1. Introduction
The menstrual cycle is regulated by progesterone and estradiol (E2) and is mainly divided into follicular, ovulatory, and luteal phases [
1]. In a previous study examining the relationship between the menstrual cycle and musculoskeletal disorders in women, anterior cruciate ligament (ACL) injuries were reported to occur more frequently during the ovulatory phase [
2]. In addition, the rate of injury to muscle tendons was reported to be 88% higher in the late follicular phase, when E2 levels are maximal, compared to the follicular phase, and muscle tears, strains, spasms, tendon disorders, and tendon ruptures occur more than twice as frequently in this phase as in other phases [
3]. Understanding the effects of female hormones on the body may be useful for preventing injuries in female athletes.
Regarding the relationship between female hormones and the myotendon structure, expression of E2 receptors has been reported in myofascia [
4], myofibers [
5], and tendons [
6]. E2 has also been reported to acts on the synthesis of type I collagen and fibroblast proliferation, along with the degradation of type I collagen by matrix metalloproteases (MMPs) [
7]. In a previous study in which fibroblasts detached from human thigh fascia were treated with E2 and cultured, when E2 concentration was increased to a level equivalent to that present in humans before ovulation, type I collagen decreased and type III collagen and fibrillin increased, increasing the elasticity of the fascia [
8]. Multiple pathways thus exist for the effect of E2 on myotendons, and different tissues are affected in different ways, suggesting that changes in mechanical properties during the menstrual cycle may vary among individuals. According to the mechanical model of skeletal muscle, musculotendons are classified into contractile elements, serial elastic elements, and parallel elastic elements, with contractile elements composed of muscle, serial elastic elements composed of tendon, and parallel elastic elements composed of fascia [
9]. In a previous in vivo study examining the mechanical properties of human muscle tendons during the early follicular, ovulatory, and luteal phases, maximal isometric voluntary contraction and muscle activation levels of the knee extensors and ankle plantar flexors (contractile elements) were not found to change significantly during the menstrual cycle [
10]. On the other hand, for tendons (elastic elements), differences in evaluation methods and variations in results depending on the measurement site have been reported, and no clear consensus has been obtained.
Recently, the MyotonPRO digital palpation device (Myoton AS, Tallinn, Estonia), which allows objective, noninvasive measurement of mechanical properties, has been used to evaluate muscle mechanical properties [
11,
12,
13]. A study using the MyotonPRO to examine changes in mechanical properties of the thigh musculature during the early follicular, ovulatory, and luteal phases reported that stiffness of the vastus medialis muscle (VM) and semitendinosus muscle (ST) was higher during the ovulatory phase compared to the luteal phase, while the vastus lateralis muscle (VL) and biceps femoris muscle (BF) did not change with the menstrual cycle [
11]. These findings suggest that changes in the mechanical properties of muscle during the menstrual cycle may be site-dependent. To the best of our knowledge, the mechanical properties of tendons during the menstrual cycle have not been examined using MyotonPRO. A previous study examining changes in the mechanical properties of lower leg muscle groups during the early follicular and ovulatory phases reported no changes during the menstrual cycle to the stiffness of the peroneus longus muscle (PL), tibialis anterior muscle (TA) or medial head of the gastrocnemius muscle (MG) [
12,
13]. Examination of the muscles and tendons of the thigh and lower leg during the same menstrual cycle is therefore needed to clarify changes in the mechanical properties of the muscle-tendon complex.
The purpose of this study was to examine changes in the mechanical properties of the muscles and tendons of the anterior aspect of the thigh and posterior aspect of the lower leg during the early follicular and ovulatory phases among female university students, and to determine correlations between muscles and tendons. We hypothesized that the mechanical properties of the thigh and lower leg musculotendons would change during the ovulatory phase compared to the early follicular phase, and that correlations would exist between the mechanical properties of the musculotendons.
