1. Introduction
The tibialis anterior muscle (TA) is divided into anterior and posterior fibers. The anterior fibers of the TA originate from the lateral condyle and anterior border of the tibia, while the posterior fibers of the TA originate from the anterolateral surface of the tibia and the anterior surface of the interosseous membrane. The TA transitions to a flat tendon while descending to the anterolateral surface of the tibia, passing under the extensor retinaculum at the anterior, lower end of the lower leg, then inserting onto the medial cuneiform bone (MCB) and first metatarsal bone (1MB) [
1]. The TA is the largest muscle in the anterior compartment of the lower leg, accounting for over 60% of dorsiflexor volume and playing an important role in human movement [
2]. The TA contributes to inversion and dorsiflexion of the ankle joint and is involved in maintaining the medial arch of the foot [
3]. During locomotion, the TA is active at heel strike and during the swing phase to control foot drop and prevent tripping, respectively [
4]. Activity of the TA increases with walking speed and decreases with the switch to running [
4]. The TA is one of the most important muscles in daily life because it is deeply involved in human movement [
5].
Rupture of the TA tendon (TAT) is a rare injury that is commonly diagnosed late due to mild clinical signs and symptoms [
6]. Despite disruption of the TAT being a rare condition, this pathology is the third most common form of tendon rupture in the lower limb, after the Achilles tendon and patellar tendon [
7]. Surgical reconstruction of the TAT is the treatment of choice in cases with severe impairment of dorsal extension and supination of the foot [
8]. Different techniques have been reported according to the severity of tendon injury or gap formation. To restore the natural lever arm of the TA, the TAT must be reinserted at the anatomical footprint [
9]. Precise anatomical description of ligament and tendon attachments is therefore crucial and can help optimize reconstruction procedures in terms of anchor placement and graft sizing. Surgical preparation of the tendon at its insertion necessitates knowledge of the anatomy [
10].
The anatomy of the TAT has long been reported in previous studies using cadavers. Regarding the attachment of the TAT to bone, types with one, two, and three fiber bundles have been reported [
8,
11,
12,
13,
14,
15,
16], with the two-bundle type reported as the most frequent [
8,
11,
12,
14,
15]. Regarding the type with a single bundle of fibers, Musial et al. [
14] and Willegger et al. [
8] found no types with a single fiber bundle in their studies of 122 and 41 fixed cadavers, respectively. However, Karauda et al. [
13] examined 100 pairs of fetal fixed cadavers and reported the one fiber bundle type as the most frequent, at 60%. For the type with three fiber bundles, Brenner et al. [
11] and Willegger et al. [
8] reported that these were not present, while Olewnik et al. [
12] and Karauda et al. [
13] reported frequencies of 2% and 4%, respectively. Such findings suggest that the frequencies of TAT attachment types remain controversial. In addition, Olewnik et al. [
12] suggested that TAT attachment types may vary by ethnicity. Further, very few studies have examined the footprint (attachment site area) of the TAT, and most studies are limited to examinations of the width and thickness of the TAT and the shape of the footprint [
8,
11,
12,
13,
14,
15,
16]. A previous study [
8] examining the footprint of the TAT reported that the mean attachment site area to MCB was 71.5 mm2 (range, 20.1–151.0 mm2) and that to 1MB was 48.1 mm2 (range, 18.5–97.0 mm2) for the type with two bundles of the TAT. However, measurement of the attachment site area in that previous study [
8] was limited to two-dimensional measurement. Since the surfaces of the MCB and 1MB, as the attachment sites of the TAT, comprise curved surfaces, measurement of attachment surface areas should be performed in three dimensions.
The purpose of this study was to clarify the attachment types of the TAT in Japanese fixed cadavers and to determine the attachment site area in three dimensions. The hypothesis for this study was that the proportions of type classifications would differ from those of previous studies, and that the area of attachment to bone would be larger than in previous studies.
2. Materials and Methods
2.1. Cadavers
We examined 100 feet from 50 Japanese cadavers (mean age at death, 80 ± 11 years; 56 sides from 28 men, 44 sides from 22 women; 50 right sides, 50 left sides) that had been switched to alcohol after placement in 10% formalin. No legs showed any sign of previous major surgery around the foot or ankle. This investigation was conducted with the approval of the ethics committee at our institute (approval no. 18867). This study complied with the Declaration of Helsinki and was conducted after informed consent was obtained from all donor families.
