Submitted:
18 January 2023
Posted:
19 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ion-Exschange Membranes
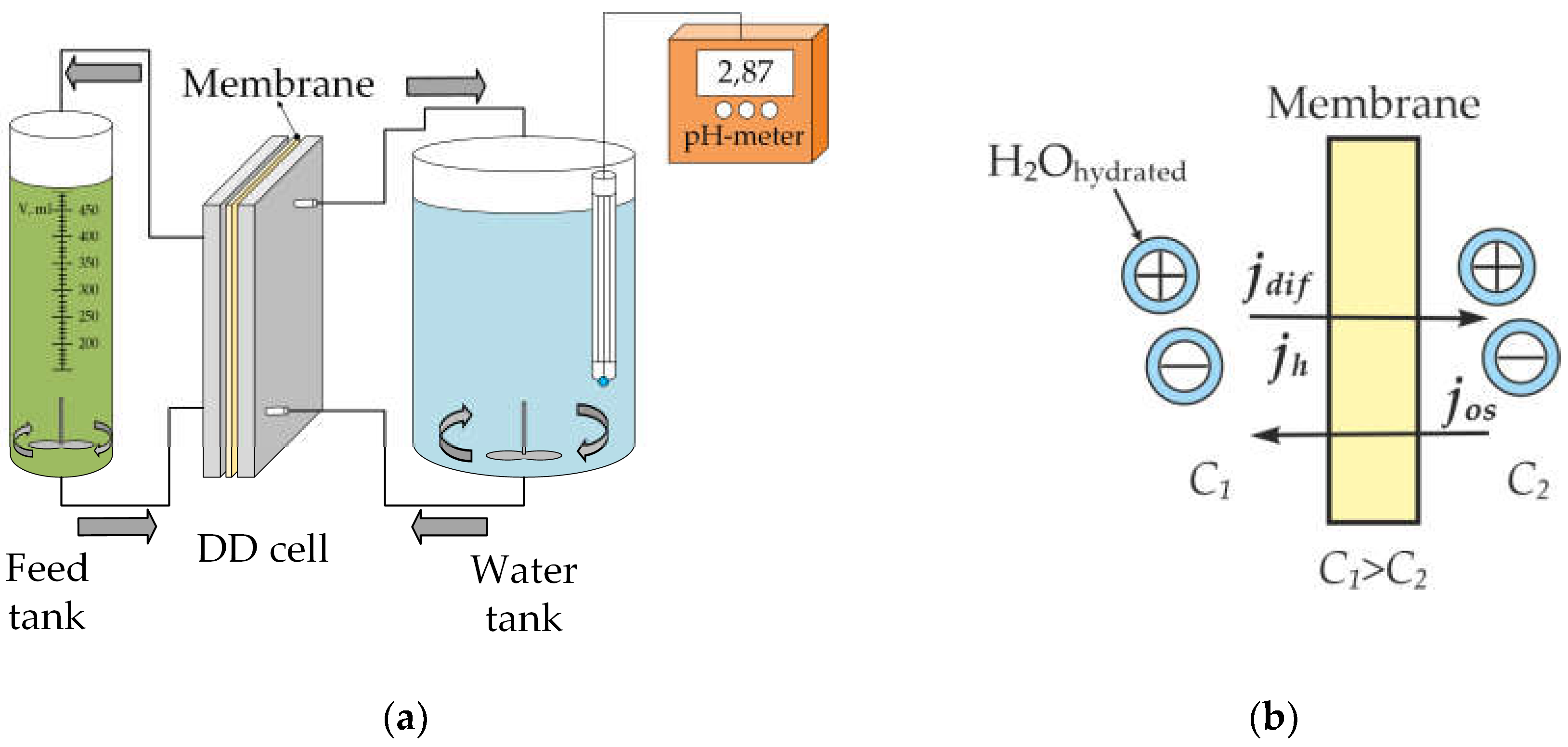
2.2. Method of Dialisys
3. Results
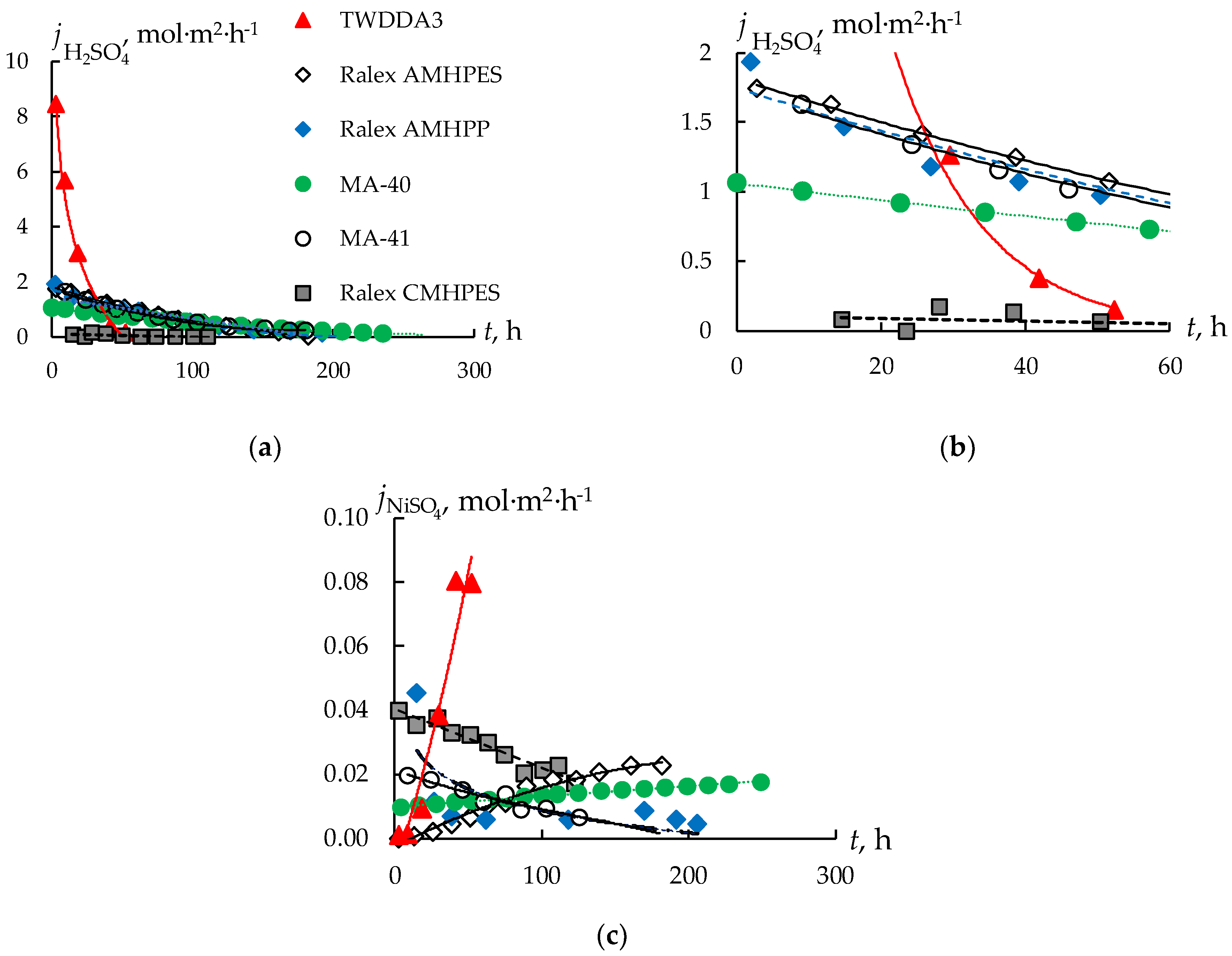
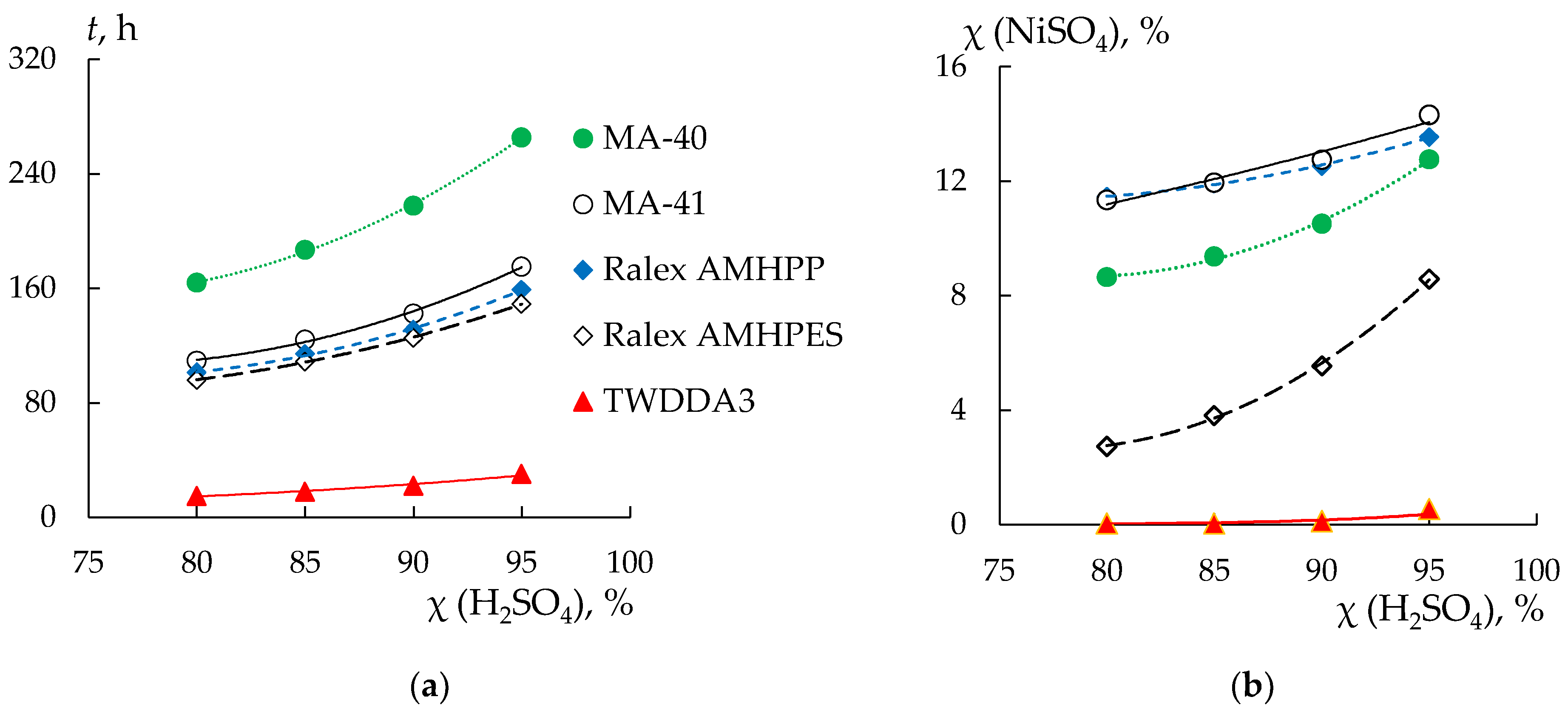
3.1. Sulfuric Acid and Nickel Sulfate Transfer through Membranes
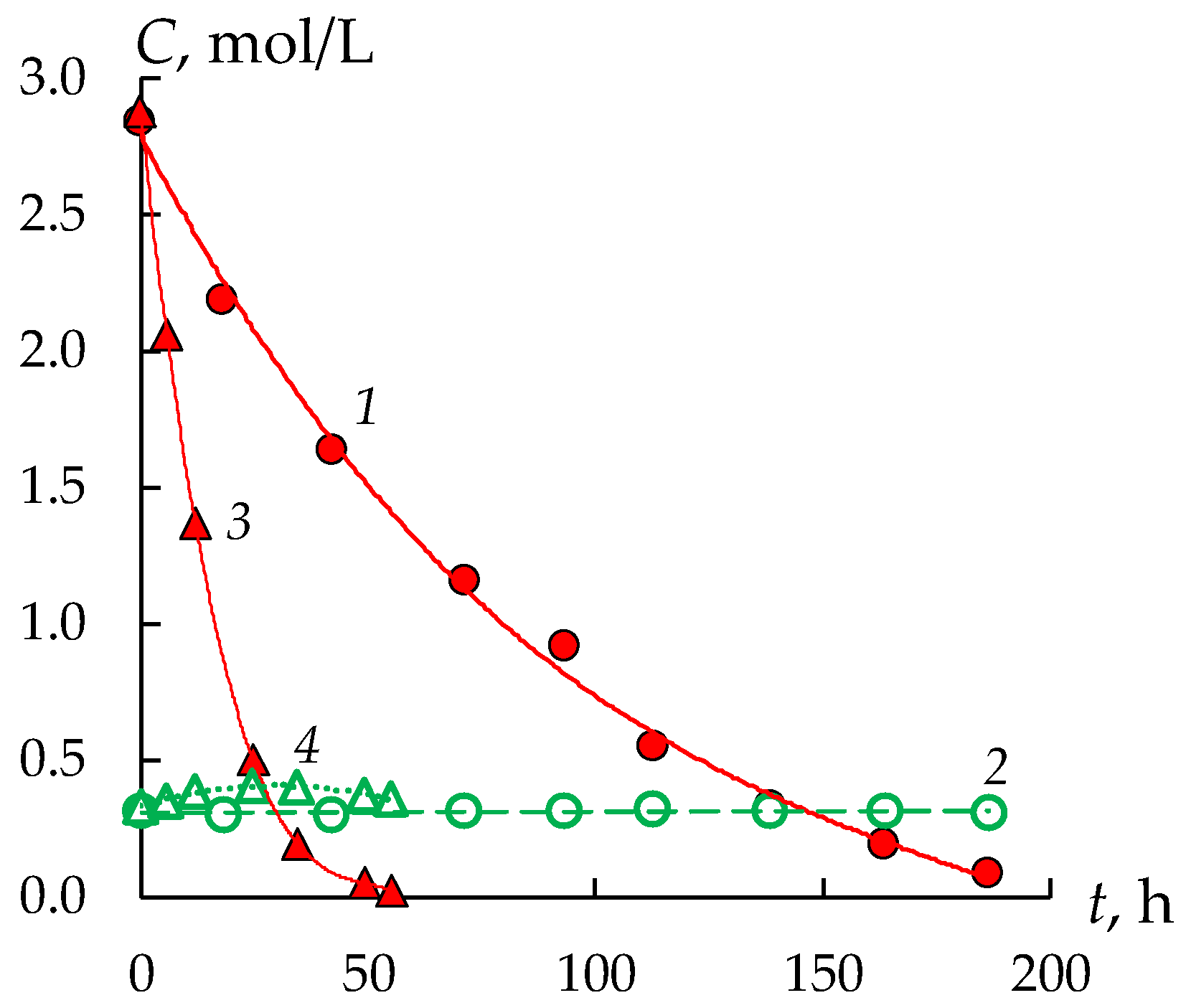
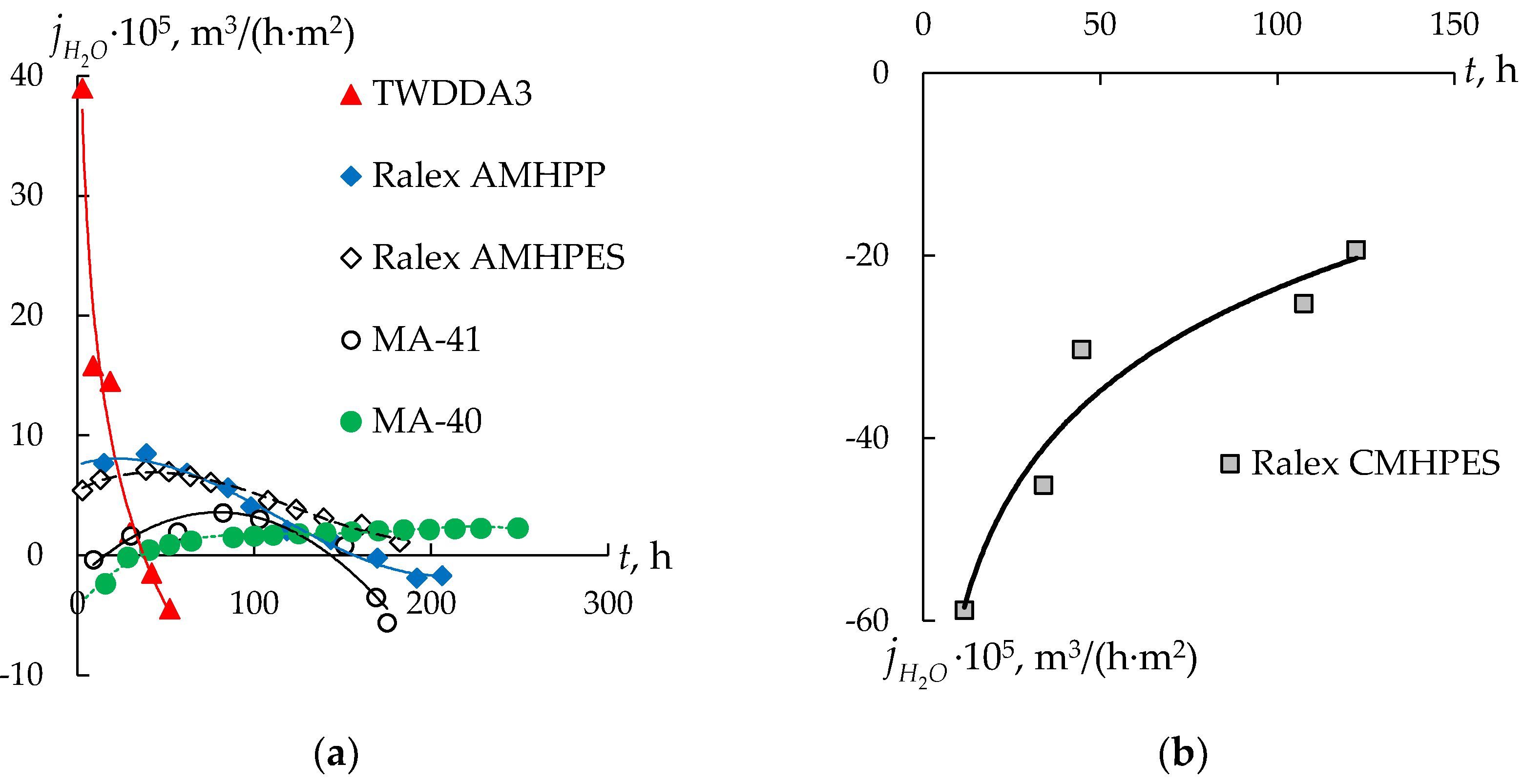
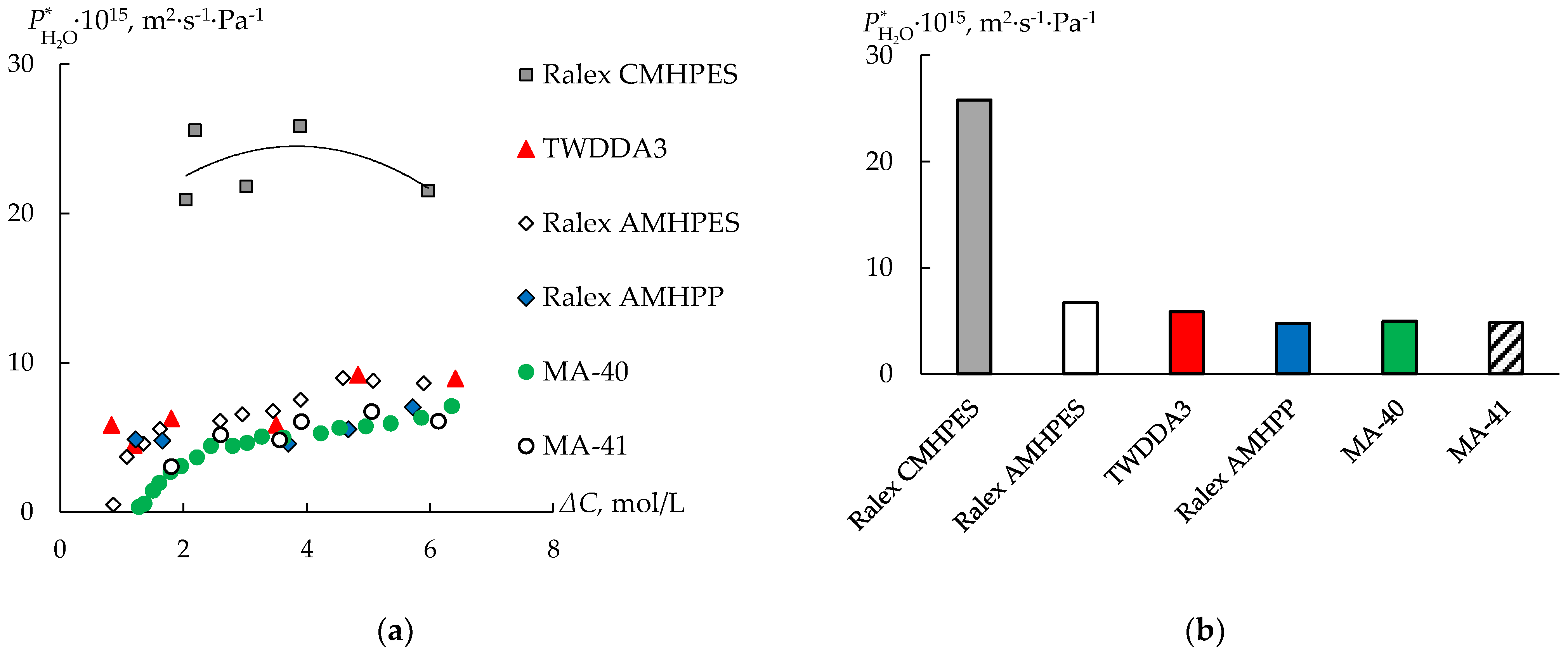
3.2. Water Transport during Diffusion Dialysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koros, W.J.; Ma, Y.H.; Shimidzu, T. Terminology for membranes and membrane processes (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 1479–1489. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Memb. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Vorob’Eva, E.A. Dynamics of the separation of amino acid and mineral salt in the stationary dialysis of solutions with an MK-40 profiled sulfo group cation exchange membrane. Russ. J. Phys. Chem. A 2012, 86, 1726–1731. [Google Scholar] [CrossRef]
