Submitted:
10 January 2023
Posted:
13 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Location and geological settings of the study area
2.2. Collection and preparation of rock, soil, and plant samples
2.3. Total selenium (tSe) determination in soil, plant, and rock samples
2.4. Chemical fractions of Se in soil and rock samples
2.5. Enzyme-soluble selenium species extraction from plant organs
2.6. Statistical analysis
3. Results and Discussion
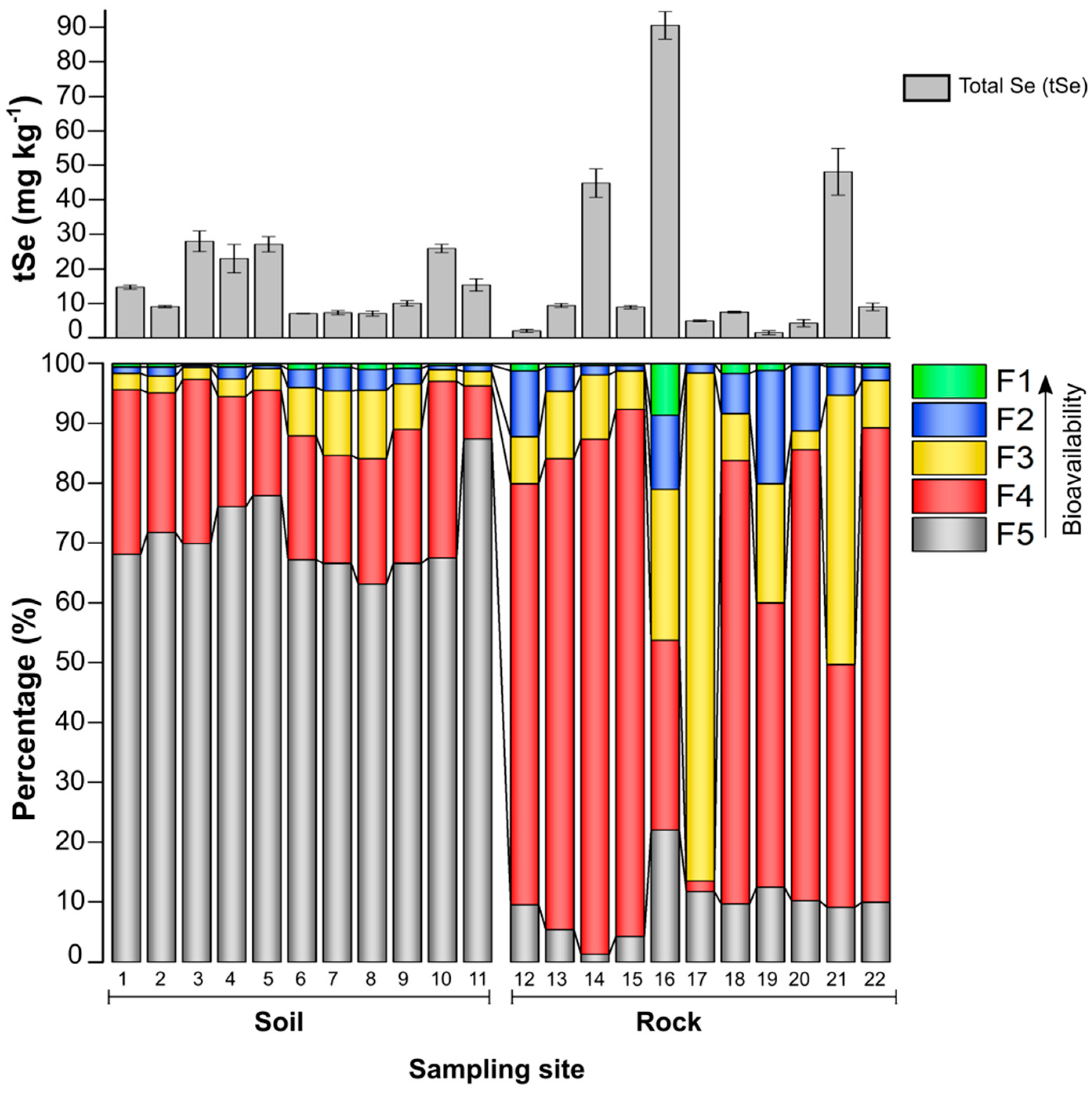
3.1. Selenium fractions in rock-soils interface and translocation potential
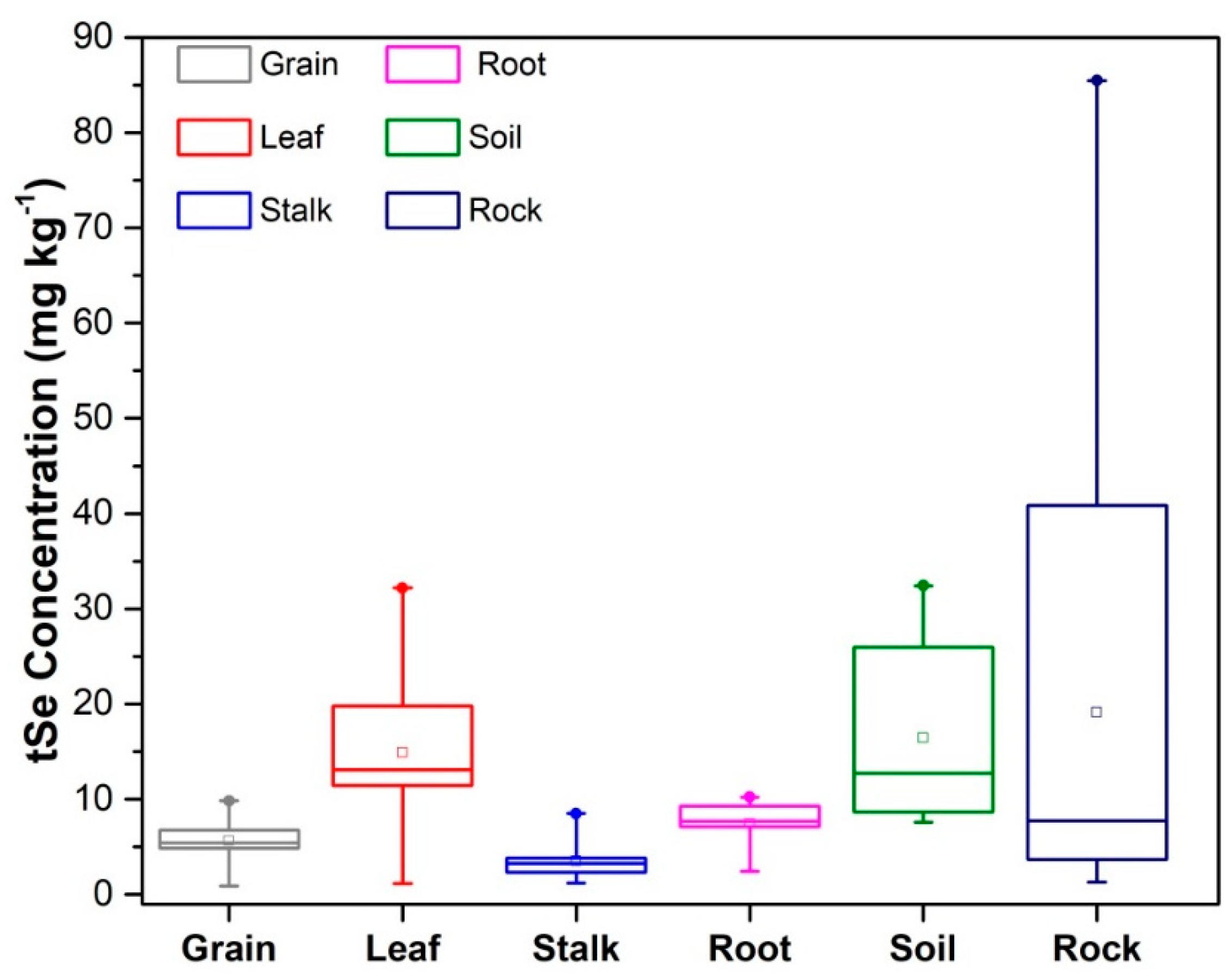
3.2. Selenium accumulation in maize depending on tSe and Se fractions in soil
3.3. Selenium organ-specific species distribution in maize
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Abiven, S.; Heim, A.; Schmidt, M.W.I. Lignin content and chemical characteristics in maize and wheat vary between plant organs and growth stages: Consequences for assessing lignin dynamics in soil. Plant Soil 2011, 343, 369–378. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; Aro, A. Effects of nationwide addition of Selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Beilstein, M.; Whanger, P.; Yang, G. Chemical forms of Selenium in corn and rice grown in a high selenium area of China. Biomed Env. Sci. 1991, 4, 392. [Google Scholar]
