1. Introduction
The management of patients with high-grade (HG) non-muscle invasive bladder cancer (NMIBC) remains a challenging issue in urological practice [
1,
2]. In particular, it is still debated when is appropriate to perform an immediate radical cystectomy (RC) in this subgroup of patients. Indeed, RC could be an effective treatment in selected pT1 HG, while it might represent a potential overtreatment for others. Over the past decades, many efforts have been made to improve risk stratification and identify those patients who may benefit from immediate radical treatment; some features have demonstrated a solid predictive role and are currently employed [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14].
Recently, the European Association of Urology (EAU) guidelines introduced an updated risk group system based on the risk of disease progression [
2,
15]. The updated system introduced the group of very high-risk (VHR) patients in addition to the three already existing low, intermediate and high-risk groups. Guidelines suggest discussing immediate RC with VHR patients [
16].
Although not yet included in any guideline, a consensus was reached on the prognostic value of assessing the extent of lamina propria (LP) invasion in transurethral resection of bladder tumor (TURBT) specimens. Therefore, the newest World Health Organization (WHO) Classification of the Urinary and Male Genital Tumors strongly encourages pathologists to report this feature [
17]. Despite this recommendation, validation of a gold standard method able to produce a reliable pT1 substaging is still needed. Indeed, over the last years, different pT1 substaging approaches have been proposed [
14,
15,
16,
17,
18,
19,
20,
21]. To date, the anatomy-based method is one of the most applied, employing the histological landmark of the
muscularis mucosae layer to produce a three- or a simplified two-tiered- system (T1a/b/c or T1a/b) [
18]. On the other hand, the size-based approaches adopt micrometric measurements of the maximum extent of LP invasion in any direction [
21,
22,
23,
24,
25]. These systems showed clinical significance and overcome the challenging evaluation of
muscularis mucosae layer in TURBT specimens due to lack of orientation, possible hyperplastic appearance and anatomical variations, or total absence [
20]. However, the most effective size-based method has not yet been identified [
26,
27].
Our approach is called Rete Oncologica Lombarda (ROL) system; it has been developed over the last decade thanks to the collaboration of three large institutions in northern Italy. ROL is a size-based system employing a simple 1.0 mm threshold, corresponding approximately to the diameter of a 20-power field (objective 20x). We have recently demonstrated that ROL was more feasible compared to other substaging methods and showed a high predictive value for tumor progression after TURBT [
28,
29]. Nevertheless, our previous analyses were limited by their retrospective nature. Thus, in this work we present the results of a prospective study aiming to validate ROL system predictive value on a large mono-institutional series of primary pT1 HG BC treated with intravesical Bacillus Calmette-Guerin (BCG).
2. Materials and Methods
2.1. Patients
From January 2016 to December 2020, we prospectively maintained a database of all patients with first diagnosis of pT1 HG BC and treated with BCG in a tertiary research hospital. Cases with histotype different from transitional, muscle-invasion (pT2) at re-staging TUR (reTURBT), incorrect grading or incomplete follow-up data were excluded. The maintenance scheme with BCG and follow-up was provided in accordance with the updated European guidelines (16). All patients completed BCG induction course. Detailed clinico-pathologic data were registered in the database.