- Vasil’eva, V.; Goleva, E.; Pismenskaya, N.; Kozmai, A.; Nikonenko, V. Effect of surface profiling of a cation-exchange membrane on the phenylalanine and NaCl separation performances in diffusion dialysis. Sep. Purif. Technol. 2019, 210, 48–59. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Wang, Y. Diffusion dialysis for acid recovery from acidic waste solutions: Anion exchange membranes and technology integration. Membranes (Basel). 2020, 10, 1–23. [Google Scholar] [CrossRef]
- Sonoc, A.C.; Jeswiet, J.; Murayama, N.; Shibata, J. A study of the application of Donnan dialysis to the recycling of lithium ion batteries. Hydrometallurgy 2018, 175, 133–143. [Google Scholar] [CrossRef]
- Bendova, H.; Weidlich, T. Application of diffusion dialysis in hydrometallurgical separation of nickel from spent Raney Ni catalyst. Sep. Sci. Technol. 2018, 53, 1218–1222. [Google Scholar] [CrossRef]
- Ng, P.K.; Snyder, D. Mass Transport Characterization of Donnan Dialysis: the Nickel Sulfate System. Proc. - Electrochem. Soc. 1981, 81–2, 71–87. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Fu, R.; Fu, R.; Li, R.; Chen, B.; Jiang, C.; Ge, L.; Liu, Z.; Wang, Y.; et al. Ion exchange membranes for acid recovery: Diffusion Dialysis (DD) or Selective Electrodialysis (SED)? Desalination 2022, 531. [Google Scholar] [CrossRef]
- Merkel, A.; Čopák, L.; Dvořák, L.; Golubenko, D.; Šeda, L. Recovery of spent sulphuric acid by diffusion dialysis using a spiral wound module. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Lopez, J.; Gueccia, R.; Randazzo, S.; Cipollina, A.; Cortina, J.L.; Micale, G. Diffusion dialysis for the treatment of H2SO4-CuSO4 solutions from electroplating plants: Ions membrane transport characterization and modelling. Sep. Purif. Technol. 2021, 266, 118215. [Google Scholar] [CrossRef]
