1. Introduction
Gliomas regroup all tumors that originate from glial cells, astrocytic tumors, oligodendroglioma and ependymoma. Gliomas are the common primary malignant brain tumors (with around 80%), where Glioblastoma (GBM) is the most frequent one and the one, which represents the worst prognosis for patients [
1].
Immunotherapy is taking place as a strong approach for the treatment of cancer. Several studies suggest the potential of targeting immune checkpoints pathways to overcome tumor progression [
2,
3]. However, the current two main targeted immunosuppressive molecules in treating cancer, CTLA-4 & PD-1, did not result in favorable outcomes in glioma patients [
4,
5]. It is therefore, important to explore new potential immunotherapeutic targets.
Human gliomas are characterized by an immunosuppressive microenvironment [
6,
7]. Several ongoing clinical studies encourage the use of immune checkpoint inhibitors to overcome gliomas. However, Nivolumab and Ipilimumab, the two most tested immunotherapeutic antibodies, resulted in a very limited outcome [
8]. It was therefore important to identify novel molecules, which function as immune checkpoints and which could be used as new targets either solely or in combination with others, in order to improve the anti-tumor immune response in glioma patients.
T cell immunoglobulin and ITIM domain (TIGIT), also known as Washington University Cell Adhesion Molecule (WUCAM), V-set and transmembrane domain-containing protein 3 (Vstm3) and V-set and immunoglobulin domain-containing protein 9 (VSIG9) [
9,
10,
11], is an immune checkpoint identified on the surface of T cells [
12,
13] and NK cells [
14]. This molecule is composed of three domains, an extracellular, which is made up of an immunoglobulin-like domain, a transmembrane type 1 domain and a cytoplasmic domain that contains both ITIM
(Immunoreceptor Tyrosine-based Inhibitory Motif) and ITT (
Ig Tail-Tyrosine like motif) motifs, which are responsible for the negative signaling [
10]. TIGIT delivers co-inhibitory signals following the stimulation with the ligand CD155, and after a competition with CD96 and CD226 that also bind to the same ligand CD155 [
11]. Several studies reported that a higher expression of TIGIT is correlated with a bad prognosis in different cancer types [
15,
16,
17,
18], and the blockade of this molecule would allow the immune system to regain its defensive capacities [
19,
20,
21].
Regulatory T cells are known to be an immunosuppressive subtype of CD4+ T-cells. In the tumor microenvironment, these cells produce immunosuppressive cytokines that cooper in the inhibition of the immune system. In the context of glioma, we showed that FoxP3, which is a regulatory T cell specific transcription factor [
22], is positively associated with higher grades of glioma [
23].
In this study, we report evidence for the implication of TIGIT in human glioma progression. We started by evaluating TIGITs’ levels of expression and its association with few clinical features including grades, IDH mutation status. We then explored the potential link of TIGIT with markers of immunosuppression, including those responsible for CD8 T-cell dysfunction, and those related to regulatory T-cells. Our results showed that high expression of TIGIT is related to poor prognosis in human glioma patients and impacts the overall survival. These findings support a therapeutic targeting of TIGIT in glioma patients.
2. Materials and Methods
Patients:
A total of 53 glioma patients approved their involvement in our study with written informed consent for the use and experimentation of their clinical data and biopsies. Fresh specimens were isolated at the time of the removal of the tumor at the Neurosurgery Service of the University Hospital Center Ibn Rochd, Casablanca Morocco. Metastatic tumors were excluded.
TCGA Data Analysis:
A total of 667 including 152 Glioblastoma Multiform (GBM) and 515 Low-Grade Glioma (LGG) patients were explored through the cBioportal for Cancer Genomics https://www.cbioportal.org/ , by evaluating clinical data and converted Log2 mRNA-seq database of The Cancer Genome Atlas (TCGA).
Multivariate logistic regression and multivariate COX analysis
Logistic regression was performed to investigate the association between TIGIT expression and the clinical characteristics. P<0.05 was considered to indicate a statistically significant difference. Multivariate Cox analysis was used to evaluate the influence of TIGIT expression and other clinic-pathological factors (age, gender, IDH1 status, grade and histological type) on survival.
Cell type-specific gene expression profiles
CIBERSORT R code and cell type-specific gene expression profiles module (Newman et al., 2015) was used to identify cell marker genes between high and low TIGIT patients from TCGA cohort. This analysis allows us to identify those cell marker genes from a bulk RNAseq transcriptomes without the need for physical cell sorting. P-value was used as a parameter of CIBERSORT, which measured the confidence of the results. Paired t-test to compare those cell marker genes between these two groups.
Real-Time RT-PCR assays:
Total RNA was extracted from conserved biopsies using a home-developed protocol using TRIzol solution (Bioline, France). After being dosed on a NanoVueTM plus Spectrophotometer (GE Healthcare, UK), 0.5µg of total RNA was then converted to cDNA in two steps. Step one was for inhibiting RNA’s secondary structures by incubation of RNA for 10min at 70°C with Random Hexamer Primers (1µl; Bioline, France) and RNase Free Water (4µl). The step two was to synthesis the cDNA by incubating the product from step one for 10min at 25°C, 30min at 45°C and 5min at 85°C with 4 μl Tetro Reverse Transcriptase buffer, 4 μl of dNTP (10 mM), 0.5 μl of RNase Inhibitor (40U/µl; Invitrogen, France), 0.5 μl Tetro Reverse Transcriptase Enzyme (10000U; Bioline, France) and 1 μl of RNase-Free Water. To evaluate mRNA gene expression, qPCR experiment was performed using Fast 7500 machine using 10µl of fluorescent dye SYBR ™ Green PCR Master Mix (Thermo Fischer), 7µl of H2O, and 0.5µl of each primer (forward and reverse).
The primers used for experimentations were:
beta-actin Forward: 5’-TGGAATCCTGTGGCATCCATGAAAC-3’
beta-actin Reverse: 5’-TAAAACGCAGCTCAGTAACAGTCCG-3’
TIGIT Forward: 5’-CGTGAACGATACAGGGGAGT-3’,
TIGIT Reverse: 5’-ACGATGACTGCTGTGCAGAT-3’,
Forward: 5’TCTTCCTTGAACCCCATGCC-3’
Reverse: 5’GCATGAAATGTGGCCTGTCC-3’
Statistical analysis:
IBM SPSS Statistics (version 26) predictive analytics software was used to check the normality of distribution of each cohort. Statistical analyses and graph designs were performed on GraphPad Prism 6.0 software (GraphPad Software, USA). Mann-Whitney t-test was used to compare differences between medians for the expression of distinct sets of genes. Correlations were performed by Spearman test and ANOVA was used to analyze the differences among means. All analysis were conducted by two independent researchers before validating results.
3. Results
TIGIT was significantly up-regulated in advanced human glioma.
When analyzing the expression of TIGIT depending on clinical and histological parameters available in TCGA database (Table 1), the results of non-parametric t-test showed that TIGIT was linked to sex, age, high grade and IDH wild type status (p=0.0145; p = 0.0006; p < 0,0001; p = 0,0041 respectively) but not with the overall survival status (p = 0.1896).
