1. Introduction
Despite advances in periodontal disease (PD) research and treatments, nearly 42% of adults in the United States (US) suffer from PD (Eke et al., 2018). If PD is left untreated, it can lead to tooth loss and poor quality of life (Genco & Borgnakke, 2013). Research has demonstrated that PD can be prevented if the associated risk factors are controlled at the right time (Koshi et al., 2012; Lang et al., 2015; Tonetti et al., 2017). For example, researchers evaluated the long-term effect of risk factors such as smoking, diabetes, age and calculus on PD initiation and progression (Ramseier et al., 2017; Schätzle et al., 2010) (Schatzle et al., 2004; Schätzle et al., 2003, 2009, 2010). While these studies provided meaningful insights most study cohorts were from 1969 to 1988, which may not represent the current patient population (Loe et al., 1986; Needleman et al., 2018; Ramseier et al., 2017). In addition, these studied were conducted on non-US patient population and did not have long follow-up due to loss to follow-up (Ramseier et al., 2017). Observing disease change over time is necessary especially for PD, a chronic disease which progresses slowly.(Worthington et al., 2013). However, it is difficult to conduct such studies because it is expensive, laborious, time-consuming, and difficult to retain patients for a long time (T. P. Thyvalikakath et al., 2020).
The high usage of electronic dental record (EDR) systems to document patient care information provides a huge opportunity to study the clinical course of PD, as well as the influence of risk factors (J. Patel et al., 2017; T Thyvalikakath et al., 2022; Wang et al., 2017). The EDR has many advantages for conducting longitudinal studies. For example, the EDR can provide a longer follow-up study period, provide patients' up-to-date clinical information, and provide real-world evidence (Song et al., 2013; St Sauver et al., 2017; Thankam Thyvalikakath et al., 2018). Despite this promising potential, EDR data has challenges, such as questionable quality, fragmented information documented in different sections of the EDR, and missing information. For example, to study the clinical course of a disease using EDR data, the t0 (no disease state) progressing to t1…tn (disease stage) is critical. However, many patients visit dentists when the disease is already in the active stage. As a result, it is unknown whether EDR data has the potential to provide patients' important t0 information (Cowie et al., 2017).
Several studies have utilized longitudinal electronic health record (EHR) data to predict hospitalization rate and risk of cardiovascular disease in medicine (Zhang et al., 2018) Zhao et al. 2019. Similarly, in dentistry, researchers have utilized automated approaches to compare the completeness of periodontal charting information in four large US dental academic institutes’ EDR data and automated diagnosis (using SQL) (Mullins et al., 2021). The authors also determined new periodontitis cases and tooth loss leveraging EDR data from three of these institutions (Tokede et al., 2022). Another study reported a deep learning model that automated staging and grading of periodontitis (Chang et al., 2020). Yet, to the best of our knowledge, no study has utilized longitudinal EDR data to study PD change and its clinical course over time.
Therefore, our long-term goal is to utilize longitudinal EDR data to examine the clinical course of PD and assess long-term treatment outcomes of surgical and non-surgical periodontal treatments. The objective of this study was to determine the feasibility of utilizing longitudinal EDR data to track patients' PD change over time and generate three patient cohorts 1) patients whose disease did not change over time, 2) patients whose PD progressed, and 3) patients whose disease improved over time. The secondary objective of this study was to develop automated algorithms to track patients’ PD progression overtime. This study determines the quality of the longitudinal EDR to inform the feasibility of using EDR data to study the clinical course of PD. This study also presents a step-by-step process to generate a longitudinal cohort of patients that researchers from other institutes could utilize for their PD related research. Finally, the automated algorithm developed in this study will be made publicly available for other researchers and clinicians’ use.
