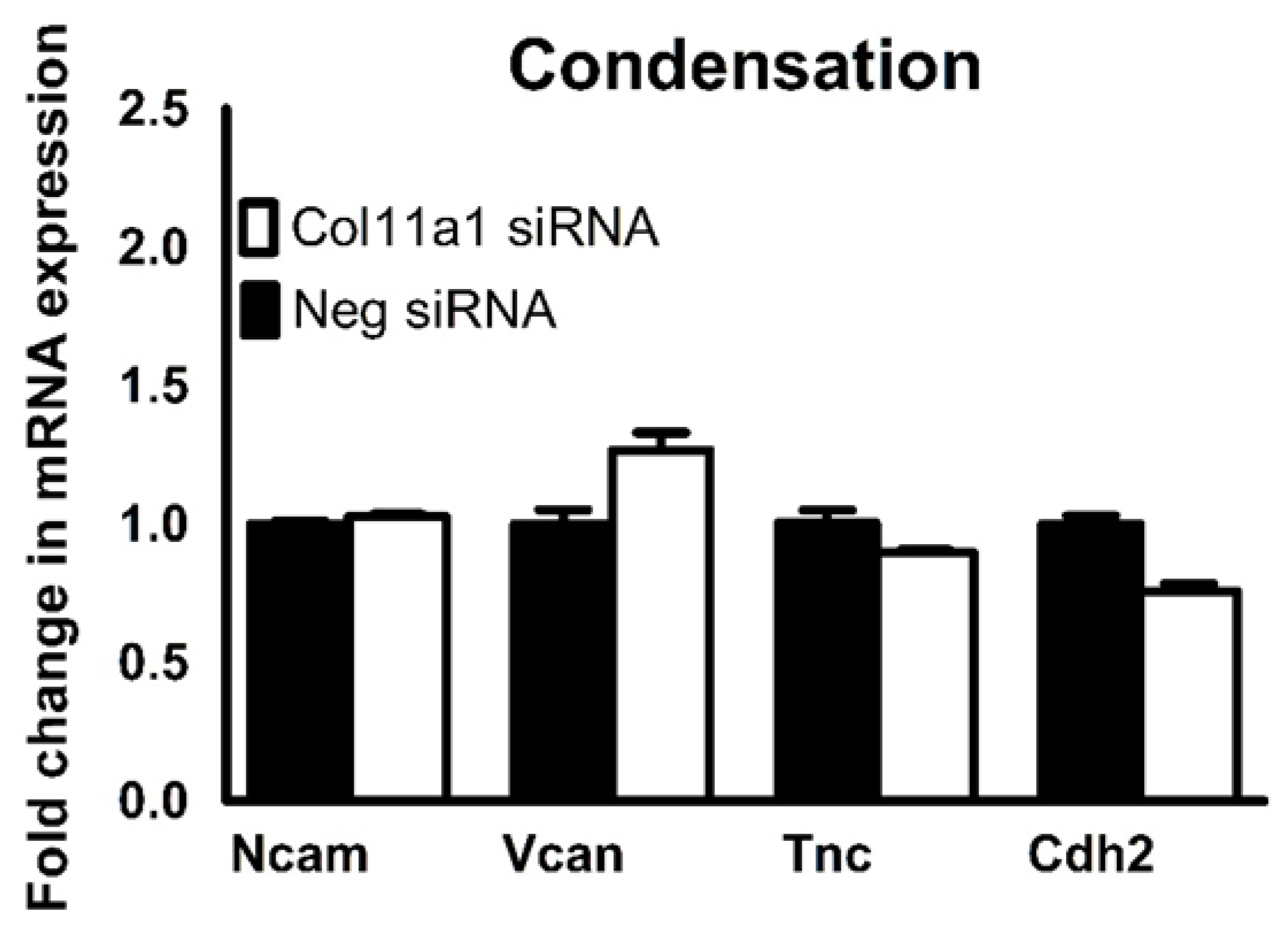
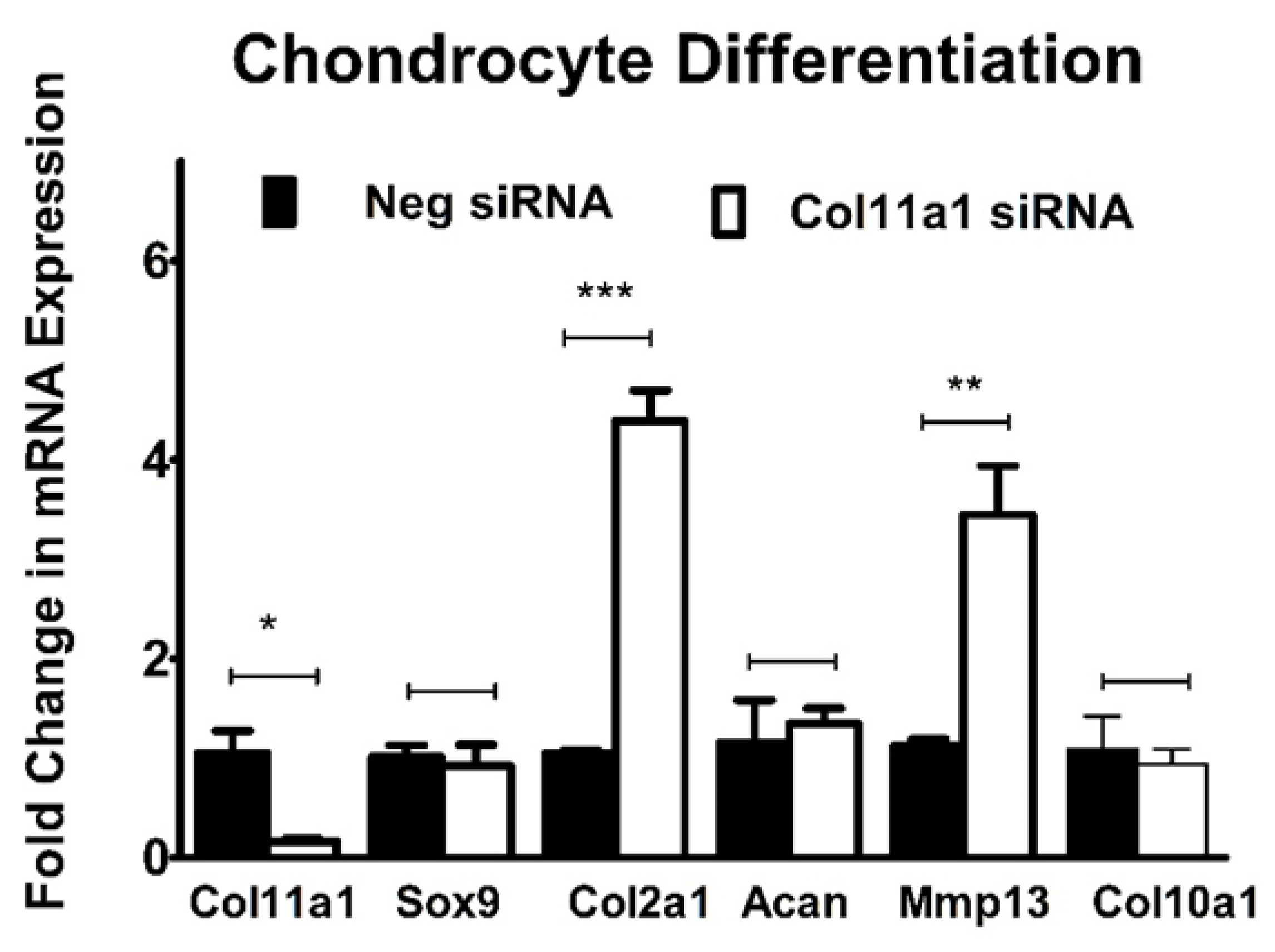
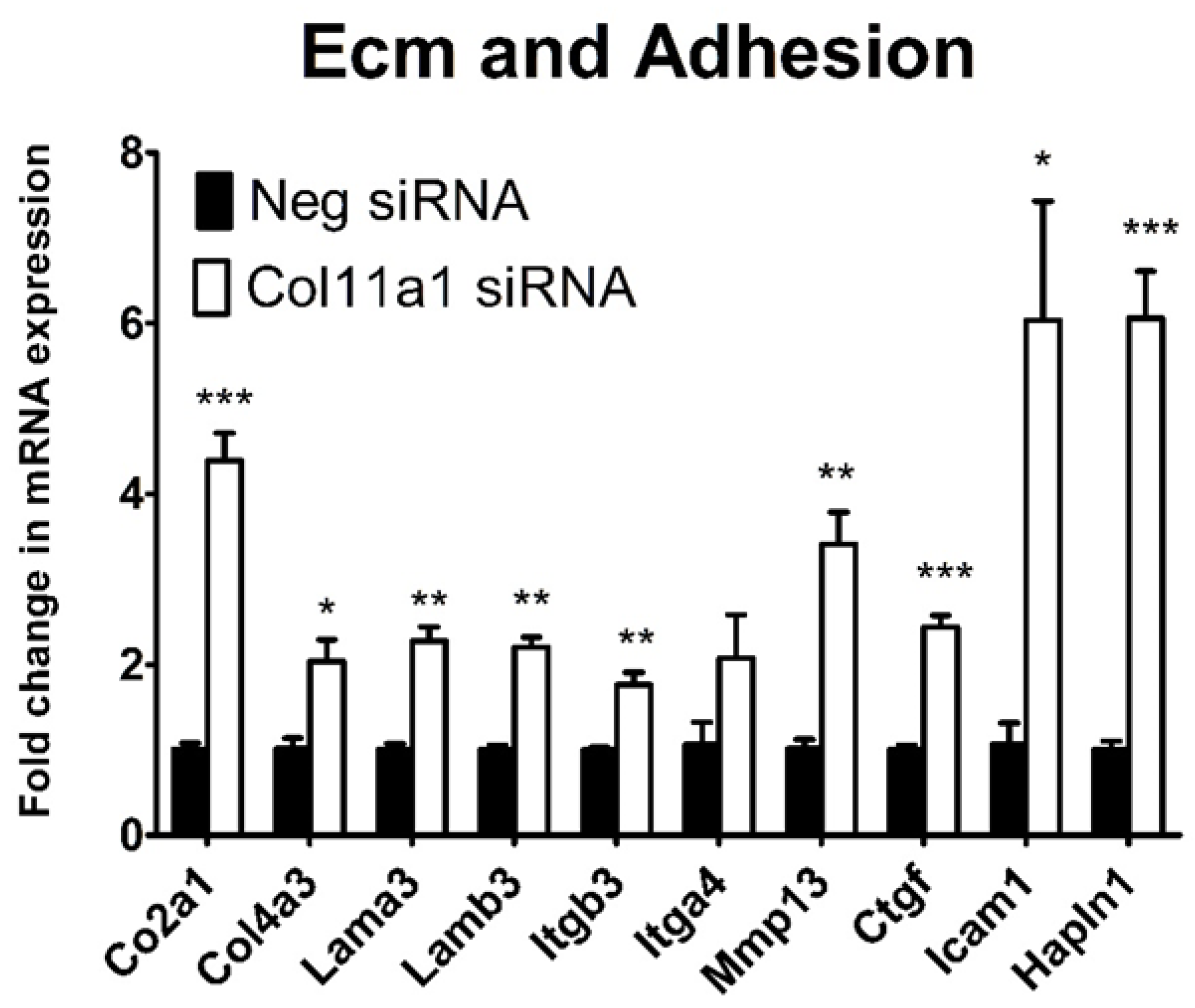
2.3. Inhibition of Col11a1 Expression Does Not Affect Genes Involved in Mesenchymal Condensation But Does Affect Genes