- Carey, A.M.; Scheckel, K.G.; Lombi, E.; Newville, M.; Choi, Y.; Norton, G.J.; Price, A.H.; Meharg, A.A. Grain accumulation of selenium species in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 5557–5564. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Cuderman, P.; Stibilj, V. Stability of Se species in plant extracts rich in phenolic substances. Anal. Bioanal. Chem. 2010, 396, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Huang, J.; Peng, Q.; Yu, D.; Wang, S.; Liang, D. Risk assessment for human health in a seleniferous area, Shuang'an, China. Environ. Sci. Pollut. Res. 2017, 24, 17701–17710. [Google Scholar] [CrossRef] [PubMed]
- De Feudis, M.; D’Amato, R.; Businelli, D.; Guiducci, M. Fate of selenium in soil: A case study in a maize (Zea mays L.) field under two irrigation regimes and fertilized with sodium selenite. Sci. Total Environ. 2019, 659, 131–139. [Google Scholar] [CrossRef]
- Duncan, E.G.; Maher, W.A.; Jagtap, R.; Krikowa, F.; Roper, M.M.; O'Sullivan, C.A. Selenium speciation in wheat grain varies in the presence of nitrogen and sulphur fertilisers. Environ. Geochem. Health 2017, 39, 955–966. [Google Scholar] [CrossRef]
- Ellis, D.R.; Salt, D.E. Plants, Selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- ERM_certificate, ERM_certificate: http://www.speciation.net/Database/Materials/LGC-Ltd/ERMBC210a--Wheat-Flour--Selenium-and-Selenomethionine-;i1203 (available on 90.09.2022). Google Scholar.
- Food and Agriculture Organization of the United Nations (FAO)., 2014. World reference base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps. FAO, Rome.
