Submitted:
07 January 2023
Posted:
10 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
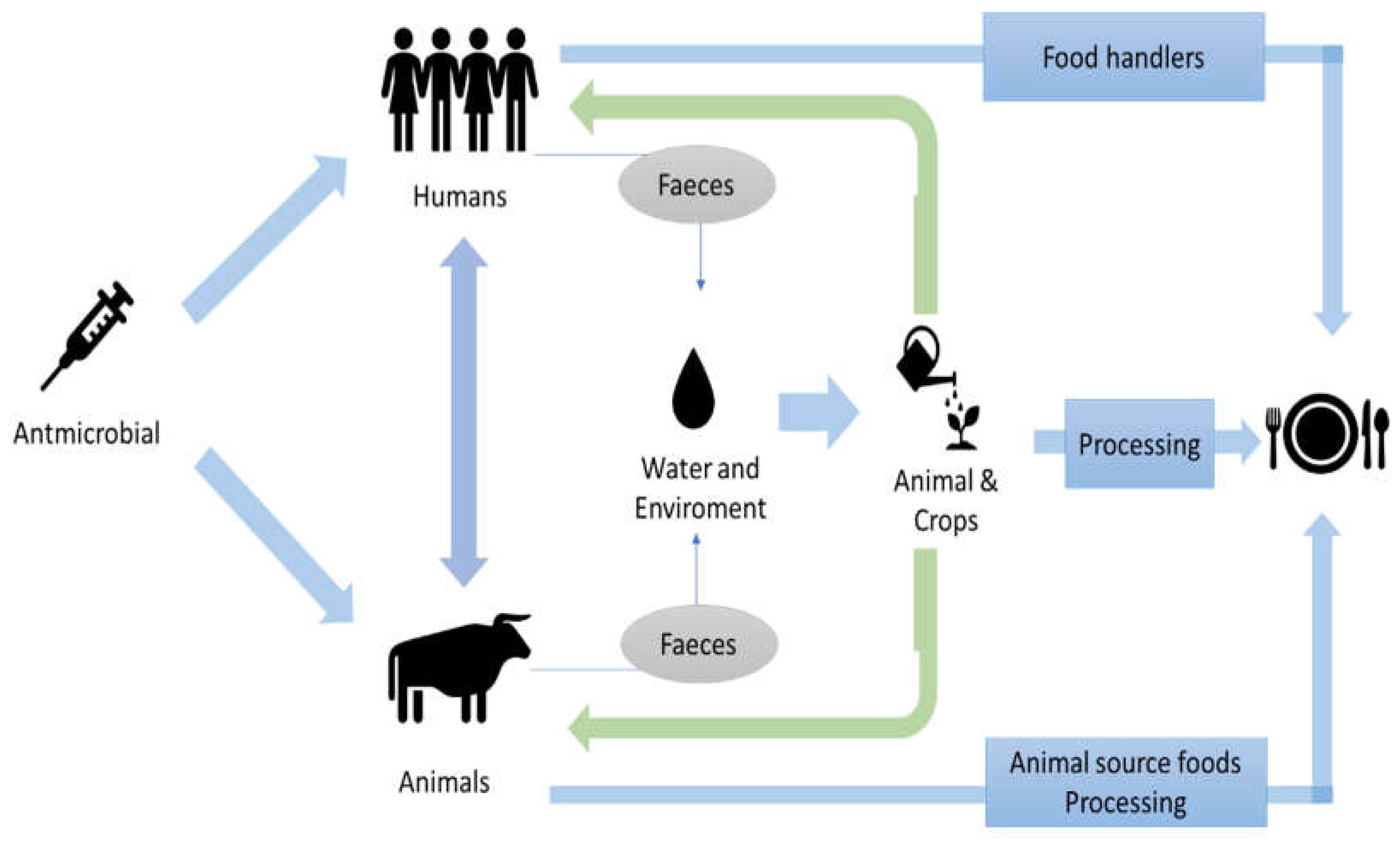
2. Antibiotic Resistance in the Food Chain
2.1. Antibiotic Resistance Acquisition and Transference Mechanisms
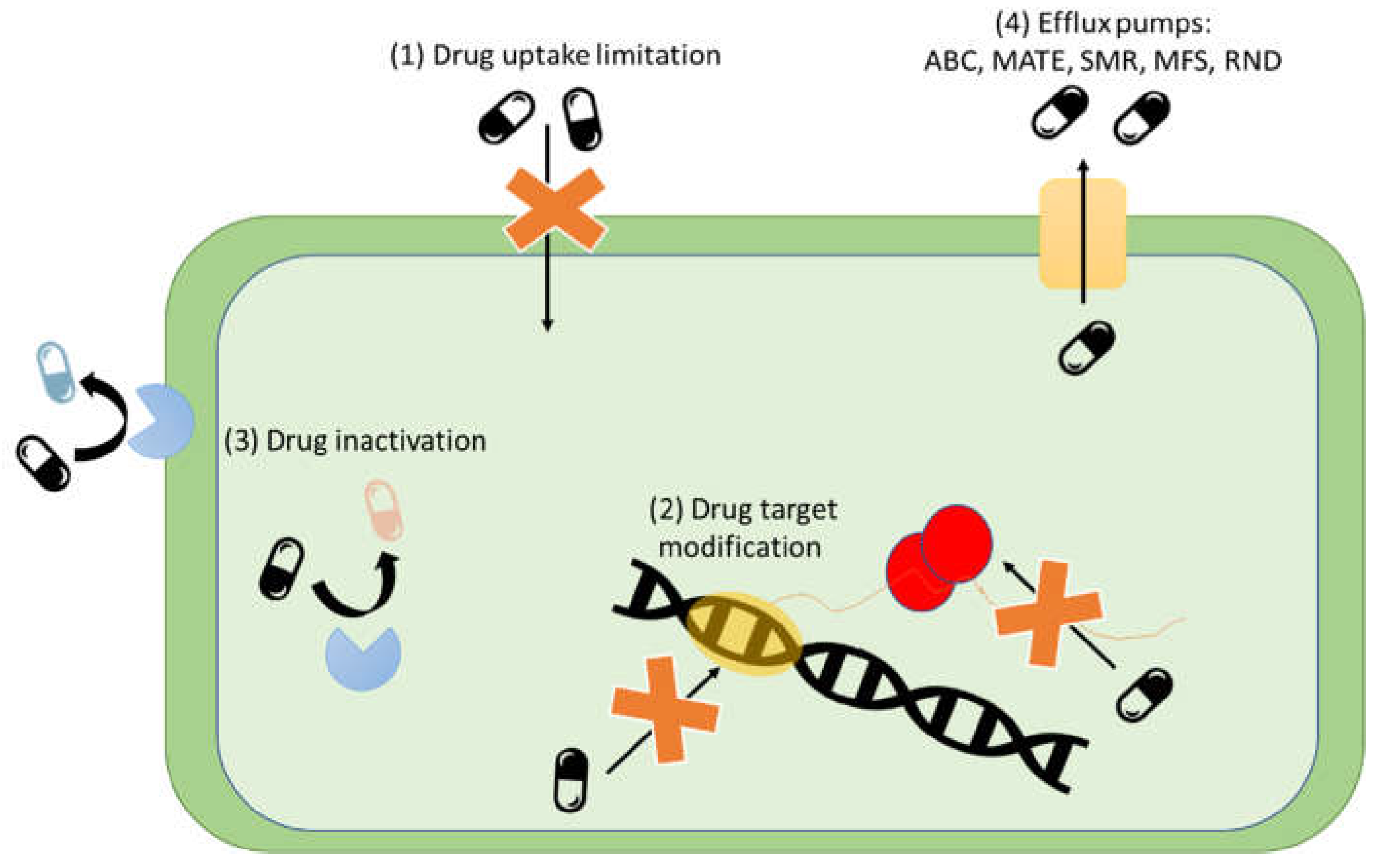
2.2. Mechanism Antibiotic Resistance
2.2.1. Drug Uptake Limitation
2.2.2. Drug Target Modification
2.2.3. Drug Inactivation
2.2.4. Drug Efflux
2.2.4.1. ABC Transporter Family
2.2.4.2. MATE Transporter Family
2.2.4.3. SMR Transporter Family
2.2.4.4. MFS Transporter Family
2.2.4.5. RND Transport Family
3. Potential Routes of Transmission and Prevalence of ABR in the Food Chain
5. Antibiotic Resistance and Food Safety: Implications on Public Health
6. New Alternatives to Antibiotics: Bacteriocins and Their Physicochemical Properties
8. Conclusions
Acknowledgments
References
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front Microbiol 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Roe, E.; Hocknell, S. Food Supply Chains and the Antimicrobial Resistance Challenge: On the Framing, Accomplishments and Limitations of Corporate Responsibility. Environment and Planning A: Economy and Space 2021, 53, 1373–1390. [Google Scholar] [CrossRef]
- Nelson, D.W.; Moore, J.E.; Rao, J.R. Antimicrobial Resistance (AMR): Significance to Food Quality and Safety. Food Quality and Safety 2019, 3, 15–22. [Google Scholar] [CrossRef]
- Nji, E.; Kazibwe, J.; Hambridge, T.; Joko, C.A.; Larbi, A.A.; Damptey, L.A.O.; Nkansa-Gyamfi, N.A.; Stålsby Lundborg, C.; Lien, L.T.Q. High Prevalence of Antibiotic Resistance in Commensal Escherichia Coli from Healthy Human Sources in Community Settings. Sci Rep 2021, 11, 3372. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The Threat of Antimicrobial Resistance in Developing Countries: Causes and Control Strategies. Antimicrob Resist Infect Control 2017, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.E.; Hammami, R. Recent Insights into Structure–Function Relationships of Antimicrobial Peptides. J Food Biochem 2019, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Tackling Antibiotic Resistance from a Food Safety Perspetive in Europe; Denmark, 2011; ISBN 978 92 890 1422 9.