Involved in Matrix Production and Remodeling
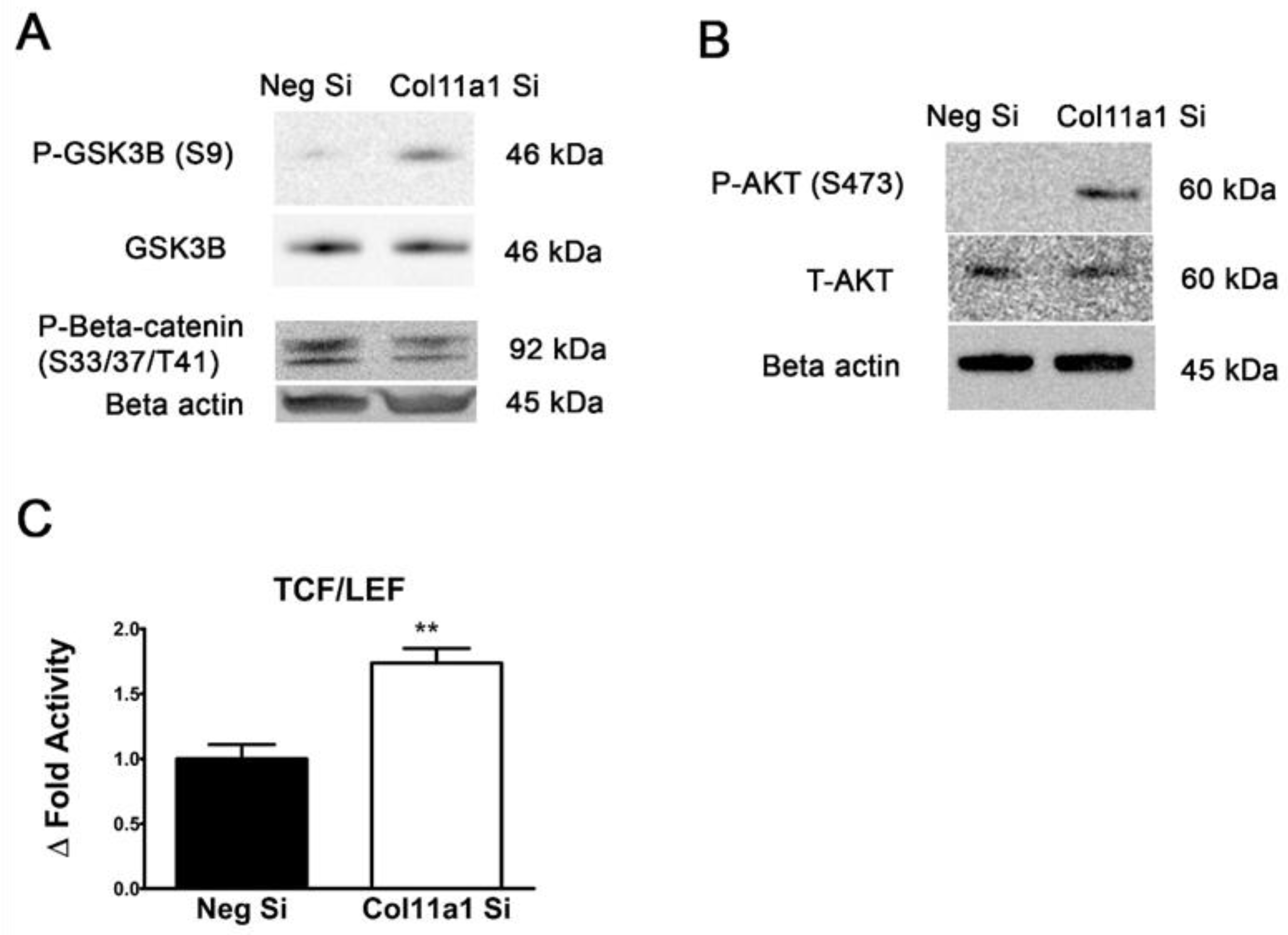
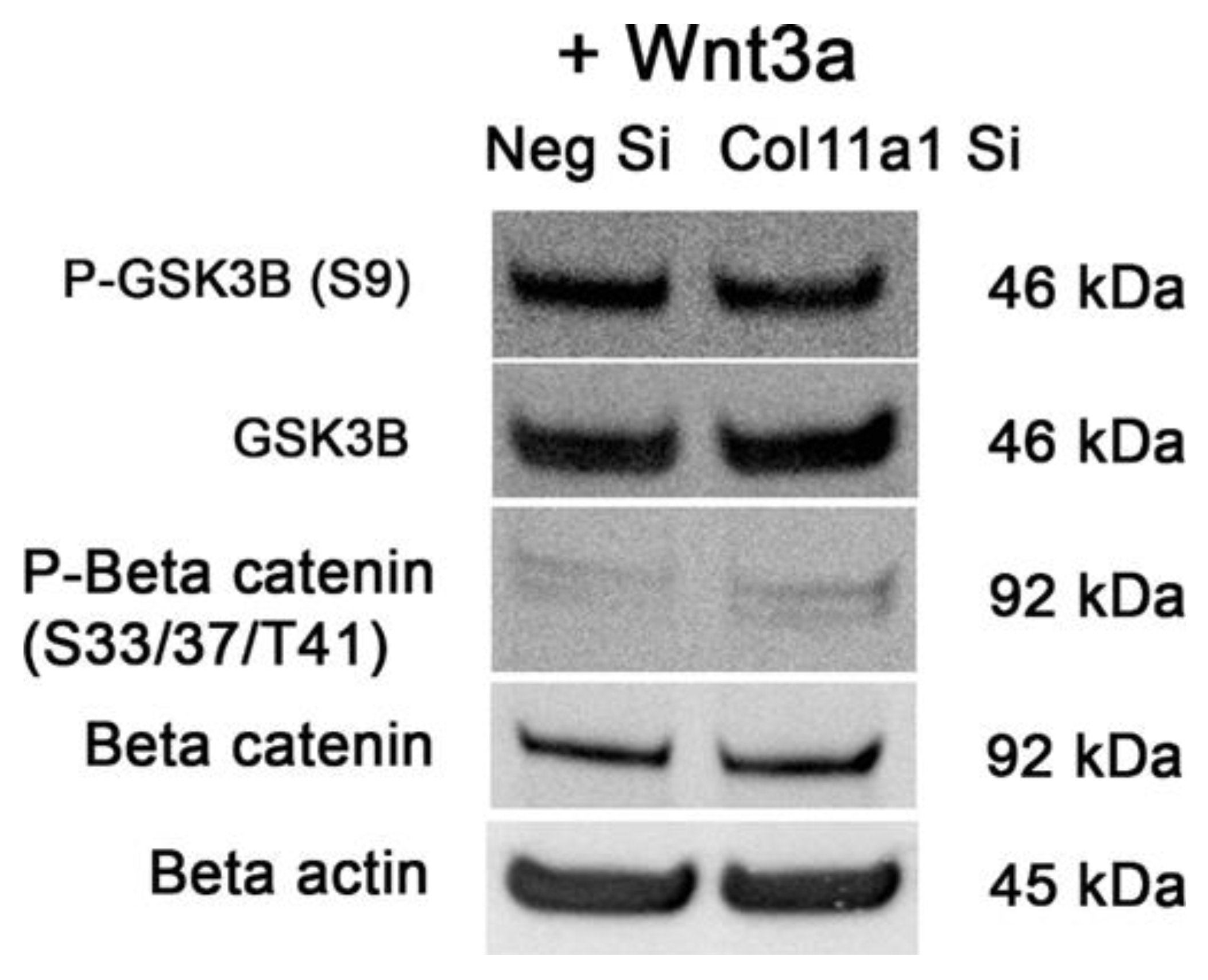
2.4. β-Catenin Signaling Pathway Activity Is Increased When Col11a1 Expression Is Inhibited during Chondrogenesis and Is Independent of Wnt Induction
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Col11a1 Knockdown by siRNA Transfection
4.3. 3D Micromass Cultures
4.4. Quantitative RT-PCR (qPCR) Analysis
4.5. Luciferase Assays
4.6. Western Blot Analysis
4.7. Alcian Blue and Alizarin Red Staining
4.8. Immunocytochemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, B., and Miyake, T. 2000. All for one and one for all: condensations and the initiation of skeletal development. BioEssays 22(2): 138–147.
