1. Introduction
Genome instability is considered one of the hallmarks of aging [
1]. Telomeres are one of several key elements required for genome stability. Telomeric DNA consists of tandem repeats of a simple, often G-rich, sequence. This sequence is determined by the action of telomerase, which lengthens terminal regions of eukaryotic telomeric DNA by RNA-templated addition of the repeated DNA sequence [
2]. However, with advancing age, telomerase activity is affected, and telomeres start shortening, which compromises cell function and lifespan [
2]. This shortening has been associated with multiple age-related diseases, including cardiovascular disease, malignancies, dementia, osteosarcopenia, frailty, and other conditions [
2,
3,
4].
Vitamin D is a micronutrient with an important role in inflammation, cell growth & differentiation, and apoptosis [
5]. Vitamin D insufficiency, defined as serum levels of 25-hydroxyvitamin D [25(OH)D] concentrations below 50 nmol/L, has also been associated with the age-related diseases listed above [
6]. Therefore, a link between serum 25OHD levels and telomere shortening has been proposed but remains partially explored [
7,
8]. In a recent study analyzing data from 1,542 younger adults (aged 20-39 years), 1,336 middle-aged adults (aged 40-59 years), and 1,382 older adults (aged ≥60 years) participants in the US NHANES 2001-2002, Beilfuss et al. [
9] reported that serum 25(OH)D was positively associated with leukocyte telomere length (LTL) in middle-aged participants (aged 40-59 years) only, independently of other factors. However, this association was discrete and not observed in older participants. Liu et al., [
10] examined the cross-sectional association between serum 25OHD concentration in plasma and LTL in 1,154 US radiologic technologists aged 48-93 (373 white females, 278 white males, 338 black females, 165 black males) and found a weak positive association between 25(OH)D and LTL over the entire range of 25(OH)D level in the overall study population and subgroups as a function of sex and race. Other studies have shown conflicting results and are difficult to interpret because of their small sample size [
2].
In addition to the deleterious effect of vitamin D deficiency and its known association with multiple age-related conditions [
5], the impact of very high 25OHD serum levels on the pathogenesis of those conditions remains to be elucidated. Recent research has identified a “U-shaped association”, with morbidity and mortality risks at both high and low 25 (OH) D levels [
11,
12]. However, the underlying mechanisms explaining the harmful effects of having too much or too little circulating vitamin D levels remain elusive. Interestingly, there is growing evidence suggesting that higher circulating vitamin D levels are associated with longer LTL, thus having a potentially beneficial effect on aging and age-related diseases [
2]. If this hypothesis proved to be correct, longer LTL, induced in part by vitamin D, would be responsible for improved cell differentiation, function and survival, and overall healthy aging. To test this hypothesis, the present study examined the relationship between 25OHD levels and LTL in the very large and well established UK Biobank.
2. Materials and Methods
UK Biobank
Over 500,000 participants aged 40-70 years were recruited between 2006 and 2010. Participants visited one of 22 assessment centers near their residences. Baseline assessments (at recruitment) included physical measures such as grip strength, heel ultrasound, and bioimpedance measurements, biological samples (blood, saliva, and urine samples) for various assays, and surveys on demographics, lifestyle, and environmental factors, plus personal and family medical history [
13]. UK Biobank received ethical approval from the Northwest Centre for Research Ethics Committee (11/NW/0382), and all participants provided written informed consent.
Data
Data used to examine the association between LTL and serum 25OHD included: 1) LTL from the baseline visit, 2) 25OHD levels at the baseline visit, 3) baseline covariates: serum calcium, anthropometric, demographic, or socioeconomic variables, and lifestyle factors. The UK Biobank field IDs used to find the data above are provided in
Table 1.
