1. Introduction
Food intake and energy balance is controlled mostly by the central nervous system (CNS). An increase or decrease in food consumption is regulated by several orexigenic or anorexigenic neurons located in the CNS. These neurons are activated or deactivated through signals elicited by hormones secreted from the CNS or periphery, such as stomach, intestine, and pancreas. These neurons have ties with the ventral tegmental area (VTA) via the paraventricular nucleus (PVN). The dopaminergic neurons in the VTA project to the nucleus accumbens (NAc) [
1], a brain area often referred to as the hedonic hotspot [
2]. Intake of food to fulfil the energy requirements of the body is referred to as homeostatic feeding. However, when energy requirement is met, consumption of palatable food beyond the energy requirement activates reward circuit, causing dopamine release in the NAc [
3], thereby leading to binge eating and possibly food addiction. However, the underlying mechanism of food reward and addiction is not fully understood.
Both palatable diet and addictive drugs activate the mesolimbic dopaminergic system [
4]. The endogenous opioid system, consists of opioid peptides and opioid receptors, has also been implicated in food reward and food addiction [
5]. Along this line, palatable food exposure has been shown to upregulate MOR among obese rats [
6]. Likewise, excessive high fat diet consumption has been implicated in dysregulated dopamine and opioid gene expression [
7]. Similarly, overeating of palatable food has been shown to involve the endogenous opioid system [
8]. Moreover, consumption of either palatable or non-palatable/regular food has been reported to induce the release of endogenous opioids [
9]. Additionally, local administration of MOR antagonists, such as naltrexone, in the NAc, amygdala has been reported to reduce palatable diet intake [
10,
11]. Overall, these observations suggest that endogenous opioids can regulate food consumption and may be involved in food reward. Thus, in the current research, we have combined the place conditioning paradigm with food intake to assess if conditioning with a high-fat diet (HFD) induces reward in mice and if the duration of conditioning with HFD would be important in the acquisition of a conditioned place preference (CPP). Considering that MOR is implicated in palatability of food, we also determined the role of MOR in HFD-induced CPP. To address these issues, we used MOR knockout and their wildtype controls to investigate if there is any difference in HFD consumption and HFD-induced reward between mice of the two genotypes.
Not only endogenous opioids, but also exogenous opioids may be involved in food intake and food reward. For instance, local administration of a selective mu opioid receptor (MOR) agonist in the NAc, amygdala, hypothalamus, or VTA has been shown to increase palatable diet intake [
12,
13,
14]. Conversely, a previous report has shown lowering of food liking after treatment with MOR antagonist [
15]. Furthermore, MORs have strong correlation with control of palatable diet intake [
16]. Together, these findings suggest that there is a crosstalk between food reward and the rewarding actions of opioids. We hypothesized that prior exposure to HFD induces sensitization via the release of endogenous opioids acting at MORs. Thus, we also assessed if a prior conditioning with HFD would alter the rewarding action of oxycodone, a relatively selective MOR agonist.
Evidence exists to suggest for a strong relationship between sex hormones and opioids. Interestingly, populations of neurons contining proopiomelanocortin (POMC, a precursor for the the synthesis of beta-edorphin, an endogenous opioid) have been reported to express estrogen receptors (αERs). In addition, POMC mRNA fluctuates throughout the different phases of the estrus cycle, and estrogen depletion via ovariectomy in females, has been reported to decrease POMC mRNA [
17]. Furthermore, knocking out the αER on POMC neurons leads to an increase in food consumption and body weight in female but not male mice [
17]. Moreover, both MOR and αER are present in the ARC and NAc [
1,
18,
19], raising the possibility that an interaction between these two receptor types is likely and thus male/female differences may be present in food intake and food reward or in the involvement of MORs in food reward. Considering that the rewarding actions of drugs of abuse can be different between males and females [
20], we also assessed if sex-related differences exist in HFD-induced reward, crosstalk between food reward and oxycodone reward or in the involvement of MORs in these responses.
2. Materials and Methods
2.1. Materials. Subjects
Age-matched male (25-30 g) and female (20-21 g) C57BL/6J mice as well as male (30-32 g) and female (20 -21 g) mice lacking MOR backcrossed for 12 generations on a C57BL/6J mouse strain bred in-house were used throughout. Each group consisted of 6 male and 6 female C57BL/6J mice or mice lacking MOR and their wildtype littermates. The original breeders of each line were purchased from Jackson Laboratories (Bar harbor ME, USA). Subjects were kept one mouse per cage in a temperature-controlled (22±3 °C) under a 12 h/12h light/dark cycle (6 am light on and 6 pm light off). Animals had access to regular laboratory chow and water ad libitum except during the experiment. All the procedures were in accord with the NIH guideline for the use of animals in research and approved by the Institutional Animal Care and Use Committee (IACUC) at Western University of Health Sciences (Pomona, California, USA).
2.2. Drug
Oxycodone hydrochloride, purchased from Sigma Aldrich (St. Louise, MO, United States), was dissolved in normal saline (0.9% sodium chloride solution) and injected intraperitoneally to each mouse during the conditioning at a dose of (5 mg/kg, i.p.) per body weight.
2.3. Diets
High fat diet (HFD) for rodents was purchased from Research Diets (New Brunwick, NJ 08901, USA). The diet contains 60 Kcal % fat (Code name D12492, which contains 245 gm of fat generated from lard, 200 gm lactic protein, and 125 gm carbohydrate in each 773.85 gm). The regular chow diet (RCD) for rodents contains 18% protein (protein 18.6 %, fat 6.2 %, and carbohydrate 44.2 %) purchased from Teklad Global Diet (Madison, Wisconsin, United States).
