1. Introduction
Today's comprehensive knowledge and applied science about HPV as the causative agent for cervical cancer offers enormous potential for improved prevention of cervical cancer [
1]. For decades, cytology-based screening has been the most important strategy, reducing cervical cancer incidences and mortality by detection and treatment of precancerous lesions [
2]. However, the availability of new technologies and HPV-vaccination urges policymakers to review current screening policies and adapt practices. The transition to HPV primary screening is a logical step considering the overwhelming evidence from clinical data that HPV testing has higher sensitivity than cytology for cervical cancer and its precursors, implying increased safety [
3]. However, its specificity for CIN2+ is lower than cytology [
4].
Understanding the natural history of the HPV infection and progression to cervical cancer is key to tailoring an optimal program for prevention, balancing the benefits and harms of screening [
5]. HPV is a common infection, highly prevalent among the general population, declining with age. The risk of future cell abnormalities, linked to the overexpression of the E6/E7 viral oncogenes affecting cellular pathways, varies strongly by HPV genotype and duration of infection [
6]. Most of the infections are transient and will be eliminated by the immune system during 1 to 2 years, resulting in a considerable risk of overdiagnosis and overtreatment of the HPV primary screening positives [
7].
Several countries in Europe have implemented molecular testing while it is still debated how to best manage the HPV screened positives [
8]. To date, cytology alone or in combination with partial genotyping are the recommended triage options. However, cervical cytology is an imperfect test due to its subjectivity, poor reproducibility where its sensitivity varies widely, reducing the overall program sensitivity [
9].
Given the above, onus is still on the expert community to search for alternatives for how to improve management of the HPV-positive women, being especially important in the post-vaccination era where the change of HPV prevalence is inevitable. In a recent work by Nygård et al. they performed a large population-based HPV prevalence study in the Nordic region, investigating the associated risk by different genotypes. Based on risk profiles, they concluded that current screening of HPV-vaccinated cohorts might perform better if restricted to the HPV types in the nonavalent vaccine [
10]. Literature describes the seven genotypes to be the most oncogenic HPV-types, identified in 90% of cervical cancer tissue worldwide [
11]. Wentzensen et al. further discuss how clinical management of women with cervical cancer screening results is moving to use risk thresholds rather than individual test results, describing the goals of screening this way: «An optimal integrated screening and triage strategy should reassure the vast majority of women that they are at very low risk of cervical cancer, send the women at highest risk to colposcopy at the right time, when disease can be colposcopically detected, and minimize the intermediate risk group that requires continued surveillance» [
12]. Among the promising biomarkers of interest, detection of mRNA E6/E7 could serve as a risk-based approach to differentiate between women warranted for immediate colposcopy and biopsy from women that could safely return to follow-up [
13].
In this study we compared the performance of a 7-type HPV mRNA test, detecting and individually genotyping the seven oncogenic types as included in the HPV vaccine, to cervical cytology being the approved strategy in triage of the HPV-DNA primary screen positive women, focusing on the number of colposcopies per CIN2+ detected.
2. Materials and Methods
The Norwegian Cervical Cancer Screening program (NCCSP) invites all women between the ages of 25- and 69-years to participate. Recently, HPV DNA primary screening every 5th year was implemented, starting from the age of 34 years while primary cytology every 3rd year is maintained for women 25-33 years of age [
14]. The department of Clinical Pathology at the University Hospital of North Norway receives samples from all women participating in screening in the northernmost county in Norway, Troms and Finnmark [
15]. Following the governmental roll-out plan for a gradual introduction of HPV-DNA test in primary screening, 50% of the women 34-69 years of age were selected for HPV testing during 2019 throughout 2020, being fully implemented from 2021 [
16]. During January 1.
st, 2019, until December 31.
