1. Introduction
The COVID-19 pandemic has affected the daily lives of citizens worldwide, and social restrictions have altered different health behaviors such as eating habits and physical activity [
1]. Recent data has shown that physical inactivity (PI) increases considerably during home confinement [
2,
3,
4,
5]. Physical inactivity and the adoption of unhealthy eating habits may have influenced weight gain during the pandemic [
6], which may have affected glycemic levels [
7]. Although studies have shown the negative effects of the pandemic on the general health of the population [
7,
8], it is important to understand how physical inactivity during social restrictions can be associated with glycemic changes, thus posing a threat to the increased incidence of type 2 diabetes mellitus (T2DM) worldwide.
T2DM has emerged as a serious public health problem due to a modern lifestyle characterized by increased sedentary behavior and the consumption of ultra-processed foods [
9]. The International Diabetes Federation has reported that approximately 422 million people worldwide live with diabetes mellitus [
9,
10]. One of the measures used to determine glycemic control is the HbA1c test, which is considered the gold standard for assessing glycemic control, and an indicator of the average plasma glucose level during the previous 120 days [
11].
Abnormalities in serum HbA1c concentrations are implicated in disease progression and physical disability, and result in microvascular and macrovascular risks, as well as neuropathy, retinopathy, renal dysfunction, and cardiovascular disease [
9,
12].
Some behaviors increase the risk of HbA1c changes, including smoking, poor eating habits, being overweight, and physical inactivity [
13]. However, studies suggest that physical activity is an important non-pharmacological intervention for the treatment of diabetes mellitus, acting significantly on insulin resistance, glycemic control, and reducing HbA1c [
14,
15]. The recommendation for adults to involve in regular physical activity is, at least 150 minutes of moderate physical activity or 75 minutes of vigorous physical activity, weekly [
13].
Faced with the scarcity of biochemical data during the period of social restriction due to the COVID-19 pandemic and the prospect of intense modification in lifestyle and health behavior, especially physical activity, which may have influenced weight gain and consequently glycemic change, it is important to understand the mechanisms associated with this clinical condition to try to minimize negative health impacts, in addition to a higher prevalence of diabetes in the medium/long term. Therefore, this study aimed to evaluate serum HbA1c levels and their association with physical inactivity mediated by overweight. We hypothesized that glycemic alterations may be associated with physical inactivity at leisure during social restriction, and that being overweight may be a mediating variable in this association.
2. Materials and Methods
2.1. Study design and sampling
This was a cross-sectional study, using data from the COVID-Inconfidentes project, a population-based household epidemiological survey conducted between October and December 2020 in two municipalities (Ouro Preto and Mariana) in the state of Minas Gerais, Brazil. Participants eligible for the study were permanent residents of households in the urban area of the municipalities, aged 18 years or older, and who consented to participate in the study. Individuals with impaired cognitive function, difficulty answering the questionnaire or inability to provide blood samples due to difficulties in venous access were excluded.
For data collection, a sample calculation was performed based on the 2010 population census for the urban area of each city, adopting a confidence level of 95% and an effect size equal to 1.5. Furthermore, 20% was added to the sample size of each city for possible refusals, absence of the resident selected, or people not at home during the visit. The sample size was calculated using OpenEpi (
https://www.openepi.com/Menu/OE_Menu.htm), with 732 interviews for each municipality. Three-stage conglomerate sampling was used: census sector (selected with probability proportional to the number of households), household (selected from systematic sampling) and residents (≥ 18 years old, randomly selected by applying Sorteador de Nomes). The sample weight of each selected unit (census sector, household, and individual) was calculated to correlate with the 2019 population projections (DATASUS) [
16]. In this calculation, adjustments were made to compensate for interview losses due to non-response. For more details on sample calculation and field logistics, see Meireles et al. 2021 [
17].
2.2. Data Collection
In each municipality, data were collected on three weekends (Friday, Saturday, and Sunday), with intervals of 21 days between each. In the week preceding the data collection weekend, actions were carried out to disseminate the survey in selected census sectors, draw households, draw lots, and approach households, with the aim of increasing awareness and adherence to the survey. On the days of data collection, a resident was selected, followed by blood collection and face-to-face interviews. For venous blood collection, a 2.7 mL S-Monovette (Sarstedt) tube containing sodium fluoride/EDTA was used for the analysis of serum HbA1c levels.
Face-to-face interviews lasted 30 to 45 minutes, using the questionnaire in the DataGoal application via tablets. The interviewers maintained a minimum distance of 1.5 meters from the interviewee as a protective measure against COVID-19, and physical contact was restricted to the point of collection of biological material.
The questionnaire included sociodemographic and economic variables, lifestyle habits, and general health status.
2.3. Outcome variable: Glycated hemoglobin (HbA1c)
HbA1c was measured in the Clinical Analysis Pilot Laboratory (LAPAC) of the School of Pharmacy/Federal University of Ouro Preto using the immunoturbidimetry method in the COBAS INTEGRA 400 plus automatic analyzer (Roche, Germany), following a protocol standardized by the manufacturer. Before each analysis, the device was calibrated with quality controls (HbA1c Control N and HbA1c Control P, Roche). A minimum volume of 400 µL of whole blood was used for the samples. The normal range adopted for the HbA1c level was ≤ 6.4%, and levels ≥ 6.5% were classified as having glycemic alterations [
18].