2.2. Pathological Evaluation and Substage Attribution
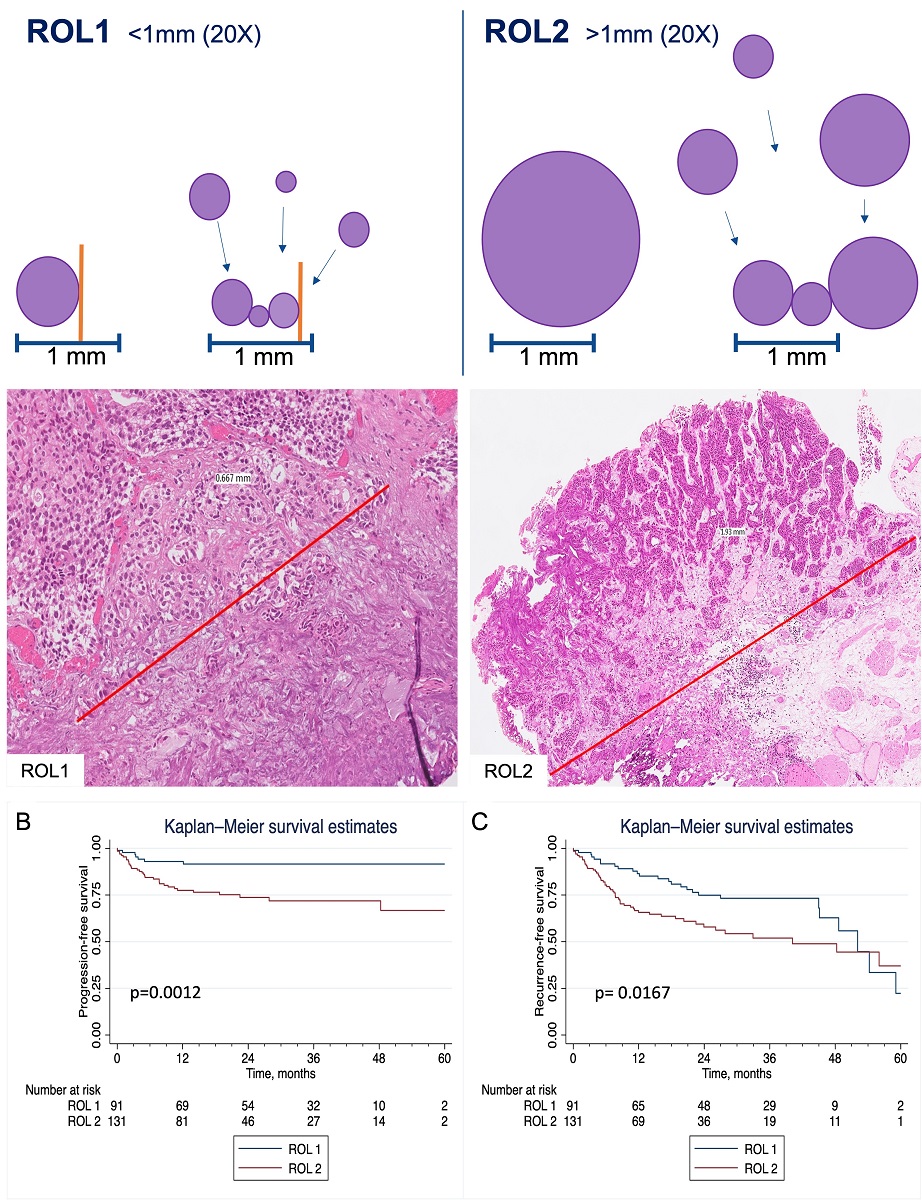
Using the ROL system for assessing LP invasion and substaging pT1, we adopted a cut-off of 1 mm (corresponding to the diameter of a high-power field, HPF, objective 20x, ocular 10x/field 22, diameter 1x1mm) on Hematoxylin and Eosin slides. Tumors were stratified in ROL1 and ROL2 (
Figure 1). ROL1 was defined as follows: 1) a single focus of LP invasion extending for ≤ 1.0 mm or 2) multiple foci of LP invasion extending for ≤ 1.0 mm summed together. ROL2 presented 1) a single focus of LP invasion >1.0 mm or 2) multiple foci of LP invasion extending for >1.0 mm summed together. The number of slides ranged from one to 14, depending on size of tumor resected. All slides were reviewed independently by three expert uropathologists to attribute substaging and record pathologic features. Cases with discordant results were collectively discussed to reach a consensus.
2.3. Statistical Analysis
The endpoint was to assess and confirm ROL predictive value in terms of progression to muscle-invasive bladder cancer (MIBC) and recurrence free survival after TURBT. Progression was defined as the diagnosis of a MIBC or distant metastasis either at TURBT or RC. Recurrence was defined as relapsing pT1 or lower stage tumor.
