1. Introduction
The avocado is a highly nutritious and tasty fruit, characteristics that have led to a high global demand for this fruit [
1,
2]. The market for avocado processing has increased significantly in recent years and its value is expected to continue to increase beyond 2024 with new products entering the market such as guacamole, frozen slices, sauces, purees, canned avocados, dried avocados, and avocado oil [
2]. Increasing evidence of the health benefits of avocado is leading to increased consumption and stimulating research [
3,
4,
5,
6], not only on the potential health benefits, but also on all other factors that may influence the external quality of the product in the final market. To extend the shelf life of fresh fruit after harvest, cold storage is the first issue, whether in combination with controlled atmosphere or not. During storage, postharvest losses occur due to mechanical damage, diseases and physiological disorders [
7]. Important structural and metabolic components of plant/fruit cells include fatty acids and lipids. Changes in the lipid composition of the membrane frequently have detrimental effects on the cell’s capacity to adapt to high temperatures and other stressful situations, which in fruit can result in a variety of physiological storage diseases [
7].
Plant organ senescence, both naturally occurring and brought on by stress, has long been linked to loss of membrane function caused by lipid catabolism and peroxidation. The suggested se-nescence cascade of phospholipid catabolism involves two major enzymes: phospholipase D (PLD) and lipoxygenase (LOX), the former of which starts the process and the latter of which produces hydroperoxides from free di- and tri-enoic fatty acids. Both PLD and LOX are now known to comprise vast gene families that play a crucial role in responses to biotic and abiotic stress via synthesis of lipid messengers and defense chemicals, aside from their damaging effects on membrane structure. Redistribution of phytosterols between the free and conjugated pools of sterols is another, less researched modification in membrane structure related to stress responses in plants [
8].
Plant polyphenols are a sizable class of specialized plant metabolites that can be further separated into a number of sizable subclasses of important phyto-antioxidants, such as flavonoids and hydroxycinnamates. In other fruits like the tomato, it has already been noted that polyphenols tend to concentrate more in the fruit skin than in the pericarp because they play a part in how plants respond to stress [
9]. In the post-genomic era, metabolite analysis, also known as metabolomics, is a crucial aspect of systems biology. All of a plant’s main metabolites are polar substances. They include sugars, amino acids, organic acids, and a broad variety of other chemistries. In plant metabolism-related research initiatives, quantitative study of such compounds is frequently necessary [
10]. Understanding how fatty acids, polar metabolites, and phenolics alter during storage is essential for resolving the postharvest storage issues that the Hass avocado currently faces. This study looked at changes in fatty acids, polar metabolites, and phenolics in Hass avocado fruits that had been stored under two distinct settings.
2. Materials and Methods
2.1. Sampling and Storage Conditions
Based on a prior pilot study that used ten orchards from various agro-climatic zones, two orchards were chosen [
5]. Hass avocado fruit (400 per orchard) were gathered throughout the course of two harvests (2018/2019 and 2019/2020), which were characterized as early 23–26% dry matter and late >27-30% dry matter. Fruit samples were collected and delivered to the lab facilities. Fruit was numbered before storage, and four small batches of 50 fruits each were randomly marked. The other 200 fruits were kept in regular air (RA) at 5 °C for 30 days while the other 200 were kept in controlled atmosphere (CA) at 5 °C, 4 kPa O
2, and 6 kPa CO
2 for 55 days (simulating export travel conditions to Asia) (simulating the internal market storage). Each harvest resulted in the evaluation of 1600 independent fruits, for a total of 3200 fruits. Small batches (50 fruit) that had been randomly marked and collected for subsequent studies at harvest (day zero), 20, 35, and 55 days following storage were used for the CA storage condition. Samples were taken under RA storage conditions at harvest (day zero), and after 10, 20, and 30 days of storage. The fruit peel from each batch was removed; promptly freeze dried under liquid nitrogen, and kept at -80
0C for later metabolic profiling study and other biochemical measurements.
2.2. Polar Metabolites
Polar metabolites (sugars, amino acids, and organic acids) were measured using the Hatoum [
14] technique, as completely described by Uarrota [
13]. Relative quantities were used to express the results. Briefly, the sample was incubated at 70 °C for 15 min with shaking after being mixed with 50 µL of cold methanol and 20 µL of 2910 ng L
-1 phenyl -D-glucopyranoside (Labnet International Inc., Edison, NJ, USA). Labnet International Inc., Edison, New Jersey, USA, centrifuged the supernatant at 17000 g for 20 minutes before drying it with a stream of nitrogen gas using 100 µL. (Indura, Santiago, Chile). Methoximation and trimethylsilylation processes made up the derivatization. Two GC-MS techniques were used, one for less concentrated chemicals like organic and amino acids and the other for highly concentrated compounds like sugars. 1 µL of sample was injected into the injector for each method, which had injector and interface temperatures of 220 °C and 280 °C, respectively. As a carrier gas, helium (Indura, Santiago, Chile) was employed at a constant flow rate of 1 mL min
-1. At a scanning rate of 2.66 scan cycles per second, mass spectra in the 50–600 m/z region were captured. Temperatures for the quadrupole and MS ion source were 150 °C and 230 °C, respectively. The procedure for more abundant chemicals included an injection with a split ratio of 1:150, and the oven temperature was set to begin at 120 °C (for 1 min), increase to 300 °C at a rate of 10 °C per minute, and then hold for 6 min. The splitless injection mode was utilized for the approach for less abundant compounds, and the oven temperature was set to begin at 50 °C (for 1 min), increase to 310 °C at a rate of 10 °C per minute, and then hold for 13 min. Using Mass Hunter Quantitative software, the chromatographic peaks were deconvolved and identified by comparing retention durations and mass spectra to a custom library of commercial standards and the NIST14 library (Agilent Technologies, Santa Clara, CA, USA). Using the peak area of phenyl β-D-glucopyranoside (as an internal standard), the sample fresh weight, and a quality control (QC) sample representative of all samples, the peak area data were corrected to determine the relative response of each molecule discovered.