- Fordyce, F. Selenium Geochemestry. Int. Symp. Med. Geol. 2007, 18–19. [Google Scholar] [CrossRef]
- Gergely, V.; Kubachka, K.M.; Mounicou, S.; Fodor, P.; Caruso, J.A. Selenium speciation in Agaricus bisporus and Lentinula edodes mushroom proteins using multi-dimensional chromatography coupled to inductively coupled plasma mass spectrometry. J. Chromatogr. A 2006, 1101, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, H. Biogeochemistry of Selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.J.; Fairweather-Tait, S.J.; Broadley, M.R.; Dickinson, S.J.; Foot, I.; Knott, P.; McGrath, S.P.; Mowat, H.; Norman, K.; Scott, P.R.; Stroud, J.L.; Tucker, M.; White, P.J.; Zhao, F.J.; Hurst, R. Selenium concentration and speciation in biofortified flour and bread: Retention of Selenium during grain biofortification, processing and production of Se-enriched food. FOOD CHEM 2011, 126, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B. Effect of Selenium on Salt Tolerance in Maize Plants. J. Environ. Sci. 2008, 49, 91–124. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na + accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kulp, T.R.; Pratt, L.M. Speciation and weathering of Selenium in Upper Cretaceous chalk and shale from South Dakota and Wyoming, USA. Geochim. Cosmochim. Acta 2004, 68, 3687–3701. [Google Scholar] [CrossRef]
- Lai, J.; Messing, J. Increasing maize seed methionine by mRNA stability. Plant J. 2002, 30, 395–402. [Google Scholar] [CrossRef]
- Li, J.; Peng, Q.; Liang, D.; Liang, S.; Chen, J.; Sun, H.; Li, S.; Lei, P. Effects of aging on the fraction distribution and bioavailability of Selenium in three different soils. Chemosphere 2016, 144, 2351–2359. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Luo, K.; Li, H. Environmental behaviors of selenium in soil of typical selenosis area, China. J. Environ. Sci. 2008, 20, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X. Effects of Chinese cooking methods on the content and speciation of selenium in selenium bio-fortified cereals and soybeans. Nutrients 2018, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luo, K. The Lujiaping Formtion of Northern Daba Mountain. J. Stratigraphy, 2006, 30(2): 149–156 (in Chinese with English Abstract).
- Luo, K.; Xu, L.; Tan, J.; Wang, D.; Xiang, L. Selenium source in the selenosis area of the Daba region, South Qinling Mountain, China. Environ. Geol. 2004, 45, 426–432. [Google Scholar] [CrossRef]
- Martens, Dean A.; Suarez, Donald L. Selenium Speciation of Soil/ Sediment Determined with Sequential Extractions and Hydride Generation Atomic Absorption Spectrophotometry. Environ. Sci. Technol. 1997, 31, 133-139. Retrieved from https://digitalcommons.unl.edu/usdaarsfacpub/502.
- Ministry of Ecology and Environment of the People's Republic of China (MEEPRC)., 2004. Technical specification for soil environmental monitoring (HJ/T 166-2004). Beijing, 44 pp. (in Chinese).
- Ministry of Natural Resources of the People's Republic of China (MNRPRC)., 2016. Specification of land quality geochemical assessment (DZ/T 0295-2016). Beijing, 52 pp. (in Chinese).
- Muleya, M.; Young, S.D.; Reina, S.V.; Ligowe, I.S.; Broadley, M.R.; Joy, E.J.M.; Chopera, P.; Bailey, E.H. 2021. Selenium speciation and bioaccessibility in Se-fertilised crops of dietary importance in Malawi. J. Food Compos. Anal. 103841. [CrossRef]
- Nelson, D.W.; Sommers, L.E. 1996. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 3 Chem. Methods 961–1010. [CrossRef]
- Neuhierl, B.; Thanbichler, M.; Lottspeich, F.; Böck, A. A family of S-methylmethionine-dependent thiol/selenol methyltransferases. Role in selenium tolerance and evolutionary relation. J. Biol. Chem. 1999, 274, 5407–5414. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcón, M.; López-Martínez, M.C. Essentiality of Selenium in the human body: Relationship with different diseases. Sci. Total Environ. 2000, 249, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Pilon-smits, E.A.H.; Quinn, C.F. 2010. Selenium Metabolism in Plants In Progress in botany (pp. 93–107). Cham, Switzerland.Springer. [CrossRef]
- Płaczek, A.; Patorczyk-Pytlik, B. The dynamics of selenium uptake by maize (Zea mays L.). Agronomy 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Qin, H. B, Zhu, J. ming, Liang, L.; Wang, M. shi, Su, H. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ. Int. 2013, 52, 66–74. [CrossRef]
- Qin, H.B.; Zhu, J.M.; Lin, Z.Q.; Xu, W.P.; Tan, D.C.; Zheng, L.R.; Takahashi, Y. Selenium speciation in seleniferous agricultural soils under different cropping systems using sequential extraction and X-ray absorption spectroscopy. Environ. Pollut. 2017, 225, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.B.; Zhu, J.M.; Su, H. Selenium fractions in organic matter from Se-rich soils and weathered stone coal in selenosis areas of China. Chemosphere 2011, 86, 626–633. [Google Scholar] [CrossRef]
- Rayman, M.P. The influence of Selenium on human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Rudnick, R.L. and Gao, S. 2003 Composition of the Continental Crust. In: Rudnick, R.L.; Ed., The Crust, Elsevier-Pergamon, 1-64. [CrossRef]
- Salinas-Moreno, Y.; García-Salinas, C.; Ramírez-Díaz, J.L.; Alemán-de la Torre, I. 2017. Phenolic Compounds in Maize Grains and Its Nixtamalized Products. Phenolic Compd. - Nat. Sources, Importance Appl. [CrossRef]
- SCF (Scientific Committee on Food)., 2000. Opinion on the Scientific Committee on Food on the tolerable upper intake level of selenium. SCF/CS/NUT/UPPLEV/25 Final. 18 pp.