Time to event was calculated as the number of months from TUR to the event.Patients who did not recur or progress were censored at the date of death or the last follow-up visit. The characteristics of the patients were reported as descriptive statistics. Pearson’s chi-squared test and Wilcoxon’s rank sum test were used to comparing categorical and continuous variables, respectively. Univariable and multivariable Cox regression analyses were used to identify significant independent predictors of progression after TUR. Kaplan-Meier (KM) survival estimates were used to investigate ROL’s correlation with progression-free survival (PFS) and recurrence-free survival (RFS). Two-sided p-value (p) < 0.05 was considered statistically significant. Analyses were performed with STATA 17.0 (StataCorp, College Station, Texas).
3. Results
A total of 284 patients with a new diagnosis of pT1 HG BC between January 2016 and December 2020 entered the prospective study. Of these, ten patients staged pT2 at second TUR were initially excluded. At slides review, 25 cases were excluded for incorrect grading (n=12), absence of clear LP infiltration (n=4), histotype other than transitional (n=9). Eventually, 13 patients were lost due to incomplete follow-up data and 14 were excluded due to incomplete BCG induction. As a result, 222 patients with confirmed urothelial pT1HGBC and available complete follow-up data were analyzed. Clinico-pathologic features of the patients are summarized in
Table 1. Median age was 74 years (Interquartile range (IQR):67-80), and most patients were male (73.8%). Sixty-nine patients presented with multifocal tumors (31.7%), and 33 cases presented divergent differentiation (15%). Concomitant carcinoma in situ (CIS) and lymphovascular invasion (LVI) occurred in 31 (13%) and 18 (8.1%) cases, respectively.
ROL system was feasible in all cases; 91 tumors were classified as ROL1 (41%), while 131 were substaged as ROL2 (59%). LVI, necrosis and the presence of multiple foci of LP invasion were more present in ROL 2 patients.