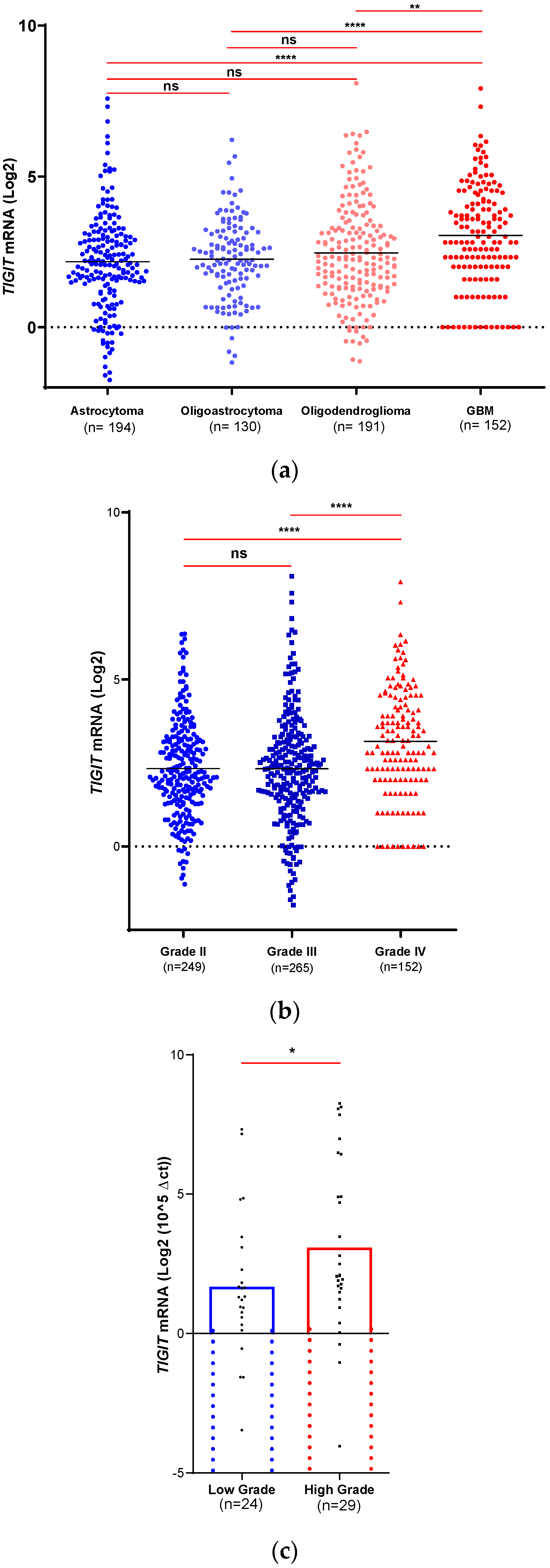
According to the TCGA database, and depending on the 2016’ WHO classification of histological status (Figure 1.a), TIGIT was highly expressed in GBM compared to astrocytoma (GII & GIII) (p < 0.0001), oligoastrocytoma (GII & GIII) (p < 0.0001) and oligodendroglioma (GII & GIII) (p = 0.0016). In contrast, no significant differences were detected when comparing the other subtypes (astrocytoma vs oligoastrocytoma; p = 0.6269), (astrocytoma vs oligodendroglioma; p = 0.1618) and (oligoastrocytoma vs oligodendroglioma p = 0.5209). These data indicated that high expression of TIGIT was positively associated with the most malignant subtypes of human gliomas.
ANOVA test revealed a significant difference between the means of the three available grades of Astrocytoma in TCGA database (GII, GIII and GIV/GBM) (p < 0.0001) (Table 1). Also, t-test was performed to evaluate the expression of TIGIT depending on the grades from TCGA database. Results showed higher expression in glioma Grade IV (GBM) compared to grade II (Astrocytoma, Oligoastrocytoma and Oligodendroglioma) (p < 0.0001) and grade III (Astrocytoma, Oligoastrocytoma and Oligodendroglioma) (p < 0.0001). In contrast, no statistical difference was observed between grade II and grade III (p = 0.1639) (Figure 1.b). These results corroborated those reported in (Figure 1.a) and showed that elevated levels of TIGIT were associated to the most advanced grade of human gliomas.
Table 1.
Expression of TIGIT depending on distinct patients’ pathophysiological parameters in the TCGA cohort.
Table 1.
Expression of TIGIT depending on distinct patients’ pathophysiological parameters in the TCGA cohort.
| Clinical parameters |
TCGA Cohorts (n) |
Median of TIGIT mRNA |
pvalue
|
| Sex |
Women (n= 285) |
2.284 |
0.0145 |
| Man (n= 382) |
2.557 |
| Age |
< 40 (n= 262) |
2.179 |
0.0006 |
| > 40 (n= 393) |
2.585 |
| IDH mutation |
Wild Type (n= 169) |
2.972 |
0.0041 |
| Mutant (n= 99) |
2.362 |
| Overall Survival Status |
Living (n=441) |
2.337 |
0.1896 |
| Deceased (n=225) |
2.585 |
| Grade |
Low grade (GII-GII) (n=514) |
2.221 |
< 0.0001 |
| High grade (GIV) (n=152) |
3.000 |
| Expression Subtype (GBM) |
Mesenchymal (n=49) |
3.700 |
0,0101
|
| Classical (n=39) |
2.585 |
| Neural (n=26) |
2.989 |
| Proneural (n=29) |
2.322 |
| G-CIMP (n=8) |
2.953 |
| Histological Diagnosis |
Astrocytoma GII (n=63) |
1.958 |
<0,0001 |
| Astrocytoma GIII (n=131) |
2.312 |
| Astrocytoma GIV (GBM) (n=152) |
3.170 |
| Oligoastrocytoma GII (n=74) |
2.350 |
0.1223 |
| Oligoastrocytoma GIII (n=55) |
2.219 |
| Oligodendroglioma GII (n=112) |
2.274 |
0.9708 |
| Oligodendroglioma GIII (n=79) |
2.323 |
The second cohort used in this study was related to the Moroccan patients, and which was composed of 53 human glioma patients (Table 2). In this cohort, TIGIT showed similar trends as those observed in the TCGA cohort. However, TIGIT expression did not reach significant difference when comparing groups of patients depending on age and sex (p = 0.8734; p = 0,1268 respectively).
Table 2.
Expression of TIGIT depending on distinct patients’ pathophysiological parameters in the Moroccan cohort.
Table 2.
Expression of TIGIT depending on distinct patients’ pathophysiological parameters in the Moroccan cohort.
| Clinical data |
Cohorts (n) |
Median of TIGIT mRNA |
p value |
| Sex |
Female (n=23) |
1.480 |
0.1268
|
| Male (n=30) |
1.955 |
| Age |
< 40 (n=28) |
1.705 |
0,8734
|
| > 40 (n=24) |
1.820 |
| Grade |
Low grade* (n=24) |
1.310 |
0.0423
|
| High grade* (n=29) |
2.060 |
Interestingly, when exploring the 53 Moroccan glioma biopsies (Figure 1.c), TIGIT expression was significantly higher in advanced grades (GIII & GIV, n = 29) compared to lower grades of gliomas (GI & GII, n = 24) (p = 0.0423), confirming data from the TCGA cohort.
Figure 1.
TIGIT is positively associated with severe malignant glioma. mRNA expression of TIGIT was analyzed in two different cohorts to explore its levels depending on histological subtypes and grades a) Tumors from glioblastoma multiform patients of the TCGA dataset exhibited higher expression of TIGIT. b) TIGIT expression in TCGA glioma patients (GII: n= 249; GIII: n= 265; GIV: n= 152) exhibited its highest levels in GIV. c). TIGIT expression in our home cohort is highly expressed in high grade (n=29) compared to low grade (n=24) glioma. Significant differences are indicated (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01, * for p < 0.05).
Figure 1.
TIGIT is positively associated with severe malignant glioma. mRNA expression of TIGIT was analyzed in two different cohorts to explore its levels depending on histological subtypes and grades a) Tumors from glioblastoma multiform patients of the TCGA dataset exhibited higher expression of TIGIT. b) TIGIT expression in TCGA glioma patients (GII: n= 249; GIII: n= 265; GIV: n= 152) exhibited its highest levels in GIV. c). TIGIT expression in our home cohort is highly expressed in high grade (n=29) compared to low grade (n=24) glioma. Significant differences are indicated (**** for p < 0.0001, *** for p < 0.001, ** for p < 0.01, * for p < 0.05).
TIGIT expression was higher in IDH-wildtype glioma and was associated to mesenchymal-molecular subtype.
TIGIT expression was assessed according to the glioma molecular status. The expression of TIGIT was first evaluated depending on IDH gene status, IDH wildtype versus mutant in patients from TCGA database (Table 1). The results showed that IDH-wildtype patients displayed higher expressions of TIGIT compared to patients with IDH-mutant (p = 0.0041). This analysis could not be achieved in the second cohort (the Moroccan cohort) because of the small sample size (Wildtype, n=3; Mutant, n=4).
Subsequently, Kruskal-Wallis test was used to assess the difference in the expression of GBM molecular subtypes (Mesenchymal, Classical, Neural, Proneural and G-CIMP) and reported a significant difference between these groups (p = 0,0101) (Figure S1). Furthermore, when performing several t-tests between the five subtypes above, we found that TIGIT was significantly higher in the mesenchymal molecular subtype compared to Proneural and Classical (p=0,0073; p=0,0043 respectively) (Table S1).
Higher TIGIT expression was linked to higher expression of immune T cell markers in the microenvironment of human glioma.