- World Health Organization Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 7 September 2022).
- Cahill, S.M.; Desmarchelier, P.; Fattori, V.; Bruno, A.; Cannavan, A. Techniques in Food and Agriculture Global Perspectives on Antimicrobial Resistance in the Food Chain; 2017; Vol. 37.
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. The Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Balkhy, H.H.; Goossens, H.; Jarlier, V.; Kluytmans, J.; Laxminarayan, R.; Saam, M.; van Belkum, A.; Pittet, D. Antimicrobial Resistance: One World, One Fight! Antimicrob Resist Infect Control 2015, 4, 49. [Google Scholar] [CrossRef]
- Ochman, H.; Lawrence2, J.G.; Groisman3, E.A. Lateral Gene Transfer and the Nature of Bacterial Innovation; 2000; Vol. 405.
- Croucher, N.J.; Mostowy, R.; Wymant, C.; Turner, P.; Bentley, S.D.; Fraser, C. Horizontal DNA Transfer Mechanisms of Bacteria as Weapons of Intragenomic Conflict. PLoS Biol 2016, 14, e1002394. [Google Scholar] [CrossRef]
- Chiang, Y.N.; Penadés, J.R.; Chen, J. Genetic Transduction by Phages and Chromosomal Islands: The New and Noncanonical. PLoS Pathog 2019, 15. [Google Scholar] [CrossRef]
- Raleigh, E.A.; Low, K.B. Conjugation. In Brenner’s Encyclopedia of Genetics; Elsevier, 2013; pp. 144–151.
- Hall, R.M. Mobile Gene Cassettes and Integrons: Moving Antibiotic Resistance Genes in Gram-Negative Bacteria. In; 2007; pp. 192–205.
- van Bambeke, F.; Balzi, E.; Tulkens, P.M. Antibiotic Efflux Pumps. Biochem Pharmacol 2000, 60, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Richmond, G.E.; Piddock, L.J. Multidrug Efflux Pumps in Gram-Negative Bacteria and Their Role in Antibiotic Resistance. Future Microbiol 2014, 9, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol 1900, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Mahon, C.; Lehman, D.; Manuselis, G. Antimicrobial Agent Mechanism of Action and Resistance. In Textbook of diagnostic microbiology; Saunders: St. Louis, 2014; pp. 254–273. [Google Scholar]
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol Med 2020, 26, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Andreoletti, O.; Budka, H.; Buncic, S.; Colin, P.; Collins, J.D.; de Koeijer, A.; Griffin, J.; Havelaar, A.; Hope, J.; Klein, G.; et al. Foodborne Antimicrobial Resistance as a Biological Hazard - Scientific Opinion of the Panel on Biological Hazards. EFSA Journal 2008, 6, 765. [Google Scholar] [CrossRef]
- McDermott, P.F.; Zhao, S.; Wagner, D.D.; Simjee, S.; Walker, R.D.; White, D.G. The Food Safety Perspective of Antibiotic Resistance. Anim Biotechnol 2002, 13, 71–84. [Google Scholar] [CrossRef]
- You, Y.; Silbergeld, E.K. Learning from Agriculture: Understanding Low-Dose Antimicrobials as Drivers of Resistome Expansion. Front Microbiol 2014. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) Tackling Antibiotic from a food Safety perspective in Europe. 2011, 8–12.
- FAO-WHO Foodborne Antimicrobial Resistance; FAO.; WHO;, 2022; ISBN 978-92-5-135734-7.
- Kumar, K.; C. Gupta, S.; Chander, Y.; Singh, A.K. Antibiotic Use in Agriculture and Its Impact on the Terrestrial Environment. In; 2005; pp. 1–54.