Table 2 shows patients’ characteristics stratified according to ROL status. Representative cases are depicted in
Figure 2.
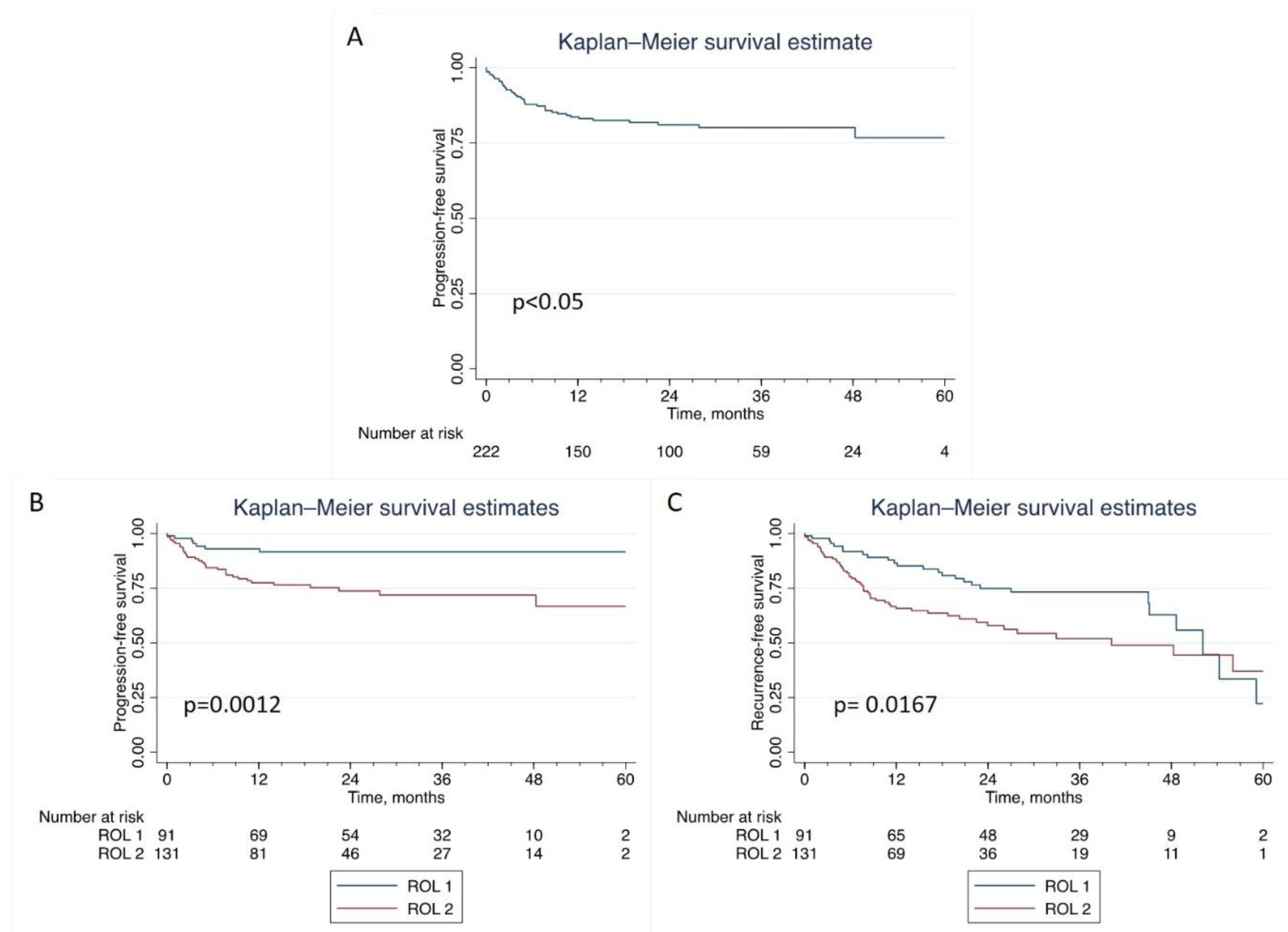
At a median follow up of 26.9 months (IQR 13.8-40.6), 80 patients recurred while 40 patients progressed to MIBC. The 1-yr-PFS rates were 93% (95%CI: 84.9-96.7) and 77% (95%CI: 69.0-83.9) for ROL1 and ROL2, respectively, while the 3-yr-PFS rates were 92% (95%CI: 83.1-95.9) for ROL1 and 72% (95%CI: 62.0-79.6) for ROL2 (p=0.0012). As for recurrence, 1-yr-RFS and 3-yr-RFS were 86% (95%CI: 76.8-92.3) and 73% (95%CI: 61.5-81.9) for ROL1 and 66% (95%CI: 56.5-73.4) and 52% (95%CI: 40.7-62.0) for ROL 2, respectively. We found a significant statistical difference in time to recurrence between ROL1 and ROL2 (p=0.0167).
At univariate Cox regression analysis, ROL emerged as a significant predictor of tumor progression (HR 3.53; CI 95% 1.56 – 7.99; p<0.01). This was confirmed at multivariate analysis (HR 2.90; CI 95% 1.25 – 6.75; p=0.01) (
Table 3). At KM estimates for PFS (
Figure 3), we prospectively confirmed ROL significant correlation with progression (p=0.0012) (
Figure 3b). Additionally, ROL reached significance also for RFS (p=0.0167) (
Figure 3c).
4. Discussion
Over the last decades, some clinical, pathological and molecular features have shown a reliable negative predictive role and are currently used in the risk stratification of these patients [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. Among the investigated histological characteristics, the value of assessing LP invasion has been extensively debated. The main concerns regarded interobserver variability and diagnostic pitfalls in staging superficial urothelial carcinoma in TURBT specimens, such as poor orientation, tangential sectioning, thermic injury, iatrogenic changes or deceptive features like involvement of von Brunn’s nests, brisk inflammation and pseudo-invasion [
30,
31,
32,
33].