- Schilling, K.; Johnson, T.M.; Dhillon, K.S.; Mason, P.R.D. Fate of Selenium in Soils at a Seleniferous Site Recorded by High Precision Se Isotope Measurements. Environ. Sci. Technol. 2015, 49, 9690–9698. [Google Scholar] [CrossRef]
- Smrkolj, P.; Stibilj, V.; Kreft, I.; Kapolna, E. Selenium species determination in selenium-enriched pumpkin (Cucurbita pepo L.) seeds by HPLC-UV-HG-AFS. Anal. Sci. 2005, 21, 1501–1504. [Google Scholar] [CrossRef]
- Sun, C.; Yang, Y.; Zeeshan, M.; Qin, S.; Ma, J.; Liu, L.; Yang, J.; Zhou, X.; Huang, J. Arbuscular mycorrhizal fungi reverse selenium stress in Zea mays seedlings by improving plant and soil characteristics. Ecotoxicol. Environ. Saf. 2021, 228, 113000. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Liu, X.; Williams, P.N.; Zhu, Y.G. Distribution and translocation of Selenium from soil to grain and its speciation in Paddy Rice (Oryza sativa L.). Environ. Sci. Technol. 2010, 44, 6706–6711. [Google Scholar] [CrossRef] [PubMed]
- Supriatin, S.; Weng, L.; Comans, R.N.J. Selenium speciation and extractability in Dutch agricultural soils. Sci. Total Environ. 2015, 532, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Supriatin, S.; Weng, L.; Comans, R.N.J. Selenium-rich dissolved organic matter determines selenium uptake in wheat grown on Low-selenium arable land soils. Plant Soil 2016, 408, 73–94. [Google Scholar] [CrossRef]