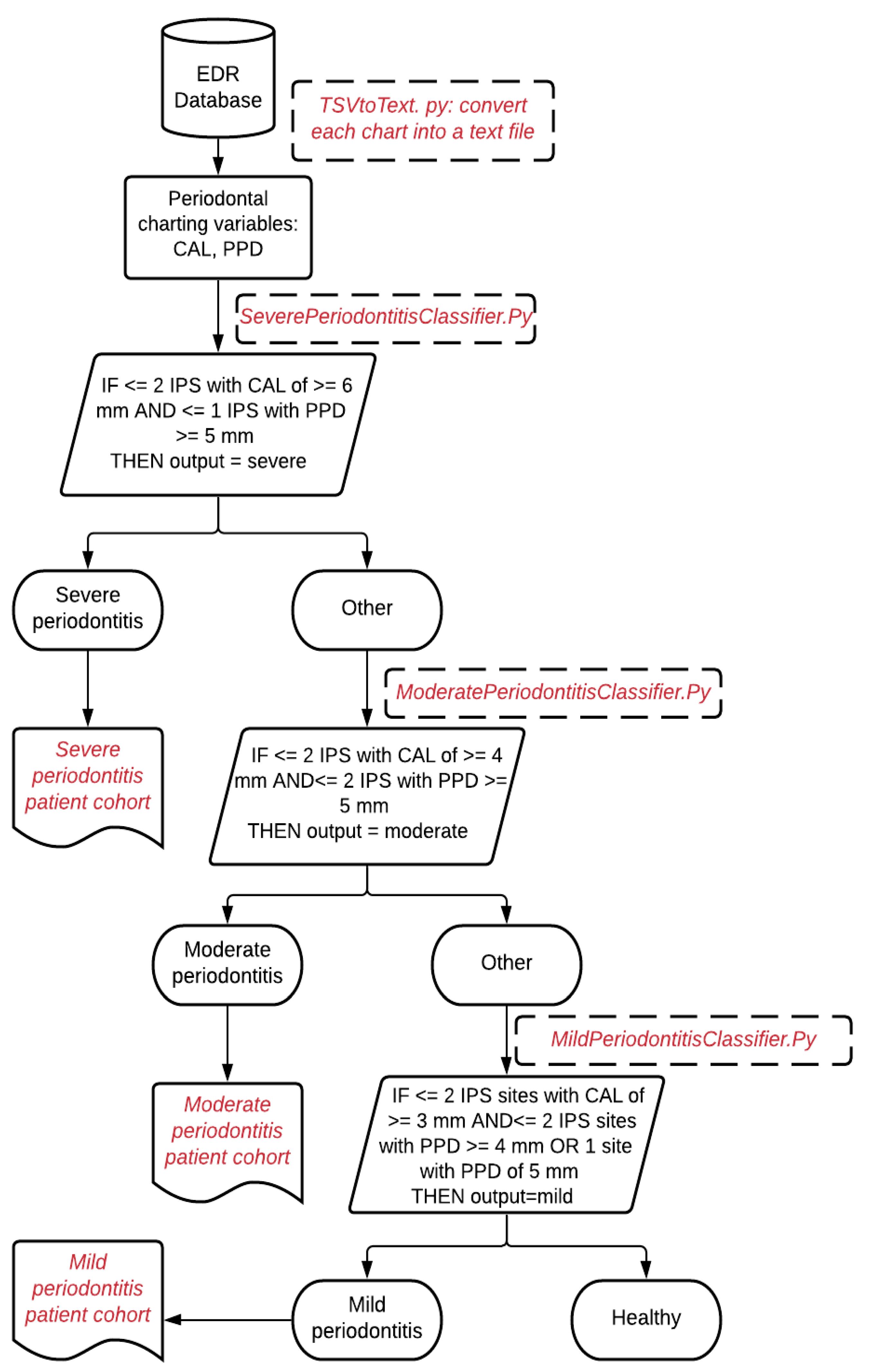
Figure 1.
Illustration of determining a patient's periodontitis diagnosis into healthy, mild, moderate, and severe cases.
Figure 1.
Illustration of determining a patient's periodontitis diagnosis into healthy, mild, moderate, and severe cases.
2. Materials and Methods
2.1. Data source
We utilized EDR (axiUm®, Exan software, Las Vegas, Nevada, USA) data from the Indiana University School of Dentistry (IUSD) predoctoral clinics to conduct this study. The data included periodontal examination findings (charting) through periodontal clinical notes of patients who underwent comprehensive oral examination (COE) between January 1, 2009, and December 31, 2014, and who were 18 years or older during their first completed COE during this period. The patients' visit information that may fall outside this period was also included in this study. For example, if the patient received COE in 2010 and received treatments in 2007 and 2015, information from 2007 and 2015 would also be included.
