Introduction
Viruses are numerically abundant and an integral part of life on Earth. However, there is very little known about viruses across many of Earth’s environments, including the many extreme habitats that serve as astrobiology analogs, and even less about viral response to the space environment beyond Earth's atmosphere. Understanding viruses and virus-host interactions will inform our grasp of their role(s) in human space flight missions, including environmental control and life support systems (ECLSS) and the search for life elsewhere. Here, we review what is known about viruses on Earth and in space and lay out a roadmap for future research.
Viruses are sometimes defined as “very small obligate intracellular parasites” (Acheson, 2011), and more broadly as “entities whose genomes are elements of nucleic acids that replicate inside living cells using the cellular synthetic machinery and causing the synthesis of specialized elements that can transfer the viral genome to other cells.” (Luria et al., 1978). These ‘specialized elements’ are crucial to the definition of the virus. Viruses contain genetic information that can be transferred at massive scales due to the very large numbers of virus particles (virions) on Earth.
Viruses were first discovered as the causative agent of plant and then animal diseases (Loeffler and Frosch 1897; Beijerinck, 1898), and are astronomically abundant, with up to 1031 viruses in the Earth’s oceans alone (Suttle, 2005; Mushegian 2020). The vast majority of these viruses infect microbes (Suttle, 2005; Parikka et al. 2017), affecting many biological processes, including encoding photosynthesis genes that substantially contribute to atmospheric oxygen, stimulating development of immune systems and regulating global nutrient cycling (Greene and Reid, 2014). Viruses can infect all three domains of life and can even infect other viruses. Viral infections have played a key role in the evolution of life on Earth via the exchange of genes between viruses and their hosts, providing genetic diversity, lysing dominant hosts thus maintaining balanced populations, and possibly inventing DNA itself (Forterre and Prangishvili, 2009; Enard et al., 2016). Virus remnants are present in most cellular genomes, indicating that viruses have been present in almost all environments which contain life for a very long time. This is also true in humans, where viruses play key roles in human physiology (Bannert and Kurth 2004; Arneth 2021).
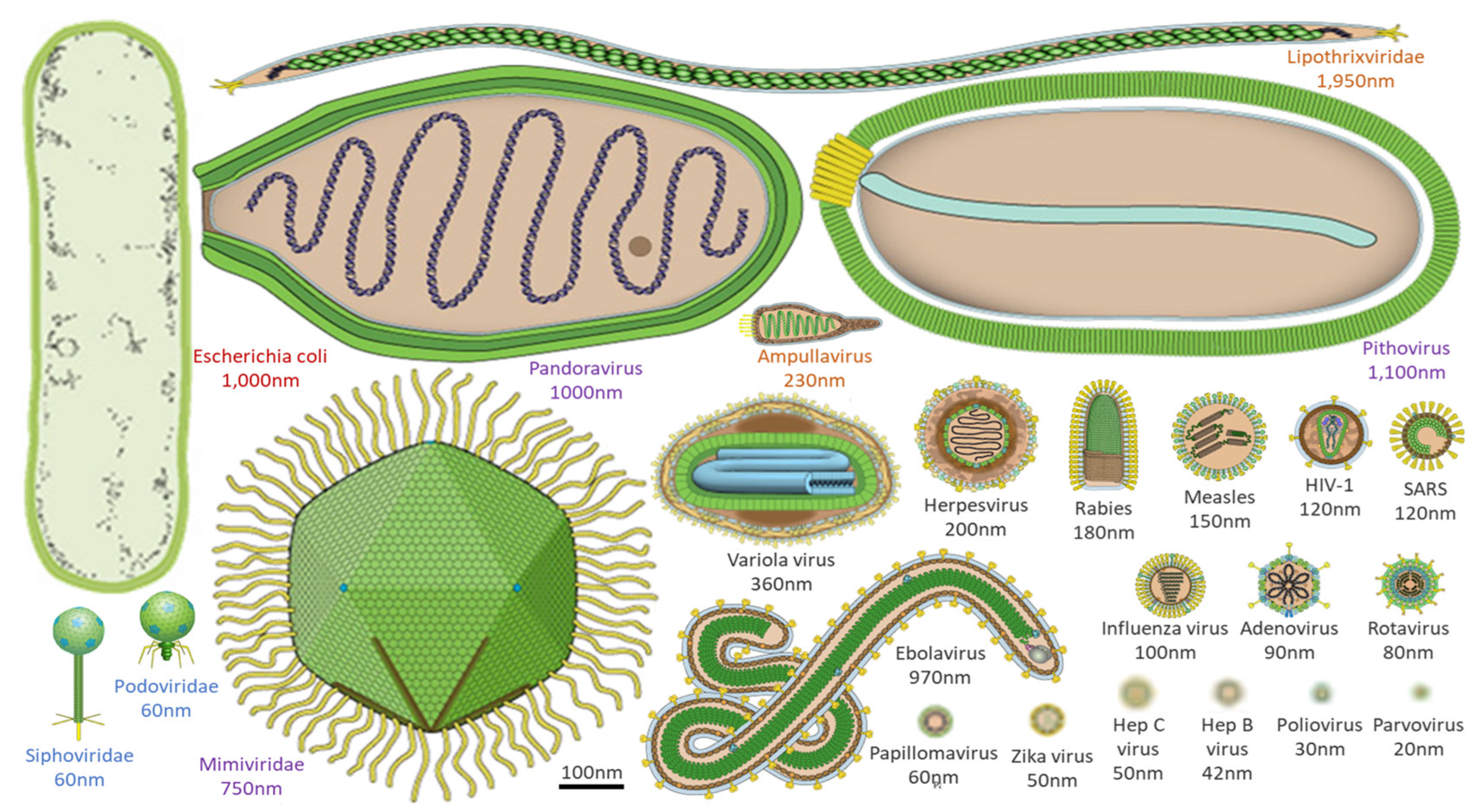
Notably, the diverse morphology of virions–which is unlike that found in cellular life (Fig. 1)–and the presence of unique genes for the virion coat or capsid, have been used by some researchers as a definition of “other” life: “capsid-encoding organisms” (Forterre and Prangishvili 2009). Virions of viruses that infect bacteria and archaea typically range in size from tens to a few hundred nanometers, while virions of viruses that infect eukaryotes organisms (e.g., amoebae or humans) can range from tens to thousands of nanometers (Fig. 1). These so-called giant viruses can be larger than some bacteria or archaea and can be infected by viruses themselves (Sommers et al., 2021). For example, virophages have been observed to infect giant viruses that are themselves infecting cellular organisms (e.g., amoebae). In these cases, the virophage infection co-opts the giant virus and may improve the condition of the host organism (Roux et al., 2017; Backstrom et al. 2019; Schulz et al. 2020). Viruses can also alter the genetic architecture, phenotypic characteristics, reproduction strategy, infection dynamics, and evolution of nearby viruses, which in turn influences how viruses interact with their hosts and ultimately, ecosystems. This spectrum of virus behaviors across all domains of life is also reflected in the productivity of their infected host cells (i.e., modulation of host-cell metabolism and tens to thousands of progeny virus particles per cell) and timing of single-cycle infection (e.g., tens to thousands of minutes). Notably, the timing for one cycle of virus growth correlates with the doubling time of healthy host cells (Jin et al., 2021).
The diversity of unique viral qualities compels us to better understand the potential roles of viruses and virus-like entities in both astrobiology and space biology. There are multiple critical knowledge gaps in our understanding of viruses in the space environment and their potential role in other hypothetical biospheres in our Solar System and beyond. This multifaceted gap has major consequences for our understanding of 1) the origins and evolution of life, 2) human space flight and crewmember health, 3) life support systems involving organisms besides humans, 4) life detection, and 5) planetary protection.
New virus research here on Earth should contribute to addressing key scientific questions in planetary science, space biology, and astrobiology, such as:
How did life (and viruses) originate and what were the mechanisms and potential timelines of evolution in cellular and complex multicellular organisms?
What environmental conditions and processes are most conducive to the preservation and propagation of information molecules or molecular signatures of life, and what conditions are incompatible with those processes?
What role(s) could acellular informational molecules play in potential biospheres elsewhere, and how might those roles be detectable as biosignatures?
Can the detection of informational molecules beyond Earth be interpreted as a sign of life?
Can understanding of how natural versus artificial environments drive changes in the virome lead to a better understanding of how viruses impact and are shaped by their ecosystems?
Figure 1.
Morphological comparison of viruses, and a common bacterium. Viral morphology and size are highly diverse, ranging from a few to thousands of nanometers. Here, an example bacterium (Escherichia coli, red text) is contrasted with viruses that infect humans (black text), amoebae (purple text), archaea (orange text), and bacteria (blue text). Virus images adapted from ViralZone, Swiss Institute of Bioinformatics.
Figure 1.
Morphological comparison of viruses, and a common bacterium. Viral morphology and size are highly diverse, ranging from a few to thousands of nanometers. Here, an example bacterium (Escherichia coli, red text) is contrasted with viruses that infect humans (black text), amoebae (purple text), archaea (orange text), and bacteria (blue text). Virus images adapted from ViralZone, Swiss Institute of Bioinformatics.