- Tan, J.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Lu, G.; Fan, B.; Xiang, W.; Bao, Z. Bioaccumulation and risk assessment of heavy metals in soil-crop systems in Liujiang karst area, Southwestern China. Environ. Sci. Pollut. Res. 2021, 28, 9657–9669. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xie, S.; Carranza, E.J.M.; Bao, Z.; Zhang, H.; Wu, S.; Wei, C.; Ma, Z. Distributions of Selenium and related elements in high pyrite and Se-enriched rocks from Ziyang, Central China. J. Geochemical Explor. 2020, 212, 106506. [Google Scholar] [CrossRef]
- Wang, M.; Cui, Z.; Xue, M.; Peng, Q.; Zhou, F.; Wang, D.; Dinh, Q.T.; Liu, Y.; Liang, D. Assessing the uptake of selenium from naturally enriched soils by maize (Zea mays L.) using diffusive gradients in thin-films technique (DGT) and traditional extractions. Sci. Total Environ. 2019, 689, 1–9. [Google Scholar] [CrossRef]
- Wang, S.; Liang, D.; Wang, D.; Wei, W.; Fu, D.; Lin, Z. Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci. Total Environ. 2012, 427–428, 159–164. [CrossRef]
- Wang, Y.; Shi, X.; Huang, X.; Huang, C.; Wang, H.; Yin, H.; Shao, Y.; Li, P. Linking microbial community composition to farming pattern in selenium-enriched region: Potential role of microorganisms on Se geochemistry. J. Environ. Sci. (China) 2022, 112, 269–279. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yang, Z.; Lv, Y.; Hou, Q.; Xia, X.; Feng, H.; Zhang, M.; Jin, L.; Kan, Z. The origin and geochemical cycle of soil selenium in a Se-rich area of China. J. Geochemical Explor. 