2.2. Natural language processing (NLP) application (PD Extractor.py) to extract PD diagnoses from periodontal evaluation forms
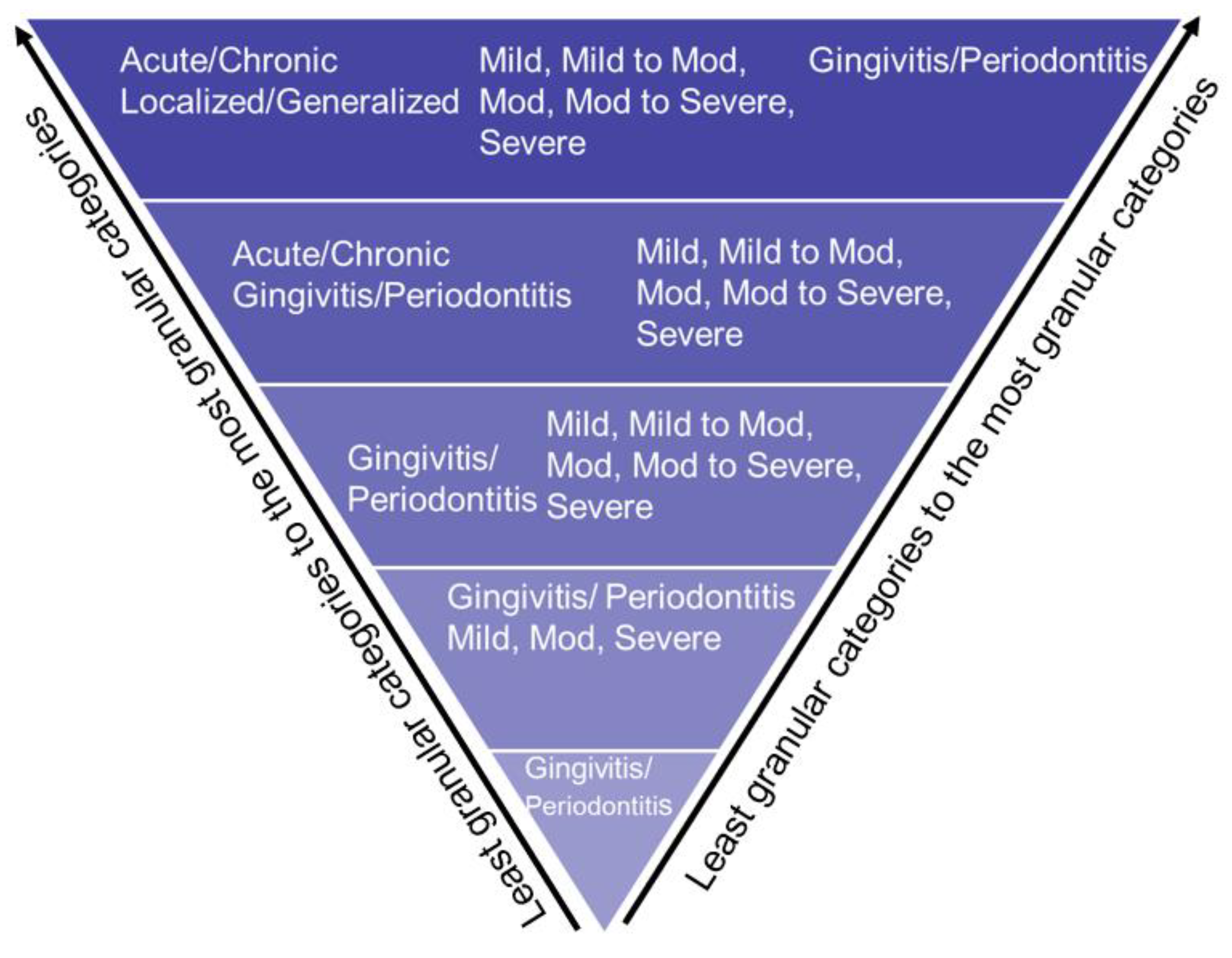
We developed an NLP application PD Extractor.py to retrieve PD diagnosis written as free text in the periodontal evaluation form. Clinicians typically write PD type (gingivitis or periodontitis), severity (mild, mild to moderate, moderate, moderate to severe, and severe), location (maxilla, mandible, tooth number), onset (acute or chronic), and extent (localized or generalized). The PD Extractor.py used the approximate string-matching function (ASM) to retrieve these concepts from the periodontal evaluation form (PEF) and convert each disease concept into a structured format. Details of the NLP application development are described elsewhere (J. S. Patel, 2020). During processing this step, we encountered one limitation.
Not all records contained patients' detailed PD information such as PD type, severity, location, onset, and extent. Therefore, we used the stepwise bottom-up processing approach, as demonstrated in Figure 2, in which the application would consider as maximum information detail. However, if any detailed structured categories are missing, the PD Extractor.py will not throw an error and extract the limited information present in the clinical note. For example, suppose the clinical note contains "mild periodontitis" and does not contain information on the location or extension. In that case, the program will still extract this information in a structured format and leave other categories such as location, onset, and extent empty.
2.4. A computer application (PD Change Classifier.py) that automatically determines PD change overtime
Lastly, we developed a computer application PD Change Classifier.py that examines patients' PD diagnoses information in each consecutive visit and classifies it into one of the following categories:
PD progression: —e.g., from mild gingivitis to mild periodontitis, from mild periodontitis to moderate periodontitis, etc.
No change in disease status: —e.g., from mild gingivitis to mild gingivitis, from mild periodontitis to mild periodontitis, etc.
Disease improvement: —e.g., from moderate periodontitis to mild periodontitis, from severe periodontitis to mild periodontitis, etc.