Potential role(s) of viruses in the early stages of life
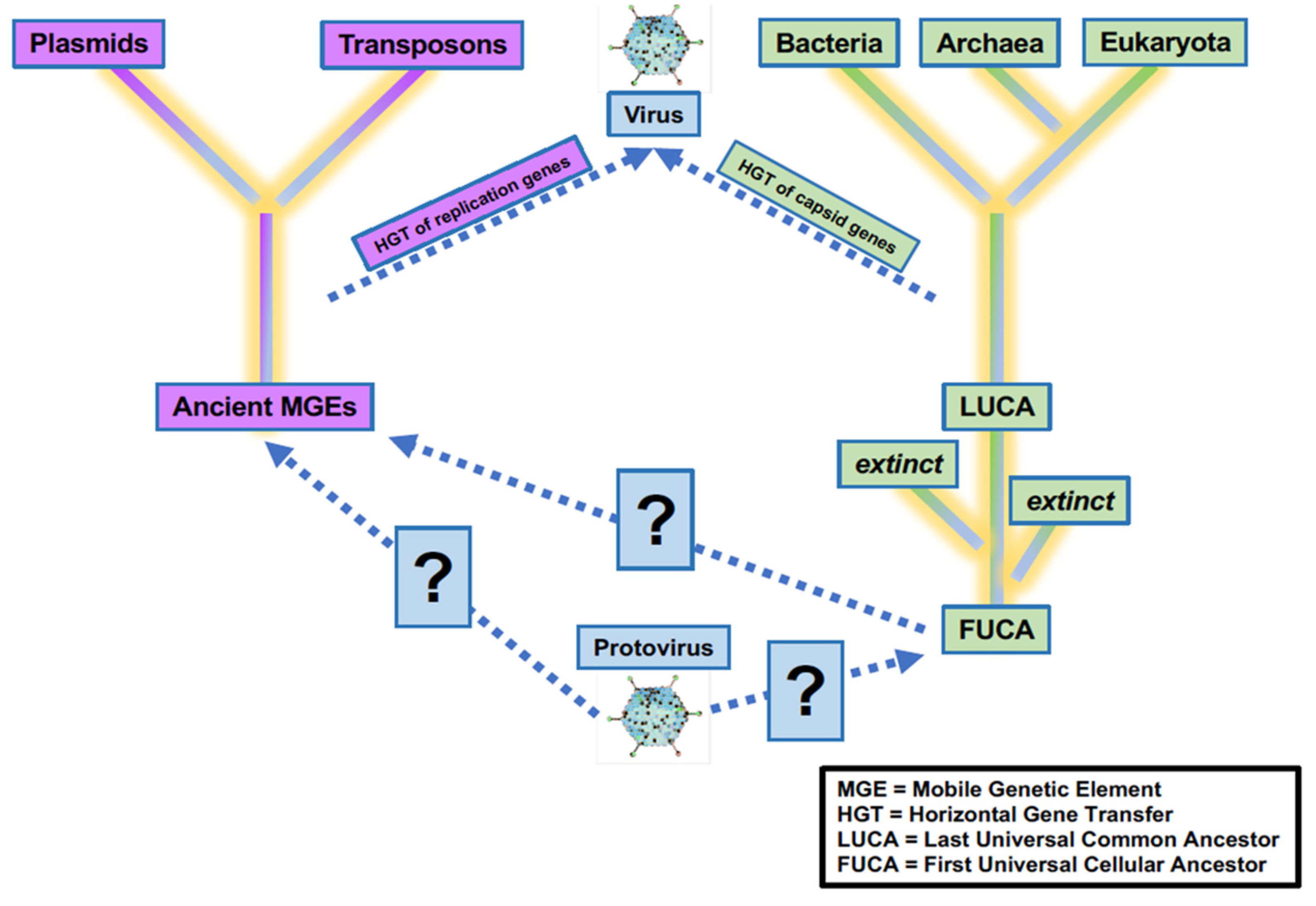
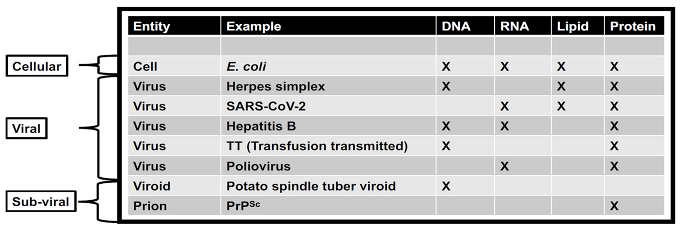
Viruses have and continue to play a critical role in the evolution of life on Earth. Precursors of virus-like entities may even mark the beginning of life itself, making the virosphere as old and significant as the cellular biosphere (Fig. 2; Janjic 2018; Moelling and Broecker, 2019). Viral signatures may be pivotal in the search for life elsewhere and in understanding evolutionary mechanics of other biospheres. Theoretical models predict that (depending on the information-transfer properties of the system) parasitic replicators will emerge anywhere in the universe that life-like processes evolve (Eigen 1971; Bresch, Niesert, and Harnasch, 1980; Vignuzzi and López 2019; Vlok, Gibbs, and Suttle, 2019). Indeed, even viruses themselves fall prey to parasitic replicators in the form of defective virus genomes that multiply at the expense of fully intact virus genomes (Perrault, 1981; Vignuzzi and López, 2019). The ‘RNA-world' hypothesis characterizes the origin of life with self-replicating RNA, followed by ribonucleoproteins that later evolved into DNA and larger proteins. Viruses and virus-like replicators are the only known extant biological entities that contain all types of genomes (
Table 1), including single-stranded and double-stranded RNA and DNA or mixtures thereof. RNA viruses serve as models for how RNA and ribonucleoproteins could have propagated via simple self-replicating RNA structures and ribozyme activity (Landweber, Simon, and Wagner, 1998; Koonin, Senkevich, and Dolja, 2006; Tyler 2008; Durzyńska, and Goździcka-Józefiak, 2015; Matsumura et al. 2016; Weinberg, Weinberg, and Hammann, 2019;). Viruses may have helped support the transition from the early RNA-world to the current DNA-world (Forterre 2006; Diemer and Stedman, 2012). Further, viruses played a role in the origins of the placenta (Mi et al. 2000) and of the mitochondrion (Shutt and Gray, 2006). They may also have been critical in the evolution of eukaryotes and multicellularity (Forterre and Gaïa 2016; Lee et al. 2018; Guglielmini et al. 2019).
Figure 2.
Uncertainty in the origin(s) and evolution of life on Earth. Viruses play a critical role in the evolution of life as we know it, and viruses or precursors of virus-like entities must be considered in origin experiments. Adapted from Harris and Hill (2021).
Figure 2.
Uncertainty in the origin(s) and evolution of life on Earth. Viruses play a critical role in the evolution of life as we know it, and viruses or precursors of virus-like entities must be considered in origin experiments. Adapted from Harris and Hill (2021).
While uncovering the history of viruses is key to understanding the origin of life on Earth, an additional benefit is the development of new technologies that could be valuable for characterizing the diversity and history of non-terran life. For instance, comparative analysis of 3D atomic structures is a non-genomic method being used to interrogate virus origins that could also be applied to non-terran samples that may lack nucleic acids (Bamford et al. 2005). This method is used to detect and classify viruses since there are no genes shared by all extant viruses (Roux et al. 2019; Tisza et al. 2020). In comparison, all organismal cells conserve some genomic features such as ribosomal RNA (
Table 1). Thus, the search for life elsewhere will benefit from understanding the role of viruses in the origin of life on Earth.
Future directions
- a)
Investigate evolutionary relationships between RNA viruses and ribozymes (ribonucleotide enzymes) to evaluate the potential role of viruses in the RNA-world, and transition to the DNA-world.
- b)
Create tools and methods to make inferences about life in the Archaean Eon through comparative analysis of extant viral life.
- c)
Determine the origin of viruses on Earth and the role viruses played in the emergence of life by reconstructing ancient events.
- d)
Develop phylogenies that consider all biological entities to better constrain the shared and/or divergent evolutionary histories of viruses and cellular organisms.
Habitability and persistence and process limits of viruses and cellular life
Understanding the extreme ranges of conditions in which viruses and their hosts can survive informs both the search for life on other worlds and also the operational considerations for planetary protection and viral monitoring during human spaceflight. Viruses persist and interact with their hosts under a wide range of extreme conditions on Earth (Gil et al., 2021), including extremes in temperature [e.g., −12 to +8ºC (Colangelo-Lillis and Deming, 2012); 81–96ºC (Baquero et al., 2020), pH [e.g., pH 1 (Baquero et al., 2020); pH 10 (Brum and Steward, 2010)], pressure [e.g., ~381 mbsf (Engelhardt et al., 2013)], and salinity [e.g., hypersalinity (Atanasova et al., 2011)]. These conditions manifest in both natural and built environments, and viruses have been observed in a variety of settings, such as domestic hot water heaters (Dublineau et al., 2011), acid mine drainages (Tapia et al., 2012, Nagayoshi et al. 2016, Gil et al. 2021), droplets suspended in the atmosphere (Reche et al., 2018), permafrost-associated soils (Trubl G., et al., 2018; Emerson et al., 2018; Trubl et al., 2021), ice cores or cryoconite holes (Wells and Deming 2006; Zhong et al., 2020; Sommers et al., 2020), ice cubes (Jalava et al., 2018), hot springs (Laidler and Stedman 2010; Laidler et al., 2013), chemically harsh conditions (Gupta, Lee, and Yin, 1995; Olofsson et al., 2001), and deep-sea sediments (Engelhardt, Orsi, and Jørgensen 2015; Cai et al., 2019).
Environmental factors drive the ability of viruses to persist and maintain their integrity for extended periods of time. For instance, viruses that adsorb to clays are protected from inactivation, enabling them to persist in soils for longer periods of time in the absence of a host (Bitton 1975; Lance and Gerba 1984; Lipson and Stotzky 1985; Syngouna and Chrysikopoulos 2010). Additionally, viruses have the potential to aid in the preservation of biological signatures. Viruses that infect microbial mats can potentially cause or expedite their fossilization into a stromatolite by acting as a nucleation site for organomineralization, altering microbial host metabolism for carbonate precipitation, or increasing the production of microbial extracellular substances (Pacton et al., 2014; White, Visscher, and Burns 2020). Virus particles in the environment can be preserved via silicification and can become reactivated (i.e., infectious) when the silica is removed (Laidler et al., 2013). Further, upon thawing, viruses preserved in frozen permafrost for ~30,000 years were able to infect modern day versions of their hosts (Legendre 2014). Overall, these findings clearly indicate that viruses (especially viral capsids) are key structures for preservation and propagation of biological information, especially in extreme environments. In this context, a better understanding of the key environmental and molecular factors driving this long-term persistence will inform future searches for virus-like elements outside of Earth.
By persisting through extreme environmental conditions, viruses can also extend protection to their host. For instance, viruses can confer heat tolerance to their host (Márquez et al., 2007), carry genes for sporulation that may drive their host to form an inactive spore that is robust to unfavorable conditions (Trubl et al. 2018; Van Goethem et al., 2019; Pelusi et al., 2021; Travers et al., 2022), or allow photosynthesis in cyanobacteria under desiccating conditions (Azua-Bustos et al., 2012). Harsh environments, such as polar regions or hydrothermal vents, also have a high incidence of temperate viruses, which can reside within their microbial hosts (lysogeny) until conditions are favorable for viral replication (Anderson et al., 2014; Brum et al., 2016). While in this lysogenic state, viruses can express genes that alter their microbial host’s physiology and metabolism, increasing the host cell’s ability to survive conditions in which resources are limited, including sea ice, dry soil, or acidic environments (Chen et al., 2005; Yu et al., 2015; Howard-Varona et al., 2017; Wu et al., 2021; Lee et al. 2021).
By integrating into hosts that are capable of dormancy, viruses can persist through conditions that are incompatible with activity, and later become viable when conditions improve. Extreme examples of organisms regaining function after dormancy include: cyanobacteria reviving after a 672-day exposure to space outside of the ISS (Laranjeiro et al., 2021), cyanobacteria in Antarctica metabolizing within a week after rewetting following 20–30 years without stream flow (McKnight et al., 2007), nematodes being revived from 30,000 year old permafrost in Siberia (Shatilovich et al., 2018) and rotifers in northeastern Siberia revived from 24,000 year old permafrost (Shmakova et al., 2021). Microbes living in low-energy conditions in the South Pacific Gyre have even retained their metabolic response after 101.5 million years (Morono et al., 2020). Investigation of the viruses, if any, have hitchhiked along with these long-persisting hosts and how viral activity interacts with long host dormancies is a ripe research opportunity. The diverse evidence of microbial and eukaryote dormancy noted above shows the potential for organisms to survive and even grow in space-like environments and planetary analogs. However, there is no comparable body of research on viruses, or virus-virus and virus-host interactions.
It is critical to evaluate the effect of viruses on organisms in extreme environments because virus-host interactions are fundamentally different in harsh environments when compared to ideal environments (Dávila-Ramos et al., 2019). It is now feasible to computationally integrate how functions encoded in virus genomes interact with material and energy resources of their hosts, and thereby predict the timing and levels of virus growth (Yin and Redovich 2018). This capability could characterize viral activity in human-supporting environments in space. We posit that similar virus-host interactions are likely wherever life exists, and it is thus critical to expand our understanding of these interactions on Earth to enable the detection and identification of such phenomena during space missions.
Investigations into viral activity in low-energy environments can benefit from development of high sensitivity tracer approaches. Bulk tracer methods, such as nucleic acid stable isotope probing (aka SIP; Manefield et al., 2002; Radajewski et al., 2000), can provide detailed information about the level of stable isotope incorporation by microbes and have been applied to investigate virus activity in complex environments (Pasulka et al. 2018; Starr et al., 2021; Trubl et al. 2021; Lee et al. 2021; Lee et al. 2022). However, bulk methods like SIP integrate over relatively large sample mass (Nuccio et al. 2022), thus requiring more enriched substrate in complex systems. However, investigators have recently developed imaging mass spectrometry approaches that can detect viral replication at the single particle level based on incorporation of rare stable carbon and nitrogen isotopes (Pasulka et al. 2018; Gates et al. 2018; Mayali et al., 2019). Because virion enrichment has been detected in individual particles using nanometer-scale secondary ion mass spectrometry (nanoSIMS), the approach is clearly equally viable for other very small samples. These studies demonstrated sensitivity down to 100 nm-diameter virions. Further research is necessary to extend these methods to bacteriophage in the 50 nm-diameter range.