2.4. To Determine if Binge Eating of a High-Fat Diet (HFD) Induces Conditioned Place Preference or Alter the Rewarding Action Oxycodone, a Mu Opioid Receptor Agonist, and if Sex-Related Differences Exist in These Responses
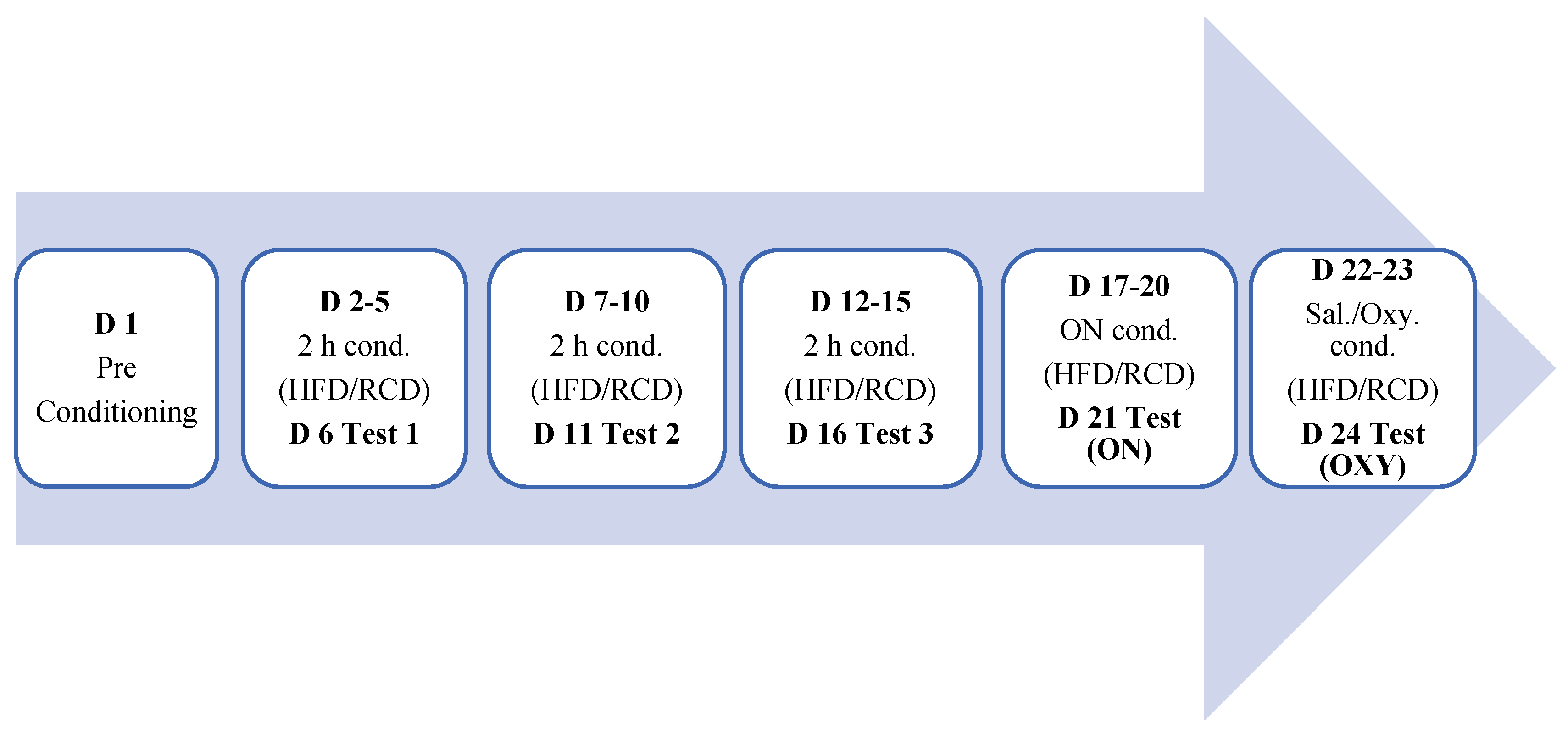
We used the place conditioning paradigm, widely used as an animal model of reward [
21], to determine if high fat diet would induce reward or alter the rewarding effect of oxycodone, a mu opioid receptor agonist. We also used both male and female mice and assessed if sex-related differences exist in these responses. The place conditioning protocol consisted of three phases: (1) preconditioning, (2) conditioning, and (3) postconditioning. The preconditioning test was conducted on day 1 to assess the baseline place preference of each mouse toward the conditioning chambers. On this day, each mouse was placed in the gray central neutral chamber with both guillotine doors opened and allowed to explore the three chambers for 15 min. The amount of time that mice spent in each chamber was recorded. Subjects who spent more than 67% or less than 33% of the total time (900s) in any of the chambers were excluded from the remaining of the study, according to an earlier report [
22]. Mice were then divided into two groups: (1) treated group and (2) control group. The treated group received conditioning in the next four days, two with the HFD in one of the conditioning chambers or paired chamber (PCh) and two with the RCD in the opposite chamber or non-paired chamber (NPCh). The control group received conditioning with RCD in both chambers but one of the conditioning chambers was considered as the PCh and the other as NPCh. The conditioning was carried out from 3-5 pm (2 hours) each day. On day 6, mice were then tested for place preference for 15 minutes (test 1), as described for day 1. Given that we did not observe any preference toward the PCh, we continued with two additional sets of conditioning and tested mice for placed preference. After the third test for place preference, they received conditioning for 16 hours (6 pm – 10 am) for the next four days. Mice were then tested for place preference. The rationale for overnight conditioning was that we hypothesized that a longer conditioning with HFD would induce reward.
We then tested if HFD conditioning for the rewarding action of oxycodone to assess if prior conditioning with HFD would alter the rewarding action of oxycodone, a relatively selective MOR agonist. To this end, the day after the last conditioning, mice were conditioned with oxycodone (5 mg/kg, i.p.) in the PCh and saline in the NPCh for one hour. On the following day, mice received the alternate treatment and were conditioned to the opposite chamber. Twenty-four h later, mice were tested for a place preference toward the chambers, as described for day 1. A schematic presentation of the place conditioning protocol is provided below.
Figure 1.
A schematic presentation of the experimental procedure in mice. Abbreviations: D: day; Cond.: conditioning; ON: overnight; OXY: test after oxycodone conditioning; HFD: high fat diet; RCD: regular chow diet; sal: saline, oxy: oxycodone.
Figure 1.
A schematic presentation of the experimental procedure in mice. Abbreviations: D: day; Cond.: conditioning; ON: overnight; OXY: test after oxycodone conditioning; HFD: high fat diet; RCD: regular chow diet; sal: saline, oxy: oxycodone.
2.5. To Assess the Role of MOR in High-Fat Diet (HFD) Induced Reward and if Sex-Related Differences Exist in These Responses
The experimental procedure was the same as described above except MOR knockout and wildtype mice were used and mice of both genotypes were exposed to HFD or RCD on alternative days. Given that we did not observe any place preference in the control mice and to order to reduce the number of mice used, we did not include the control group in this experiment.
2.6. Data Analysis
The data are presented as means ± standard errors of the mean (± SEM) of the amount of time that mice spent in the PCh and NPCh. Data were analyzed using three-way repeated measures analysis of variance (ANOVA) followed by the Fisher’s LSD post hoc test for multiple comparison. For the C57BL/6J mice, the between factors were group (control vs. treated), context and time, and for the MOR knockout and their wildtype controls, the between factors were genotype, context and time (for the MOR wildtype and knockout mice) with time as the within factor. P ≤ 0.05 was considered statistically significant. Each group contained 6 male and 6 female mice per group or genotype.