PD Change Classifier.py application consisted of several Python libraries that include Natural Library Toolkit (NLTK), string, regular expression, and Pandas. By using these libraries, first, the
PD Change Classifier.py read the text file and saved disease type, severity, and disease extent in temporary variables. Next, the classifier created two temporary variables, "From" and "To" and determined the date difference between the two visit dates. If these two dates were different (differences have to be 90 days apart), then the diagnosis from the first date was placed in the "From" temporary variable. Similarly, the diagnosis recorded at the latest date was placed in the "To" temporary variable. Next, it determined if these two dates recorded in the "From" and "To" variables were similar or not. The application skipped these records and went to the next available date if they were identical. If there was no other diagnosis present, it went to the next row (patient ID).
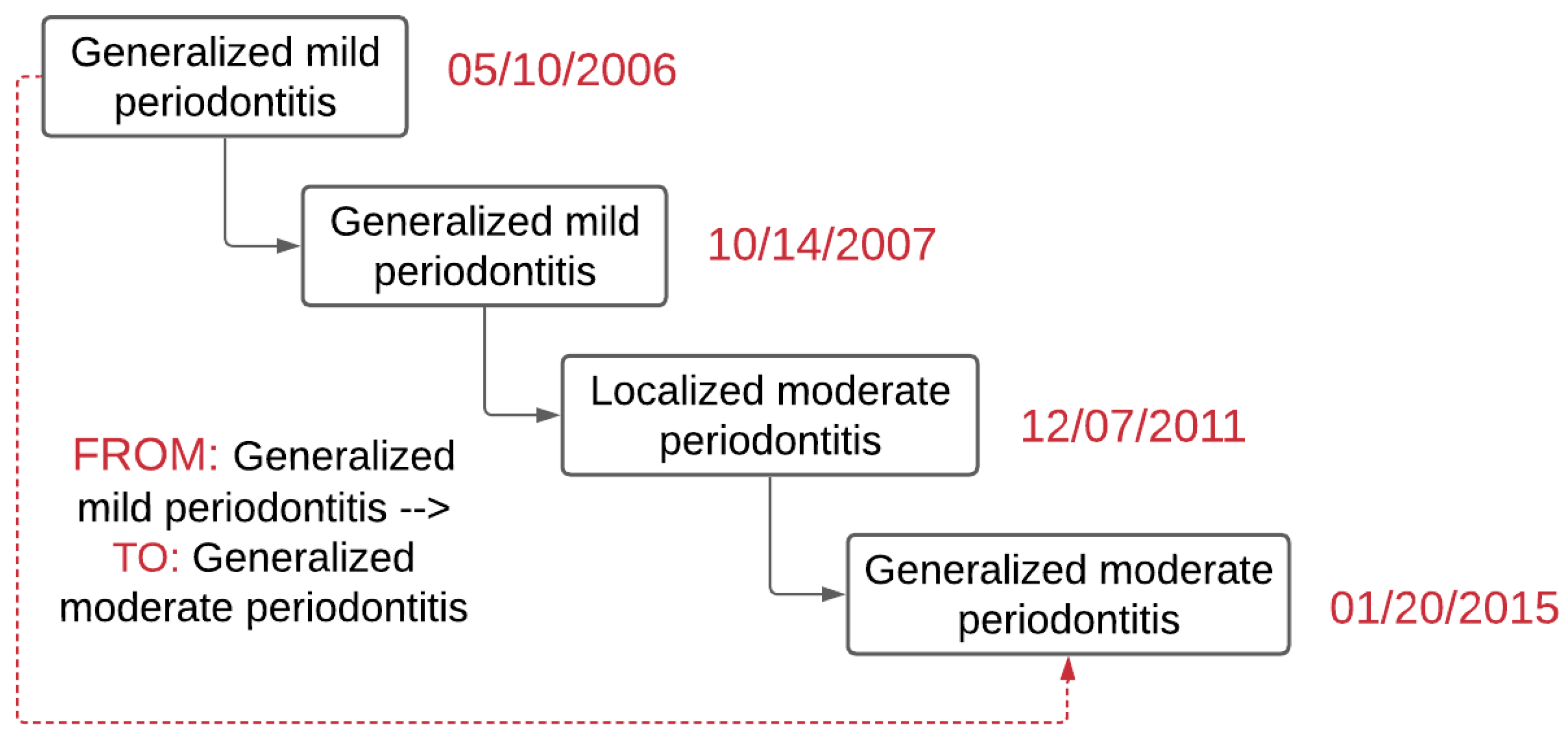
Figure 3 demonstrates an example of the output of the
PD Change Classifier.py.
Figure 2.
Bottom-Up approach to extract PD based on disease type, disease severity, disease location, and disease extension.
Figure 2.
Bottom-Up approach to extract PD based on disease type, disease severity, disease location, and disease extension.