Inclusion and exclusion criteria
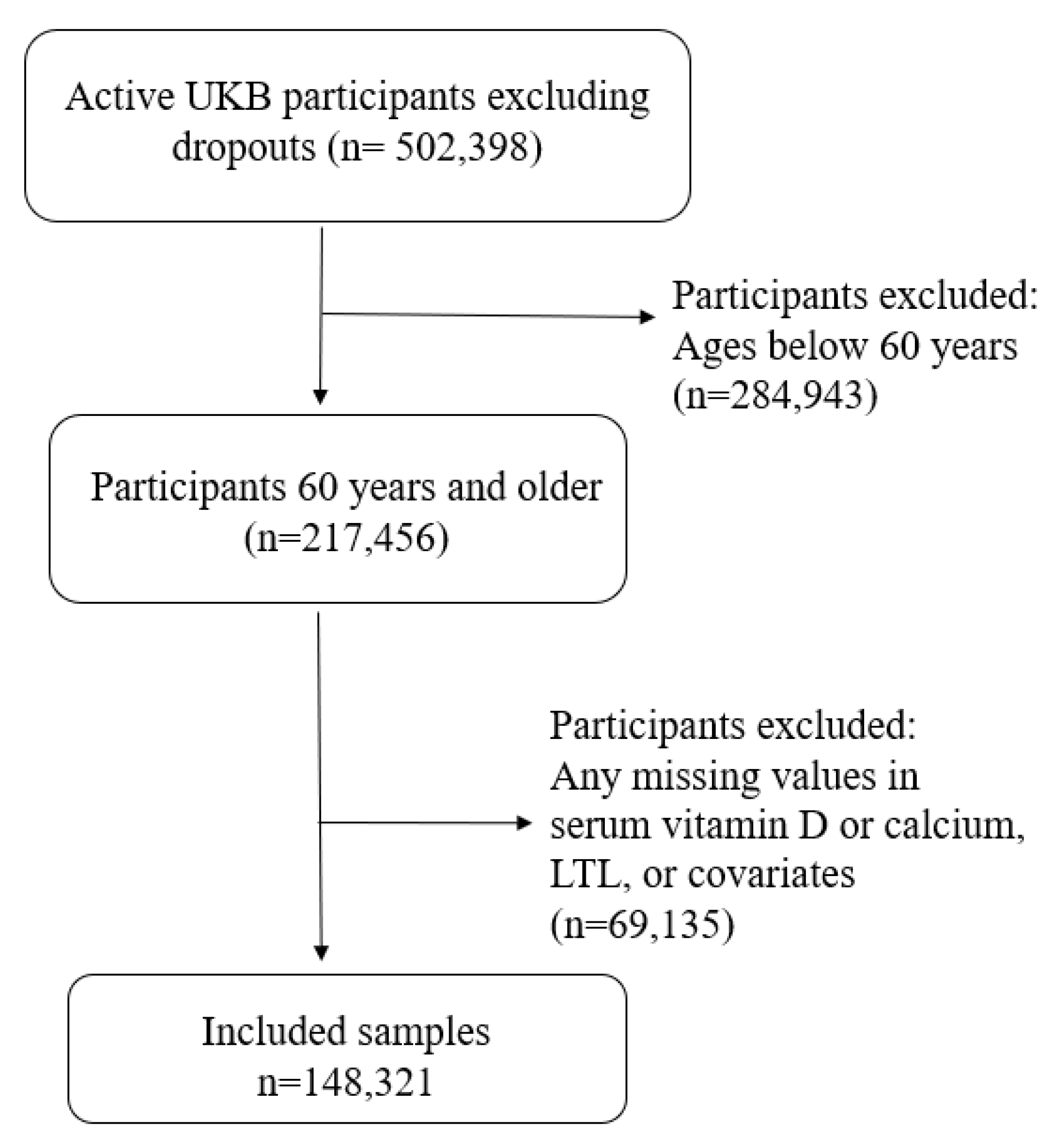
UK Biobank participants attending the baseline visit were included regardless of self-reported ethnicities; we chose 60 years as a cut-off due to the high prevalence and clinical significance of vitamin D deficiency in this population [
5,
7]. Additionally, participants with any missing data were excluded, leaving a total of 148,321 participants for analysis (
Figure 1).
Leukocyte Telomere Length
DNA was extracted from peripheral blood leukocytes. LTL was measured using a multiplex qPCR-based technique by comparing the amount of the telomere amplification product (T) to that of a single-copy gene (S). A T/S ratio was derived representing the mean LTL. LTL adjusted for the influence of technical parameters was released by UK Biobank and used in this project.
