Submitted:
21 January 2023
Posted:
23 January 2023
You are already at the latest version
Abstract
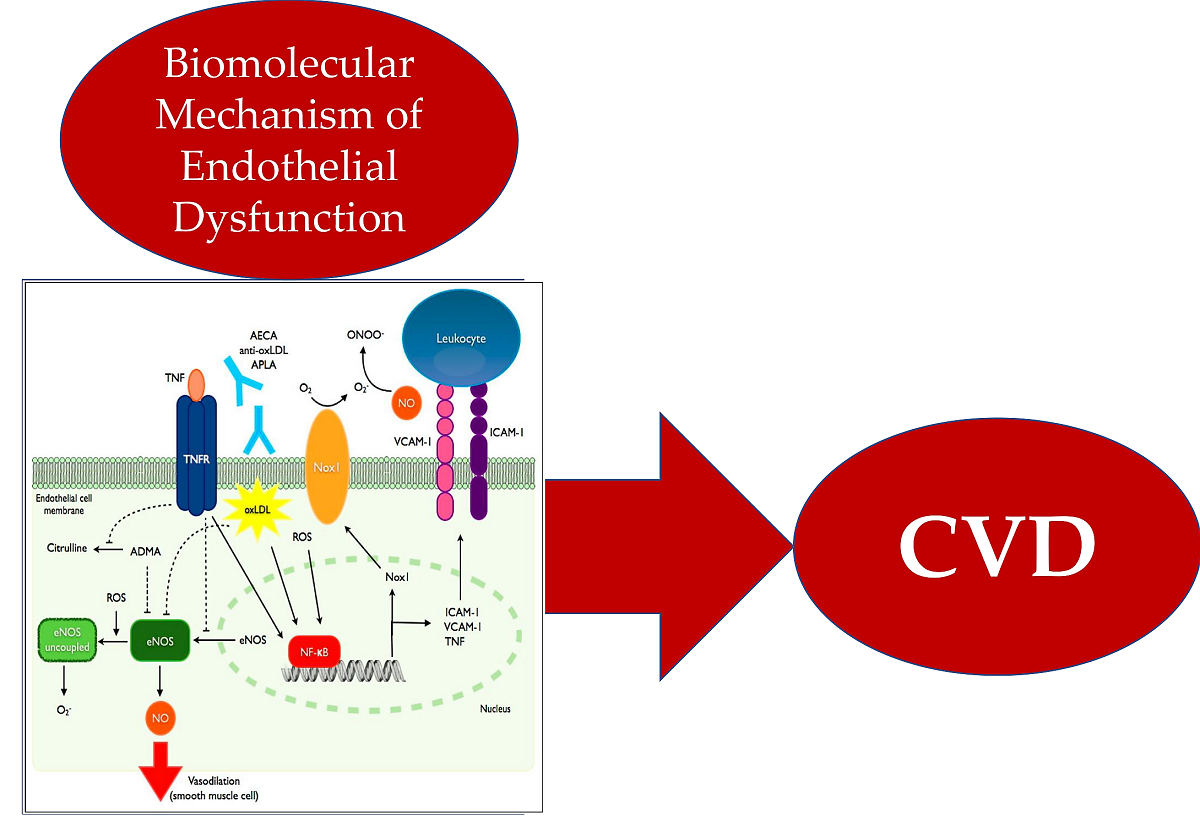
Keywords:
1. Introduction
2. Methods
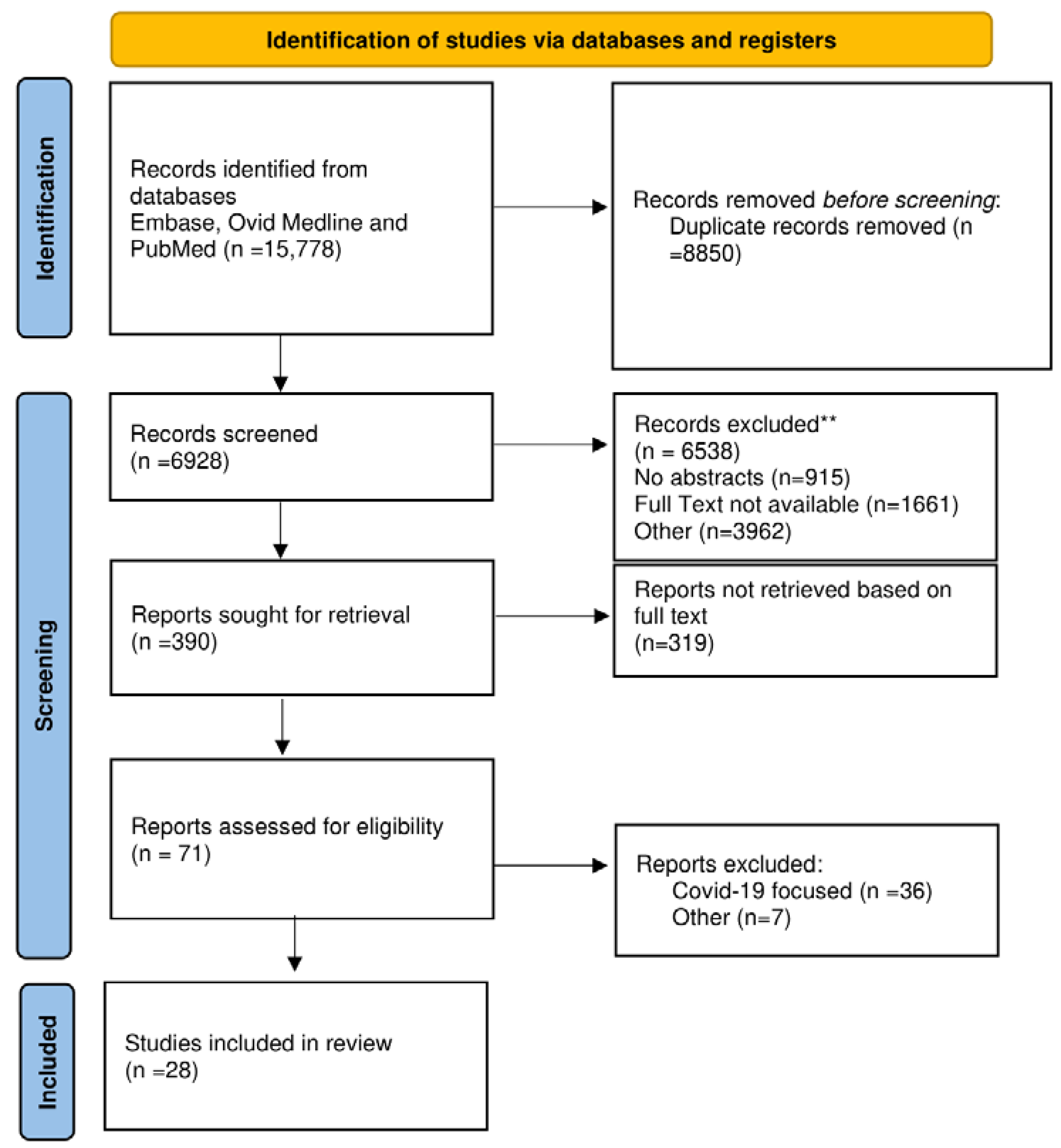
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.3. Endpoints and Effect Summary
3. Results
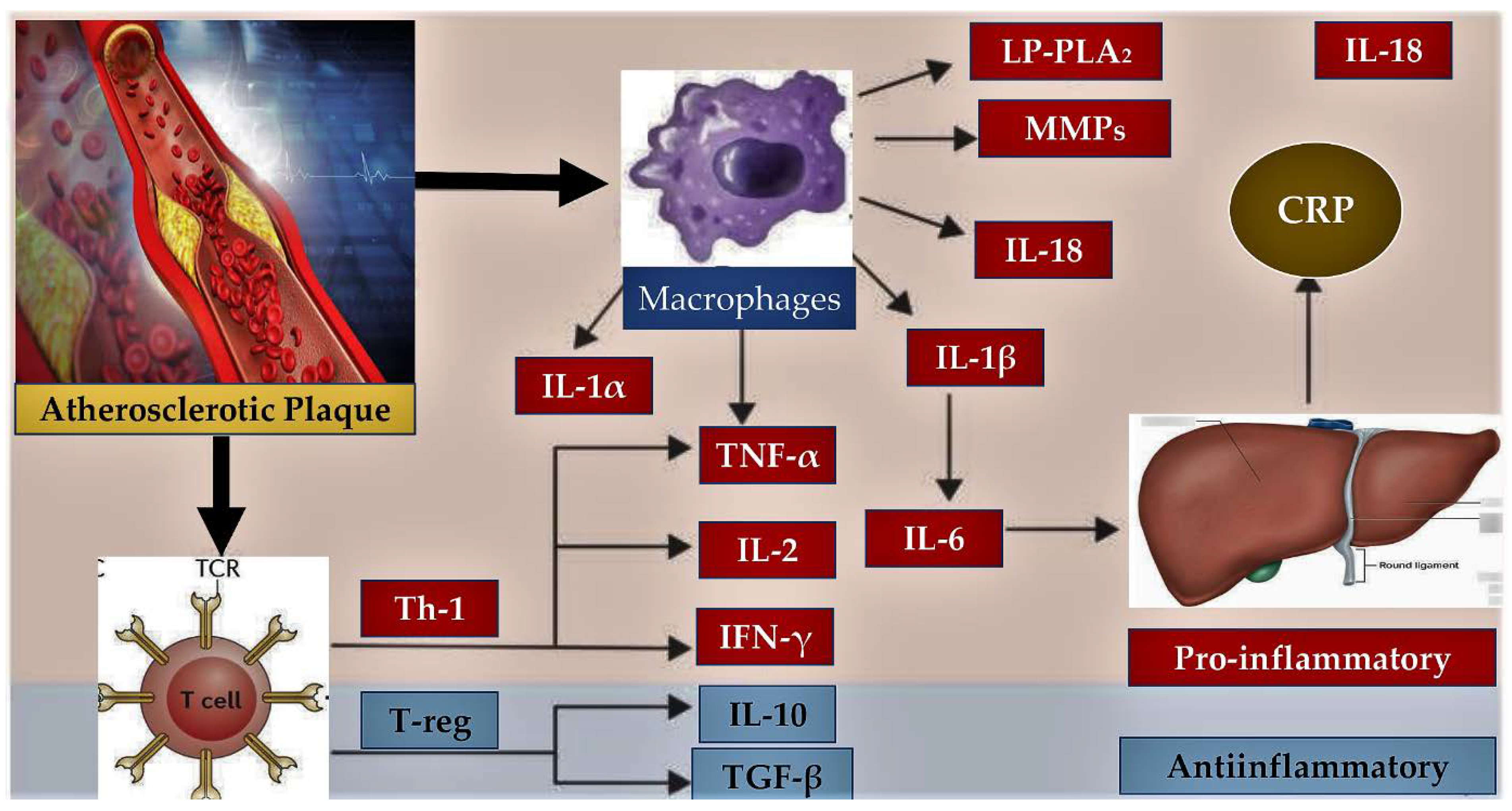
3.1. Presence of Inflammation in Endothelial Dysfunction
3.2. Pulmonary Hypertension Mediates Endothelial Dysfunction
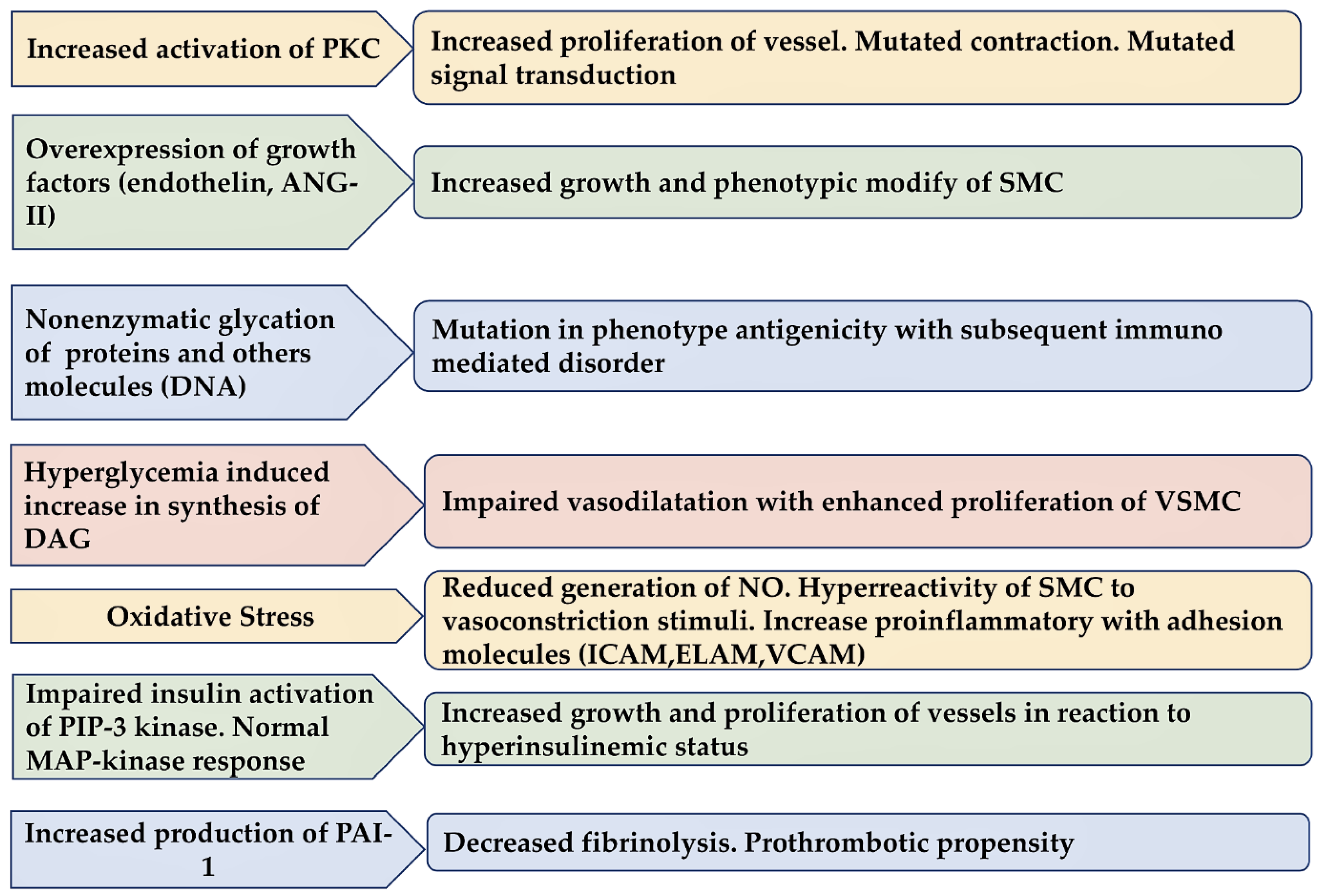
3.3. Diabetes Induce Injury in Endothelium
3.4. Endothelial Dysfunction and Fabry Disease
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nappi F, Fiore A, Masiglat J, Cavuoti T, Romandini M, Nappi P, Avtaar Singh SS, Couetil JP. Endothelium-Derived Relaxing Factors and Endothelial Function: A Systematic Review. Biomedicines. 2022 Nov 10;10(11):2884. [CrossRef]
- Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf). 2017; 219:22–96. [CrossRef]
- Shimokawa H. 2014 Williams Harvey lecture: importance of coronary vasomotion abnormalities-from bench to bedside. Eur Heart J. 2014; 35:3180–3193. [CrossRef]
- Godo S, Takahashi J, Yasuda S, Shimokawa H. Endothelium in Coronary Macrovascular and Microvascular Diseases. J Cardiovasc Pharmacol. 2021 Dec 3;78(Suppl 6): S19-S29. [CrossRef]