2.5. Evaluate the performance of automated computer applications
Two clinical faculty manually reviewed 250 patients' periodontal charts and diagnosed patients using the PD diagnosis categories reported in a previous US population PD prevalence study (Eke et al., 2018). Similarly, they also reviewed 250 clinical notes containing patients' PD diagnoses. The final inter-rater agreement was one that demonstrated excellent agreement (Cohen’s Kappa=1). These manually reviewed datasets were compared against the computer-generated outputs. Next, a confusion matrix containing true positive (TP), false positive (FP), true negative (TN), and false negative (FN) were created for both algorithms (Van Stralen et al., 2009). Using this confusion matrix, we calculated precision (correctly predicted positive observations to the total predicted positive observations), recall (correctly predicted positive observations to all observations in actual class), and F-1 measure (weighted average of Precision and Recall) to assess performances (Lalkhen & McCluskey, 2008).
2.6. Data Analysis
Descriptive statistics with 95% confidential intervals were performed on the clinician-documented diagnoses between June 1, 2005, to August 1, 2019. The average days, months, and years between patients' first, second, third, and first and fourth visits were calculated. This test helped us identify how frequently patients' clinician-documented diagnoses were available to determine their disease change over time. The frequency count and the number of patients by the observation time between their first and last visits were generated. The frequency counts were generated in the following four follow-up categories: 1) none, 2) up to 5 years, 3) >5 and <=10 years, and 4) >10 and <=15 years. Last, the frequency count of the number of patients whose disease status did not change, disease status progressed, and disease status improved from their first to the last visit using patients' clinician-recorded diagnoses was also generated.
3. Results
3.1. Patient Demographics
The EDR data included 28,908 distinct patients who received at least one COE between January 1, 2009, and December 31, 2014. Fifty-four percent of patients were females with a mean age of 46 years (standard deviation=16.74). Seventy-nine percent (N=22,880) of patients had at least one full-mouth periodontal finding, and 13,219 patients had both clinician-documented diagnoses in the EDR.
3.2. Periodontitis cases automatically classified by Periodontitis_Diagnoser.py and PD Extractor.py
Table 1 demonstrates the automated generated diagnosis using the NLP program from periodontal clinical notes. The clinician-documented diagnoses were available for 13,219 patients (46%). Among these patients, 3,193 patients (24%) were diagnosed with mild gingivitis, 1,607 (12%) with moderate gingivitis, and 143 (1%) with severe gingivitis out of 13,219 available periodontal evaluation forms. Eighteen percent of patients (2,430) were diagnosed with mild periodontitis, 1,899 (14%) with moderate periodontitis, and 554 (4%) with severe periodontitis cases.
3.3. Observation time of longitudinal EDR data
There were 63,552 periodontal charts documented for 22,880 unique patients. The observation time of patients who had at least one periodontal charting dataset ranged from 0 to 15 years. A total of 15,217 (53%) patients out of 28,908 (100%) had no follow-up visits, 9,954 (34%) patients had up to 5 years of observation time, 3,203 (11%) had 5 years to 10 years of observation time, and 534 (2%) patients had 10 years to 15 years of observation time. For the periodontal charting findings, the average visit was 2.78 (median=2, standard deviation=2.9) (see
Table 2).
There were 20,152 clinician-documented diagnoses for 13,219 unique patients. The average documented PD diagnosis was 1.52 (median=1, standard deviation=1) for 13,114 unique patients. We found that 7,657 (58%) of patients had exclusively one clinician-documented PD diagnosis, 3, 197 (24%) had exclusively two diagnoses, 1,052 (8%) had three diagnoses, and 1,313 (10%) patients had 4 to 28 PD diagnoses. There were 5,562 patients who had more than one clinician-documented diagnosis available to determine their disease change between their first and last visits (see
Table 3).
Among the 5,562 patients who had more than two diagnoses available, the average time period between their first and second visit was 0.9 years (approximately 11 months [346 days]) (standard deviation of 584 days); the first and third visit was 1.6 years (approximately 19 months [588 days]) (standard deviation of 709 days); and first and fourth visit was 3 years (approximately 35 months [1,072 days]) (standard deviation of 855 days).
3.4. Number of patients whose periodontal diagnosis changed over time
When considering clinician-documented diagnoses, 72% percent of patients (n=3,919) out of 5,562 (100%) did not have a disease status change between their first and last visits. See Supplementary Table S1 for detailed categories.
We found 669 (13%) patients' disease status progressed between their first and last visit. The top three categories in disease progression include the following:
77 (12%) out of 669 (100%) patients: progression from generalized mild periodontitis to localized moderate periodontitis,
66 (10%): progression from generalized moderate periodontitis to localized severe periodontitis, and
56 (9%): generalized mild periodontitis to generalized moderate periodontitis. See Supplementary Table S2 for detailed categories.