3. Results
3.1. Conditioning with a High-Fat Diet (HFD) for 16 h but Not 2 h Induced Conditioned Place Preference and Enhanced the Rewarding Action of Oxycodone in Male and Female C57BL/6J Mice
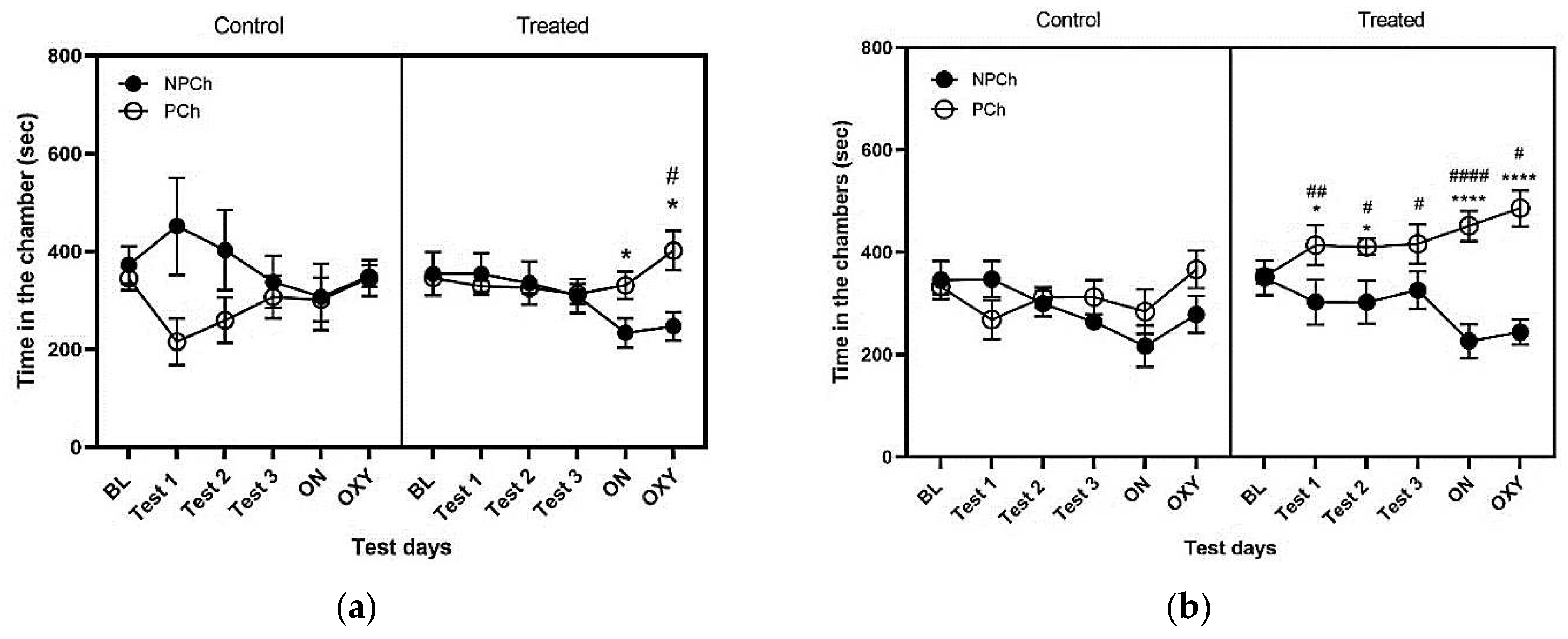
Figure 2 illustrates the amount of time that female (2a) and male (2b) mice spent in the PCh and NPCh. Female mice only showed preference toward the PCh over the NPCh following the LAC (Fig. 2a
). Three-way repeated measures ANOVA revealed a significant interaction between context and treatment (F (1, 59) = 7.062; P<0.05). The Fisher’s LSD
post hoc test showed that SAC with HFD failed to induce CPP in these mice. On the other hand, when mice were exposed to LAC with HFD, they spent more time in the PCh than NPCh (P<0.05; Fig. 2a, right panel, ON). In contrast, control mice did not exhibit a preference for the PCh over NPCh in response to SAC or LAC (P>0.05; Fig. 2a, left panel). Likewise, oxycodone failed to induce CPP in control mice (Fig. 2a, left panel). In contrast, treated mice exhibited more preference toward the PCh over NPCh (P<0.05; Fig. 2a
, right panel).
The amount of time that male mice spent in the PCh and NPCh is shown in
Figure 2b
. Three-way repeated measures ANOVA showed a significant interaction between context and treatment (F (1, 60) = 14.23; P<0.001) as well as a significant effect of treatment (F (1, 60) = 18.93, P<0.0001) and context (F (1, 60) = 27.09; P<0.0001). The Fisher LSD
post hoc test revealed that there was no difference between time spent in the two chambers by control male mice (P>0.05). On the other hand, mice exposed to HFD in one chamber and RCD in the opposite chamber spent more time in the PCh than NPCh on the test days following the first and second sets of conditionings (P<0.05; Fig. 2b, right panel). While control mice showed no preference towards the PCh vs. NPCh (Fig. 2b, left panel), the treated mice continued to display a significant (P<0.0001) preference towards PCh vs. NPCh after conditioning with oxycodone (Fig. 2b, right panel). This result demonstrates that LAC with HFD induced a CPP response and potentiated the rewarding action of oxycodone.
3.2. LAC but Not SAC with HFD Induced CPP and Enhanced the Rewarding Action of Oxycodone in Wild-Type (MOR+/+) but Not MOR Knockout (MOR-/-) Mice
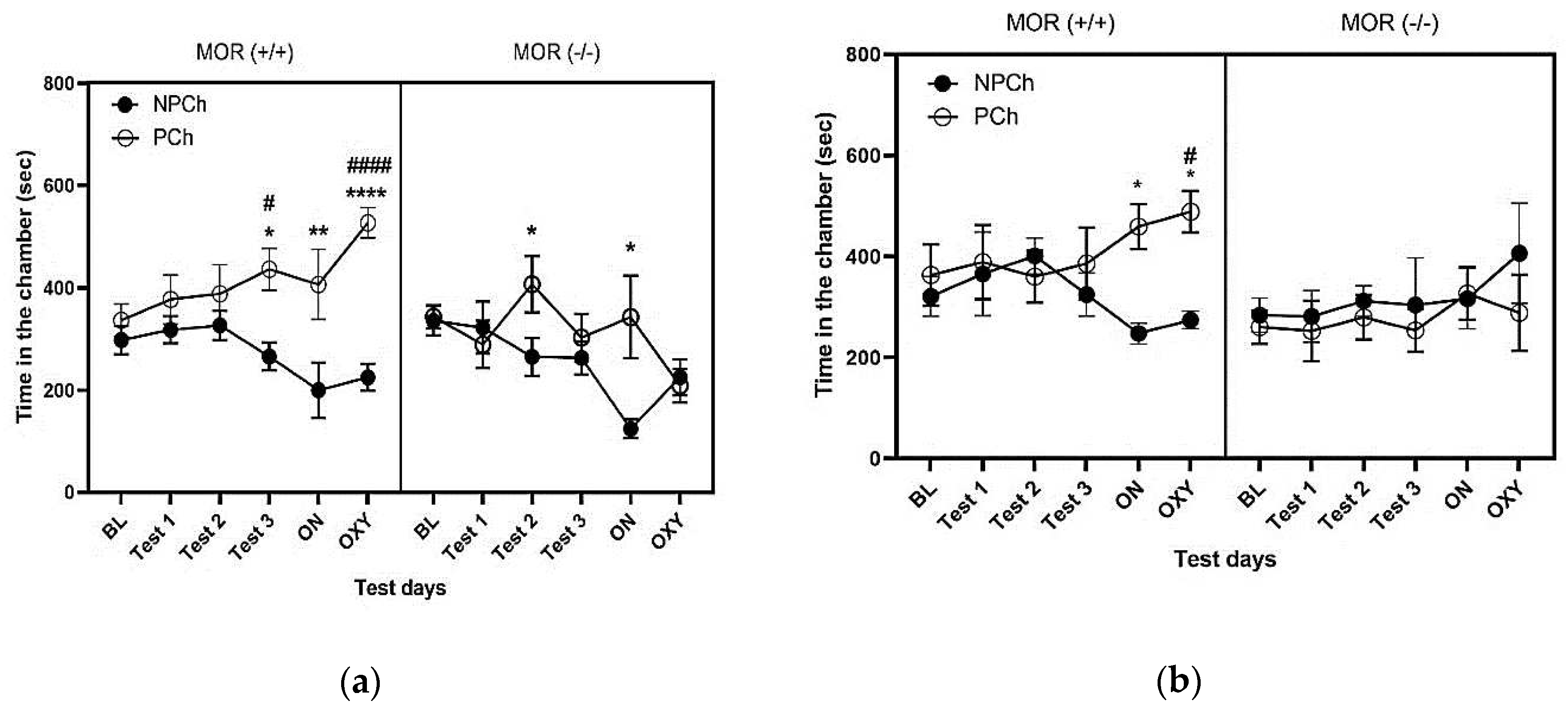
Figure 3 illustrates the amount of time that female
(a) and male
(b) mice lacking MOR (right panel) and their wildtype controls (left panel) spent in the PCh and NPCh. Three-way repeated measure analysis of variance (ANOVA) revealed a significant effect of genotype (F (1, 87) = 19.88, P<0.0001) and treatment (F (1, 87) = 21.92, P<0.0001). Also, a significant interaction between time and genotype (F (5, 87) = 3.737, P<0.01) and time and treatment (F (5, 87) =2.859; P<0.05) was observed. Fisher’s LSD
post hoc test showed that female wildtype mice spent more time in the PCh than NPCh during test 3 (P<0.05) as well as following the LAC (P<0.01), and oxycodone conditioning (OXY) (P<0.0001) (Fig. 3a, left panel). While mice lacking MOR did not exhibit consistent CPP response. Fisher’s LSD
post hoc test revealed that MOR (-/-) mice showed no CPP during test 1 but during test 2 but not test 3 they showed a modest CPP response (P<0.05) (Fig. 3 a, right panel). However, they showed a CPP response after LAC with HFD but not following the oxycodone conditioning (Fig. 3 a, right panel).