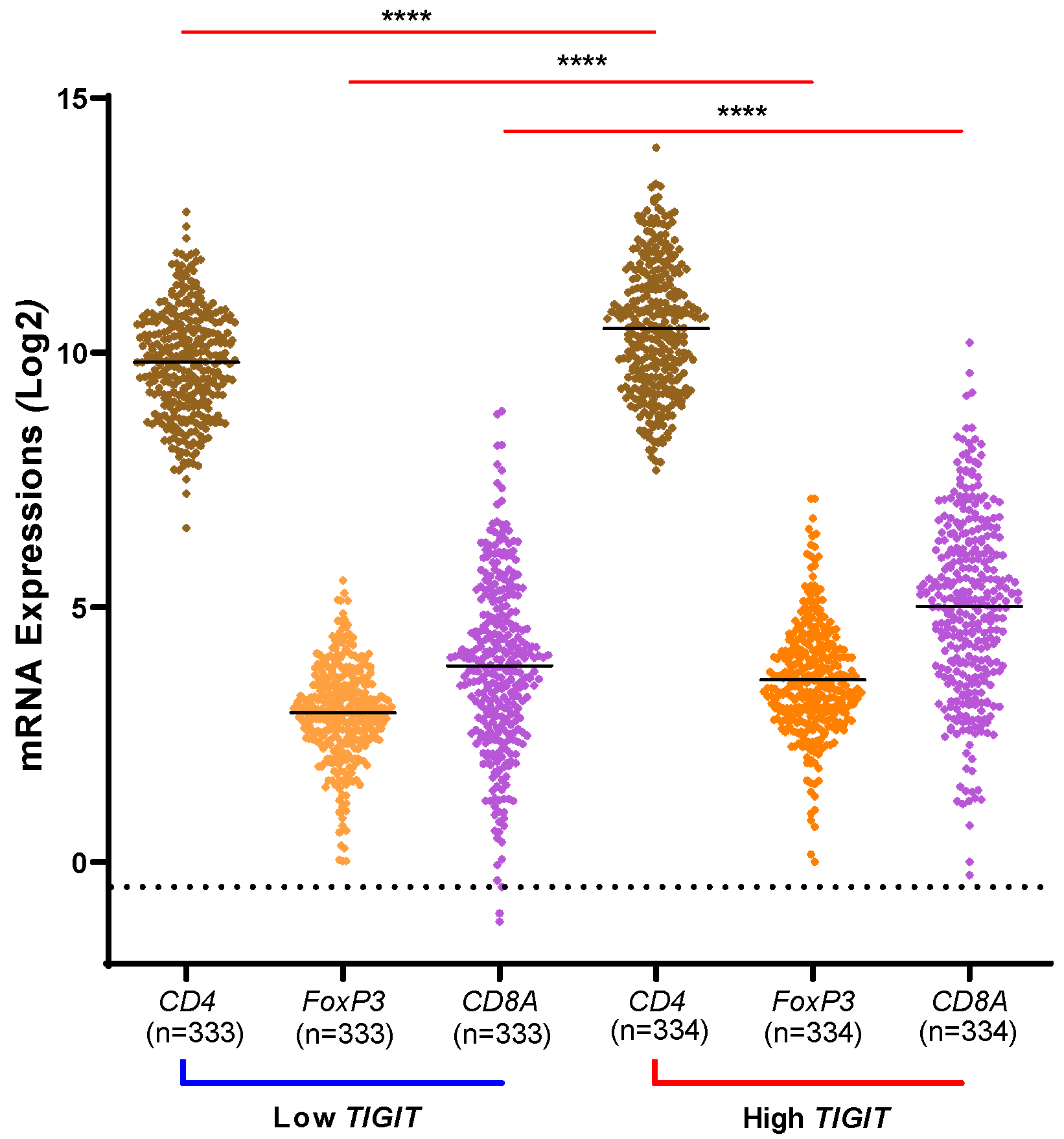
As previously mentioned in the introduction, several studies confirmed a higher expression of TIGIT on T-cells in advanced grades of different tumor sites. While exploring TCGA database, we wanted to assess whether a high expression of TIGIT could be associated with a higher infiltration of CD4+ T-cells, CD8+ T-cells and regulatory T-cells (via FoxP3) in glioma (Figure 2). Our results showed that indeed, markers of CD4, CD8 and regulatory T-cells were upregulated in patients with high expression of TIGIT versus cases with lower expression of TIGIT in human glioma (p < 0.0001). This suggested that high expression of TIGIT, whose expression was found to be associated with advanced subtypes of glioma from previous results, also was positively associated with high infiltration of T cells.
Figure 2.
T cell subtypes marker expression (CD4, CD8A and Tregs/FoxP3) depending TIGIT expression status. Patients from TCGA database were distributed into two cohorts for each marker of T-cells and depending on the level of expression of TIGIT. For each marker, non-parametric t-test was used to compare the level of expression depending on high versus low profile of TIGIT. The three markers of T-cells exhibited their higher expression levels in the condition of high TIGIT, compared to the condition of low expression of TIGIT where their expressions were at lower levels. Significant statistical differences are indicated (**** for p < 0.0001).
Figure 2.
T cell subtypes marker expression (CD4, CD8A and Tregs/FoxP3) depending TIGIT expression status. Patients from TCGA database were distributed into two cohorts for each marker of T-cells and depending on the level of expression of TIGIT. For each marker, non-parametric t-test was used to compare the level of expression depending on high versus low profile of TIGIT. The three markers of T-cells exhibited their higher expression levels in the condition of high TIGIT, compared to the condition of low expression of TIGIT where their expressions were at lower levels. Significant statistical differences are indicated (**** for p < 0.0001).
TIGIT was associated with an immunosuppressive microenvironment in human glioma.
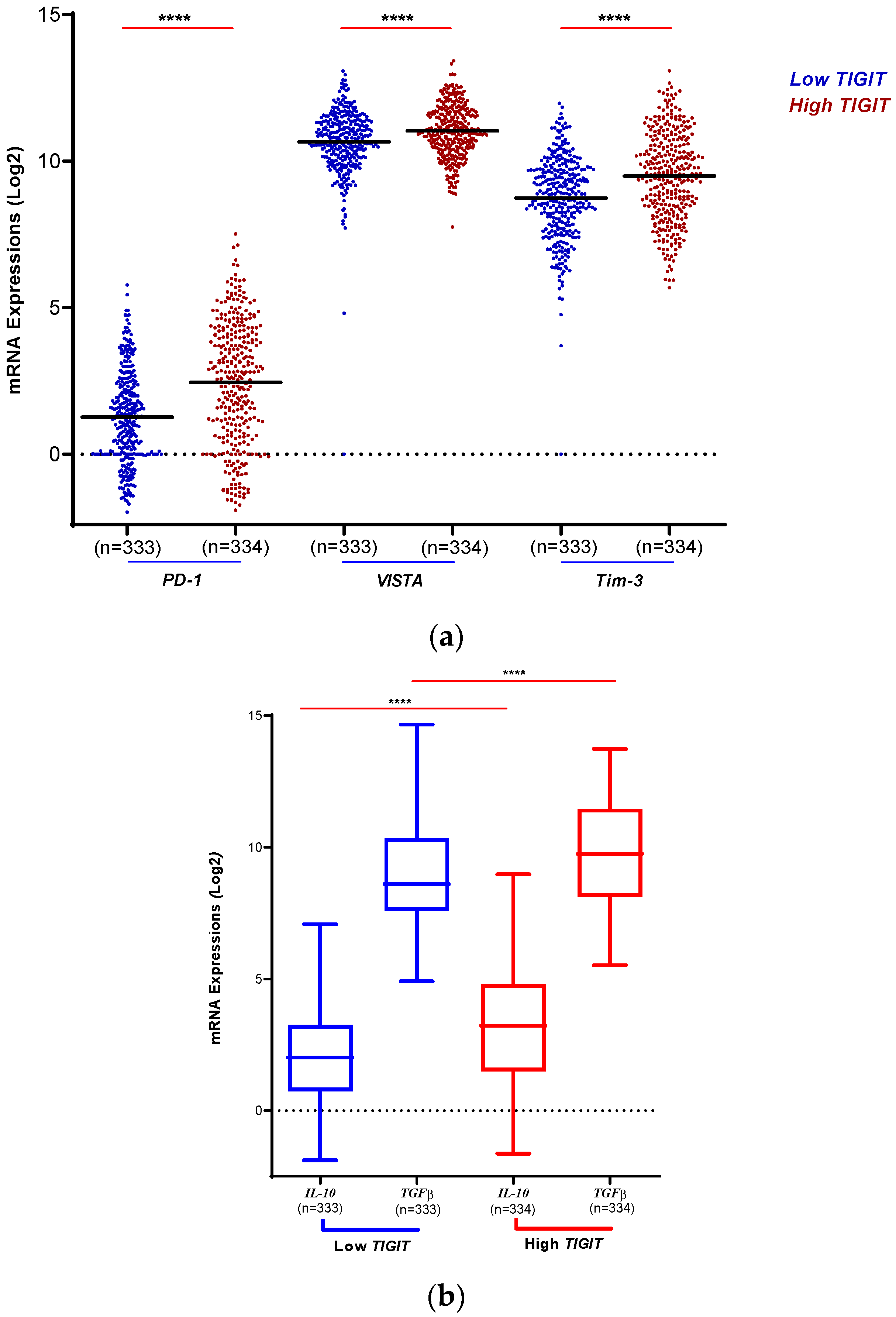
To assess the immunosuppressive status of human gliomas according to TIGIT expression levels (Figure 3), three immune checkpoints (PD-1, VISTA, Tim-3) were explored. Interestingly, and according to TCGA database (Figure 3.a), we found that in the context of low TIGIT, the immune checkpoints PD-1, VISTA and Tim-3 exhibited lower expression compared to the context of high TIGIT expression where all the three immune checkpoints were upregulated (p < 0.0001). This showed that in human glioma’s microenvironment, the expression level of the three inhibitory molecules PD-1, VISTA and Tim-3 goes along with the level of expression of TIGIT, supporting the important role of TIGIT as a potential potent immunosuppressive immune checkpoint in human glioma.