Future directions
- a)
Increase of environmental surveys and long-term monitoring of viral persistence and activity in extreme environments that can be used as analogs of celestial bodies and early life on Earth.
- b)
Experimental work on the lower and upper limits to viral persistence and activity under a variety of conditions (e.g., aridity, oxygen content, humidity, or trapping in amber).
- c)
Evaluation of the persistence and activity of viruses in dormant and active hosts, combining techniques (e.g., stable isotope probing with metagenomics) to better characterize viruses and detect low-level host activity.
Biogeochemical cycling and biosignature detection
Our understanding of viruses as major players in Earth’s biogeochemical cycles has been reshaped by the advent of metagenomic approaches that have enabled the study of uncultivated viruses (Kristensen et al. 2010; Roux et al. 2016; Roux et al. 2019). Marine virology has been particularly intensely studied, highlighting the potential for worlds with liquid oceans, such as Enceladus or Europa, to contain informational molecules like virus genomes and proteins among their organic compounds (Postberg et al. 2018). On Earth, viruses have a huge impact on oxygen concentrations by infecting the marine cyanobacteria responsible for about 25% of the oxygen we breathe. Since, at any moment, about half of marine cyanobacteria are infected, leading to either a decrease in oxygen production (i.e., cells lysed by viruses) or an increase in the efficiency of their oxygen production (Sieradzki et al. 2019). Other work has demonstrated the activity of viruses in frozen soils (Trubl et al. 2021) and illustrates the potential for viruses in biogeochemical processes of terrestrial worlds, such as Mars. Terrestrial worlds, or at least partly rocky bodies, such as comets, may host ice-lidded cryoconite holes in their polar ice caps that could result in the presence of liquid water to support active life (Zawierucha, Ostrowska, and Kolicka 2017). This includes locations such as Venus, which could harbor within its thick mid-deck atmosphere containing clouds of sulfuric acid droplets) iron and sulfur driven metabolism that viruses have been identified to augment on Earth (Anantharaman et al. 2014; Roux et al. 2016; Bonnain, Breitbart and Buck 2016; Martins et al. 2018).
The widespread and frequent detection of genes used by viruses to hijack the metabolism of their host cell(s) and manipulate them to produce progeny viruses strengthens the need for a conceptual shift to call virus-infected cells “virocells”, in order to emphasize their differences from uninfected cells (Forterre 2011; Forterre 2013). Viral infections can change a microbe’s metabolic outputs, impacting the composition and quantity of their biosignatures. Combined experimental and in silico studies of virus growth on bacterial hosts have shown how the physiological state of the host cell can be reflected in the timing and level of virus production, whereas virus production is intimately linked to the availability of resources for protein synthesis (Mahmoudabadi, Milo, and Phillips 2017). Since nutrient availability in the host’s environment impacts its ability to produce viruses, the productivity of virus infection can provide a readout of the metabolic demands of the living host (You, Suthers, and Yin 2002). Further, computational modeling suggests that viruses evolve to optimally use the finite metabolic energy resources of their host cells (Kim and Yin 2004). Virus growth may also be linked to host physiology in ways not previously appreciated; studies of viruses that infect Bacteria, Eukarya, and Archaea exhibit delay times for virus production that correlate with the doubling times of their host cells (Jin and Yin, 2021). Similar virus-host interactions could be at play in other systems, and it is thus critical to expand our understanding of these interactions on Earth to enable the detection and identification of such phenomena in other biospheres.
A key way to evaluate virus-host interactions is via stable isotopic signatures. Autotrophic microbial life preferentially incorporates the lighter isotope available for each biogenic element, a feature often used to identify ancient microbial life. Yet the exact signatures of this process depend on the organism(s) performing a given metabolic process, how many enzymatic reactions take place in relevant metabolic pathways, and how the enzyme(s) work. For example, fractionation factors are different for denitrification via fungi versus bacteria (Ostrom and Ostrom 2017), or for methanogenesis depending on the initial substrate and the organisms involved (Whiticar 1999; Hornibrook, Longstaffe, and Fyfe, 2000; Penning et al. 2006). When a virus infects a microbial host, it redirects its metabolism, thus possibly impacting these isotopic values. This could be more dramatic if a virus were to encode an auxiliary metabolic gene that has a different fractionation factor than the host version of that gene. For example, kinetic measurements of virus and host versions of the same enzyme have revealed that the virus enzyme had a significantly lower kcat/KM value than the host enzyme (Thompson et al. 2011). Such viral influences can lead to large variations in isotopic signatures, leading to uncertainty when distinguishing between abiotic and biological processes and in the utility of a particular biosignature (Schwieterman et al. 2018). Isotopic fractionation thus has the potential to reveal fundamental characteristics of biosphere metabolism, including the impact and contribution of viruses.
Isotopic fractionation measurements can be applied to the geologic record on Earth, as well as on Mars, to infer characteristics of the metabolisms of past biospheres and changes over time (Johnson et al. 2017). The isotope history of marine ecosystems throughout Earth’s history is well-studied (Zerkle and Mikhail, 2017; Krissansen-Totton et al., 2018). The isotopic composition of icy environments through time on Earth is less well-characterized but may provide analog environments to other planetary bodies such as Mars (Havig and Hamilton 2019). Distinguishing viral influences on both present isotopic signatures and on their variation over multiple timescales may therefore provide a reference for interpreting isotopic biosignatures of past life on other worlds.
Future directions
- a)
Improve understanding of how different viruses hijack their host cellular machinery.
- b)
Further explore the broad range of virus-host interactions and dynamics in various ecosystems.
- c)
Quantitatively estimate the role and impact(s) of virocell metabolism on Earth’s different biogeochemical cycles.
- d)
Improve the understanding of viral impacts on biosignatures for major Earth biogeochemical cycles, including the magnitude of such effects.
- e)
Search for the existence of generalizable biosignatures associated with viral metabolism reprogramming to reduce uncertainty associated with biosignatures for life detection.
Impact of viruses on human space exploration
As human spaceflight includes sustained missions beyond Low Earth Orbit (LEO), nearly every aspect of the mission (including ECLSS, human health and performance, lander operations and extravehicular activities) must consider the impact of virus and host interactions. Otherwise, the impacts would be akin to the current concern of volatile contamination on the ISS where instrument capabilities and measurements are less sensitive or erroneous due to different environmental conditions that were not tested (Wallace et al., 2022).
Viral response to spaceflight environments
Viral studies in microgravity have been very limited, and most microbiology investigations are focused on bacteria. Bacteria have shown a variety of species-specific and even strain-specific morphological and physiological responses to the low fluid shear force and lack of liquid media convection in microgravity (as reviewed in Diaz et al. 2019; Acres et al., 2021; Sharma et al.). Without externally applied forces, nearly everything in a microgravity environment can stay suspended or quiescent. Brownian motion dominates, reducing the ability for host cells to gather nutrients (Zea et al. 2016), thereby influencing the size, structure, and organization of these host cells and potentially modifying the infiltration capabilities of viruses. The lack of buoyancy effects in microgravity may also influence viral dispersal and host encounter rates in both the spacecraft cabin atmosphere and quiescent fluid systems. Another response to microgravity is aggregation (Zea et al., 2017; Domnin et al., 2022), which might limit the ability of viruses outside an aggregate to encounter a host surface or increase host encounter rate once one host in an aggregate is lysed. Even with external forces (fans, pumps, etc.) moving liquid and gas loops, it is still unknown how well viruses adhere to surfaces that they encounter. Simulated microgravity has been associated with a thicker cell membrane envelope in E. coli (Zea et al., 2017), which could inhibit the ability of viruses to infect hosts. In contrast, lower membrane integrity was observed in Vibrio fischeri (now known as Aliivibrio fischeri,Vroom et al., 2021), which could make hosts more susceptible to membrane-disrupting events.
The effects of microgravity on virus dynamics can be measured through environmental sequencing of the various surfaces aboard space stations and crewmember microbiomes. Though they come with limitations in sampling size, frequency, and depth of coverage due to the technical and logistical challenges of obtaining samples, several such data sets from ISS are already available for reanalysis (Be et al., 2017; Urbaniak et al., 2018 & 2022; Singh et al., 2018; Checinska Sielaff et al., 2019; Avila-Herrera et al., 2020). The virus-to-host ratio for known virus-host pairs can also be estimated and its distribution explored spatially and across time or as a response to perturbation, natural or experimental. Targeted and untargeted metagenomic sequencing of microgravity samples would have complementary strengths allowing for studies ranging from broad surveys at species and genus taxonomic levels to narrowly focused studies modeling specific strain and variant population dynamics. The dynamics of viral dispersal, host adsorption, infection, and progeny productivity in spaceflight environments are extremely understudied and deserve further work.
Future directions
- a)
Characterize differences in dispersal, aggregation, and adsorption processes of viruses in a microgravity fluid or air environment.
- b)
Compare the ability of viruses to adsorb to and infect hosts during microgravity-induced cellular changes.
- c)
Estimate statistics that describe virus-host interactions to understand how viruses and hosts behave under nominal microgravity spaceflight conditions and how they respond to perturbations, for example virus-to-host ratios.
- d)
Continue to develop capabilities for onboard biosurveillance.