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, Vol. 11, Page 1430 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Kimera, Z.I.; Mdegela, R.H.; Mhaiki, C.J.N.; Karimuribo, E.D.; Mabiki, F.; Nonga, H.E.; Mwesongo, J. Determination of Oxytetracycline Residues in Cattle Meat Marketed in the Kilosa District, Tanzania. Onderstepoort J Vet Res 2015, 82. [Google Scholar] [CrossRef] [PubMed]
- Olatoye, I.O.; Ehinmowo, A.A. Oxytetracycline Residues in Edible Tissues of Cattle Slaughtered in Akure, Nigeria. Internet Journal of Food Safety 2009, 11, 62–66. [Google Scholar] [CrossRef]
- Tavakoli, H.R.; Safaeefirouzabadi, M.S.; Afsharfarnia, S.; Joneidijafari, N.; Saadat, S. Detecting Antibiotic Residues by HPLC Method in Chicken and Calves Meat in Diet of a Military Center in Tehran. Acta Medica Mediterranea 2015, 31, 1427–1433. [Google Scholar]
- Er, B.; Onurdağ, F.K.; Demirhan, B.; Özgacar, S.Ö.; Öktem, A.B.; Abbasoğlu, U. Screening of Quinolone Antibiotic Residues in Chicken Meat and Beef Sold in the Markets of Ankara, Turkey. Poult Sci 2013, 92, 2212–2215. [Google Scholar] [CrossRef]
- Chowdhury, S.; Hassan, M.M.; Alam, M.; Sattar, S.; Bari, Md.S.; Saifuddin, A.K.M.; Hoque, Md.A. Antibiotic Residues in Milk and Eggs of Commercial and Local Farms at Chittagong, Bangladesh. Vet World 2015, 8, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Wang, J.; Han, R.; Xu, X.; Zhen, Y.; Qu, X.; Sun, P.; Li, S.; Yu, Z. Occurrence of Several Main Antibiotic Residues in Raw Milk in 10 Provinces of China. Food Additives and Contaminants: Part B 2013, 6, 84–89. [Google Scholar] [CrossRef]
- Tao, Q.; Wu, Q.; Zhang, Z.; Liu, J.; Tian, C.; Huang, Z.; Malakar, P.K.; Pan, Y.; Zhao, Y. Meta-Analysis for the Global Prevalence of Foodborne Pathogens Exhibiting Antibiotic Resistance and Biofilm Formation. Front Microbiol 2022, 13. [Google Scholar] [CrossRef]
- Mc Carlie, S.; Boucher, C.E.; Bragg, R.R. Molecular Basis of Bacterial Disinfectant Resistance. Drug Resistance Updates 2020, 48, 100672. [Google Scholar] [CrossRef]
- Hassani, S.; Moosavy, M.-H.; Gharajalar, S.N.; Khatibi, S.A.; Hajibemani, A.; Barabadi, Z. High Prevalence of Antibiotic Resistance in Pathogenic Foodborne Bacteria Isolated from Bovine Milk. Sci Rep 2022, 12, 3878. [Google Scholar] [CrossRef] [PubMed]
- Boonyasiri, A.; Tangkoskul, T.; Seenama, C.; Saiyarin, J.; Tiengrim, S.; Thamlikitkul, V. Prevalence of Antibiotic Resistant Bacteria in Healthy Adults, Foods, Food Animals, and the Environment in Selected Areas in Thailand. Pathog Glob Health 2014, 108, 235–245. [Google Scholar] [CrossRef]
- Frana, T.S.; Beahm, A.R.; Hanson, B.M.; Kinyon, J.M.; Layman, L.L.; Karriker, L.A.; Ramirez, A.; Smith, T.C. Isolation and Characterization of Methicillin-Resistant Staphylococcus Aureus from Pork Farms and Visiting Veterinary Students. PLoS One 2013, 8, e53738. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, M.; Kawamori, F.; Harada, T.; Sano, Y.; Miwa, N.; Sugiyama, K.; Hara-Kudo, Y.; Masuda, T. Antibiotic Resistance in Bacterial Pathogens from Retail Raw Meats and Food-Producing Animals in Japan. J Food Prot 2012, 75, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- FDA No Title. Available online: https://www.fda.gov/food/hazard-analysis-critical-control-point-haccp/haccp-principles-application-guidelines.
- Brenes, A. Estado De La Nación En Desarrollo; 2013.
- Fernández, A. Guía Para La Prevención Del Fraude En La Indústria Agroalimentaria. Premiumlab 2017, 01–41. [Google Scholar]
- Fabiana Meijon Fadul FAO; 2019.