Eventually, the prognostic value of assessing the extent of LP invasion in TURBT specimens recently reached a consensus in the scientific community. In keeping with this achievement, the authors of the newest WHO classification strongly recommend conveying the extent of LP invasion in the pathology reports using any of the methods
proposed in the literature over the last years [
13,
14,
15,
16,
17,
18,
19,
20,
21], since a gold standard method has not been validated yet [
26,
27]. One of the most applied approaches is the anatomy-based method that uses
muscularis mucosae layer as a landmark to produce a substaging system (T1a/b/c or T1a/b) [
18]. Nevertheless, the evaluation of
muscularis mucosae in TURBT specimens is often challenging. Although some clues have been identified in the definition of the muscle layers and LP of the urinary bladder, lack of orientation of the fragments, hyperplastic
muscularis mucosae hardly distinguishable from
muscularis propria, and anatomic variations limit the reproducibility of this method [
20]. In contrast, micrometric systems measuring the maximum extent of LP invasion at any direction overcome the anatomic issues and proved clinical significance. Interestingly, a recent retrospective study conducted on 73 patients compared 6 different substaging methods and showed that reporting the extent and/or the number of invasive foci represented the most practical approach not conditioned by orientation or artifacts [
34]. The one proposed by Van Rhijn et al. applies a 0.5 mm cut-off to classify tumors in T1m (microinvasive) and T1e (extensively invasive) [
21]. Bertz et al. employed the same approach [
22], but the micrometric systems that appeared to be the most feasible for standardized use were those employing the cut-off of 1.0 mm (23–25). Recently, de Jong et al. showed that T1 substaging (T1m/e) was an independent predictor of high-grade recurrence-free survival and progression-free survival in 264 patients treated with intravesical BCG [
35].
In this setting, ROL system, developed by our group, is a very simple micrometric approach, based on a 1.0 mm cut-off, with a more detailed and objective definition of the extent of LP invasion assessment, favoring reproducibility. Based on our experience, we believed that the daily practice might benefit from the possibility to adopt a 20x HPF diameter as a simplified threshold. In our large retrospective series of 314 patients with pT1 HG BC after TURBT, ROL impact on survival was compared to the anatomy-based approach (T1a/b) and the van Rhijn method (T1m/e). ROL and T1m/e were feasible in all cases, in contrast to T1a/b with only 72.3%, mainly due to the difficult identification of
muscularis mucosae in the specimen. ROL system alone correlated with PFS, while none of them predicted RFS [
28]. Remarkably, in 2018 we reported similar results for a multi-institutional retrospective series of 250 transitional pT1 HG BC, with ROL and van Rhijn systems being applicable in 99.6% of cases, whereas the feasibility of the anatomic approach was 76%. Consistently with the previous study, no system correlated with recurrence and ROL was the only statistically significant predictor of progression [
29]. These results were limited by the retrospective design of the studies. Therefore, we decided to conduct a prospective study aiming to confirm ROL predictive value for progression, adopting ROL system from 2016 to 2020.
We here reported results of a prospective validation of ROL system on a mono-institutional series of 222 primary pT1 HG urothelial carcinomas of the bladder treated with BCG [
36]. ROL confirmed its high feasibility since it was applicable in 100% of cases. In retrospective studies, ROL was a significant predictor of progression at univariable analysis [
28,
29]. In this study, this evidence was supported for the first time by a reliable multivariate regression analysis (HR 2.90, p=0.01). Importantly, ROL independently and significantly predicted progression also when including LVI in the analysis, which showed strong statistical significance in univariable analysis for progression (HR 3.55, p<0.01). At KM estimates we prospectively confirmed that ROL significantly correlates with PFS (p=0.0012). In addition, and in contrast with our previous findings, our results show a significant correlation also with RFS (p=0.0167). Possibly, our results benefit from the prospective nature of the study and the consequent more accurate data registration and follow-up.