- Gueccia, R.; Randazzo, S.; Chillura Martino, D.; Cipollina, A.; Micale, G. Experimental investigation and modeling of diffusion dialysis for HCl recovery from waste pickling solution. J. Environ. Manage. 2019, 235, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Gueccia, R.; Aguirre, A.R.; Randazzo, S.; Cipollina, A.; Micale, G. Diffusion dialysis for separation of hydrochloric acid, iron and zinc ions from highly concentrated pickling solutions. Membranes (Basel). 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Xu, T.; Weihua, Y. Tuning the diffusion dialysis performance by surface cross-linking of PPO anion exchange membranes—simultaneous recovery of sulfuric acid and nickel from electrolysis spent liquor of relatively low acid concentration. J. Hazard. Mater. 2004, 109, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Memb. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Robinson, R.A.; Stokes, R.H. Electrolyte solutions. The Measurement and Interpretation of Conductance, Chemical Potential and Diffusion in Solutions of Simple Electrolytes.; Second edi.; Butterworths Scientific Publications: London, 1959. [CrossRef]
- Pitzer, K.S.; Roy, R.N.; Silvester, L.F. Thermodynamics of Electrolytes. 7. Sulfuric Acid. J. Am. Chem. Soc. 1977, 99, 4930–4936. [Google Scholar] [CrossRef]
- Pitzer, K.S. Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 1972, 77, 268–277. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Mayorga, G. Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 1973, 77, 2300–2308. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Mayorga, G. Thermodynamics of electrolytes. III. Activity and osmotic coefficients for 2-2 electrolytes. J. Solution Chem. 1974, 3, 539–546. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Kim, J.J. Thermodynamics of Electrolytes. IV. Activity and Osmotic Coefficients for Mixed Electrolytes. J. Am. Chem. Soc. 1974, 96, 5701–5707. [Google Scholar] [CrossRef]
- Van Gauwbergen, D.; Baeyens, J.; Creemers, C. Modeling osmotic pressures for aqueous solutions for 2-1 and 2-2 electrolytes. Desalination 1997, 109, 57–65. [Google Scholar] [CrossRef]
