1. Introduction
Ungulate overpopulation is an important issue in the northern hemisphere [
1], including northern America [
2] and Europe [
3]. Ungulate populations are regulated by bottom-up, top-down, and abiotic factors [
4]. A lack of certain nutrients/elements may limit ungulate population growth, especially at a high population density.
Sodium (Na), calcium (Ca), and magnesium (Mg) may be limiting elements for ungulates at a high population density because livestock are often deficient in these elements, especially Na, which is supplemented artificially [
5]. A mineral deficiency can also occur in nature based on the fact that various animals use salt licks [
6,
7,
8]. Geophagy (i.e., eating soil) is common in ungulates [
9,
10,
11], probably for mineral supplementation. Various species were reported to lick natural springs, presumably for obtaining minerals [
12]. Ceacero et al. (2014) [
13] reported that Iberian red deer (
Cervus elaphus) feed on sea weeds, possibly to acquire minerals. The use of salt licks has often been attributed to a requirement for Na [
8,
9,
14], which is important for maintaining osmotic pressure [
15]. However, it may also be due to a requirement for other minerals, such as Ca, which is a major component of bones [
16], and Mg, which has important roles in enzyme activity and bone development [
17]. Several studies have argued that natural salt licks are important sources of Ca and Mg [
18].
Sika deer (
Cervus nippon) are native to eastern Asia and have been introduced into many parts of the world, such as Europe, North America, and New Zealand [
19]. In some areas, the deer populations have increased enough that they cause serious browsing damage to natural and anthropogenic environments [
19,
20]. In Japan, especially, the sika deer population has increased dramatically in recent decades [
21]. According to the Ministry of the Environment (2015), the distribution of sika deer expanded more than 2.5 times from 1978 to 2015 [
22]. Since this substantial increase in the sika deer population and distribution and subsequent overbrowsing could lead to mineral deficiencies in sika deer, we considered that the deficient minerals in overabuntant populations act as an attractant to cull deer. Indeed, sika deer also lick salt [
23]. In addition, sika deer are being culled in many parts of Japan to reduce overabundant populations, and the development of effective culling methods is desirable. Because selective culling of female deer is reported to be effective in reducing sika deer populations [
24,
25], it is particularly important to clarify the differences in mineral deficiency between male and female. However, no study has evaluated whether sika deer in Japan obtain sufficient minerals, such as Na, Ca, and Mg, through food plants by comparing the minerals provided by food plants and mineral requirements of sika deer. Mineral deficiencies between male and female deer have also not been compared. In this study, we evaluated whether food plants can provide sika deer sufficient Na, Ca, and Mg by comparing the minerals provided by food plants, calculated from leaf mineral contents, and mineral requirements of sika deer, simulated by several scenarios of daily dry matter intake (DMI), body weight (BW), and sex.
2. Materials and Methods
Dataset
A dataset of leaf nutrient contents of sika deer food plants in Japan was constructed using plant trait data from the TRY Plant Trait Database [
26]. From TRY, we extracted the leaf Na, Ca, and Mg contents per leaf dry mass (Trait IDs 260, 252, and 257, respectively) for plant species previously documented as sika deer food in Japan [
27]. The final dataset included 3179 values for 191 plant species (Table S1) from five original publications [
28,
29,
30,
31,
32]. The leaf mineral content data were averaged for each species, and the averaged values were used for further analysis.
Analysis
Using the leaf mineral concentration data for sika deer food plants in Japan, we simulated whether each plant species could provide sufficient minerals for sika deer. We considered four sika deer body weight (BW) scenarios based on the BW range of sika deer in Japan (i.e., 30, 60, 90, and 120 kg) and four daily dry matter intake (DMI) scenarios considering a wider range than reported from feeding experiments (i.e., 0.5, 1.5, 3, and 4.5 kg) [
33,
34,
35]. Unrealistic scenarios (BW 30 kg with DMI 3 or 4.5 kg; BW 60 kg with DMI 4.5 kg; and BW 120 kg with DMI 0.5 kg) were excluded from our analysis. Mineral contents provided by leaves, which were calculated by multiplying DMI and leaf mineral concentration, were compared with the mineral requirement of sika deer calculated as follows. The Na maintenance requirement was calculated based on the following equation for
Cervus [
16]:
, where
Na_req_maintain is the Na requirement for maintenance and
BW is body weight (kg). The Na requirement for males was calculated as
Na_req_maintain plus the requirement for antlers, assuming that antler growth in sika deer requires 150 days, as follows [
16]:
The equation for determining the Na requirement of females during lactation [
16] was modified for sika deer as follows:
, where
Na_milk_sika is the Na content in sika deer milk (1.00 g L
-1, [
36]). We also calculated the Na requirement of female during non-lactating period (equal to
Na_req_maintain) by omitting the term expressing the Na requirement for milk production from the equation. The above equations do not include the Na requirements for body growth, assuming that body growth is limited by a lack of food plants. The maintenance Mg requirement (
Mg_req_maintain) was calculated based on the following equation for goats [
16], because equations for goats were recommended to calculate values for cervids (equation for cervids were not available) [
16]:
Without considering Mg requirement for body growth, the Mg requirement for males was assumed to be equal to
Mg_req_maintain [
16]. The Mg requirement for lactating females was calculated using equations prepared for goats as recommended [
16], but modified as follows:
, where
Mg_milk_sika is the Mg content of sika deer milk (0.0819 g L
-1, [
36]). Mg requirement for gestation was not taken into consideration, because this was not included equations for Na and Ca. Thus, Mg requirement of female during non-lactating period was considered equal to Mg requirement of male. The maintenance Ca requirement (
Ca_req_maintain) was calculated based on the following equation for
Cervus [
16]:
The Ca requirement for males was calculated as
Ca_req_maintain plus the Ca requirement for antlers as [
16]:
The Ca requirement for lactating females was calculated as
Ca_req_maintain plus the Ca requirement for lactation [
16] with a minor modification:
, where
Ca_milk_sika is the Ca content of sika deer milk (1.62 g L
-1, [
36]). The Ca requirements for body growth were also not included. Ca requirement of female during non-lactating period (equal to
Ca_req_maintain) was also calculated by omitting the term expressing the Ca requirement for milk production from the equation.