- Kronenberg, H.M. 2003. Developmental regulation of the growth plate. Nature 423(6937): 332–336. [CrossRef]
- Berendsen, A., and Olsen, B. 2015. Bone development. Bone 80: 14–8.
- Li, Y., Lacerda, D., Warman, M., Beier, D., Yoshioka, H., Ninomiya, Y., Oxford, J., Morris, N., et al. 1995. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell 80(3): 423–30. [CrossRef]
- Hafez, A., Squires, R., Pedracini, A., Joshi, A., Seegmiller, R., and Oxford, J. 2015. Col11a1 Regulates Bone Microarchitecture during Embryonic Development. J. Dev. Biol. 3(4): 158–176. [CrossRef]
- Tompson, S., Bacino, C., Safina, N., Bober, M., Proud, V., Funari, T., Wangler, M., Nevarez, L., Ala-Kokko, L., Wilcox, W., Eyre, D., Krakow, D., and Cohn, D. 2010. Fibrochondrogenesis results from mutations in the COL11A1 type XI collagen gene. Am. J. Hum. Genet. 87(5): 708–12. [CrossRef]
- Hufnagel, S., Weaver, K., Hufnagel, R., Bader, P., Schorry, E., and Hopkin, R. 2014. A novel dominant COL11A1 mutation resulting in a severe skeletal dysplasia. Am. J. Med. Genet. A 164A(10): 2607–12. [CrossRef]
- Noponen-Hietala, N., Kyllönen, E., Männikkö, M., Ilkko, E., Karppinen, J., Ott, J., and Ala-Kokko, L. 2003. Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann. Rheum. Dis. 62(12): 1208–14. [CrossRef]
- Rodriguez, R., Seegmiller, R., Stark, M., and Bridgewater, L. 2004. A type XI collagen mutation leads to increased degradation of type II collagen in articular cartilage11. Osteoarthr. Cartil. 12(4): 314–320. [CrossRef]
- Raine, E., Dodd, A., Reynard, L., and Loughlin, J. 2013. Allelic expression analysis of the osteoarthritis susceptibility gene COL11A1 in human joint tissues. BMC Musculoskelet. Disord. 14(1): 85. [CrossRef]
- Morris, N., and Bächinger, H. 1987. Type XI collagen is a heterotrimer with the composition (1 alpha, 2 alpha, 3 alpha) retaining non-triple-helical domains. J. Biol. Chem. 262(23): 11345–50. [CrossRef]
- Fernandes, R., Weis, M., Scott, M., Seegmiller, R., and Eyre, D. 2007. Collagen XI chain misassembly in cartilage of the chondrodysplasia (cho) mouse. Matrix Biol. 26(8): 597–603. [CrossRef]
- Blaschke, U., Eikenberry, E., Hulmes, D., Galla, H., and Bruckner, P. 2000. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J. Biol. Chem. 275(14): 10370–8. [CrossRef]
- Gregory, K., Oxford, J., Chen, Y., Gambee, J., Gygi, S., Aebersold, R., Neame, P., Mechling, D., Bächinger, H., and Morris, N. 2000. Structural organization of distinct domains within the non-collagenous N-terminal region of collagen type XI. J. Biol. Chem. 275(15): 11498–506. [CrossRef]
- Holmes, D., and Kadler, K. 2006. The 10+4 microfibril structure of thin cartilage fibrils. Proc. Natl. Acad. Sci. 103(46): 17249–17254. [CrossRef]
- Bernard, M., Yoshioka, H., Rodriguez, E., Van der Rest, M., Kimura, T., Ninomiya, Y., Olsen, B., and Ramirez, F. 1988. Cloning and sequencing of pro-alpha 1 (XI) collagen cDNA demonstrates that type XI belongs to the fibrillar class of collagens and reveals that the expression of the gene is not restricted to cartilagenous tissue. J. Biol. Chem. 263(32): 17159–66. [CrossRef]
- Oxford, J., DeScala, J., Morris, N., Gregory, K., Medeck, R., Irwin, K., Oxford, R., Brown, R., Mercer, L., and Cusack, S. 2004. Interaction between amino propeptides of type XI procollagen alpha1 chains. J. Biol. Chem. 279(12): 10939–45. [CrossRef]
- Warner, L., Brown, R., Yingst, S., and Oxford, J. 2006. Isoform-specific Heparan Sulfate Binding within the Amino-terminal Noncollagenous Domain of Collagen α1(XI). J. Biol. Chem. 281(51): 39507–39516. [CrossRef]