2. Materials and Methods
2.1. Subjects
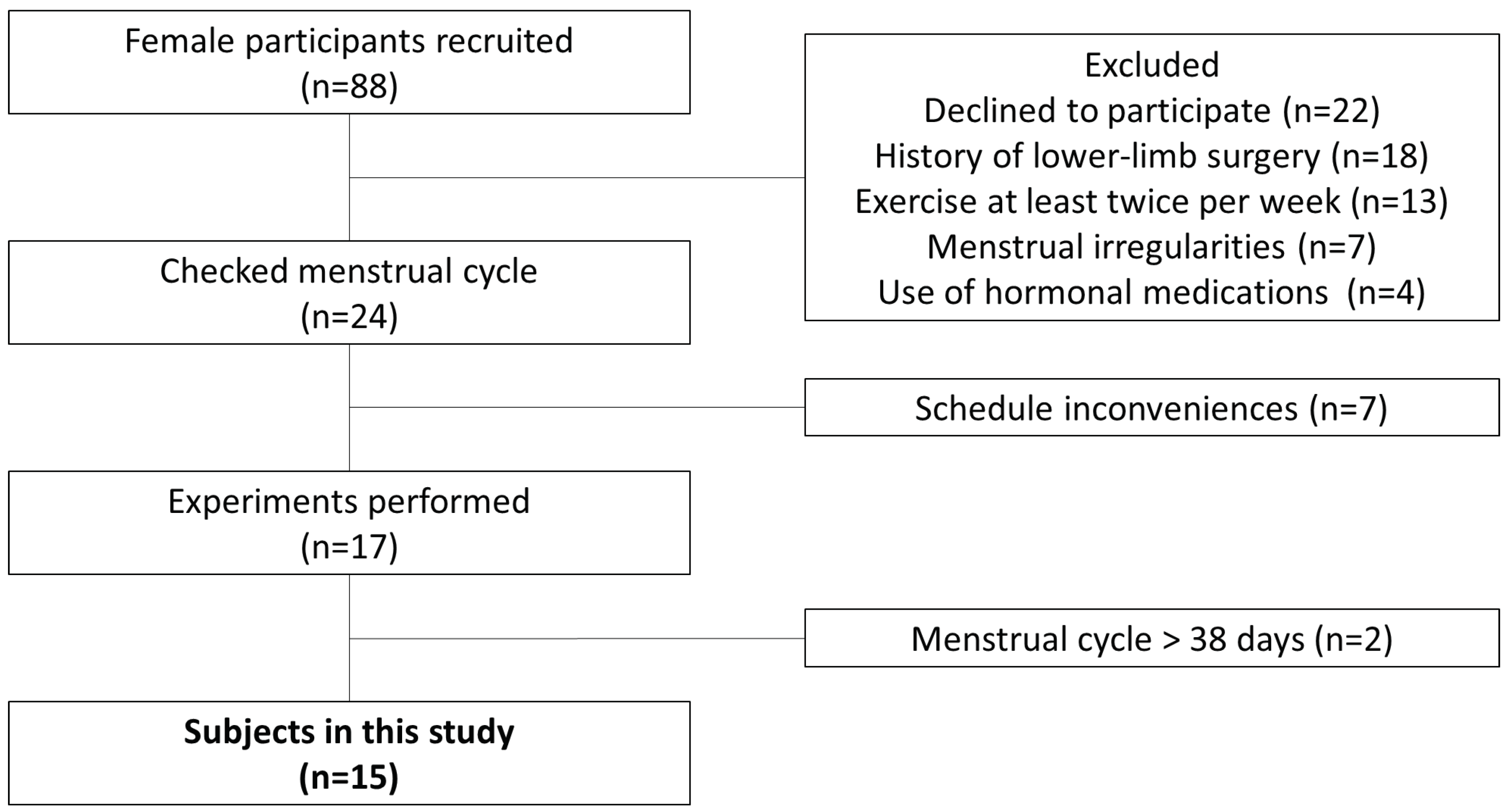
A questionnaire was administered to 88 female university students. Inclusion criteria were as follows: 1) menstruation approximately 10 times/year, with a menstrual cycle of 25–38 days [
14]; 2) no membership in a designated strength club or athletic club and no current history of exercise more than twice a week [
15]; 3) no use of oral contraceptives or other hormonal agents within the past 6 months [
16]; and 4) no history of doctor-diagnosed disorders or surgery on the lower-limb. Fifteen subjects (mean age, 20 ± 0.5 years; height, 159.2 ± 7.1 cm; weight, 55.9 ± 7.5 kg) who met inclusion criteria 1–4 and consented to participate in the study were included (
Figure 1). All study protocols were performed according to the Declaration of Helsinki, after receiving approval from the ethics committee at our institution (approval no. 17946). The study content was fully explained to each subject and provided in written form; informed consent was obtained from all subjects prior to participation in the study.
2.2. Recording the Menstrual Cycle
Subjects were instructed to use a basal body thermometer (CTEB503L electronic thermometer; Citizen Systems Co., Tokyo, Japan) to assess basal body temperature every morning. An ovulation prediction kit (Doctor's Choice One Step Ovulation Test Clear; Beauty and Health Research, Torrance, CA, USA) was used to estimate the day of ovulation, starting the day after the end of period bleeding, and continuing until a positive result was obtained. Subjects were asked to use the ONE TAP SPORTS athlete condition management system (Euphoria Co., Tokyo, Japan) to record the start and end of the period, basal body temperature, and ovulation prediction kit results.
