1. Introduction
The worldwide prevalence of diabetes mellitus (DM) over the last three decades has at least tripled making this disease a critical public health and economic issue for most nations [
1,
2]. About 90-95% of those with DM have type 2 DM (T2D) which is rapidly increasing in children, adolescents, and young adults along with an increasing rate of prediabetes, a major risk factor for T2D [
3]. About one in three USA adults have prediabetes which is undiagnosed in eight out of ten people [
4] [
5]. The total USA cost of DM in 2017 was
$327 billion with each diagnosed individual incurring about
$16,000 in annual medical expenses [
4].
While the US Prevention Services Task Force currently recommends screening adults for prediabetes and T2D, it suggests that current evidence is insufficient to assess the balance of benefits and harms of screening for children or adolescents [
5]. Of the 37.1 million USA adolescents and adults with DM, it is estimated that about one in five is “undiagnosed” [
6]. While individual lifestyle alterations for T2D and prediabetes are generally considered the treatments of choice, this option can be problematic for those without a diagnosis. Moreover, children and adolescents with T2D show a high dropout from the medical care system suggesting the need for some “remodeling of current healthcare practices” [
7].
As detailed below, considerable evidence supports the role of damaging oxidative stress (OxS) as a key factor in both the initiation and progression of T2D. This review will explore the hypothesis that minimizing physiological factors that induce damaging OxS and maximizing natural antioxidant factors (i.e., the OptRedox strategy) may provide a means of preventing or slowing T2D progression. Both adults and the pediatric population could potentially benefit from this OptRedox strategy. Since early intervention is critical, this strategy would be optimally effective if implemented as a public health policy in the pediatric population. School-based T2D interventions are a logical focal point for exploring future OptRedox strategy implementation and research [
8].
Considering the large number of individuals that have undiagnosed prediabetes or T2D, a “blanket” implementation of OptRedox must be justified by evidence-based medicine. Moreover, some individuals are likely at high risk for OxS-driven T2D, and a systems medicine approach with a complementary emphasis on redoxomics might identify such individuals and predict if they could be responders to OptRedox or other medical treatments [
9]. Before detailing the natural OptRedox factors for combating T2D, we will first summarize the dynamic involvement of OxS in T2D pathogenesis.
2. The Role of Oxidative Stress and T2D Pathogenesis
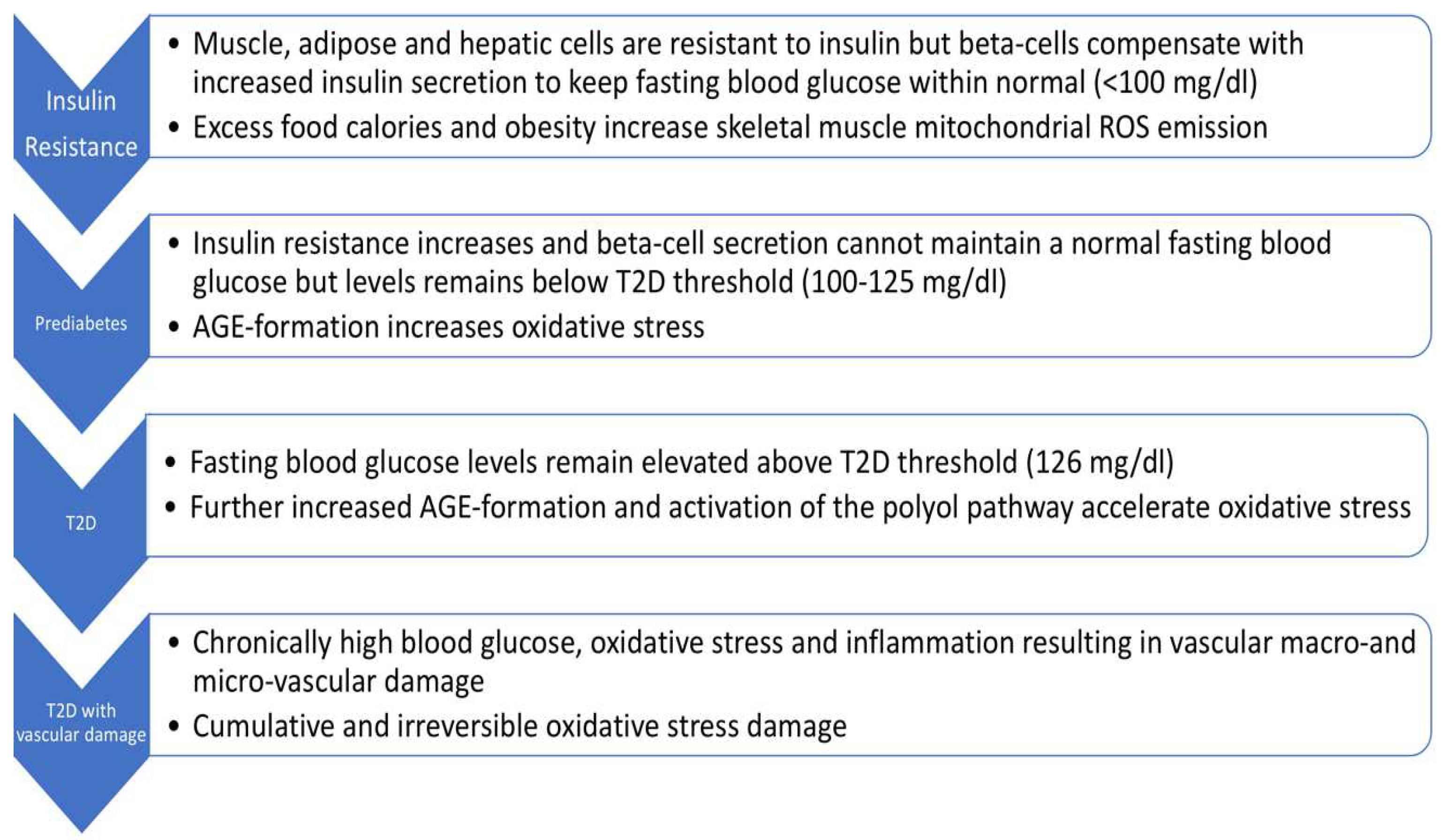
As shown in
Figure 1, there are four stages to T2D progression, i.e., insulin resistance, prediabetes, T2D, and T2D with vascular damage [
10,
11]. As indicated, fasting blood glucose levels (and/or HbA1c) levels are the main criteria for distinguishing these stages. T2D pathogenesis is multifactorial with very complex alterations in carbohydrate, lipid, and protein metabolism [
12]. A comprehensive review of T2D pathogenesis is beyond the scope of this review. Viewing T2D as a continuum (as in
Figure 1) is, however, useful for promoting interventions at the earliest stage. Fortunately, this is also where the OptRedox strategy may be particularly effective.