- Brown, R., Mallory, C., McDougal, O., and Oxford, J. 2011. Proteomic analysis of Col11a1-associated protein complexes. Proteomics 11(24): 4660–76. [CrossRef]
- Goldring, M., Tsuchimochi, K., and Ijiri, K. 2006. The control of chondrogenesis. J. Cell. Biochem. 97(1): 33–44. [CrossRef]
- Chen, L., Fink, T., Zhang, X., Ebbesen, P., and Zachar, V. 2005. Quantitative transcriptional profiling of ATDC5 mouse progenitor cells during chondrogenesis. Differentiation 73(7): 350–363. [CrossRef]
- Altaf, F., Hering, T., Kazmi, N., Yoo, J., and Johnstone, B. 2006. Ascorbate-enhanced chondrogenesis of ATDC5 cells. Eur. Cell. Mater. 12: 64-9-70. [CrossRef]
- Yao, Y., and Wang, Y. 2013. ATDC5: An excellent in vitro model cell line for skeletal development. J. Cell. Biochem. 114(6): 1223–1229. [CrossRef]
- Verzijl, N., DeGroot, J., Thorpe, S., Bank, R., Shaw, J., Lyons, T., Bijlsma, J., Lafeber, F., Baynes, J., and TeKoppele, J. 2000. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 275(50): 39027–31. [CrossRef]
- Toyama, B., and Hetzer, M. 2013. Protein homeostasis: live long, won’t prosper. Nat. Rev. Mol. Cell Biol. 14(1): 55–61. [CrossRef]
- Tamamura, Y., Otani, T., Kanatani, N., Koyama, E., Kitagaki, J., Komori, T., Yamada, Y., Costantini, F., Wakisaka, S., Pacifici, M., Iwamoto, M., and Enomoto-Iwamoto, M. 2005. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J. Biol. Chem. 280(19): 19185–95.
- Dao, D., Jonason, J., Zhang, Y., Hsu, W., Chen, D., Hilton, M., and O’Keefe, R. 2012. Cartilage-specific β-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J. Bone Miner. Res. 27(8): 1680–94.
- Usami, Y., Gunawardena, A., Iwamoto, M., and Enomoto-Iwamoto, M. 2016. Wnt signaling in cartilage development and diseases: lessons from animal studies. Lab. Investig. 96(2): 186–196. [CrossRef]
- Doble, B., and Woodgett, J. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116(7). [CrossRef]
- Miclea, R., Siebelt, M., Finos, L., Goeman, J., Löwik, C., Oostdijk, W., Weinans, H., Wit, J., Robanus-Maandag, E., and Karperien, M. 2011. Inhibition of Gsk3β in cartilage induces osteoarthritic features through activation of the canonical Wnt signaling pathway. Osteoarthr. Cartil. 19(11): 1363–1372. [CrossRef]
- Kawasaki, Y., Kugimiya, F., Chikuda, H., Kamekura, S., Ikeda, T., Kawamura, N., Saito, T., Shinoda, Y., Higashikawa, A., Yano, F., Ogasawara, T., Ogata, N., Hoshi, K., Hofmann, F., Woodgett, J.R., Nakamura, K., Chung, U., and Kawaguchi, H. 2008. Phosphorylation of GSK-3beta by cGMP-dependent protein kinase II promotes hypertrophic differentiation of murine chondrocytes. J. Clin. Invest. 118(7): 2506–15.
- Aberle, H., Bauer, A., Stappert, J., Kispert, A., Kemler, R., Aberle, H., Butz, S., Stappert, J., Weissig, H., Kemler, R., Hoschützky, H., Aberle, H., and Moon, R. 1997. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16(13): 3797–804.
- Delcommenne, M., Tan, C., Gray, V., Rue, L., Woodgett, J., and Dedhar, S. 1998. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. U. S. A. 95(19): 11211–6.
- Stevenson, D., Vanzo, R., Damjanovich, K., Hanson, H., Muntz, H., Hoffman, R., and Bayrak-Toydemir, P. 2012. Mosaicism in Stickler syndrome. Eur. J. Med. Genet. 55(6–7): 418–22.
- Faletra, F., D’Adamo, A.P., Bruno, I., Athanasakis, E., Biskup, S., Esposito, L., and Gasparini, P. 2014. Autosomal recessive Stickler syndrome due to a loss of function mutation in the COL9A3 gene. Am. J. Med. Genet. A 164A(1): 42–7.