- Sergievskii, V. V.; Rudakov, A.M. Dependence of the osmotic coefficients and average ionic activity coefficients on hydrophobic hydration in solutions. Russ. J. Phys. Chem. A 2016, 90, 1567–1573. [Google Scholar] [CrossRef]
- Rudakov, A.M.; Sergievskii, V. V.; Nagovitsyna, O.A. Dependences of the osmotic coefficients of aqueous calcium chloride solutions on concentration at different temperatures. Russ. J. Phys. Chem. A 2017, 91, 2361–2365. [Google Scholar] [CrossRef]
- Adapa, S.; Malani, A. Cation hydration by confined water and framework-atoms have crucial role on thermodynamics of clay swelling. Sci. Rep. 2022, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kiriukhin, M.Y.; Collins, K.D. Dynamic hydration numbers for biologically important ions. 2002, 99, 155–168. [CrossRef]
- Ohtaki, H.; Radnai, T. Structure and Dynamics of Hydrated Ions. Chem. Rev. 1993, 93, 1157–1204. [Google Scholar] [CrossRef]
- Vchirawongkwin, V.; Rode, B.M.; Persson, I. Structure and dynamics of sulfate ion in aqueous solution-an ab initio QMCF MD simulation and large angle X-ray scattering study. J. Phys. Chem. B 2007, 111, 4150–4155. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M. Basic Principles of Membrane Technology; 2nd ed.; Springer Dordrecht: Dordrecht, 1996; ISBN 978-0-7923-4248-9.
- Demina, O.A.; Kononenko, N.A.; Falina, I. V.; Demin, A. V. Theoretical estimation of differential coefficients of ion-exchange membrane diffusion permeability. Colloid J. 2017, 79, 317–327. [Google Scholar] [CrossRef]
- Schlögl, R. Elektrodiffusion in freier Lösung und geladenen Membranen. Zeitschrift fur Phys. Chemie 1889, 1, 305–339. [Google Scholar] [CrossRef]
| Sample No. | Membrane | Ionogenic groups | Reinforcing mesh |
Q, mmol/g | l, μm |
|---|---|---|---|---|---|
| 1 | Ralex AMHPES | quaternary amino groups |
polyester | 1.05 | 550 |
| 2 | Ralex AMHPP | quaternary amino groups |
polypropylene | 0.97 | 550 |
| 3 | Ralex CMHPES | sulfo groups | polyester | 1.16 | 530 |
