1. Introduction
Gout is the most common inflammatory arthritic condition. Sustained higher serum uric acid (SUA) levels, a condition known as hyperuricemia, leads to the formation of monosodium urate crystals- the hallmark of developing gout [
1]. Formation and deposition of monosodium urate crystals in and around the joints are responsible for acute gout flares. Reports on the prevalence and incidence of gout vary widely depending on the population studied and the methods employed; but the prevalence rates range from <1% to 6.8% and incidence at 0.58-2.89 per 1,000 person-years [
2]. For recurring gout flare prevention and treatment, the American College of Rheumatology guidelines recommend urate-lowering therapy (ULT) with xanthine oxidase inhibitors, allopurinol or febuxostat as first-line options to achieve a goal serum urate level of less than 6mg/dL [
3]. Probenecid is a second-line agent and can be combined with xanthine oxidase inhibitors in cases where monotherapy is not enough to reach treat-to-target urate levels. Despite the high incidence of gout in the United States population, studies suggest that most patients with gout do not receive appropriate treatment to manage their disease. An observational study published in 2019 using data from the National Health and Nutrition Examination Survey using 2007-2016 survey waves showed that only one-third of those diagnosed with gout are treated with ULT [
2]. Additionally, it was estimated that only 37.7% of gout patients were meeting their therapeutic serum urate goal [
4]. Despite strong recommendations, pharmacologic treatment of gout is often limited to anti-inflammatory agents for symptomatic relief. This approach can provide comfort to the patient but ignores the underlying cause of elevated serum urate, leading to recurring flares and further joint damage. Short-term complications of gout include the development of subcutaneous tophi, a granulomatous reaction to the monosodium urate crystals deposited in the joints [
5], and kidney stones [
6].
Patients with gout (or chronic hyperuricemia) also have a higher risk of developing cardiovascular diseases and are at an increased risk of major adverse cardiovascular events (acute coronary syndrome, stroke, arrhythmia, peripheral artery disease) [
7,
8]. Furthermore, gout is an independent risk factor for developing type 2 diabetes and hypertension [
7,
9]. A limited number of studies have evaluated the impact of ULT and long-term clinical outcomes among patients with gout. Allopurinol and febuxostat have been associated with improved treatment outcomes in heart failure patients and reduced risk of acute cardiovascular events [
10,
11]. The American College of Rheumatology conditionally recommends ULT for those experiencing their first gout flare and with CKD ≥3 and SU> 9mg/dL or urolithiasis. While a recent meta-analysis found that the use of allopurinol was associated with changes in blood glucose parameters [
12]; the association between ULT use and clinical biomarkers such as hemoglobin A1C, C-reactive protein (CRP), and lipid traits remains ill-defined. Evaluating these clinical outcomes is critical to improve adherence to ULT and provide real-world evidence for optimal gout management, especially in the context of multiple comorbidities [
13]. Despite the forementioned observations, the relationship between gout treatment status and the prevalence of chronic conditions such as ischemic heart disease, and heart failure remains unknown, and no recent studies have explored the association between the prevalence of these conditions and gout treatment status. To address these gaps, we evaluated the relationship between the prevalence of coronary heart disease, heart failure, hyperlipidemia, hypertension, chronic kidney disease, and clinical biomarkers by gout treatment status among US adults with a diagnosis of gout.
2. Materials and Methods
This observational cohort study used data from the National Health and Nutrition Examination Survey (NHANES). NHANES is a cross-sectional prospective continuous health survey that aims to assess the health and nutrition of the non-institutionalized civilian population in the United States. NHANES conducts at-home health interviews and physical examinations at mobile centers. Questionnaire, laboratory, and physical examination data are available in 2-year survey waves. Data from the NHANES survey waves of 2013-2014, 2015-2016, and 2017-2018 were combined in this study. We included adults 30 years or older who had answered ‘yes’ when being asked whether a doctor had told him/her they had gout. All data used in the study is publicly available and deidentified and no institutional review board approval was required (45 CFR §46.102(f)).