3. Insulin Resistance in Skeletal Muscle is Considered the Initiating Defect Leading to T2D
Considerable evidence supports the view that damaging OxS plays a central role in both initiating and accelerating the progression of T2D [
9,
13,
14]. We will focus on the role of OxS in promoting insulin resistance and prediabetes. Insulin resistance in skeletal muscle is considered the initiating or primary defect leading to T2D [
15]. GLUT4 is the primary glucose transporter for skeletal muscle. Under normal circumstances, insulin binding to the insulin receptor on skeletal muscle cells activates AKT (serine/threonine-specific protein kinases) phosphorylation eventually resulting in the translocation of GLUT4 to the plasma membrane thereby promoting glucose transport from plasma into skeletal muscle cells. Skeletal muscle insulin resistance occurs when the normal amount of insulin is inadequate for promoting the expected uptake of glucose. Over time, insulin resistance may result in prediabetes with an increased level of blood glucose. Skeletal muscle accounts for about 80% of insulin-stimulated glucose uptake [
15]. Decreased insulin-stimulated translocation of GLUT4 to the skeletal muscle surface, and/or decreased expression of GLUT4, are well-accepted causes of insulin resistance [
16,
17,
18].
4. Skeletal Muscle Mitochondrial Hydrogen Peroxide (H2O2) Emission Results in Insulin Resistance
The pioneering work of Anderson
et al. [
19], in normal rodents and humans, shows that excess dietary calories promote skeletal muscle mitochondrial hydrogen peroxide (H
2O
2) emission resulting in transient insulin resistance. While excess dietary carbohydrates and fat both promote this effect, it is markedly more pronounced with a high-fat diet. In obese but otherwise healthy human subjects, skeletal muscle mitochondrial H
2O
2 emission was twice the level observed in lean human subjects [
19].
A synthetic mitochondrial-specific antioxidant, SS31, was able to block skeletal muscle mitochondrial H
2O
2 emitting potential as well as the subsequent development of insulin resistance [
19]. Mechanistically, a high-fat diet prevented the AKT-mediated translocation of GLUT4 to the skeletal muscle surface as detailed above (other mechanisms could also be involved) [
19]. Increased mitochondrial hydrogen H
2O
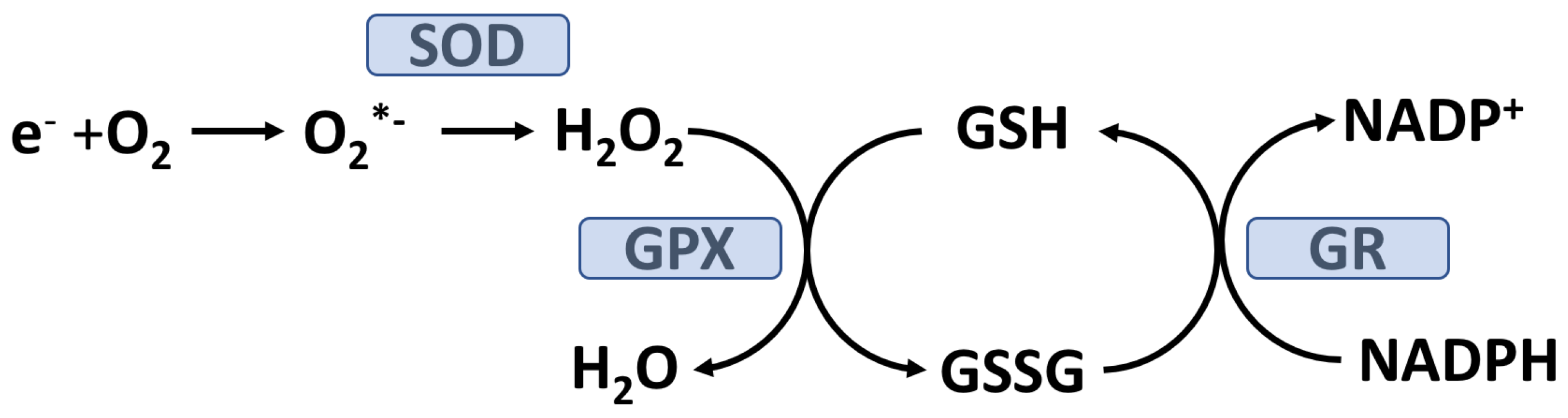
2 emission was accompanied by a reduction in the skeletal muscle ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) which would be expected by the glutathione peroxidase (GPX) reduction of H
2O
2 to H
2O (see
Figure 2).
Collectively, the results of Anderson
et al. suggest that excess calories, particularly excess fat calories, can result in transitory insulin resistance in normal humans. More recent
in vitro and
in vivo work by Fazakerley
et al. [
20] indicates that specifically inducing mitochondrial production of O
2-. /H
2O
2, by use of paraquat, will also induce insulin resistance in adipocytes and muscle cells by blocking insulin-stimulated GLUT4 translocation to the plasma membrane. Moreover, Fazakerley
et al. present evidence suggesting that mitochondrial OxS, in the absence of cytosolic OxS, is sufficient to cause insulin resistance [
20]. Natural antioxidants that can inhibit mitochondrial-induced insulin resistance are, therefore, high-priority candidates for OptRedox since they have the potential to slow or prevent a key T2D-initiating event. This hypothesis will be explored further below.