2.3. Fatty Acids
Following the earlier O’Fallon [
15] and Uarrota [
16] methodology, fatty acid methyl ester production was carried out. In a nutshell, freeze-dried peel samples were ground in a coffee grinder, and 1g was then introduced to screw-cap culture tubes along with 5.3 ml of methanol and 0.7 ml of 10N KOH in water. To properly penetrate, dissolve, and hydrolyze the sample, the tube was incubated in a 55 °C water bath for 1.5 hours while being vigorously shaken every 20 minutes. 0.58 ml of H
2SO
4 24N in water was added once the mixture had cooled to below room temperature in the cold tap water bath. By inversion, the tube was mixed before being incubated once again in a water bath at 55 °C for 1.5 hours, shaking the tube every 20 minutes. The tube was chilled in a bath of ice-cold tap water following FAME synthesis. The tube was filled with 3 ml of hexane, vortexed for 5 minutes, centrifuged for 5 minutes at 3000 rpm, and the FAME-containing hexane layer was recovered and transferred to GC vials. Prior to GC analysis, the vial was sealed and stored at 20 °C. An automatic injection system-equipped Thermo Focus Gas Chromatograph (GC) was used to identify and measure the fatty acid mixture (AS3000auto-sampler). The following settings/parameters were applied: GC column: Varian CP-FFAP (free fatty acids), 25 m x 0.32 mm x 0.25 m, detector: FID at 280 °C, injection port temperature 250 °C, injection volume 1 μl, split ratio 1:20, column pressure 150 kPa helium. GC program: ramp 7 °C/min to 150 °C for 1 min, ramp 4 °C/min to 250 °C for 20 min, then hold at 250 °C. By comparing the peak regions of fatty acid standards, identification and quantification were performed. Injection errors were then fixed by using an internal standard (tetradecane) in each sample injected. Similarly, calibration curves for the various fatty acids found in avocados that have already been published were developed (oleic 18:1, palmitic 16:0, palmitoleic 16:1, stearic 18:0, linoleic 18:2, linolenic 18:3). Relative quantity was used to express the results.
2.4. Phenolic Contents
With few adjustments, Kosnska’s [