st, 2021, cervical samples from 58,029 women were sent to the department of Clinical Pathology for examination. 30.5% (17,684/58,029) of the women fulfilled the criteria for HPV-DNA primary screening, representing a low-risk population. The department utilised ThinPrep liquid-based cytology (Hologic) and Cobas 4800 HPV-test (Roche) in daily routine. According to national guidelines, HPV-DNA primary screen positives are triaged by cytology and reported using the Bethesda classification system [
17]. Cervical biopsies are diagnosed using the WHO classification [
18]. Our study population comprised the 17,684 women selected for primary HPV-DNA-testing during 2019-2021, with follow-up until December 31st, 2021. All test positive samples were triaged by cytology being the standard practice and followed-up according to national guidelines [
14]. Additionally, all positive HPV-DNA samples were triaged by a commercially available 7-type HPV mRNA test. Study endpoint was histologically confirmed cervical intraepithelial neoplasia grade 2 (CIN2+).
HPV mRNA testing: After processing for HPV-DNA and LBC, the leftover material was sent for HPV mRNA testing at an accredited laboratory (PreTect AS). Nucleic acids were isolated from 1 ml of the sample material using PreTect X (PreTect AS, Klokkarstua, Norway) and subsequently analyzed for HPV mRNA E6/E7 expression from types 