3. Results
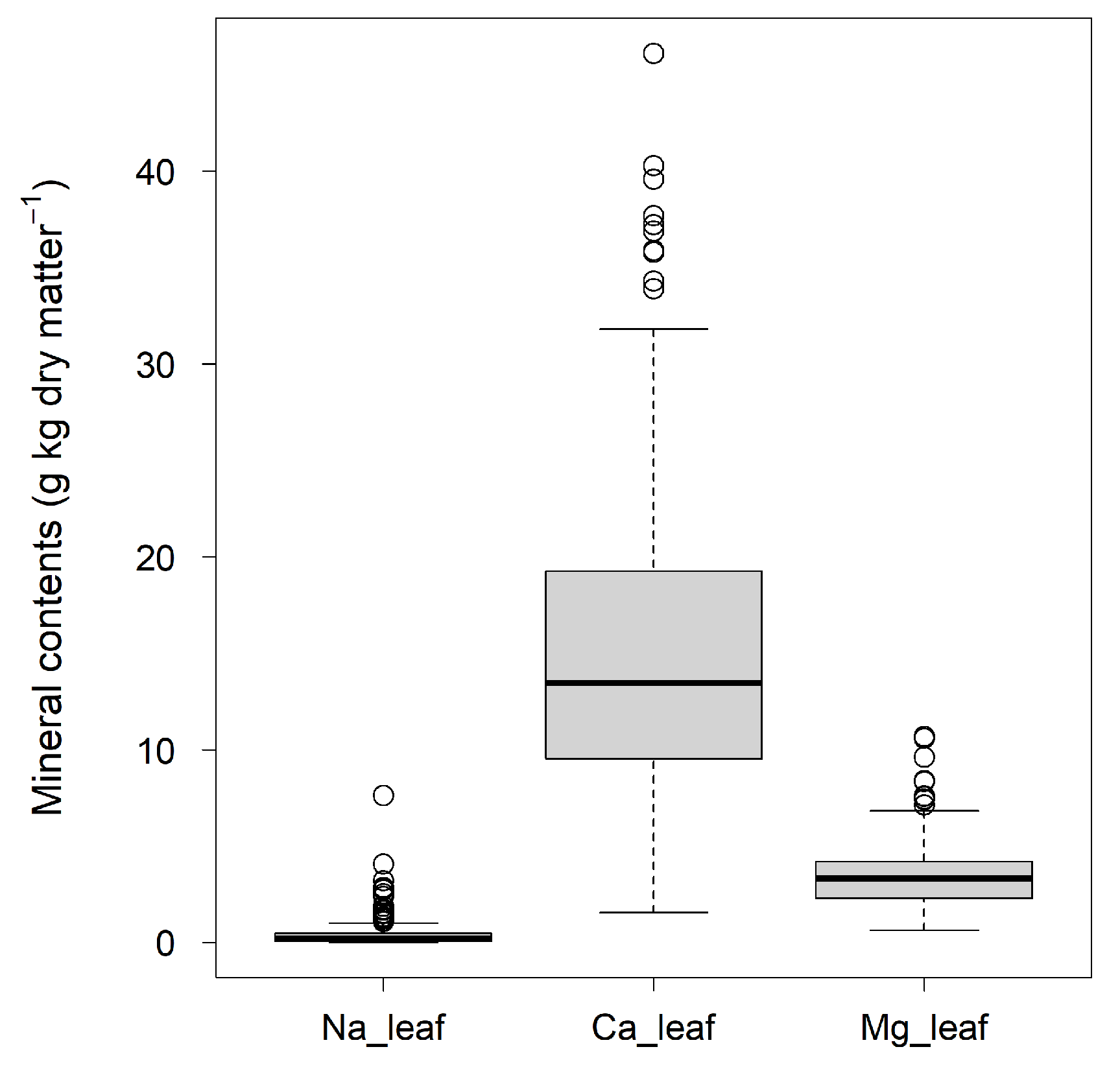
The sika deer food plant dataset used for this analysis showed large variance (
Fig. 1). The average leaf contents of Na, Ca, and Mg were 0.47 ± 0.82, 15.3 ± 8.4, and 3.5 ± 1.8 (g element kg-1 dry matter ± standard deviation), respectively.