Assessment of Urate-Lowering Therapy Use
Urate-lowering therapy (ULT) was defined as the use of any ULT medication (yes/no), including allopurinol, febuxostat, probenecid, or combination agents. Combination products were lesinurad/allopurinol and colchicine/probenecid. ULT use data was obtained from the medication file for each 2-year cycle which collects bottle-verified use of prescribed medications within the 30 days before the survey interview.
Evaluation of Chronic Conditions and Clinical Biomarkers
Participants with comorbid dyslipidemia, coronary heart disease, heart failure, hypertension, and chronic kidney disease were identified by individual health survey questions asking whether a doctor has ever told them they had either of those conditions. Biomarkers of interest were serum uric acid levels, C-reactive protein (CRP), glycohemoglobin, high-density lipoprotein, low-density lipoprotein, and total cholesterol levels which were obtained from the laboratory component. C-reactive protein levels were only available in the 2015-2016 and 2017-2018 survey waves. NHANES uses standardized methods to collect blood and urine samples. Other biomarkers included eGFR and systolic and diastolic blood pressure. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation and the respective demographic and laboratory data. Systolic and diastolic blood pressure levels were obtained from the physical examination and the average of two different readings for each participant were used in this study.
Statistical Analyses
Prevalence use of ULT overall and among participants with the chronic conditions were estimated and reported using an unweighted sample count and nationally weighted percentage. Count and national percent were also used to describe the use of ULT by individual agent, prevalence of comorbid chronic conditions and the proportion of participants meeting the uric acid treatment goal (serum uric acid <6mg/dL or ≥6mg/dL) by gout treatment status. Mean and standard error (SE) were used to report all continuous laboratory marker values by gout treatment status. Chi-square tests were used to evaluate the association between ULT use and biannual survey years and to compare socio-demographic factors (sex (male/female), race/ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Other races), education (high school or less, some college, college graduate or higher), health insurance coverage (yes/no), and body mass index (<25 kg/m2, ≥25 kg/m2) by gout treatment status. Independent t-tests were used to assess the relationship between continuous laboratory markers (CRP, hemoglobin A1C, lipids, blood pressure, and eGFR), age, and duration of gout (in years) by gout treatment status. Univariable and multivariable logistic regression models were developed to evaluate the association between ULT use and the prevalence of comorbid conditions. Multivariable models used age, sex, race/ethnicity, diagnosis of type 2 diabetes and duration of gout as covariates. Patients diagnosed with diabetes may garner added benefits due to the use the of various medications that may influence the outcomes of interest. Additionally, diabetes diagnosis may introduce bias by virtue of routine care, and therefore, adjusting for diabetes was warranted. Crude and adjusted odds ratios and their respective 95% confidence intervals were reported. An alpha value of 5% was used to determine statistical significance. All statistical tests were adjusted for complex sampling design and estimates are nationally representative. IBM-SPSS version 27 was used to run all statistical analyses.