16, 18, 31, 33, 45, 52 and 58 (PreTect HPV-Proofer`7, PreTect AS, Klokkarstua, Norway) according to the manufacturer’s instructions. The assay is based on real time Nucleic Acid Sequence Based Amplification (NASBA) technology, an isothermal RNA amplification method targeting full-length E6/E7 transcripts providing qualitative results. Assay validation comprised positive and negative controls corresponding to the viral mRNA for all targets, including an intrinsic sample control confirming sample adequacy. Results were interpreted and presented by the PreTect Analysis Software.
Statistical analysis: Data were analyzed in Statistical Package for Social Sciences (SPSS) version 28.0 with Chi-square test and Chi-square test for trend with p-values < 0.05 as significance level.
Ethical approval: The Regional Committee for Medical and Health Research Ethics (REC North) has approved the protocol as quality assurance (REK nord 203384). Norwegian regulations exempt quality assurance studies from written informed consent from the patients.
3. Results
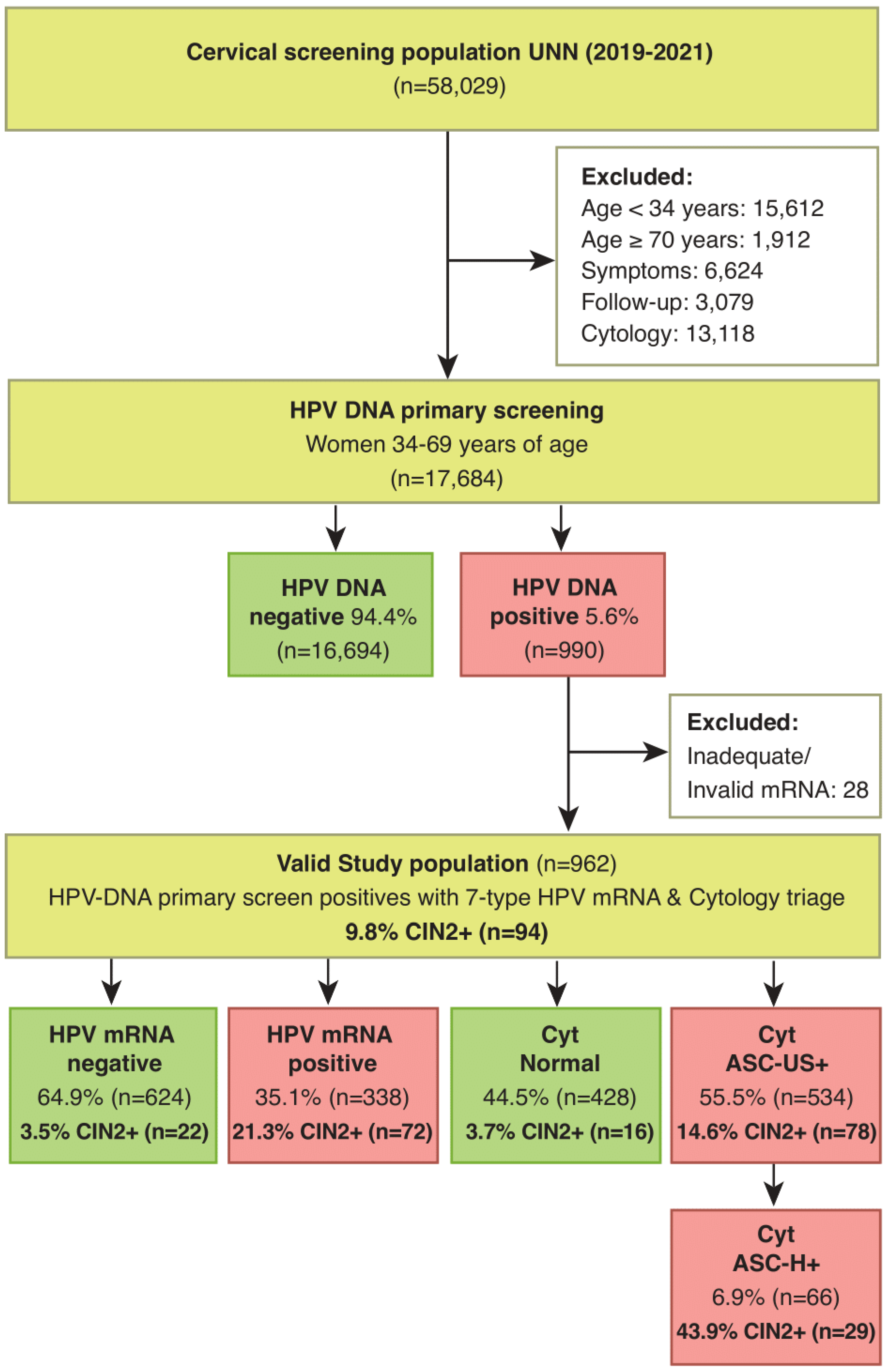
Of the 17,684 women with an HPV-DNA test as primary screening, 990 women (5.6%) had a positive HPV-DNA test. Included were 962 women with a positive HPV-DNA test and a valid HPV mRNA test. In triage of HPV-DNA positive women 55.5% (534/962) had abnormal cytology (ASC-US+) and 35.1% (338/962) had a positive HPV mRNA test (