2.4. Explanatory variable: Physical inactivity in leisure time
The self-reported physical activity during leisure time was evaluated by weekly frequency, duration, and type of physical exercise. Moderate physical activity was defined as walking, treadmill walking, weight training, water aerobics, Pilates, volleyball, and dancing, and vigorous physical activity included running, cycling, swimming, treadmill running, aerobics in general, wrestling, soccer/futsal, basketball, and tennis (AINSWORTH [
19,
20,
21,
22]. Then the weekly frequency (0 to 7 days) was multiplied by the daily time (in minutes) to obtain the weekly amount of physical activity during leisure time in minutes. This amount was categorically evaluated using the cut-off points established by the World Health Organization (WHO) and the Physical Activity Guide for the Brazilian Population [
23,
24] into physically active (≥150 min/week of moderate physical activity, ≥75 min/week of vigorous physical activity, or a combination of both) or physically inactive (<150 min/week of moderate physical activity or <75 min/week of vigorous physical activity).
2.5. Mediating variable: overweight
Overweight was used as a mediation variable, measured by body mass index (BMI), calculated from self-reported weight and height according to the following formula: BMI = body weight (kg) / height (m²). kg/m2 if < 60 years to WHO cut-off points into "not overweight" (BMI <25.0 kg/m2 if < 60 years or BMI <28.0 kg/m2 if ≥ 60 years) and "overweight" (BMI ≥ 25.0 kg/m² if < 60 years or BMI ≥ 28.0 kg/m2 if ≥ 60 years) [
25,
26].
2.6. Adjustment of variables
Sociodemographic covariates, tobacco use, and morbidities were included. The sociodemographic covariates determined were sex (women and men), age group (18-34 years; 35-59 years; ≥ 60 years), race/ethnicity categories was self-reported (white, black, brown and others: yellow and indigenous), marital status (single or married), current family income (≤ 2 wages; >2 to ≤ 4 wages; >4 wages), and education (non-literate; < 9 years; ≥ 9 years).
Tobacco consumption was determined using the question, "Do you smoke, or have you ever smoked cigarettes or any other tobacco product?" The answer options were yes and no.
Reported morbidity was determined by self-reporting of the following diseases: high blood pressure, asthma, lung disease, chronic kidney disease, depression, anxiety disorder, obstructive sleep apnea, cancer, heart disease, or thyroid disease. Individuals who reported having at least one of the diseases were classified as having morbidity, and those with no disease as having no morbidity.
2.7. Ethical considerations