3. Results
Sample characteristics and ULT use
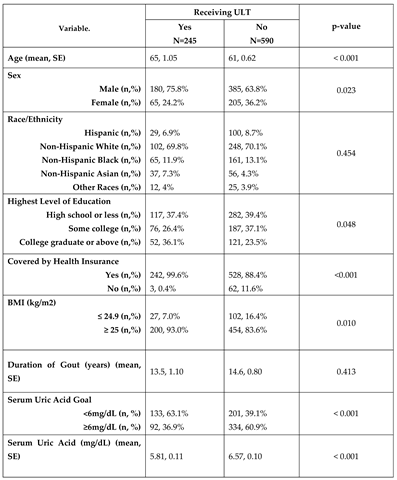
The study cohort included 835 adults 30 years or older who had been told by a doctor they had gout. Sample characteristics are shown in
Table 1. Individuals taking ULT had a higher mean age (64.86 ± 1.05 versus 60.77 ± 0.62, p<0.001) and were more likely to be male (75.8% versus 63.8%, p=0.023). There was no difference by racial/ethnic group (p>0.05). Individuals on ULT were more likely to have a higher level of education, health insurance coverage, and a body mass index (BMI) of 25 kg/m2 or greater (p<0.05) (
Table 1). The mean duration of gout was 13.4 years and did not significantly differ between groups (p>0.05). Among the 835 participants in our cohort, 245 were on ULT (28.9%; 95%CI 24.3–33.9). Use of ULT did not change significantly over time from 2013-2018 (p>0.05, data not shown). The most reported ULT medication was allopurinol (91.4%; 95%CI 84.9-95.3), followed by febuxostat (7.3%; 95%CI 3.5-14.8), probenecid (1%; 95%CI 0.3-3.7), and combinations drugs (0.2%; 95%CI 0-1.7). There was a significant difference in urate levels by ULT treatment status, being lower for those on treatment (5.81mg/dL versus 6.57mg/dL, p<0.001). Of those on ULT, 63.9% met their serum uric acid level goal compared to 39.1% who reached their goal without the use of ULT (p<0.001).
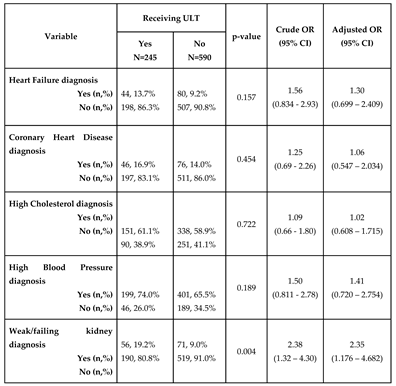
Prevalence of chronic comorbid conditions by gout treatment status
Among those meeting inclusion criteria, 40.8%, 14.6%, 14.9%, ,71.9% and 15.0% reported having been diagnosed with dyslipidemia, coronary heart disease, heart failure, hypertension, and chronic kidney disease, respectively. There was no significant association between ULT use and dyslipidemia, coronary heart disease, heart failure and hypertension in the univariable model or the multivariable model (p>0.05). There was a significant association between chronic kidney disease (CKD) and ULT treatment status (OR 2.38, 95% CI 1.32 - 4.30). The association remained significant after adjusting for age, race/ethnicity, sex, duration of gout and diagnosis of type 2 diabetes (OR 2.35, 95% CI 1.18-4.68,
Table 2).
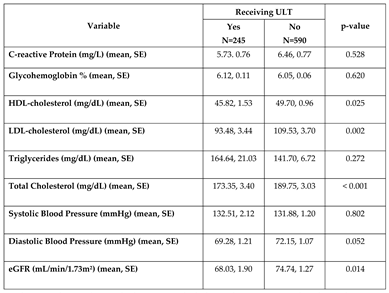
Evaluation of clinical biomarkers by gout treatment status
Total, LDL- and HDL- cholesterol levels were significantly higher among those not on ULT, but no difference was observed across triglycerides. Those on ULT also had significantly lower mean eGFR of 68.03 mL/min/1.73m² compared to 74.74 mL/min/1.73m² for those not on ULT (p=0.014). There were no statistically significant differences in glycohemoglobin, CRP, systolic, or diastolic blood pressure by gout treatment status (
Table 3).
4. Discussion
This is the first pharmacoepidemiologic study to assess the prevalence of chronic comorbid conditions by gout treatment status. In this nationally representative sample of US adults with gout, the prevalence of ULT use was 28.9%. This estimate falls in the lower end of what has been previously reported in published epidemiologic studies [
2]. The relatively low prevalence of ULT use among adults with gout and the difference in serum uric acid levels between those on ULT and not on ULT suggests that gout remains sub-optimally managed in a sizable proportion of patients. Poor gout management translates into a higher risk of frequent gout flares and the development of gout-related complications, such as nephrolithiasis, hypertension, and cardiovascular events [
11]. Augmented gout care could improve gout management and increase the uptake of ULT among patients with gout. Different care models involving nurses and pharmacists have shown a great potential to increase the proportion of patients receiving ULT and achieving SU level target [
14]. Efforts focused on patient understanding of the disease and adherence to ULT are needed to minimize the burden of suboptimal gout management.