| 4 | МА-40 | secondary and tertiary amino groups |
nylon | 2.15 | 520 |
| 5 | МА-41 | quaternary amino groups |
nylon | 0.54 | 420 |
| 6 | TWDDA3 | quaternary amino groups |
Polyphenylene oxide |
0.49 | 145 |
| Substance | Concentration, g/L |
|---|---|
| Н2SO4 | 252.3 |
| Ni | 20.9 |
| Zn | 2.3 |
| Fe | 1.08 |
| Cu | 0.90 |
| Sn | 0.080 |
| Sb | 0.050 |
| Cd | 0.045 |
| Pb | 0.015 |
| As | 0.003 |
| χ (H2SO4), % | 80 | 85 | 90 | 95 |
|---|---|---|---|---|
| TWDDA3 | ||||
| H2SO4 | 4.63 | 4.64 | 4.48 | 3.95 |
| NiSO4 | 0.001 | 0.003 | 0.004 | 0.010 |
| MA-41 | ||||
| H2SO4 | 1.02 | 0.96 | 0.88 | 0.77 |
| NiSO4 | 0.016 | 0.016 | 0.015 | 0.010 |
| MA-40 | ||||
| H2SO4 | 0.66 | 0.62 | 0.56 | 0.49 |
| NiSO4 | 0.0003 | 0.001 | 0.002 | 0.003 |
| Ralex AMHPES | ||||
| H2SO4 | 1.12 | 1.06 | 0.98 | 0.87 |
| NiSO4 | 0.001 | 0.003 | 0.004 | 0.007 |
| Ralex AMHPP | ||||
| H2SO4 | 1.08 | 1.03 | 0.94 | 0.82 |
| NiSO4 | 0.012 | 0.011 | 0.010 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