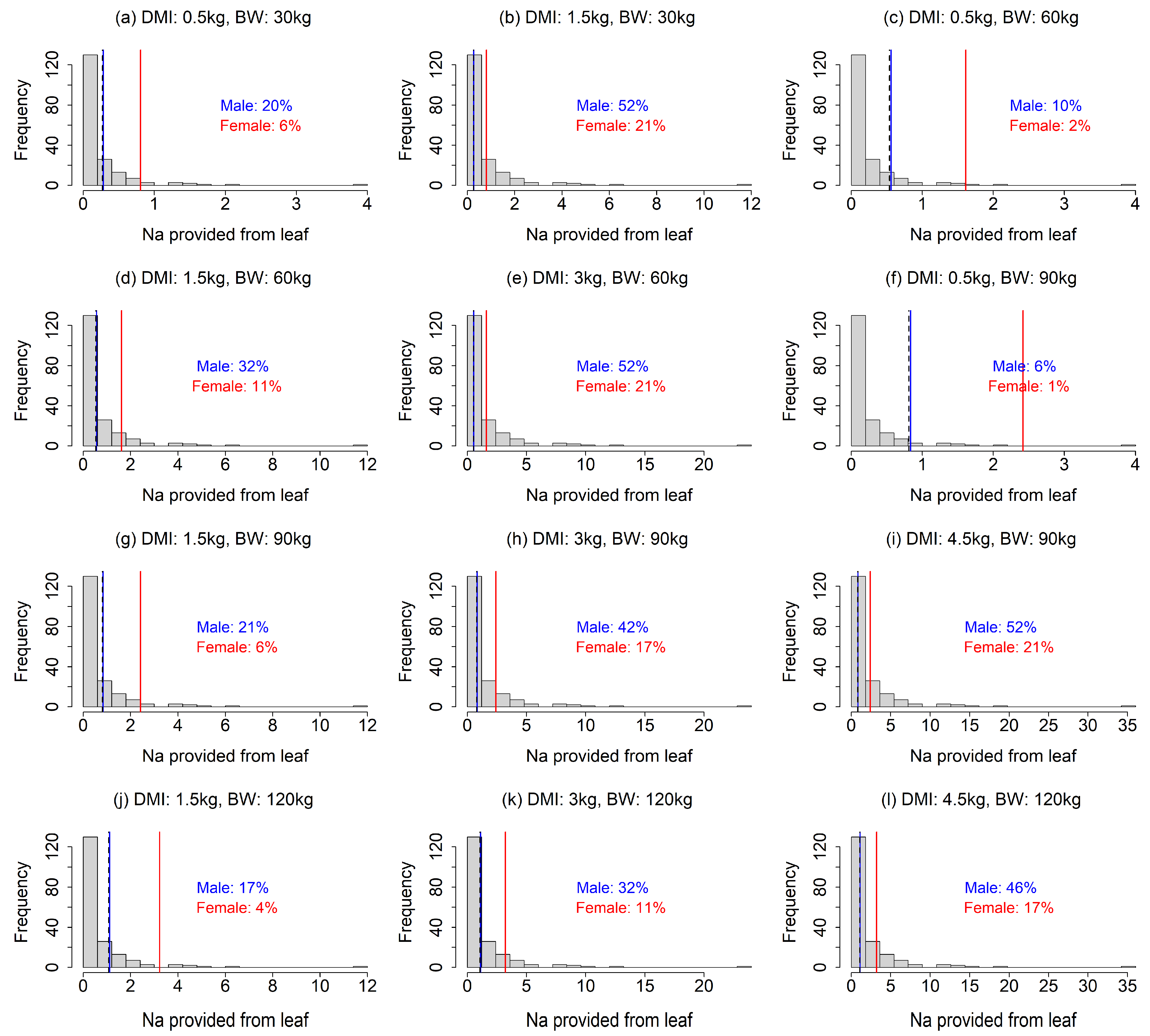
Our analysis showed that the Na requirements were rarely achieved by food plants, especially under the scenarios with a smaller DMI and larger BW (
Fig. 2). In most of the 12 scenarios, less than 50% of the food plant species provided sufficient Na (
Fig. 2a, c, d, f–h, j–l). The results also indicated that a Na deficiency would be more intense for lactating females than males (
Fig. 2), as the Na requirement for females was more than double that of males (