- Strayer- Easter, S.; Everstine, K.; Kennedy, S. Economically Motivated Adulteration of Honey: Quality Control Vulnerabilities in the International Honey Market. Food Prot Trends.
- Arsène, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D.M. The Public Health Issue of Antibiotic Residues in Food and Feed: Causes, Consequences, and Potential Solutions. Vet World 2022, 662–671. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food Safety Impacts of Antimicrobial Use and Their Residues in Aquaculture. Public Health Rev 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Silveira, R.F.; Roque-Borda, C.A.; Vicente, E.F. Antimicrobial Peptides as a Feed Additive Alternative to Animal Production, Food Safety and Public Health Implications: An Overview. Animal Nutrition 2021, 7, 896–904. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of Antibiotic Residues in Animal Food. Food and Chemical Toxicology 2019, 125, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Rastogi, A.; Pandey, S.; Gupta, S.; Sohal, J.S. Multidrug-Resistant Bacteria: Their Mechanism of Action and Prophylaxis. BioMed Research International 2022, 2022. [Google Scholar] [CrossRef]
- Hughes, A.; Roe, E.; Hocknell, S. Food Supply Chains and the Antimicrobial Resistance Challenge: On the Framing, Accomplishments and Limitations of Corporate Responsibility. Environment and Planning A: Economy and Space 2021, 53, 1373–1390. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jacxsens, L.; Membré, J.M.; Nauta, M.; Peterz, M. Relevance of Microbial Finished Product Testing in Food Safety Management. Food Control 2016, 60, 31–43. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. Journal of Agricultural and Food Chemistry 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Ben, Y.; Hu, M.; Zhong, F.; Du, E.; Li, Y.; Zhang, H.; Andrews, C.B.; Zheng, C. Human Daily Dietary Intakes of Antibiotic Residues: Dominant Sources and Health Risks. Environ Res 2022, 212, 113387. [Google Scholar] [CrossRef]
- Beyene, T. Veterinary Drug Residues in Food-Animal Products: Its Risk Factors and Potential Effects on Public Health. J Vet Sci Technol 2015, 07. [Google Scholar] [CrossRef]
- Kyuchukova, R. Antibiotic Residues and Human Health Hazard -Review. Bulgarian Journal of Agricultural Science 2020, 26, 664–668. [Google Scholar]
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health Concerns and Management of Select Veterinary Drug Residues. Food and Chemical Toxicology 2016, 88, 112–122. [Google Scholar] [CrossRef]
- Raison-Peyron, N.; Messaad, D.; Bousquet, J.; Demoly, P. Anaphylaxis to Beef in Penicillin-Allergic Patient. Allergy 2001, 56, 796–797. [Google Scholar] [CrossRef]
- Donkor, E.S.; Newman, M.J.; Tay, S.C.K.; Dayie, N.T.K.D.; Bannerman, E.; Olu-Taiwo, M. Investigation into the Risk of Exposure to Antibiotic Residues Contaminating Meat and Egg in Ghana. Food Control 2011, 22, 869–873. [Google Scholar] [CrossRef]
- Thiim, M.; Friedman, L.S. Hepatotoxicity of Antibiotics and Antifungals. Clin Liver Dis 2003, 7, 381–399. [Google Scholar] [CrossRef]