Defining serum vitamin D status
Biochemical assays were performed on blood samples collected during the baseline evaluation at the assessment centers. Samples were collected in a silica clot accelerator tube and stored at −80°C. These samples were later processed in a central laboratory using an automated dispensing system [
14]. Serum 25(OH)D status was measured by chemiluminescence immunoassay (DiaSorin LIAISON XL, Italy), which was certified by the Vitamin D Standardization-Certification Program of the Centers for Disease Control and Prevention [
15]. To ensure the precision of analysis, quality control samples at different concentrations were analyzed [
16], and the accuracy of 25OHD was verified through the RIQAS Immunoassay Specialty I EQA program (Randox Laboratories), an external quality assurance scheme [
17].
Covariates
Race included White, Black, South Asian, and Other ethnicities. Education ranged from none to college or university degree (higher education). Townsend deprivation index was a measure of material deprivation at the postcode level based on the preceding national census data (mean 0 in the UK population), with higher scores representing greater levels of deprivation. Whole body fat mass was measured using bioelectrical impedance analysis. Smoking status (never, previous, or current) was accessed through a touchscreen questionnaire, and similarly for alcohol intake frequency (daily or almost daily, three or four times a week, once or twice a week, one to three times a month, special occasion only). Physical activity was assessed by adapted questions from the short International Physical Activity Questionnaire (IPAQ) [
18]. Time spent in vigorous, moderate, and walking activities was weighted by their intensity levels to derive the total metabolic equivalent task (MET) minutes per week, which along with days of each activity for a certain duration were used to determine low, moderate, or high physical activity level, following the IPAQ guidelines. Serum calcium was measured using a Beckman Coulter (UK) Ltd assay and Beckman Coulter AUS800 platform using colorimetric analysis methodology. Units of measurement were mmol/L and the manufacturer’s analytical range was 1-3.5 mmol/L. Extensive QC procedures were followed to identify invalid results, dilution issues and laboratory drift as previously described [
17].
Statistical Methods
Serum 25OHD was linked to LTL in a linear regression model adjusting for covariates. Serum 25OHD and LTL were z-transformed using the rank-based inverse normal transformation prior to the association analysis. The transformed 25OHD and LTL followed a standard normal distribution with the mean 0 and standard deviation 1. To capture a non-linear relationship of 25OHD with LTL, 25OHD was categorized into five groups with z-scores in the ranges of ≤ -2, (-2, -1], (-1, 1] (reference), (1, 2], and > 2, corresponding to the ranges of 25OHD in the original scale: ≤16.6 nmol/L, (16.6 nmol/L, 29.7 nmol/L], (29.7 nmol/L, 71.8 nmol/L] (reference), (71.8 nmol/L, 95.9 nmol/L], and > 95.9 nmol/L. For convenience, the five 25OHD groups were named as follows: extremely low, low, medium, moderately high, and high. The unadjusted associations between 25OHD and LTL were reported as well as associations adjusting for demographic/socioeconomic variables (age, sex, ethnicity, Townsend deprivation index, education), whole body fat mass (z-transformed by the rank-based inverse normal distribution), and lifestyle factors (smoking status, alcohol intake frequency, and IPAQ activity group), and serum calcium (z-transformed by the rank-based inverse normal transformation). P-values smaller than 5% were considered statistically significant. All the statistical analyses were performed in R version 4.1.2.
3. Results
Population characteristics
Data were obtained at the baseline visit when the mean age of the included samples was 64.13 years (SD: 2.85). Fifty percent of participants were women, and the vast majority were of European ancestry (97.3%). 27.5% of the participants received a college or university degree whereas 25.5% had no degree. Overall, according to the Townsend deprivation index, the included samples were less materially deprived than the population on average. Fifty percent had never smoked, 92% drank more or less, and 86.3% reported moderate to high physical activity levels (
Table 2).