- Davies, G., Oxford, J., Hausafus, L., Smoody, B., and Morris, N. 1998. Temporal and spatial expression of alternative splice-forms of the α1(XI) collagen gene in fetal rat cartilage. Dev. Dyn. 213(1): 12–26.
- Eteson, D., Adomian, G., Ornoy, A., Koide, T., Sugiura, Y., Calabro, A., Lungarotti, S., Mastroiacovo, P., Lachman, R.S., and Rimoin, D.L. 1984. Fibrochondrogenesis: radiologic and histologic studies. Am. J. Med. Genet. 19(2): 277–90.
- Whitley, C., Langer, L., Ophoven, J., Gilbert, E., Gonzalez, C., Mammel, M., Coleman, M., Rosemberg, S., Rodriques, C., Sibley, R., Horton, W., Opitz, J., and Gorlin, R. 1984. Fibrochondrogenesis: Lethal, autosomal recessive chondrodysplasia with distinctive cartilage histopathology. Am. J. Med. Genet. 19(2): 265–275.
- Komori, T., Yagi, H., Nomura, S., Yamaguchi, A., Sasaki, K., Deguchi, K., Shimizu, Y., Bronson, R., Gao, Y.-H., Inada, M., Sato, M., Okamoto, R., Kitamura, Y., Yoshiki, S., and Kishimoto, T. 1997. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell 89(5): 755–764.
- Modarresi, R., Lafond, T., Roman-Blas, J.A., Danielson, K.G., Tuan, R.S., and Seghatoleslami, M. 2005. N-cadherin mediated distribution of β-catenin alters MAP kinase and BMP-2 signaling on chondrogenesis-related gene expression. J. Cell. Biochem. 95(1): 53–63.
- Gao, L., McBeath, R., and Chen, C. 2010. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells 28(3): 564–72.
- Marie, P., Haÿ, E., Modrowski, D., Revollo, L., Mbalaviele, G., and Civitelli, R. 2014. Cadherin-mediated cell-cell adhesion and signaling in the skeleton. Calcif. Tissue Int. 94(1): 46–54.
- Ballock, R., Heydemann, A., Wakefield, L., Flanders, K., Roberts, A., and Sporn, M. 1993. TGF-β1 Prevents Hypertrophy of Epiphyseal Chondrocytes: Regulation of Gene Expression for Cartilage Matrix Proteins and Metalloproteases. Dev. Biol. 158(2): 414–429.
- Vega, R., Matsuda, K., Oh, J., Barbosa, A., Yang, X., Meadows, E., McAnally, J., Pomajzl, C., Shelton, J., Richardson, J., Karsenty, G., and Olson, E. 2004. Histone Deacetylase 4 Controls Chondrocyte Hypertrophy during Skeletogenesis. Cell 119(4): 555–566.
- van der Kraan, P.M., and van den Berg, W.B. 2012. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 20(3): 223–232.
- Jayasuriya, C., Zhou, F., Pei, M., Wang, Z., Lemme, N., Haines, P., and Chen, Q. 2014. Matrilin-3 chondrodysplasia mutations cause attenuated chondrogenesis, premature hypertrophy and aberrant response to TGF-β in chondroprogenitor cells. Int. J. Mol. Sci. 15(8): 14555–73.
- Yang, L., Tsang, K., Tang, H., Chan, D., and Cheah, K. 2014. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. 111(33): 12097–12102.
- Xu, L., Flahiff, C., Waldman, B., Wu, D., Olsen, B., Setton, L., and Li, Y. 2003. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho). Arthritis Rheum. 48(9): 2509–2518.
- Xu, L., Servais, J., Polur, I., Kim, D., Lee, P.L., Chung, K., and Li, Y. 2010. Attenuation of osteoarthritis progression by reduction of discoidin domain receptor 2 in mice. Arthritis Rheum. 62(9): 2736–2744.
- Hering, T., Wirthlin, L., Ravindran, S., and McAlinden, A. 2014. Changes in type II procollagen isoform expression during chondrogenesis by disruption of an alternative 5’ splice site within Col2a1 exon 2. Matrix Biol. 36: 51–63.
- McAlinden, A., Johnstone, B., Kollar, J., Kazmi, N., and Hering, T. 2008. Expression of two novel alternatively spliced COL2A1 isoforms during chondrocyte differentiation. Matrix Biol. 27(3): 254–66.
- Sandell, L., Morris, N., Robbins, J., and Goldring, M. 1991. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J. Cell Biol. 114(6): 1307-19.
- Sandell, L., Nalin, A., and Reife, R. 1994. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev. Dyn. 199(2): 129–140.
- Yamada, Y., and Watanabe, H. 1999. Mice lacking link protein develop dwarfism and craniofacial abnormalities. Nat. Genet. 21(2): 225–229.