The study is not devoid of limitations. First, the study involved a tertiary university hospital with a dedicated genitourinary pathology service. Consequently, it is impossible to draw any conclusions on the replicability of the ROL system on a daily basis among not-dedicated pathologists. Therefore, a multi-institutional prospective study is needed. Second, several T1 HG BC patients were treated with immediate RC and thus were not included in the study. This could represent a selection bias that may have excluded patients with very adverse outcomes. Furthermore, the decision to perform RC or bladder-sparing therapies following BCG failure was at the discretion of the clinician and, therefore, not standardized. Updated results after a longer FUP time may further confirm our findings. Moreover, it could be helpful trying to identify any difference in ROL application between “en bloc” and fragmented specimens, to date considered together, as well as any prognostic impact of separating ROL2 cases with small multiple foci of LP invasion extending for >1 mm from cases with a massive LP-invasion, sometimes close to
muscularis propria [
37]. From a technical point of view, although not applied in daily practice, it could be intriguing to analyze borderline cases (such as ROL1 with LP invasion close to 1 mm) through multiple sections at different levels in tumor blocks: the possible uncovering of a larger LP invasion might result in a substaging shift from ROL1 to ROL2. Additionally, ongoing studies are attempting to correlate pT1 substaging with the molecular subtypes included in the newest WHO classification, aiming to identify further predictors of progression and recurrence [
38].
5. Conclusions
In conclusion and in keeping with the suggestions of the newest WHO classification (17), we encourage the application of ROL system for reporting the extent of LP invasion and for substaging pT1HGBC. ROL is a simple and feasible method that might identify high risk patients, and eventually improve risk stratification and urological decision making.
Author Contributions
Conceptualization, P.C., R.H. and M.V.; writing—original draft preparation, M.V.; review and editing, R.C., M.V., P.C., R.H.; collecting data, M.C, G.M.E., C.D.C. and A.B; methodology, R.C. and V.F.; analysis and validation M.V., R.C, P.C., R.H. and M.L.; data curation, M.V., R.C., C.S., A.G., P.A., V.D. and G.T; supervision L.M.T, P.C and R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of IRCCS Humanitas Clinical and Research Hospital.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, due to ethical considerations related to the privacy of medical data of patients included in this study.
Acknowledgments
The sturdy has been in part presented as oral presentation at European Congress of pathology, Basel 2022 and European Congress of Urology, Amsterdam 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Malavaud B. T1G3 bladder tumours: The case for radical cystectomy. Eur Urol. 2004;45(4):406–10.
- Contieri R, Hurle R, Paciotti M, Casale P, Saita A, Porpiglia F, et al. Accuracy of the European Association of Urology (EAU) NMIBC 2021 scoring model in predicting progression in a large cohort of HG T1 NMIBC patients treated with BCG. Minerva Urol Nephrol. 2022 Oct.
- De Carlo C, Valeri M, Corbitt DN, Cieri M, Colombo P. Non-muscle invasive bladder cancer biomarkers beyond morphology. Vol. 12, Frontiers in Oncology. Frontiers Media S.A.; 2022.
- Kamat AM, Flaig TW, Grossman HB, Konety B, Lamm D, O’Donnell MA, et al. Expert consensus document: Consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Vol. 12, Nature Reviews Urology. Nature Publishing Group; 2015. p. 225–35.
- Pellucchi F, Freschi M, Moschini M, Rocchini L, Maccagnano C, Nazareno S, et al. Oncological predictive value of the 2004 World Health Organisation grading classification in primary T1 non-muscle-invasive bladder cancer. A step forward or back? BJU Int. 2015 Feb 1;115(2):267–73.
- Martin-Doyle W, Leow JJ, Orsola A, Chang SL, Bellmunt J. Improving selection criteria for early cystectomy in high-grade T1 bladder cancer: A meta-analysis of 15,215 patients. Vol. 33, Journal of Clinical Oncology. American Society of Clinical Oncology; 2015. p. 643–50.
- Soria F, Dutto D, Gontero P. Clinical and biological markers for risk-stratification of T1 high-grade non-muscle invasive bladder cancer. Vol. 32, Current Opinion in Urology. Lippincott Williams and Wilkins; 2022. p. 517–22.
- Mariappan P, Fineron P, O’Donnell M, Gailer RM, Watson DJ, Smith G, et al. Combining two grading systems: the clinical validity and inter-observer variability of the 1973 and 2004 WHO bladder cancer classification systems assessed in a UK cohort with 15 years of prospective follow-up. World J Urol. 2021 Feb 1;39(2):425–31.