5. Avoiding High-Fat, High-calorie Meals Could Be an Effective OptRedox Strategy
The Anderson
et al. research strongly suggests that avoiding high-fat, high-calorie meals (and the attendant OxS) could be an effective OptRedox strategy by inhibiting early T2D initiation [
19]. Encouragingly, there is evidence showing that a low-calorie diet combined with long-term weight loss support has the potential to reverse T2D in adults [
21,
22]. The potential of low-calorie diets in slowing or preventing T2D progression in children has not been reported, underlining the critical need for research in this population. The United States Department of Agriculture (USDA)- National School Lunch Program (NSLP) serves over 30 million children and would be an ideal agency to explore research into the option of providing diets optimized to prevent T2D initiation and progression. Such an intervention would need a careful research design and the voluntary participation of children and their parents/guardians.
6. Hyperglycemia, Oxidative Stress (OxS), and T2D Progression
In susceptible individuals, skeletal muscle insulin resistance can be a contributing factor to increased postprandial glycemia (PPG) [
23,
24]. Postprandial glucose can eventually exceed 140 mg/dl (2 hours after a carbohydrate-containing meal) and become postprandial hyperglycemia (PPH), which is one of the earliest abnormalities associated with T2D [
23].
High levels of PPG are problematic for multiple reasons. PPG, 2 hours after lunch, is a better predictor of cardiovascular events and all-cause mortality in T2D than fasting blood glucose levels [
25,
26]. In an excellent and extensive review, Sottero [
24]
et al. concluded that chronic postprandial OxS resulting from PPH is a key factor driving T2D progression. The mechanisms connecting hyperglycemia and OxS are detailed below.
7. Hyperglycemia, AGE Formation, the Polyol Pathway, and Oxidative Stress
Glucose can covalently react with lysine, arginine, and N-terminal residues on proteins to form glycation adducts which can further react to form advanced glycation endproducts (AGEs) [
27]. Fasting hyperglycemia or PPH increases AGE formation in plasma proteins as well as proteins on the outer plasma membrane surface of most cells [
28,
29,
30]. Covalent modification of proteins by glucose can alter their structure and, potentially, their functions. For cells relying on insulin-independent GLUT transporters (e.g. GLUT1) intracellular glucose concentration will increase in response to hyperglycemia thereby promoting AGE-modification of cytosolic proteins. Both red blood cells and vascular endothelial cells rely on GLUT1 and glucose in these cell types will equilibrate with plasma glucose. Under PPH or fasting hyperglycemia, cells primarily relying on GLUT1 will utilize the polyol pathway and thereby lower intracellular glucose by converting it to fructose. The polyol pathway consumes NADPH which is needed to keep GSH is the reduced form (see
Figure 2) required to detoxify H
2O
2 (and lipid hydroperoxides) by the GPX system. Under hyperglycemic conditions, it has been estimated that about 30% of blood glucose goes through the polyol pathway [
31].
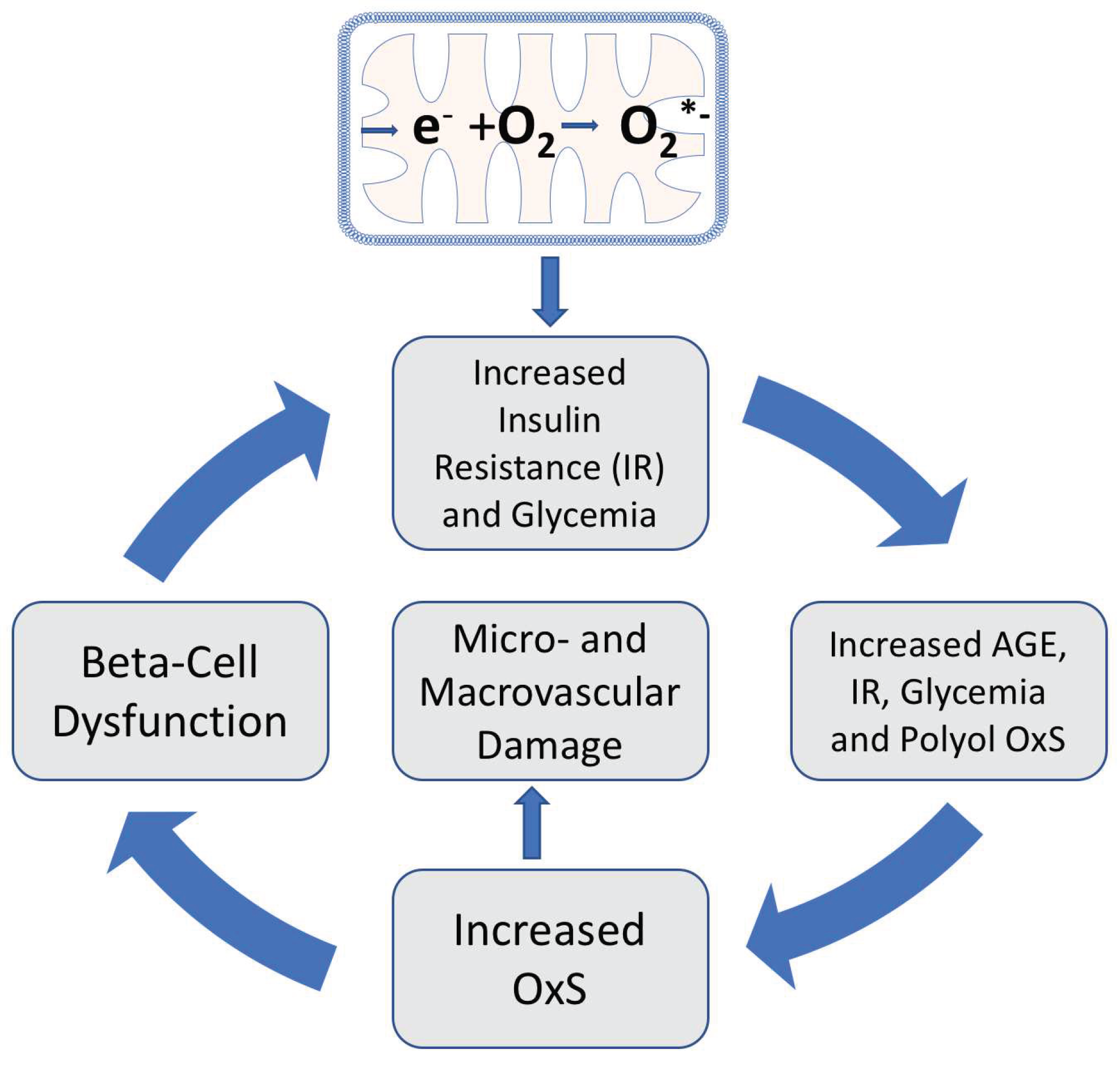
As indicated in
Figure 3, mitochondrial OxS induces insulin resistance leading to increased plasma glucose, increased plasma AGEs and increased polyol-induced NADPH consumption: all of which contribute to systemic OxS and inflammation. Moreover, both
in vitro and
in vivo evidence indicate that plasma protein-AGEs can induce insulin resistance by repression of GLUT4 expression in skeletal muscle [
32]. This AGE-induced insulin resistance is an example of an amplifying OxS feedback loop, i.e., plasma-AGEs further increase insulin resistance by amplifying glycemia and OxS. OxS can also contribute to beta-cell dysfunction and reduced glucose-induced insulin secretion thereby further increasing the risk of hyperglycemia and T2D progression [
33]. Both AGE-formation and systemic OxS give rise to an increased risk of both macrovascular and microvascular damage [
34,
35].