This study was approved by the Ethics Committee on Human Research of the Federal University of Minas Gerais (protocol number:32815620.0.1001.5149). All procedures followed the Brazilian guidelines and standards for human research. Participants were informed about the research objectives, the steps to be taken, and the risks and benefits of their participation. Those who agreed to participate signed an informed consent form.
2.8. Statistical analysis
The study population was characterized using descriptive calculations, such as relative frequencies, mean values, and 95% confidence intervals (CI). Pearson chi-square test was used to verify the relationship between glycemic alterations and the sociodemographic characteristics of the study population. Univariate and multivariate logistic regression analyzes were performed to assess the association between physical inactivity and glycemic changes.
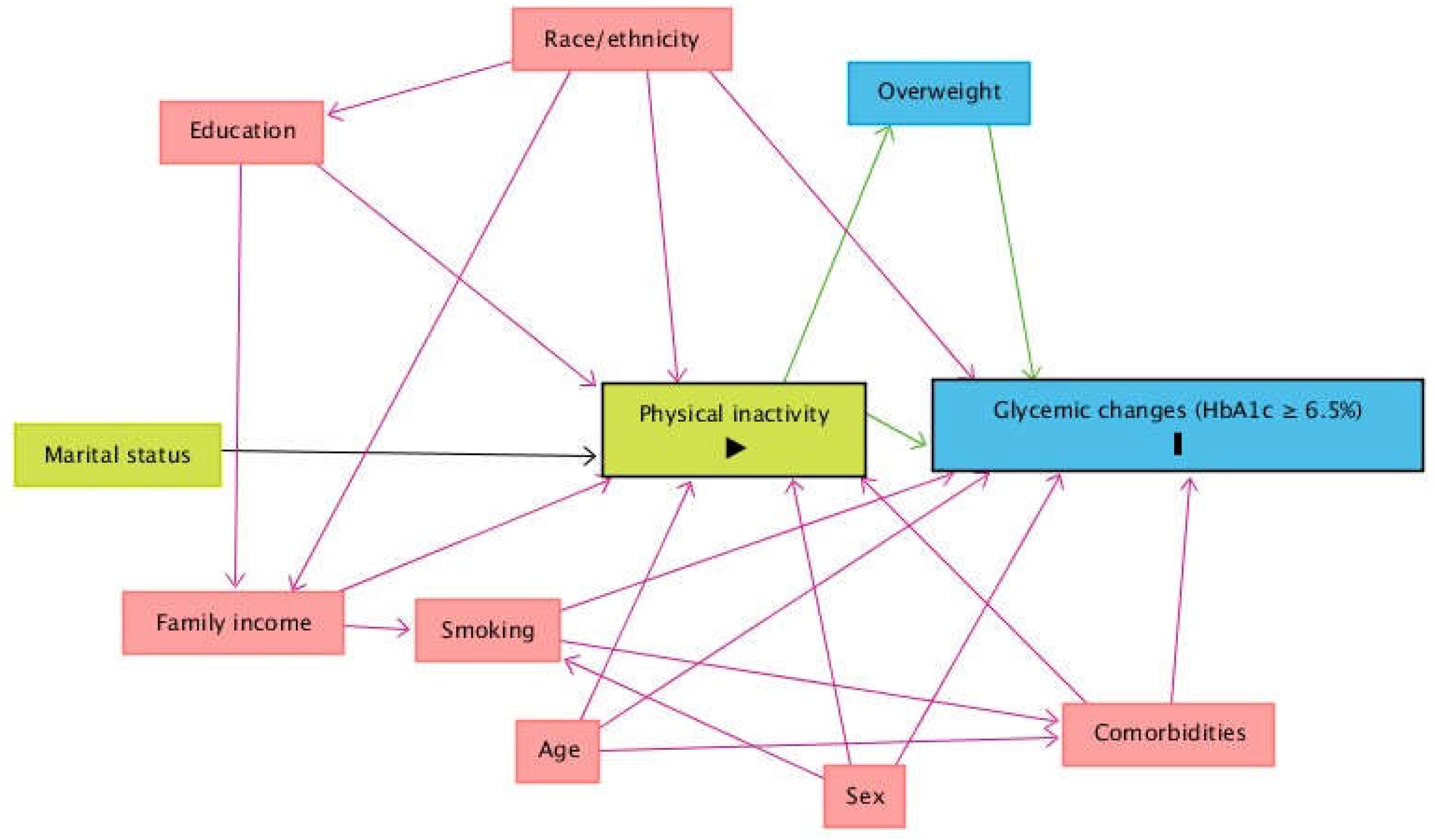
A theoretical causality model based on a directed acyclic graph (DAG) was developed based on exposure (physical inactivity), outcome (glycemic changes, HbA1c ≥ 6.5%), and covariate variables using online Dagitty software, software version 3.2 [
27] (
Figure 1). DAG was used to avoid unnecessary adjustments, spurious associations, and estimation errors. The backdoor criterion was used to select a minimum set of confounders to fit the analyzes [
28]. The model was fitted using the following minimal and sufficient variables: sex, age, family income, race and referred morbidities.
Legend: The variable in green and with the symbol ">" inside the rectangle is the exposure variable. The variable in blue and with the letter "I" inside the rectangle is the outcome variable; Key: The variable and with symbol ">" inside the rectangle is the exposure variable. In blue and with the letter I is the outcome variable; variables in blue are the antecedents of the outcome variable; and those in red are the antecedents of the outcome and exposure variables. Black arrows are non-causal and unbiased paths; green arrows are causal paths between the explanatory variable and outcome variable or antecedents; red arrows are biased paths.
To verify whether overweight could be a mediating variable between the association of PI and glycemic changes, we used mediation analysis using the Karlson-Holm-Breen method, package "khb" in Stata [
29]. This method estimates total, direct, and indirect associations between the explanatory variable (leisure-time PI) and the outcome variable (glycemic changes: serum HbA1c levels ≥ 6.5%). Using logistic regression models, the method decomposed the total effect of a variable into a direct effect (direct association of leisure-time physical inactivity and glycemic changes) and an indirect effect (the mediating effect of overweight on glycemic changes).