This study found no association between chronic comorbidities associated with gout and ULT treatment status with the exception of chronic kidney disease. Those on ULT were more likely to have been diagnosed with CKD and have a lower mean eGFR compared to those not on ULT. Prompt treatment with ULT in individuals with impaired kidney function, especially in those with moderate-to-severe CKD, is conditionally recommended by the most recent American College of Rheumatology gout management guidelines and may aid in preventing frequent gout flares or worsening kidney function [
3]. While the nephrotoxic effect of allopurinol is rare and yet to be determined, there is growing evidence to suggest that ULT may preserve kidney function [
15]. This might explain the higher use of ULT among patients with impaired kidney function. Despite the relationship between gout and chronic diseases reported in the literature [
7,
8], there was no association between ULT use and heart failure, coronary heart disease, hypertension, and dyslipidemia comorbidity in this study.
The association between gout and cardiovascular disease (CVD) remains too controversial to recommend SUA monitoring for following an individual’s risk of CVD or using ULT for hypertension. Although SUA can be an independent risk factor for developing CVD and kidney disease, SUA may not be the direct cause of kidney disease. However, SUA concentrations are believed to lead to hypertension, which may more significantly affect the kidney than SUA itself. Therefore, the causal relationship between SUA and CVD requires a reappraisal and further studies to show the direct effect of SUA on CVD and kidney disease [
16]. A randomized crossover-controlled trial of relatively healthy young adults (18-40 years) without chronic kidney disease showed that allopurinol therapy did not affect systolic and diastolic blood pressure [
17]. However, the study found a significant effect of allopurinol treatment on flow mediated dilation, supporting the hypothesis that SUA affects endothelial function [
17]. In our current analysis, there was some evidence that ULT treatment status was associated with a lower diastolic blood pressure compared those not receiving ULT (69 vs. 72 mm Hg); however, the association did not reach statistical significance (p=0.052).
This study showed the impact of ULT use on select clinical biomarkers. Moreover, despite the lack of an observable association between ULT use and the diagnosis of dyslipidemia, those on ULT had significantly lower LDL, HDL, and total cholesterol levels when compared to those not on ULT. Lower lipid levels among those on ULT suggest that gout patients with comorbid dyslipidemia may see an added benefit from using ULT. Although those on ULT showed lower mean HDL levels, the reduction is not likely to be clinically impactful when compared to the reductions observed in LDL and total cholesterol. The association between ULT use and lower lipid levels may be confounded due to possibly dietary habits among individuals diagnosed with gout. Our findings are in line with previous report, suggesting that ULT is associated with a significant reduction in cholesterol and triglyceride at 3-5 weeks in Chinese patients, even when lipid-lowering therapy (LLT) was required [
18]. Particularly, febuxostat was only ULT that reduced both total cholesterol and triglyceride in the absence of LLT [
18].
Another large retrospective study identified that Taiwanese patients without antigout treatments had greater than three-fold higher risk of hyperlipidemia compared with patients without gout [
19]. Patients receiving ULT had a significantly lower risk of hyperlipidemia than gout patients without ULT (aHR <0.90) [
16]. Furthermore, in vitro studies have shown that antigout treatment decreased the expression of hepatic genes related to lipogenesis in hepatic cells, indicating that gout patients receiving ULT could have a lower risk for developing hyperlipidemia [
19]. Another potential mechanism to explain these results is the genetic intersection between the risk of developing gout and response to LLT. To explain, genetic polymorphism in ABCG2 (rs2231142C>T) is associated with 3-4-fold higher risk of developing gout [
20]. The same genetic polymorphism has been implicated in greater reduction in LDL-C among patients receiving statin, especially rosuvastatin [
21,
22]. Collectively, the combined effects of reduced lipogenesis, because of antigout treatment, and the genetic predisposition for gout may render patient with gout to garner greater benefits from LLT. Nonetheless, it is important to bear in mind that we examined the association between ULT and lipid changes on a cross-sectional basis so further research should evaluate this association longitudinally.