2.3. Timing of Measurement
Salivary E2 concentration and muscle tendon mechanical properties were measured once each in the early follicular phase and once each in the ovulatory phase. The timing of measurement in the early follicular phase was on the second to fourth day after the onset of menstruation, and that in the ovulatory phase was on the second to fourth day after a positive result from the ovulation kit [
14]. To allow for diurnal variations, all measurements were conducted from 08:00 to 12:00. Room temperature was 20–25°C.
2.4. Measurement Methods
2.4.1. Estradiol concentration
E2 concentrations were measured from saliva. Because of the possibility of influences on E2 concentration, subjects were asked to strictly observe the following six points before saliva collection, based on previous studies [
15,
16]: 1) no alcohol consumption for 12 h prior to measurement; 2) no food consumption for 60 min; 3) no brushing the teeth for 45 min; 4) no consumption of dairy products for 20 min; 5) no beverages with high sugar or acid content or caffeine before saliva collection; and 6) in case of dental treatment, no saliva collection within 48 h after the treatment. Subjects were asked to rinse their mouth before starting the experiment, so that no food particles remained in the mouth. Saliva was collected at least 10 min after rinsing the mouth to prevent causing a decrease in E2 concentration. Saliva was collected using a special straw (Saliva Collection Aid; SAL) and placed in a saliva collection vessel (Cryovial; SAL) after collection in the mouth for 1 min. Saliva samples were immediately frozen in a freezer at -80°C or lower. After all samples were collected, E2 concentrations were analyzed by Funakoshi Corporation (Tokyo, Japan). Samples were thawed at room temperature, mixed by vortexing, centrifuged at 1500×g for 15 min, and analyzed by enzyme-linked immunosorbent assay using high-sensitivity salivary immunoassay kits (17β-Estradiol Enzyme Immunoassay Kit and Salivary Progesterone Enzyme Immunoassay Kit; Salimetrics). The dilution factor was uniformly 1-fold (undiluted).
2.4.2. Mechanical properties of muscle and tendon