8. The OptRedox Strategy for Preventing or Slowing the Progression of T2D
Most publications on the roles of natural antioxidants in T2D progression tend to focus on individual antioxidant molecules such as vitamin E and their roles in blocking OxS. There are, however, benefits that arise from reframing this focus to also include OptRedox strategies/factors for preventing or slowing the progression of T2D. An OptRedox approach is more nuanced by also embracing dynamic physiological processes such as glycemic control and physical activity. As outlined in
Figure 3, factors that increase insulin resistance and glycemia can also promote damaging OxS and T2D progression. In contrast, factors that decrease insulin resistance and glycemia can be considered as factors promoting an OptRedox strategy. As will be detailed below, both glycemic control and exercise can be viewed as natural factors promoting an OptRedox status [
13]. Exercise, despite causing a transient oxidative OxS, subsequently induces skeletal muscle antioxidant enzymes that are beneficial for preventing T2D progression [
36,
37].
8.1. Glycemic Control as a Natural OptRedox Factor
In those with T2D, glycemia control is clinically defined as maintaining an HbA1C level of less than 7.0% or 6.5% for more strict control [
38]. Both fasting and PPG impact HbA1C levels. Woerle
et al. have found that lowering PPG is essential for optimizing glycemic control. In contrast to good glycemic control, poor glycemic control in subjects with T2D or prediabetes results in decreased plasma total antioxidant capacity and increased lipid peroxidation (a marker of OxS) [
39]. Physical activity/exercise is well a recognized and critical factor for promoting glycemic control in individuals with T2D or prediabetes [
40,
41].
8.2. Physical Activity/Exercise as an OptRedox Lifestyle Factor
As emphasized by the American Diabetes Association (ADA), physical activity in T2D and prediabetes improves glycemic control while also reducing cardiovascular risk and promoting weight loss [
40]. Moreover, the ADA suggests that “regular exercise may prevent or delay T2D development”. Light exercise provides an excellent example of an OptRedox lifestyle factor with an untapped potential to reduce OxS and slow T2D progression and, could potentially be cost-effectively implemented in a school setting [
42].
8.3. Light-Intensity Walking as an OptRedox Lifestyle Factor that Reduces Postprandial Glycemia (PPG)
A recent meta-analysis in predominantly adults with overweight or obesity showed that as little as two to five minutes of light-intensity walking significantly reduced PPG levels compared to prolonged sitting [
43]. This meta-analysis also found that the light-exercise-induced lowering of PPG was more effective in overweight individuals compared to individuals with obesity, suggesting that obesity imposes additional metabolic dysfunction [
43,
44]. Given the acknowledged importance of early intervention, it is critically important that this research be repeated in a pediatric population, perhaps, in a school setting. The potential near-future availability of real-time noninvasive blood glucose sensors on consumer smartwatches will rapidly accelerate research efforts focused on PPG as well as fasting hyperglycemia [
45,
46]. Twenty-four-hour glycemic profiles have shown a significant reduction in PPG in T2D subjects performing 15 minutes of strolling during each postprandial period compared to sedentary T2D subjects [
41,
47].
Short-duration light-intensity walking can lower PPG simply by promoting skeletal muscle consumption of glucose. Exercise can, however, also increase skeletal muscle uptake of glucose by promoting GLUT4 translocation to the plasma membrane as well as GLUT4 expression [
48]. Both low- and high-intensity exercise can equally and immediately induce GLUT4 expression in human skeletal muscle [
49,
50]. The signaling pathway for exercise-induced translocation of GLUT4 is distinct from that of insulin (see above) and likely involves AMP-activated protein kinase (AMPK) and increased sarcoplasmic calcium [
48].
9. Exercise, Reactive Oxygen Species (ROS), and OptRedox Status
As reviewed by Kawamura [
51] there is a “consensus that exercise increases the production of free radicals.” It is recognized, however, that exercise duration and intensity are key determinants of measurable OxS [
51,
52]. Moreover, transient exercise-induced OxS can evoke both beneficial and adverse physiological depending on the intensity/duration of the OxS and body conditioning [
36]. High levels of exercise-induced OxS can result in adverse damage to skeletal muscle, particularly in untrained individuals [
53]. It has long been recognized that physiological levels of ROS play essential roles in cell signaling pathways [
54,
55]. This raises the possibility that high levels of exogenous chemical antioxidant supplements could interfere with cell signaling pathways (see below) [
56].