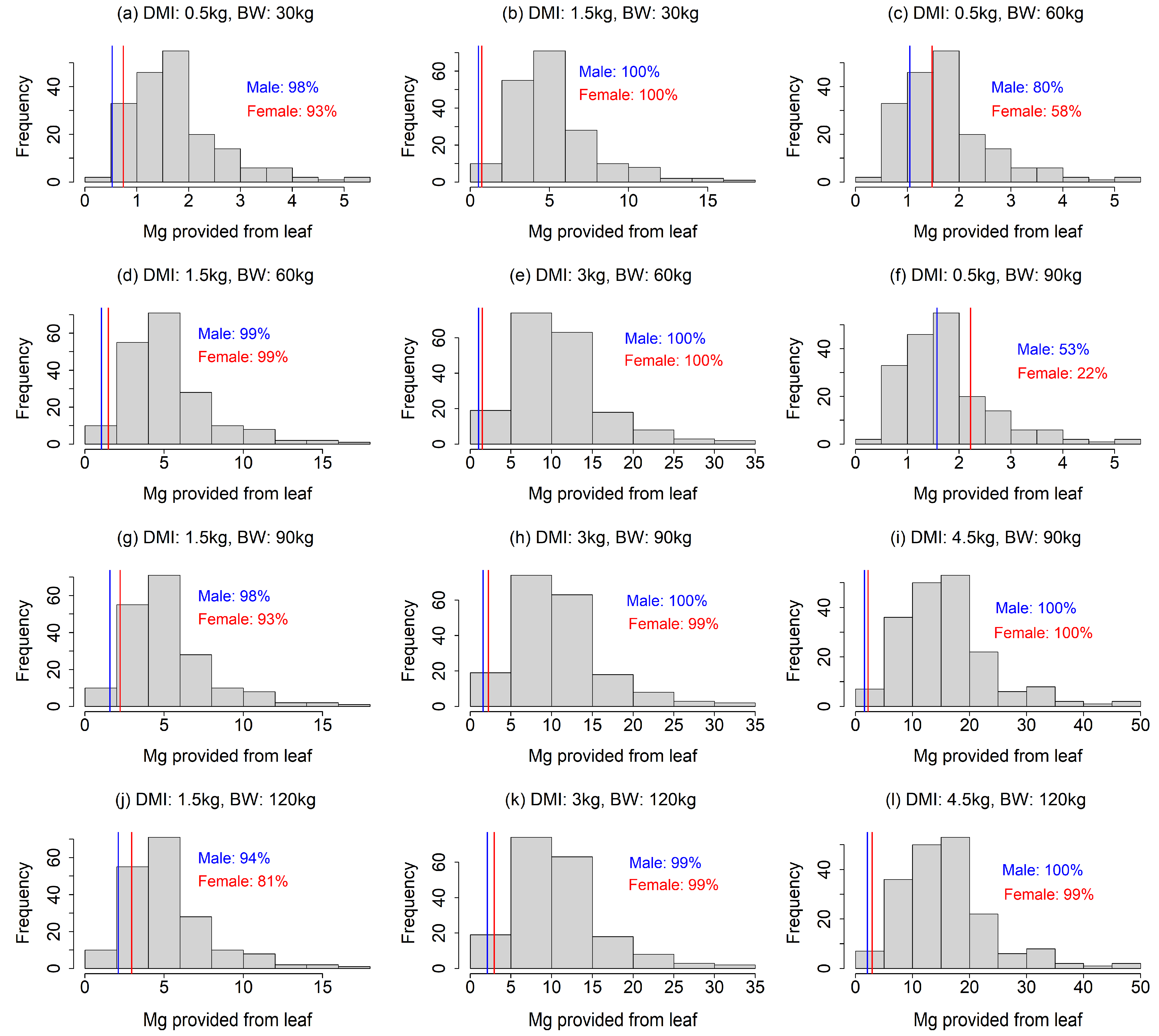
Fig. 2). Our finding that Na requirements often exceed the provision by food plants contrasted to that for Mg; the leaf Mg contents were higher than required in most of the food plants in our analysis, except for the scenarios with combinations of large BW and small DMI (
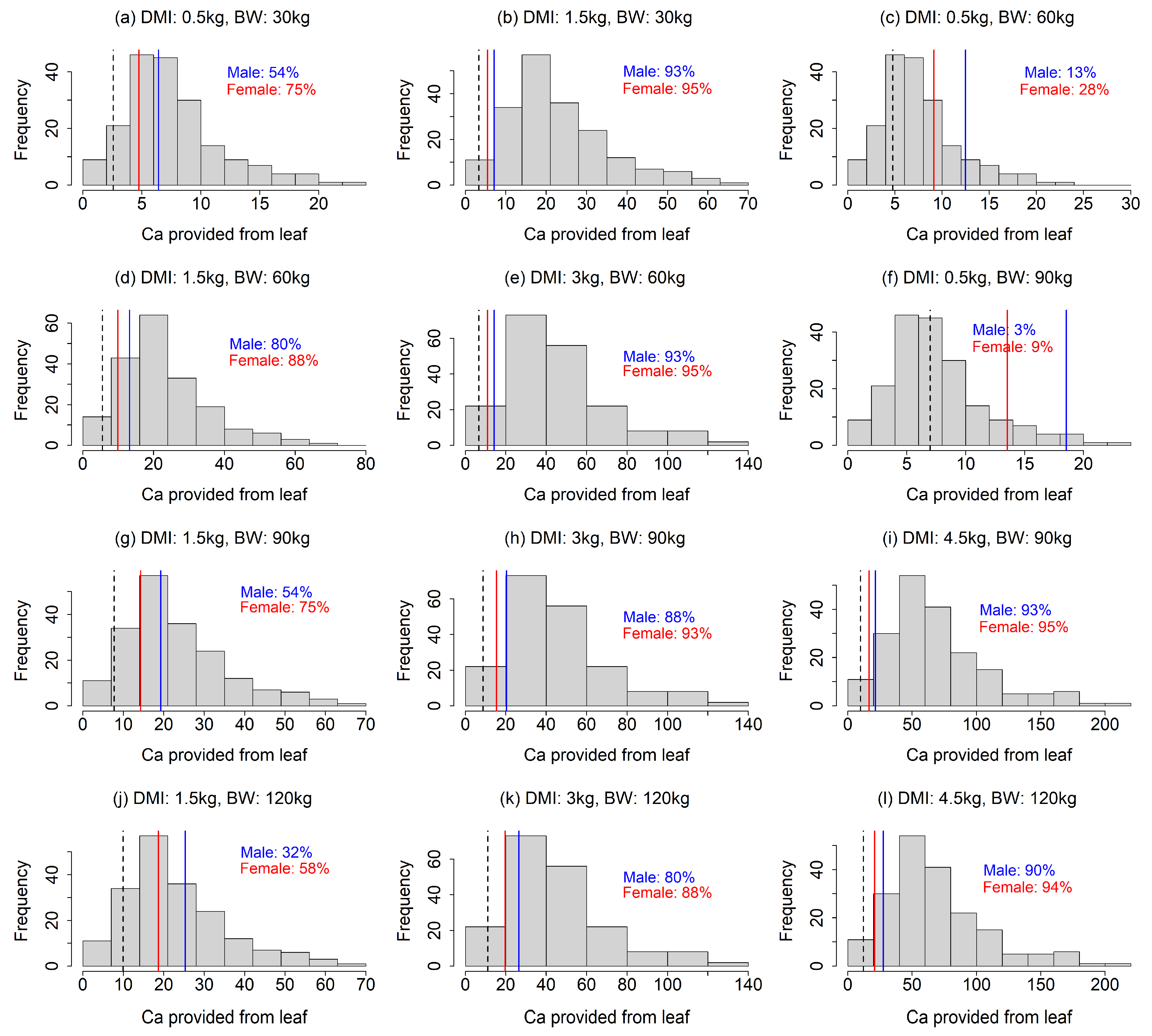
Fig. 3c, f), indicating that Mg deficiency is less likely. The fulfillment of the Ca requirement depended largely on the scenario (
Fig. 4). A lower DMI resulted in insufficient Ca provision in a large portion of the food plant species, especially for greater BWs (
Fig. 4a, c, g, f, j), whereas Ca requirements were met in a large portion of the food plant species when the DMI was ≥ 3 kg (
Fig. 4). Ca requirement for males was larger than that of females (
Fig. 4). The sika deer food plant dataset used for this analysis showed large variance (
Fig. 1). The average leaf contents of Na, Ca, and Mg were 0.47 ± 0.82, 15.3 ± 8.4, and 3.5 ± 1.8 (g element kg
-1 dry matter ± standard deviation), respectively.