2014, 139, 97–108. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, L.; Yang, K.; Zhang, S. Influence of mycorrhizal inoculation on the accumulation and speciation of Selenium in maize growing in selenite and selenate spiked soils. Pedobiologia (Jena). 2011, 54, 267–272. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Qi, S.; Yin, X. The threshold effect between the soil bioavailable molar Se:Cd ratio and the accumulation of Cd in corn (Zea mays L.) from natural Se-Cd rich soils. Sci. Total Environ. 2019, 688, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yang, W.; Wang, M.; Miao, Y.; Cui, Z.; Li, Z.; Liang, D. Effects of selenium application on Se content and speciation in Lentinula edodes. Food Chem. 2018, 265, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, B.; Wang, Z.; Xiao, H.; Mao, D.; Su, H. Distribution of Selenium in corn and its relationship with soil selenium in yutangba mini-landscape. Chinese J. Geochemistry 2000, 20, 161–166. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, N.; Li, S.; Li, L.; Su, H.; Liu, C. Distribution and transport of Selenium in Yutangba, China: Impact of human activities. Sci. Total Environ. 2008, 392, 252–261. [Google Scholar] [CrossRef]
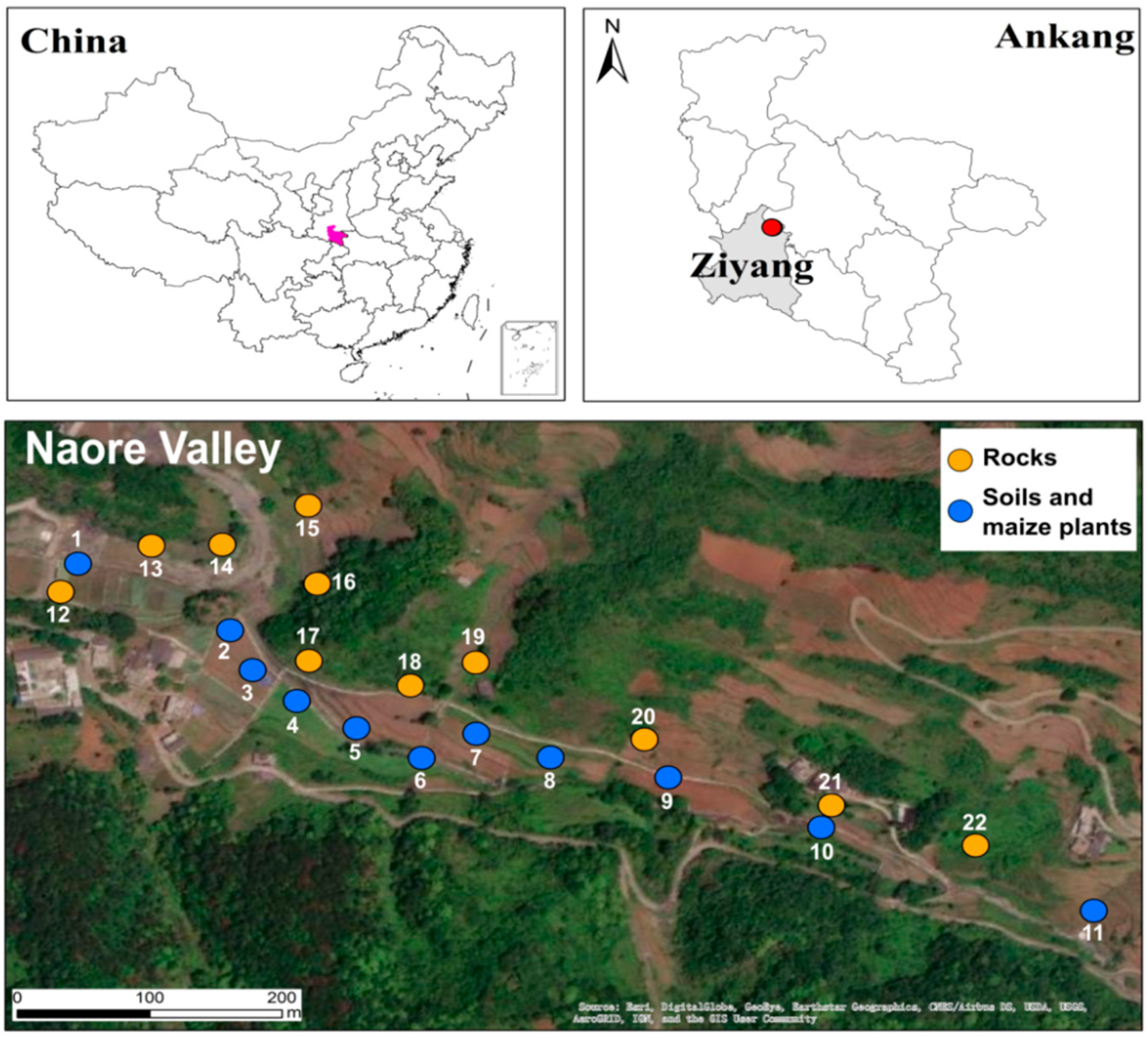
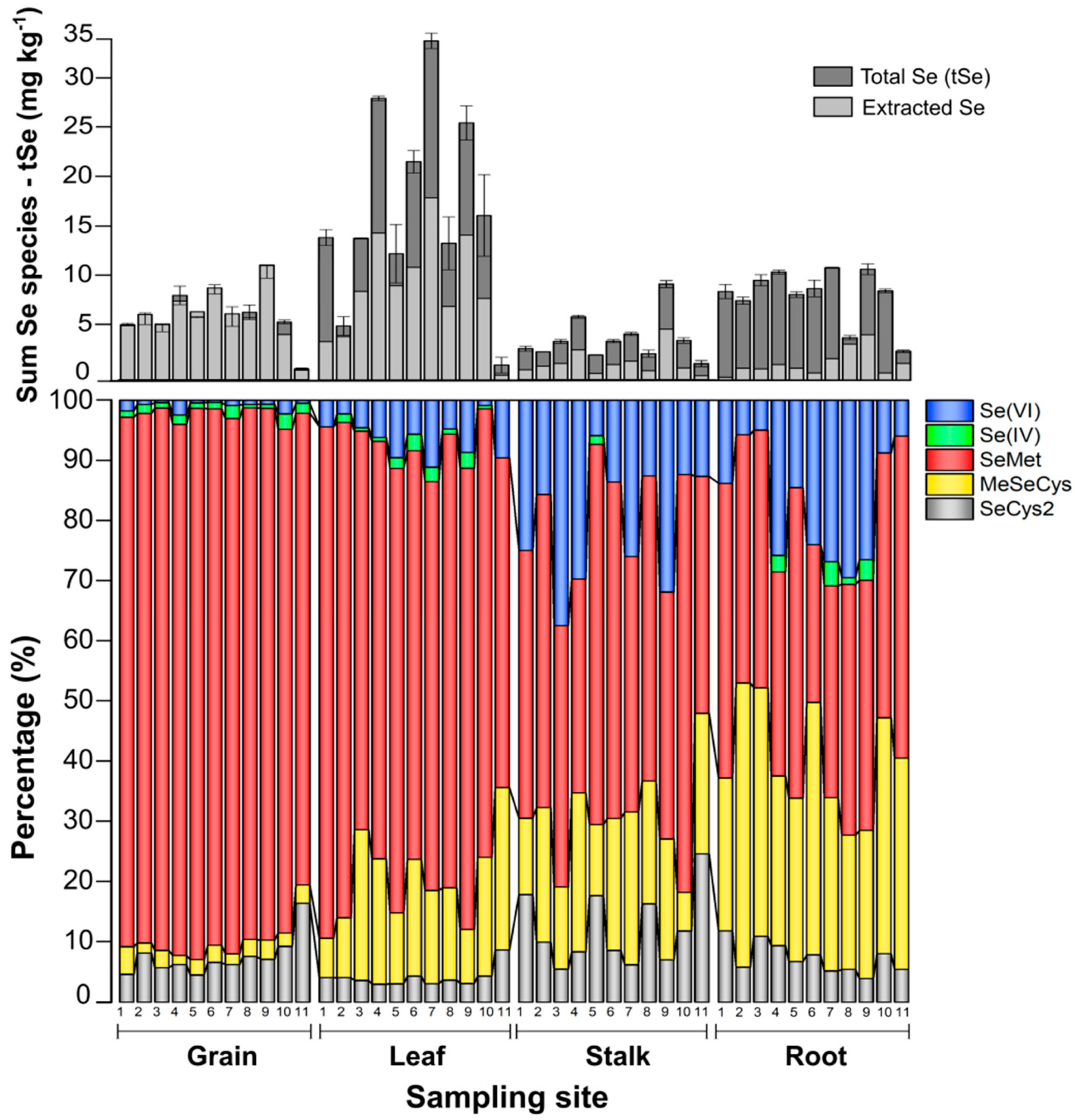
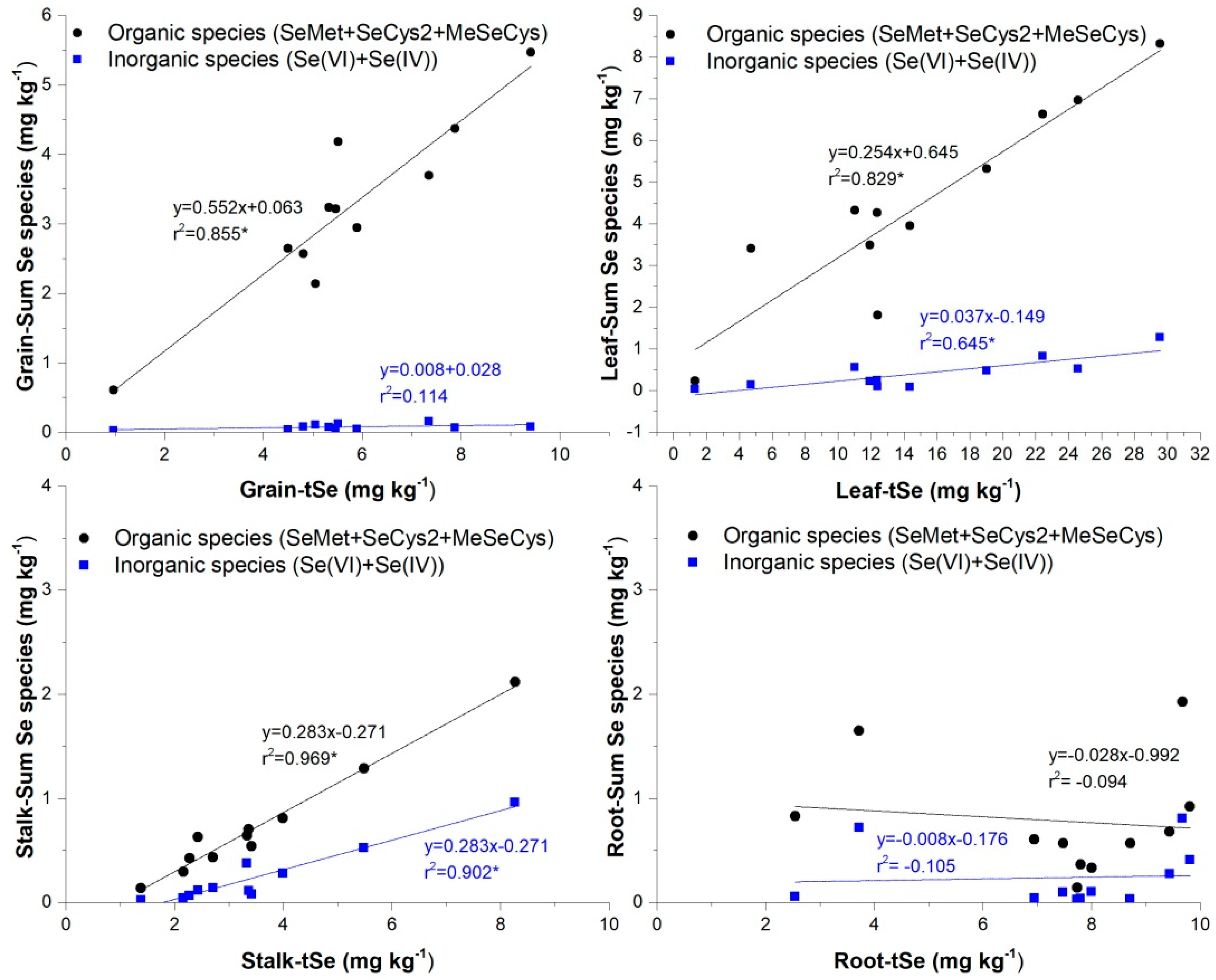
| Samples | Parameter | Plot 1 | Plot 2 | Plot 3 | Plot 4 | Plot 5 | Plot 6 | Plot 7 | Plot 8 | Plot 9 | Plot 10 | Plot 11 |