Associations between telomere length and serum 25OHD
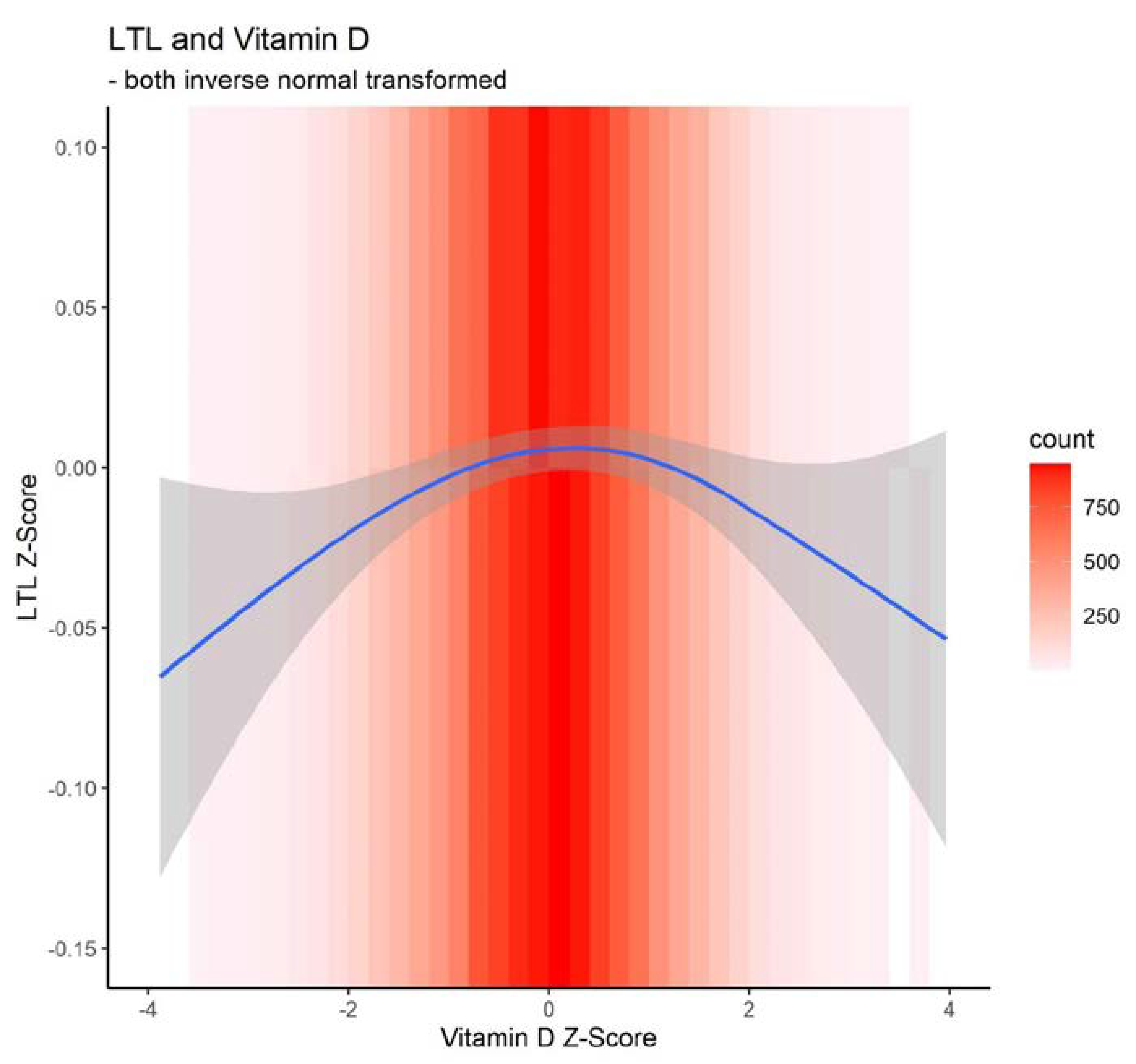
As shown in
Figure 2, there was an inverted U-shaped relationship between z-transformed 25OHD and LTL. Low and extremely low 25OHD levels were significantly associated with shorter LTL as well as high 25OHD levels in both unadjusted and adjusted association analyses (
Table 3). In the adjusted association analysis (
Table 3), a low (z score in (-1, 1] or serum 25OHD in (29.7 nmol/L, 71.8 nmol/L]) or extremely low (z score ≤ -2 or serum 25OHD ≤ 16.6 nmol/L) level of serum 25OHD compared to the medium level (z score in (-1, 1] or serum 25OHD in (29.7 nmol/L, 71.8 nmol/L]) was associated with shorter LTL: 0.018 SD (standardized
β= -0.018, 95% CI -0.033 to -0.003,
P=0.022) and 0.048 SD (standardized
β= -0.048, 95% CI -0.083 to -0.014,
P=0.006) shorter mean LTL, respectively. Additionally, the high serum 25OHD group (z score >2 or serum 25OHD > 95.9 nmol/L) had 0.038 SD shorter mean LTL than the group with medium 25OHD levels (z score in (-1, 1] or serum 25OHD in (29.7 nmol/L, 71.8 nmol/L]). As expected, the adjusted model (
Table 3) showed that older age and males were associated with shorter LTL. Longer LTL was observed in Blacks than in Whites and South Asians. Higher education, lower whole body fat mass, never smoking, and physical activity was associated with longer LTL, but lower Townsend deprivation or alcohol intake frequency was not significantly associated with LTL. Calcium in the blood also was not significantly associated with LTL.
4. Discussion
In this population-based study, we examined the association between LTL and vitamin D status. Shorter LTL was associated with high and low 25OHD levels in older individuals aged 60 and older. Interestingly, we found an inverted U-shaped association between shorter LTL and serum 25OHD at very low and high serum levels.
The clinical implications of vitamin D deficiency on the musculoskeletal system [
6], which include osteosarcopenia [
19] and rheumatic conditions [
20], are well established. In addition, vitamin D deficiency is also associated with the development of age-related conditions such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, and cardiovascular disease [
6]. Although the biological mechanisms explaining these associations remain partially elucidated, there is mounting evidence to propose that normal vitamin D levels are required to delay the appearance of several of the major biological hallmarks of aging [1, 6], including genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled autophagy, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and chronic inflammation [
6,
21].