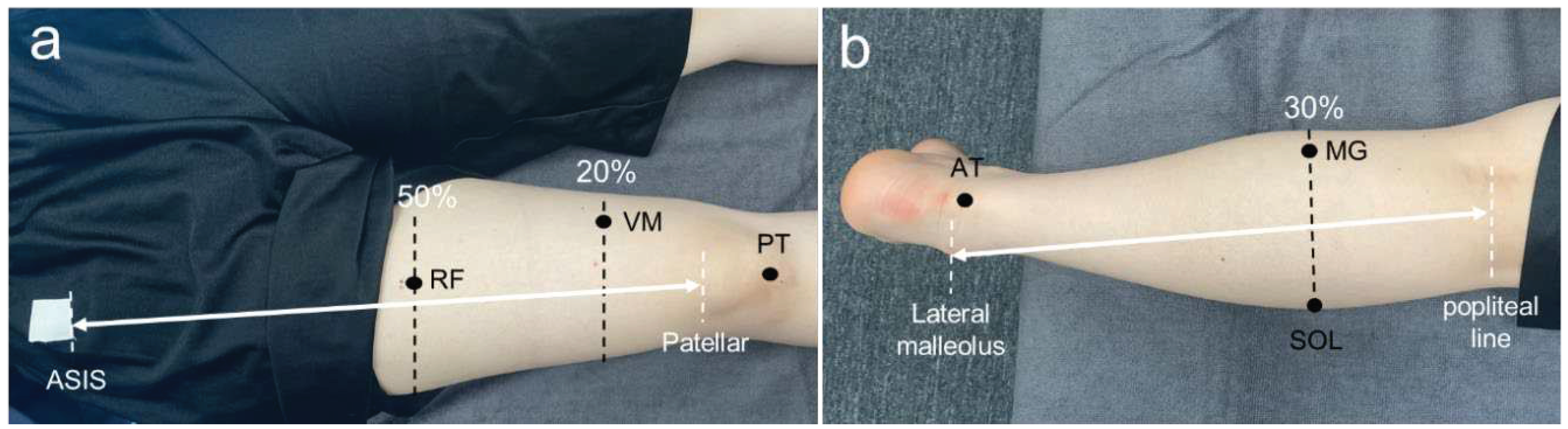
Muscle-tendon mechanical properties were measured at six sites: rectus femoris muscle (RF), VM, patellar tendon (PT), MG, soleus muscle (SOL), and Achilles tendon (AT). Measurement positions were identified using an ultrasound imaging system (Aplio500; Canon Medical Systems Corporation, Tochigi, Japan) and a linear probe (PL0081; Canon Medical Systems Corporation, Tochigi, Japan) with reference to previous studies. RF was identified at 50% of the length from the anterior superior iliac spine (ASIS) to the bottom of the patella [
17], VM was identified at the distal 20% of the length from the ASIS to the bottom of the patella [
18], PT was identified at the midpoint between the patellar apex and tibial rough surface [
19], MG and SOL were identified at the proximal 30% of the length from the orbital skin line to the external capsule [
20], and the AT was identified at 3 cm [
21] proximal to the tendon attachment site (calcaneal tuberosity) (
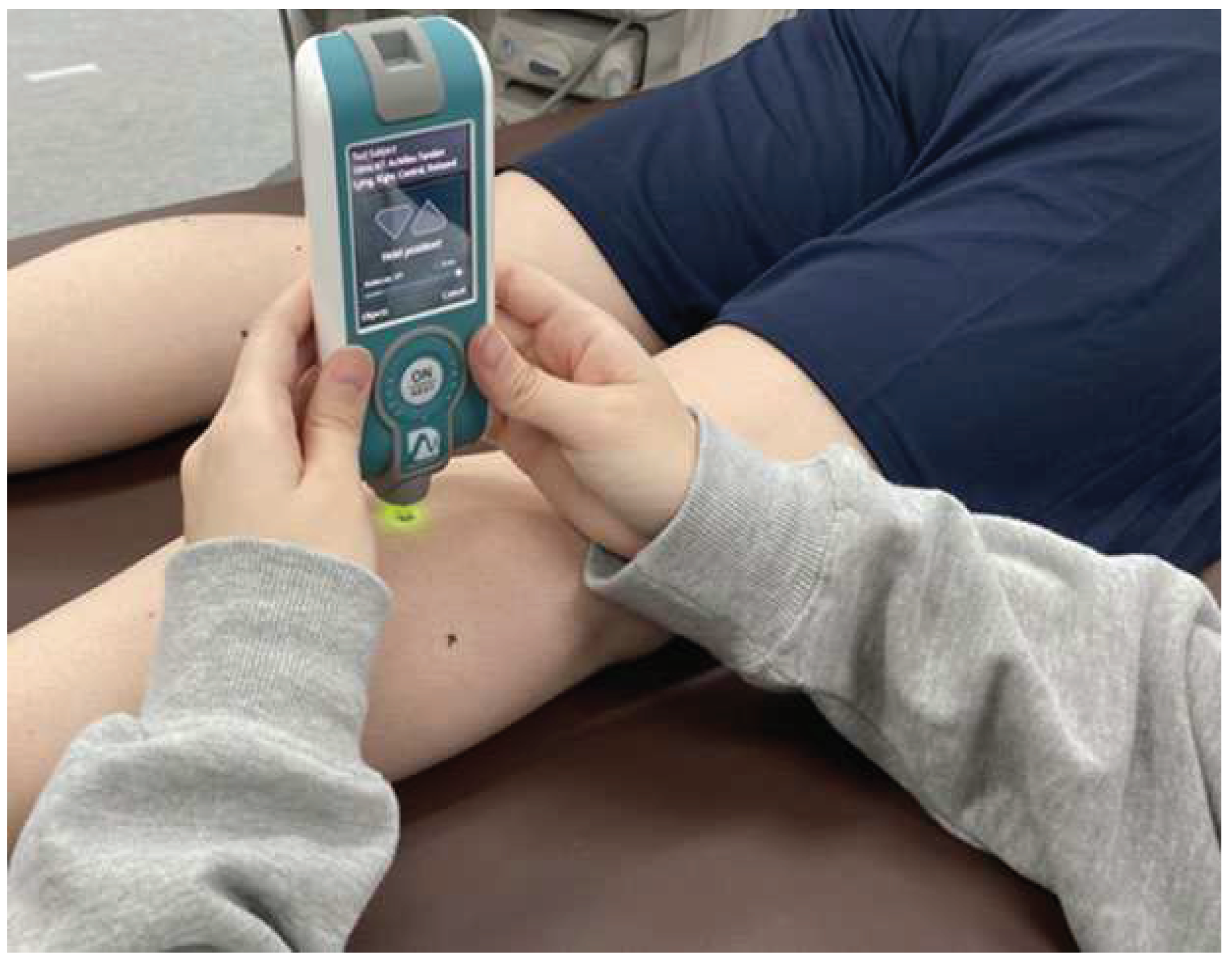
Figure 2). Mechanical properties of the muscles and tendons were measured using MyotonPRO by making a mark with a pen just above the muscle belly or central part of the tendon (
Figure 3). Stiffness was used as a parameter of mechanical properties in this study. The highest and lowest data of five measurements were discarded, then the three remaining measurements were averaged for each site. Measurements were taken with the axial foot (opposite to the foot that kicks a ball). The thigh was measured in the prone position with the knee joint in extension and the lower leg was measured in the supine position with the knee joint in extension and the foot hanging down over the edge of the bed (ankle-joint resting position). The order of measurement was randomized for the lower leg, thigh, and each part of the body. Considering the possibility that changes in position during measurement may affect the values of mechanical properties, subjects were instructed to rest for 10 min after identification of the muscle tendons and after changes in position of the lower leg and thigh.