| Soil | No. | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 |
| tSe (mg/kg) | 13.3 ± 0.6 | 8.4 ± 0.3 | 29.4 ± 3.0 | 22.0 ± 4.1 | 29.9 ± 2.2 | 7.8 ± 0.1 | 8.0 ± 0.6 | 8.8 ± 0.7 | 11.1 ± 0.8 | 27.8 ± 1.2 | 14.2 ± 1.8 | |
| pH | 7.5 | 7.7 | 7.9 | 8.7 | 7.7 | 7.6 | 7.7 | 7.3 | 6.5 | 7.5 | 7.6 | |
| TOC | 4.5 | 3.3 | 4.9 | 5.7 | 7.0 | 2.2 | 5.0 | 3.2 | 5.2 | 4.2 | 5.1 | |
| Rock | No. | R12 | R13 | R14 | R15 | R16 | R17 | R18 | R19 | R20 | R21 | R22 |
| tSe (mg/kg) | 2.0 ± 0.4 | 10.0 ± 0.5 | 41.5 ±4.1 | 7.7 ±0.5 | 85.5 ± 4.0 | 3.8 ± 1.0 | 6.1 ± 0.2 | 1.3±0.3 | 3.7 ± 0.6 | 40.9 ± 6.8 | 7.7 ± 1.1 | |
| pH | 9.3 | 9.0 | 8.5 | 8.7 | 8.0 | 8.3 | 9.1 | 9.3 | 9.3 | 6.5 | 8.8 | |
| TOC | 2.1 | 2.0 | 1.2 | 8.4 | 13.1 | 3.4 | 3.1 | 8.1 | 3.8 | 15.0 | 0.2 | |
| Maize | No. | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 |
| tSe-grain (mg/kg) | 4.8 ± 0.1 | 5.3 ± 0.5 | 4.5 ± 0.3 | 7.3 ± 0.8 | 5.5 ± 0.05 | 7.9 ± 0.4 | 5.5 ± 0.8 | 5.9 ± 0.63 | 9.4 ± 0.5 | 5.0 ± 0.2 | 1.0 ± 0.08 | |
| tSe-leaf (mg/kg) | 12.4 ± 0.8 | 4.7 ± 0.05 | 12.4 ± 0.2 | 24.6 ± 2.5 | 11.0 ± 1.0 | 19.0 ± 0.7 | 29.6 ± 2.3 | 11.9 ± 1.5 | 22.4 ± 3.5 | 14.3 ± 0.7 | 1.3 ± 0.2 | |
| tSe-stalk (mg/kg) | 2.7 ± 0.2 | 2.4 ± 0.2 | 3.3 ± 0.1 | 5.5 ± 0.03 | 2.2 ± 0.13 | 3.4 ± 0.1 | 4.0 ± 0.3 | 2.3 ± 0.06 | 8.3 ± 0.2 | 3.4 ± 0.04 | 1.4 ± 0.2 | |
| tSe-root (mg/kg) | 7.7 ± 0.6 | 6.9 ± 0.3 | 8.7 ± 0.5 | 9.4 ± 0.2 | 7.5 ± 0.2 | 8.0 ± 0.7 | 9.8 ± 0.05 | 3.7 ± 0.2 | 9.7 ± 0.5 | 7.8 ± 0.2 | 2.5 ±0.1 | |