- Chen, R.-A.; Wu, W.-K.; Panyod, S.; Liu, P.-Y.; Chuang, H.-L.; Chen, Y.-H.; Lyu, Q.; Hsu, H.-C.; Lin, T.-L.; Shen, T.-C.D.; et al. Dietary Exposure to Antibiotic Residues Facilitates Metabolic Disorder by Altering the Gut Microbiota and Bile Acid Composition. mSystems 2022, 7. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, L.; Wang, J.; Hao, M.; Che, H. Antibiotic-Induced Gut Microbiota Dysbiosis Damages the Intestinal Barrier, Increasing Food Allergy in Adult Mice. Nutrients 2021, 13, 3315. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Balzi, E.; Tulkens, P.M. Antibiotic Efflux Pumps. Biochemical Pharmacology 2000, 60, 457–470. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The Antimicrobial Peptides and Their Potential Clinical Applications. Am J Transl Res 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial Peptides as Natural Bio-Preservative to Enhance the Shelf-Life of Food. J Food Sci Technol 2016, 53, 3381–3394. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Deb, B.; Chakraborty, S. A Crosstalk on Antimicrobial Peptides. Int J Pept Res Ther 2021, 27, 229–244. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide Antimicrobial Agents. Clin Microbiol Rev 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The Antimicrobial Peptides and Their Potential Clinical Applications. Am J Transl Res 2019, 11, 3919–3931. [Google Scholar]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front Cell Infect Microbiol 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-Membrane Permeabilizing Modes of Action of Antimicrobial Peptides on Bacteria. Curr Top Med Chem 2015, 16, 76–88. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L. Ben; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a New Generation of Antimicrobials: Toxicity Aspects and Regulations. FEMS Microbiol Rev 2021, 45, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in Vivo Models. Front Microbiol 2021, 12. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L. ben; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a New Generation of Antimicrobials: Toxicity Aspects and Regulations. FEMS Microbiol Rev 2021, 45. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front Microbiol 2021, 12. [Google Scholar] [CrossRef]
- Cao, L.T.; Wu, J.Q.; Xie, F.; Hu, S.H.; Mo, Y. Efficacy of Nisin in Treatment of Clinical Mastitis in Lactating Dairy Cows. J Dairy Sci 2007, 90, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- de Kwaadsteniet, M.; Doeschate, K.T.; Dicks, L.M.T. Nisin F in the Treatment of Respiratory Tract Infections Caused by Staphylococcus Aureus. Lett Appl Microbiol 2009, 48, 65–70. [Google Scholar] [CrossRef]
- Heunis, T.D.J.; Smith, C.; Dicks, L.M.T. Evaluation of a Nisin-Eluting Nanofiber Scaffold To Treat Staphylococcus Aureus-Induced Skin Infections in Mice. Antimicrob Agents Chemother 2013, 57, 3928–3935. [Google Scholar] [CrossRef]
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R.; Ahmed, T.A.E.; Hammami, R.; Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G.; et al. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in Vivo Models. Antimicrob Resist Infect Control 2019, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Importance of Probiotics in Cancer Prevention and Treatment. In Recent Developments in Applied Microbiology and Biochemistry; 2018; pp. 33–45 ISBN 9780128163283.