17] colorimetric test with the Folin-Ciocalteu phenol reagent was used to measure the total phenolic content. In a nutshell, a 100 mg ground avocado peel sample was extracted with 80% methanol in a thermostatic shaking water bath at 60 0C for 15 min. The supernatant was then centrifuged at 12000 g for 10 min., filtered through 0.45 µm cellulose filters, and evaporated under N
2 flux. The dried extract was then dissolved in 1 mL pure methanol for further analysis. 240µL of distilled water, 20µL of extract, 20µL of Folin-Ciocalteau reagent 1N, and 20µL of sodium carbonate 5% made up the reaction mixture. The mixture was incubated for 30 minutes at room temperature, shielded from light, and the absorbance at 765 nm was measured. Gallic acid (5–200 µg mL
-1, y=0.0012x+0.002, r2=0.99) was employed as the standard, and phenolics were expressed as µg of gallic acid per gram of dry weight. Triplicate measurements were made.
2.5. Statistical Analysis
Data of polar metabolites was summarized and submitted to multivariate analysis to see the significant variables connected to storage period. Data of fatty acids and phenolic contents were summarized and submitted to analysis of variance and expressed as mean and standard error of the mean. The most effective method for dimension reduction was principal component analysis. R program 4.2.2 was used to do all statistics [
18].
3. Results and Discussion
3.1. Fatty Acids
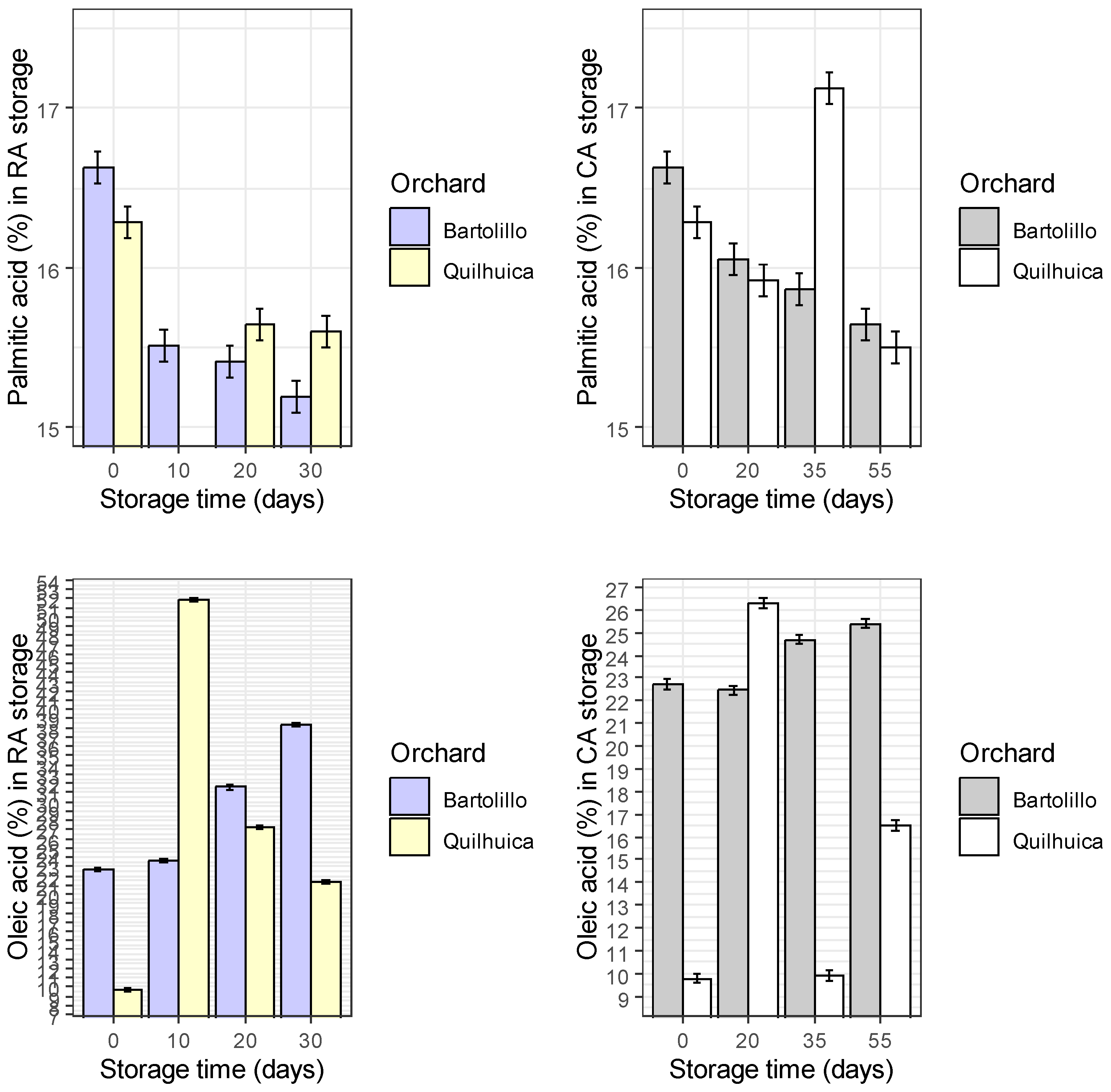
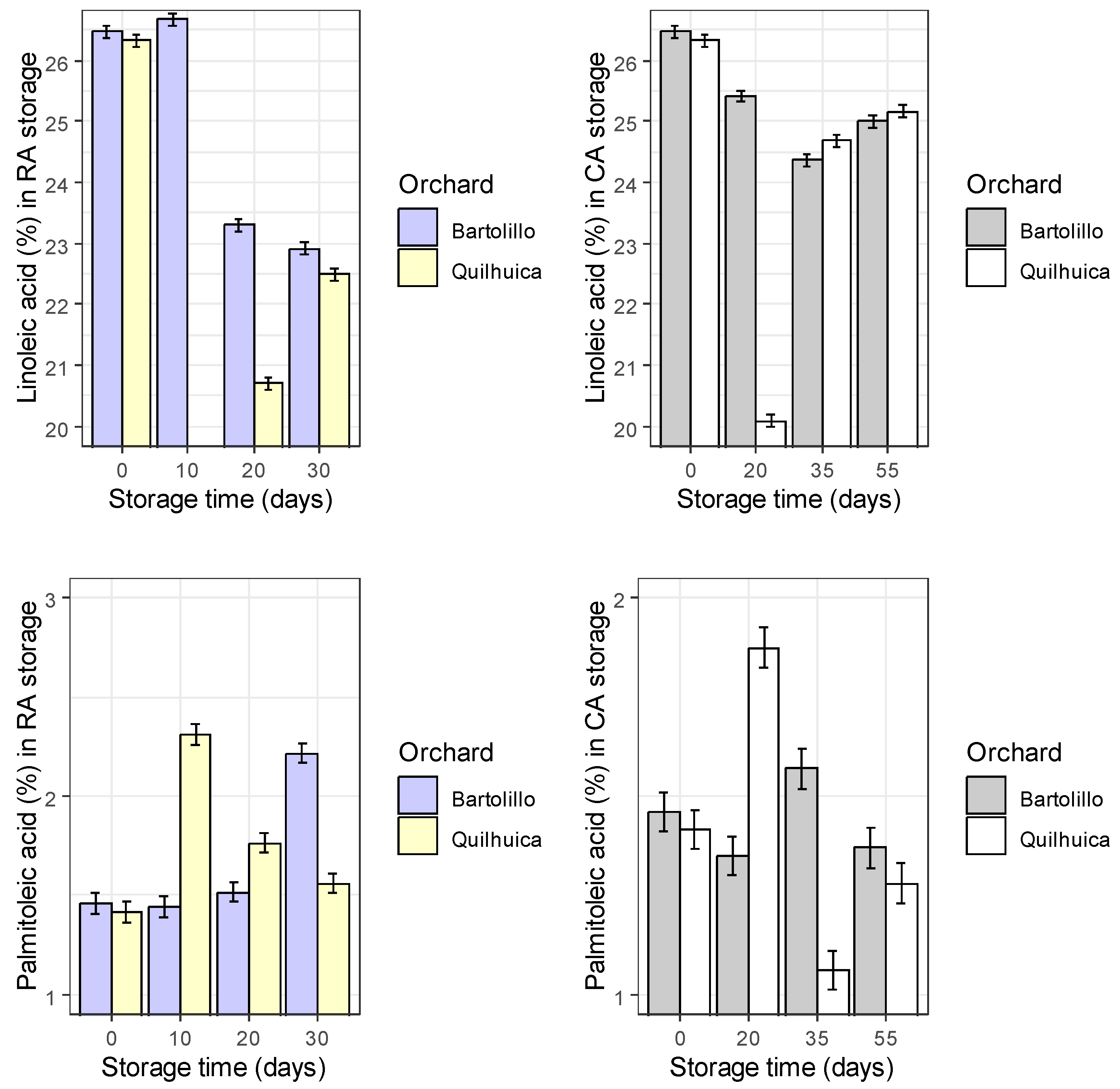
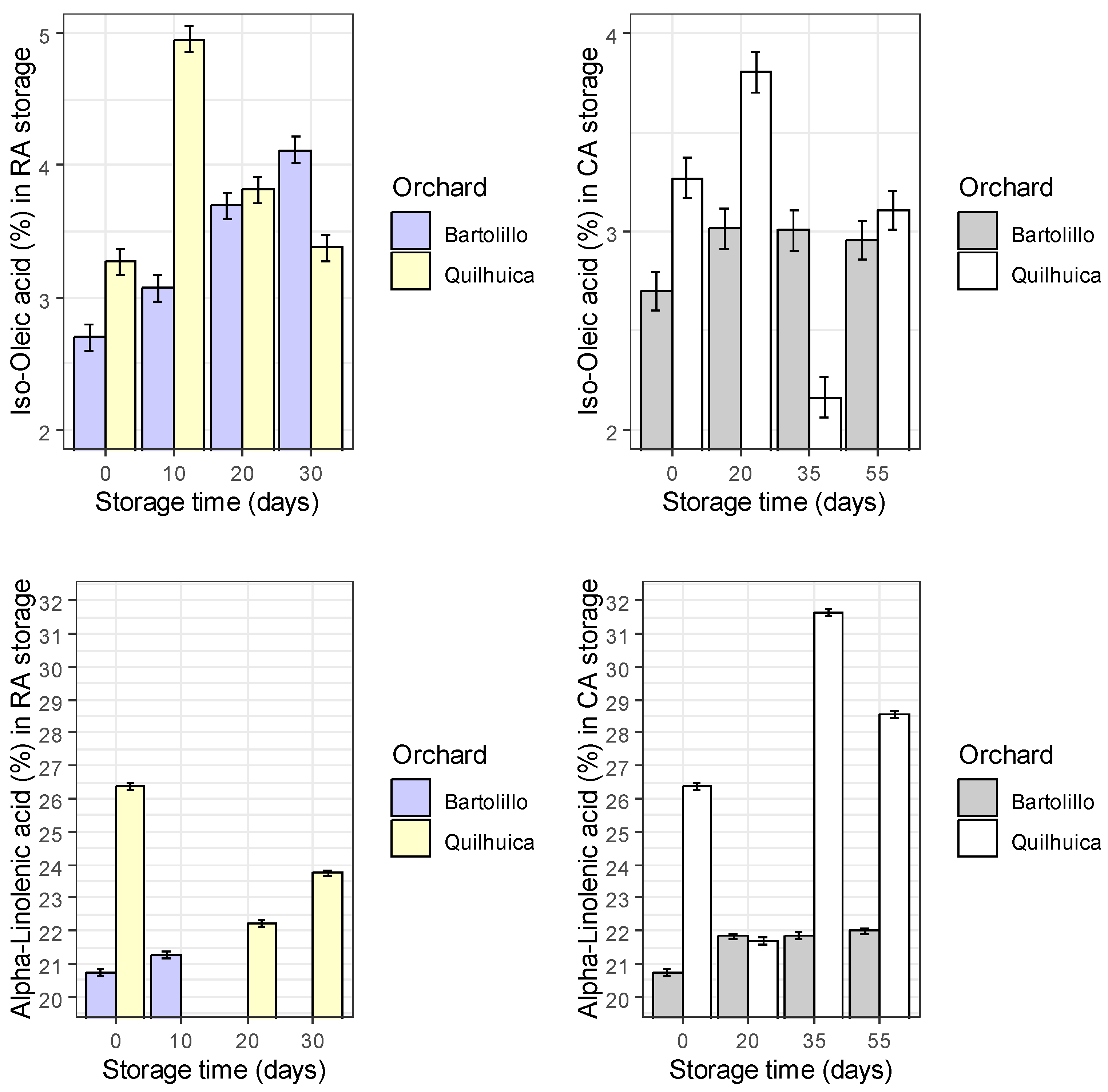
Results of fatty acids are represented in the
Figure 1. Palmitic acid was observed to be higher at harvest and decreased during storage in both conditions and orchards evaluated (regular air –RA and controlled atmosphere—CA), except in CA at day 35 for Quilhuica orchard which higher levels were observed. Oleic acid, increased in both storage conditions for Bartolillo while for Quilhuica orchard, oleic increased in the first days of storage both in RA and CA and the decreased. Linoleic acid in RA storage decreased for Bartolillo while for Quilhuica such decreased was observed only until day 20 and then increased. In CA, a decrease until day 20 and 35 for Quilhuica and Bartolillo respectively was observed and the an