Our analysis showed that the Na requirements were rarely achieved by food plants, especially under the scenarios with a smaller DMI and larger BW (
Fig. 2). In most of the 12 scenarios, less than 50% of the food plant species provided sufficient Na (
Fig. 2a, c, d, f–h, j–l). The results also indicated that a Na deficiency would be more intense for lactating females than males (
Fig. 2), as the Na requirement for females was more than double that of males (
Fig. 2). Our finding that Na requirements often exceed the provision by food plants contrasted to that for Mg; the leaf Mg contents were higher than required in most of the food plants in our analysis, except for the scenarios with combinations of large BW and small DMI (
Fig. 3c, f), indicating that Mg deficiency is less likely. The fulfillment of the Ca requirement depended largely on the scenario (
Fig. 4). A lower DMI resulted in insufficient Ca provision in a large portion of the food plant species, especially for greater BWs (
Fig. 4a, c, g, f, j), whereas Ca requirements were met in a large portion of the food plant species when the DMI was ≥ 3 kg (
Fig. 4). Ca requirement for males was larger than that of females (
Fig. 4).
4. Discussion
Our results suggest that food plants in Japan are unlikely to provide sufficient Na, which agrees with the traditional view that Na is the main deficient mineral leading to the use of salt licks [
8,
9,
14]. By comparing several salt solutions as attractants experimentally, Fraser and Reardon (1980) demonstrated that Na, but not K, Ca, or Mg, attracted moose (
Alces alces) and white-tailed deer (
Odocoileus virginianus), indicating that Na was the primary attractant at their study site [
6]. Sika deer were also reported to be attracted by Na [
14,
23,
37]. The larger Na deficiency for lactating females than males (
Fig. 2) is consistent with reports that female sika deer were attracted by salt, especially during pregnancy or lactation [
14,
23].
This study suggests the importance of Ca as a potentially limiting element of sika deer in Japan. Although natural salt licks have been attributed to Na acquisition, several authors have reported that Ca could also be a salt lick attractant [
18]. Kitahara et al. (2005) reported that sika deer in Hokkaido, Japan, may be Ca-deficient [
38]. Tsujii and Tokumoto (1997) reported that several natural salt licks in Japan d
id not have high Na contents [
39], but they had tended to have high Ca, Fe, Mg, and Mn contents. Miyazaki and Kohara (2017) reported that sika deer licked an antifreeze containing calcium chloride (CaCl
2) as the main component, proposing that antifreezes accelerate the overpopulation of sika deer in Japan by providing Ca [
40]. Our simulation supported the hypothesis that sika deer in Japan are potentially deficient in Ca, demonstrating that Ca requirements may not be met by food plants, at least in certain situations (
Fig. 4).
Our finding might be beneficial for efficiently culling sika deer using minerals as attractants. Culling is one of the important management tools for overabundant sika deer populations [
24,
41], and suitable attractants have been verified to increase culling efficiency [
42,
43]. We suggest that Na can be an effective attractant for selectively culling female deer, which has been reported to be effective in reducing sika deer populations [
24,
25], especially during lactating period (
Fig. 2). We also suggest that Ca could be a potential attractant for sika deer (
Fig. 4), as well as Na, which has been used as an effective attractant for hunting sika deer [
23]. However, since Ca deficiency is stronger in male deer, Ca may not be an effective attractant for selective culling of female. Given the different mineral requirement patterns observed for male and female (
Fig. 2 and 4) and potential seasonal changes in the effects of minerals to attract male and female [
14], combination of multiple mineral usage depending on seasons might be more effective for sika deer culling.
In summary, our results indicate that food plants are unlikely to always provide sufficient Na and Ca, especially under current conditions in Japan, where sika deer overpopulation has intensified herbivorous pressure and the choice of food plant species is often limited. Furthermore, we suggested that Na can be an effective attractant for selectively culling female deer, whereas Ca could be an attractant for male deer. Nevertheless, this study has several limitations. First, the data were obtained from a global database, not in Japan. Since plant mineral contents are affected by soil conditions, data collection in Japan is required in the future. Second, the equations used to calculate the Na, Ca, and Mg requirements were not constructed for sika deer, although a minor modification was made for sika deer (see Materials and Methods). Equations for sika deer are required for a more robust estimation. Despite those limitations, this study demonstrates that sika deer in Japan might require extra Ca and Na sources in addition to food plants, and therefore these minerals could be useful for developing effective culling methods.
Author Contributions
Conceptualization, T.M. and S.I.; methodology, T.M.; software, T.M.; validation, K.K.S.; formal analysis, T.M. and K.K.S.; investigation, T.M.; resources, T.M.; data curation, T.M.; writing—original draft preparation, T.M. and K.K.S.; writing—review and editing, T.M., S.I., H.Y., and K.K.S.; visualization, T.M.; supervision, K.K.S.; project administration, K.K.S.; funding acquisition, K.K.S. All authors have read and agreed to the published version of the manuscript.”