Figure 1). Of the women with a positive HPV-DNA test, 318 women had biopsy during follow-up.
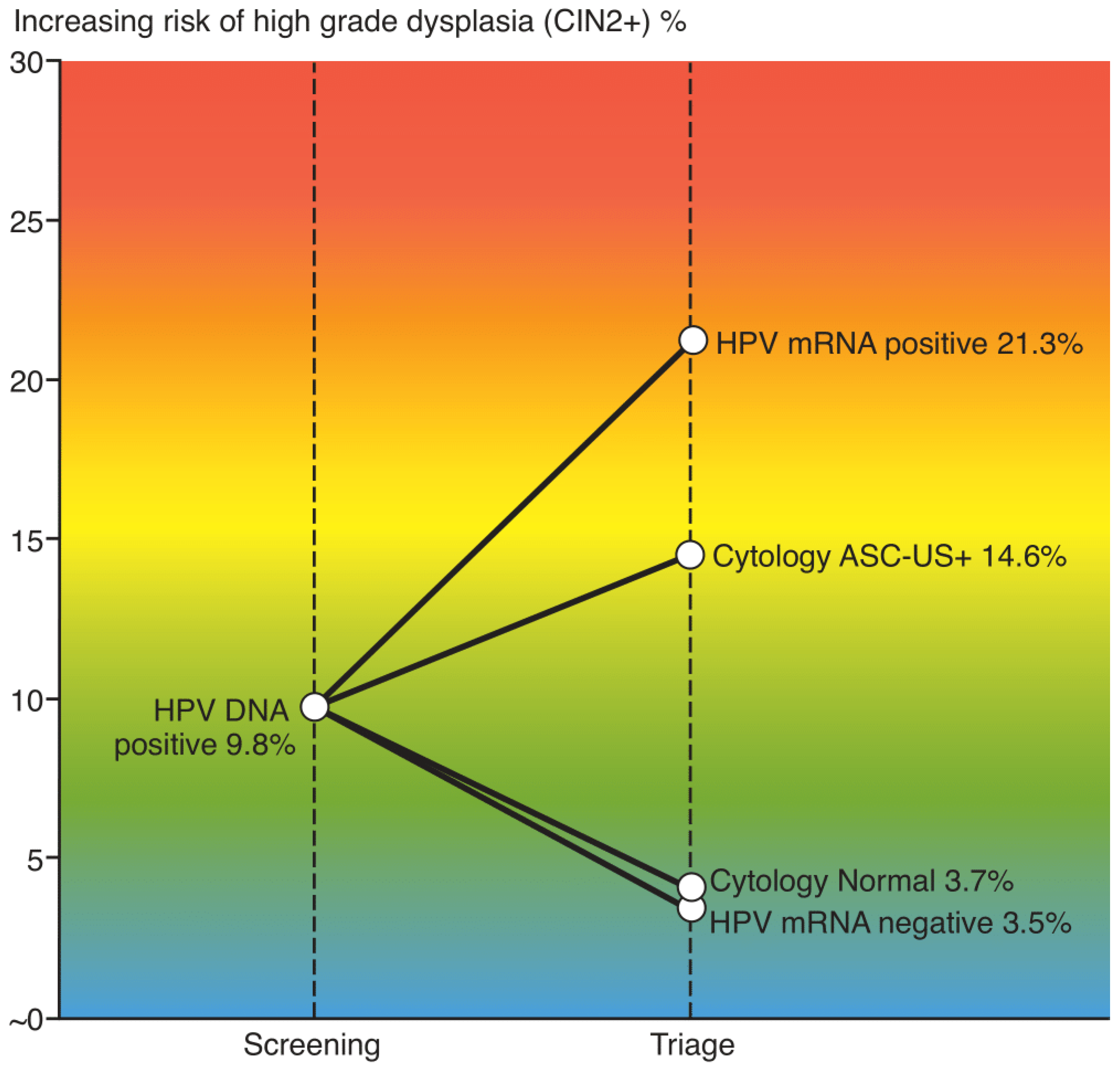
In total 94 women 9.8% (94/962) had CIN2+. The sensitivity of cytology versus the HPV mRNA test in triage of HPV-DNA positive women was 83.0% (78/94) versus 76.6% (72/94), p=0.36. The specificity of cytology versus the HPV mRNA test was 47.5% (412/868) versus 69.4% (602/868), p<0.001. The PPV for CIN2+ was 14.6% (78/534) for cytology and 21.3% (72/338) for the HPV mRNA test, p=0.014. NPV was 96.3 (412/428) and 96.5% (602/624) respectively.
Using high grade cytology (ASC-H+) as cut-off instead of ASC-US+, the number of triage positives was reduced from 55.5% (534/962) to 6.9% (66/962), while the sensitivity for CIN2+ dropped from 83.0% (78/94) to 30.9% (29/94).
Restricting the number of HPV-types in the HPV mRNA test from seven (16, 18, 31, 33, 45, 52 and 58) to five (16, 18, 31, 33 and 45), the number of triage positives was reduced from 35.1% (338/962) to 27.9% (268/962), p<0.001, and the sensitivity for CIN2+ dropped from 76.6% (72/94) to 69.1% (65/94), p=0.325, (
Table 1). The number of colposcopies per CIN2+ detected by cytology and HPV mRNA test were 6.8 and 4.7 respectively (
Table 2).
The increased risk of high-grade dysplasia across the triage strategies are illustrated in
Figure 2.