There were 589 (11%) patients out of 5,562 (100%) patients whose disease improved between their first and last visits. The top three categories in disease improvement included:
76 (13%) out of 537 (100%) patients: from generalized moderate periodontitis to generalized mild periodontitis,
32 (5%): generalized mild periodontitis to generalized mild gingivitis, and
30 (5%): generalized mild periodontitis to localized mild periodontitis. See Supplementary Table S3 for detailed categories.
There were 437 (7%) patients out of 5,486 (100%) patients in the unknown category. See Supplementary Table S4 for detailed categories.
3.5. Performance of the automated applications
As demonstrated in the article (J. S. Patel, 2020) we achieved excellent results with 99% precision, 100% recall, and 99.5% F-measure for the Periodontitis_Diagnoser.py and an average of 98% precision, recall, and F-measure of the PD Extractor.py. For the PD Change Classifier.py application, we achieved excellent results with 97% precision, 99% recall, and 98% F-measure.
4. Discussion
This retrospective study demonstrated the feasibility of using longitudinal EDR data to track change in PD diagnosis and determined the quality of the longitudinal EDR data for clinical research. We found 34% of our patients (n=9,954) had up to five years of follow-up visits with an average visit of 2.78 when their periodontal charting information was utilized. We found an average of 3 patient visits per year when periodontal charts (63,552) were utilized to obtain their periodontal diagnosis. Similarly, when clinician-documented diagnoses were considered, we found 42% patients (n=5,562) who had at least two PD diagnoses to determine the disease change. Moreover, we were able to successfully classify the cohort of patients whose disease statuses changed over time. This diagnosis information with patients' other medical history, dental history, and social history would allow us to study the clinical course of PD.
4.1. No disease change group
Our automated application PD Change Classifier.py found seventy-two percent of patients (n=3,919) into the "no disease change" category between their first and last dental appointments. We believe that the patients falling in this category may have had received periodontal treatment, oral prophylaxis, and preventive treatments regularly. As a result, the treatments provided at the right interval would have prevented the disease progression in these patients. Further studies determining treatment outcomes among these patients would allow us to determine the effectiveness of periodontal treatments. It is also interesting to note that most of these patients' disease stages were still mild-moderate periodontitis cases. This provides us some insight: if PD can be diagnosed early, we can retain patients for the long-term in milder PD stages and prevent tooth loss. More studies are essential to examine the effectiveness of periodontal treatments in mild-moderate periodontitis cases. One other reason for “no disease change” could be because patient visits were clustered close to their initial COE date. For example, if the patient had ten years of follow-up, most visits were clustered either in the beginning or later period. This may not provide the complete picture of disease progress over the ten longitudinal years.
4.2. Disease progression group
The disease progression group included 669 (13%) patients whose disease status progressed between their first and last visit. The progression could be due to various reasons that require further investigation. Risk factors such as smoking, diabetes, and other common inflammatory systemic diseases may contribute to the patients’ disease progression. Next, home-based oral healthcare and compliance are major contributors to the success of periodontal treatments. These patients may or may not be compliant with the home hygiene instruction, and their disease could have progressed. Therefore, future studies should also evaluate the influence patient compliance on their oral health.
4.3. Disease improvement group
In the disease improvement group, 589 (11%) patients’ s disease status improved between their first and last visits. Many reasons may have improved these patients' disease status. First, most of the improved disease categories were mild to moderate periodontitis. Typically, when patients are on long-term periodontal maintenance therapy, their periodontal pocket depth and clinical attachment are expected to improve over time. Next, research studies have demonstrated that if periodontitis is diagnosed and treated in early stages (mild to moderate), then a good prognosis can be achieved, and the patient can remain progression-free (Tonetti et al., 2018). Last, the maximum improvement was observed in the extent of periodontitis. For example, many patients who had generalized periodontitis improved to localized periodontitis after receiving periodontal treatments.
Like any study, we encountered some limitations. First, these study results may not be generalizable because it included EDR data only from one institution Nevertheless, this study demonstrated a step-by-step approach to evaluate the quality of longitudinal EDR data that future studies can adopt and expand further. To facilitate further studies, the computational programs are shared through this paper . Next, the NLP algorithm used in this study may or may not work optimally on other datasets due to variations in documentation across different institutions. Regardless, researchers would be able to use the basic NLP framework as demonstrated in this paper to retrieve their clinician-documented diagnoses.
5. Conclusions
This study demonstrated the feasibility of utilizing longitudinal EDR data to track the disease change over 15 years. We successfully generated three different cohorts of PD patients (no disease change, disease progression, disease improvement) to study the clinical course of PD. We discovered moderate data quality when clinician-documented diagnoses were considered. The results of this study demonstrated a step-by-step process on how to utilize informatics methods to process patients' longitudinal EDR data for PD clinical research. This approach can be used to investigate longitudinal EDR data for PD response to different treatments.