We therefore analyzed the level of expression of known and potent regulatory T cell secreting immunosuppressive cytokines (Figure 3.b), and found that the expression of both IL-10 and TGF-beta was upregulated in the case of high TIGIT compared to low TIGIT conditions (p < 0.0001 and p < 0.0001, respectively). This supports the hypothesis that TIGIT highly contributes to the setting up of a potent immunosuppressive microenvironment in human glioma.
Figure 3.
High expression of TIGIT was linked to an Immunosuppressive microenvironment in human glioma. a): TIGIT expression is positively associated with critical immune checkpoint regulators. TCGA dataset was explored to evaluate the levels of expression of three inhibitory molecules. Results showed an upregulation of PD-1, VISTA and Tim-3 along with the upregulation of TIGIT in the TCGA database. The blue color indicates patients expressing low levels of TIGIT. The red color indicates patients expressing high levels of TIGIT. Significant differences are indicated for each molecule (**** for p < 0.0001). b): High expression of TIGIT transcripts is associated to high expression of anti-inflammatory cytokines. According to TCGA database, IL-10 and TGF-beta showed strong levels of expression in glioma patients in the context of high versus low TIGIT expression profile, indicating the strong link between these molecules in inhibiting the anti-tumor response. The statistical significance of the two tests was indicated (**** for p < 0.0001).
Figure 3.
High expression of TIGIT was linked to an Immunosuppressive microenvironment in human glioma. a): TIGIT expression is positively associated with critical immune checkpoint regulators. TCGA dataset was explored to evaluate the levels of expression of three inhibitory molecules. Results showed an upregulation of PD-1, VISTA and Tim-3 along with the upregulation of TIGIT in the TCGA database. The blue color indicates patients expressing low levels of TIGIT. The red color indicates patients expressing high levels of TIGIT. Significant differences are indicated for each molecule (**** for p < 0.0001). b): High expression of TIGIT transcripts is associated to high expression of anti-inflammatory cytokines. According to TCGA database, IL-10 and TGF-beta showed strong levels of expression in glioma patients in the context of high versus low TIGIT expression profile, indicating the strong link between these molecules in inhibiting the anti-tumor response. The statistical significance of the two tests was indicated (**** for p < 0.0001).
Human glioma microenvironment displayed exhausted T cells under high TIGIT expression conditions.
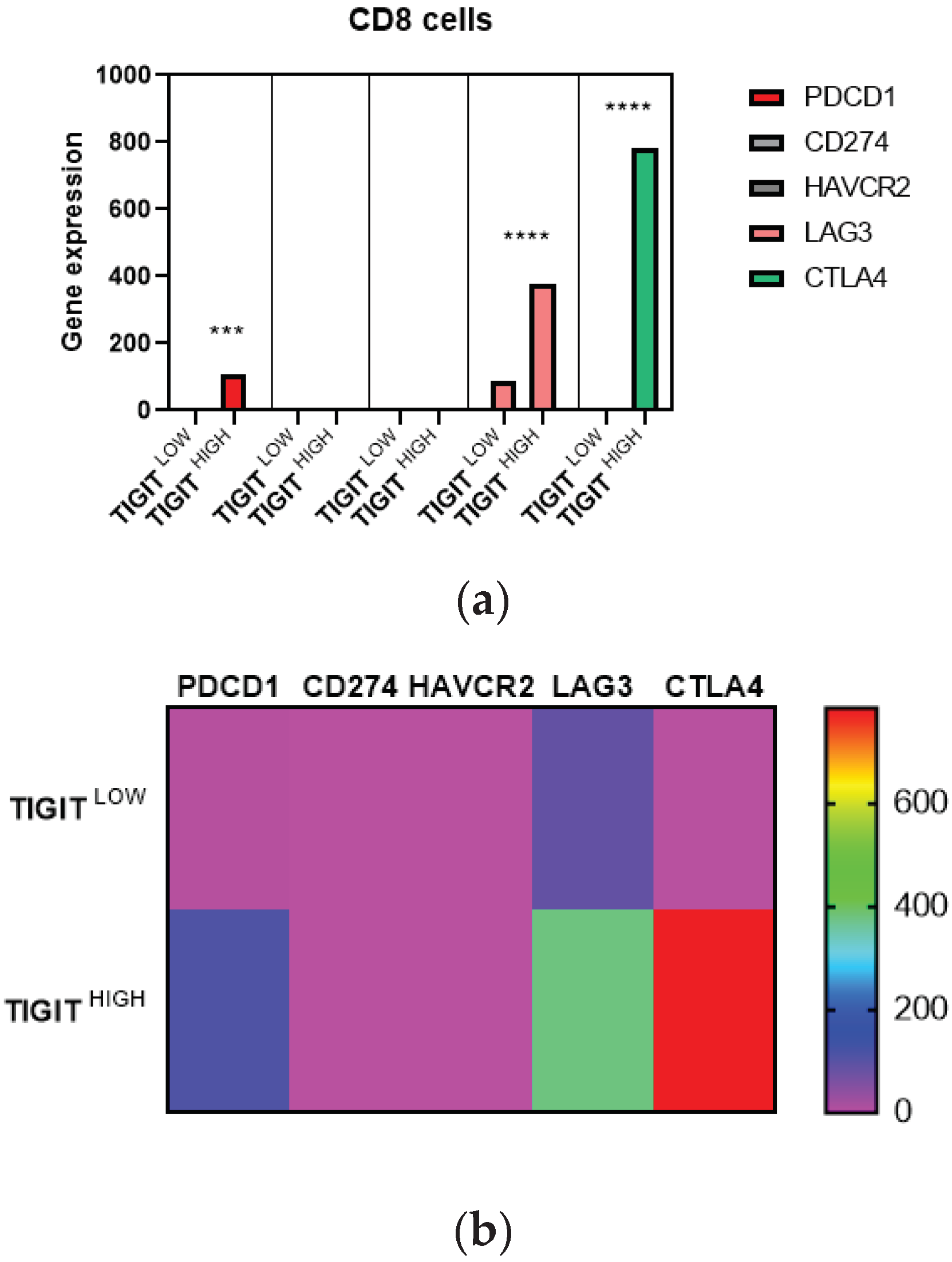
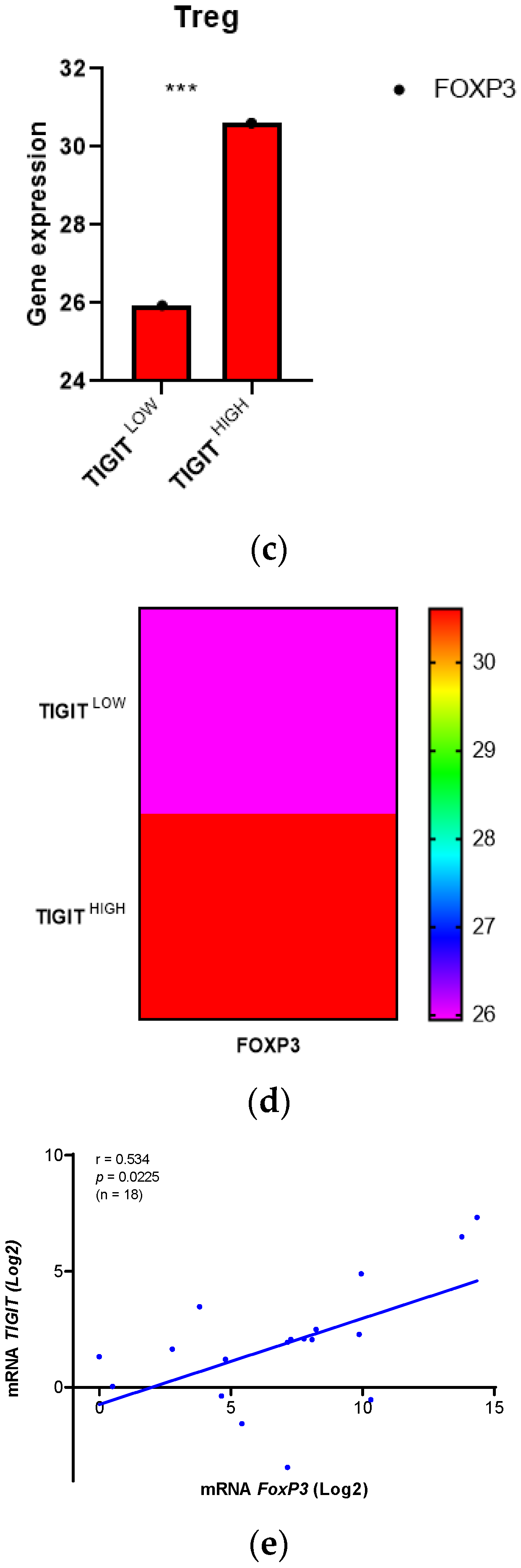
In order to identify cell type-specific gene expression profiles, we used CIBERSORTx and R algorithms. We explored precisely T cell CD8 marker genes, PD1 (PDCD1), PDL1 (CD274), Tim-3 (HAVCR2), CTLA4 and LAG3, we also explored Treg marker gene, FoxP3, in glioma patients from TCGA database, depending on high and low expression of TIGIT (Figure 4). Paired T-test was used to compare the expression of cell marker genes between the two groups (high and low TIGIT). We assessed the expression of PD1, PDL1, Tim-3, CTLA4 and LAG3 genes in CD8 T cells, genes known to play critical roles in suppressing CD8 T cells (Figure 4 a&b). Results showed that patients with high TIGIT expression displayed high expression of PD1, CTLA4 and LAGMoreover, to examine the pro-tumoral activity of TIGIT in glioma TME, we also evaluated FoxP3 expression in Treg cells (Figure 4 c&d), which are known to strongly suppress the anti-tumor immune response. Remarkably, patients with high expression of TIGIT had increased expression levels of FoxP3 compared to those presenting low expression of TIGIT. Subsequently, we checked if data of the Moroccan cohort were consistent with this observation. 18 patients were analyzed (Figure 4.e). Results corroborated that TIGIT was positively correlated to FoxP3 (r= 0.534; p = 0.0225). We suggest that, in the condition of high expression of TIGIT, CD8 T cells from patients suffering from glioma, would be unable to exert cytolytic activity in the presence of the aforementioned immune checkpoints (PD1, CTLA4, and LAG3) and Treg cells. We can state the hypothesis that these CD8 T cells would likely be exhausted and their ability to destroy tumor cells is abolished as a consequence.