Results were analyzed using the Stata statistical program, version 15.1, operating the "svy" command, which considers a complex sample design. Statistical significance was established at P < 0.05.
3. Results
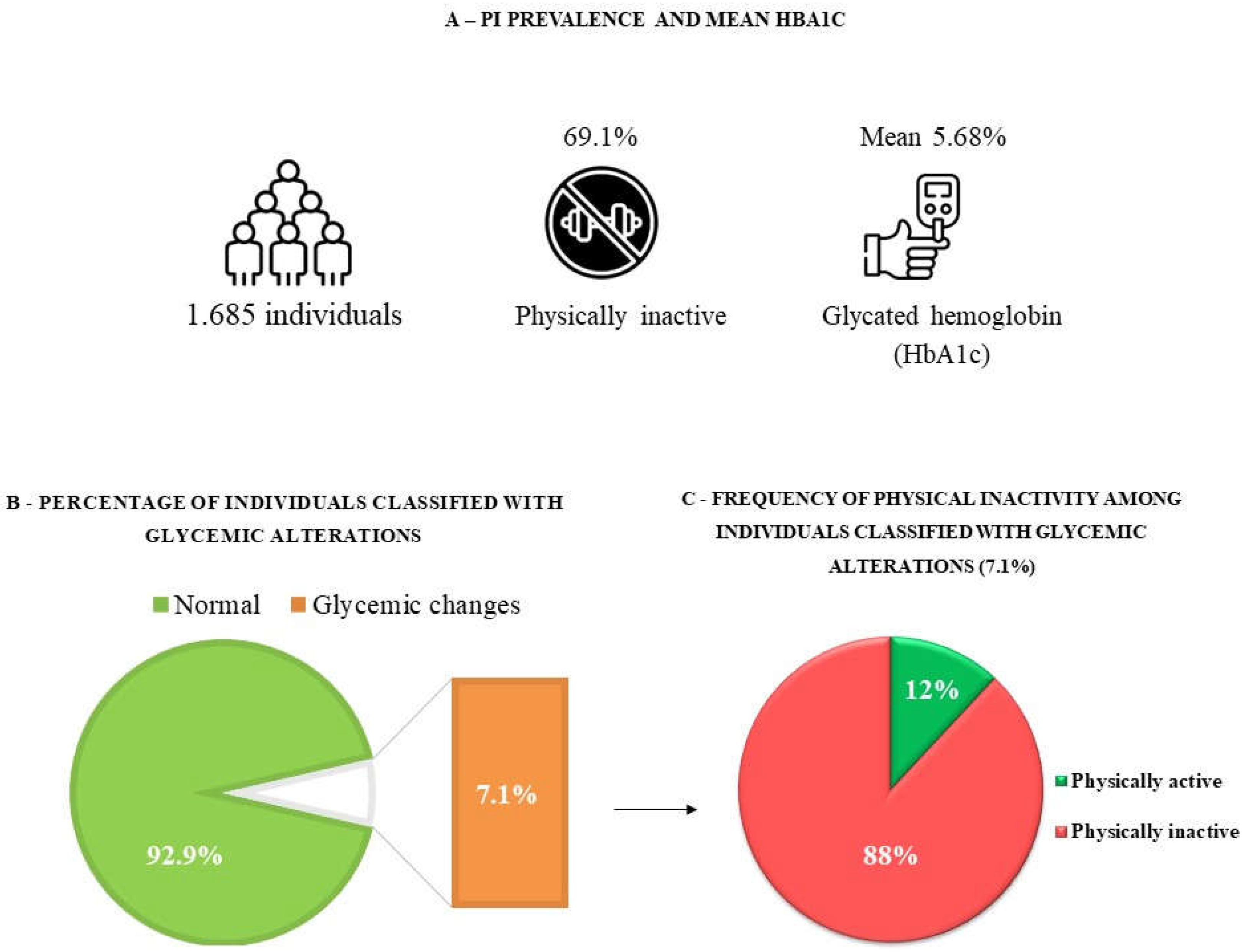
We evaluated 1,685 individuals, most of whom were woman (52.4%; 95% CI:40.5-54.8), aged 35-59 years old (45.8%; 95% CI:41.2-50.5), married (52.9%; 95% CI:46.8-58.9), self-reported as brown (48.1%; 95% CI:41. 7-54.5), less than nine years of schooling (69.1%; 95% CI:64.3-73.6), income less than or equal to two minimum wages (41.2%; 95% CI:35.6-47.1), and overweight (56.5%; 95% CI: 49.8-63.0). In addition, most of the respondents reported having one or more morbidities (55.5%; 95% CI:48.3-62.4), as shown in
Table 1.
The mean HbA1c was 5.68% (95% CI: 5.58-5.77). We observed that 92.9% (95% CI: 90.6-94.7) of the subjects had normal levels of HbA1c, and 7.1% (95% CI: 5.3-9.4) had glycemic changes. In addition, 69.2% (95% CI: 64.2-73.7) of the participants were physically inactive at leisure (
Figure 2).
Table 1 presents the characteristics of the participants according to their serum HbA1c levels. Variables related to the presence of glycemic alterations according to Pearson chi-square test (
P< 0.05) were age ≥ 60 years, female, education ≤ 9 years, overweight, black race/ethnicity, and presence of morbidities (
Table 1).
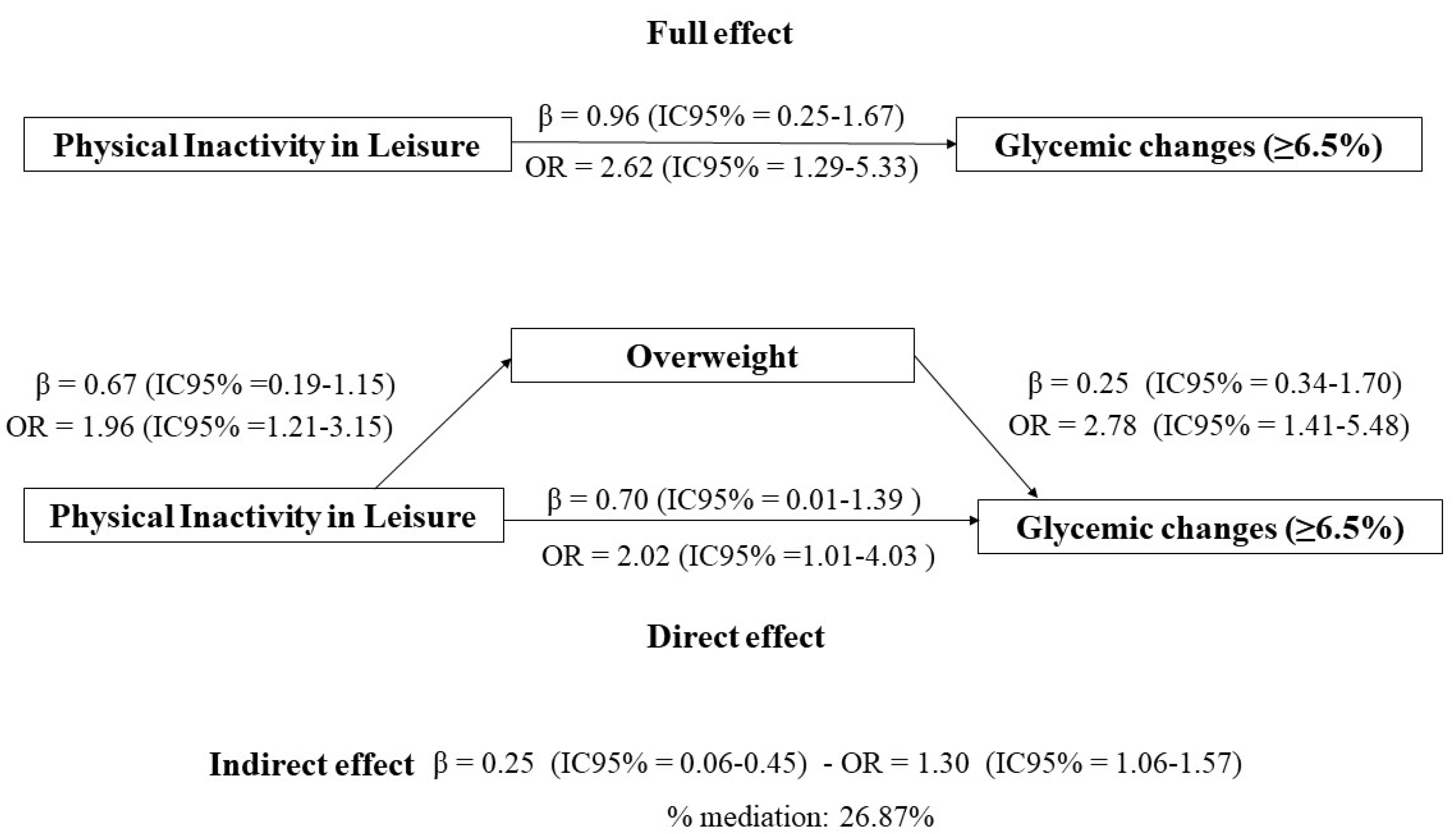
When examining the results of the logistic regression, it was observed in the multivariate analysis (adjusted for sex, age, race/ethnicity, family income, and reported morbidity) that individuals who were physically inactive during leisure time were 2.62 times more likely to have glycemic alterations (OR:2.62,95%CI:1.31-5.24), as presented in
Table 2. In the mediation analysis by overweight, it was possible to verify that physically inactive individuals during leisure time had 2.62 times greater chance of having glycemic alterations (OR: 2.62,95% CI: 1.29-5.33), and 26.87% of this effect was mediated by overweight (OR: 1.30, 95% CI: 1.06-1.57 (
Figure 3).
Legend: Explanatory variable; leisure-time physical inactivity. Outcome variable; glycated hemoglobin (HbA1c ≥ 6.5%). Mediating variable; overweight (BMI ≥ 25.0 kg/m² if < 60 years or BMI ≥ 27.0 kg/m² if ≥ 60 years). Mediation model from Karlson Holm Breen method, adjusted according to directed acyclic graph according to minimum and sufficient set: age, sex, race/ethnicity, family income, and referred morbidity.
4. Discussion
This study investigated the association between physical activity and glycemic alterations, as well as the mediation of overweight, in this association. Our findings confirm the initial hypothesis that adults who accumulate 150 min of moderate or 75 min of vigorous physical activity per week are less likely to have glycemic changes than individuals who do not reach the weekly minutes of physical activity recommended by the guidelines [