2.2. Measurement procedures
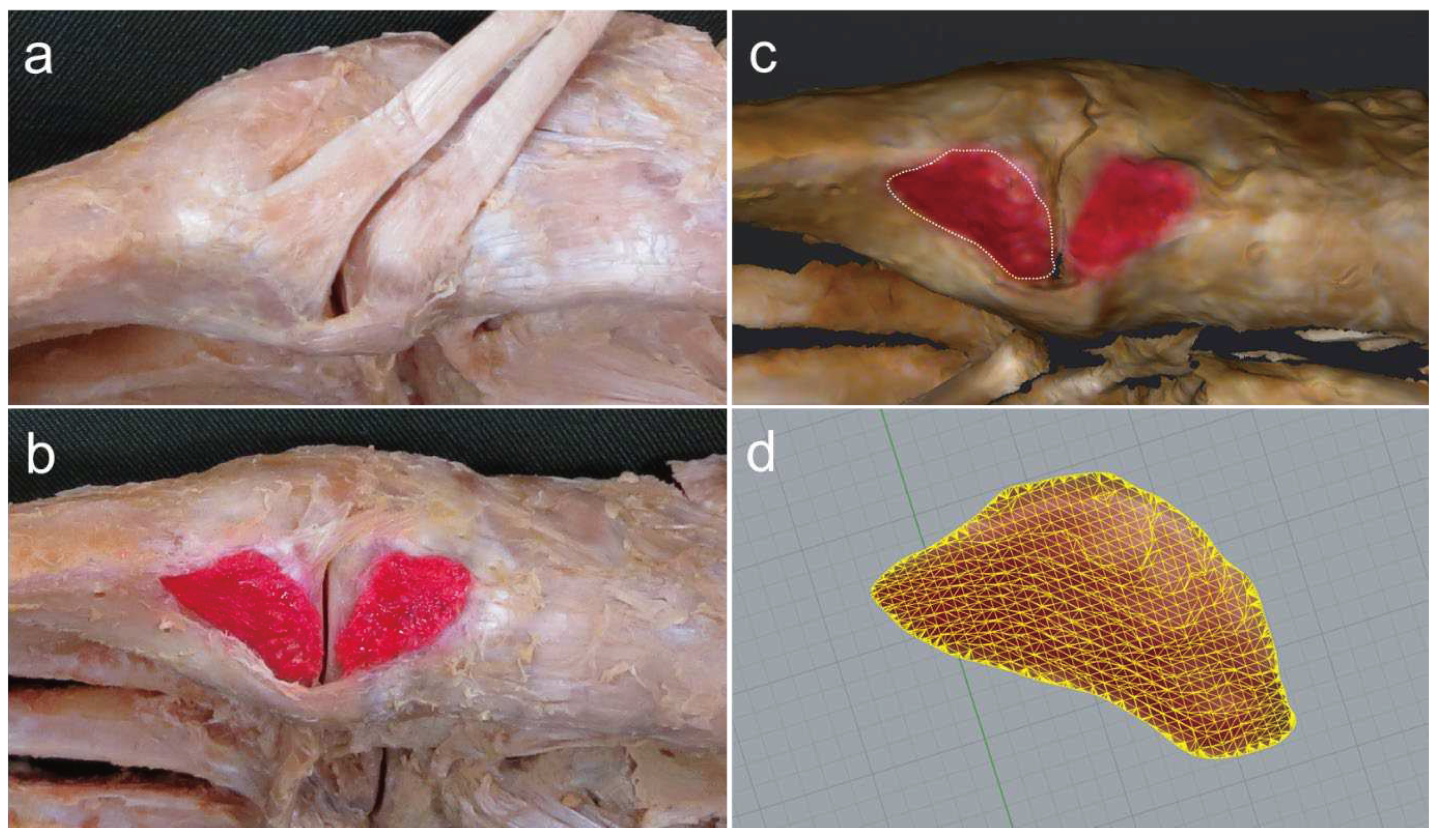
The procedure for dissecting the TAT is described below. Isolated specimens of the leg were created by transection about 10 cm above the ankle joint. The skin and subcutaneous tissue were removed, the TAT was carefully dissected out and the attachment to the bone was confirmed (
Figure 1a). The TAT was classified according to differences in the number of fiber bundles as: Type I, with one fiber bundle; Type II, with two fiber bundles; and Type III, with three fiber bundles. The attachment site area was identified by peeling away any adherent tissue, then coloring the attachment site with a pencil (
Figure 1b). The surface area was then measured using a three-dimensional (3D) scanner (EinScan Pro HD, measurement precision according to manufacturer, 0.04 mm; SHINING 3D, Hangzhou, China) to produce a 3D foot sample. The resulting data were read into Geomagic Freeform 2021 design software (3D SYSTEMS), and the boundary of the attachment site was drawn as a curve with a pen-type device (Touch; 3D SYSTEMS) (
Figure 1c). Surface area was then calculated using Rhinoceros7 3D software (McNeel) (
Figure 1d). All measurements were performed by the same physical therapist (T.H.).