Figure 4.
Overexpression of TIGIT was associated to exhausted T cells in human glioma microenvironment. Figure 4 .a&b: Gene expression profile specific to exhausted T cells CD8 in high and low TIGIT Groups. a) Bar plots illustrating the expression of markers across exhausted T cells CD8. Each bar plot represents immune cell fraction in glioma patients (Low and high grade) with low TIGIT (n =331) and high TIGIT (n =332). Paired T-test was used to compare those cell marker genes between these two groups. The statistical significance of the test was indicated too (p < 0.05). b) Heatmap showing the average expression of exhausted T cells CD8 markers across high and low TIGIT groups. Figure 4 c&d: Gene expression profile specific to regulatory T cells in glioma patients depending on high and low TIGIT. c) Bar plots illustrating the expression of markers across Tregs cells. Each bar plot represents immune cell fraction in glioma patients (Low and high grade) with low TIGIT (n =331) and high TIGIT (n =332). Paired T-test was used to compare those cell marker genes between these two groups. The statistical significance of the test was indicated too (p < 0.05). d) Heatmap showing the average expression of Tregs cells markers across high and low TIGIT groups. Figure 4.e: Correlation between expression of TIGIT and the expression of FoxP3 in human glioma patients of Morocco. 18 human glioma patients from the Moroccan cohort were included in the Spearman r test to evaluate the correlation between TIGIT and FoxPTIGIT expression was positively correlated to FoxP3 in the Moroccan cohort. Positive correlation was mentioned on the graph (r=0.534). The statistical significance of the test was indicated too (p < 0.05).
Figure 4.
Overexpression of TIGIT was associated to exhausted T cells in human glioma microenvironment. Figure 4 .a&b: Gene expression profile specific to exhausted T cells CD8 in high and low TIGIT Groups. a) Bar plots illustrating the expression of markers across exhausted T cells CD8. Each bar plot represents immune cell fraction in glioma patients (Low and high grade) with low TIGIT (n =331) and high TIGIT (n =332). Paired T-test was used to compare those cell marker genes between these two groups. The statistical significance of the test was indicated too (p < 0.05). b) Heatmap showing the average expression of exhausted T cells CD8 markers across high and low TIGIT groups. Figure 4 c&d: Gene expression profile specific to regulatory T cells in glioma patients depending on high and low TIGIT. c) Bar plots illustrating the expression of markers across Tregs cells. Each bar plot represents immune cell fraction in glioma patients (Low and high grade) with low TIGIT (n =331) and high TIGIT (n =332). Paired T-test was used to compare those cell marker genes between these two groups. The statistical significance of the test was indicated too (p < 0.05). d) Heatmap showing the average expression of Tregs cells markers across high and low TIGIT groups. Figure 4.e: Correlation between expression of TIGIT and the expression of FoxP3 in human glioma patients of Morocco. 18 human glioma patients from the Moroccan cohort were included in the Spearman r test to evaluate the correlation between TIGIT and FoxPTIGIT expression was positively correlated to FoxP3 in the Moroccan cohort. Positive correlation was mentioned on the graph (r=0.534). The statistical significance of the test was indicated too (p < 0.05).