23,
24]. To our knowledge, this is the first major epidemiological survey to investigate HbA1c and physical inactivity during leisure time during social restriction caused by the COVID-19 pandemic.
These results are important regarding public health and clinical practice, as approximately 70% of the population in this study did not meet the minimum guidelines for physical activity. Although our study did not allow us to assess the change in physical activity practice during the COVID-19 pandemic, it is believed the pandemic may have contributed substantially to the increased prevalence of physical inactivity [
3] due to the closure of recreational facilities, urban parks, and gyms to mitigate the spread of the virus. However, chronic damage caused by reduced levels of physical activity in the face of prolonged home stays has become a significant challenge [
3,
4]. The health consequences are inevitable, with deterioration of the physical and mental conditions of the population observed due to the confinement period [
30].
Thus, some studies show a worsening prevalence of physical inactivity and overweight due to social restriction and reduced urban mobility worldwide [
4,
6,
31,
32,
33]. This landscape becomes vulnerable to inflated risks for developing chronic non-communicable diseases [
34], in addition to the increased occurrence of certain disorders, such as changes in HbA1c levels. These changes in the glycemic profile may represent the progression and/or worsening of the clinical picture to type 2 diabetes mellitus [
35]. Another concern is the susceptibility to infection and the risk of COVID-19 in individuals with glycemic alterations [
36]. This emphasizes the importance of maintaining good glycemic control for overall health and to prevent worsening and complications associated with SARS-CoV-2 infection (Severe Acute Respiratory Syndrome Coronavirus 2).
Regular physical activity has long been considered a non-pharmacological way to improve general health, manage glycemic control, and treat T2DM, and its low cost has increased its appeal [
37,
38]. According to the American Diabetes Association [
39], physical activity is essential for preventing the harmful effects of T2DM [
40]. Muscle contractile activity stimulates the translocation of glucose transporter protein-4 (GLUT-4) to the plasma membrane, promoting glucose uptake and clearance [
15,
41]. Furthermore, it acts on energy balance and reduces adiposity, and overweight is one of the main risk factors for the development of T2DM [
42].