increase until last day of storage. Palmitoleic in RA increased for Bartolillo while for Quilhuica increased until day 10 and then decreased. In CA there was not a specific trend in plamitoleic. Iso-Oleic increased for Bartolillo orchard in both storage conditions and for Quilhuica increase was observed until day 10 and 20 in RA and CA storage respectively and then a decrease. Similar trend was observed was alpha-linoleic acid (
Figure 1).
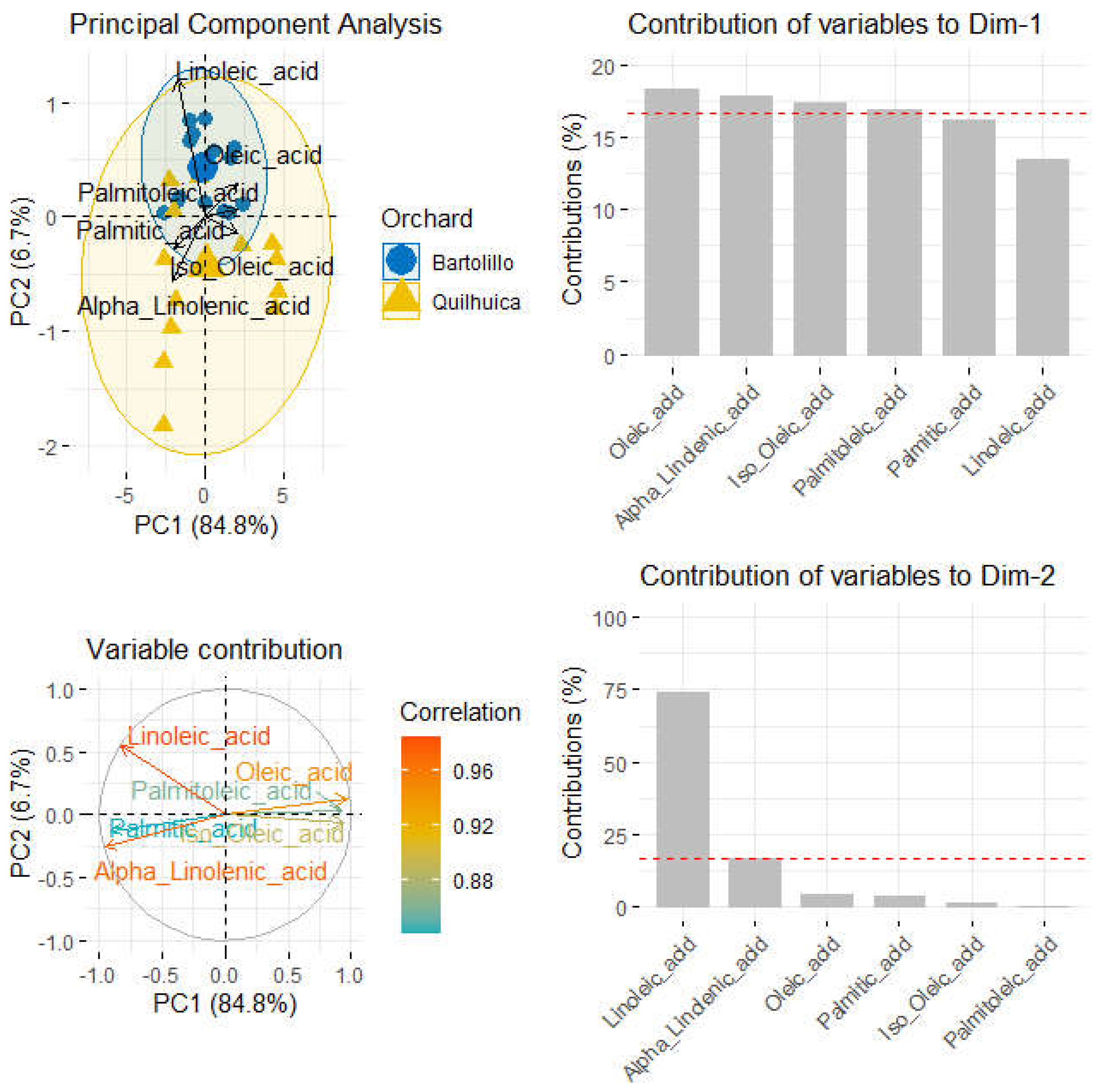
When data of fatty acids was submitted to principal component analysis (PCA), a better separation between the two orchards was observed. The total variance captured by the two first components (PCs) was 91.7%, being 84.8% and 6.7% for first and second component (
Figure 2). Most Bartolillo orchard fruits were mostly correlated to Linoleic and oleic acid as important variables. Most samples were grouped in the first component due to oleic, linolenic, iso-oleic and palmitoleic acid while those in the second component were most influenced by linoleic and alpha-linolenic acid (
Figure 2).
Avocados have fruit oils rich in monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and saturated fatty acids, with 71% MUFA, 13% PUFA, and 16% saturated fatty acids (SFA). The monounsaturated oleic acid increases and the saturated fat decreases as the avocado fruit ripen [
19].
Fruit of Bartolillo orchard stored in RA condition presented increases in palmitoleic and oleic acid while decreases in palmitic, linoleic and linolenic acids were observed. Oleic acid increased during storage, and the authors noted that this fatty acid retains levels of high density lipoproteins and functions as an antioxidant [
20].
Linoleic (C18:2) and linolenic (C18:3) acids were also shown to decrease during the storage periods [
20]. When fruits were stored at 5 °C, Nahed [
21] also saw a drop in linoleic and linolenic acids and an increase in the content of oleic acid. They claimed that the oxidation of unsaturated fatty acids into primary and secondary oxidation products was the principal cause of the decline in (C18:2) and (C18:3). While the decrease in linoleic and linolenic acids were mostly responsible for the rise in oleic acid. Only increases for palmitic and linolenic acid were seen in CA storage.