2.5. Reliability of Measurements
The reliability of measurements of muscle-tendon mechanical properties using My otonPRO was examined in 5 healthy male university students (mean age, 21 ± 0 years; height, 173.6 ± 2.2 cm; weight, 63.3 ± 3.9 kg) with no history of orthopedic disease and 10 feet. Repeat measurements were performed between 2 days and 1 week. Reliability was calculated using the intraclass correlation coefficient (ICC) (1, 3). ICCs were 0.802 for RF, 0.653 for VM, 0.968 for PT, 0.746 for MG, 0.774 for SOL, and 0.835 for AT. According to the criteria of Landis et al. [
22], reliability was considered substantial for an ICC of 0.61–0.80, representing practical reliability.
2.6. Statistical Analysis
Paired t-tests were used to compare E2 concentrations in the early follicular and ov ulatory phases and to compare the mechanical properties of each muscle tendon in the early follicular and ovulatory phases. Pearson's product-moment correlation coefficient was used to correlate thigh and lower leg tendons. Statistical significance was set at the 5% level.
3. Results
E2 concentrations were significantly higher in the ovulatory phase than in the early follicular phase (p = 0.017) (
Table 1). Stiffness of all muscle tendons did not differ significantly between early follicular and ovulatory phases (p > 0.05) (
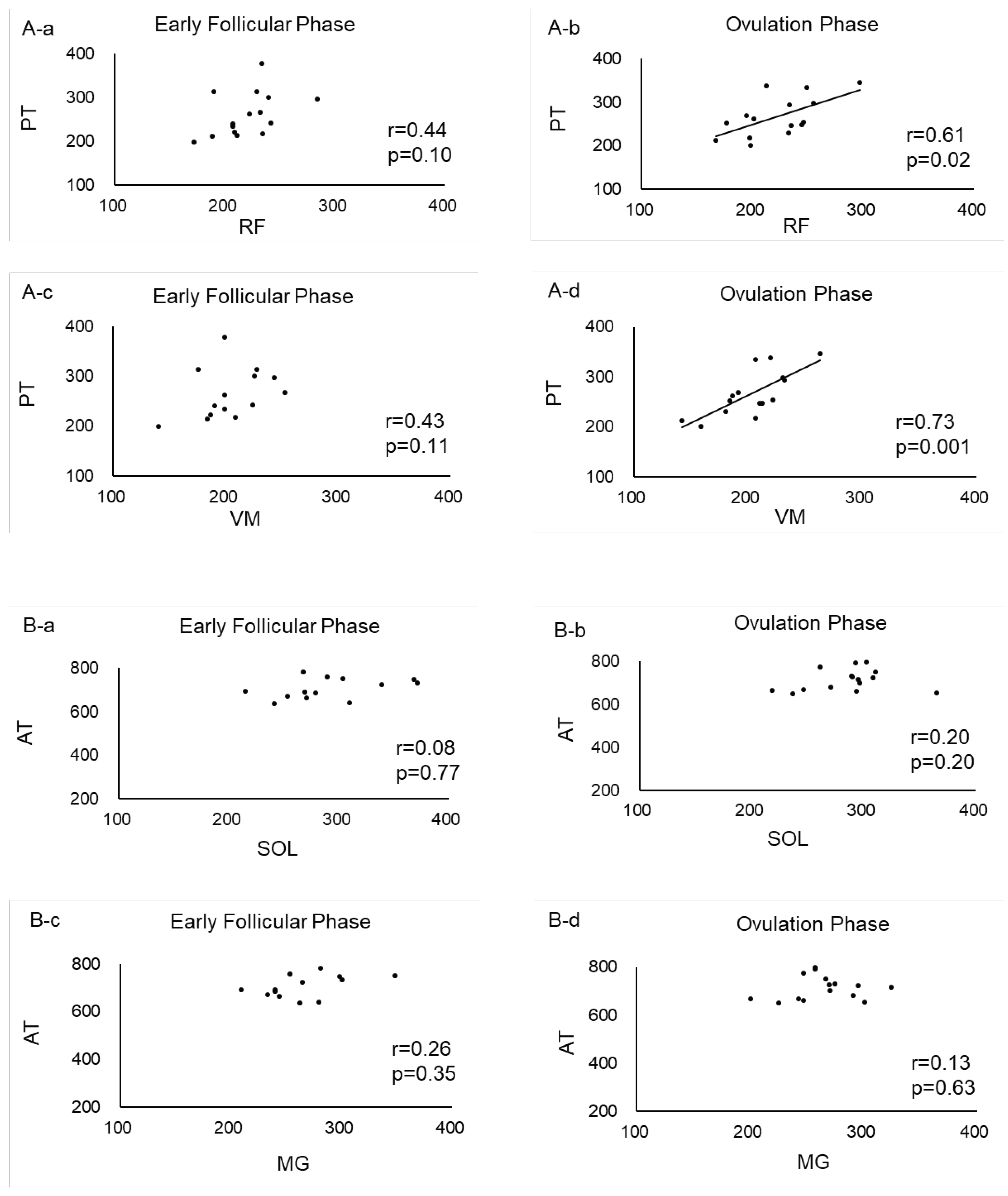
Table 1). No correlation was found between muscle tendons in the lower leg (
Figure 4A). In the thigh, significant positive correlations were found between RF and PT (r = 0.6079, p = 0.0162) and between VM and PT (r = 0.7370, p = 0.0017) during ovulation phase (
Figure 4B).