Author Contributions
Conceptualization, methodology, J.P. and T.T.; writing—original draft preparation, J.P.; writing—review and editing, T.T.; visualization, J.P.; supervision, T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by Dr. Thankam Paul Thyvalikakath's start-up funds at the Indiana University School of Dentistry.
Institutional Review Board Statement
This study was reviewed and approved by the Indiana University Institutional Review Board (IRB: 1909819686) granted authorization.
Informed Consent Statement
This study was a retrospective chart review study; therefore, obtaining patient consent was waived.
Data Availability Statement
The dataset for this study contains identifiable information and therefore, there is no permission to share publicly.
Acknowledgments
We want to thank Mr. Craig Eberhardt for generating study data sets. We also want to thank Hoa Vo for generating references.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chang, H.-J., Lee, S.-J., Yong, T.-H., Shin, N.-Y., Jang, B.-G., Kim, J.-E., Huh, K.-H., Lee, S.-S., Heo, M.-S., Choi, S.-C., Kim, T.-I., & Yi, W.-J. (2020). Deep Learning Hybrid Method to Automatically Diagnose Periodontal Bone Loss and Stage Periodontitis. Scientific Reports, 10(1), 7531. [CrossRef] [PubMed]
- Cowie, M. R. , Blomster, J. I., Curtis, L. H., Duclaux, S., Ford, I., Fritz, F., Goldman, S., Janmohamed, S., Kreuzer, J., Leenay, M., Michel, A., Ong, S., Pell, J. P., Southworth, M. R., Stough, W. G., Thoenes, M., Zannad, F., & Zalewski, A. (2017). Electronic health records to facilitate clinical research. In Clinical Research in Cardiology (Vol. 106, Issue 1, p. 1). Dr. Dietrich Steinkopff Verlag GmbH and Co. KG. [CrossRef]
- Eke, P. I., Thornton-Evans, G. O., Wei, L., Borgnakke, W. S., Dye, B. A., & Genco, R. J. (2018). Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009-2014. Journal of the American Dental Association (1939), 149(7), 576-588.e6. [CrossRef]
- Genco, R. J., & Borgnakke, W. S. (2013). Risk factors for periodontal disease. Periodontology 2000, 62(1), 59–94.
- Koshi, E., Rajesh, S., Koshi, P., & Arunima, P. R. (2012). Risk assessment for periodontal disease. Journal of Indian Society of Periodontology, 16(3), 324–328. [CrossRef] [PubMed]
- Lalkhen, A. G., & McCluskey, A. (2008). Clinical tests: sensitivity and specificity. Continuing Education in Anaesthesia Critical Care & Pain, 8(6), 221–223.
- Lang, N. P., Suvan, J. E., & Tonetti, M. S. (2015). Risk factor assessment tools for the prevention of periodontitis progression a systematic review. Journal of Clinical Periodontology, 42(S16).
- Loe, H., Anerud, A., Boysen, H., & Morrison, E. (1986). Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. Journal of Clinical Periodontology, 13(5), 431–440. [CrossRef] [PubMed]
- Mullins, J., Yansane, A., Kumar, S. V, Bangar, S., Neumann, A., Johnson, T. R., Olson, G. W., Kookal, K. K., Sedlock, E., Kim, A., Mertz, E., Brandon, R., Simmons, K., White, J. M., Kalenderian, E., & Walji, M. F. (2021). Assessing the completeness of periodontal disease documentation in the EHR: a first step in measuring the quality of care. BMC Oral Health, 21(1), 282. [CrossRef]
- Needleman, I., Garcia, R., Gkranias, N., Kirkwood, K. L., Kocher, T., Iorio, A. Di, Moreno, F., & Petrie, A. (2018). Mean annual attachment, bone level, and tooth loss: A systematic review. Journal of Periodontology, 89, S120–S139. [CrossRef]
- Patel, J. S. (2020). Utilizing Electronic Dental Record Data to Track Periodontal Disease Change [Indiana University]. In ProQuest Dissertations and Theses. https://www.proquest.com/dissertations-theses/utilizing-electronic-dental-record-data-track/docview/2441238833/se-2?accountid=14656%0Ahttp://gw2jh3xr2c.search.serialssolutions.com/directLink?atitle=Utilizing+Electronic+Dental+Record+Data+to+Track+Periodo.