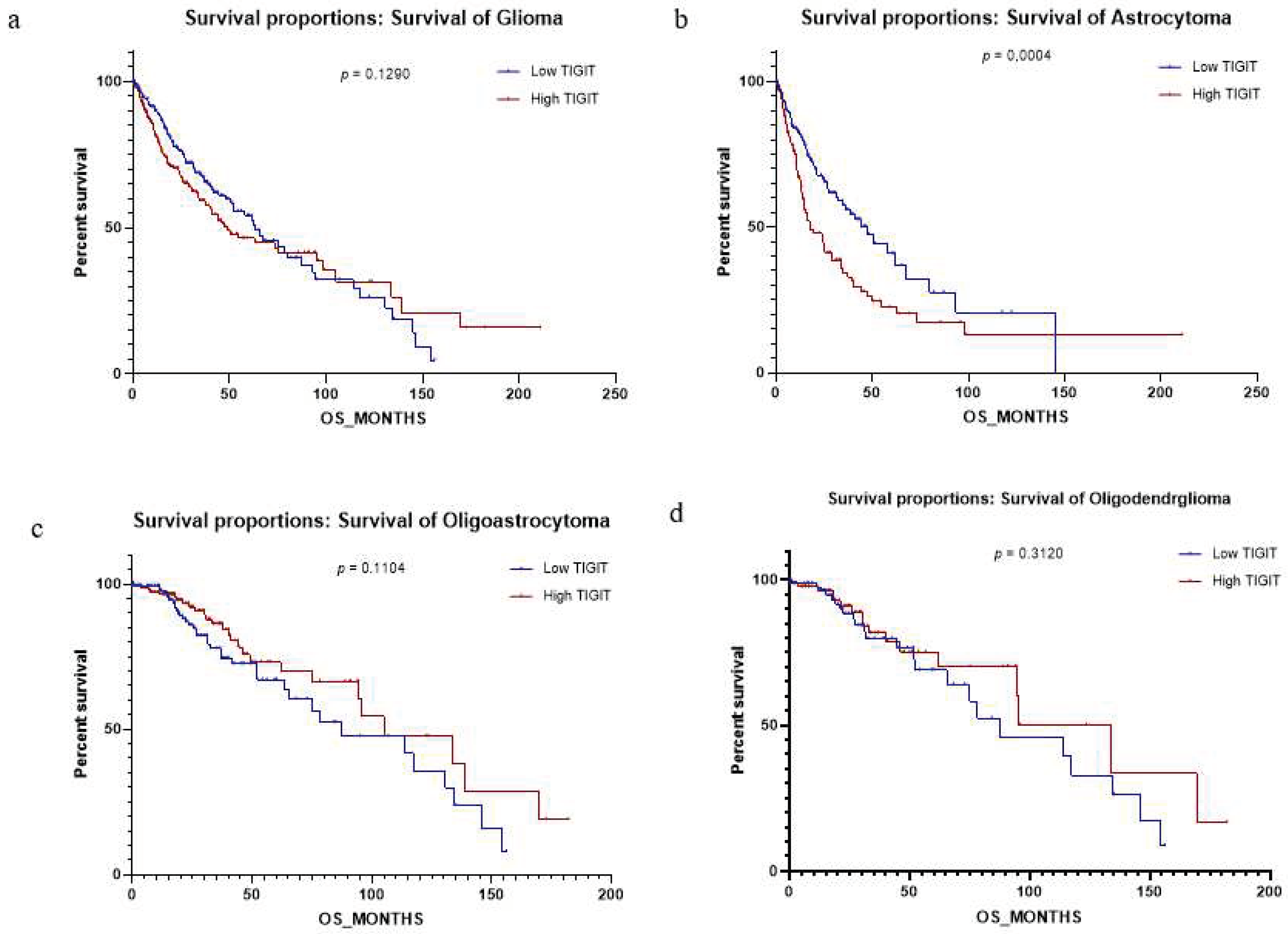
High expression of TIGIT represents a prognosis factor depending on the histological subtype of glioma.
To refine the prognosis of high expression of TIGIT on human glioma, we aimed to check if the expression of TIGIT had an impact on the overall survival. First we analyzed survival independently of glioma subtypes (Figure 5.a). The result didn’t reveal significant difference between low and high TIGIT expression (p=0.1290), which was surprising considering all the previous results. However, we previously showed (table 1) that the expression of TIGIT was significantly different between grades of Astrocytoma (p <0,0001), but not between grades of oligoastrocytoma and oligodendroglioma (p=0.1223; p=0.9708 respectively). Interestingly, when we stratified patients depending on different subtypes (Astrocytoma, Oligoastrocytoma and Oligodendroglioma), we found that TIGIT had a significant impact on the overall survival in Astrocytoma (Figure 5.b), (p=0.0004), but not in Oligoastrocytoma and oligodendroglioma (Figure 5.c&d) (p=0.1104; p=0.3120 respectively). These results showed that TIGIT could have an impact in glioma progression, especially in the Astrocytoma subtype.
Finally, multivariate logistic regression analysis between several clinical parameters was performed (Figure 5.e). Results indicated that TIGIT expression in glioma patients (high and low grades) impacted significantly the histological type (P=0,004). However, there was no impact of the expression of TIGIT on sex, age, grades and IDH status. These results (Figure 5) indicated that TIGIT could be used as a prognostic factor in the astrocytoma histological subtype.
Figure 5.
TIGIT expression level exhibited a significant impact on the Astrocytoma subtype of human glioma. Fig 5.a-d: Survival analysis depending on high or low expression of TIGIT in glioma patients from TCGA dataset. a) Survival analysis depending on TIGIT expression in all subtypes of glioma (Astrocytoma, Oligoastrocytoma, Oligodendroglioma). b) Survival analysis depending on TIGIT expression in Astrocytoma subtype (GII, GIII & GIV). c) Survival analysis depending on TIGIT expression in Oligoastrocytoma subtype (GII & GIII). d) Survival analysis depending on TIGIT expression in Oligodendroglioma subtype (GII, GIII). Fig 5.e: Multivariate logistic regression analysis of TIGIT expression in glioma patients (Low and high grade) showing low and high TIGIT profile.
Figure 5.
TIGIT expression level exhibited a significant impact on the Astrocytoma subtype of human glioma. Fig 5.a-d: Survival analysis depending on high or low expression of TIGIT in glioma patients from TCGA dataset. a) Survival analysis depending on TIGIT expression in all subtypes of glioma (Astrocytoma, Oligoastrocytoma, Oligodendroglioma). b) Survival analysis depending on TIGIT expression in Astrocytoma subtype (GII, GIII & GIV). c) Survival analysis depending on TIGIT expression in Oligoastrocytoma subtype (GII & GIII). d) Survival analysis depending on TIGIT expression in Oligodendroglioma subtype (GII, GIII). Fig 5.e: Multivariate logistic regression analysis of TIGIT expression in glioma patients (Low and high grade) showing low and high TIGIT profile.
4. Discussion
Glioma is a general term that describes different tumors that initiate from glial cells, including astrocytes, oligodendrocytes, and ependymal cells. This tumor is known to be the most common primary brain cancer and represents a very bad prognosis with high rates of recurrence [
24]. Lower median survival is registered, especially in advanced grade glioma, even with several choices of treatments, including surgery, chemotherapy and radiotherapy [
25].
In this work, we describe the involvement of TIGIT in human glioma development in two independent cohorts (TCGA database and Moroccan patients). We found that this newly identified co-inhibitory molecule is strongly associated with a malignant type of glioma.
TIGIT expression was upregulated in higher grades especially in the glioblastoma. Our study reports an elevated rate of
TIGIT in higher compared to lower age. Also, Tian et al. showed that female patients with GBM have higher cancer specific survival compared to male. The study suggests an implication of female hormones to prevent the malignity of glioma [
26]. Consistently, our results revealed an elevated rate of expression of this inhibitory molecule in male compared to female. A link between
TIGIT upregulation and hormones is likely. Further studies need to be conducted to evaluate whether there is a direct link.
IDH mutation represents a critical genomic alteration, which could indicate tumor prognosis [
27]. The
IDH-wildtype patients tends to display an aggressive phenotype compared to the
IDH mutant patients [
28]. Interestingly, our results reported that
TIGIT was significantly more prevalent in patients with the
IDH-wildtype gene compared to patients with the
IDH mutant gene. Moreover, Phillips et al. and Kafess et al. shed light on the several molecular subtypes of glioblastoma, reporting that the mesenchymal subtype represents the most aggressive phenotype [
29,
30]. Our results were consistent with these two studies. First, ANOVA test showed a significant difference of the expression pattern of
TIGIT between different molecular subtypes with an elevated expression in the mesenchymal group. This observation was subsequently confirmed using t-test. This suggests that
TIGIT could be used as a potential biomarker for the mesenchymal molecular subtype. Therefore, the two findings join previous results to confirm the association of
TIGIT with poor prognosis in human glioma.
It is believed that tumors are positively associated with immune cell infiltration, including T lymphocytes (Tumor Infiltrating Lymphocytes) [
31]. The upregulation of immune checkpoints on these TILs drive them to a state of exhaustion due to chronic activation [
32]. For this reason, we evaluated the expression of
TIGIT in different T cell subtypes,
CD4+,
CD8+ and regulatory T-cells, via their specific markers (
CD4,
CD8A and
FoxP3, respectively). Our results showed a significant upregulation of the three markers of T-cells in the case of high
TIGIT compared to low
TIGIT, indicating a likely role of
TIGIT in suppressing the immune system and limiting its anti-tumor capacity.
It has been reported that glioma displays an immunosuppressive microenvironment [
33].
PD-1,
VISTA and
Tim-3 are a set of immune checkpoints that are upregulated on TILs in different sites of tumor including glioma [
34,
35,
36]. The three studies suggest that these molecules have an impact on tumor progression via the modulation of the immune response in the tumor microenvironment. Our results show a significant association between the upregulation of
PD-1, VISTA and Tim-3 along with the upregulation of
TIGIT, suggesting that
TIGIT should also be considered as an appropriate immunotherapeutic target within the human glioma microenvironment. Moreover, we explored immunosuppressive cytokines (
IL-10 and
TGF-beta) have been confirmed in previous studies to be implicated in advanced grades of glioma [
37,
38,
39]. Our results reported that both
IL-10 and
TGF-beta were also significantly upregulated in the context of high
TGIT compared to low
TIGIT, suggesting a key role for
TIGIT in setting up an immunosuppressive environment.