Limitations
Our analyses have some limitations. First, due to the cross-sectional nature of our data, cause-effect relationships between gout treatment status and the studied conditions and clinical laboratory markers cannot be established. Additionally, data on gout severity (e.g., presence of subcutaneous tophi, frequency of gout flares, and others) was not gathered by the NHANES. History of chronic conditions relied on self-report so some misclassification error and recall bias may have occurred. Unmeasured parameters such as the use of antihyperlipidemic, antidiabetics, or antihypertensives were not evaluated and may confound the relationship studied here. This study focused on the use of urate-lowery therapy only; therefore, the use of on-demand pain medications or anti-inflammatory agents such as steroids was not evaluated. Finally, NHANES does not collect data on medication adherence, length of therapy, or medication doses; thus, assessment of optimal management in patients being treated with ULT could not be evaluated in this study. Despite the limitations described, this study produced nationally representative estimates and utilized multivariable logistic regression models to adjust for several confounding factors. Data on clinical laboratory markers was obtained from objective laboratory measurements and results are also generalizable to the ambulatory US population.
5. Conclusions
This study is the first to evaluate the association of ULT use with the prevalence of chronic comorbid conditions including cardiovascular and renal disease among adults with gout. The low prevalence of ULT use could partly explain the higher mean serum urate levels among those not on ULT, suggesting that gout remains sub-optimally managed in a large proportion of patients. The higher prevalence of ULT use among those with a lower mean eGFR is likely to be driven by recent data suggesting that ULT use may improve outcomes and help preserve kidney function in this proportion of patients besides preventing future gout flares. Despite participants on ULT being more likely to be overweight or obese when compared to those not on ULT, lower lipid levels among those on ULT were seen. Future prospective longitudinal studies should further evaluate the clinical implications of chronically elevated serum uric acid and the impact of gout treatment on the incidence and management of gout comorbidities.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nature Reviews Rheumatology. 2020, 16, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis & Rheumatology. 2019, 71, 991–999. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care & Research. 2020, 72, 744–760. [Google Scholar] [CrossRef] [PubMed]
- 4. Abhishek A, Cipolletta E, Nakafero G, Avery AJ, Mamas M, Tata LJ. Serum urate outcomes of treat-to-target urate lowering treatment: Results of a nationwide cohort study from 1997 to the COVID-19 pandemic using data from the Clinical Practice Research Datalink. Annals of the Rheumatic Diseases. [CrossRef]
- Dalbeth N, Pool B, Gamble GD, et al. Cellular characterization of the Gouty Tophus: A quantitative analysis. Arthritis & Rheumatism. 2010, 62, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- YÜ TSAI-FAN. Uric acid nephrolithiasis in gout. Annals of Internal Medicine. 1967, 67, 1133. [Google Scholar] [CrossRef] [PubMed]
- Abeles, AM. Hyperuricemia, gout, and cardiovascular disease: An update. Current Rheumatology Reports. 2015, 17. [Google Scholar] [CrossRef]
- Sandoval-Plata G, Nakafero G, Chakravorty M, Morgan K, Abhishek A. Association between serum urate, gout and comorbidities: a case-control study using data from the UK Biobank. Rheumatology (Oxford). 2021, 60, 3243–3251. [Google Scholar] [CrossRef]