- Patel, J., Siddiqui, Z., Krishnan, A., & Thyvalikakath, T. (2017). Identifying patients’ smoking status from electronic dental records data. Studies in Health Technology and Informatics, 245, 1281. [CrossRef]
- Ramseier, C. A., Anerud, A., Dulac, M., Lulic, M., Cullinan, M. P., Seymour, G. J., Faddy, M. J., Bürgin, W., Schätzle, M., & Lang, N. P. (2017). Natural history of periodontitis: Disease progression and tooth loss over 40 years. Journal of Clinical Periodontology, 44(12), 1182–1191. [CrossRef]
- Schätzle, M., Faddy, M. J., Cullinan, M. P., Seymour, G. J., Lang, N. P., Bürgin, W., Ånerud, Å., Boysen, H., & Löe, H. (2009). The clinical course of chronic periodontitis: V. Predictive factors in periodontal disease. Journal of Clinical Periodontology, 36(5), 365–371. [CrossRef]
- Schatzle, M., Loe, H., Lang, N. P., Burgin, W., Anerud, A., & Boysen, H. (2004). The clinical course of chronic periodontitis. IV. Gingival inflammation as a risk factor in tooth mortality. Journal of Clinical Periodontology, 31(12), 1122–1127. [CrossRef] [PubMed]
- Schätzle, M., Löe, H., Lang, N. P., Heitz-Mayfield, L. J. A., Bürgin, W., Anerud, A., & Boysen, H. (2003). Clinical course of chronic periodontitis. III. Patterns, variations and risks of attachment loss. Journal of Clinical Periodontology, 30(10), 909–918.
- Schätzle, M., Löe, H., Ramseier, C. A., Bürgin, W., Ånerud, Å., Boysen, H., & Lang, N. P. (2010). Clinical course of chronic periodontitis: effect of lifelong light smoking (20 years) on loss of attachment and teeth. Journal of Investigative and Clinical Dentistry, 1(1), 8–15. [CrossRef]
- Song, M. , Liu, K., Abromitis, R., & Schleyer, T. L. (2013). Reusing electronic patient data for dental clinical research: A review of current status. In Journal of Dentistry (Vol. 41, Issue 12, pp. 1148–1163). [CrossRef]
- St Sauver, J. L., Carr, A. B., Yawn, B. P., Grossardt, B. R., Bock-Goodner, C. M., Klein, L. L., Pankratz, J. J., Finney Rutten, L. J., & Rocca, W. A. (2017). Linking medical and dental health record data: A partnership with the Rochester Epidemiology Project. BMJ Open, 7(3), e012528. [CrossRef]
- Thyvalikakath, T. P., Duncan, W. D., Siddiqui, Z., Lapradd, M., Eckert, G., Schleyer, T., Rindal, D. B., Jurkovich, M., Shea, T., & Gilbert, G. H. (2020). Leveraging Electronic Dental Record Data for Clinical Research in the National Dental PBRN Practices Background and Significance. Applied Clinical Informatics, 11(2), 305–314. [CrossRef] [PubMed]
- Thyvalikakath, T, LaPradd, M., Siddiqui, Z., Duncan, W. D., Eckert, G., Medam, J. K., Rindal, D. B., Jurkovich, M., & Gilbert, G. H. (2022). Root Canal Treatment Survival Analysis in National Dental PBRN Practices. Journal of Dental Research, 101(11), 1328–1334. [CrossRef]
- Thyvalikakath, Thankam, Song, M., & Schleyer, T. (2018). Perceptions and attitudes toward performing risk assessment for periodontal disease: a focus group exploration. BMC Oral Health, 18(1), 90. [CrossRef]
- Tokede, B., Yansane, A., White, J., Bangar, S., Mullins, J., Brandon, R., Gantela, S., Kookal, K., Rindal, D., Lee, C. T., Lin, G. H., Spallek, H., Kalenderian, E., & Walji, M. (2022). Translating periodontal data to knowledge in a learning health system. Journal of the American Dental Association, 153(10), 996–1004. [CrossRef] [PubMed]
- Tonetti, M. S., Greenwell, H., & Kornman, K. S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Periodontology, 89, S159–S172. [CrossRef]
- Tonetti, M. S., Jepsen, S., Jin, L., & Otomo-Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology, 44(5), 456–462. [CrossRef]
- Van Stralen, K. J. Van Stralen, K. J., Stel, V. S., Reitsma, J. B., Dekker, F. W., Zoccali, C., & Jager, K. J. (2009). Diagnostic methods I: Sensitivity, specificity, and other measures of accuracy. Kidney International, 75(12), 1257–1263. [CrossRef]
- Wang, Y., Siddiqui, Z., Krishnan, A., Patel, J., & Thyvalikakath, T. (2017). Extraction and evaluation of medication data from electronic dental records. Studies in Health Technology and Informatics, 245(317), 1290. [CrossRef]
- Worthington, H. V. , Clarkson, J. E., Bryan, G., & Beirne, P. V. (2013). Routine scale and polish for periodontal health in adults. In Cochrane Database of Systematic Reviews (Vol. 2013, Issue 11). John Wiley and Sons Ltd. [CrossRef]
- Zhang, J., Kowsari, K., Harrison, J. H., Lobo, J. M., & Barnes, L. E. (2018). Patient2Vec: A Personalized Interpretable Deep Representation of the Longitudinal Electronic Health Record. IEEE Access, 6, 65333–65346. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).