A study of Brazilian women before (January and February 2020) and after a 16-week period of the COVID-19 pandemic (June and July 2020) showed that confinement promoted important changes in health-related parameters. HbA1c levels increased significantly by 9.7%, which was explained by the reduction in physical activity [
35]. Consequently, a cross-sectional study conducted in India revealed the negative impact of social restrictions on glycemic control. The mean blood glucose level is higher during the blockade phase [
7]. Furthermore, a systematic review concluded that the COVID-19 pandemic caused worsening glycemic control and complications related to T2DM, this could be due to the limitation of free space to exercise [
36].
Our results show an association between physical inactivity and glycemic alterations, and a part of this association can be explained by being overweight. These findings demonstrate clinical relevance, with physical activity being effective in mitigating the risk of developing T2DM, both for body weight maintenance and through metabolic mechanisms that help with glycemic control. A similar result to ours was found in a systematic review and dose-response meta-analysis, which compared risk estimates of having T2DM in relation to leisure-time physical activity, with and without BMI adjustment. Results with BMI adjustment were approximately 20%–30% weaker in their association compared to the results not adjusted for BMI [
42], thus demonstrating the mediating effect of BMI on the association between physical inactivity and glycemic changes.
Thus, a plausible mechanism involved in this association may be positive energy balance, that is, increased habitual food intake (mainly of ultraprocessed foods) and reduced energy expenditure by reducing urban mobility and physical activity. This may lead to weight gain and increase the risk of T2DM in individuals physically inactive [
43,
44]. Given this data, it is possible to postulate the lasting impact of the increased physical inactivity caused by the pandemic on the health of the population.
The findings of this study are of great value to the scientific community, leaders, and health professionals to better understand the side effects on short- and long-term health conditions of the COVID-19 pandemic and to devise strategies to mitigate these impacts. The COVID-19 pandemic may have provided a favorable scenario for morbidity and a poorer health prognosis. Furthermore, there are no structured educational programs for self-management of T2DM in Brazil [
45]. PA acts in the prevention and mitigation of complications from T2DM, in addition to mitigating the high costs to the public system, which is a burden on global health [
12]. Health professionals operating in the treatment of T2DM should encourage the practice of PA, promoting the population's understanding of the acute and chronic physiological responses to PA in T2DM.
Although robust and with relevant results in the scientific literature, this study had some limitations. The level of physical activity was obtained by self-reporting; therefore, it is subject to memory and other bias, which may underestimate or overestimate the data. Methodologically, the cross-sectional design of this study did not allow the establishment of causalities. Furthermore, calorie intake and added sugars, which are important covariates in the present association, were not assessed. However, our study has strengths, such as a robust sample methodology with probability selection and sample weight, providing statistical power to the study. The interviews were conducted face-to-face, allowing greater accuracy in the information obtained. In addition, assessing glycemic homeostasis through Hb1Ac is noteworthy, as it represents glycemic levels during the last four months, and not only at the current time. Counterfactual assumptions are highlighted to build theories that can support the direction of analysis. In this regard, the incorporation of the directed graphical model is important and robust to study [
46].
5. Conclusions
Leisure-time physical activity is a factor associated with greater glycemic alterations, and part of this association is explained by overweight. Therefore, our data suggest that physical activity may attenuate the risk of developing T2DM, partly by controlling overweight, but also independently of adiposity. Glycemic changes are metabolic changes that require regular attention, given their implications for the worsening of population health and high costs to the public health system. We believe that maintaining regular physical activity is essential for the management of glycemic control in periods of restricted social mobility.
During the pandemic, there was a great deal of uncertainty and fear around physical activity, especially in open areas since little was known about the forms of contamination. This favored an increase in physical inactivity during data collection. However, with current knowledge, the encouragement of physical activity in open environments should be encouraged in times of restricted social mobility. Taking the necessary precautions to reduce contagion, or at least the encouragement of physical activity at home, through online classes and simple exercises with body weight, will prevent the harmful effects on health, since increased physical inactivity can lead to an increase in weight and an increased risk of T2DM together with other pathophysiology.
Author Contributions
Original draft preparation: G.L.L.M.C., A.L.M.; methodology: S.S.M., L.A.A.M.J., A.M.S.R., A.P.B., M.C.M., J.C.C.C., G.L.L.M.C., A.L.M.; data preparation: S.S.M., L.A.A.M.J.; writing—original draft preparation: S.S.M.; writing—review and editing: S.S.M., L.A.A.M.J., A.M.S.R., A.P.B., M.C.M., J.C.C.C., G.L.L.M.C., A.L.M.; All authors have read and agreed to the published version of the manuscript.
Funding
O This study was supported by the Federal University of Ouro Preto (UFOP) [PROPPI/UFOP nº18/2022], Brazilian Council for Scientifc and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES) [9/2020; nº88887.504994/2020e00], Foundation for Research Support of the State of Minas Gerais (FAPEMIG) [nº001/2021; APQ02445–21], and finance code 001 for Ph.D. student scholarship.