Amongst these mechanisms, the effect of vitamin D on telomere attrition has received significant attention not only because of its biological plausibility but also because there is a clear correlation between those age-related conditions associated with vitamin D deficiency and those associated with telomere shortening [2, 6]. Vitamin D may reduce telomere shortening through anti-inflammatory and anti-cell proliferation mechanisms. Interestingly, very few clinical trials have tested the effect of vitamin D supplementation on telomere length in vitamin D-deficient populations, and the reports appear contradictory. Yang et al. [
22] tested the effect of vitamin D supplementation on cognitive function in 183 subjects randomized to an intervention group (vitamin D 800 IU/day, n = 93) or a placebo group (the matching starch granules, n = 90), and followed up for 12 months. They reported that vitamin D supplementation for 12 months improved cognitive function by reducing oxidative stress regulated by increased LTL in older adults with mild cognitive impairment. In contrast, Agirbasli et al. [
23] investigated the short-term effects of vitamin D supplementation on LTL in a cohort of vitamin D-deficient postmenopausal women (n = 102). The group was divided into supplementation those with oral vitamin D
3 (cholecalciferol) at a dose of 50,000 IU/week for eight weeks (n = 52) and placebo groups (n = 50). At the end of the study period, LTL levels were significantly increased in both groups, and this change was more prominent in the placebo group.
Although normalization of serum 25OHD levels in older individuals could have a beneficial effect on LTL and thus partially explain the therapeutic impact of vitamin D supplementation on age-related conditions, there is always a risk of administering too much supplementation and inducing harmful effects associated with high serum levels of vitamin D. Indeed, results from observational, population-based studies and randomized clinical trials have shown a U- or J-shaped curve and suggested an increased risk of adverse outcomes in those with the highest serum 25OHD levels, including falls, fractures, and frailty [
24,
25,
26]. Most studies have reported a higher risk in those participants with serum levels of 25OHD above 100 nmol/L. Although these findings have discouraged the practice of administering high loading doses of vitamin D, the mechanisms underlying the negative impact of high serum levels of 25OHD remain mostly speculative [
26]. Since telomere shortening is also associated with an increased risk of these adverse events [
25,
27,
28], it is therefore tempting to speculate that induction of LTL shortening by high levels of serum 25OHD could be among the involved mechanisms but require further studies.