9.1. Exercise-Induced ROS Production Has a Biphasic Impact on Skeletal Muscle Force Production
Powers
et al. [
53] have reviewed the evidence showing that exercise-induced ROS production has a biphasic impact on skeletal muscle force production, i.e., low levels of ROS production increase contractile force, but high ROS levels decrease force production. There is, therefore, an optimal level of ROS production (and redox status) that is required for maximal skeletal muscle contraction force. The molecular mechanism(s) for this biphasic effect is not fully understood but illustrates the need to consider complex physiological processes in determining an OptRedox status [
53,
57]. In addition to the potentially positive effect ROS can have on skeletal muscle function, ROS can also increase the expression of some enzymatic antioxidants. As will be discussed below, this effect may be blunted by natural exogenous chemical antioxidants such as vitamin E.
9.2. Exercise-Induced Oxidative Stress and Induction of Enzymatic Antioxidants
As mentioned above, exercise has long been associated with an increased activity level of some endogenous antioxidant enzyme,
e.g., skeletal muscle GPX [
58]. It has also been hypothesized that the ROS generated during exercise could be essential for this induction of antioxidant enzymes by modulating signal transduction pathways [
37,
58]. If this hypothesis is correct, it raises the concern that antioxidant supplements could interfere with this induction. The research of Ristow
et al. [
37] shows that supplementation with ascorbate (1000 mg/day) plus vitamin E (400 IU/day) blocked the exercise (four weeks) induced expression of GPX1 in skeletal muscle biopsies from healthy young men. These investigators also found that four weeks of exercise increased insulin sensitivity, but this effect was blocked by daily supplementation with ascorbate plus vitamin E. The form of vitamin E used in these experiments was RRR-alpha-tocopherol (more on this below). Ristow
et al. conclude that exercise-induced ROS are essential for promoting insulin sensitivity and the induction of GPX1 [
37]. Although discussed in the context of T2D, the subjects in this study were healthy adult males.
10. The Distinct Forms of Vitamin E and Their Effects on T2D Progression
Natural chemical antioxidants that prevent or inhibit skeletal muscle mitochondrial oxidative stress would be excellent candidates for potentially slowing or blocking the progression of insulin resistance. Vitamin E is considered the primary lipid-soluble antioxidant and is present in all biomembranes including those of mitochondria where vitamin E levels can be increased by dietary supplementation [
59].
10.1. Natural vitamin E and the Importance of Stereochemistry
According to the International Union of Pure and Applied Chemistry the term “vitamin E” refers to “all tocol and tocotrienol derivatives exhibiting qualitatively the biological activity of α-tocopherol” (
https://iupac.qmul.ac.uk/misc/toc.html). Natural vitamin E from dietary sources consists of: (1) four tocopherol vitamers (T),
i.e., alpha-T, beta-T, gamma-T, and delta-T) and; (2) four tocotrienol (T3) vitamers, i.e., alpha-T3, beta-T3, gamma-T3 and delta-T3. T3s are forms of vitamin E in which the hydrophobic “tails” have three trans double bonds [
60].
The stereochemistry of natural vitamin E vitamers is very specific. For example, natural alpha-tocopherol has three asymmetric carbons, and each has the R stereochemical designation, i.e., 2
R,4′
R,8′
R-alpha-tocopherol (or RRR-alpha-T). In contrast, synthetic alpha-tocopherol has both R and S configurations at each of the three asymmetric carbons and is, therefore, an equimolar mixture of eight compounds with only one eighth being the natural RRR-alpha-T: the other seven forms being xenobiotics with mostly unknown physiochemical properties. The synthetic form of alpha-T is most often termed all-racemic-alpha-tocopherol (or all-rac-alpha-T). Since vitamin E compounds have signal transduction properties that are independent of their antioxidant oxidant abilities, it is important to detail the form used in any experiment/trial [
61,
62]. Unfortunately, this is often not the case which makes reproducibility difficult, obscures potential physiological effects unique to specific vitamin E vitamers, and confounds meta-analyses which often lump all forms of vitamin E together.
10.2. Supplementation with “Vitamin E” May Be a Valuable Strategy for Controlling Diabetes Complications
A meta-analysis of the effects of antioxidant vitamins on T2D concluded that supplementation with vitamin E “may be a valuable strategy for controlling diabetes complications” [
63]. In this meta-analysis, the different forms of vitamin E were lumped together making it difficult to determine if positive or negative effects were unique to a form of vitamin E. In addition, the primary papers sometimes fail to distinguish all-rac-alpha-tocopherol from RRR-alpha-tocopherol. Nevertheless, all vitamin E vitamers are free radical quenchers and inhibit lipid peroxidation. It is possible, therefore, that the antioxidant properties shared by all members of the vitamin E family contribute to their positive effects on controlling T2D complications. Interestingly, increased lipid peroxidation is associated with poor glycemic control in T2D patients [
39].
10.3. All-Racemic-Alpha-Tocopherol (all-rac-alpha-T) and Rice Bran Tocopherol Concentrate Inhibit Skeletal Muscle Generation of Hydrogen Peroxide
Given the central importance of mitochondrial oxidative stress in T2D (see
Figure 3), vitamin E has been advanced as an antioxidant with great therapeutic potential for T2D treatment. As detailed above, skeletal mitochondrial hydrogen peroxide emission may be an initiating event for the development of insulin resistance. In a rat model, Chow
et al. have quite remarkedly demonstrated that dietary supplementation with either all-rac-alpha-T or a rice bran tocopherol concentrate (8.6% RRR-alpha-T, 5.5% RRR-beta-T and 15.4% gamma-T3) markedly decreases skeletal muscle hydrogen peroxide generation in a dose-dependent manner [
64]. The mechanism for this important effect is not well understood, and has not been studied in human skeletal muscle, but implies that early supplementation with vitamin E could slow the progression of T2D. Futuremechanistic research on the role of vitamin E on mitochondrial hydrogen peroxide emission is a high priority.