For Quilhuica, fruit stored in RA presented higher increase in palmitoleic and oleic acids, while in CA an increase was observed for palmitic and oleic acids. High-palmitoleic acid phenotype plants were shown to have a coordinated decrease in fatty acid synthase II activity and an increase in stearoyl-ACP desaturase activity, according to Salas [
22]. According to certain theories, palmitoleic acid may stop beta-cell apoptosis brought on by saturated fats or glucose. It has been asserted that unsaturated fatty acids can withstand biotic challenges like pathogen infection and herbivore wounding as well as abiotic factors like cold, heat, drought, and salt. They serve as components and regulators of cellular membranes in glycerolipids, a store of carbon and energy in triacylglycerol (TAG), stocks of components of the extracellular barrier, precursors of various bioactive molecules, and regulators of stress signaling, to name a few of their roles in stress defense [
23].
3.2. Phenolic Contents
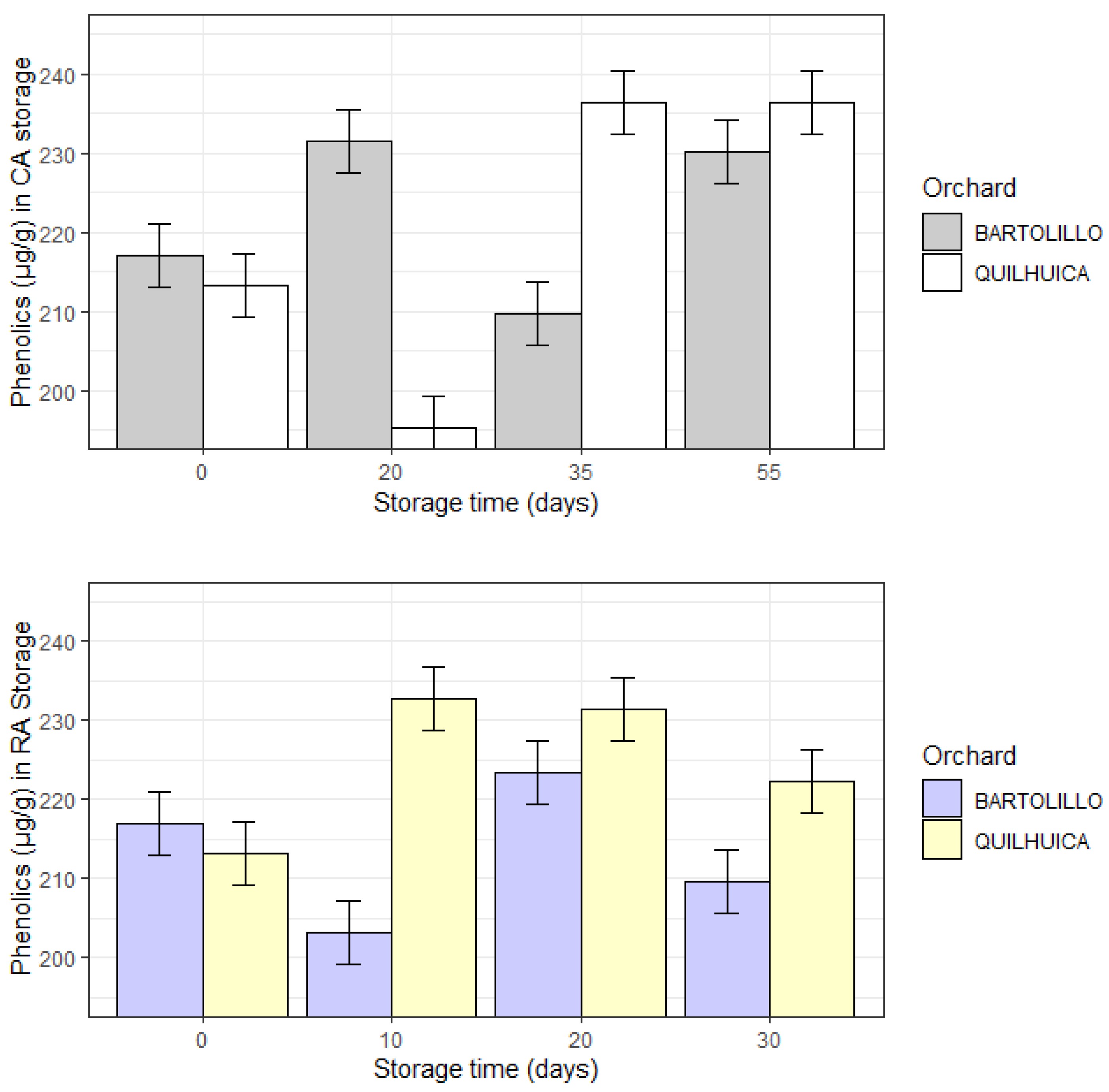
Figure 3 presents results of total phenolic contents during storage. As it can be observed, in CA storage phenolics of Quilhuica orchard decreased until day 20 and then increased, while for Bartolillo there was no typical trend. In RA storage, phenolics increased for Quilhuica while for Bartolillo no trend was observed (
Figure 3).
Fruits are a natural source of antioxidants since fruits’ phenolic chemicals are mostly responsible for their antioxidant activity. Numerous intrinsic factors, like the genus, species, and cultivar, as well as extrinsic factors, including agronomic and environmental factors, handling and storage, affect the phenolic content of food and plants [
24].
Fruit’s phenolic component composition may change depending on the climate and conditions after harvest, such as processing and storage. Processing and storage can cause phenolic compounds to undergo prolonged chemical and enzymatic oxidation, which helps to reduce them [
24]. On the other hand, Villa-Rodriguez [
25] discovered a reported rise in phenolics.