Figure 3 b depicts the amount of time that male MOR knockout mice (right panel) and their wild-type littermates/controls (left panel) spent in the PCh and NPCh. Three-way repeated measures analysis of variance (ANOVA) revealed an effect of genotype (F (1, 60) = 15.7; P<0.001) and a significant interaction between genotype and treatment (F (1,60) =5.160; P<0.05). The Fisher’s LSD
post hoc test showed that SAC with HFD failed to induce CPP response in male mice. But after LAC with HFD, they spent more time in the PCh than NPCh (P<0.05; Fig. 3 b, left panel, ON). On the other hand, MOR (-/-) mice did not exhibit any CPP in response to SAC or LAC (P>0.05; Fig. 3 b, right panel). Oxycodone failed to induce CPP in MOR knockout mice (Fig. 3 b, right panel). On the other hand, wildtype mice exposed to HFD showed a robust preference toward the PCh compared to the NPCh after conditioning with oxycodone (P<0.05). These results suggest that HFD-induced CPP and the potentiation of oxycodone reward are mediated via MOR, but sexual dimorphism exists in HFD-induced CPP.
3.3. Comparison of CPP Response between male And Female Control vs. Treated Mice
Figure 4 shows a comparison of the CPP response between males and females of treated (upper panel) and control (lower panel) groups. A three-way repeated measure ANOVA revealed a significant effect of sex (F (1, 59) = 6.646, P<0.05) and treatment (F (1, 59) = 37.41, P<0.0001). There was also a significant interaction between sex and treatment (F (1, 59) = 12.20, P<0.001). Moreover, the Fisher’s LSD
post hoc test showed that there was a significant increase in the amount of time that male treated mice spent in the PCh compared to female mice on test 3 (P<0.05; Fig. 4
, upper panel) and test after overnight (ON) conditioning (P<0.01; Fig. 4, upper panel). On the other hand, three-way repeated measure ANOVA revealed that there was a modest effect of sex (F (1, 60) = 4.012; P<0.05) and an interaction between sex and context (F (1, 60) = 5.075; P<0.05) in male and female control mice. Fisher’s LSD
post hoc test revealed no significant differences between male and female control groups in the amount of time that mice spent in the so-called PCh (Fig. 4, bottom panel).
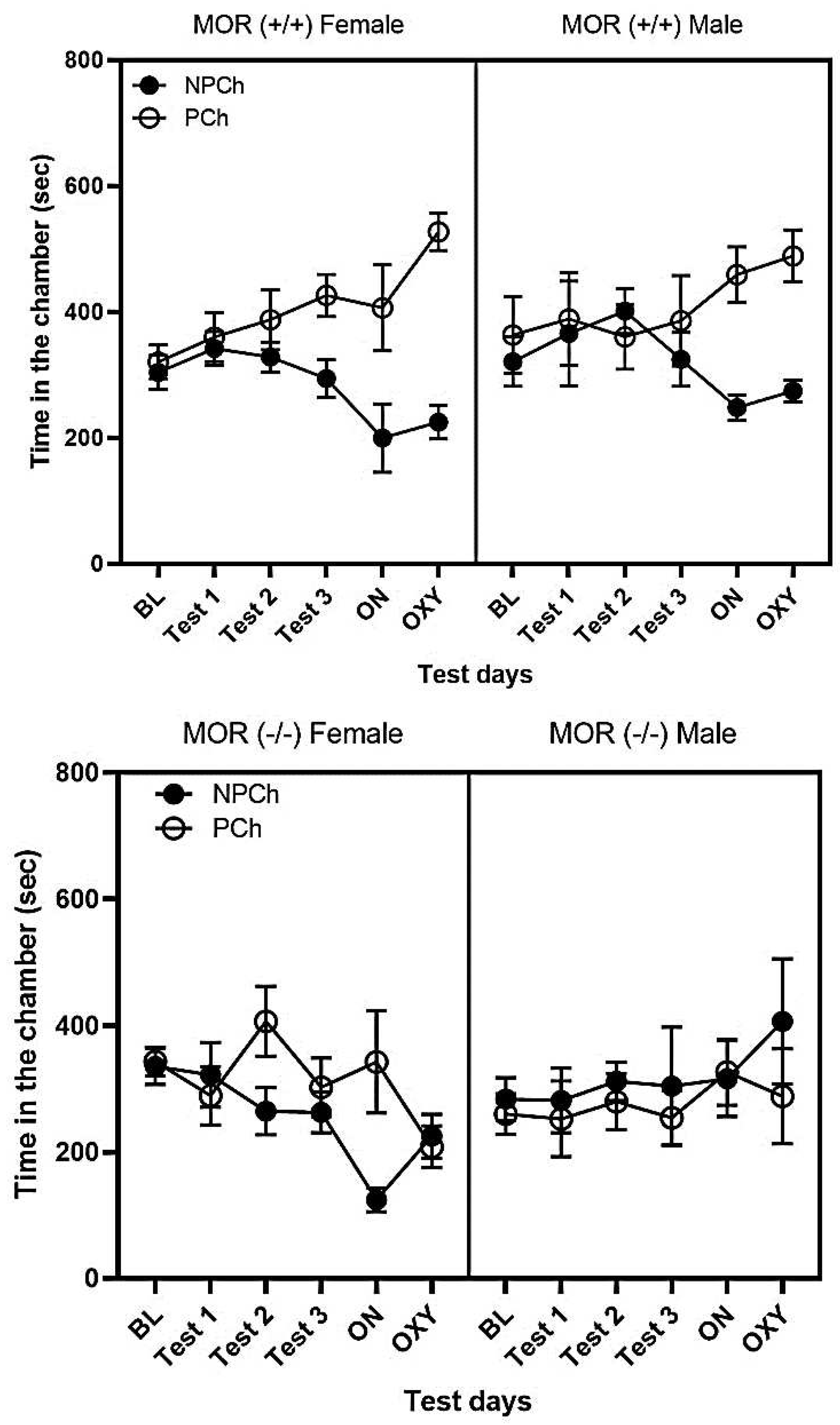
3.4. Comparison of CPP Response between Male and Female MOR Knockout and Wildtype Mice
Figure 5 compares the amount of time that male versus female MOR (-/-) and MOR (+/+) mice spent in the PCh and NPCh. A three-way repeated measure ANOVA revealed a significant effect of genotype (F (1, 80) = 20.41, P<0.0001) and interaction between time and genotype (F (1, 80) = 3.465, P<0.01) in male and female wildtype (MOR+/+) mice. Fisher’s LSD
post hoc test suggested no significant difference in the amount of time that male mice spent in the PCh than female mice (Fig. 5, top panel). For the MOR (-/-) mice, three-way repeated measure ANOVA did reveal a significant interaction between sex and genotype (F (1, 67) = 4.707, P<0.05). However, the Fisher’s LSD
post hoc test showed that there was no difference between male and female MOR (-/-) mice (P>0.05) (Fig. 5, bottom panel).