Environmental control and life support systems (ECLSS)
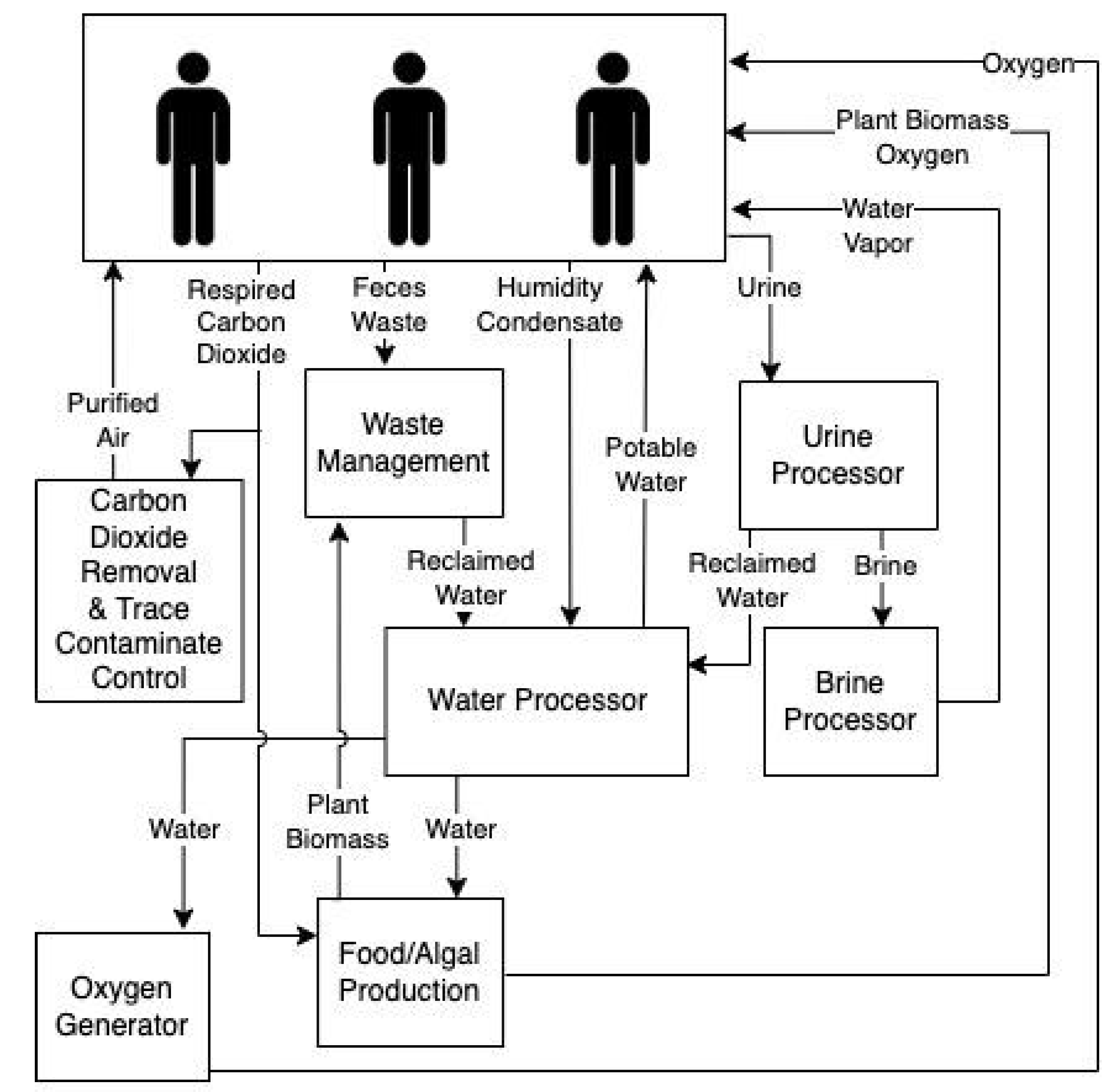
ECLSS are imperative for supporting human spaceflight. These flight-proven technologies on the International Space Station (ISS) control air composition and temperature, food and water, and waste remediation. However, long-duration missions beyond LEO will require robust alternatives that do not rely on frequent resupply missions (Anderson et al., 2019). Closing the carbon loop through bioregenerative technologies is one approach for providing ECLSS in space missions beyond LEO. Algal photobioreactors can remove CO2, create O2, remove or alter waste, and produce edible biomass (Matula and Nabity 2019; Fahrion et al., 2021). These photobioreactors can withstand the dynamic temperature environment experienced within the ISS thermal control loops (Matula, Nabity, and McKnight 2021; Matula and Nabity 2021). Preliminary spaceflight studies using algae for ECLSS observed thriving cultures (Helisch et al., 2020; Poughon et al., 2020). Likewise, extremophilic algae, lichen, cyanobacteria, and fungi included in experiments mounted on the outside of the ISS and Space Shuttle survived weeks to months-long missions (de Vera et al., 2019; Malavasi et al., 2020). However, these studies did not characterize the full microbiome within the non-axenic cultures. This is extremely important; for example, virophages can have profound implications for microbial nutrient cycling, often referred to as the microbial loop. Predator-prey simulation models indicate that the presence of virophages regulates helper virus and algal host dynamics altering carbon flux through the microbial loop in aquatic ecosystems (Yau et al., 2011). Understanding potential biome or virome shifts within these systems over long durations may elucidate the need for specific algal species selection, causes of viral or bacterial-based system failures, and operational considerations (Matula and Nabity 2019) (Fig. 3).
Bioregenerative environmental control and life support systems
On Earth, plant viruses are a threat to sustainable agriculture, and disease management relies on rapid and accurate viral identification (Rubio et al., 2020). Efforts on the ISS have focused on plants for CO2 removal and O2 production, as a source of multivitamins, urine reuse, and to promote psychological well-being (Dzhos et al., 2021). Many plants have been grown under microgravity, including flower-producing species, herbs, and vegetables. However, due to the stress of spaceflight, viral infection of these plants can be hugely influential on plant functions. A recent study found that spaceflight factors significantly affect tomato plants (Dzhos et al., 2021), increasing the productivity of the plants and the concentration of some vitamins such as carotenoids, which are important for crew members on long-term space missions. Importantly, plants grown from seeds that were in space for half a year prior to germination were resistant to viral infection. Moreover, the resilience of seeds from many plant species (among other organisms) was tested by keeping them outside the ISS for 558 or 682 days, thus exposing them to high levels of radiation (Tepfer and Leach 2017). All plant seeds could germinate after the shorter flight, but only plant seeds with a stronger coat could survive the longer radiation exposure. While these studies show promise for cultivating plants on long-term spaceflights, our understanding of plant-virus interactions on short flights is limited and we have no information at all for long-term flights.
Figure 3.
Review of spaceflight ECLSS potentially impacted by viruses. This simplified overview of environmental control life support systems for a spacecraft and surface habitat focuses on the systems that could be positively or negatively impacted by viruses.
Figure 3.
Review of spaceflight ECLSS potentially impacted by viruses. This simplified overview of environmental control life support systems for a spacecraft and surface habitat focuses on the systems that could be positively or negatively impacted by viruses.
Human physiology and the viral ecology of crewed spacecraft
In crewmembers, latent viruses are shown to reactivate more than in matched controls. Sometimes this reactivation occurs before leaving earth and is presumably stress related. There are plausible reactivation triggers associated with spaceflight itself, such as UV or ionizing radiation (Pavletíc et al., 2022), but which mechanism is responsible for increased virus reactivation in crewmembers has not been settled. Reactivation has included herpes simplex virus, Epstein-Barr virus, and Varicella zoster virus (Stowe et al., 2000; Mehta et al., 2004; Pierson 2005; Rooney et al., 2019). After 6–12 months in space, crewmembers had significant changes in their microbiome (both internal and external) that led to rashes and hypersensitivity episodes, warranting further investigation of the effects of long-term exposure from the space environment on humans and their microbes and viruses (Voorhies et al. 2019). The reaction of viruses to microgravity is poorly known, not only for human viruses, but also for viruses infecting components of the human microbiome and proposed life support organisms, such as plants or cyanobacteria.
The community composition of viruses and other organisms on the ISS is mostly comprised of viruses of non-human cells, such as bacteriophages, and this composition differs from that of terrestrial research space analogs (MDRS and HI-SEAS), thus pointing towards the space environment as a trigger (Mora 2019). Although these viruses do not infect humans, they can be transported back to Earth and released into Earth environments via plants, humans, and other entities that can harbor viruses. Continued efforts are needed for quarantining people within space stations for long-term space travel and increased screening for crewmembers returning to Earth. Although it is tempting to eliminate viruses from spacecraft wherever possible, we should be sensitive to the unanticipated roles viruses play and the amount of resources this would entail. As well, a virus’s presence can be beneficial sometimes warding off more pathogenic relatives by cross-protection or superinfection exclusion (Folimonova 2020). Immunologic “eustress” can promote a healthy organism, as is seen by comparison to the physiological deficits of germ-free animals (Round 2009). In some cases, viruses can actually be symbiotic with their host organisms (Roosinck 2011). As such, viruses are key players in crewmember health and safety and in the ecology of crewed environments. To enable long-duration crewed missions beyond low Earth orbit, it is essential to understand the influence of the space environment on viruses, their hosts, and their interactions.
Spaceflight operational impacts
To enable long-duration crewed missions beyond LEO, it is essential to understand the influence of the space environment on viruses, their hosts, and their interactions, both within the human body and in life-support systems. Given the differences in viral dispersal patterns in low-gravity environments and the extreme conditions under which viruses persist and interact with hosts (see section “Habitability and the limits to viral and life persistence and processes”), even surfaces often considered sterile, or at least clean, must be investigated. Some bacterial species—for example, Bacillus pumilis strain SAFR-032 (Tirumalai 2013)—are refractory to pre-flight sterilization, and some could be tolerant as well given the extreme diversity of viral morphotypes (including lipid-free viruses, see Fig. 1). Even with perfect sterilization of equipment (which is essentially unobtainable), it is not currently possible to achieve a virus-free environment on a manned space mission because of post-launch viral shedding by crewmembers (vide supra). The frequency of sampling, maintaining, and cleaning life support systems will impact day-to-day spaceflight operations, during both cruise phase and orbital or surface sustained missions. Extravehicular activity (EVA) and associated planetary protection protocols must also be accommodated, for example, protecting areas with high scientific value from contamination (e.g., Rummel et al 2014). Modifications of operations may entail landing further from a sample site, designing new vehicles, requiring more stringent cleaning protocols, or considering entirely new sample sites (e.g., Meyer et al 2019). Additionally, contamination restrictions during EVA will narrow the design of space suit systems (Wilson et al. 2014). Attempts to dictate planetary protection protocols without fully understanding viral dispersal and survivability will potentially result in overly restrictive constraints that ultimately hinder scientific discovery or alternatively inadequate protocols that do not achieve proper containment.
Future directions
- a)
Compare the composition and dynamics of human, algal, plant, and microbial viromes in natural vs. artificial environments.
- b)
Investigate viromes in built and artificial environments relevant to space, including the ISS.
- c)
Explore virus-host dynamics in space for human- and plant-associated microbiomes.
- d)
Assess triggers that cause latent infections to become virulent.
- e)
Obtain a better molecular understanding of how viruses hijack their host cellular machinery and transmit in a space environment.
- f)
Quantitatively estimate the role and impact(s) of virocell metabolism in space flight environments.
- g)
Identify viral loading impacts to planetary protection needs and human health preservation, and resulting modifications to spaceflight operations (travel, landing, and EVA).
- h)
Characterize viral influence on microbial biofilm composition and growth dynamics in human, algal, and plant systems under various conditions.
Detection of viruses on Earth and elsewhere
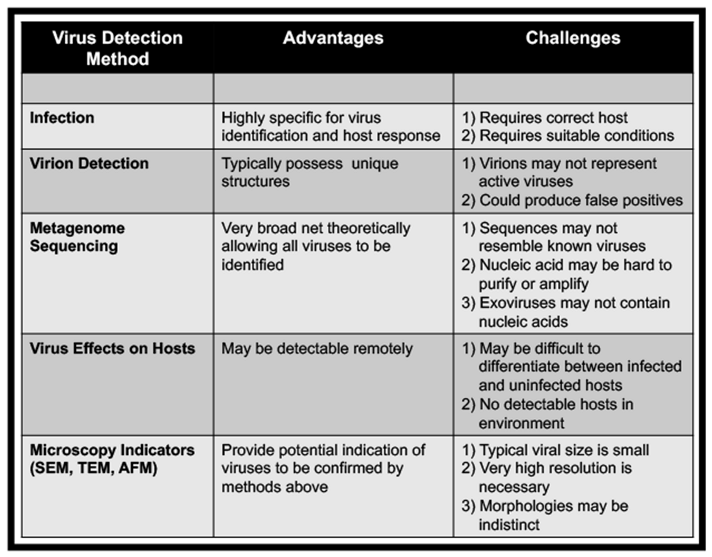
The development of flight instruments that can detect either virus particles or sequences is possible. For example, solid-state nanopore-based biosensors are being investigated for space flight and have proven capability for evaluating different biomarkers (i.e., DNA/RNA, proteins, and whole viral particles — spanning from a few nanometers to over 100 nanometers in size). Solid-state nanopores can also discriminate between viral particles by monitoring the change in electrical current as viruses pass through an electrically biased pore or by measuring the mass-density of viruses (Zhou et al., 2011; Arjmandi et al., 2014; McMullen et al., 2014; Wu, H. et al. 2016). Particle-size distributions in virus populations can also be measured by solid-state nanopores (Akpinar and Yin 2015). Methods based on the detection of individual particles in flowing streams have adapted flow cytometry down to the scale of virus particles; specifically, flow virometry employs more powerful lasers, reduced diameter fluid flows, wider-angle sampling of scattered light, and fluorescent labeling of particles (Bhat et al. 2022) Additionally, microscopy has been used to evaluate microbial and viral populations. For example, an atomic force microscope was used during the Phoenix Mars Lander to investigate Martian soils (Pike et al., 2011) and recently, a scanning electron microscope was added to the International Space Station (ISS) (Own et al., 2018). Microscopy technologies complement spectroscopic methods by providing orthogonal evidence for viral and nonviral life (Zhang et al., 2013).