10.4. Vitamin E and/or Ascorbate Supplementation Improves Glycemic Control in T2D
In contrast to the work of Ristow
et al. [
37] (see
Section 9.2) in which the subjects were adult healthy males, El-Aal
et al. [
65] conducted a clinical trial looking at the effects of vitamin E and/or ascorbate on adult T2D males who were all under metformin treatment. The results clearly show beneficial effects on glycemic control in those supplementing (for 90 days) with vitamin E alone (400 mg twice daily), ascorbate (500 mg twice daily) alone, or the combination of both. Unfortunately, it is not clear if RRR-alpha-tocopherol (RRR-alpha-T) or all-racemic-alpha-tocopherol (all-rac-alpha-T) was the form of vitamin E used in this trial. Nevertheless, this study strongly suggests that future studies should look at the potential beneficial effects of vitamin E supplementation on pediatric subjects with prediabetes or with T2D. Subjects with T2D or prediabetes with antioxidant deficiencies due to enhanced OxS may respond differently to antioxidant supplementation (or exercise) than healthy subjects with adequate antioxidant levels.
10.5. The Tocotrienol-Rich Fraction (TRF) from Palm Oil May Be Beneficial in Both Prediabetes, T2D and in Preventing Early Diabetic Retinopathy
Evidence from a randomized double-blind placebo-controlled clinical trial (the Vafa study) in T2D subjects with poor glycemic control shows that the tocotrienol-rich fraction (TRF) from palm oil (200 mg daily) for eight weeks lowered fasting blood glucose levels by 15% and improved indices of OxS [
66]. An earlier study (the Baliarsingh study) in adult T2D subjects did not show any benefit of tocotrienols (60 days, 3 mg/kg body weight, twice daily) on fasting blood glucose but these subjects had well medication-controlled blood glucose levels at the start of the trial [
67]. This earlier study did, however, show a marked decrease in atherogenic low density lipoprotein-cholesterol levels in the subjects taking the tocotrienol supplement. In both the Baliarsingh
et al. study and the Vafa
et al. study, the palm oil TRF was predominantly alpha-T3, beta-T3, gamma-T3, and RRR-alpha-T with only a small content (about 4-8%) of delta-T3. The low content of delta-T3 in the TRF is noteworthy since delta-T3 may be uniquely important in preventing T2D progression as detailed below.
A recent study by Ho, et al. [
68] showed that TRF (200 mg twice daily for 12 months) was effective in preventing early diabetic retinopathy compared to placebo. Diabetic retinopathy is a very common microvascular complication of T2D and makes a major contribution to blindness and vision loss in adults [
68].
10.6. Delta-Tocotrienol Shows Promise in Treating Prediabetes
Treatment options for prediabetes are limited, especially in the pediatric population [
69]. Encouragingly, a recent randomized-controlled pilot study looked at the effects of T3 purified from annatto seeds: this preparation was primarily delta-T3 (90%) and 10% gamma-T3 [
70]. The prediabetic adult study population was not being treated with any medications for glucose control or taking any dietary supplements. After 12 weeks of 300 mg/day of T3s, there were significant improvements in glycemic control compared to the controls taking a placebo [
70]. T3s are thought to improve glycemic control by upregulating the skeletal muscle expression of GLUT4, insulin receptor substrate 1, AKT signaling, and by activation of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) [
70,
71].
11. Conclusions
The evidence reviewed here strongly supports the role of OxS and ROS in both the initiation and progression of T2D. The role of natural antioxidant factors in combating T2D is best evaluated in the context of the complex physiological processes associated with T2D. The consumption of high-calorie, high-fat diets gives rise to transient insulin resistance resulting from mitochondrial OxS. In susceptible individuals, gradual increases in PPG and fasting blood glucose promote systemic OxS with amplifying feedback loops that eventually can cause OxS-induced beta-cell dysfunction and T2D with micro-and macro-vascular damage (see
Figure 3). Avoiding high-calorie, high-fat diets and engaging in exercise are, in effect, key frontline natural antioxidant factors. The role of natural antioxidant compounds in combating T2D must likewise be considered beyond their simple roles as inhibitors of
in vitro or
ex vivo OxS. Vitamin E illustrates this point. While all members of the vitamin E family have powerful
in vitro antioxidant properties, they can also exhibit distinct abilities to regulate cellular signal transduction pathways important to T2D progression. While most literature on the role of vitamin E vitamers in T2D or prediabetes has been limited to RRR-alpha-T or all-rac-alpha-T, emerging trials with tocotrienols show promise. As detailed in
Section 10.6, a delta-T3-rich supplement improved glycemic control in adults with prediabetes. Although there is a consensus that early dietary, exercise, and effective nutraceutical interventions are optimal for preventing or reversing T2D progression, most research has focused on adults. It is critical, therefore, that more research be promoted with pediatric populations.
An additional way of promoting the OptRedox strategy in clinical settings is utilization of the Bright Futures guidelines which recommends educating patients and parents on the importance of a healthy diet and exercise beginning at age two [
72]. Healthcare providers should assess dietary and physical activity behaviors and make age appropriate recommendations. Despite these recommendations, childhood obesity rates continue to rise which contributes to the rise in T2D. An emphasis on prevention strategies such as diet and exercise must be accompanied by a better understanding of human motivation. Lifestyle modifications, while proven to be effective, are the some of the hardest for patients and families to achieve.
Author Contributions
Conceptualization, W.L.S.; writing—review and editing, W.L.S, D.S.T., E.A.L and G.A.P. ; funding acquisition, W.L.S., and East Tennessee State University All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the National Institutes of Health grant C06RR0306551 and the East Tennessee State University Robert W. Summers Pediatric Research Endowment.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
References
- diabetes, W. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 12 July 2022).