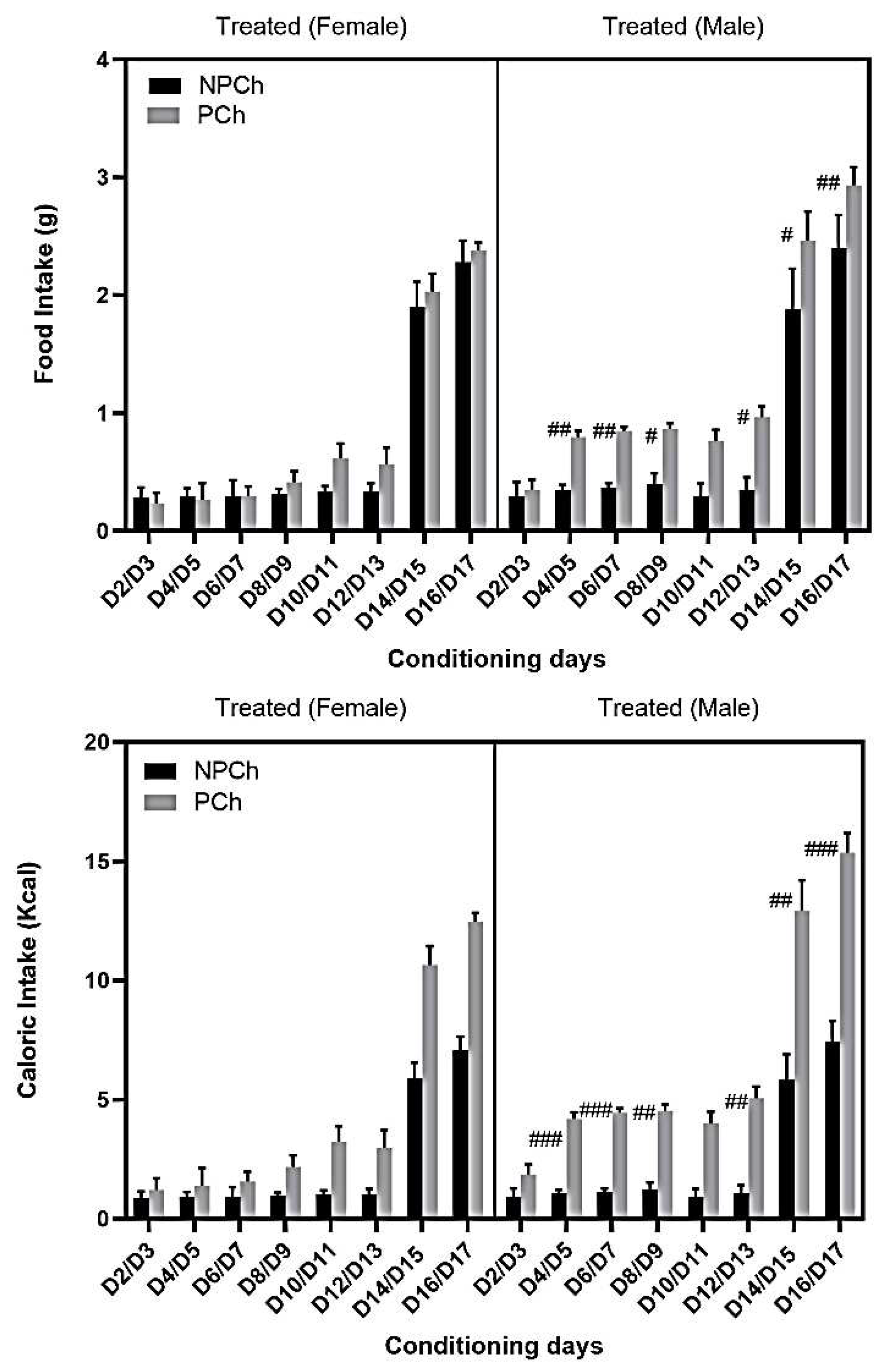
3.5. Food intake and Calorie Consumption between Treated and Control Male and Female C57BL/6J
Food intake (upper panel) and calorie consumption (lower panel) in male and female mice is shown in
Figure 6. Three-way repeated measures ANOVA revealed a significant effect of time (F (7, 80) = 119.1 P<0.0001), sex (F (1, 80) = 15.32, P<0.001) and treatment (F (1, 80) = 52.63, P<0.0001). There was also a significant interaction between sex and treatment (F (1, 80) = 22.43, P<0.0001). The Fisher’s LSD
post hoc test showed that the HFD intake was higher in males compared to females on D4-D7, D16-17 (P<0.01) and D8-9, D12-15 (P<0.05; Fig. 6; HFD intake in Male vs. Female). Likewise, calorie intake was higher in male treated mice (Fig. 6, lower panel). On the other hand, three-way repeated measure ANOVA revealed a significant effect of time (F (7, 80) =128.7, P<0.0001), sex (F (1, 80) =24.00, P<0.0001) and treatment (F (1, 80) = 406.2, P<0.0001). Fisher’s LSD
post hoc test showed that there was an increase in caloric intake in male than female mice on D4-D7 and D16/D17 (P<0.001; Fig. 6, bottom panel).