Current technologies are also being configured for future space missions to detect and characterize viruses. For example, nanoscale secondary ion mass spectroscopy (nanoSIMS) can perform in situ quantitative trace sample analysis with exceptional sensitivity and spatial resolution (Mayali 2020; Pett-Ridge and Weber 2022). NanoSIMS is currently being evaluated by the Network for Life Detection to help discern between abiotic and biological signatures based on the differences in elemental and isotopic patterns of cellular organisms versus minerals and other precipitates. This technology has recently been used to detect viruses (Gates et al., 2018) and also map their elemental and isotopic distributions in complex communities and in mineralized samples (Pacton et al., 2014). These studies suggest viruses play a role in organomineralization and may be important for how life is preserved in the geologic record. These technologies complement spectroscopic methods by providing orthogonal evidence for viral and nonviral life (Zhang et al., 2013). Overall, there are multiple approaches available to detect viruses, which might be a key sign of life beyond Earth, given their ability to persist in extreme environments and their dependence on cellular life.
Independent of
in situ investigations, the identification of virus particles and genomes can be applied to planetary protection and sample-return missions. The same technologies described above can be used in conjunction with higher resolution and fidelity sequencing methods or 3D atomic structure analysis (Bamford et al. 2005). Moreover, ‘relic’ or environmental DNA can be removed to target the detection of intact viruses and microbes, such as with the use of propidium monoazide (Wagner et al. 2008; Weinmaier et al., 2015). It must therefore be ensured in advance that methods of environmental virology will also be applied to instruments used for life detection and on returned samples (Janjic 2018; Ricciardi et al. 2022;
Table 2).
Future directions
- a)
Develop flight-ready technologies for more efficient and accurate detection and characterization of virus genomes.
- b)
Investigate innovative technologies for automated detection and identification of virus and virus-like particles.
- c)
Apply such technologies to Earth analog systems to explore prospects and limitations for detecting viruses and virus-like particles.
- d)
Incorporate measurements of viruses in standard operating procedures for return flight missions and in examination of samples from return missions.
Conclusions
For accurate and effective pursuits in the field of Astrobiology, it is critical to understand how life on Earth functions, as it is currently the only known place where life exists. Viruses are key contributors to Earth’s ecosystems; however, much remains unknown regarding their influence on cellular life, role in evolutionary history, and their fundamental physical interactions with the Earth system. Basic ecological factors, such as persistence and decay under various scenarios, also remain underexplored. Likewise, safe and effective pursuits in deep space travel will require a thorough understanding of the human microbiome in space, including viruses. Above, we have outlined ways in which focused astrovirological investigation can advance the goals of space science. Across the diverse disciplines that make up astrobiology, we highlight several classes of investigations which cannot afford to neglect viruses. Most of the diversity of life on Earth is comprised of viruses, whether diversity is defined by number of species, type of genetic information, number of individuals (Breitbart 2002; Roossinck 2012; Paez-Espino et al., 2016; Parikka et al. 2017; Mushegian 2020), absence of any universally present gene, mode of replication, or number of unique (i.e., non-homologous) genes (Koonin and Wolf 2012). Viruses should be explicitly considered in organism-level astrobiological investigations. For instance, metagenomic surveys that collect mainly ribosomal RNA data are of limited utility to astrobiology because they reflect only a subset of terran biological diversity (although other studies of the ribosome remain important to understanding how terran life emerged). The ability to detect virus-like organisms must be a point of assessment for instruments and missions to directly detect extraterrestrial organisms, and Mars sample return materials should be examined for the presence of viruses (Janjic 2018; Ricciardi et al., 2022). When organisms are used as model systems, viruses should be adequately represented. This consideration will reduce biases and increase utility of diverse astrobiological studies.
“Whether viruses are alive or not may be a moot question, but if a virion (or virus-like particle) were to be unequivocally detected in an extraterrestrial sample, very few people would claim that this would not be evidence for life—wherever that sample was from”.
– Berliner et al. (2018)
Funding
The work of G.T., J.P-R. and P.W. was supported by the US Department of Energy (DOE) Office of Science, Office of Biological and Environmental Research Genomic Science program award SCW1632, and by Lawrence Livermore National Laboratory LDRD award 21-LW-060, and under the auspices of the DOE under contract DE-AC52-07NA27344. The work conducted by S.R. was supported by the U.S. Department of Energy Joint Genome Institute (https://ror.org/04xm1d337), DOE Office of Science User Facility, and the Office of Science of the U.S. Department of Energy operated under Contract No. DE-AC02-05CH11231. Work by K.S. was supported by the U.S. National Science Foundation, MCB-1929273 and MCB-2025305. Work by J.Y. was supported by the U.S. National Science Foundation (MCB-2029281 and CBET-2030750). Work in the Kaelber lab was supported in part by the U.S. National Institutes of Health, R21GM140345.
References
- Acheson, N.H. Fundamentals of molecular virology; (No. Ed. 2). John Wiley & Sons, Inc. 2011. [Google Scholar]
- Acres, J.M.; Youngapelian, M.J.; Nadeau, J. The influence of spaceflight and simulated microgravity on bacterial motility and chemotaxis. npj Microgravity 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, F.; Yin, J. Characterization of vesicular stomatitis virus populations by tunable resistive pulse sensing. Journal of virological methods 2015, 218, 71–76. [Google Scholar] [CrossRef]
- Anderson, R.E.; Sogin, M.L.; Baross, J.A. Evolutionary strategies of viruses, bacteria and archaea in hydrothermal vent ecosystems revealed through metagenomics. PloS one 2014, 9, e109696. [Google Scholar] [CrossRef]
- Anderson, M.; Sargusingh, M.; Gatens, R.; Perry, J.; Schneider, W.; Macatangay, A.; Toomarian, N.; McKinley, M.; Shaw, L. 2019, July. NASA environmental control and life support technology development and maturation for exploration: 2018 to 2019 overview. 49th International Conference on Environmental Systems.
- Arjmandi, N.; Van Roy, W.; Lagae, L. Measuring mass of nanoparticles and viruses in liquids with nanometer-scale pores. Analytical chemistry 2014, 86, 4637–4641. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Leftovers of viruses in human physiology. Brain Structure and Function 2021, 226, 1649–1658. [Google Scholar] [CrossRef]
- Atanasova, N.S.; Roine, E.; Oren, A.; Bamford, D.H.; Oksanen, H.M. Global network of specific virus–host interactions in hypersaline environments. Environmental Microbiology 2012, 14, 426–440. [Google Scholar] [CrossRef]
- Avila-Herrera, A.; Thissen, J.; Urbaniak, C.; Be, N.A.; Smith, D.J.; Karouia, F.; Mehta, S.; Venkateswaran, K.; Jaing, C. Crewmember microbiome may influence microbial composition of ISS habitable surfaces. PloS one 2020, 15, e0231838. [Google Scholar] [CrossRef]
- Azua-Bustos, A.; González-Silva, C.; Arenas-Fajardo, C.; Vicuña, R. Extreme environments as potential drivers of convergent evolution by exaptation: the Atacama Desert Coastal Range case. Frontiers in microbiology 2012, 3, 426. [Google Scholar] [CrossRef] [PubMed]
- Backstrom, D.; Yutin, N.; Jorgensen, S.L.; Dharamshi, J.; Homa, F.; et al. Virus genomes from deep sea sediments expand the ocean megavirome and support independent origins of viral gigantism. mBio 2019, 10, e02497-18. [Google Scholar] [CrossRef]
- Bamford, D.H.; Grimes, J.M.; Stuart, D.I. What does structure tell us about virus evolution? . Current opinion in structural biology 2005, 15, 655–663. [Google Scholar] [CrossRef]
- Bannert, N.; Kurth, R. Retroelements and the human genome: new perspectives on an old relation. Proceedings of the National Academy of Sciences 2004, 101 (Suppl_2), 14572–14579. [Google Scholar] [CrossRef] [PubMed]
- Baquero, D.P.; Contursi, P.; Piochi, M.; Bartolucci, S.; Liu, Y.; Cvirkaite-Krupovic, V.; Prangishvili, D.; Krupovic, M. New virus isolates from Italian hydrothermal environments underscore the biogeographic pattern in archaeal virus communities. The ISME journal 2020, 14, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Be, N.A.; Avila-Herrera, A.; Allen, J.E.; Singh, N.; Checinska Sielaff, A.; Jaing, C.; Venkateswaran, K. Whole metagenome profiles of particulates collected from the International Space Station. Microbiome 2017, 5, 1–19. [Google Scholar]
- Beijerinck, M.W. ; 1898. Ueber ein Contagium vivum fluidum als Ursache der Fleckenkrankheit der Tabaksblatter.
- Berliner, A.J.; Mochizuki, T.; Stedman, K.M. Astrovirology: viruses at large in the universe. Astrobiology 2018, 18, 207–223. [Google Scholar] [CrossRef]
- Bhat, T.; Cao, A.; Yin, J. Virus-like Particles: Measures and Biological Functions. Viruses. 2022, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G. Adsorption of viruses onto surfaces in soil and water. Water Research 1975, 9, 473–484. [Google Scholar] [CrossRef]
- Breitbart, M.; Salamon, P.; Andresen, B.; Mahaffy, J.M.; Segall, A.M.; Mead, D.; Azam, F.; Rohwer, F. Genomic analysis of uncultured marine viral communities. Proceedings of the National Academy of Sciences 2002, 99, 14250–14255. [Google Scholar] [CrossRef] [PubMed]
- Brum, J.R.; Steward, G.F. Morphological characterization of viruses in the stratified water column of alkaline, hypersaline Mono Lake. Microbial ecology 2010, 60, 636–643. [Google Scholar] [CrossRef]
- Brum, J.R.; Hurwitz, B.L.; Schofield, O.; Ducklow, H.W.; Sullivan, M.B. Seasonal time bombs: dominant temperate viruses affect Southern Ocean microbial dynamics. The ISME journal 2016, 10, 437–449. [Google Scholar] [CrossRef]
- Cai, L.; Jørgensen, B.B.; Suttle, C.A.; He, M.; Cragg, B.A.; Jiao, N.; Zhang, R. Active and diverse viruses persist in the deep sub-seafloor sediments over thousands of years. The ISME journal 2019, 13, 1857–1864. [Google Scholar] [CrossRef]
- Castelán-Sánchez, H.G.; Lopéz-Rosas, I.; García-Suastegui, W.A.; Peralta, R.; Dobson, A.D.; Batista-García, R.A.; Dávila-Ramos, S. Extremophile deep-sea viral communities from hydrothermal vents: Structural and functional analysis. Marine genomics 2019, 46, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Checinska Sielaff, A.; Urbaniak, C.; Mohan, G.B.M.; Stepanov, V.G.; Tran, Q.; Wood, J.M.; Minich, J.; McDonald, D.; Mayer, T.; Knight, R.; Karouia, F. Characterization of the total and viable bacterial and fungal communities associated with the International Space Station surfaces. Microbiome 2019, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Golding, I.; Sawai, S.; Guo, L.; Cox, E.C. Population fitness and the regulation of Escherichia coli genes by bacterial viruses. PLoS biology 2005, 3, e229. [Google Scholar] [CrossRef] [PubMed]
- Colangelo-Lillis, J.R.; Deming, J.W. Genomic analysis of cold-active Colwelliaphage 9A and psychrophilic phage–host interactions. Extremophiles 2013, 17, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.E.; Stowe, R.P.; Pierson, D.L.; Sams, C.F. Immune system dysregulation following short-vs long-duration spaceflight. Aviation, space, and environmental medicine 2008, 79, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Ramos, S.; Castelán-Sánchez, H.G.; Martínez-Ávila, L.; Sánchez-Carbente, M.D.R.; Peralta, R.; Hernández-Mendoza, A.; Dobson, A.D.; Gonzalez, R.A.; Pastor, N.; Batista-García, R.A. A review on viral metagenomics in extreme environments. Frontiers in microbiology 2019, 10, 2403. [Google Scholar] [CrossRef]
- De Vera, J.P.; Alawi, M.; Backhaus, T.; Baqué, M.; Billi, D.; Böttger, U.; Berger, T.; Bohmeier, M.; Cockell, C.; Demets, R.; De la Torre Noetzel, R. Limits of life and the habitability of Mars: the ESA space experiment BIOMEX on the ISS. Astrobiology 2019, 19, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Li, W.; Irwin, T.; Calle, L.; Callahan, M. 2019, Investigation of Biofilm Formation and Control for Spacecraft –An Early Literature Review, 49th International Conference on Environmental Systems, Boston, MA.