- Godo S, Shimokawa H. Endothelial Functions. Arterioscler Thromb Vasc Biol. 2017 Sep;37(9): e108-e114. [CrossRef]
- Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4: e002270. [CrossRef]
- Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009; 53:323–330. [CrossRef]
- Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004; 44:2137–2141. [CrossRef]
- Chaulin AM, Sergeev AK. The Role of Fine Particles (PM 2.5) in the Genesis of Atherosclerosis and Myocardial Damage: Emphasis on Clinical and Epidemiological Data, and Pathophysiological Mechanisms. Cardiol Res. 2022 Oct;13(5):268-282. [CrossRef]
- Tremblay JC, Pyke KE. Flow-mediated dilation stimulated by sustained increases in shear stress: a useful tool for assessing endothelial function in humans? Am J Physiol Heart Circ Physiol. 2018 Mar 1;314(3):H508-H520. [CrossRef]
- Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4: e002270. [CrossRef]
- Donato AJ, Machin DR, Lesniewski LA. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ Res. 2018 Sep 14;123(7):825-848. [CrossRef]
- Paneni F, Diaz Cañestro C, Libby P, Lüscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. 2017; 69:1952–1967. [CrossRef]
- Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013 May 23;368(21):2004-13. [CrossRef]
- Honda A, Tahara N, Nitta Y, Tahara A, Igata S, Bekki M, Nakamura T, Sugiyama Y, Kaida H, Kurata S, Fujimoto K, Abe T, Enomoto M, Adachi H, Narula J, Yamagishi S, Fukumoto Y. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose-positron emission tomography/ computed tomography is associated with endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2016; 36:1980–1988. [CrossRef]
- Kang MK, Kim CJ, Choo EH, Han EJ, Hwang BH, Kim JJ, Kim SH, O JH, Chang K. Anti-inflammatory effect of statin is continuously working throughout use: a prospective three time point 18F-FDG PET/CT imaging study. Int J Cardiovasc Imaging. 2019 Sep;35(9):1745-1753. [CrossRef]
- Frauenknecht V, Thiel S, Storm L, Meier N, Arnold M, Schmid JP, Saner H, Schroeder V. Plasma levels of mannan-binding lectin (MBL)-associated serine proteases (MASPs) and MBL-associated protein in cardio- and cerebrovascular diseases. Clin Exp Immunol. 2013 Jul;173(1):112-20. [CrossRef]