| Biomass-grain (g) | 414.8 | 385.1 | 282.1 | 298.2 | 101.6 | 322.2 | 369.9 | 356.3 | 515.8 | 188.7 | 415.0 | |
| Biomass-leaf (g) | 94.9 | 106.3 | 88.9 | 63.2 | 100.1 | 146.3 | 128.7 | 125.8 | 145.1 | 84.5 | 143.6 | |
| Biomass-stalk (g) | 94.5 | 93.5 | 136.6 | 121.5 | 98.3 | 106.5 | 124.0 | 116.2 | 114.4 | 145.8 | 133.5 | |
| Biomass-root (g) | 63.0 | 109.62 | 34.2 | 33.8 | 39.3 | 99.5 | 35.8 | 49.0 | 56.2 | 85.5 | 96.2 |
| Maize organ |
tSe | SUM-Se extracted | Organic species | Inorganic species | |||||||
| SeMet | SeCys2 | MeSeCys | Se(IV)+Se(VI) | Extraction efficiency | |||||||
| (mg/kg) | (mg /Kg) | (mg/kg) | (%) | (mg/kg) | (%) | (mg/kg) | (%) | (mg/kg) | (%) | (%) | |
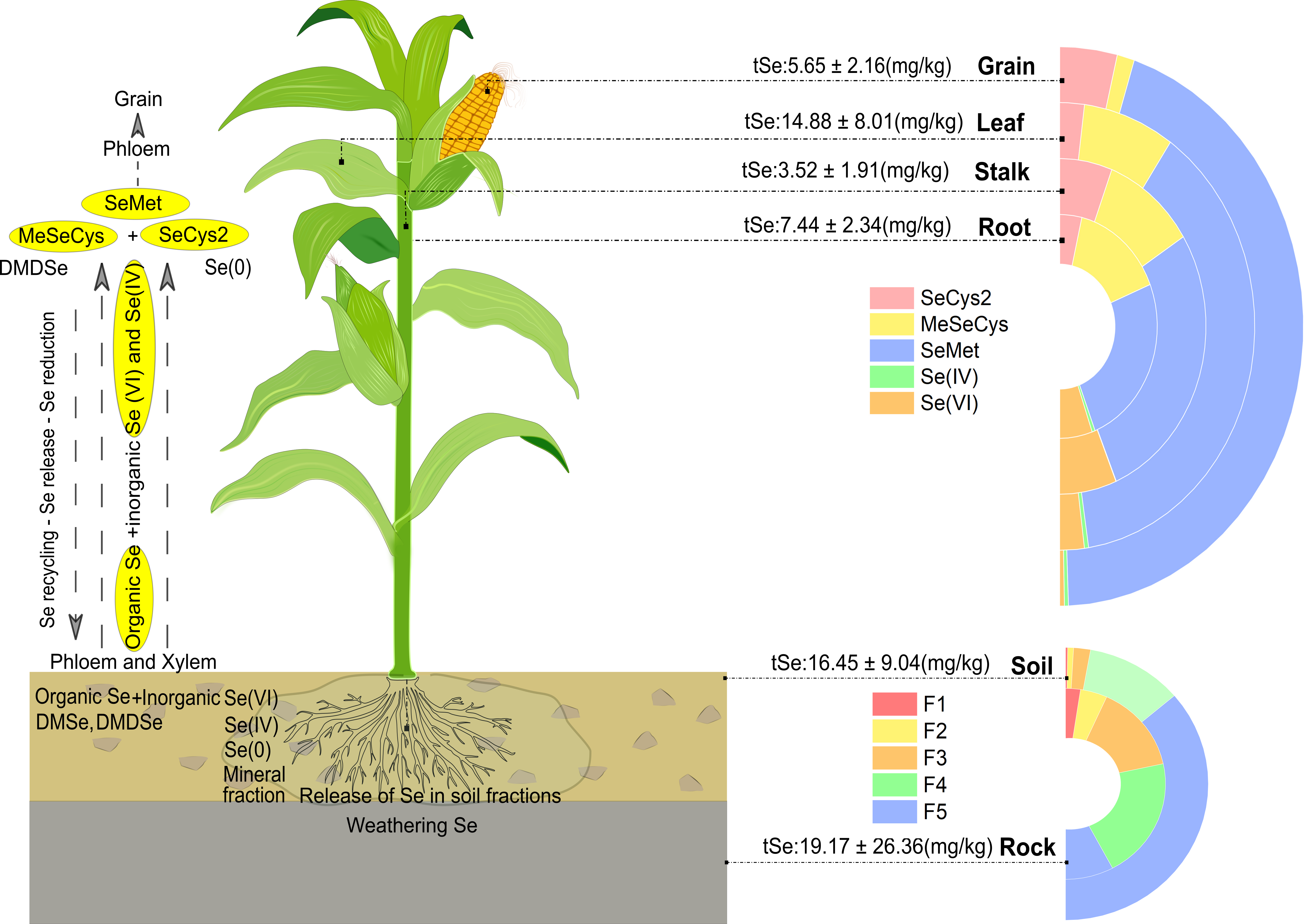
| Grain | 5.6 ± 2.2 | 5.6 ± 2.3 | 5.0 ± 2.1 | 86.7 ± 3.5 | 0.43 ± 0.17 | 7.4 ± 3.3 | 0.13 ± 0.1 | 2.62 ± 0.86 | 0.06 ± 0.03 | 2.3 ± 1.3 | 98.7 ± 9.8 |
| Leaf | 14.9 ± 8.4 | 8.0 ± 4.6 | 6.2 ± 3.5 | 80.0 ± 8.3 | 0.33 ± 0.15 | 4.0 ± 1.6 | 1.2 ± 0.82 | 16.1 ± 6.5 | 0.30 ± 0.28 | 7.3 ± 3.8 | 54.1 ± 15.5 |
| Stalk | 3.5 ± 1.9 | 1.5 ± 1.2 | 0.81 ± 0.57 | 48.1 ± 10.4 | 0.17 ± 0.1 | 12.0 ± 6.0 | 0.30 ± 0.31 | 18.3 ± 6.3 | 0.19 ± 0.21 | 20.1 ± 10.0 | 37.5 ± 10.6 |
| Root | 7.4 ± 2.3 | 1.5 ± 1.1 | 0.76 ± 0.58 | 41.8 ± 7.9 | 0.11 ± 0.07 | 7.3 ± 2.5 | 0.46 ± 0.29 | 32.7 ± 8.4 | 0.18 ± 0.21 | 17.9 ± 10.7 | 25.4 ± 25.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