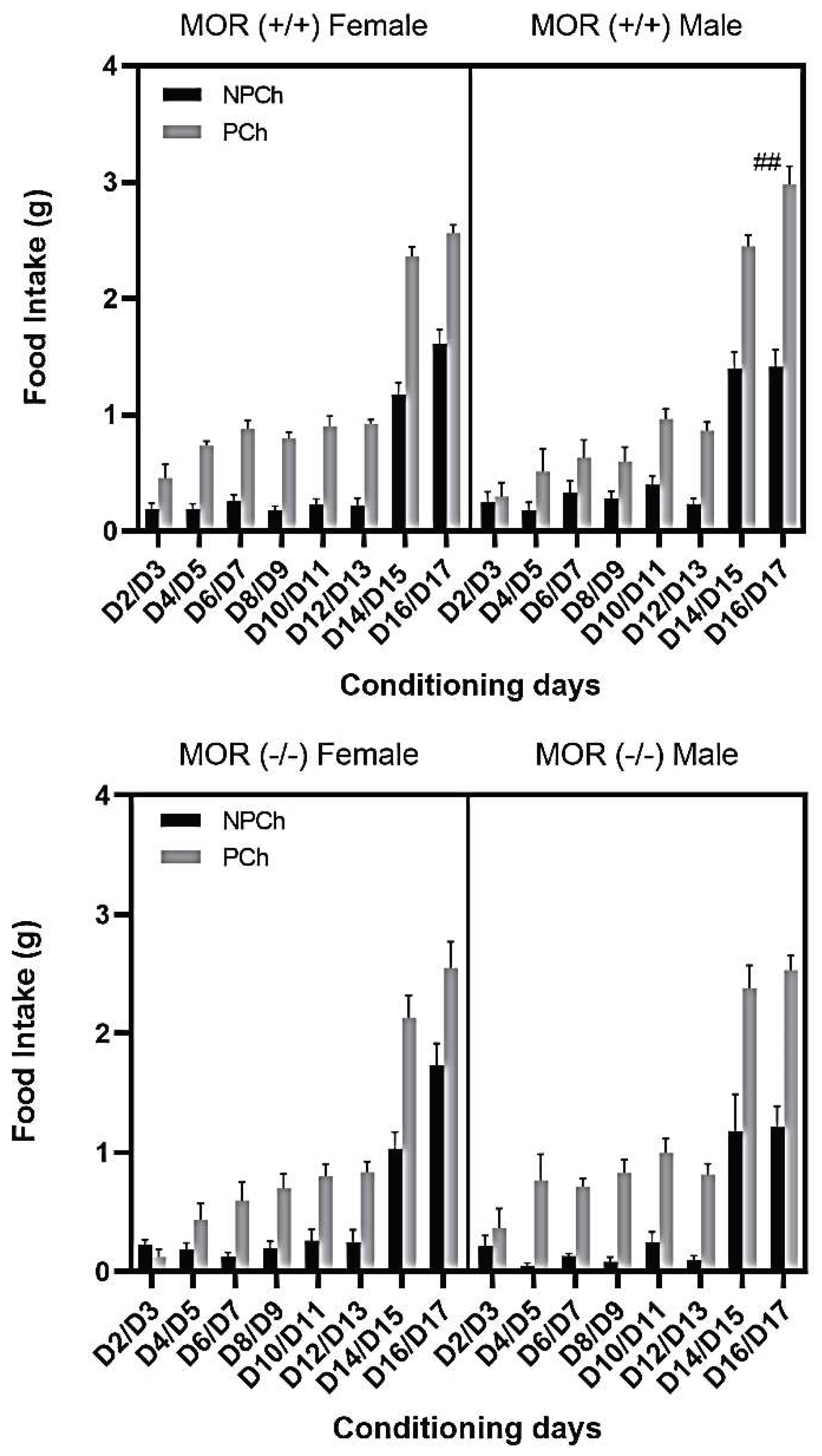
3.6. Comparison in Food Intake between MOR Male and Female Mice (Knockout vs. Wildtype)
Figure 7 depicts food consumption in male and female wildtype (upper panel) and knockout (lower panel) mice. Three-way repeated measure ANOVA of data in wildtype mice showed a significant effect of time (F (7, 108) =189.7; P<0.0001) and treatment (F (1, 108) =583.6; P<0.0001) and an interaction between time and treatment (F (7, 108) = 55.85; P<0.0001). However, Fisher’s LSD
post hoc test revealed only a significant (P<0.01) increase in food intake in male than female mice on day 17 (D17; Fig. 7, top panel). For the MOR (-/-) male and female mice, three-way repeated measures ANOVA revealed a significant effect of time (F (7, 92) = 94.67; P<0.0001) and treatment (F (1,92) = 242.0; P<0.0001) and a significant interaction between time and treatment (F (7, 92) =8.577; P<0.0001). Fisher’s LSD
post hoc test showed that there was no significant difference between food intake between male and female MOR (-/-) mice on any of the conditioning days.
4. Discussion
The main findings of the current study are that male and female mice exhibited a significant place preference only following long access to HFD in the conditioning chamber. The preference becomes more robust following conditioning with oxycodone, which failed to induce any significant preference in control mice, i.e., mice conditioned with regular chow diet (RCD) in both conditioning chambers. These results suggest that LAC with HFD induces conditioned place preference (CPP) and potentiates oxycodone-induced reward, as we only observed CPP following oxycodone in the treated but not control mice. We also observed that male mice lacking MOR failed to exhibit any CPP following SAC or LAC as well as following oxycodone conditioning. In contrast, female MOR knockout mice exhibited a variable CPP response after SAC and LAC; yet, not in response to conditioning with oxycodone. These results suggest that the rewarding action of HFD and oxycodone may involve MOR, but male/female differences may exist in the former response.
Previous studies have shown that overconsumption of fat-rich palatable diets induced drug-like reward hyposensitivity [
23]. So, we assessed if conditioning with HFD for 2 h (short-access to HFD), which may lead to binge eating in both males and females, could induce reward in C57BL/6J mice. Our data showed that short-term (2h) conditioning with HFD induced modest reward in male but not in female C57BL/6J mice. However, this response was not recapitulated in male MOR wildtype mice. When male and female mice received 16-h conditioning with HFD, it elicited a significant CPP response in both male and female mice. We link this CPP response to the increased HFD consumption compared to 2 h conditioning, when animals had access for a short time to HFD. There was a 2-3-fold increase in food intake during the LAC compared to SAC. Although SAC led to binge eating, but it did not consistently induce CPP in male and female mice. Thus, the duration of conditioning and the amount of HFD consumed may be needed to result in a significant preference toward the chamber paired with HFD.
An earlier research claims that palatable foods recruit the endogenous opioids [
24]. Another study reported intermittent sugar intake led to endogenous opioid dependence [
25]. Considering that the endogenous opioid system has been implicated in the palatability of food [
12,
26], we proposed that prior conditioning with HFD would enhance the rewarding action of oxycodone, a mu opioid receptor agonist [
27]. To test this possibility, we used a single conditioning paradigm and a low oxycodone dose (5 mg/kg, i.p.) which did not induce CPP in male or female control mice in the current study. In contrast, both male and female mice conditioned with HFD in one conditioning chamber and RCD in the opposite chamber (treated group) displayed a robust CPP response following the single conditioning with oxycodone, when paired with the HFD-paired chamber (PCh). Our results are consistent with previous reports [
28,
29,
30] suggesting that prior exposure to HFD enhances the rewarding action of addictive drugs, in this case, oxycodone. However, given that it is generally thought that palatable food is rewarding (for a review, see [
31]) and that mice already showed CPP following LAC with HFD, it is unclear whether the CPP response following oxycodone is potentiation of oxycodone reward or oxycodone conditioning increased the expression of the CPP induced by HFD. Thus, further studies are needed to delineate between these two possibilities in the future.