- Diemer, G.S.; Stedman, K.M. A novel virus genome discovered in an extreme environment suggests recombination between unrelated groups of RNA and DNA viruses. Biology direct 2012, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Domnin, P.A.; Parfenov, V.A.; Kononikhin, A.S.; Petrov, S.V.; Shevlyagina, N.V.; Arkhipova, A.Y.; Koudan, E.V.; Nezhurina, E.K.; Brzhozovskiy, A.G.; Bugrova, A.E.; et al. Combined Impact of Magnetic Force and Spaceflight Conditions on Escherichia coli Physiology. Int. J. Mol. Sci. 2022, 23, 1837. [Google Scholar] [CrossRef]
- Dublineau, A.; Batéjat, C.; Pinon, A.; Burguière, A.M.; Leclercq, I.; Manuguerra, J.C. Persistence of the 2009 pandemic influenza A (H1N1) virus in water and on non-porous surface. PLoS One 2011, 6, e28043. [Google Scholar] [CrossRef]
- Emerson, J.B.; Roux, S.; Brum, J.R.; Bolduc, B.; Woodcroft, B.J.; Jang, H.B.; Singleton, C.M.; Solden, L.M.; Naas, A.E.; Boyd, J.A.; Hodgkins, S.B. Host-linked soil viral ecology along a permafrost thaw gradient. Nature microbiology 2018, 3, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Enard, D.; Cai, L.; Gwennap, C.; Petrov, D.A. Viruses are a dominant driver of protein adaptation in mammals. elife 2016, 5, e12469. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, T.; Sahlberg, M.; Cypionka, H.; Engelen, B. Biogeography of Rhizobium radiobacter and distribution of associated temperate phages in deep subseafloor sediments. The ISME journal 2013, 7, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, T.; Orsi, W.D.; Jørgensen, B.B. Viral activities and life cycles in deep subseafloor sediments. Environmental microbiology reports 2015, 7, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Fahrion, J.; Mastroleo, F.; Dussap, C.G.; Leys, N. Use of photobioreactors in regenerative life support systems for human space exploration. Frontiers in Microbiology 2021, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Folimonova, S.Y.; Achor, D.; Bar-Joseph, M. Walking Together: Cross-Protection, Genome Conservation, and the Replication Machinery of Citrus tristeza virus. Viruses 2020, 12, 1353. [Google Scholar] [CrossRef]
- Forterre, P.; Prangishvili, D. The great billion-year war between ribosome-and capsid-encoding organisms (cells and viruses) as the major source of evolutionary novelties. Annals of the New York Academy of Sciences 2009, 1178, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, F.; Scaillet, B. The sulfur content of volcanic gases on Mars. Earth and Planetary Science Letters, 2009, 279, 34–43. [Google Scholar] [CrossRef]
- Gates, S.D.; Condit, R.C.; Moussatche, N.; Stewart, B.J.; Malkin, A.J.; Weber, P.K. High initial sputter rate found for vaccinia virions using isotopic labeling, nanoSIMS, and AFM. Analytical chemistry 2018, 90, 1613–1620. [Google Scholar] [CrossRef]
- Gil, J.F.; Mesa, V.; Estrada-Ortiz, N.; Lopez-Obando, M.; Gómez, A.; Plácido, J. Viruses in extreme environments, current overview, and biotechnological potential. Viruses 2021, 13, 81. [Google Scholar] [CrossRef]
- Greene, S.E.; Reid, A. 2013. Viruses throughout life & time: friends, foes, change agents.
- Gupta, K.; Lee, Y.; Yin, J. Extremo-phage: in vitro selection of tolerance to a hostile environment. Journal of Molecular Evolution 1995, 41, 113–114. [Google Scholar] [CrossRef]
- Harris, H.M.; Hill, C. A place for viruses on the tree of life. Frontiers in Microbiology 2021, 11, 604048. [Google Scholar] [CrossRef]
- Helisch, H.; Keppler, J.; Detrell, G.; Belz, S.; Ewald, R.; Fasoulas, S.; Heyer, A.G. High density long-term cultivation of Chlorella vulgaris SAG 211-12 in a novel microgravity-capable membrane raceway photobioreactor for future bioregenerative life support in SPACE. Life Sciences in Space Research 2020, 24, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Hespeels, B.; Penninckx, S.; Cornet, V.; Bruneau, L.; Bopp, C.; Baumlé, V.; Redivo, B.; Heuskin, A.C.; Moeller, R.; Fujimori, A.; Lucas, S. Iron Ladies–How Desiccated Asexual Rotifer Adineta vaga Deal With X-Rays and Heavy Ions? Frontiers in microbiology 2020, 11, 1792. [Google Scholar] [CrossRef] [PubMed]
- Hulo, C.; De Castro, E.; Masson, P.; Bougueleret, L.; Bairoch, A.; Xenarios, I.; Le Mercier, P. ViralZone: a knowledge resource to understand virus diversity. Nucleic acids research 2011, 39 (Suppl_1), D576–D582. [Google Scholar] [CrossRef] [PubMed]
- Jalava, K.; Kauppinen, A.; Al-Hello, H.; Räsänen, S. An outbreak of norovirus infection caused by ice cubes and a leaking air ventilation valve. Epidemiology & Infection 2019, 147. [Google Scholar]
- Jin, T.; Yin, J. Patterns of virus growth across the diversity of life. Integrative Biology. 2021, 13, 44–59. [Google Scholar] [CrossRef]
- Johnson, P.A.; Johnson, J.C.; Mardon, A.A. 2021, March. Where to Look Next: Extant Life Niches and Biomarkers on Mars. In 52nd Lunar and Planetary Science Conference (No. 2548, p. 1081).
- Jönsson, K.I.; Rabbow, E.; Schill, R.O.; Harms-Ringdahl, M.; Rettberg, P. Tardigrades survive exposure to space in low Earth orbit. Current biology 2008, 18, R729–R731. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, K.I.; Wojcik, A. Tolerance to X-rays and heavy ions (Fe, He) in the tardigrade Richtersius coronifer and the bdelloid rotifer Mniobia russeola. Astrobiology 2017, 17, 163–167. [Google Scholar] [CrossRef]
- Kaplan, F.; Shapiro-Ilan, D.; Schiller, K.C. Dynamics of entomopathogenic nematode foraging and infectivity in microgravity. npj Microgravity 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Koonin, E.V.; Wolf, Y.I. Evolution of microbes and viruses: a paradigm shift in evolutionary biology? Frontiers in cellular and infection microbiology 2012, 2, 119. [Google Scholar] [CrossRef] [PubMed]
- Laidler, J.R.; Stedman, K.M. Virus silicification under simulated hot spring conditions. Astrobiology 2010, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Lance, J.C.; Gerba, C.P. Virus movement in soil during saturated and unsaturated flow. Applied and Environmental Microbiology 1984, 47, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Laranjeiro, R.; Harinath, G.; Pollard, A.K.; Gaffney, C.J.; Deane, C.S.; Vanapalli, S.A.; Etheridge, T.; Szewczyk, N.J.; Driscoll, M. Spaceflight affects neuronal morphology and alters transcellular degradation of neuronal debris in adult Caenorhabditis elegans. Iscience 2021, 24, 102105. [Google Scholar] [CrossRef] [PubMed]
- Lipson, S.M.; Stotzky, G. Specificity of virus adsorption to clay minerals. Canadian journal of microbiology 1985, 31, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Mosca, C.; Fagliarone, C.; Napoli, A.; Rabbow, E.; Rettberg, P.; Billi, D. Revival of anhydrobiotic cyanobacterium biofilms exposed to space vacuum and prolonged dryness: implications for future missions beyond low Earth orbit. Astrobiology 2021, 21, 541–550. [Google Scholar] [CrossRef]
- McKnight, D.M.; Tate, C.M.; Andrews, E.D.; Niyogi, D.K.; Cozzetto, K.; Welch, K.; Lyons, W.B.; Capone, D.G. Reactivation of a cryptobiotic stream ecosystem in the McMurdo Dry Valleys, Antarctica: a long-term geomorphological experiment. Geomorphology 2007, 89, 186–204. [Google Scholar] [CrossRef]
- McMullen, A.; De Haan, H.W.; Tang, J.X.; Stein, D. Stiff filamentous virus translocations through solid-state nanopores. Nature Communications 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Laidler, J.R.; Shugart, J.A.; Cady, S.L.; Bahjat, K.S.; Stedman, K.M. Reversible inactivation and desiccation tolerance of silicified viruses. Journal of Virology 2013, 87, 13927–13929. [Google Scholar] [CrossRef]
- Lee, S.; Sieradzki, E.T.; Nicol, G.W.; et al. Propagation of viral genomes by replicating ammonia-oxidising archaea during soil nitrification. ISME J 2022. [Google Scholar] [CrossRef]
- Lee, S.; Sieradzki, E.T.; Nicolas, A.M.; Walker, R.L.; Firestone, M.K.; Hazard, C.; Nicol, G.W. Methane-derived carbon flows into host–virus networks at different trophic levels in soil. Proceedings of the National Academy of Sciences 2021, 118, e2105124118. [Google Scholar] [CrossRef]
- Legendre, M.; Bartoli, J.; Shmakova, L.; Jeudy, S.; Labadie, K.; Adrait, A.; Lescot, M.; Poirot, O.; Bertaux, L.; Bruley, C.; Couté, Y. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proceedings of the National Academy of Sciences 2014, 111, 4274–4279. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, F.; Frosch, P. Summarischer bericht uber die ergebnisse der untersuchungen zur erforschung der maul-und klauenseuche. Zentbl. Bakteriol. Parasitenkd Abt. I 1897, 22, 257–259. [Google Scholar]
- Lossouarn, J.; Dupont, S.; Gorlas, A.; Mercier, C.; Bienvenu, N.; Marguet, E.; Forterre, P.; Geslin, C. An abyssal mobilome: viruses, plasmids and vesicles from deep-sea hydrothermal vents. Research in microbiology 2015, 166, 742–752. [Google Scholar] [CrossRef]
- Luria, S.E.; Darnell Jr, J.E.; Baltimore, D.; Campbell, A. Animal virus multiplication: the RNA viruses. In General virology; 3rd ed. John Wiley & Sons, Inc.; New York, 1978; pp. 317–327.