According to reports, the total phenolic content rose up until the sixth day of storage before declining when senescence set in [
24]. The decline was related to a number of chemical and enzymatic modifications that take place throughout the fruit’s rapid maturity phase. Glycoside hydrolysis by glycosidases, phenol oxidation by phenol oxidases, and polymerization of free phenols are some examples of these modifications [
24]. The results of the present study are in accordance with those reported by Golucku and Ozdemir [
26] who reported that total phenolic content increased at the beginning of the harvesting period up to the second harvesting time, and it decreased at the end of the harvesting time (third harvesting time). The phenolic composition of avocado fruits showed significantly differences in terms of the cultivars and their storage condition [
12].
3.3. Polar Metabolites
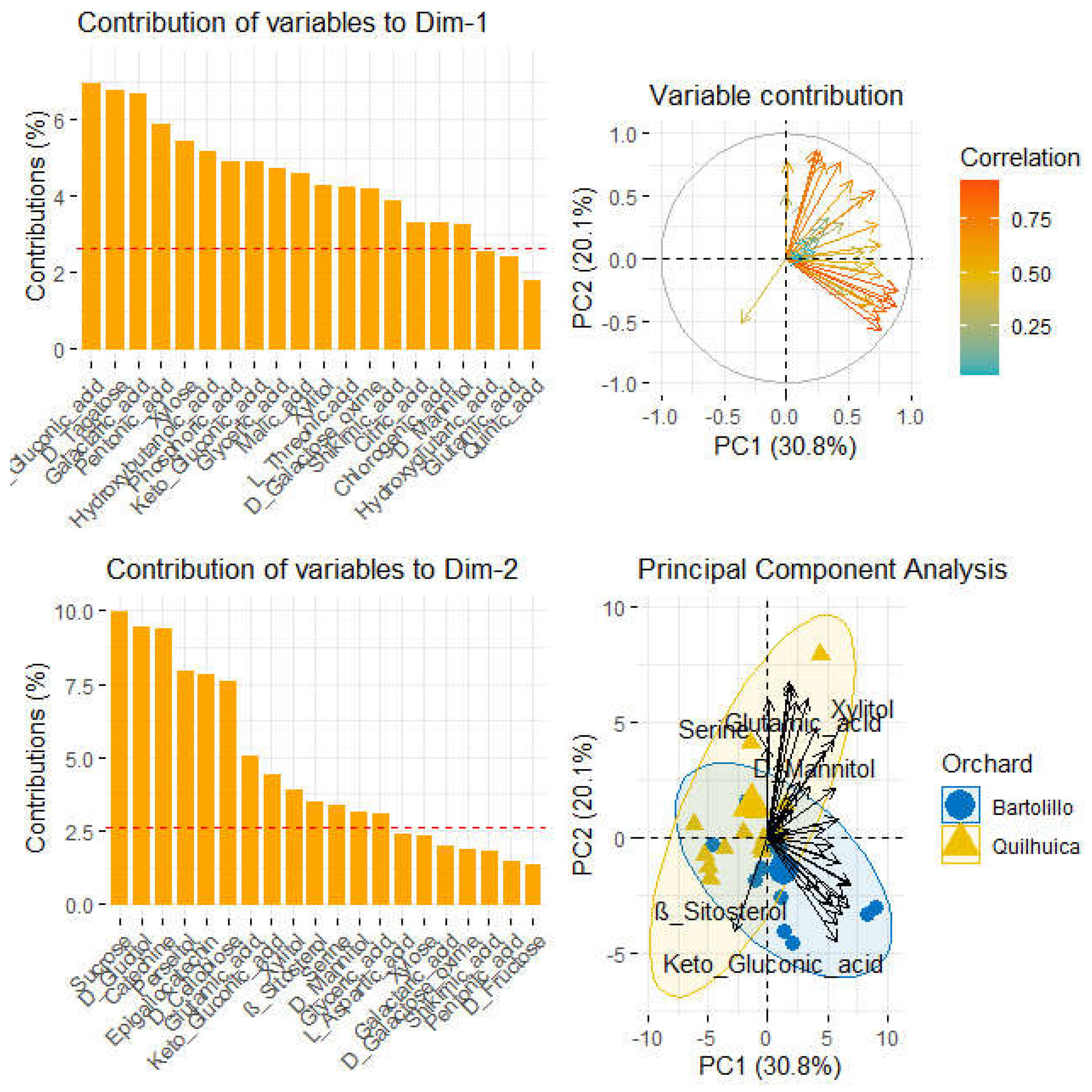
Analyses of polar metabolites (
Figure 4) are also presented. Principal component analysis accounted 50.9% of total variance captured by the two first components, being 30.8% and 20.1% for component 1 (PC1) and component 2 (PC2) respectively. Orchards were also better classified by polar metabolites and component 1. Serine, glutaric acid, xylitol and D-mannitol were the metabolites that most correlated with fruits of Quilhuica orchard. Contrarily, For Bartolillo, ß-sitosterol and gluconic were the compounds related to fruits of Bartolillo orchard. Samples in the first component were most influenced with gluconic, tagatose, pentonic, xylose, hydroxybutanoic acid, glyceric, malic, xylitol,threonic, galactose, shikimic acid, citric and chlorogenic acid. Samples in the second component were influenced by sucrose, glucitol, catechine, perseitol, epigallocatechin, glutamic, gluconic, xylitol, ß-sitosterol, serine and mannitol (
Figure 4).
The scientific field of metabolomics examines the intricate chemical signature of a biological system to determine its state. The term “metabolome” refers to any chemical species created, consumed, or present in the biological system that has a molecular weight between 1000 and 1500 Da. The direct results of gene expression, metabolism, dietary patterns, and environmental exposure are metabolites [