4. Discussion
Molecular HPV testing has shown to be an objective, preferential method in preventing cervical cancer. HPV DNA tests detect the presence of virus, in both transient and persistent state, while mRNA tests identify only transcriptionally active viruses. The established cause of cervical cancer is not the infection per se, but an overexpression of the E6/E7 viral oncogenes affecting cellular pathways, thus enabling HPV mRNA tests to inform of a high-risk condition.
In view of HPV being highly prevalent (8-10% in western countries) and the lifetime risk for HPV estimated to be up to 80% for sexually active people combined with the biology of the viral infections where most are self-resolving [
19], the benefits of a sensitive 14-type HPV test in primary screening are challenged by the low specificity [
4]. The risk of clinically significant disease (CIN3+) among HPV-DNA positive women is only 5-8% [
20], a contrast that might result in unnecessary follow up and over treatment of test positive women. Ideally, optimal screening strategies would detect as many CIN3+ as possible while keeping the number of colposcopies at an acceptable level. Thus, requiring a highly specific test in triage of the HPV screen positive women [
21].
Evaluating which triage approach that might perform better for HPV DNA primary screen positive women, we compared the performance of liquid-based cytology to a 7-type HPV mRNA test. Our data confirms the 7-type HPV mRNA test to be significantly more specific than cervical cytology (69.4% versus 47.5%). Our findings are in line with the previously reported advantage of mRNA testing, verifying the E6/E7 mRNA transcripts to have a higher specificity, with the potential to limit the addressed harms of screening for cervical cancer in a population of healthy women [
13,
15,
22,
23,
24,
25,
26]. The discussion of whether a lower sensitivity for the mRNA-test compared to cytology (76.6% versus 83.0%, p=0.36) might negatively affect the effectiveness of screening is of minor importance as long as triage negative women are followed up with a new HPV DNA test after 12 months. Realistically no test is 100% perfect, and we must own up to the need for periodically repeated testing for triage negative women, informed by the tests’ safety (NPV). In the present analysis, NPV for the approved triage algorithm cytology was 96.3% while the mRNA test was providing equal safety, 96.5%, justifying return to follow-up for triage negative women.
The recognition that persistent HPV infection is necessary for developing precancer and cancer and the characteristics of infections including which genotype and duration of infection, underlies the new US risk-based approach for assessing a patient’s immediate risk of precancer [
27]. The value of HPV extended genotyping for clinical management of abnormal screening results is well established in the literature [
21]. Scientific data over decades have been informing of the risk profile per genotype and their cancer-causing strengths and have identified seven hrHPV-types (HPV 16, 18, 31, 33, 45, 52, 58) as the most prevalent and aggressive types associated with cervical cancer [
11]. These data have helped to improve the HPV vaccine by including the same seven types for maximal protection and informed the design of new molecular tests entailing extended genotyping. Literature describes the E6/E7 mRNA biomarkers to have a good correlation with the risk of cervical cancer development [
13,
15,
22,
23,
24,
25,
26]. Additionally, in contrast to cervical cytology, triage by molecular biomarkers allows the use of HPV-self-sampling [
28]. Importantly, self-sampling has been anticipated to offering great potential to increased participation of hard-to-reach women, thus improving prevention of cervical cancer [
29].
Most countries having implemented HPV DNA test in primary screening use cytology in triage of screen positive women, applying ASC-US as cut-off. When tailoring an optimal screening algorithm, it is important to carefully choose a threshold for referral to colposcopy based on the balance between benefits and harms. In countries with limited colposcopy resources, it might be justified to make use of ASC-H as a cut-off to reduce the number of colposcopies, while still being able to identify the few women warranted for immediate colposcopy and treatment. In present material, this strategy would reduce the referral rate at baseline from 55.5% to 6.9%, at the expense of a substantial reduction of sensitivity for CIN2+ from 83.0% to 30.9% and lowering the number of colposcopies per CIN2+ from a ratio of 6.8 for ASC-US+ to 2.3 for ASC-H+. It must be noted that in developed countries a sensitivity for CIN2+ at baseline of 30.9% is considered far too low for implementation of such strategy.