In the present study, our data show a non-linear relationship between 25OHD and LTL after modeling vitamin D by the serum 25OHD z-score groups: ≤ -2, (-2, -1], (-1, 1], (1, 2], and >2. A z score of -2 corresponds to serum 25OHD 16.6 nmol/L in the original scale, and a z score of 2 corresponds to 95.9 nmol/L. These extremely low and moderately high 25OHD serum levels approach those previously associated with adverse outcomes in clinical trials (25). Whether a dose response could explain these findings goes beyond the scope of this study.
5. Conclusion
In conclusion, our study is the first of its kind to demonstrate an inverted U-shape relationship between LTL and vitamin D status in a large sample of community-dwelling individuals. We conducted this analysis adjusting for various demographic/socioeconomic variables and lifestyle factors known to affect serum vitamin D and/or telomere length. We also controlled for whole body fat since obese and overweight individuals tend to demonstrate blunted responses to vitamin D replacement [
29]. Nonetheless, the cross-sectional nature of our study prohibits any causal inferences, limiting our ability to exclude the role of unknown confounders or the existence of reverse causation. Nevertheless, non-linear Mendelian randomization methods are useful tools to test causality [
30]. Further mechanistic studies in animal models and human subjects are still required.
Author Contributions
Conceptualization, CL.K., B.K., M.X, and G.D.; methodology, CL.K., B.K., M.X, and G.D.; formal analysis, CL.K.; data curation, CL.K.; writing—original draft preparation, CK.K. and G.D..; writing—review and editing, CL.K., B.K., M.X, LC.P., GA.K., R.K., and G.D.; supervision, G.K. and G.D.; funding acquisition, CL.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an R21 grant (NR018963-01A1) funded by the National Institute of Nursing Research, National Institute of Health, USA to CL.K..
Institutional Review Board Statement
UK Biobank received ethical approval from the Northwest Centre for Research Ethics Committee (11/NW/0382).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors acknowledge the UK Biobank for their role in data collection. This study was conducted using the UK Biobank Resource Application Number: 14631.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2022 Dec 26:S0092-8674(22)01377-0. [CrossRef]
- Gruber HJ, Semeraro MD, Renner W, Herrmann M. Telomeres and Age-Related Diseases. Biomedicines. 2021 Sep 27;9(10):1335. [CrossRef]
- Kirk B, Al Saedi A, Duque G. Osteosarcopenia: A case of geroscience. Aging Med (Milton). 2019 Sep 8;2(3):147-156. [CrossRef]
- Lorenzi M, Bonassi S, Lorenzi T, Giovannini S, Bernabei R, Onder G. A review of telomere length in sarcopenia and frailty. Biogerontology. 2018 Jul;19(3-4):209-221. [CrossRef]
- Umar M, Sastry KS, Chouchane AI. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int J Mol Sci. 2018 May 30;19(6):1618. [CrossRef]
- Berridge MJ. Vitamin D deficiency accelerates ageing and age-related diseases: A novel hypothesis. J Physiol. 2017 Nov 15;595(22):6825-6836. [CrossRef]
- Zarei M, Zarezadeh M, Hamedi Kalajahi F, Javanbakht MH. The Relationship Between Vitamin D and Telomere/Telomerase: A Comprehensive Review. J Frailty Aging. 2021;10(1):2-9. [CrossRef]
- Mazidi M, Mikhailidis DP, Banach M, Dehghan A. Impact of serum 25-hydroxyvitamin D 25(OH) on telomere attrition: A Mendelian Randomization study. Clin Nutr. 2020 Sep;39(9):2730-2733. [CrossRef]