- Krogh SS, Holt CB, Steffensen R, Funck KL, Høyem P, Laugesen E, Poulsen PL, Thiel S, Hansen TK. Plasma levels of MASP-1, MASP-3 and MAp44 in patients with type 2 diabetes: influence of glycaemic control, body composition and polymorphisms in the MASP1 gene. Clin Exp Immunol. 2017 Jul;189(1):103-112. [CrossRef]
- Hertle E, Arts IC, van der Kallen CJ, Feskens EJ, Schalkwijk CG, Hoffmann-Petersen IT, Thiel S, Stehouwer CD, van Greevenbroek MM. Distinct longitudinal associations of MBL, MASP-1, MASP-2, MASP- 3, and MAp44 with endothelial dysfunction and intima-media thickness: the cohort on diabetes and atherosclerosis maastricht (CODAM) study. Arterioscler Thromb Vasc Biol. 2016 ; 36 :1278–1285. [CrossRef]
- Hertle E, Arts ICW, van der Kallen CJH, Feskens EJM, Schalkwijk CG, Stehouwer CDA, van Greevenbroek MMJ. Classical Pathway of Complement Activation: Longitudinal Associations of C1q and C1-INH With Cardiovascular Outcomes: The CODAM Study (Cohort on Diabetes and Atherosclerosis Maastricht)-Brief Report. Arterioscler Thromb Vasc Biol. 2018 May;38(5):1242-1244. [CrossRef]
- Emeny RT, Zierer A, Lacruz ME, Baumert J, Herder C, Gornitzka G, Koenig W, Thorand B, Ladwig KH; KORA Investigators. Job strain-associated inflammatory burden and long-term risk of coronary events: findings from the MONICA/KORA Augsburg case-cohort study. Psychosom Med. 2013 Apr;75(3):317-25. [CrossRef]