Institutional Review Board Statement
All procedures adopted in this study followed the Declaration of Helsinki and the Brazilian guidelines and norms for research involving humans and approved by the Research Ethics Committee of the Federal University of Ouro Preto, under protocol number 32815620.0.1001.5149.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and/or analyzed as part of the current study are not publicly available due to confdentiality agreements with subjects. However, they can be made available solely for the purpose of review and not for the purpose of publication from the corresponding author upon reasonable request.
Acknowledgements
The researchers thank the Federal University of Ouro Preto, the Municipal Health Secretariats of Ouro Preto and Mariana. The group for Research and Teaching in Nutrition and Collective Health at the Federal University of Ouro Preto and the Laboratory of Epidemiology at the School of Medicine at the Federal University of Ouro Preto and the Pilot Clinical Analysis Laboratory at the School of Pharmacy at the Federal University of Ouro Preto for the biochemical dosages.
Conflict of interest: The authors declare that they have no competing interests.
References
- Romero-Blanco, C.; Rodríguez-Almagro, J.; Onieva-Zafra, M.D.; Parra-Fernández, M.L.; Prado-Laguna, M.D.C.; Hernández-Martínez, A. Physical activity and sedentary lifestyle in university students: Changes during confinement due to the Covid-19 pandemic. International Journal of Environmental Research and Public Health 2020, 17, 6567. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.A.; Macêdo, G.A.; Cabral, L.L.; Oliveira, G.T.; Vivas, A.; Fontes, E.B.; Elsangedy, H.M.; Costa, E.C. Initial impact of the COVID-19 pandemic on physical activity and sedentary behavior in hypertensive older adults: An accelerometer-based analysis. Experimental gerontology 2020, 142, 111121. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Babarro, A.; Arbillaga-Etxarri, A.; Gutiérrez-Santamaría, B.; Coca, A. Physical activity change during COVID-19 confinement. International Journal of Environmental Research and Public Health 2020, 17, 6878. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; McDowell, C.; Lansing, J.; Brower, C.; Smith, L.; Tully, M.; Herring, M. Changes in physical activity and sedentary behavior in response to COVID-19 and their associations with mental health in 3052 US adults. International Journal of Environmental Research and Public Health 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Tison, G.H.; Avram, R.; Kuhar, P.; Abreau, S.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Worldwide effect of COVID-19 on physical activity: a descriptive study. Annals of internal medicine 2020, 173, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. Journal of translational medicine 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Rajput, R.; Verma, S.; Balania, V.K.; Jangra, B. Impact of lockdown in COVID 19 on glycemic control in patients with type 1 Diabetes Mellitus. Diabetes metabolic syndrome: clinical research reviews 2020, 14, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International journal of antimicrobial agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Rajakumar, G.; Subramanian, U.; Venkidasamy, B.; Khanna, V.G.; Thiruvengadam, M. Insights on the current status and advancement of diabetes mellitus type 2 and to avert complications: an overview. Biotechnology applied biochemistry 2020, 67, 920–928. [Google Scholar] [CrossRef]
- Atlas, D.J.H.e.h.w.d.o.r.p.-e.h. International Diabetes Federation (IDF). 2000. 2017.
- Higgins, T. HbA1c—An analyte of increasing importance. Clinical biochemistry 2012, 45, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-M.; Shen, F.-C.; Chen, J.-F.; Chang, W.-D.; Chang, N.-J. Effects of resistance exercise on glycated hemoglobin and functional performance in older patients with comorbid diabetes mellitus and knee osteoarthritis: a randomized trial. International Journal of Environmental Research Public Health 2020, 17, 224. [Google Scholar] [CrossRef] [PubMed]
- Ofori, E.K.; Angmorterh, S.K. Relationship between physical activity, body mass index (BMI) and lipid profile of students in Ghana. The Pan African Medical Journal 2019, 33. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, M.; Brandou, F.; Perez-Martin, A.; Fedou, C.; Mercier, J.; Brun, J. Low intensity endurance exercise targeted for lipid oxidation improves body composition and insulin sensitivity in patients with the metabolic syndrome. Diabetes metabolism 2003, 29, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Jorge, M.L.M.P.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.D.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Saúde, B.M.d.S.P.d. Informações de Saúde (TABNET)--Demográficas e Socioeconômicas. o Ministério Brasília: 2010.
- Meireles, A.L.; Lourenção, L.G.; Menezes Júnior, L.A.A.d.; Coletro, H.N.; Justiniano, I.C.S.; Moura, S.S.d.; Diniz, A.P.; Sabião, T.d.S.; Rocha, A.M.S.; Batista, A.P.; et al. COVID-Inconfidentes - SARS-CoV-2 seroprevalence in twoBrazilian urban areas during the pandemic first wave: studyprotocol and initial results. SciELO Preprints 2021. [Google Scholar] [CrossRef]
- Golbert, A.; Vasques, A.C.J.; Faria, A.; Lottenberg, A.M.P.; Joaquim, A.G.; Vianna, A.G.D. Diretrizes da Sociedade Brasileira de Diabetes 2019-2020. São Paulo: Clannad 2019, 1–491. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs Jr, D.R.; Montoye, H.J.; Sallis, J.F.; Paffenbarger Jr, R.S. Compendium of physical activities: classification of energy costs of human physical activities. Medicine science in sports exercise 1993, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O. Compendium of physical activities: an update of activity codes and MET intensities. Medicine science in sports exercise 2000, 32, S498–S504. [Google Scholar] [CrossRef] [PubMed]
- Farinatti, P.d.T.V. Apresentação de uma versão em português do compêndio de atividades físicas: uma contribuição aos pesquisadores e profissionais em fisiologia do exercício. Rev Bras Fisiol Exerc 2003, 2, 177–208. [Google Scholar]
- BRASIL. Guia de atividade física para população brasileira. Availabe online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_atividade_fisica_populacao_brasileira.pdf (accessed on 31 de março de 2022).