Chronic HFD exposure has been reported to alter MOR gene expression differentially between male and female mice [
22,
32]. Previous research has reported that fat-rich palatable foods are rewarding [
31] and may cause the release of endogenous opioids known to govern palatable food intake [
33]. Thus, we hypothesized that HFD induces reward, and MOR is involved in this response. We used MOR knockout mice and their wildtype littermates to test our hypothesis. We also determined if any sex-related difference exists in the CPP response induced by HFD or potentiation of oxycodone reward. We discovered a modest CPP response on the third test in female wildtype mice, but we did not observe any significant CPP response in male wildtype mice following SAC with HFD. Nevertheless, we observed a significant CPP response in both male and female wildtype mice after LAC with oxycodone and following oxycodone conditioning. In contrast, only female mice lacking MOR did exhibit a CPP response on the second test, but the response was not sustained on the third test. Again, they expressed a significant CPP after LAC with HFD, which was absent after oxycodone conditioning, showing that the rewarding action of oxycodone is mediated via the MOR. Male wildtype mice did only exhibit CPP after LAC and after conditioning with oxycodone. But the male knockout mice did not express any CPP at all. Despite this, the food intake was the same between mice of the two genotypes. Thus, the lack of CPP in knockout mice was not due to a decrease food intake in these mice, as they were consuming an equal amount of HFD compared their wildtype littermates. Together, these findings suggest that MOR is necessary for the expression of CPP induced by overnight conditioning with HFD and oxycodone in mice, but sexual dimorphism is observed in MOR knockout mice, which requires further investigation. For example, it would be necessary to determine the interaction between sex hormones and MOR, as its expression can be altered during different phases of the estrous cycle [
34]. The NAc can be a potential target to assess for the interaction between MOR and sex hormones, as both MOR and αER are present in the NAc, and this brain area is implicated in food reward and drug reward [
1,
18,
19].
5. Conclusions
This is the first report to reveal that 16 h but not 2 h conditioning with HFD induces reward and enhances the rewarding action of oxycodone in both male and female C57BL/6J mice as well as in MOR wildtype mice. These responses were blunted in male MOR knockout mice. However, female MOR knockout mice demonstrated a variable CPP following conditioning with HFD, suggesting that a sexual dimorphism in HFD-induced reward in the absence of MOR may exist. Thus, further studies are needed to delineate the underlying mechanism of the crosstalk between HFD and oxycodone reward and sexual dimorphism in these responses.
Author Contributions
Conceptualization, K.L.; methodology, A.I., A.H. and S.M.A.; software, A.I.; validation, A.I. and K.L.; formal analysis, A.I. and K.L.; investigation, A.I.; resources, K.L.; data curation, A.I.; writing—original draft preparation, A.I.; writing—review and editing, K.L.; supervision, K.L.; project administration, A.I. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the National Institute of Health for the Care and Use of Animals in Research and approved by the Institutional Animal Care and Use Committee (IACUC) at Western University of Health Sciences (R20IACUC024).
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data are stored in a OneDrive folder belong to WesternU and will be made available upon reasonable request.
Acknowledgments
This study is part of a thesis project of Mr. Asif Iqbal, the first author of this manuscript, that he completed and submitted to the University Library. The authors wish to express their gratitude to the Department of Pharmaceutical Sciences Graduate Program for providing financial support to Mr. Asif Iqbal while he was completing his thesis project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nogueiras, R., et al., The opioid system and food intake: homeostatic and hedonic mechanisms. Obes Facts, 2012. 5(2): p. 196-207. [CrossRef]
- Castro, D.C. and K.C. Berridge, Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness "liking" and "wanting". J Neurosci, 2014. 34(12): p. 4239-50.
- Spanagel, R., A. Herz, and T.S. Shippenberg, Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A, 1992. 89(6): p. 2046-50. [CrossRef]
- Nestler, E.J., Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci, 2001. 2(2): p. 119-28. [CrossRef]
- Mendez, I.A., et al., Involvement of Endogenous Enkephalins and β-Endorphin in Feeding and Diet-Induced Obesity. Neuropsychopharmacology, 2015. 40(9): p. 2103-12. [CrossRef]
- Barnes, M.J., et al., Increased expression of mu opioid receptors in animals susceptible to diet-induced obesity. Peptides, 2006. 27(12): p. 3292-8. [CrossRef]
- Reyes, T.M., High-fat diet alters the dopamine and opioid systems: effects across development. Int J Obes Suppl, 2012. 2(Suppl 2): p. S25-8. [CrossRef]
- Ziauddeen, H., et al., Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry, 2013. 18(12): p. 1287-93. [CrossRef]
- Tuulari, J.J., et al., Feeding Releases Endogenous Opioids in Humans. J Neurosci, 2017. 37(34): p. 8284-8291. [CrossRef]
- Glass, M.J., C.J. Billington, and A.S. Levine, Naltrexone administered to central nucleus of amygdala or PVN: neural dissociation of diet and energy. Am J Physiol Regul Integr Comp Physiol, 2000. 279(1): p. R86-92. [CrossRef]
- Lenard, N.R., H. Zheng, and H.R. Berthoud, Chronic suppression of μ-opioid receptor signaling in the nucleus accumbens attenuates development of diet-induced obesity in rats. Int J Obes (Lond), 2010. 34(6): p. 1001-10. [CrossRef]
- Yeomans, M.R. and R.W. Gray, Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev, 2002. 26(6): p. 713-28. [CrossRef]
- Bodnar, R.J., Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides, 2004. 25(4): p. 697-725.
- Gosnell, B.A. and A.S. Levine, Reward systems and food intake: role of opioids. Int J Obes (Lond), 2009. 33 Suppl 2: p. S54-8. [CrossRef]
- Eikemo, M., et al., Sweet taste pleasantness is modulated by morphine and naltrexone. Psychopharmacology (Berl), 2016. 233(21-22): p. 3711-3723. [CrossRef]
- Wang, M., et al., Activation of orexin-1 receptors in the amygdala enhances feeding in the diet-induced obesity rats: Blockade with μ-opioid antagonist. Biochem Biophys Res Commun, 2018. 503(4): p. 3186-3191. [CrossRef]
- Frank, A., L.M. Brown, and D.J. Clegg, The role of hypothalamic estrogen receptors in metabolic regulation. Front Neuroendocrinol, 2014. 35(4): p. 550-7. [CrossRef]
- Becker, J.B. and E. Chartoff, Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology, 2019. 44(1): p. 166-183. [CrossRef]
- Liu, X. and H. Shi, Regulation of Estrogen Receptor alpha Expression in the Hypothalamus by Sex Steroids: Implication in the Regulation of Energy Homeostasis. Int J Endocrinol, 2015. 2015: p. 949085.
- MacNicol, B., The biology of addiction. Can J Anaesth, 2017. 64(2): p. 141-148. [CrossRef]
- Bardo, M.T. and R.A. Bevins, Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl), 2000. 153(1): p. 31-43.
- Blanco-Gandía, M.C., et al., The rewarding effects of ethanol are modulated by binge eating of a high-fat diet during adolescence. Neuropharmacology, 2017. 121: p. 219-230. [CrossRef]
- Kenny, P.J., G. Voren, and P.M. Johnson, Dopamine D2 receptors and striatopallidal transmission in addiction and obesity. Curr Opin Neurobiol, 2013. 23(4): p. 535-8. [CrossRef]
- Le Merrer, J., et al., Reward processing by the opioid system in the brain. Physiol Rev, 2009. 89(4): p. 1379-412. [CrossRef]
- Colantuoni, C., et al., Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res, 2002. 10(6): p. 478-88. [CrossRef]
- Kelley, A.E., et al., Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci, 2003. 18(9): p. 2592-8.