4. Discussion
This study investigated changes in the mechanical properties of muscles and tendons of the anterior thigh and posterior lower leg among female college students with normal menstrual cycles during the early follicular and ovulatory phases using MyotonPRO. Previous studies have not provided a consistent view of changes in muscle mechanical properties during the menstrual cycle. To the best of our knowledge, this represents the first study to determine the relationships between muscle and tendon mechanical properties during the menstrual cycle using MyotonPRO.
In this study, no significant differences in stiffness of any muscles or tendons were apparent between the early follicular and ovulatory phases. A previous study reported that anterior knee laxity increased during the ovulatory phase compared to the early follicular phase in females with genu recurvatum [
23]. In addition, in the foot, increased elasticity of the plantar fascia during the ovulatory phase has been suggested to increase postural sway and increase the risk of falling [
24]. Furthermore, it has been reported that exercise and mechanical stimulation increase collagen synthesis [
25]. These findings suggest that increased joint laxity in the knee and ankle joints during the ovulatory phase may result in increased load on the musculotendons to compensate for instability and stiffening of the tissues. On the other hand, E2 has been reported to act on the degradation of type I collagen by MMPs, which are degrading enzymes [
7], and tissue softening may be influenced by hormonal changes during the menstrual cycle. Therefore, the response to maintain joint stability may have been counteracted by the action of E2.
In the present study, significant positive correlations were found between RF and PT and between VM and PT during the ovulatory phase. A previous study using MyotonPRO reported that in the thigh muscle group, stiffness of the VM and ST was higher during the ovulatory phase compared to the luteal phase, while VL and BF were unaffected by the cycle [
11]. This suggests that the quadriceps and medial site of hamstrings (ST) may be affected during the ovulatory phase, when E2 concentrations are higher [
11]. On the other hand, in the lower leg muscle group, no periodic changes in stiffness of the PL, TA, or MG were seen [
13]. Previous studies also showed site-specific variations, suggesting that mechanical properties may exhibit site-specific changes.
ACL injuries are reported to occur more frequently in women [
26]. Multiple factors contribute to such differences between sexes, one of which appears to be differences in landing strategy [
27]. Potential energy during one-leg hop performance was reportedly greater in males, but vertical floor reaction force and force loading rate were higher in females, and the ability to attenuate force was decreased [
28]. Stiff landing increases the risk of ACL injury [
29], and the present results may suggest that restriction of smooth knee flexion motion and decreased shock-absorbing capacity occur during ovulation, making the individual more susceptible to stiff landing. Our results thus support the study by Wojtys et al. [
2], who found an increased risk of ACL injury during the ovulatory period.
Several limitations to the present study must be considered when interpreting the results. First, only one part of each muscle-tendon mechanical properties were examined in the thigh and lower leg. The relationships between anterior and posterior sites of muscles are related to the stabilization mechanism of the lower limb and the risk of disability, so changes in the overall relationship need to be investigated in the future. Second, MyotonPRO measures the surface layer of the muscle and a portion of the same muscle at rest, so changes in the entire muscle or deeper layers may not be captured.
5. Conclusions
The present study found no significant differences in muscle or tendon stiffness between anterior sites of the thigh and posterior sites of the lower thigh between the early follicular and ovulatory phases. Only anterior sites of the thigh showed a significant positive correlation during the ovulatory phase. The results of this study suggest that stiffness of the muscles and tendons of the anterior thigh and posterior lower leg may not change between the early follicular and ovulatory phases. During the ovulatory phase, tendons may also be stiffer in individuals with stiffer anterior thigh muscles.