- Herder C, Kannenberg JM, Carstensen-Kirberg M, Huth C, Meisinger C, Koenig W, Peters A, Rathmann W, Roden M, Thorand B. Serum levels of interleukin-22, cardiometabolic risk factors and incident type 2 diabetes: KORA F4/FF4 study. Cardiovasc Diabetol. 2017 Jan 31;16(1):17. [CrossRef]
- Herder C, de Las Heras Gala T, Carstensen-Kirberg M, et al. Circulating levels of interleukin 1-receptor antagonist and risk of cardiovascular disease: meta-analysis of six population-based cohorts. Arterioscler Thromb Vasc Biol. 2017; 37:1222–1227. [CrossRef]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017 Sep 21;377(12):1119-1131. [CrossRef]
- Choi BJ, Matsuo Y, Aoki T, Kwon TG, Prasad A, Gulati R, Lennon RJ, Lerman LO, Lerman A. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014; 34:2473–2477. [CrossRef]
- Zaric B, Obradovic M, Trpkovic A, Banach M, Mikhailidis DP, Isenovic ER. Endothelial Dysfunction in Dyslipidaemia: Molecular Mechanisms and Clinical Implications. Curr Med Chem. 2020;27(7):1021-1040. [CrossRef]
- Feletou M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells-Focus on Endothelium-Derived Vasoactive Mediators. San Rafael, CA: Morgan & Claypool Life Sciences Publishers; 2011.