27] Because of the wide range of physicochemical properties that the metabolites have, comprehensive or untargeted metabolomics involves the entire compartment of the metabolites. Polar metabolite chemicals have also been observed to be impacted by variety, climate, and soil type [
28]. In an exploratory research using GC-MS, Hurtado-Fernandez [
29] revealed, for instance, the quantities of glyceric acid in ripening avocados. This study concluded that Serine, glutaric acid, xylitol, D-mannitol, ß-sitosterol and gluconic are highly changed during the storage.
4. Conclusions
According to the orchard and storage conditions, fatty acids differed. Linoleic and oleic acid were mainly connected with most Bartolillo orc-hard fruits as significant variables. Mostly with palmitoleic, palmitic, and oleic acids for Quilhuica. For one orchard, the phenolic content increased at the start of storage and declined at the conclusion, whereas the opposite was true for another, showing that the outcome was depending on the orchard and storage conditions. The polar metabolites that most closely connected with the fruits of the Quilhuica orchard were serine, glutaric acid, xylitol, and D-mannitol, whereas ß-sitosterol and gluconic were related to the fruits of the Bartolillo orchard. The state of the orchard and storage of the fruits determines changes in lipid metabolism, polar metabolites, and phenolics during postharvest storage of Hass avocado.
Author Contributions
V.U.: Conceptualization, Methodology, Software, Data curation, Writing—Original draft preparation.
Funding
This research was fully funded by ANID-FONDECYT (Chile) postdoctoral project 3190055.
Data Availability Statement
Datasets presented in this study are available to the user on request.
Acknowledgments
Virgilio Uarrota thanks Pontificia Universidad Católica de Valparaíso for providing the research facilities and associated avocado producers and exporters for providing the fruit during the project. The authors also acknowledge Sociedad Gardiazabal y Mena Ltd.a and Instituto de Investigaciones Agropecuarias (INIA) La Platina.
Conflicts of Interest
None declared.