- Mahmoudabadi, G.; Milo, R.; Phillips, R. Energetic cost of building a virus. Proceedings of the National Academy of Sciences 2017, 114, E4324–E4333. [Google Scholar] [CrossRef]
- Márquez, L.M.; Redman, R.S.; Rodriguez, R.J.; Roossinck, M.J. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. science 2007, 315, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, V.; Soru, S.; Cao, G. Extremophile Microalgae: the potential for biotechnological application. Journal of phycology 2020, 56, 559–573. [Google Scholar] [CrossRef]
- Matula, E.E.; Nabity, J.A. Failure modes, causes, and effects of algal photobioreactors used to control a spacecraft environment. Life sciences in space research 2019, 20, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Matula, E.E.; Nabity, J.A.; McKnight, D.M. Supporting Simultaneous Air Revitalization and Thermal Control in a Crewed Habitat With Temperate Chlorella vulgaris and Eurythermic Antarctic Chlorophyta. Frontiers in Microbiology 2021, 2348. [Google Scholar] [CrossRef]
- Matula, E.E.; Nabity, J.A. Effects of stepwise changes in dissolved carbon dioxide concentrations on metabolic activity in Chlorella for spaceflight applications. Life Sciences in Space Research 2021, 29, 73–84. [Google Scholar] [CrossRef]
- Mayali, X.; Weber, P.K.; Nuccio, E.; Lietard, J.; Somoza, M.; Blazewicz, S.J.; Pett-Ridge, J. 2019. Chip-SIP: stable isotope probing analyzed with rRNA-targeted microarrays and NanoSIMS. In Stable Isotope Probing (pp. 71-87). Humana, New York, NY.
- Mayali, X. 2020. NanoSIMS: microscale quantification of biogeochemical activity with large-scale impacts. Annual Review of Marine Science, 12.
- Mehta, S.K.; Cohrs, R.J.; Forghani, B.; Zerbe, G.; Gilden, D.H.; Pierson, D.L. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. Journal of medical virology 2004, 72, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the extremes: extremophiles and the limits of life in a planetary context. Frontiers in microbiology 2019, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; et al. . Report of the Joint Workshop on Induced Special Regions. Life Sciences in Space Research 2019, 23, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Wink, L.; Kögler, I.; Mahnert, A.; Rettberg, P.; Schwendner, P.; Demets, R.; Cockell, C.; Alekhova, T.; Klingl, A.; Krause, R.; Zolotariof, A.; Alexandrova, A.; Moissl-Eichinger, C. Space Station conditions are selective but do not alter microbial characteristics relevant to human health. Nat Commun 2019, 10, 3990. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, Y.; Kumagae, K.; Mori, K.; Tashiro, K.; Nakamura, A.; Fujino, Y.; Hiromasa, Y.; Iwamoto, T.; Kuhara, S.; Khshima, T.; et al. Physiological properties and genome structure of the hyperthermophilic filamentous phage ϕOH3 which infects thermus thermophilus HB8. Front. Microbiol. 2016, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Nuccio, E.E.; Blazewicz, S.J.; Lafler, M.; Campbell, A.N.; Kakouridis, A.; Kimbrel, J.A.; Wollard, J.; Vyshenska, D.; Riley, R.; Tomatsu, A.; Hestrin, R. HT-SIP: a semi-automated stable isotope probing pipeline identifies cross-kingdom interactions in the hyphosphere of arbuscular mycorrhizal fungi. Microbiome. 2022, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, L.; Ankarloo, J.; Andersson, P.O.; Nicholls, I.A. Filamentous bacteriophage stability in non-aqueous media. Chemistry & Biology. 2001 Jul 1;8(7):661-71. Chemistry & Biology 2001, 8, 661-71. [Google Scholar]
- Parikka, K.J.; Le Romancer, M.; Wauters, N.; Jacquet, S. Deciphering the virus-to-prokaryote ratio (VPR): insights into virus–host relationships in a variety of ecosystems. Biological reviews 2017, 92, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Pavletić, B.; Runzheimer, K.; Siems, K.; Koch, S.; Cortesão, M.; Ramos-Nascimento, A.; Moeller, R. Spaceflight Virology: What Do We Know about Viral Threats in the Spaceflight Environment? Astrobiology 2022, 22, 210–224. [Google Scholar] [CrossRef]
- Morono, Y.; Ito, M.; Hoshino, T.; Terada, T.; Hori, T.; Ikehara, M.; D’Hondt, S.; Inagaki, F. Aerobic microbial life persists in oxic marine sediment as old as 101.5 million years. Nature communications 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Mushegian, A.R. Are there 1031 virus particles on earth, or more, or fewer? Journal of bacteriology 2020, 202, e00052-20. [Google Scholar] [CrossRef] [PubMed]
- Pasulka, A.L.; Thamatrakoln, K.; Kopf, S.H.; Guan, Y.; Poulos, B.; Moradian, A.; Sweredoski, M.J.; Hess, S.; Sullivan, M.B.; Bidle, K.D.; Orphan, V.J. Interrogating marine virus-host interactions and elemental transfer with BONCAT and nanoSIMS-based methods. Environmental microbiology 2018, 20, 671–692. [Google Scholar] [CrossRef] [PubMed]
- Pelusi, A.; De Luca, P.; Manfellotto, F.; Thamatrakoln, K.; Bidle, K.D.; Montresor, M. Virus-induced spore formation as a defense mechanism in marine diatoms. New Phytologist 2021, 229, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Pett-Ridge, J.; Weber, P.K. 2022. NanoSIP: NanoSIMS applications for microbial biology. In Microbial systems biology (pp. 91-136). Humana, New York, NY.Pike, W.T.; Staufer, U.; Hecht, M.H.; Goetz, W.; Parrat, D.; Sykulska-Lawrence, H.; Vijendran, S.; Madsen, M.B. Quantification of the dry history of the Martian soil inferred from in situ microscopy. Geophysical Research Letters 2011, 38. [Google Scholar]
- Own, C.S.; Martinez, J.; Cushing, J.; DeRego, T.; Own, L.S.; Weppelman, G.; Thomas-Keprta, K.T.; Rahman, Z.; Pettit, D.R. ; 2018, July. Portable Electron Microscopy for ISS and Beyond. In ISSR&D Conference 2018 (No. JSC-E-DAA-TN57264).
- Pacton, M.; Wacey, D.; Corinaldesi, C.; Tangherlini, M.; Kilburn, M.R.; Gorin, G.E.; Danovaro, R.; Vasconcelos, C. Viruses as new agents of organomineralization in the geological record. Nature communications 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paez-Espino, D.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Thomas, A.D.; Huntemann, M.; Mikhailova, N.; Rubin, E.; Ivanova, N.N.; Kyrpides, N.C. Uncovering Earth’s virome. Nature 2016, 536, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Poughon, L.; Laroche, C.; Creuly, C.; Dussap, C.G.; Paille, C.; Lasseur, C.; Monsieurs, P.; Heylen, W.; Coninx, I.; Mastroleo, F.; Leys, N. Limnospira indica PCC8005 growth in photobioreactor: model and simulation of the ISS and ground experiments. Life sciences in space research 2020, 25, 53–65. [Google Scholar] [CrossRef]
- Rastelli, E.; Corinaldesi, C.; Dell'Anno, A.; Tangherlini, M.; Martorelli, E.; Ingrassia, M.; Chiocci, F.L.; Lo Martire, M.; Danovaro, R. High potential for temperate viruses to drive carbon cycling in chemoautotrophy-dominated shallow-water hydrothermal vents. Environmental microbiology 2017, 19, 4432–4446. [Google Scholar] [CrossRef]
- Reche, I.; D’Orta, G.; Mladenov, N.; Winget, D.M.; Suttle, C.A. Deposition rates of viruses and bacteria above the atmospheric boundary layer. The ISME journal 2018, 12, 1154–1162. [Google Scholar] [CrossRef]
- Ricci, C.; Caprioli, M.; Santo, N. Feeding and anhydrobiosis in bdelloid rotifers: A preparatory study for an experiment aboard the International Space Station. Invertebrate Biology 2004, 123, 283–288. [Google Scholar] [CrossRef]
- Ricciardi, A.; Cassey, P.; Leuko, S.; Woolnough, A.P. Planetary Biosecurity: Applying Invasion Science to Prevent Biological Contamination from Space Travel. BioScience 2022, 72, 247–253. [Google Scholar] [CrossRef]
- Rooney, B.V.; Crucian, B.E.; Pierson, D.L.; Laudenslager, M.L.; Mehta, S.K. Herpes virus reactivation in astronauts during spaceflight and its application on earth. Frontiers in microbiology 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Roosinck, M.J. The good viruses: viral mutualistic symbioses. Nat Rev Microbiol 2011, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Roossinck, M.J. Plant virus metagenomics: biodiversity and ecology. Annual review of genetics 2012, 46, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009, 9, 313–23. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Adriaenssens, E.M.; Dutilh, B.E.; Koonin, E.V.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Lavigne, R.; Brister, J.R.; Varsani, A.; Amid, C. Minimum information about an uncultivated virus genome (MIUViG). Nature biotechnology 2019, 37, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Chan, L.K.; Egan, R.; Malmstrom, R.R.; McMahon, K.D.; Sullivan, M.B. Ecogenomics of virophages and their giant virus hosts assessed through time series metagenomics. Nat. Commun. 2017, 8, 858. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Galipienso, L.; Ferriol, I. Detection of Plant Viruses and Disease Management: Relevance of Genetic Diversity and Evolution. Frontiers in Plant Science 11. [CrossRef]
- Rummel, J.D.; Beaty, D.W.; Jones, M.A.; Bakermans, C.; Barlow, N.G.; Boston, P.J.; et al. A new analysis of Mars "Special Regions": Findings of the Second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 2014, 14, 887–968. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.K.; Gendron, E.M.S.; Vincent, K.; Solon, A.J.; Sommers, P.; Schubert, Z.R.; Vimercati, L.; Porazinska, D.L.; Darcy, J.L.; Sowell, P. Life at extreme elevations on Atacama volcanoes: the closest thing to Mars on Earth? Antonie van Leeuwenhoek 2018, 111, 1389–1401. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Vimercati, L.; Darcy, J.L.; Arán, P.; Gendron, E.M.; Solon, A.J.; Porazinska, D.; Dorador, C. A Naganishia in high places: functioning populations or dormant cells from the atmosphere? Mycology 2017, 8, 153–163. [Google Scholar] [CrossRef]
- Schulz, F.; Roux, S.; Paez-Espino, D.; Jungbluth, S.; Walsh, D.A.; et al. Giant virus diversity and host interactions through global metagenomics. Nature 2020, 578, 432–36. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Curtis, P.D. The Impacts of Microgravity on Bacterial Metabolism. Life 2022, 12, 774. [Google Scholar] [CrossRef]
- Shatilovich, A.V.; Tchesunov, A.V.; Neretina, T.V.; Grabarnik, I.P.; Gubin, S.V.; Vishnivetskaya, T.A.; Onstott, T.C.; Rivkina, E.M. ; 2018, May. Viable nematodes from late Pleistocene permafrost of the Kolyma river lowland. In Doklady Biological Sciences (Vol. 480, No. 1, pp. 100–102). Pleiades Publishing.