- Johnson, P.M. and P.J. Kenny, Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci, 2010. 13(5): p. 635-41.
- Cruz, B., et al., The emergence of insulin resistance following a chronic high-fat diet regimen coincides with an increase in the reinforcing effects of nicotine in a sex-dependent manner. Neuropharmacology, 2021. 200: p. 108787. [CrossRef]
- O'Dell, L.E., et al., Enhanced nicotine self-administration and suppressed dopaminergic systems in a rat model of diabetes. Addict Biol, 2014. 19(6): p. 1006-19. [CrossRef]
- Richardson, J.R., et al., Insulin resistant rats display enhanced rewarding effects of nicotine. Drug Alcohol Depend, 2014. 140: p. 205-7. [CrossRef]
- Volkow, N.D., et al., Obesity and addiction: neurobiological overlaps. Obes Rev, 2013. 14(1): p. 2-18. [CrossRef]
- Vucetic, Z., J. Kimmel, and T.M. Reyes, Chronic high-fat diet drives postnatal epigenetic regulation of μ-opioid receptor in the brain. Neuropsychopharmacology, 2011. 36(6): p. 1199-206. [CrossRef]
- Benarroch, E.E., Endogenous opioid systems: current concepts and clinical correlations. Neurology, 2012. 79(8): p. 807-14.
- Maggi, R., et al., Binding characteristics of hypothalamic mu opioid receptors throughout the estrous cycle in the rat. Neuroendocrinology, 1993. 58(3): p. 366-72. [CrossRef]
Figure 2.
The amount of time that female (a) and male (b) control (left panel) and treated (right panel) mice spent in the HFD-paired chamber (PCh) vs. non-paired chamber (NPCh) on the test days. Data represent mean ± standard error of the mean (±S.E.M) of the amount of time that treated (n = 6) and control (n = 6) mice spent in the conditioning chambers on the preconditioning (BL) and postconditioning (Tests 1, 2, 3, ON and OXY) days. *P<0.05, PCh vs. NPCh for the overnight (ON) and oxycodone (OXY) test. *P<0.05, ****P<0.0001, PCh vs. NPCh for each group, #P = 0.05, ##P<0.01, ####P<0.0001, PCh for Treated vs. PCh for Control mice, based on three-way repeated measure ANOVA.
Figure 2.
The amount of time that female (a) and male (b) control (left panel) and treated (right panel) mice spent in the HFD-paired chamber (PCh) vs. non-paired chamber (NPCh) on the test days. Data represent mean ± standard error of the mean (±S.E.M) of the amount of time that treated (n = 6) and control (n = 6) mice spent in the conditioning chambers on the preconditioning (BL) and postconditioning (Tests 1, 2, 3, ON and OXY) days. *P<0.05, PCh vs. NPCh for the overnight (ON) and oxycodone (OXY) test. *P<0.05, ****P<0.0001, PCh vs. NPCh for each group, #P = 0.05, ##P<0.01, ####P<0.0001, PCh for Treated vs. PCh for Control mice, based on three-way repeated measure ANOVA.
Figure 3.
The amount of time that female (a) and male (b) MOR knockout mice (right panel) and their wildtype littermates (left panel) spent in the paired-chamber (PCh) and non-paired chamber (NPCh). Data are represented as mean ± standard error of the mean (± S.E.M) of the amount of time that mice spent in the HFD-paired chamber (PCh) or RCD-paired chamber (NPCh) in mice lacking MOR (-/-) (n = 6 mice per sex) and their wildtype controls (n = 6-8 mice per sex). *P<0.05, **P<0.01, ****P<0.0001 for PCh vs. NPCh; #P<0.05, ####P<0.0001, for PCh in wildtype mice vs. knockout mice, based on three-way repeated measure ANOVA.
Figure 3.
The amount of time that female (a) and male (b) MOR knockout mice (right panel) and their wildtype littermates (left panel) spent in the paired-chamber (PCh) and non-paired chamber (NPCh). Data are represented as mean ± standard error of the mean (± S.E.M) of the amount of time that mice spent in the HFD-paired chamber (PCh) or RCD-paired chamber (NPCh) in mice lacking MOR (-/-) (n = 6 mice per sex) and their wildtype controls (n = 6-8 mice per sex). *P<0.05, **P<0.01, ****P<0.0001 for PCh vs. NPCh; #P<0.05, ####P<0.0001, for PCh in wildtype mice vs. knockout mice, based on three-way repeated measure ANOVA.
Figure 4.
Comparison of male vs. female treated (upper panel) and control (bottom panel) mice. #P<0.05, ##P<0.01, PCh male vs. female mice of the treated group (top panel). Data represent mean ± standard error of the mean (±S.E.M) time spent in PCh and NPCh, based on three-way repeated measure ANOVA.
Figure 4.
Comparison of male vs. female treated (upper panel) and control (bottom panel) mice. #P<0.05, ##P<0.01, PCh male vs. female mice of the treated group (top panel). Data represent mean ± standard error of the mean (±S.E.M) time spent in PCh and NPCh, based on three-way repeated measure ANOVA.
Figure 5.
The amount of time that male and female mice of each genotype spent in the PCh vs. NPCh. Data represents mean ± standard error of the mean (±S.E.M) of time that mice (n = 6 mice per genotype of each sex) spent in the conditioning chambers.
Figure 5.
The amount of time that male and female mice of each genotype spent in the PCh vs. NPCh. Data represents mean ± standard error of the mean (±S.E.M) of time that mice (n = 6 mice per genotype of each sex) spent in the conditioning chambers.
Figure 6.
Comparison of food and caloric intake between female and male mice. Data represents mean ± standard error of the mean (±S.E.M) of food intake (upper panel) and calorie intake (lower panel) in the PCh in female (n = 6) vs. male (n=6) treated mice. #P<0.05, ##P<0.01, food intake/calorie consumption in the PCh for the treated male vs. female mice, based on three-way repeated measure ANOVA.
Figure 6.
Comparison of food and caloric intake between female and male mice. Data represents mean ± standard error of the mean (±S.E.M) of food intake (upper panel) and calorie intake (lower panel) in the PCh in female (n = 6) vs. male (n=6) treated mice. #P<0.05, ##P<0.01, food intake/calorie consumption in the PCh for the treated male vs. female mice, based on three-way repeated measure ANOVA.
Figure 7.
Food consumption in male vs. female wild-type (upper panel) and knockout (lower panel) mice. Data represent mean ± standard error of the mean (±S.E.M) of food intake in male and female mice lacking MOR and their wild-type littermates. ##P<0.01, a significant increase in food intake on d17 in male (n = 6) vs. female (n = 6) MOR (+/+) mice, based on three-way repeated measure ANOVA.
Figure 7.
Food consumption in male vs. female wild-type (upper panel) and knockout (lower panel) mice. Data represent mean ± standard error of the mean (±S.E.M) of food intake in male and female mice lacking MOR and their wild-type littermates. ##P<0.01, a significant increase in food intake on d17 in male (n = 6) vs. female (n = 6) MOR (+/+) mice, based on three-way repeated measure ANOVA.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).