- Mebarki, S., Désert, R., Sulpice, L., Sicard, M., Desille, M., Canal, F., Dubois-Pot Schneider, H., Bergeat, D., Turlin, B., Bellaud, P., Lavergne, E., Le Guével, R., Corlu, A., Perret, C., Coulouarn, C., Clément, B., and Musso, O. 2016. De novo HAPLN1 expression hallmarks Wnt-induced stem cell and fibrogenic networks leading to aggressive human hepatocellular carcinomas. Oncotarget 7(26): 39026–39043.
- Nishida, T., Kubota, S., Fukunaga, T., Kondo, S., Yosimichi, G., Nakanishi, T., Takano-Yamamoto, T., and Takigawa, M. 2003. CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J. Cell. Physiol. 196(2): 265–275.
- Zhou, Y., Capuco, A., and Jiang, H. 2008. Involvement of connective tissue growth factor (CTGF) in insulin-like growth factor-I (IGF1) stimulation of proliferation of a bovine mammary epithelial cell line. Domest. Anim. Endocrinol. 35(2): 180–189.
- Huang, B., Brugger, S., and Lyons, K. 2010. Stage-specific control of connective tissue growth factor (CTGF/CCN2) expression in chondrocytes by Sox9 and beta-catenin. J. Biol. Chem. 285(36): 27702–12.
- Oh, C., Yasuda, H., Zhao, W., Henry, S., Zhang, Z., Xue, M., de Crombrugghe, B., and Chen, D. 2016. SOX9 directly Regulates CTGF/CCN2 Transcription in Growth Plate Chondrocytes and in Nucleus Pulposus Cells of Intervertebral Disc. Sci. Rep. 6: 29916.
- Saegusa, J., Yamaji, S., Ieguchi, K., Wu, C., Lam, K., Liu, F., Takada, Y., and Takada, Y. 2009. The Direct Binding of Insulin-like Growth Factor-1 (IGF-1) to Integrin v 3 Is Involved in IGF-1 Signaling. J. Biol. Chem. 284(36): 24106–24114.
- Arioka, M., Takahashi-Yanaga, F., Sasaki, M., Yoshihara, T., Morimoto, S., Takashima, A., Mori, Y., and Sasaguri, T. 2013. Acceleration of bone development and regeneration through the Wnt/β-catenin signaling pathway in mice heterozygously deficient for GSK-3β. Biochem. Biophys. Res. Commun. 440(4): 677–682.
- Tatsumoto, N., Arioka, M., Yamada, S., Takahashi-Yanaga, F., Tokumoto, M., Tsuruya, K., Kitazono, T., and Sasaguri, T. 2016. Inhibition of GSK-3 β increases trabecular bone volume but not cortical bone volume in adenine-induced uremic mice with severe hyperparathyroidism. Physiol. Rep. 4(21): e13010.
- Sisask, G., Marsell, R., Sundgren-Andersson, A., Larsson, S., Nilsson, O., Ljunggren, Ö., and Jonsson, K. 2013. Rats treated with AZD2858, a GSK3 inhibitor, heal fractures rapidly without endochondral bone formation. Bone 54(1): 126–132.
- Kahler, R., Yingst, S., Hoeppner, L., Jensen, E., Krawczak, D., Oxford, J., and Westendorf, J. 2008. Collagen 11a1 is indirectly activated by lymphocyte enhancer-binding factor 1 (Lef1) and negatively regulates osteoblast maturation. Matrix Biol. 27(4): 330–8.
- Ciorba, A., Corazzi, V., Melegatti, M., Morgan, A., Pelliccione, G., Girotto, G., & Bigoni, S. (2021). Non-Syndromic Sensorineural Prelingual and Postlingual Hearing Loss due to COL11A1 Gene Mutation. The journal of international advanced otology, 17(1), 81–83. [CrossRef]
- Schmittgen, T., and Livak, K. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3(6): 1101–8.

| Target | Forward Sequence | Reverse Sequence |
|---|---|---|
| Mouse Col2a1 | ACGAAGCGGTGGCAACCTCA | CCCTCGGCCCTCATCTCTACATCA |
| Mouse HPRT | CTGGTGAAAAGGACCTCTCGAA | CTGAAGTACTCATTATAGTCAAGGGCAT |
| Mouse PPIA | CGCGTCTCCTTCGAGCTGTTTG | TGTAAAGTCACCACCCTGGCACAT |
|
Mouse Col10a1 |
TGCCCGTGTCTGCTTTTACTGTCA | TCAAATGGGATGGGGGCACCTACT |
|
Mouse Sox9 |
GAGGCCACGGAACAGACTCA | CAGCGCCTTGAAGATAGCATT |
|
Mouse Acan |
CCTCGGGCAGAAGAAAGA | GTCTCATGCTCCGCTTCTGT |
|
Mouse Mmp13 |
AGTTGACAGGCTCCGAGAAA | GGCACTCCACATCTTGGTTT |
| Mouse Col11a1 | TGGAAACCCACACCGGAAA | TGCCTCTGTTTGTGCTACTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