Author Contributions
Conceptualization, R.S. and M.E.; methodology, R.S. and M.E.; validation, R.S., M.S., Y.S., T.H., K.K., C.S., H.Y.,R.H., T.I., H.A., R.T., Y.Y., H.O. and M.E.; formal analysis, R.S. and M.E. investigation, R.S., M.S., Y.S. and M.E.; data curation, R.S. and M.E.; writing—original draft preparation, R.S. and M.E.; writing—review and editing, R.S., M.S., Y.S., T.H., K.K., C.S., H.Y.,R.H., T.I., H.A., R.T., Y.Y., H.O. and M.E.; visualization, R.S.; project administration, M.E.; funding acquisition, M.E.; supervision, M.E. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This study was supported by a Grant-in-Aid for Scientific Research (22K19739) from the Japan Society for the Promotion of Science (JSPS), commissioned by the Japan Sports Agency (Female Athletes Development and Support Projects 2022), and a Grant-in-Aid program from Niigata University of Health and Welfare.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Niigata University of Health and Welfare (18671—20 July 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting the study's conclusions are accessible from the author.
Acknowledgments
The researchers appreciate and thank all the female volunteers who took part in this study. And the researchers would like to thank FORTE (
https://forte-science.co.jp/) for the English language review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beynnon, B.D.; Johnson, R.J.; Braun, S.; Sargent, M.; Bernstein, I.M.; Skelly, J.M.; Vacek, P.M. The relationship between menstrual cycle phase and anterior cruciate ligament injury: a case-control study of recreational alpine skiers. Am J Sports Med 2006, 34, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Wojtys, E.M.; Huston, L.J.; Lindenfeld, T.N.; Hewett, T.E.; Greenfield, M.L. Association between the menstrual cycle and anterior cruciate ligament injuries in female athletes. Am J Sports Med 1998, 26, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Timmins, K.; Cowie, C.; Alty, J.; Mehta, R.; Tang, A.; Varley, I. Injury Incidence Across the Menstrual Cycle in International Footballers. Front Sports Act Living 2021, 3, 616999. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Albertin, G.; Petrelli, L.; Sfriso, M.M.; Biz, C.; De Caro, R.; Stecco, C. Hormone receptor expression in human fascial tissue. Eur J Histochem 2016, 60, 2710. [Google Scholar] [CrossRef] [PubMed]
- Wiik, A.; Ekman, M.; Morgan, G.; Johansson, O.; Jansson, E.; Esbjörnsson, M. Oestrogen receptor beta is present in both muscle fibres and endothelial cells within human skeletal muscle tissue. Histochem Cell Biol 2005, 124, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, J.T.; Zhang, Y.; Donahue, H.; Wade, A.M.; Juliano, P.J. Estrogen receptor expression in posterior tibial tendon dysfunction: a pilot study. Foot & ankle international. / American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society 2010, 31, 1081–1084. [Google Scholar] [CrossRef]
- Leblanc, D.R.; Schneider, M.; Angele, P.; Vollmer, G.; Docheva, D. The effect of estrogen on tendon and ligament metabolism and function. J Steroid Biochem Mol Biol 2017, 172, 106–116. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Fan, C.; Albertin, G.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Sensitivity of the fasciae to sex hormone levels: Modulation of collagen-I, collagen-III and fibrillin production. PloS one 2019, 14, e0223195. [Google Scholar] [CrossRef] [PubMed]
- Borg, T.K.; Caulfield, J.B. Morphology of connective tissue in skeletal muscle. Tissue Cell 1980, 12, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Miyamoto, M.; Tanaka, S.; Maki, A.; Tsunoda, N.; Kanehisa, H. Muscle and tendon properties during menstrual cycle. International journal of sports medicine 2009, 30, 139–143. [Google Scholar] [CrossRef]
- Sung, E.S.; Kim, J.H. The difference effect of estrogen on muscle tone of medial and lateral thigh muscle during ovulation. J Exerc Rehabil 2018, 14, 419–423. [Google Scholar] [CrossRef]
- Khowailed, I.A.; Lee, H. Neuromuscular Control of Ankle-stabilizing Muscles-specific Effects of Sex and Menstrual Cycle. International journal of sports medicine 2021, 42, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Khowailed, I.A.; Lee, Y.; Lee, H. Assessing the differences in muscle stiffness measured with shear wave elastography and myotonometer during the menstrual cycle in young women. Clinical physiology and functional imaging 2022, 42, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Sekine, C.; Shagawa, M.; Yokota, H.; Hirabayashi, R.; Togashi, R.; Yamada, Y.; Hamano, R.; Ito, A.; Sato, D.; et al. Menstrual Cycle Changes Joint Laxity in Females-Differences between Eumenorrhea and Oligomenorrhea. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Maruyama, S.; Sekine, C.; Shagawa, M.; Yokota, H.; Hirabayashi, R.; Togashi, R.; Yamada, Y.; Hamano, R.; Ito, A.; Sato, D.; et al. Menstrual Cycle Changes Joint Laxity in Females—Differences between Eumenorrhea and Oligomenorrhea. In Journal of Clinical Medicine, 2022; Vol. 11.