In Europe, most cases of cervical cancer are caused by only five hrHPV-types (HPV 16, 18, 31, 33 and 45) [
11]. Restricting the number of HPV-types in triage to mRNA expression from these five types in this study, the referral rate was reduced from 35.1% to 27.9% (p<0.001). The sensitivity for CIN2+ was reduced from 76.6% to 69.1%, (p=0.325) and the corresponding number of colposcopies per CIN2+ was ratio 4.7 using seven HPV mRNA-types and 4.1 using five HPV mRNA-types as a cut-off. Given the significant reduction of number of referrals and insignificant drop of sensitivity, the five-type approach might be worth considering. However, the slight reduction of number of colposcopies per CIN2+ (4.7-4.1) must be weighed against the increased detection of CIN2 in a prospective cost-benefit analysis.
In the previous work of Stoler et al., various triage strategies including combinations of genotypes and dual-stained cytology were evaluated for a subset of women with hrHPV infections who were participating in the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study [
21]. Among the HPV-DNA genotype combinations evaluated, the 7-type (HPV16/18/31/33/45/52/58) were found to be more sensitive than the FDA approved algorithm (HPV16/18+ or 12-other hrHPV+ AND Pap+) but also to slightly increase the number of colposcopies per CIN3 detected from a ratio of 8.4 to 8.9. Taken in consideration that the reported HPV types were detecting DNA, identified using the LINEAR ARRAY HPV Genotyping Test, a direct comparison to our material is not possible. However, utilizing mRNA E6/E7 extended genotyping, it is presumed a lower relative positivity rate for mRNA types versus the corresponding DNA, especially in a low-risk population such as women attending primary screening. In Predictors 3, Cuzick et al. reported a rel. positivity rate for HPV DNA 16 at 3,5% (Cobas 4800, Roche) versus 2.1% detected by PreTect HPV-Proofer [
30]. In present material, HPV 16+ cases were 0.7% versus 0.4% for mRNA16 positives. Integrating a 7-type mRNA test in triage would most likely improve the clinical utility over what has been reported for DNA extended genotyping, having higher specificity, and lowering the number of colposcopies per detected CIN2.
In 2015, Norway ran a pilot using an HPV DNA test in primary screening in four counties [
31]. All HPV DNA positive women with abnormal cytology (ASC-US+) were referred to colposcopy and biopsy, giving a three times higher referral rate for colposcopy at baseline in the HPV-arm compared to the cytology arm. Experiences from the pilot demonstrated that HPV 16/18 genotyping provided further risk stratification and reduced over referral of all women who had an HPV positive and low-grade cytology (ASC-US / LSIL) result to colposcopy and biopsy. Women with low-grade cytology and HPV 16/18 should be referred to colposcopy, while women with low-grade cytology and other HPV-types should be followed-up with a new HPV-test after 12 months [
14]. From 2021, this approach was fully implemented in the Norwegian algorithm, expected to inevitably reduce the referral rate of HPV DNA positive women, but also reduce the sensitivity of CIN2+ at baseline. Given the high inter-laboratory differences for cytology results observed in Norway [
9,
14], an objective biomarker as a 7-type HPV mRNA test might serve as a better alternative to reach the goal of screening, hereby maximizing the number of immediate CIN3+ detections and minimize the number of colposcopies. Still, prospective studies addressing the associated costs of shifting from an already established practice such as cytology to a molecular triage test are required.
4.1. Strengths and limitations
One of the strengths of this study is the continued enrollment of all women attending screening in one county in Norway, still ongoing. All results have been derived from the original liquid-based cytology sample, making a direct comparison of the triage alternatives possible. A major limitation is that follow up of test positive women has been according to cytology only, complying with the approved Norwegian algorithm. Cytology negative, 7-type mRNA positive women were not scheduled to colposcopy/biopsy and the reported performance of the mRNA test in this analysis might be underestimated accordingly.