- Satoh K, Matoba T, Suzuki J, O'Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin ZG, Yan C, Abe J, Berk. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008 Jun 17;117(24):3088-98. [CrossRef]
- Satoh K, Satoh T, Kikuchi N, Omura J, Kurosawa R, Suzuki K, Sugimura K, Aoki T, Nochioka K, Tatebe S, Miyamichi-Yamamoto S, Miura M, Shimizu T, et al. Basigin mediates pulmonary hypertension by promoting inflammation and vascular smooth muscle cell proliferation. Circ Res. 2014 Sep 26;115(8):738-50. [CrossRef]
- Xue C, Sowden M, Berk BC. Extracellular Cyclophilin A, Especially Acetylated, Causes Pulmonary Hypertension by Stimulating Endothelial Apoptosis, Redox Stress, and Inflammation. Arterioscler Thromb Vasc Biol. 2017 Jun;37(6):1138-1146. [CrossRef]
- Xue C, Senchanthisai S, Sowden M, Pang J, White RJ, Berk BC. Endothelial-to-Mesenchymal Transition and Inflammation Play Key Roles in Cyclophilin A-Induced Pulmonary Arterial Hypertension. Hypertension. 2020 Oct;76(4):1113-1123. [CrossRef]
- Rosa A, Butt E, Hopper CP, Loroch S, Bender M, Schulze H, Sickmann A, Vorlova S, Seizer P, Heinzmann D, Zernecke A Cyclophilin A Is Not Acetylated at Lysine-82 and Lysine-125 in Resting and Stimulated Platelets. Int J Mol Sci. 2022 Jan 27;23(3):1469. [CrossRef]
- Liu SF, Nambiar Veetil N, Li Q, Kucherenko MM, Knosalla C, Kuebler WM. Pulmonary hypertension: Linking inflammation and pulmonary arterial stiffening. Front Immunol. 2022 Oct 5; 13:959209. [CrossRef]
- Rogula S, Pomirski B, Czyżak N, Eyileten C, Postuła M, Szarpak Ł, Filipiak KJ, Kurzyna M, Jaguszewski M, Mazurek T, Grabowski M, Gąsecka A. Biomarker-based approach to determine etiology and severity of pulmonary hypertension: Focus on microRNA. Front Cardiovasc Med. 2022 Oct 6 ;9 :980718. [CrossRef]
- Kherbeck N, Tamby MC, Bussone G, Dib H, Perros F, Humbert M, Mouthon L. The role of inflammation and autoimmunity in the pathophysiology of pulmonary arterial hypertension. Clin Rev Allergy Immunol. 2013 Feb ;44(1) :31-8. [CrossRef]
- Dutta P, Gomez D, Gladwin MT. Do BRD (4)S of a Feather Flock Together? How an Inflammation-Driven Epigenetic Regulator May Link Pulmonary Hypertension and Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2017 Aug;37(8):1428-1430. [CrossRef]
- Borck PC, Guo LW, Plutzky J. BET Epigenetic Reader Proteins in Cardiovascular Transcriptional Programs. Circ Res. 2020 Apr 24;126(9):1190-1208. [CrossRef]
- Li MX, Jiang DQ, Wang Y, Chen QZ, Ma YJ, Yu SS, Wang Y. Signal Mechanisms of Vascular Remodeling in the Development of Pulmonary Arterial Hypertension. J Cardiovasc Pharmacol. 2016 Feb;67(2):182-90. [CrossRef]
- Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, Couture C, Michelakis ED, Provencher S, Bonnet S. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014 Feb 18;129(7):786-97. [CrossRef]
- Meloche J, Le Guen M, Potus F, Vinck J, Ranchoux B, Johnson I, Antigny F, Tremblay E, Breuils-Bonnet S, Perros F, Provencher S, Bonnet S. miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2015 Sep 15 ;309(6):C363-72. [CrossRef]
- Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thébaud B, Husain AN, Cipriani N, Rehman. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010 Jun 22;121(24):2661-71. [CrossRef]
- Yamaji-Kegan K, Su Q, Angelini DJ, Myers AC, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J Immunol. 2010 Nov 1;185(9):5539-48. [CrossRef]
- Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Poloczek A, El-Haddad H, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMα) in chronic hypoxia- and antigen-mediated pulmonary vascular remodeling. Respir Res. 2013 Jan 4;14(1):1. [CrossRef]
- Yamaji-Kegan K, Takimoto E, Zhang A, Weiner NC, Meuchel LW, Berger AE, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (FIZZ1/RELMα) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014 Jun 15;306(12): L1090-103. [CrossRef]
- Johns RA, Takimoto E, Meuchel LW, Elsaigh E, Zhang A, Heller NM, Semenza GL, Yamaji-Kegan K. Hypoxia-inducible factor 1α is a critical downstream mediator for hypoxia-induced mitogenic factor (FIZZ1/RELMα)-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2016; 36:134–144. [CrossRef]