The reliability of surface area measurement by 3D scanner was calculated using the intraclass correlation coefficient (ICC) (1,1) for 10 of the 100 feet, yielding an ICC of 0.98. Surface area measurements in the present study showed almost perfect reliability, which is consistent with previous results [
17].
2.3. Statistical analysis
Statistical analyses were performed using SPSS version 24.0 (SPSS Japan, Tokyo, Japan). For Type II (with two fiber bundles), the difference in attachment site area between the fiber bundle attached to the MCB and that attached to the 1MB was compared using paired t-tests. A significance level of 5% was used.
3. Results
3.1. Type classification by number of TAT fiber bundles
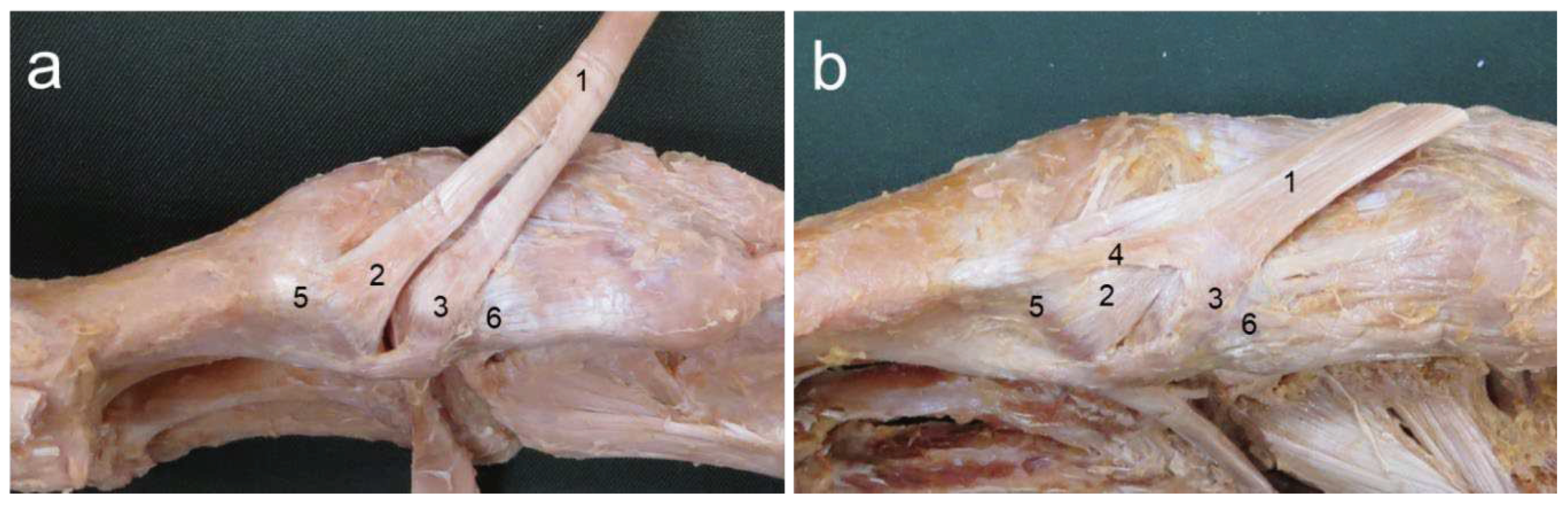
No feet showed Type I (with one fiber bundle). Type II (with two fiber bundles) was seen in 95 legs (95%) and Type III (with three fiber bundles) was seen in 5 legs (5%) (
Figure 2).
3.2. Surface area of TAT attachment site
In Type II, mean attachment site area for the MCB was 85.2 ± 18.2 mm2, and that for 1MB was 72.4 ± 19.0 mm2. Mean attachment site area to MCB was significantly larger than that to 1MB (p < 0.01).
4. Discussion
The purpose of this study was to clarify the attachment types of the TAT in Japanese fixed cadavers and to determine 3D attachment site areas. To the best of our knowledge, this represents the first study to clarify the type of classification by number of TAT fiber bundles using Japanese cadavers and 3D attachment site area.
In this study, for type classification by the number of TAT fiber bundles, Type I (with one fiber bundle) was not present, while Type II (with two fiber bundles) was present in 95 pairs (95%) and Type III (with three fiber bundles) in 5 pairs (5%). Type II was definitively the most common result.