- Sharma, A. Importance of Probiotics in Cancer Prevention and Treatment. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier, 2019; pp. 33–45.
- Leisner, J.J.; Laursen, B.G.; Prévost, H.; Drider, D.; Dalgaard, P. Carnobacterium: Positive and Negative Effects in the Environment and in Foods. FEMS Microbiol Rev 2007, 31, 592–613. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, C.A.; Koo, O.K.; Sostrin, M.L.; Ricke, S.C.; Crandall, P.G.; Johnson, M.G. Characteristics of Bacteriocins and Use as Food Antimicrobials in the United States. In Food and Feed Safety Systems and Analysis; 2018; pp. 273–286 ISBN 9780128498880.
- Kasimin, M.E.; Shamsuddin, S.; Molujin, A.M.; Sabullah, M.K.; Gansau, J.A.; Jawan, R. Enterocin: Promising Biopreservative Produced by Enterococcus Sp. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
| Antimicrobial group | Resistance mechanism |
|---|---|
| Aminoglycocides Gentamicin Streptomycin Kanamycin |
Enzyme modification Decreased permeability Target resistance (ribosome) Efflux bombs |
| β- Lactams Cephalothin Cefoxitin Ceftiofur Cefquinome |
Reduced permeability Altered penicillin-binding proteins (PBPs) β- Lactamases, cephalosporinases Efflux bombs |
| Folate pathway inhibitors Sulfonamides |
Decreased permeability Production of drug-insensitive enzymes |
| Macrolide-lincosamide-streptgramin B Erythromycin Lincomycin Virginiamycin |
Enzyme modification Decreased permeability Decreased ribosomal binding |
| Phenicols Chloramphenicol Florfenicol |
Enzyme modification Decreased permeability Decreased ribosomal binding Efflux bombs |
| Quinolones and fluoroquinolones Nalidixic acid Ciprofloxacin Enrofloxacin |
Target resistance (DNA gyrase, topoisomerase IV) Efflux bombs Decreased permeability |
| Tetracyclines Chlortetaracycline Tetracycline Doxicycline |
Target resistance (ribosome) Drug detoxification Efflux bombs |
| Antibiotic residue | Concentration | Food product | Associated health concern risk | Source |
|---|---|---|---|---|
| Oxytetracycline | 2604.1 ± 703.7 μg/kg | Chicken muscle | Allergic hypersensitivity reactions or toxic effects (phototoxic skin reactions, chondrotoxic) | [29] |
| 3434.4 ± 604.4 μg/kg | Chicken liver | Carcinogenicity, cytotoxicity | ||
| 51.8 ± 90.53 μg/kg | Beef | Carcinogenicity, cytotoxicity | [30] | |
| Enrofloxacin | 0.73 - 2.57 μg/kg | Chicken meat | Allergic hypersensitivity reactions or toxic effects, phototoxic skin reactions, chondrotoxic. | [31] |
| Chloramphenicol | 1.34 - 13.9 μg/kg | Chicken | Bone marrow toxicity, optic neuropathy, brain abscess | |
| Penicillin | 0.87 - 1.3 μg/kg | Calves | Allergy, affect starter cultures to produce fermented milk product | |
| Oxytetracycline | 3.5 - 4.61 μg/kg | Chicken meat | Carcinogenicity, cytotoxicity in the bones of broiler chickens | |
| Quinolones | 30.81± 0.45 μg/kg μg/kg | Chicken meat | Allergic hypersensitivity reactions or toxic effects (phototoxic skin reactions, chondrotoxic) | [32] |
| 6.64 ±1.11 μg/kg | Beef | |||
| Amoxicilin | 9.8 -56.16 μg/mL | Milk | Carcinogenic, teratogenic, and mutagenic effects | [33] |
| 10.46 -48.8 μg/g | Eggs | |||