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol 2011, 8, 228–236. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, D.; Yi, S.S. Trends in Prediabetes Among Youths in the US From 1999 Through 2018. JAMA Pediatr 2022, 176, 608–611. [Google Scholar] [CrossRef]
- workforce, C. How Type 2 Diabetes Affects Your Workforce. Available online: https://www.cdc.gov/diabetes/prevention/how-type2-affects-workforce.htm#:~:text=Diabetes%20Is%20Costly,over%20a%205%2Dyear%20period (accessed on 13 July 2022).
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; Kubik, M.; et al. Screening for Prediabetes and Type 2 Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 326, 736–743. [Google Scholar] [CrossRef] [PubMed]
- DM, C.p. Prevalence of Both Diagnosed and Undiagnosed Diabetes, Available online: https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html (accessed on.
- Reinehr, T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes 2013, 4, 270–281. [Google Scholar] [CrossRef]
- Pansier, B.; Schulz, P.J. School-based diabetes interventions and their outcomes: a systematic literature review. J Public Health Res 2015, 4, 467. [Google Scholar] [CrossRef]
- Alu, S.N.; Los, E.A.; Ford, G.A.; Stone, W.L. Oxidative Stress in Type 2 Diabetes: The Case for Future Pediatric Redoxomics Studies. Antioxidants 2022, 11, 1336. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE COMPREHENSIVE TYPE 2 DIABETES MANAGEMENT ALGORITHM - 2018 EXECUTIVE SUMMARY. Endocr Pract 2018, 24, 91–120. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Garber, A.J.; Grunberger, G.; Handelsman, Y.; Garvey, W.T. DYSGLYCEMIA-BASED CHRONIC DISEASE: AN AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS POSITION STATEMENT. Endocr Pract 2018, 24, 995–1011. [Google Scholar] [CrossRef]
- Hansen, T. Type 2 diabetes mellitus--a multifactorial disease. Ann Univ Mariae Curie Sklodowska Med 2002, 57, 544–549. [Google Scholar]
- Wright, E.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract 2006, 60, 308–314. [Google Scholar] [CrossRef]
- Chikezie, P.C.; Ojiako, O.A.; Ogbuji, A.C. Oxidative Stress in Diabetes Mellitus. International Journal of Biological Chemistry 2015, 9, 92–109. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 Suppl 2, S157–163. [Google Scholar] [CrossRef]
- Mueckler, M. Insulin resistance and the disruption of Glut4 trafficking in skeletal muscle. J Clin Invest 2001, 107, 1211–1213. [Google Scholar] [CrossRef]
- Maier, V.H.; Gould, G.W. Long-term insulin treatment of 3T3-L1 adipocytes results in mis-targeting of GLUT4: implications for insulin-stimulated glucose transport. Diabetologia 2000, 43, 1273–1281. [Google Scholar] [CrossRef]
- Kampmann, U.; Christensen, B.; Nielsen, T.S.; Pedersen, S.B.; Ørskov, L.; Lund, S.; Møller, N.; Jessen, N. GLUT4 and UBC9 protein expression is reduced in muscle from type 2 diabetic patients with severe insulin resistance. PLoS One 2011, 6, e27854. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C.; et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J Biol Chem 2018, 293, 7315–7328. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Juray, S.; Axen, K.V.; Trasino, S.E. Remission of Type 2 Diabetes with Very Low-Calorie Diets-A Narrative Review. Nutrients 2021, 13, 2086. [Google Scholar] [CrossRef]
- Maffettone, A.; Rinaldi, M.; Fontanella, A. Postprandial hyperglycemia: a new frontier in diabetes management? Italian Journal of Medicine 2018, 12, 108–115. [Google Scholar] [CrossRef]
- Sottero, B.; Gargiulo, S.; Russo, I.; Barale, C.; Poli, G.; Cavalot, F. Postprandial Dysmetabolism and Oxidative Stress in Type 2 Diabetes: Pathogenetic Mechanisms and Therapeutic Strategies. Med Res Rev 2015, 35, 968–1031. [Google Scholar] [CrossRef] [PubMed]
- Cavalot, F.; Petrelli, A.; Traversa, M.; Bonomo, K.; Fiora, E.; Conti, M.; Anfossi, G.; Costa, G.; Trovati, M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006, 91, 813–819. [Google Scholar] [CrossRef]
- Cavalot, F.; Pagliarino, A.; Valle, M.; Di Martino, L.; Bonomo, K.; Massucco, P.; Anfossi, G.; Trovati, M. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011, 34, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: a review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep 2014, 14, 453. [Google Scholar] [CrossRef]
- Greifenhagen, U.; Frolov, A.; Blüher, M.; Hoffmann, R. Plasma Proteins Modified by Advanced Glycation End Products (AGEs) Reveal Site-specific Susceptibilities to Glycemic Control in Patients with Type 2 Diabetes. J Biol Chem 2016, 291, 9610–9616. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Beppu, M.; Kikugawa, K.; Nagai, R.; Horiuchi, S. Membrane proteins of human erythrocytes are modified by advanced glycation end products during aging in the circulation. Biochemical and biophysical research communications 1999, 258, 123–127. [Google Scholar] [CrossRef]
- Yan, L.J. Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Animal Model Exp Med 2018, 1, 7–13. [Google Scholar] [CrossRef]
- Pinto-Junior, D.C.; Silva, K.S.; Michalani, M.L.; Yonamine, C.Y.; Esteves, J.V.; Fabre, N.T.; Thieme, K.; Catanozi, S.; Okamoto, M.M.; Seraphim, P.M.; et al. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci Rep 2018, 8, 8109. [Google Scholar] [CrossRef]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative stress and beta-cell dysfunction. Pflugers Arch 2010, 460, 703–718. [Google Scholar] [CrossRef]
- Stirban, A.; Gawlowski, T.; Roden, M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol Metab 2014, 3, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.; Friess, U. Oxidative stress, AGE, and atherosclerosis. Kidney Int Suppl 2007, S17–S26. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: past, present and future. J Physiol 2016, 594, 5081–5092. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Woerle, H.J.; Neumann, C.; Zschau, S.; Tenner, S.; Irsigler, A.; Schirra, J.; Gerich, J.E.; Göke, B. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007, 77, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, H.P.; Silva, R.; Sivakanesan, R.; Ranasinghe, P.; Katulanda, P. Poor Glycaemic Control Is Associated with Increased Lipid Peroxidation and Glutathione Peroxidase Activity in Type 2 Diabetes Patients. Oxid Med Cell Longev 2019, 2019, 9471697. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.W.; van Loon, L.J. Exercise strategies to optimize glycemic control in type 2 diabetes: a continuing glucose monitoring perspective. Diabetes Spectr 2015, 28, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Velicer, C. Available online: https://thrivingschools.kaiserpermanente.org/kids-and-type-2-diabetes-how-parents-and-teachers-can-help-curb-the-tide/ (accessed on 13 Sept 2022).