- Shmakova, L.; Malavin, S.; Iakovenko, N.; Vishnivetskaya, T.; Shain, D.; Plewka, M.; Rivkina, E. A living bdelloid rotifer from 24,000-year-old Arctic permafrost. Current Biology 2021, 31, R712–R713. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Wood, J.M.; Karouia, F.; Venkateswaran, K. Succession and persistence of microbial communities and antimicrobial resistance genes associated with International Space Station environmental surfaces. Microbiome 2018, 6, 1–23. [Google Scholar]
- Smith, C. Chemosynthesis in the deep-sea: life without the sun. Biogeosciences Discussions 2012, 9, 17037–17052. [Google Scholar]
- Sommers, P.; Chatterjee, A.; Varsani, A.; Trubl, G. Integrating viral metagenomics into an ecological framework. Annual Review of Virology 2021, 8, 133–1588. [Google Scholar] [CrossRef] [PubMed]
- Sommers, P.; Fontenele, R.S.; Kringen, T.; Kraberger, S.; Porazinska, D.L.; Darcy, J.L.; Schmidt, S.K.; Varsani, A. Single-stranded DNA viruses in antarctic cryoconite holes. Viruses 2019, 11, 1022. [Google Scholar] [CrossRef]
- Starr, E.P.; Shi, S.; Blazewicz, S.J.; Koch, B.J.; Probst, A.J.; Hungate, B.A.; Pett-Ridge, J.; Firestone, M.K.; Banfield, J.F. Stable-isotope-informed, genome-resolved metagenomics uncovers potential cross-kingdom interactions in rhizosphere soil. Msphere 2021, 6, e00085-21. [Google Scholar] [CrossRef] [PubMed]
- Stowe, R.P.; Pierson, D.L.; Feeback, D.L.; Barrett, A.D. Stress-induced reactivation of Epstein-Barr virus in astronauts. Neuroimmunomodulation 2000, 8, 51–58. [Google Scholar] [CrossRef]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Interaction between viruses and clays in static and dynamic batch systems. Environmental science & technology 2010, 44, 4539–4544. [Google Scholar]
- Tapia, P.; Flores, F.M.; Covarrubias, P.C.; Acuña, L.G.; Holmes, D.S.; Quatrini, R. ; 2012. Complete Genome Sequence of Temperate Bacteriophage Aca ML1 from the Extreme Acidophile Acidithiobacillus caldus ATCC 5 1756. [CrossRef]
- Thomas, E.; Anderson, R.E.; Li, V.; Rogan, L.J.; Huber, J.A. Diverse viruses in deep-sea hydrothermal vent fluids have restricted dispersal across ocean basins. Msystems 2021, 6, e00068-21. [Google Scholar] [CrossRef] [PubMed]
- Travers Cook, T.J.; Skirgaila, C.; Martin, O.Y.; Buser, C.C. Infection by dsRNA viruses is associated with enhanced sporulation efficiency in Saccharomyces cerevisiae. Ecology and Evolution 2022, 12, e8558. [Google Scholar] [CrossRef] [PubMed]
- Trubl, G.; Jang, H.B.; Roux, S.; Emerson, J.B.; Solonenko, N.; Vik, D.R.; Solden, L.; Ellenbogen, J.; Runyon, A.T.; Bolduc, B.; Woodcroft, B.J. Soil viruses are underexplored players in ecosystem carbon processing. MSystems 2018, 3, e00076-18. [Google Scholar] [CrossRef] [PubMed]
- Trubl, G.; Kimbrel, J.A.; Liquet-Gonzalez, J.; Nuccio, E.E.; Weber, P.K.; Pett-Ridge, J.; Jansson, J.K.; Waldrop, M.P.; Blazewicz, S.J. Active virus-host interactions at sub-freezing temperatures in Arctic peat soil. Microbiome 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Urbaniak, C.; Morrison, M.D.; Thissen, J.B.; Karouia, F.; Smith, D.J.; Mehta, S.; Jaing, C.; Venkateswaran, K. Microbial Tracking-2, a metagenomics analysis of bacteria and fungi onboard the International Space Station. Microbiome 2022, 10, 1–19. [Google Scholar] [CrossRef]
- Urbaniak, C.; Sielaff, A.C.; Frey, K.G.; Allen, J.E.; Singh, N.; Jaing, C.; Wheeler, K.; Venkateswaran, K. Detection of antimicrobial resistance genes associated with the International Space Station environmental surfaces. Scientific reports 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Van Goethem, M.W.; Swenson, T.L.; Trubl, G.; Roux, S.; Northen, T.R. Characteristics of wetting-induced bacteriophage blooms in biological soil crust. MBio 2019, 10, e02287-19. [Google Scholar] [CrossRef]
- Vignuzzi, M.; López, C.B. Defective viral genomes are key drivers of the virus–host interaction. Nature microbiology 2019, 4, 1075–1087. [Google Scholar] [CrossRef]
- Vimercati, L.; Hamsher, S.; Schubert, Z.; Schmidt, S.K. Growth of high-elevation Cryptococcus sp. during extreme freeze–thaw cycles. Extremophiles 2016, 20, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Voorhies, A.A.; Mark Ott, C.; Mehta, S.; Pierson, D.L.; Crucian, B.E.; Feiveson, A.; Oubre, C.M.; Torralba, M.; Moncera, K.; Zhang, Y.; Zurek, E. Study of the impact of long-duration space missions at the International Space Station on the astronaut microbiome. Scientific reports 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Vroom, M.M.; Rodriguez-Ocasio, Y.; Lynch, J.B. Modeled microgravity alters lipopolysaccharide and outer membrane vesicle production of the beneficial symbiont Vibrio fischeri. npj Microgravity 7, 8 (2021). [CrossRef]
- Wagner, A.O.; Malin, C.; Knapp, B.A.; Illmer, P. Removal of free extracellular DNA from environmental samples by ethidium monoazide and propidium monoazide. Applied and Environmental Microbiology 2008, 74, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Wells, L.E.; Deming, J.W. Modelled and measured dynamics of viruses in Arctic winter sea-ice brines. Environmental Microbiology 2006, 8, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Wełnicz, W.; Grohme, M.A.; Kaczmarek, Ł.; Schill, R.O.; Frohme, M. Anhydrobiosis in tardigrades—the last decade. Journal of insect physiology 2011, 57, 577–583. [Google Scholar] [CrossRef] [PubMed]
- White III, R.A.; Visscher, P.T.; Burns, B.P. Between a rock and a soft place: the role of viruses in lithification of modern microbial mats. Trends in Microbiology 2021, 29, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.J.; Cary, S.C.; Williamson, K.E.; Helton, R.R.; Bench, S.R.; Winget, D.; Wommack, K.E. 2008. Lysogenic virus–host interactions predominate at deep-sea diffuse-flow hydrothermal vents. Weronika, E.; Łukasz, K. Tardigrades in space research-past and future. Origins of Life and Evolution of Biospheres 2017, 47, 545–553. [Google Scholar]
- Willis, C.R.; Szewczyk, N.J.; Costes, S.V.; Udranszky, I.A.; Reinsch, S.S.; Etheridge, T.; Conley, C.A. Comparative transcriptomics identifies neuronal and metabolic adaptations to hypergravity and microgravity in Caenorhabditis elegans. Iscience 2020, 23, 101734. [Google Scholar] [CrossRef] [PubMed]
- Willson, D.; Rask, J.C.; George, S.C.; deLeon, P.; Bonaccors, R.; Blank, J.; Slocombe, J.; Silburn, K.; Steele, H.; Gargarno, M.; .McKay, C.P. The performance of field scientists undertaking observations of early life fossils while in simulated space suit. Acta Astronautica 2014, 93, 193–206. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Zhou, Q.; Wang, R.; Xia, B.; Ma, D.; Luo, K.; Liu, Q. Translocation of rigid rod-shaped virus through various solid-state nanopores. Analytical chemistry 2016, 88, 2502–2510. [Google Scholar] [CrossRef]
- Wu, R.; Davison, M.R.; Gao, Y.; Nicora, C.D.; Mcdermott, J.E.; Burnum-Johnson, K.E.; Hofmockel, K.S.; Jansson, J.K. Moisture modulates soil reservoirs of active DNA and RNA viruses. Communications biology 2021, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.; Lauro, F.M.; DeMaere, M.Z.; Brown, M.V.; Thomas, T.; et al. 2011. Virophage control of antarctic algal host-virus dynamics. PNAS 108:6163–68Yin, J.; Redovich, J. Kinetic modeling of virus growth in cells. Microbiology and Molecular Biology Reviews 2018, 82, e00066-17. [Google Scholar]
- Yu, Z.C.; Chen, X.L.; Shen, Q.T.; Zhao, D.L.; Tang, B.L.; Su, H.N.; Wu, Z.Y.; Qin, Q.L.; Xie, B.B.; Zhang, X.Y.; Yu, Y. Filamentous phages prevalent in Pseudoalteromonas spp. confer properties advantageous to host survival in Arctic sea ice. The ISME journal 2015, 9, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Zea, L.; Prasad, N.; Levy, S.E.; Stodieck, L.; Jones, A.; Shrestha, S.; et al. A Molecular Genetic Basis Explaining Altered Bacterial Behavior in Space. PLoS ONE 2016, 11, e0164359. [Google Scholar] [CrossRef]
- Zea, L.; Nisar, Z.; Rubin, P.; Cortesão, M.; Luo, J.; McBride, S.A.; Moeller, R.; Klaus, D.; Müller, D.; Varanasi, K.K.; Muecklich, F. Design of a spaceflight biofilm experiment. Acta astronautica 2018, 148, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hung, T.; Song, J.; He, J. Electron microscopy: essentials for viral structure, morphogenesis and rapid diagnosis. Science China Life Sciences 2013, 56, 421–430. [Google Scholar] [CrossRef]
- Zhong, Z.P.; Rapp, J.Z.; Wainaina, J.M.; Solonenko, N.E.; Maughan, H.; Carpenter, S.D.; Cooper, Z.S.; Jang, H.B.; Bolduc, B.; Deming, J.W.; Sullivan, M.B. Viral ecogenomics of arctic cryopeg brine and sea ice. Msystems 2020, 5, e00246-20. [Google Scholar] [CrossRef]
- Zhou, K.; Li, L.; Tan, Z.; Zlotnick, A.; Jacobson, S.C. Characterization of hepatitis B virus capsids by resistive-pulse sensing. Journal of the American Chemical Society 2011, 133, 1618–1621. [Google Scholar] [CrossRef]
Table 1.
Comparison of Cellular and Non-Cellular Entities – Cells possess all four significant genetic and structural materials. In the case of viruses and subviral elements, only reduced suites of these key molecules are present. Since non-cellular entities can possess either DNA or non-DNA genomes, the search for DNA, RNA or lipid alone would fail to detect some of these entities.
Table 1.
Comparison of Cellular and Non-Cellular Entities – Cells possess all four significant genetic and structural materials. In the case of viruses and subviral elements, only reduced suites of these key molecules are present. Since non-cellular entities can possess either DNA or non-DNA genomes, the search for DNA, RNA or lipid alone would fail to detect some of these entities.
Table 2.
How can we detect viruses? Of the currently known methods of detecting the presence of viruses, those suitable for astrobiology applications are challenging to imagine. This is an area of technology in need of exploration and development.
Table 2.
How can we detect viruses? Of the currently known methods of detecting the presence of viruses, those suitable for astrobiology applications are challenging to imagine. This is an area of technology in need of exploration and development.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).