- Lin Q, Fan C, Gomez-Arroyo J, Van Raemdonck K, Meuchel LW, Skinner JT, Everett AD, Fang X, Macdonald AA, Yamaji-Kegan K, Johns RA. HIMF (Hypoxia-Induced Mitogenic Factor) Signaling Mediates the HMGB1 (High Mobility Group Box 1)-Dependent Endothelial and Smooth Muscle Cell Crosstalk in Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2019 Dec;39(12):2505-2519. [CrossRef]
- Félétou M, Köhler R, Vanhoutte PM. Endothelium-derived vasoactive factors and hypertension: possible roles in pathogenesis and as treatment targets. Curr Hypertens Rep. 2010 Aug;12(4):267-75. [CrossRef]
- Yaoita N, Shirakawa R, Fukumoto Y, Sugimura K, Miyata S, MiuraY, Nochioka K, Miura M, Tatebe S, Aoki T, Yamamoto S, Satoh K, Kimura T, Shimokawa H, Horiuchi H. Platelets are highly activated in patients of chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2014; 34:2486–2494. [CrossRef]
- Yaoita N, Satoh K, Satoh T, Sugimura K, Tatebe S, Yamamoto S, Aoki T, Miura M, Miyata S, Kawamura T, Horiuchi H, Fukumoto Y, Shimokawa H. Thrombin-activatable fibrinolysis inhibitor in chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2016; 36:1293– 1301. [CrossRef]
- Loader J, Montero D, Lorenzen C, Watts R, Méziat C, Reboul C, Stewart S, Walther G. Acute hyperglycemia impairs vascular function in healthy and cardiometabolic diseased subjects: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015; 35:2060–2072. [CrossRef]
- Lespagnol E, Dauchet L, Pawlak-Chaouch M, Balestra C, Berthoin S, Feelisch M, Roustit M, Boissière J, Fontaine P, Heyman E. Early Endothelial Dysfunction in Type 1 Diabetes Is Accompanied by an Impairment of Vascular Smooth Muscle Function: A Meta-Analysis. Front Endocrinol (Lausanne). 2020 Apr 17; 11:203. [CrossRef]
- Horton WB, Jahn LA, Hartline LM, Aylor KW, Patrie JT, Barrett EJ. Acute hyperglycaemia enhances both vascular endothelial function and cardiac and skeletal muscle microvascular function in healthy humans. J Physiol. 2022 Feb;600(4):949-962. [CrossRef]
- Loader J, Meziat C, Watts R, Lorenzen C, Sigaudo-Roussel D, Stewart S, Reboul C, Meyer G, Walther G. Effects of sugar-sweetened beverage consumption on microvascular and macrovascular function in a healthy population. Arterioscler Thromb Vasc Biol. 2017; 37:1250–1260. [CrossRef]
- Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA, Hamburg NM. Protein kinase C-β contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013 Jan 1;127(1):86-95. [CrossRef]
- Farb MG, Karki S, Park SY, Saggese SM, Carmine B, Hess DT, Apovian C, Fetterman JL, Bretón-Romero R, Hamburg NM, Fuster JJ, Zuriaga MA, Walsh K, Gokce N. WNT5A-JNK regulation of vascular insulin resistance in human obesity. Vasc Med. 2016 Dec;21(6):489-496. [CrossRef]
- Walther G, Obert P, Dutheil F, Chapier R, Lesourd B, Naughton G, Courteix D, Vinet A. Metabolic syndrome individuals with and without type 2 diabetes mellitus present generalized vascular dysfunction: crosssectional study. Arterioscler Thromb Vasc Biol. 2015; 35:1022–1029. [CrossRef]
- Bretón-Romero R, Feng B, Holbrook M, Farb MG, Fetterman JL, Linder EA, Berk BD, Masaki N, Weisbrod RM, Inagaki E, Gokce N, Fuster JJ, Walsh K, Hamburg NM. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler Thromb Vasc Biol. 2016; 36:561–569. [CrossRef]
- Cho YK, Kang YM, Lee SE, Lee Y, Seol SM, Lee WJ, Park JY, Jung CH. Effect of SFRP5 (Secreted Frizzled-Related Protein 5) on the WNT5A (Wingless-Type Family Member 5A)-Induced Endothelial Dysfunction and Its Relevance With Arterial Stiffness in Human Subjects. Arterioscler Thromb Vasc Biol. 2018 Jun;38(6):1358-1367. [CrossRef]
- Park S, Kim JA, Joo KY, Choi S, Choi EN, Shin JA, Han KH, Jung SC, Suh SH. Globotriaosylceramide leads to K(Ca)3.1 channel dysfunction: a new insight into endothelial dysfunction in Fabry disease. Cardiovasc Res. 2011 Feb 1;89(2):290-9. [CrossRef]
- Satoh K. Globotriaosylceramide induces endothelial dysfunction in fabry disease Arterioscler Thromb Vasc Biol. 2014 Jan;34(1):2-4. [CrossRef]