- Buffey, A.J.; Herring, M.P.; Langley, C.K.; Donnelly, A.E.; Carson, B.P. The Acute Effects of Interrupting Prolonged Sitting Time in Adults with Standing and Light-Intensity Walking on Biomarkers of Cardiometabolic Health in Adults: A Systematic Review and Meta-analysis. Sports Med 2022, 52, 1765–1787. [Google Scholar] [CrossRef]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef]
- Chang, T.; Li, H.; Zhang, N.; Jiang, X.; Yu, X.; Yang, Q.; Jin, Z.; Meng, H.; Chang, L. Highly integrated watch for noninvasive continual glucose monitoring. Microsyst Nanoeng 2022, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-León, C.; Villalonga, C.; Munoz-Torres, M.; Ruiz, J.R.; Banos, O. Mobile and Wearable Technology for the Monitoring of Diabetes-Related Parameters: Systematic Review. JMIR Mhealth Uhealth 2021, 9, e25138. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.W.; Venema, M.; van Mechelen, W.; Stehouwer, C.D.; Hartgens, F.; van Loon, L.J. Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care 2013, 36, 3448–3453. [Google Scholar] [CrossRef] [PubMed]
- Flores-Opazo, M.; McGee, S.L.; Hargreaves, M. Exercise and GLUT4. Exerc Sport Sci Rev 2020, 48, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Kraniou, G.N.; Cameron-Smith, D.; Hargreaves, M. Acute exercise and GLUT4 expression in human skeletal muscle: influence of exercise intensity. J Appl Physiol (1985) 2006, 101, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants (Basel) 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Quindry, J.; Stone, W.; King, J.; Broeder, C. The effects of acute exercise on neutrophils and plasma oxidative stress. Med Sci Sports Exerc 2003, 35, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J Sport Health Sci 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans 2001, 29, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; McHardy, H.; Morton, J.P.; Nikolaidis, M.G.; Close, G.L. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic Biol Med 2015, 84, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc 2001, 33, 371–376. [Google Scholar] [CrossRef]
- Ji, L.L. Antioxidant enzyme response to exercise and aging. Med Sci Sports Exerc 1993, 25, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C.; Jensen, S.K. α-Tocopherol incorporation in mitochondria and microsomes upon supranutritional vitamin E supplementation. Genes Nutr 2012, 7, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med 2007, 28, 692–728. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Gysin, R.; Kempna, P.; Munteanu, A.; Negis, Y.; Villacorta, L.; Visarius, T.; Zingg, J.M. Vitamin E mediates cell signaling and regulation of gene expression. Ann N Y Acad Sci 2004, 1031, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M. Vitamin E: A Role in Signal Transduction. Annu Rev Nutr 2015, 35, 135–173. [Google Scholar] [CrossRef] [PubMed]
- Balbi, M.E.; Tonin, F.S.; Mendes, A.M.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Antioxidant effects of vitamins in type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetol Metab Syndr 2018, 10, 18. [Google Scholar] [CrossRef]
- Chow, C.K.; Ibrahim, W.; Wei, Z.; Chan, A.C. Vitamin E regulates mitochondrial hydrogen peroxide generation. Free Radic Biol Med 1999, 27, 580–587. [Google Scholar] [CrossRef]
- El-Aal, A.A.; El-Ghffar, E.A.A.; Ghali, A.A.; Zughbur, M.R.; Sirdah, M.M. The effect of vitamin C and/or E supplementations on type 2 diabetic adult males under metformin treatment: A single-blinded randomized controlled clinical trial. Diabetes Metab Syndr 2018, 12, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Vafa, M.; Haghighat, N.; Moslehi, N.; Eghtesadi, S.; Heydari, I. Effect of Tocotrienols enriched canola oil on glycemic control and oxidative status in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. J Res Med Sci 2015, 20, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Baliarsingh, S.; Beg, Z.H.; Ahmad, J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis 2005, 182, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.I.; Ng, E.Y.; Chiew, Y.; Koay, Y.Y.; Chuar, P.F.; Phang, S.C.W.; Ahmad, B.; Kadir, K.A. The effects of vitamin E on non-proliferative diabetic retinopathy in type 2 diabetes mellitus: Are they sustainable with 12 months of therapy. SAGE Open Med 2022, 10, 20503121221095324. [Google Scholar] [CrossRef] [PubMed]
- Kalvaitus, K.; Portnoy, S.A. Available online: https://www.healio.com/news/endocrinology/20120325/experts-recommend-two-pronged-approach-to-treating-prediabetes (accessed on 11 Jan 2023).
- Suleman, F.; Khan, D.A.; Pervez, M.A.; Aamir, M. Effects of delta-tocotrienol supplementation on glycaemic control in individuals with prediabetes: A randomized controlled study. J Pak Med Assoc 2022, 72, 4–7. [Google Scholar] [CrossRef]
- Fang, F.; Kang, Z.; Wong, C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Molecular nutrition & food research 2010, 54, 345–352. [Google Scholar] [CrossRef]
- Pediatrics, A.A.o. Available online: https://www.aap.org/en/practice-management/bright-futures/bright-futures-materials-and-tools/bright-futures-guidelines-and-pocket-guide/ (accessed on 11 Jan 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).