Also, taken in consideration that present data do not include an HPV-vaccinated population, performance might be directly impacted by vaccinated women entering screening in 2031. Inevitably, debates on how to adapt screening practices in future must recognize the importance of triage alternatives addressing a high-risk condition among a low prevalence disease population.
5. Conclusions
In summary, this analysis found the 7-type HPV mRNA test to be significantly more specific than cervical cytology in triage of the HPV DNA positive women. Using this biomarker as a threshold for referral to colposcopy may better balance the benefits and harms of screening. Women with a positive HPV DNA test, but negative HPV mRNA test can safely be followed up with repeat HPV DNA testing after 12 months.
Author Contributions
Conceptualization, S.W.S. and B.M.F.; methodology, S.W.S. and B.M.F.; formal analysis, S.W.S.; investigation, S.W.S., M.A and B.M.F.; resources, S.W.S., M.A and B.M.F.; data curation, S.W.S.; writing—original draft preparation, S.W.S. and B.M.F.; writing—review and editing, S.W.S., M.A. and B.M.F.; visualization, S.W.S. and B.M.F.; supervision, S.W.S.; project administration, S.W.S. and B.M.F.; funding acquisition, S.W.S. and B.M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. HPV mRNA test kits were provided FOC by PreTect AS.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Regional Committee for Medical and Health Research Ethics (REC North, 203384, 18th December 2020) for studies involving humans.
Informed Consent Statement
Patient consent was waived due to Norwegian regulations which exempt quality assurance studies from written informed consent.
Data Availability Statement
All data presented are available upon request.
Acknowledgments
The authors would like to extend our gratitude to the staff at the department of Clinical Pathology at the University Hospital of North Norway and to all the laboratory staff performing HPV DNA/mRNA testing, cytology and histopathology evaluation for their great work and collaboration during this study.
Conflicts of Interest
S.W.S and M.A declare no conflict of interest. B.M.F is an employee of PreTect AS.
References
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12-9.
- Denny L. Cytological screening for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2012;26(2):189-96. [CrossRef]
- Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524-32. [CrossRef]
- Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8:CD008587. [CrossRef]
- de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2-13. [CrossRef]
- zur HH. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342-50.
- Sroczynski G, Esteban E, Widschwendter A, Oberaigner W, Borena W, von Laer D, et al. Reducing overtreatment associated with overdiagnosis in cervical cancer screening-A model-based benefit-harm analysis for Austria. Int J Cancer. 2020;147(4):1131-42. [CrossRef]
- Cuschieri K, Ronco G, Lorincz A, Smith L, Ogilvie G, Mirabello L, et al. Eurogin roadmap 2017: Triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer. 2018;143(4):735-45. [CrossRef]
- Sorbye SW, Suhrke P, Reva BW, Berland J, Maurseth RJ, Al-Shibli K. Accuracy of cervical cytology: comparison of diagnoses of 100 Pap smears read by four pathologists at three hospitals in Norway. BMC Clin Pathol. 2017;17:18. [CrossRef]
- Nygard M, Hansen BT, Kjaer SK, Hortlund M, Tryggvadottir L, Munk C, et al. Human papillomavirus genotype-specific risks for cervical intraepithelial lesions. Hum Vaccin Immunother. 2021;17(4):972-81. [CrossRef]
- Arbyn M, Tommasino M, Depuydt C, Dillner J. Are 20 human papillomavirus types causing cervical cancer? J Pathol. 2014;234(4):431-5.
- Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J Clin Virol. 2016;76 Suppl 1:S49-S55. [CrossRef]
- Derbie A, Mekonnen D, Woldeamanuel Y, Van Ostade X, Abebe T. HPV E6/E7 mRNA test for the detection of high grade cervical intraepithelial neoplasia (CIN2+): a systematic review. Infect Agent Cancer. 2020;15:9.