- Martini A, Afferi L, Zamboni S, Schultz JG, Lonati C, Mattei A, et al. Oncologic Surveillance for Variant Histology Bladder Cancer after Radical Cystectomy. J Urol. 2021 Oct 1;206(4):885–93.
- Lonati C, Baumeister P, Afferi L, Mari A, Minervini A, Krajewski W, et al. Survival Outcomes After Immediate Radical Cystectomy Versus Conservative Management with Bacillus Calmette-Guérin Among T1 High-grade Micropapillary Bladder Cancer Patients: Results from a Multicentre Collaboration. Eur Urol Focus. 2022 Sep 1.
- Park J, Song C, Hong JH, Park BH, Cho YM, Kim CS, et al. Prognostic significance of non-papillary tumor morphology as a predictor of cancer progression and survival in patients with primary T1G3 bladder cancer. World J Urol. 2009;27(2):277–83.
- Tilki D, Shariat SF, Lotan Y, Rink M, Karakiewicz PI, Schoenberg MP, et al. Lymphovascular invasion is independently associated with bladder cancer recurrence and survival in patients with final stage T1 disease and negative lymph nodes after radical cystectomy. BJU Int. 2013 Jun;111(8):1215–21.
- Yafi FA, Aprikian AG, Chin JL, Fradet Y, Izawa J, Estey E, et al. Impact of concomitant carcinoma in situ on upstaging and outcome following radical cystectomy for bladder cancer. World J Urol. 2014 Oct 1;32(5):1295–301.
- Chade DC, Shariat SF, Adamy A, Bochner BH, Donat SMH, Herr HW, et al. Clinical outcome of primary versus secondary bladder carcinoma in situ. J Urol. 2010;184(2):464–9.
- Sylvester RJ, Rodríguez O, Hernández V, Turturica D, Bauerová L, Bruins HM, et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non–muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel[Formula present. Eur Urol. 2021;79(4):480–8.
- Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75–94.
- WHO Classification of Tumours Editorial Board. Urinary and male genital tumours. Lyon (France): International Agency for Research on Cancer; 2022. (WHO classification of tumours series 5th ed.; vol. 8). https://publications.iarc.fr. No Title.
- Orsola A, Trias I, Raventós CX, Español I, Cecchini L, Búcar S, et al. Initial high-grade T1 urothelial cell carcinoma: Feasibility and prognostic significance of lamina propria invasion microstaging (T1a/b/c) in BCG-treated and BCG-non-treated patients. Eur Urol. 2005;48(2):231–8.
- Van Der Aa MNM, Van Leenders GJLH, Steyerberg EW, Van Rhijn BW, Jöbsis AC, Zwarthoff EC, et al. A new system for substaging pT1 papillary bladder cancer: A prognostic evaluation. Hum Pathol. 2005 Sep;36(9):981–6.
- Paner GP, Ro JY, Wojcik EM, Venkataraman G, Datta MW, Amin MB. Further Characterization of the Muscle Layers and Lamina Propria of the Urinary Bladder by Systematic Histologic Mapping Implications for Pathologic Staging of Invasive Urothelial Carcinoma. 2007.
- Van Rhijn BWG, Van Der Kwast TH, Alkhateeb SS, Fleshner NE, Van Leenders GJLH, Bostrom PJ, et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol. 2012;61(2):378–84.
- Bertz S, Denzinger S, Otto W, Wieland WF, Stoehr R, Hofstaedter F, et al. Substaging by estimating the size of invasive tumour can improve risk stratification in pT1 urothelial bladder cancer-evaluation of a large hospital-based single-centre series. Histopathology. 2011;59(4):722–32.
- Cheng BL, Neumann RM, Weaver AL, Spotts BE, Bostwick DG. P r e di ctin g Ca n ce r P rog r e s s i o n i n Pa t i e n t s W i t h S t a g e T 1 Bla dde r C a r c ino m a. J Clin Oncol. 1999;17(10):3182–7.
- Chang WC, Chang YH, Pan CC. Prognostic significance in substaging of T1 urinary bladder urothelial carcinoma on transurethral resection. Am J Surg Pathol. 2012;36(3):454–61.