Type classification by the number of TAT fiber bundles has long been reported in many cadaveric studies [
8,
11,
12,
13,
14,
15,
16]. For Type I, Olewnik et al. [
12] examined the attachment site area of the TAT using 100 fixed Caucasian cadavers and reported that 32 cadavers (32%) showed a single bundle of fibers. Karauda et al. [
13] also examined TAT attachment sites using 100 European fetal remains and reported that 60 fetuses (60%) showed a single bundle of fibers, a result differing markedly from that of the present study. As for Type II, other than the previous study using European fixed fetal cadavers [
13], the type with two bundles was uniformly reported as the most common, as in the present study [
8,
11,
12,
14,
15]. Type III was reported as either absent [
8,
11] or present in only very small numbers (2–4%) [
12,
13]. Furthermore, variations in the attachment of foot and ankle muscles such as the tibialis posterior, peroneus longus, and extensor digitorum longus muscles may vary by ethnicity [
18,
19,
20,
21]. The present findings thus suggest the possibility of ethnic differences in TAT attachment types and suggest that the TAT attachment type in Japanese is highly likely to be Type II (with two fiber bundles), with rare cases of Type III (with three fiber bundles).
Mean attachment site areas for the TAT in the present study were 85.2 ± 18.2 mm
2 and 72.4 ± 72.4 mm
2 for MCB and 1MB, respectively, showing a significantly larger area of fiber bundle attachment to the MCB. Willegger et al. [
8] measured the attachment site area of the TAT by photographing the specimen with a camera then making measurements in two dimensions using Image J image analysis software. They reported the attachment site areas for the TAT as 71.5 mm
2 (range, 20.1–151.0 mm
2) and 48.1 mm
2 (range, 18.5–97.0 mm
2) for MCB and 1MB, respectively, again showing a larger attachment site area for MCB than for 1MB. Iwama et al. [
22] examined the attachment site area of the anterior cruciate ligament in the knee using 39 cadaveric knees and stated that the attachment site area could be more appropriately evaluated with a 3D camera or computer modeling software. The attachment site area of the TAT lies along curved and uneven surfaces of bone, so two-dimensional measurements are unlikely to accurately capture the surface area. The attachment site area in the present study was therefore larger than in previous studies, and more accurate measurement of attachment site area was possible because of the appropriate 3D measurements.
A key limitation of this study was that only the attachment site area of the TAT was considered. In the Achilles tendons, the mechanical prosperities of tendons are mainly based on maximum rupture strength and cross-sectional area of the tendon [
23]. However, in this study, tensile strength at breakage was difficult to assess because formalin-fixed cadavers were used. In the future, the mechanical prosperities of the TAT and the effects of different attachment types and attachment site areas of the TAT in vivo on TAT function will need to be examined.
5. Conclusions
The findings of the present study suggest the possibility of ethnic differences in TAT attachment types and suggest that the TAT attachment type in Japanese is most likely to be Type II (with two fiber bundles), with rare cases of Type III (with three fiber bundles). Attachment site area was larger than in previous studies, with more accurate measurement of attachment site area possible with appropriate 3D measurements.
Author Contributions
T.H. contributed to conceptualization, data curation, formal analysis, investigation, methodology, and writing—original draft; M.E. contributed to conceptualization, funding acquisition, supervision, and writing—original draft; R.T. and HO contributed to validation and writing—review and editing; R.S., K.K., M.S., C.S., H.Y., R.H., T.I., H.A., Y.Y. and T.T. contributed to writing—review and editing; I.K. supervised the study and contributed to writing—original draft. All authors read and approved the final manuscript prior to submission.
Funding
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP22K19739.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Niigata University of Health and Welfare (18867-220720).