| Suldonamides | 16.28 μg/g | Raw milk | Carcinogenicity, allergic reaction | [34] |
| Quinolones | 23.25 μg/g | Allergic hypersensitivity reactions or toxic effects (phototoxic skin reactions, chondrotoxic). |
| Microorganism | Sample source | Antibiotic resistance | Prevalence (%) | Source |
|---|---|---|---|---|
| Escherichia coli | Bovine milk sample | Azithromycin | 53 | [37] |
| Chloramphenicol | 15 | |||
| Ceftriaxone | 17 | |||
| Penicillin | 69 | |||
| Gentamicin | 6 | |||
| Amoxicillin | 55 | |||
| Tetracycline | 20 | |||
| Cephalexin | 64 | |||
| Listeria monocytogenes | Bovine milk sample | Azithromycin | 12 | |
| Chloramphenicol | 22 | |||
| Ceftriaxone | 17 | |||
| Penicillin | 46 | |||
| Gentamicin | 24 | |||
| Amoxicillin | 46 | |||
| Tetracycline | 23 | |||
| Cephalexin | 46 | |||
| Salmonella spp. | Bovine milk sample | Azithromycin | 8 | |
| Chloramphenicol | 6 | |||
| Ceftriaxone | 5 | |||
| Penicillin | 21 | |||
| Amoxicillin | 15 | |||
| Tetracycline | 5 | |||
| Cephalexin | 21 | |||
| Staphyloccocus aureus | Bovine milk sample | Azithromycin | 8 | |
| Chloramphenicol | 6 | |||
| Ceftriaxone | 6 | |||
| Penicillin | 21 | |||
| Gentamicin | 3 | |||
| Amoxicillin | 25 | |||
| Tetracycline | 7 | |||
| Cephalexin | 25 | |||
| E.coli | Healthy farm workers | β-lactams | 77.3 | [38] |
| Pigs | 76.7 | |||
| Poultry broilers | 40 | |||
| S. aureus | Pigs | Methicillin | 30 | [39] |
| Campylobacter jejuni | Chicken | Ampicillin | 5 | [40] |
| Tetracycline | 31.7 | |||
| Ciprofloxacin | 23.3 | |||
| C. coli | Pork | Ampicillin | 33.3 | |
| Erythromycin | 73.3 | |||
| Tetracycline | 73.3 | |||
| Chloramphenicol | 6.7 | |||
| Ciprofloxacin | 46.7 |
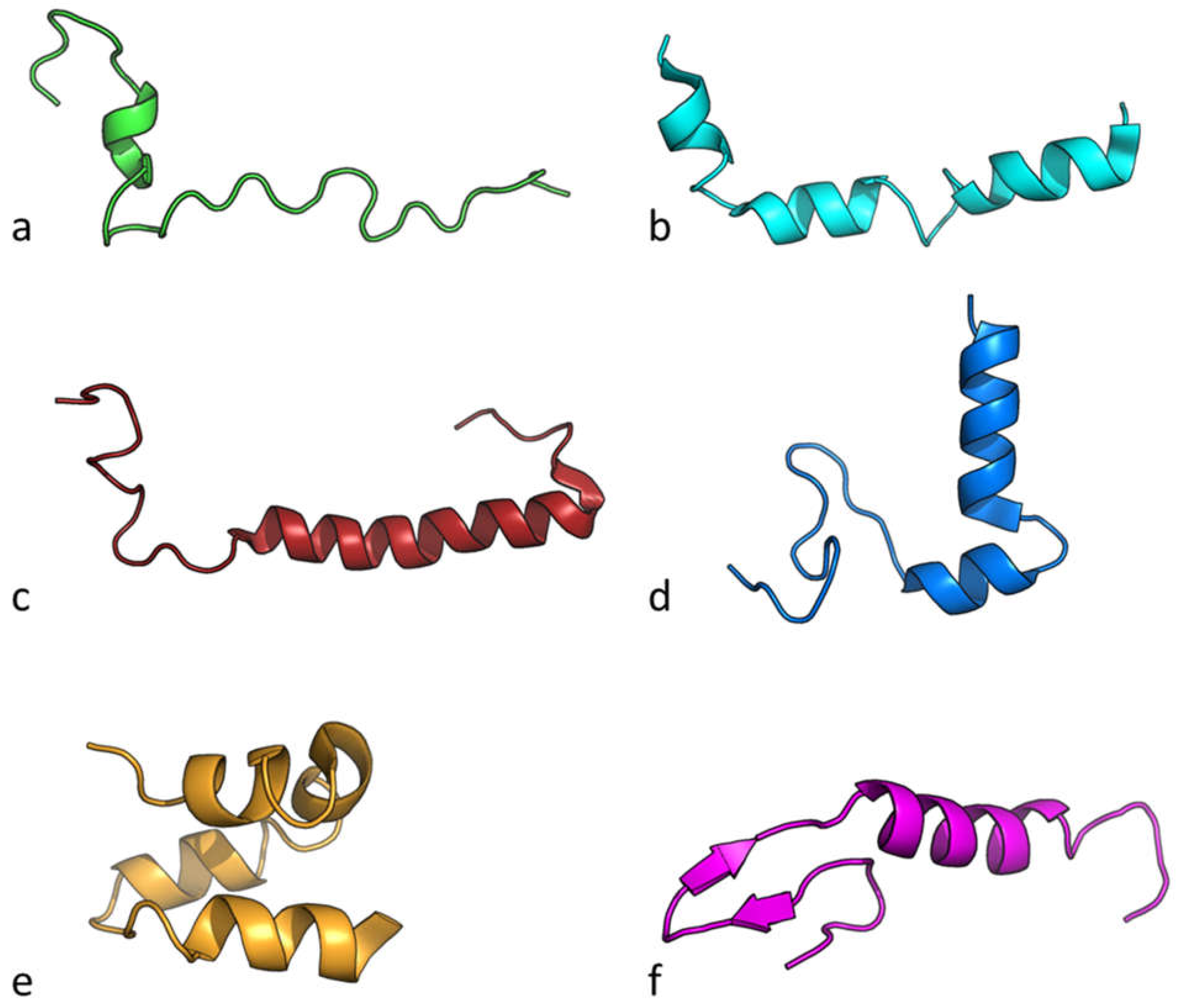
| Bacteriocin | Source | Food use | Reference |
|---|---|---|---|
| Nisin & Nisin Z | Lactococcus lactis | Prevents food-spoilage caused by Lactobacillus spp, L. monocytogens, S. aureus and Clostridum spp | [78] |
| lactococcin-G β | Lactococcus lactis | Activity against Listeria monocytogenes in yogurt, cheese, and sauerkraut | [79] |
| Leucocin A | Leuconostoc gelidum | Activity against E. coli and L. monocytogenes in meat and fish products. | [80] |
| Carnobacteriocin B2 | Carnobacterium maltaromaticum | Activity against Listeria monocytogenes in dairy, meat or fish food and feed products | [81] |
| Curvacin A | Latilactobacillus curvatus | Activity against Listeria monocytogenes | [82] |
| Enterocin 7A | Enterococcus faecalis | Activity against Listeria monocytogenes in meat and meat-based products | [83] |
| Name | Source organism | Molecular weight (Da) |
Net Charge pH 7 | Isoelectric point | Hydrophobicity | Hydrophobic Moment |
|---|---|---|---|---|---|---|
| Nisin | L. lactis | 3456.62 | 3 | 8.52 | -0.29 | 0.48 |
| lactococcin-G β | L.lactis | 4107.19 | 4 | 10.42 | 0.25 | 0.71 |
| Leucocin A | L. gelidum | 3929.80 | 2 | 8.77 | 0.26 | 1.58 |
| Carnobacteriocin B2 | C. maltaromaticum | 4966.40 | 4 | 9.96 | 0.00 | 1.60 |
| Curvacin A | L. curvatus | 4306.03 | 3 | 9.37 | 0.11 | 1.69 |
| Enterocin 7A | E. faecalis | 5172.91 | 6 | 10.68 | 0.20 | 2.12 |
| Averague | 4815.12 | 4 | 10.00 | 0.10 | 1.81 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