Funding
The present study was financially supported by research grant #202205 of the Forestry and Forest Products Research Institute.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Dr. M. Yasuda at Forestry and Forest Products Research Institute for his helpful comments on this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Côté, S.D.; Rooney, T.P.; Tremblay, J.P.; Dussault, C.; Waller, D.M. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef]
- Mcshea, W.J. Ecology and management of white-tailed deer in a changing world. Ann. N. Y. Acad. Sci. 2012, 1249, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Carpio, A.J.; Apollonio, M.; Acevedo, P. Wild ungulate overabundance in Europe: contexts, causes, monitoring and management recommendations. Mamm. Rev. 2021, 51, 95–108. [Google Scholar] [CrossRef]
- Vucetich, J.A.; Peterson, R.O. The influence of top-down, bottom-up and abiotic factors on the moose (Alces alces) population of Isle Royale. Proc. R. Soc. B Biol. Sci. 2004, 271, 183–189. [Google Scholar] [CrossRef]
- Rauch, R.E.; Robinson, P.H.; Erasmus, L.J. Effects of sodium bicarbonate and calcium magnesium carbonate supplementation on performance of high producing dairy cows. Anim. Feed Sci. Technol. 2012, 177, 180–193. [Google Scholar] [CrossRef]
- Fraser, A.D.; Reardon, E. Attraction of Wild Ungulates to Mineral-Rich Springs in Central Canada Published by : Wiley on behalf of Nordic Society Oikos Stable URL : https://www. jstor.org/stable/3682481 Attraction of wild ungulates to mineral-rich springs in central. 1980, 3, 36–39. [Google Scholar]
- Panichev, A.M.; Zaumyslova, O.Y.U.; Aramilev, V. V The importance of salt licks and other sources of sodium in the ecology of the Ussuri moose (Alces alces cameloides). Alces 2002, 38, 99–103. [Google Scholar]
- Matsubayashi, H.; Lagan, P.; Majalap, N.; Tangah, J.; Sukor, J.R.A.; Kitayama, K. Importance of natural licks for the mammals in Bornean inland tropical rain forests. Ecol. Res. 2007, 22, 742–748. [Google Scholar] [CrossRef]
- Tracy, B.F.; McNaughton, S.J. Elemental analysis of mineral lick soils from the Serengeti National Park, the Konza Prairie and Yellowstone National Park. Ecography (Cop.). 1995, 18, 91–94. [Google Scholar] [CrossRef]
- Klaus, G.; Klaus-Hügi, C.; Schmid, B. Geophagy by large mammals at natural licks in the rain forest of the Dzanga National Park, Central African Republic. J. Trop. Ecol. 1998, 14, 829–839. [Google Scholar] [CrossRef]
- Ayotte, J.B.; Parker, K.L.; Arocena, J.M.; Gillingham, M.P. Chemical composition of lick soils: Functions of soil ingestion by four ungulate species. J. Mammal. 2006, 87, 878–888. [Google Scholar] [CrossRef]
- Fraser, D.; Hristienko, H. Activity of Moose and White-Tailed Deer at Mineral Springs. Can. J. Zool. 2000, 59, 1991–2000. [Google Scholar] [CrossRef]
- Ceacero, F.; Landete-Castillejos, T.; Miranda, M.; García, A.J.; Martínez, A.; Gallego, L. Why do cervids feed on aquatic vegetation? Behav. Processes 2014, 103, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ping, X.; Li, C.; Jiang, Z.; Liu, W.; Zhu, H. Sexual difference in seasonal patterns of salt lick use by south China sika deer Cervus nippon. Mamm. Biol. 2011, 76, 196–200. [Google Scholar] [CrossRef]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th Edition ed; 2010; ISBN 978-1845934729. [Google Scholar]
- NRC, N.R.C. Nutrient Requirements of Small Ruminants. National; Academic Press: Washington; DC, 2007. [Google Scholar]
- Pinotti, L.; Manoni, M.; Ferrari, L.; Tretola, M.; Cazzola, R.; Givens, I. Human Nutrition. Nutrients 2021, 13, 509. [Google Scholar] [CrossRef]
- Jones, R.L.; Hanson, H.C. Mineral licks, geophagy, and biogeochemistry of North American ungulates.; The Iowa State Univ. Press: Ames, 1985. [Google Scholar]
- McCullough, D.; Takatsuki, S.; Kaji, K. Sika Deer: Biology and Management of Native and Introduced Populations; Springer: Tokyo, Japan, 2009. [Google Scholar]
- Putman, R.J.; Moore, N.P. Impact of deer in lowland Britain on agriculture, forestry and conservation habitats. Mamm. Rev. 1998, 28, 141–164. [Google Scholar] [CrossRef]
- Takatsuki, S. Effects of sika deer on vegetation in Japan: A review. Biol. Conserv. 2009, 142, 1922–1929. [Google Scholar] [CrossRef]
- Ministry of the Environment Estimation of sika deer population in Japan. 2015, p. 100922. Available online: http://www.env.go.jp/press/100922.html.