- Hashim D, Engesaeter B, Baadstrand SG, Castle PE, Bjorge T, Trope A, et al. Real-world data on cervical cancer risk stratification by cytology and HPV genotype to inform the management of HPV-positive women in routine cervical screening. Br J Cancer. 2020;122(11):1715-23. [CrossRef]
- Sorbye SW, Fismen S, Gutteberg TJ, Mortensen ES, Skjeldestad FE. HPV mRNA is more specific than HPV DNA in triage of women with minor cervical lesions. PLoS One. 2014;9(11):e112934. [CrossRef]
- Maver PJ, Poljak M. Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect. 2020;26(5):579-83. [CrossRef]
- Nayar R, Wilbur DC. The Bethesda System for Reporting Cervical Cytology: A Historical Perspective. Acta Cytol. 2017;61(4-5):359-72. [CrossRef]
- Richart RM. Cervical intraepithelial neoplasia. Pathol Annu. 1973;8:301-28.
- Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda GA, Zhou Y, et al. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front Public Health. 2020;8:552028. [CrossRef]
- Castle PE, Glass AG, Rush BB, Scott DR, Wentzensen N, Gage JC, et al. Clinical human papillomavirus detection forecasts cervical cancer risk in women over 18 years of follow-up. J Clin Oncol. 2012;30(25):3044-50. [CrossRef]
- Stoler MH, Baker E, Boyle S, Aslam S, Ridder R, Huh WK, et al. Approaches to triage optimization in HPV primary screening: Extended genotyping and p16/Ki-67 dual-stained cytology-Retrospective insights from ATHENA. Int J Cancer. 2020;146(9):2599-607.
- Rijkaart DC, Berkhof J, van Kemenade FJ, Coupe VM, Hesselink AT, Rozendaal L, et al. Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int J Cancer. 2012;130(3):602-10. [CrossRef]
- Benevolo M, Vocaturo A, Caraceni D, French D, Rosini S, Zappacosta R, et al. Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J Clin Microbiol. 2011;49(7):2643-50.
- Origoni M, Cristoforoni P, Carminati G, Stefani C, Costa S, Sandri MT, et al. E6/E7 mRNA testing for human papilloma virus-induced high-grade cervical intraepithelial disease (CIN2/CIN3): a promising perspective. Ecancermedicalscience. 2015;9:533.
- Westre B, Giske A, Guttormsen H, Sorbye SW, Skjeldestad FE. 5-type HPV mRNA versus 14-type HPV DNA test: test performance, over-diagnosis and overtreatment in triage of women with minor cervical lesions. BMC Clin Pathol. 2016;16:9. [CrossRef]
- Sorbye SW, Fismen S, Gutteberg T, Mortensen ES. Triage of women with minor cervical lesions: data suggesting a "test and treat" approach for HPV E6/E7 mRNA testing. PLoS One. 2010;5(9):e12724.
- Demarco M, Egemen D, Raine-Bennett TR, Cheung LC, Befano B, Poitras NE, et al. A Study of Partial Human Papillomavirus Genotyping in Support of the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis. 2020;24(2):144-7. [CrossRef]
- Aranda Flores CE, Gomez Gutierrez G, Ortiz Leon JM, Cruz Rodriguez D, Sorbye SW. Self-collected versus clinician-collected cervical samples for the detection of HPV infections by 14-type DNA and 7-type mRNA tests. BMC Infect Dis. 2021;21(1):504.
- Giorgi Rossi P, Fortunato C, Barbarino P, Boveri S, Caroli S, Del Mistro A, et al. Self-sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. Br J Cancer. 2015;112(4):667-75. [CrossRef]
- Cuzick J, Cadman L, Mesher D, Austin J, Ashdown-Barr L, Ho L, et al. Comparing the performance of six human papillomavirus tests in a screening population. Br J Cancer. 2013;108(4):908-13. [CrossRef]
- Engesaeter B, van Diermen Hidle B, Hansen M, Moltu P, Staby KM, Borchgrevink-Persen S, et al. Quality assurance of human papillomavirus (HPV) testing in the implementation of HPV primary screening in Norway: an inter-laboratory reproducibility study. BMC Infect Dis. 2016;16(1):698. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).