- WHO. WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization. 2020.
- OPAS, O.P.-A.d.S. XXXVI Reunión del Comitê Asesor de Investigaciones en Salud - Encuestra Multicêntrica - Salud Beinestar y Envejecimeiento (SABE) en América Latina e el Caribe. 2001.
- WHO. Obesity: preventing and managing the global epidemic: report of a WHO consultation on obesity, Geneva, 3-5 June 1997; World Health Organization: 1998.
- Shrier, I.; Platt, R.W. Reducing bias through directed acyclic graphs. BMC medical research methodology 2008, 8, 1–15. [Google Scholar] [CrossRef]
- Cortes, T.R.; Faerstein, E.; Struchiner, C.J. Use of causal diagrams in Epidemiology: application to a situation with confounding. Cadernos de saude publica 2016, 32. [Google Scholar]
- Breen, R.; Karlson, K.B.; Holm, A. Total, direct, and indirect effects in logit and probit models. Sociological Methods Research 2013, 42, 164–191. [Google Scholar] [CrossRef]
- Lesser, I.A.; Nienhuis, C.P. The impact of COVID-19 on physical activity behavior and well-being of Canadians. International Journal of Environmental Research Public Health 2020, 17, 3899. [Google Scholar] [CrossRef] [PubMed]
- de Melo Souza, T.C.; Oliveira, L.A.; Daniel, M.M.; Ferreira, L.G.; Della Lucia, C.M.; Liboredo, J.C.; Anastácio, L.R. Lifestyle and eating habits before and during COVID-19 quarantine in Brazil. Public Health Nutrition 2021, 1–29. [Google Scholar]
- Peçanha, T.; Goessler, K.F.; Roschel, H.; Gualano, B. Integrative Cardiovascular Physiology and Pathophysiology: Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. American Journal of Physiology-Heart Circulatory Physiology 2020, 318, H1441. [Google Scholar] [CrossRef]
- Sidor, A.; Rzymski, P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef]
- León-Latre, M.; Moreno-Franco, B.; Andrés-Esteban, E.M.; Ledesma, M.; Laclaustra, M.; Alcalde, V.; Peñalvo, J.L.; Ordovás, J.M.; Casasnovas, J.A. Sedentary lifestyle and its relation to cardiovascular risk factors, insulin resistance and inflammatory profile. Revista Española de Cardiología 2014, 67, 449–455. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.G.R.; Abud, G.F.; de Freitas, E.C.; Júnior, C.R.B. Effects of the COVID-19 pandemic on the global health of women aged 50 to 70 years. Experimental Gerontology 2021, 150, 111349. [Google Scholar] [CrossRef]
- Ghosal, S.; Sinha, B.; Majumder, M.; Misra, A. Estimation of effects of nationwide lockdown for containing coronavirus infection on worsening of glycosylated haemoglobin and increase in diabetes-related complications: a simulation model using multivariate regression analysis. Diabetes metabolic syndrome: clinical research reviews 2020, 14, 319–323. [Google Scholar] [CrossRef]
- Colberg, S.R. Exercise and diabetes: a clinician's guide to prescribing physical activity; American Diabetes Association: 2013.
- King, K.M.; Jaggers, J.R.; Della, L.J.; McKay, T.; Watson, S.; Kozerski, A.E.; Hartson, K.R.; Wintergerst, K.A. Association between physical activity and sport participation on hemoglobin A1c among children and adolescents with type 1 diabetes. International Journal of Environmental Research Public Health 2021, 18, 7490. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, G.; Hermassi, S.; Bragazzi, N. Impact of the COVID-19 Pandemic on the Physical Activity Profile and Glycemic Control Among Qatari Adults With Type 1 Diabetes: Effect of Vaccination Status. Frontiers in Public Health 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.; Pal, S. Acute exercise improves postprandial cardiovascular risk factors in overweight and obese individuals. Atherosclerosis 2011, 214, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis. European journal of epidemiology 2015, 30, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ferran, M.; de la Guía-Galipienso, F.; Sanchis-Gomar, F.; Pareja-Galeano, H. Metabolic impacts of confinement during the COVID-19 pandemic due to modified diet and physical activity habits. Nutrients 2020, 12, 1549. [Google Scholar] [CrossRef] [PubMed]
- Munekawa, C.; Hosomi, Y.; Hashimoto, Y.; Okamura, T.; Takahashi, F.; Kawano, R.; Nakajima, H.; Osaka, T.; Okada, H.; Majima, S. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: a cross-section and retrospective cohort study. Endocrine Journal 2021, 68, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.S.; Pavin, E.J.; Cardoso, E.B.; Fanti, P.; Abdoli, S. Emotional burden and care of adults with type 1 diabetes during the COVID-19 pandemic in Brazilian regions. Journal of Diabetes its Complications 2021, 35, 108053. [Google Scholar] [CrossRef]
- PEARL, J. Causality: models, reasoning and inference.; 2009.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).