- Yasuda, M.; Suzuki, K.K. Dear and minerals. J. Jpn. Wildl. Res. Soc. 2022, 39, 35–39. [Google Scholar]
- Ueno, M.; Kaji, K.; Saitoh, T. Culling Versus Density Effects in Management of a Deer Population. J. Wildl. Manage. 2010, 74, 1472–1483. [Google Scholar] [CrossRef]
- Suzuki, K. .; Kuwano, Y.; Yasuda, M. A 17 year study of the response of populations to different patterns in antlerless proportion of imposed culls: Antlerless culling reduces overabundant deer population. Biology (Basel). 2022, 11, 2020. [Google Scholar]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database – enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Fujiki, D. List of food plants and unpalatable plants of sika deer (Cervus nippon) in Japan Yoshinobu. Humans Nat. 2014, 25, 133–160. [Google Scholar]
- Nakanishi, T.; Atarashi-Andoh, M.; Koarashi, J.; Saito-Kokubu, Y.; Hirai, K. Carbon isotopes of water-extractable organic carbon in a depth profile of forest soil imply a dynamic relationship with soil carbon. Eur. J. Soil Sci. 2012, 63, 495–500. [Google Scholar] [CrossRef]
- Watanabe, T.; Broadley, M.R.; Jansen, S.; White, P.J.; Takada, J.; Satake, K.; Takamatsu, T.; Tuah, S.J.; Osaki, M. Evolutionary control of leaf element composition in plants: Rapid report. New Phytol. 2007, 174, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Sardans, J.; Llusià, J.; Owen, S.M.; Carnicer, J.; Giambelluca, T.W.; Rezende, E.L.; Waite, M.; Niinemets, Ü. Faster returns on “leaf economics” and different biogeochemical niche in invasive compared with native plant species. Glob. Chang. Biol. 2010, 16, 2171–2185. [Google Scholar] [CrossRef]
- Herz, K.; Dietz, S.; Haider, S.; Jandt, U.; Scheel, D.; Bruelheide, H. Drivers of intraspecific trait variation of grass and forb species in German meadows and pastures. J. Veg. Sci. 2017, 28, 705–716. [Google Scholar] [CrossRef]
- Schmitt, M.; Mehltreter, K.; Sundue, M.; Testo, W.; Watanabe, T.; Jansen, S. The evolution of aluminum accumulation in ferns and lycophytes. Am. J. Bot. 2017, 104, 573–583. [Google Scholar] [CrossRef]
- Souma, K.; Masuko, T.; Kobayashi, Y.; Ishijima, Y. Seasonal alteration of hay intake in the Yeso sika deer (Cervus nippon yesoensis). Hokushinetsu J. Anim. Sci. 1998, 40, 27–30. [Google Scholar]
- Yamane, M. Over-winter foraging activities of supplementally fed free-ranging Sika deer ( Cervus nippon ). Bull. Kanagawa Prefect. Nat. Environ. Conserv. Cent. 2015, 13, 5–14. [Google Scholar]
- Tamura, T.; Terasaki, T.; Oikawa, M.; Kaji, K.; Nara, M.; Arai, K.; Nakamura, K. The effect of seasonal transformation on feed intake in Sika deer Tetsuo. Bull. Tokyo Metrop. Agric. For. Res. Cent. 2012, 7, 69–78. [Google Scholar]
- Ishida, M.; Meguro, J.; Ikeda, S.; Takeda, T. Mineral analysis of meat, milk and velvet of Japanese deer. Sci. Rep. Miyagi Agric. Coll. 1995, 43, 91–94. [Google Scholar]
- Yasuda, M.; Yayota, C. A trial creating artificial salt lick in forest. Kyushu J. For. Res. 2020, 73, 121–122. [Google Scholar]
- Kitahara, R.; Komatsu, T.; Masuko, T. Effects of mineral requirements on food selection by sika deer ( Cervus nippon yesoensis ). Rep. PRO Nat. FUND 2005. [Google Scholar]
- Tsujii, H.; Tokumoto, T. Soil Licking Phenomenon of Wild Sika Deer(Cervus nippon). Around the origin of Gohukuji river at Matsumoto City. Hokuriku J. Zootech. Sci. 1997, 74, 77–80. [Google Scholar]
- Miyazaki, M.; Kohara, M. Mori no tantei; Aki Shobo, 2017. [Google Scholar]
- Suzuki, K.K.; Yasuda, M.; Sonoda, M. Spatially biased reduction of browsing damage by sika deer through culling. J. Wildl. Manage. 2022, 1–13. [Google Scholar] [CrossRef]
- Iijima, H.; Oochi, J. Examination of useful bait to attract sika deer. Mamm. Sci. (in Japanese with English Abstr. 2016, 56, 145–149. [Google Scholar]
- Ikeda, T.; Shirakawa, T.; Suzuki, M. Comparison of the attractiveness of five baits in sika deer (Cervus nippon) and wild boar (Sus scrofa). Wildl. Hum. Soc. (in Japanese with English Abstr. 2018, 6, 13–20. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).