- Brimo F, Wu C, Zeizafoun N, Tanguay S, Aprikian A, Mansure JJ, et al. Prognostic factors in T1 bladder urothelial carcinoma: The value of recording millimetric depth of invasion, diameter of invasive carcinoma, and muscularis mucosa invasion. Hum Pathol [Internet]. 2013;44(1):95–102 . [CrossRef]
- Paner GP, Montironi R, Amin MB. Challenges in Pathologic Staging of Bladder Cancer: Proposals for Fresh Approaches of Assessing Pathologic Stage in Light of Recent Studies and Observations Pertaining to Bladder Histoanatomic Variances. Adv Anat Pathol. 2017;24(3):113–27.
- Compérat E, Amin MB, Berney DM, Cree I, Menon S, Moch H, et al. What’s new in WHO fifth edition – urinary tract. Histopathology. 2022;81(4):439–46.
- Patriarca C, Hurle R, Moschini M, Freschi M, Colombo P, Colecchia M, et al. Usefulness of pT1 substaging in papillary urothelial bladder carcinoma. Diagn Pathol [Internet]. 2016;11(1):1–9. [CrossRef]
- Colombo R, Hurle R, Moschini M, Freschi M, Colombo P, Colecchia M, et al. Feasibility and Clinical Roles of Different Substaging Systems at First and Second Transurethral Resection in Patients with T1 High-Grade Bladder Cancer. Eur Urol Focus. 2018;4(1):87–93.
- Van Der Meijden A, Sylvester R, Collette L, Bono A, Ten Kate F. The role and impact of pathology review on stage and grade assessment of stages Ta and T1 bladder tumors: A combined analysis of 5 European Organization for Research and Treatment of Cancer trials. J Urol. 2000;164(5):1533–7.
- Bol MGW, Baak JPA, Buhr-Wildhagen S, Kruse AJ, Kjellevold KH, Janssen EAM, et al. Reproducibility and prognostic variability of grade and lamina propria invasion in stages Ta, T1 urothelial carcinoma of the bladder. J Urol. 2003;169(4):1291–4.
- Compérat E, Egevad L, Lopez-Beltran A, Camparo P, Algaba F, Amin M, et al. An interobserver reproducibility study on invasiveness of bladder cancer using virtual microscopy and heatmaps. Histopathology. 2013;63(6):756–66.
- Raspollini MR, Montironi R, Mazzucchelli R, Cimadamore A, Cheng L, Lopez-Beltran A. pT1 high-grade bladder cancer: histologic criteria, pitfalls in the assessment of invasion, and substaging. Vol. 477, Virchows Archiv. Springer; 2020. p. 3–16.
- Budina A, Farahani SJ, Lal P, Nayak A. Subcategorization of T1 Bladder Cancer on Biopsy and Transurethral Resection Specimens for Predicting Progression. Arch Pathol Lab Med. 2022;146(9):1131–9.
- de Jong FC, Hoedemaeker RF, Kvikstad V, Mensink JTM, de Jong JJ, Boevé ER, et al. T1 Substaging of Nonmuscle Invasive Bladder Cancer is Associated with bacillus Calmette-Guérin Failure and Improves Patient Stratification at Diagnosis. J Urol. 2021 Mar 1;205(3):701–8.
- Valeri M, Contieri R, Fasulo V, Cieri M, Elefante G, De Carlo C, et al. A novel pT1 substaging system for high-grade urothelial bladder carcinoma: a prospective mono-institutional confirmatory progression risk analysis. Virchows Archiv. 2022 Sep 1 (Vol. 481, No. SUPPL 1, pp. S20-S21).
- Hurle R, Casale P, Lazzeri M, Paciotti M, Saita A, Colombo P, et al. En bloc re-resection of high-risk NMIBC after en bloc resection: results of a multicenter observational study. World J Urol. 2020 Mar 1;38(3):703–8.
- De Carlo C, Valeri M, Rudini N, Zucali PA, Cieri M, Elefante GM, et al. Intratumoral Switch of Molecular Phenotype and Overall Survival in Muscle Invasive Bladder Cancer. Cancers (Basel). 2022;14(13).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).