Several studies explored the role of
TIGIT in different cancer sites such as bladder, colorectal, melanoma and blood [
13,
15,
16,
40].
TIGIT was also explored to show difference in expression between GBM and Multiple Sclerosis [
41], and as a potential therapeutic target upon a dual blockade of anti-TIGIT with anti-PD-1 in a murine GBM model [
21,
42]. At the best of our knowledge, our study is the first one to assess the role of
TIGIT in the progression of human glioma.
It is known that T-cell exhaustion can be determined phenotypically by the overexpression of inhibitory molecules on their surfaces [
43,
44]. In our study, we wanted to evaluate, via a gene expression profile approach, the dysfunction of T-cells in glioma. We found that high expression of TIGIT was associated with the upregulation of PD-1, LAG-3 and CTLA-4, which are known to be inhibitory molecules that are involved in T cell exhaustion [
44,
45]. Also, Treg -cells, which are supposed to maintain a physiological state of immune response in the microenvironment, where shown to play a critical role in inhibiting CD8 T-cells function[
46,
47]. Add to this, since we gave evidence that TIGIT is upregulated in advanced forms of glioma, our results concorded with a study where it was shown that FoxP3 was upregulated in advanced grades compared to lower grades of glioma [
48]. Altogether, our data suggest that the presence of several immune checkpoints and high levels of Treg cells represent an immuno-suppressive and hence, unfavorable tumor microenvironment to glioma patients.
Therefore, we wanted to check in our Moroccan cohort, whether
TIGIT could also represent a bad prognosis to glioma patients. We evaluated the potential association between
TIGIT and
FoxP3, as a regulatory T cell marker [
49]. We found that
TIGIT was positively correlated to
FoxP3+-regulatory T cells in the Moroccan patients’ cohort.
Finally, when we wanted to test whether TIGIT could be a prognosis factor in glioma, we started by evaluating the impact of high expression of TIGIT on the overall survival. We found that elevated rates of this molecule represented bad prognosis to glioma patients, especially those presenting with astrocytoma subtype. Also, we proceeded to a multivariate logistic regression analysis of TIGIT. The latter had a significant impact only on astrocytoma, supporting the fact that TIGIT could play a key role in determining the prognosis in glioma patients, especially in the case of astrocytoma.
As we mentioned previously, current immunotherapeutic targets show only limited outcome.
TIGIT exhibited a significant association to tumor proliferation in several tumor locations. Furthermore, the blockade of
TIGIT was associated with an inhibition of tumor progression and an improvement of the overall survival in a murine model [
19,
50].
Nevertheless, our study has also limitations. A major one is related to the small size of the Moroccan cohort, which did not help to better assess the possible association of TIGIT with several other clinical features. In addition, our findings need to be confirmed by assessing TIGIT protein expression. Finally, further studies need to evaluate the impact of blocking TIGIT in human glioma, especially in improving patients’ overall survival.
5. Conclusions
In summary, our data suggest that TIGIT expression was associated to human glioma progression and that the blockade of TIGIT could help reducing the immunosuppression state in the glioma microenvironment and therefore, allow the reactivation of the anti-tumor immune response.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Figure S1: Molecular subtypes of GBM exhibited different levels of expression of TIGIT. ANOVA test was assessed to analyze differences between means of expressions of different molecular subtypes of TCGA GBM patients. The Mesenchymal molecular subtype exhibited the highest rate of expression of TIGIT compared to the other molecular subtypes (Classical, G-CIMP, Neural and Proneural). Significant difference is indicated (* for p < 0.05).; Table S1: Differences between molecular subtypes of glioblastoma patients from the TCGA cohort depending on the expression of TIGIT
Author Contributions
All the authors cooperated sufficiently to take part of the content. A. Q. collected, analyzed and interpreted data, also wrote the manuscript. O. N. collected and analyzed data, A. G. collected and analyzed data, A. K. analyzed data A. L. collected and analyzed data, A. B. designed and supervised the study, analyzed and interpreted data and revised the final draft of the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by the Moroccan Ministry of Higher Education, Research and innovation through a “PPR1” project and by the Moroccan Ministry of Higher Education, Research and innovation and the Digital Development Agency "ADD” through an “Al-khawarizmi” project, coordinated both by Abdallah Badou. Ahmed Qandouci was supported by a national Moroccan scholarship
Informed Consent Statement
The consent was verified and approved by the ethical committee of the Ibn Rochd University Hospital Center, Casablanca, according to the guidelines of the Declaration of Helsinki. Written informed consent was provided and signed by all patients.
Data Availability Statement
Datasets analyzed during the current study are freely available via
https://www.cbioportal.org/. Other data are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank all the team members of the Neurosurgery Department of University Hospital Center Ibn Rochd, Casablanca, Morocco
Conflicts of Interest
The authors declare no conflict of interest in this work.
Abbreviations:
ANOVA: Analysis Of Variances
CD: Cluster of Differentiation
cDNA: Complementary Deoxyribonucleic Acid
CTLA-4: cytotoxic T-lymphocyte-associated protein 4
FoxP3: Forkhead box P3
GBM: Glioblastoma Multiform
G-CIMP: Glioma CpG Island Methylator Phenotype
IDH : Isocitrate Dehydrogenase
IL-10 : Interleukin-10
LAG3: Lymphocyte Activation Gene 3
LGG: Low Grade Glioma
mRNA: messenger Ribonucleic Acid
NK: Natural Killer
PD-1: Programmed cell Death 1
PDL-1: Programmed cell Death Ligand 1
qPCR : quantitative Polymerase Chain Reaction
RT-PCR : Reverse Transcription Polymerase Chain Reaction
SPSS: Statistical Package for the Social Science
TCGA: The Cancer Genome Atlas
TIGIT: T-cell immunoglobulin and ITIM domain
TILs: Tumor Infiltrating Lymphocyte
Tim-3: T-cell Immunoglobulin and mucin domain
TGF-b: Transforming growth factor-beta
VISTA: V-domain Ig suppressor of T-cell Activation
WHO: World Health Organization.