- Beilfuss J, Camargo CA Jr, Kamycheva E. Serum 25-Hydroxyvitamin D Has a Modest Positive Association with Leukocyte Telomere Length in Middle-Aged US Adults. J Nutr. 2017 Apr;147(4):514-520. [CrossRef]
- Liu JJ, Cahoon EK, Linet MS, Little MP, Dagnall CL, Higson H, Savage SA, Freedman DM. Relationship between plasma 25-hydroxyvitamin D and leucocyte telomere length by sex and race in a US study. Br J Nutr. 2016 Sep;116(6):953-60. [CrossRef]
- Grant WB, Karras SN, Bischoff-Ferrari HA, Annweiler C, Boucher BJ, Juzeniene A, Garland CF, Holick MF. Do studies reporting ‘U’-shaped serum 25-hydroxyvitamin D-health outcome relationships reflect adverse effects? Dermatoendocrinol. 2016;8(1):e1187349. [CrossRef]
- Davis CD. Vitamin D and health: Can too much be harmful? Am J Lifestyle Med. 2009;3(5):407–408. [CrossRef]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015 Mar 31;12(3):e1001779. [CrossRef]
- Elliott P, Peakman TC, Biobank UK, UK Biobank . The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol 2008;37:234–44. [CrossRef]
- CDC CDC vitamin D Standardization-Certification program (CDC VDSCP) 2020.
- UK Biobank Biomarker assay quality procedures: Approaches used to minimise systematic and random errors. 1.2 ed, 2019.
- UK Biobank Companion document for serum biomarker data. 1 ed, 2019.
- Cassidy S, Chau JY, Catt M, Bauman A, Trenell MI. Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233,110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ Open. 2016 Mar 15;6(3):e010038. [CrossRef]
- Bruyère O, Cavalier E, Reginster JY. Vitamin D and osteosarcopenia: An update from epidemiological studies. Curr Opin Clin Nutr Metab Care. 2017 Nov;20(6):498-503. [CrossRef]
- Charoenngam N. Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence. Int J Mol Sci. 2021 Oct 1;22(19):10659. [CrossRef]
- Giudici KV. Nutrition and the Hallmarks of Aging. J Nutr Health Aging 25, 1039–1041 (2021). [CrossRef]
- Yang T, Wang H, Xiong Y, Chen C, Duan K, Jia J, Ma F. Vitamin D Supplementation Improves Cognitive Function Through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J Alzheimers Dis. 2020;78(4):1509-1518. [CrossRef]
- Agirbasli D, Kalyoncu M, Muftuoglu M, Aksungar FB, Agirbasli M. Leukocyte telomere length as a compensatory mechanism in vitamin D metabolism. PLoS One. 2022 Feb 24;17(2):e0264337. [CrossRef]
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA. 2010 May 12;303(18):1815-22. [CrossRef]
- Kojima G, Iliffe S, Tanabe M. Vitamin D supplementation as a potential cause of U-shaped associations between vitamin D levels and negative health outcomes: A decision tree analysis for risk of frailty. BMC Geriatr. 2017 Oct 16;17(1):236. [CrossRef]
- Sanders KM, Nicholson GC, Ebeling PR. Is high dose vitamin D harmful? Calcif Tissue Int. 2013 Feb;92(2):191-206. [CrossRef]
- Wong SK, Ima-Nirwana S, Chin KY. Can telomere length predict bone health? A review of current evidence. Bosn J Basic Med Sci. 2020 Nov 2;20(4):423-429. [CrossRef]
- Rippberger PL, Emeny RT, Mackenzie TA, Bartels SJ, Batsis JA. The association of sarcopenia, telomere length, and mortality: Data from the NHANES 1999-2002. Eur J Clin Nutr. 2018 Feb;72(2):255-263. [CrossRef]
- Tobias DK, Luttmann-Gibson H, Mora S, Danik J, Bubes V, Copeland T, LeBoff MS, Cook NR, Lee IM, Buring JE, Manson JE. Association of Body Weight With Response to Vitamin D Supplementation and Metabolism. JAMA Netw Open. 2023 Jan 3;6(1):e2250681.
- Staley JR, Burgess S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol. 2017 May;41(4):341-352. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).