Informed Consent Statement
Informed consent was obtained from the families of all subjects.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to acknowledge and thank those anonymous individuals who generously donated their bodies so that this study could be performed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kimata, K.; Otsuka, S.; Yokota, H.; Shan, X.; Hatayama, N.; Naito, M. Relationship between attachment site of tibialis anterior muscle and shape of tibia: anatomical study of cadavers. Journal of foot and ankle research 2022, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Roy, R.R.; Shellock, F.G.; Hodgson, J.A.; Day, M.K.; Lee, P.L.; Kwong-Fu, H.; Edgerton, V.R. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 1992, 10, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Basmajian, J.V.; Stecko, G. THE ROLE OF MUSCLES IN ARCH SUPPORT OF THE FOOT. J Bone Joint Surg Am 1963, 45, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.L.; Kram, R. Changing the demand on specific muscle groups affects the walk-run transition speed. The Journal of experimental biology 2008, 211, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Korff, T.; Waugh, C.; Fath, F.; Blazevich, A.J. Tibialis anterior moment arm: effects of measurement errors and assumptions. Med Sci Sports Exerc 2015, 47, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Vosoughi, A.R.; Heyes, G.; Molloy, A.P.; Mason, L.W.; Hoveidaei, A.H. Management of tibialis anterior tendon rupture: Recommendations based on the literature review. Foot and ankle surgery: official journal of the European Society of Foot and Ankle Surgeons 2020, 26, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Harkin, E.; Pinzur, M.; Schiff, A. Treatment of Acute and Chronic Tibialis Anterior Tendon Rupture and Tendinopathy. Foot and ankle clinics 2017, 22, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Willegger, M.; Seyidova, N.; Schuh, R.; Windhager, R.; Hirtler, L. Anatomical Footprint of the Tibialis Anterior Tendon: Surgical Implications for Foot and Ankle Reconstructions. Biomed Res Int 2017, 2017, 9542125. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Bachelier, F.; Fürst, O.A.; Kelm, J. Rupture of the anterior tibial tendon: three clinical cases, anatomical study, and literature review. Foot & ankle international. / American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society 2006, 27, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.B.; Oji, D.E.; Yack, H.J.; Morcuende, J.A. Long-term results of tibialis anterior tendon transfer for relapsed idiopathic clubfoot treated with the Ponseti method: a follow-up of thirty-seven to fifty-five years. J Bone Joint Surg Am 2015, 97, 47–55. [Google Scholar] [CrossRef]
- Brenner, E. Insertion of the tendon of the tibialis anterior muscle in feet with and without hallux valgus. Clin Anat 2002, 15, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Olewnik, Ł.; Podgórski, M.; Polguj, M.; Topol, M. A cadaveric and sonographic study of the morphology of the tibialis anterior tendon - a proposal for a new classification. Journal of foot and ankle research 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Karauda, P.; Podgórski, M.; Paulsen, F.; Polguj, M.; Olewnik, Ł. Anatomical variations of the tibialis anterior tendon. Clin Anat 2021, 34, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Musiał, W. Variations of the terminal insertions of the anterior and posterior tibial muscles in man. Folia Morphol. 1963, 26, 294–302. [Google Scholar]
- Arthornthurasook, A.; Gaew Im, K. Anterior tibial tendon insertion: an anatomical study. J Med Assoc Thai 1990, 73, 692–696. [Google Scholar] [PubMed]
- Zielinska, N.; Tubbs, R.S.; Paulsen, F.; Szewczyk, B.; Podgórski, M.; Borowski, A.; Olewnik, Ł. Anatomical Variations of the Tibialis Anterior Tendon Insertion: An Updated and Comprehensive Review. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Willegger, M.; Seyidova, N.; Schuh, R.; Windhager, R.; Hirtler, L. The tibialis posterior tendon footprint: an anatomical dissection study. J Foot Ankle Res 2020, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, N.; Tubbs, R.S.; Ruzik, K.; Olewnik, Ł. Classifications of the extensor hallucis longus tendon variations: Updated and comprehensive narrative review. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft 2021, 238, 151762. [Google Scholar] [CrossRef]
- Uchiyama, I.; Edama, M.; Yokota, H.; Hirabayashi, R.; Sekine, C.; Maruyama, S.; Shagawa, M.; Togashi, R.; Yamada, Y.; Kageyama, I. Anatomical Study of Sites and Surface Area of the Attachment Region of Tibial Posterior Tendon Attachment. Int J Environ Res Public Health 2022, 19, 16510. [Google Scholar] [CrossRef]
- Edama, M.; Takabayashi, T.; Hirabayashi, R.; Yokota, H.; Inai, T.; Sekine, C.; Matsuzawa, K.; Otsuki, T.; Maruyama, S.; Kageyama, I. Anatomical variations in the insertion of the peroneus longus tendon. Surgical and radiologic anatomy: SRA 2020, 42, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Iwama, G.; Iriuchishima, T.; Horaguchi, T.; Aizawa, S. Measurement of the Whole and Midsubstance Femoral Insertion of the Anterior Cruciate Ligament: The Comparison with the Elliptically Calculated Femoral Anterior Cruciate Ligament Footprint Area. Indian J Orthop 2019, 53, 727–731. [Google Scholar] [CrossRef]
- Doral, M.N.; Alam, M.; Bozkurt, M.; Turhan, E.; Atay, O.A.; Dönmez, G.; Maffulli, N. Functional anatomy of the Achilles tendon. Knee Surg Sports Traumatol Arthrosc 2010, 18, 638–643. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).