References
- Schwartzbaum, J.A.; Fisher, J.L.; Aldape, K.D.; Wrensch, M. Epidemiology and molecular pathology of glioma. Nat Rev Neurol. 2006, 2, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Kone, A.; Ait Ssi, S.; Sahraoui, S.; Badou, A. BTN3A: A Promising Immune Checkpoint for Cancer Prognosis and Treatment. International Journal of Molecular Sciences. 2022, 23, 13424. [Google Scholar] [CrossRef] [PubMed]
- Ghouzlani, A.; Kandoussi, S.; Tall, M.; Reddy, K.P.; Rafii, S.; Badou, A. Immune Checkpoint Inhibitors in Human Glioma Microenvironment. Frontiers in Immunology. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; Brahmer, J.R.; McDermott, D.F.; Smith, D.C.; Gettinger, S.N. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with advanced solid tumors: Survival and long-term safety in a phase I trial. JCO. 2013, 31 (Suppl. 15), 3002–3002. [Google Scholar] [CrossRef]
- Heimberger, A.B.; Kong, L.-Y.; Abou-Ghazal, M.; Reina-Ortiz, C.; Yang, D.S.; Wei, J.; et al. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin Neurosurg. 2009, 56, 98–106. [Google Scholar] [PubMed]
- Ait Ssi, S.; Chraa, D.; El Azhary, K.; Sahraoui, S.; Olive, D.; Badou, A. Prognostic Gene Expression Signature in Patients With Distinct Glioma Grades. Front Immunol. 2021, 12, 685213. [Google Scholar] [CrossRef]
- Tan, A.C.; Heimberger, A.B.; Khasraw, M. Immune Checkpoint Inhibitors in Gliomas. Curr Oncol Rep. 2017, 19, 23. [Google Scholar] [CrossRef]
- Boles, K.S.; Vermi, W.; Facchetti, F.; Fuchs, A.; Wilson, T.J.; Diacovo, T.G.; et al. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC: HIGHLIGHTS. Eur J Immunol. 2009, 39, 695–703. [Google Scholar] [CrossRef]
- Levin, S.D.; Taft, D.W.; Brandt, C.S.; Bucher, C.; Howard, E.D.; Chadwick, E.M.; et al. Vstm3 is a Member of the CD28 Family and an Important Modulator of T Cell Function. Eur J Immunol. 2011, 41, 902–915. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nature Immunology. 2009, 10, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; et al. Cutting Edge: TIGIT Has T Cell-Intrinsic Inhibitory Functions. JI. 2011, 186, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.-F.; Joller, N.; Tan, D.J.; Teng, M.W.L.; et al. TIGIT predominantly regulates the immune response via regulatory T cells. Journal of Clinical Investigation. 2015, 125, 4053–4062. [Google Scholar] [CrossRef] [PubMed]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proceedings of the National Academy of Sciences. 2009, 106, 17858–17863. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.-M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; et al. TIGIT and PD-1 impair tumor antigen–specific CD8+ T cells in melanoma patients. J Clin Invest. 2015, 125, 2046–2058. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhu, L.; Schell, T.D.; Zhang, J.; Claxton, D.F.; Ehmann, W.C.; et al. T-Cell Immunoglobulin and ITIM Domain (TIGIT) Associates with CD8+ T-Cell Exhaustion and Poor Clinical Outcome in AML Patients. Clin Cancer Res. 2016, 22, 3057–3066. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, J.; Chen, Y.; Cui, J.; Lei, Y.; Cui, Y.; et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma). International Immunopharmacology. 2020, 80, 106198. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Li, W.-Q.; Wu, Y.-H.; Han, L.; Cao, X.-G.; Yang, X.-M.; et al. Intrinsic Expression of Immune Checkpoint Molecule TIGIT Could Help Tumor Growth in vivo by Suppressing the Function of NK and CD8+ T Cells. Front Immunol. 2018, 9, 2821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nature Immunology. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Xu, F.; Sunderland, A.; Zhou, Y.; Schulick, R.D.; Edil, B.H.; Zhu, Y. Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions. Cancer Immunol Immunother. 2017, 66, 1367–1375. [Google Scholar] [CrossRef]
- Hung, A.L.; Maxwell, R.; Theodros, D.; Belcaid, Z.; Mathios, D.; Luksik, A.S.; et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. OncoImmunology. 2018, e1466769. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Wing, K.; Miyara, M. Regulatory T cells – a brief history and perspective. European Journal of Immunology. 2007, 37, S116–S123. [Google Scholar] [CrossRef] [PubMed]
- Ghouzlani, A.; Kandoussi, S.; Rafii, S.; Lakhdar, A.; Badou, A. High Expression Levels of Foxp3 and VISTA in Advanced Human Gliomas and Impact on Patient’s Prognosis. Arch Clin Biomed Res. 2020, 04. [Google Scholar] [CrossRef]
- Brandes, A.A.; Tosoni, A.; Franceschi, E.; Sotti, G.; Frezza, G.; Amistà, P.; et al. Recurrence Pattern After Temozolomide Concomitant With and Adjuvant to Radiotherapy in Newly Diagnosed Patients With Glioblastoma: Correlation With MGMT Promoter Methylation Status. JCO. 2009, 27, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Bent, M.J.v.d.; Taphoorn, M.J.; Janzer, R.C.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Ma, W.; Chen, Y.; Yu, Y.; Zhu, D.; Shi, J.; et al. Impact of gender on the survival of patients with glioblastoma. Biosci Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, Z.; Peng, Y.; Li, K.; Wang, X.; Huang, G.; et al. Classification of glioma based on prognostic alternative splicing. BMC Med Genomics. 2019, 12. [Google Scholar] [CrossRef]
- Hartmann, C.; Hentschel, B.; Wick, W.; Capper, D.; Felsberg, J.; Simon, M.; et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010, 120, 707–718. [Google Scholar] [CrossRef]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006, 9, 157–173. [Google Scholar] [CrossRef]
- Kaffes, I.; Szulzewsky, F.; Chen, Z.; Herting, C.J.; Gabanic, B.; Velázquez Vega, J.E.; et al. Human Mesenchymal glioblastomas are characterized by an increased immune cell presence compared to Proneural and Classical tumors. Oncoimmunology. 2019, 8. [Google Scholar] [CrossRef]
- Orrego, E.; Castaneda, C.A.; Castillo, M.; Bernabe, L.A.; Casavilca, S.; Chakravarti, A.; et al. Distribution of tumor-infiltrating immune cells in glioblastoma. CNS Oncol. 2018, 7. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; et al. Coregulation of CD8+ T cell exhaustion during chronic viral infection by multiple inhibitory receptors. Nat Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef]
- Perng, P.; Lim, M. Immunosuppressive Mechanisms of Malignant Gliomas: Parallels at Non-CNS Sites. Front Oncol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Lowther, D.E.; Goods, B.A.; Lucca, L.E.; Lerner, B.A.; Raddassi, K.; van Dijk, D. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight. 1. [CrossRef]
- Li, G.; Wang, Z.; Zhang, C.; Liu, X.; Cai, J.; Wang, Z.; et al. Molecular and clinical characterization of TIM-3 in glioma through 1,024 samples. Oncoimmunology. 2017, 6. [Google Scholar] [CrossRef]
- Ghouzlani, A.; Lakhdar, A.; Rafii, S.; Karkouri, M.; Badou, A. The immune checkpoint VISTA exhibits high expression levels in human gliomas and associates with a poor prognosis. Sci Rep. 2021, 11, 21504. [Google Scholar] [CrossRef] [PubMed]
- Huettner, C.; Paulus, W.; Roggendorf, W. Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am J Pathol. 1995, 146, 317–322. [Google Scholar]
- Zhang. Interleukin 10 promotes growth and invasion of glioma cells by up-regulating KPNA 2 in vitro. https://www.cancerjournal.net/article.asp?issn=0973-1482;year=2019;volume=15;issue=4;spage=927;epage=932;aulast=Zhang. Accessed 30 Apr 2021.
- Platten, M.; Wick, W.; Weller, M. Malignant glioma biology: Role for TGF-β in growth, motility, angiogenesis, and immune escape. Microscopy Research and Technique. 2001, 52, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Audenet, F.; Farkas, A.M.; Anastos, H.; Galsky, M.D.; Bhardwaj, N.; Sfakianos, J.P. Immune phenotype of peripheral blood mononuclear cells in patients with high-risk non-muscle invasive bladder cancer. World J Urol. 2018, 36, 1741–1748. [Google Scholar] [CrossRef]
- Lucca, L.E.; Lerner, B.A.; Park, C.; DeBartolo, D.; Harnett, B.; Kumar, V.P.; et al. Differential expression of the T-cell inhibitor TIGIT in glioblastoma and MS. Neurology - Neuroimmunology Neuroinflammation. 2020, 7. [Google Scholar] [CrossRef]
- Raphael, I.; Kumar, R.; McCarl, L.H.; Shoger, K.; Wang, L.; Sandlesh, P.; et al. TIGIT and PD-1 Immune Checkpoint Pathways Are Associated With Patient Outcome and Anti-Tumor Immunity in Glioblastoma. Frontiers in Immunology. 2021, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792–e1792. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.I.; Rhodin, K.E.; Chongsathidkiet, P.; Keith, K.A.; Fecci, P.E. T-cell Dysfunction in Glioblastoma: Applying a New Framework. Clinical Cancer Research. 2018, 24, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; et al. T Cell Exhaustion Signatures Vary with Tumor Type and are Severe in Glioblastoma. Clin Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef]
- Chen, M.-L.; Pittet, M.J.; Gorelik, L.; Flavell, R.A.; Weissleder, R.; von Boehmer, H.; et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo. Proceedings of the National Academy of Sciences. 2005, 102, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Humphries, W.; Wei, J.; Sampson, J.H.; Heimberger, A.B. The Role of Tregs in Glioma-Mediated Immunosuppression: Potential Target for Intervention. Neurosurgery Clinics. 2010, 21, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Heimberger, A.B.; Abou-Ghazal, M.; Reina-Ortiz, C.; Yang, D.S.; Sun, W.; Qiao, W.; et al. Incidence and Prognostic Impact of FoxP3+ Regulatory T Cells in Human Gliomas. Clinical Cancer Research. 2008, 14, 5166–5172. [Google Scholar] [CrossRef]
- Roncarolo, M.-G.; Gregori, S. Is FOXP3 a bona fide marker for human regulatory T cells? European Journal of Immunology. 2008, 38, 925–927. [Google Scholar] [CrossRef]
- Dixon, K.O.; Schorer, M.; Nevin, J.; Amoozgar, Z.; Kondo, T.; Kassam, N.; et al. Functional Anti-TIGIT Antibodies Regulate Development of Autoimmunity and Antitumor Immunity. :9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).