- Choi HK, De Vera MA, Krishnan E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology. 2008, 47, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Thanassoulis, G. Gout, allopurinol use, and heart failure outcomes. Archives of Internal Medicine. 2010, 170, 1358. [Google Scholar] [CrossRef]
- Singh JA, Ramachandaran R, Yu S, Curtis JR. Allopurinol use and the risk of acute cardiovascular events in patients with gout and diabetes. BMC Cardiovascular Disorders. 2017, 17. [Google Scholar] [CrossRef]
- Chen J, Ge J, Zha M, Miao J-J, Sun Z-L, Yu J-Y. Effects of uric acid-lowering treatment on glycemia: A systematic review and meta-analysis. Frontiers in Endocrinology. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Roman, YM. Moving the needle in gout management: The role of culture, diet, genetics, and personalized patient care practices. Nutrients. 2022, 14, 3590. [Google Scholar] [CrossRef] [PubMed]
- Gill I, Dalbeth N, 'Ofanoa M, Goodyear-Smith F. Interventions to improve uptake of urate-lowering therapy in patients with gout: a systematic review. BJGP Open. 2020, 4, bjgpopen20X101051. [Google Scholar] [CrossRef] [PubMed]
- 15. Sharma G, Dubey A, Nolkha N, Singh J. Hyperuricemia, urate-lowering therapy, and renal outcomes: A systematic review and meta-analysis. SSRN Electronic Journal. 2018. [CrossRef]
- Johnson RJ, Bakris GL, Borghi C, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: Report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Gaffo AL, Calhoun DA, Rahn EJ, et al. Effect of serum urate lowering with allopurinol on blood pressure in young ydults: A randomized, controlled, crossover trial. Arthritis Rheumatol. 2021, 73, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Wu J, Zhang YP, Qu Y, Jie LG, Deng JX, Yu QH. Efficacy of uric acid-lowering therapy on hypercholesterolemia and hypertriglyceridemia in gouty patients. Int J Rheum Dis. 2019, 22, 1445–1451. [Google Scholar] [CrossRef]
- 19. Fang YJ, Wu TY, Lin CL, Su CY, Li JR, Chung YL, Tien N, Lim YP. Effects of urate-lowering therapy on risk of hyperlipidemia in gout by a population-based cohort study and on in vitro hepatic lipogenesis-related gene expression. Mediators Inflamm. 2020, 2020, 8890300. [CrossRef] [PubMed]
- Hoque KM, Dixon EE, Lewis RM, Allan J, Gamble GD, Phipps-Green AJ, Halperin Kuhns VL, Horne AM, Stamp LK, Merriman TR, Dalbeth N, Woodward OM. The ABCG2 Q141K hyperuricemia and gout associated variant illuminates the physiology of human urate excretion. Nat Commun. 2020, 11, 2767. [Google Scholar] [CrossRef]
- Cooper-DeHoff RM, Niemi M, Ramsey LB, Luzum JA, Tarkiainen EK, Straka RJ, Gong L, Tuteja S, Wilke RA, Wadelius M, Larson EA, Roden DM, Klein TE, Yee SW, Krauss RM, Turner RM, Palaniappan L, Gaedigk A, Giacomini KM, Caudle KE, Voora D. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and statin-associated musculoskeletal symptoms. Clin Pharmacol Ther. 2022, 111, 1007–1021. [Google Scholar] [CrossRef]
- Hu M, To KK, Mak VW, Tomlinson B. The ABCG2 transporter and its relations with the pharmacokinetics, drug interaction and lipid-lowering effects of statins. Expert Opin Drug Metab Toxicol. 2011, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Sample characteristics of U.S. adults 30 years or older being told by a physician they have gout.
Table 1.
Sample characteristics of U.S. adults 30 years or older being told by a physician they have gout.
Table 2.
Odds ratios for the prevalence of comorbid conditions among those on urate-lowering therapy among adults 30 year or older having been told by a doctor they have gout.
Table 2.
Odds ratios for the prevalence of comorbid conditions among those on urate-lowering therapy among adults 30 year or older having been told by a doctor they have gout.
Table 3.
Clinical biomarkers among adults 30 years or older having been told by a doctor they have gout .
Table 3.
Clinical biomarkers among adults 30 years or older having been told by a doctor they have gout .
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).