References
- Uarrota, V.G.; Pedreschi, R. Mathematical modelling of Hass avocado firmness by using destructive and non-destructive devices at different maturity stages and under two storage conditions. Folia Hortic. 2022, 34, 1–12. [Google Scholar] [CrossRef]
- Ramos-Aguilar, A.; Ornelas-Paz, J.; Tapia-Vargas, L.; Gardea-Béjar, A.; Yahia, E.; Ornelas-Paz, J.; et al. Comparative study on the phytochemical and nutrient composition of ripe fruit of Hass and Hass type avocado cultivars. J. Food Com-Position Anal. 2021, 97, 103796. [Google Scholar] [CrossRef]
- Hernández, I.; Uarrota, V.; Paredes, D.; Fuentealba, C.; Defilippi, B.; Campos-Vargas, R.; et al. Can metabolites at harvest be used as physiological markers for modelling the softening behaviour of Chilean “Hass” avocados destined to local and distant markets? Postharvest Biol. Technol. 2021, 174, 111457. [Google Scholar] [CrossRef]
- Lindh, V.; Uarrota, V.; Zulueta, C.; Alvaro, J.; Valdenegro, M.; Cuneo, I.; Mery, D.; Pedreschi, R. Image Analysis Reveals That Lenticel Damage Does Not Result in Black Spot Development but Enhances Dehydration in Persea americana Mill. cv. Hass during Prolonged Storage. Agronomy 2021, 11, 1699. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Hernandez, I.; Ponce Guequen, E.; Vidal Cruz, J.; Fuentealba, C.; Defilippi, B.; Lindh, V.; Zulueta, C.; Chi-rinos, R.; Campos, D.; Pedreschi, R. Unravelling factors associated with ‘blackspot’ disorder in stored Hass avocado (Persea americana Mill) fruit. J. Hortic. Sci. Biotechnol. 2020, 95, 804–815. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Wang, Y.; Wang, D.; Lee, R.; Gao, K.; et al. California Hass Avocado: Profiling of Carotenoids, Toco-pherol, Fatty Acid, and Fat Content during Maturation and from Different Growing Areas. J. Agric. Food Chem. 2009, 57, 10408–10413. [Google Scholar] [CrossRef]
- Antunes, M.D.; Guimarães, A.C.; Gago, C.; Guerreiro, A.; Panagopoulos, J.; Vilas Boas, E.; Miguel, M.G. Membrane fatty acids and physiological disorders in cold stored ‘golden delicious’ apples treated with 1-MCP and calcium chloride. Horticulturae 2022, 8, 162. [Google Scholar] [CrossRef]
- Whitaker, B.D. Membrane lipid metabolism and oxidative stress involved in postharvest ripening, senescence, and storage disorders of fruits. Acta Hortic. 2012, 945, 269–282. [Google Scholar] [CrossRef]
- Calumpang, C.L.; Saigo, T.; Watanabe, M.; Tohge, T. Cross-species comparison of fruit-metabolomics to elucidate metabolic regulation of Fruit Polyphenolics among solanaceous crops. Metabolites 2020, 10, 209. [Google Scholar] [CrossRef]
- Liu, Z.; Rochfort, S. Recent progress in polar metabolite quantification in plants using liquid chromatography-mass spectrometry. J. Integr. Plant Biol. 2014, 56, 816–825. [Google Scholar] [CrossRef]
- Lindh, V.; Uarrota, V.; Zulueta, C.; Alvaro, J.; Valdenegro, M.; Cuneo, I.; Mery, D.; Pedreschi, R. Image Analysis Reveals That Lenticel Damage Does Not Result in Black Spot Development but Enhances Dehydration in Persea americana Mill. cv. Hass during Prolonged Storage. Agronomy 2021, 11, 1699. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Hernandez, I.; Ponce Guequen, E.; Vidal Cruz, J.; Fuentealba, C.; Defilippi, B.; Lindh, V.; Zulueta, C.; Chi-rinos, R.; Campos, D.; Pedreschi, R. Unravelling factors associated with ‘blackspot’ disorder in stored Hass avocado (Persea americana Mill) fruit. J. Hortic. Sci. Biotechnol. 2020, 95, 804–815. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Fuentealba, C.; Hernández, I.; Defilippi-Bruzzone, B.; Meneses, C.; Campos-Vargas, R.; Pedreschi, R. Integration of proteomics and metabolomics data of early and middle season Hass avocados under heat treatment. Food Chem. 2019, 289, 512–521. [Google Scholar] [CrossRef]
- Hatoum, D.; Annaratone, C.; Hertog, M.; Geeraerd, A.; Nicolai, B. Targeted metabolomics study of ‘Braeburn’ apples during long-term storage. Postharvest Biol. Technol. 2014, 96, 33–41. [Google Scholar] [CrossRef]
- O’Fallon, J.; Busboom, J.; Nelson, M.; Gaskins, C. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Segatto, C.; Voytena, A.; Maraschin, M.; Avila, L.; Kazama, D.; Souza, C.A. Metabolic fingerprinting of water-stressed soybean cultivars by gas chromatography, near-infrared and UV-visible spectroscopy combined with chemometrics. J. Agron. Crop Sci. 2019, 205, 141–156. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G. Phenolic Compound Profiles and Antioxidant Capacity of Persea americana Mill. Peels and Seeds of Two Varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef]
- R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/.
- Dreher, M.; Davenport, A. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Yassin, N.; Shaaban, F.; Eletreby, S. Effect of postharvest thermal treatments on reducing external chilling injury in avocado‘fuerte’cv. Fruits. Egypt. J. Agric. Res. 2017, 95, 167–182. [Google Scholar] [CrossRef]
- Nahed, M.M.; Atta AA, A.; Awatif, I.I. Changes in the orgnoleptic characteristics and quality of olive oil during fruits storage at low temperature. Minufiya J. Agric. Res. 2011, 36, 829–843. [Google Scholar]
- Salas, J.; Martínez-Force, E.; Garcés, R. Biochemical characterization of a high-palmitoleic acid Helianthus annuus mutant. Plant Physiol. Biochem. 2004, 42, 373–381. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef] [PubMed]
- Daiuto, E.R.; Fumes, J.G.F.; Vieites RLCabia, N.C.; Castro, R.S.D. Antioxidant capacity and total phenolic content of hydrothermally-treated ‘Fuerte’ avocado. Adv. Hortic. Sci. 2011, 25, 75–80. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A. , Yahia, E.M., González-León, A., Ifie, I., Robles-Zepeda, R.E., Domínguez-Avila, J.D., González-Aguilar,G.A. Ripening of ‘Hass’ avocado mesocarp alters its phytochemical profile and the in vitro cytotoxic activity of its methanolic extracts. South Afr. J. Bot. 2020, 128, 1–8. [Google Scholar] [CrossRef]
- Golukcu, M. , Ozdemir, F. Changes in phenolic composition of avocado cultivars during harvesting time. Chem Nat Compd 2010, 46, 112–115. [Google Scholar] [CrossRef]
- Drouin, N.; Rudaz, S.; Schappler, J. Sample preparation for polar metabolites in bioanalysis. Anal. 2018, 143, 16–20. [Google Scholar] [CrossRef]
- Nasri, C.; Halabi, Y.; Harhar, H.; Mohammed, F.; Bellaouchou, A.; Guenbour, A.; Tabyaoui, M. Chemical characterization of oil from four Avocado varieties cultivated in Morocco. OCL 2021, 28, 19. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Bajoub, A.; Morales, J.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Exploratory analysis of avocado extracts by GC-MS: New insights into the avocado fruit ripening process. Anal. Methods 2015, 7, 7318–7326. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).