- Shagawa, M.; Maruyama, S.; Sekine, C.; Yokota, H.; Hirabayashi, R.; Hirata, A.; Yokoyama, M.; Edama, M. Comparison of anterior knee laxity, stiffness, genu recurvatum, and general joint laxity in the late follicular phase and the ovulatory phase of the menstrual cycle. BMC musculoskeletal disorders 2021, 22, 886. [Google Scholar] [CrossRef] [PubMed]
- Caresio, C.; Salvi, M.; Molinari, F.; Meiburger, K.M.; Minetto, M.A. Fully Automated Muscle Ultrasound Analysis (MUSA): Robust and Accurate Muscle Thickness Measurement. Ultrasound Med Biol 2017, 43, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Fukumoto, Y.; Kobayashi, M.; Kawasaki, T.; Maegawa, S.; Ibuki, S.; Ichihashi, N. Quantity and Quality of the Lower Extremity Muscles in Women with Knee Osteoarthritis. Ultrasound Med Biol 2015, 41, 2567–2574. [Google Scholar] [CrossRef]
- Chen, G.; Wu, J.; Lu, Y.; Ren, W.; Xu, W.; Xu, X.; Wu, Z.; Guan, Y.; Zheng, Y.; Qiu, B. Reliability of a portable device for quantifying tone and stiffness of quadriceps femoris and patellar tendon at different knee flexion angles. PLoS One 2019, 14, e0220521. [Google Scholar] [CrossRef]
- Saeki, J.; Ikezoe, T.; Yoshimi, S.; Nakamura, M.; Ichihashi, N. Menstrual cycle variation and gender difference in muscle stiffness of triceps surae. Clinical biomechanics (Bristol, Avon) 2019, 61, 222–226. [Google Scholar] [CrossRef]
- Rasmussen, O.S. Sonography of tendons. Scand J Med Sci Sports 2000, 10, 360–364. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Yamazaki, T.; Sato, Y.; Suzuki, Y.; Shimizu, S.; Ikezu, M.; Kaneko, F.; Matsuzawa, K.; Hirabayashi, R.; Edama, M. Relationship Between Anterior Knee Laxity and General Joint Laxity During the Menstrual Cycle. Orthop J Sports Med 2021, 9, 2325967121993045. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.; Lee, H. Greater Reduction of Balance as a Result of Increased Plantar Fascia Elasticity at Ovulation during the Menstrual Cycle. Tohoku J Exp Med 2015, 237, 219–226. [Google Scholar] [CrossRef]
- Miller, B.F.; Olesen, J.L.; Hansen, M.; Døssing, S.; Crameri, R.M.; Welling, R.J.; Langberg, H.; Flyvbjerg, A.; Kjaer, M.; Babraj, J.A.; et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 2005, 567, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Vacek, P.M.; Newell, M.K.; Tourville, T.W.; Smith, H.C.; Shultz, S.J.; Slauterbeck, J.R.; Johnson, R.J. The Effects of Level of Competition, Sport, and Sex on the Incidence of First-Time Noncontact Anterior Cruciate Ligament Injury. Am J Sports Med 2014, 42, 1806–1812. [Google Scholar] [CrossRef]
- Otsuki, R.; Del Bel, M.J.; Benoit, D.L. Sex differences in muscle activation patterns associated with anterior cruciate ligament injury during landing and cutting tasks: A systematic review. Journal of electromyography and kinesiology : official journal of the International Society of Electrophysiological Kinesiology 2021, 60, 102583. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.D.; Ford, K.R.; Myer, G.D.; Hewett, T.E. Sex differences in force attenuation: a clinical assessment of single-leg hop performance on a portable force plate. Br J Sports Med 2011, 45, 198–202. [Google Scholar] [CrossRef]
- Leppänen, M.; Pasanen, K.; Kujala, U.M.; Vasankari, T.; Kannus, P.; Äyrämö, S.; Krosshaug, T.; Bahr, R.; Avela, J.; Perttunen, J.; et al. Stiff Landings Are Associated With Increased ACL Injury Risk in Young Female Basketball and Floorball Players. Am J Sports Med 2017, 45, 386–393. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).