- Choi S, Kim JA, Na HY, Cho SE, Park S, Jung SC, Suh SH. Globotriaosylceramide induces lysosomal degradation of endothelial. KCa3.1 in fabry disease. Arterioscler Thromb Vasc Biol. 2014; 34:81–89. [CrossRef]
| First Author/Year Ref | Type of Study | Cohort | Aims | Finding |
| Honda et al (2016) Arterioscler Thromb Vasc Biol [15] |
Human Prospective Single Center (USA ) |
145 pts ((95 men and 50 women) | To evaluate the relationship between endothelial function and vascular inflammation by mean of flow-mediated dilation (FMD) and 18FDG-PET | Vascular inflammation in the carotid arteries evaluated was independently correlates to decreased %FMD suggesting the association of vascular inflammation with endothelial dysfunction |
| Kang et al (2019) Front Immunol [16] |
Prospective Single Center (Korea) |
Nine statin-naïve SA patients with inflammatory carotid plaques | To evaluate anti-inflammatory effects of statin initiation to 3 and 3 months to 1 year) by mean o 18F-FDG PET/CT | The anti-inflammatory effect of the statin continues to maintain an effect for up to 1 year. However, stable plasma LDL-C levels below the 3-month target were produced. |
| Krog et al (2017) Clin Exp Immunol [18] |
Human/Animal model Prospective Single Center (Denmark.) |
100 pts with type 2 diabetes Vs 100 sex- and age-matched controls Streptozotocin-induced diabetes mouse |
To study SNPs in the MASP1 gene and altered MASP-1, MASP-3 and MAp44 | Higher levels of MASP-1 levels among pts with type 2 diabetes and diabetic mice |
| Hertle et al (2016) Arterioscler Thromb Vasc Biol [19] |
Prospective Multicenter (Netherlands, Denmark) CODAM study (Cohort on Diabetes and Atherosclerosis Maastricht) |
574pts cIMT 73 pts CVD |
To study MBL-associated proteases (MASPs) and MBL-associated proteins (MAps) in complement activation and CVD | High MBL may contribute to low cIMT. MASP-1 and MASP-2 were not associated with adverse cardiovascular outcomes. MASP-3 and MAp44 crucial role in endothelial dysfunction |
| Hertle et al (2018) Arterioscler Thromb Vasc Biol [20] |
Prospective Multicenter (Netherlands Denmark) CODAM study (Cohort on Diabetes and Atherosclerosis Maastricht) |
574pts cIMT 73 pts CVD |
To determine the associations between factor C1q its regulator C1-INH and CVD | Nonlinear association between C1q and incident CVD |
| Herder et al (2017) Cardiovasc Diabetol. [22] |
Prospective Single Center (Germany) |
1107 pts KORA F4 study. |
Whether higher IL-22 levels are associated with lower diabetes incidence. | High serum levels of IL-22 were inversely associated with cardiometabolic risk factors. these associations did not translate into an increased risk for type 2 diabetes |
| Herder et al (2017) Arterioscler Thromb Vasc Biol [23] |
Meta-Analysis Single Center (Germany) |
5 cohort studies IL-1RA MONICA/KORA Augsburg case-cohort study 1855 pts CVD 18 745 noncases CVD |
To evaluate circulating IL-1RA and incident of CVD | Serum IL-1RA levels were associated with risk of CVD after adjustment for multiple confounders. IL-1RA lead to subclinical inflammation, oxidative stress, and endothelial activation. |
| Ridker et al (2017) NEJM J [24] |
RCT Multicenter Center CANTOS Trial |
10,061 patients canakinumab (50 mg, 150 mg, and 300 mg) 3,344 placebo group |
To compare therapeutic effect of monoclonal antibody targeting interleukin-1β | Canakinumab at a dose of 150 mg every 3 months was effective as anti-inflammatory against interleukin-1β laeding to a significantly lower rate of recurrent cardiovascular events than placebo, |
| Choi et al (2014) Arterioscler Thromb Vasc Biol [25] |
Multicenter Center (USA/Korea) |
40 ptswith mild coronary atherosclerosis | To study endothelial dysfunction in pts with early CAD presenting macrophages and vasa vasorum infiltrates. | Epicardial endothelial dysfunction was associated with optical coherence tomography -identified macrophages and microchannels in mild coronary atherosclerosis. |
| First Author/Year Ref | Type of Study | Cohort | Aims | Finding |
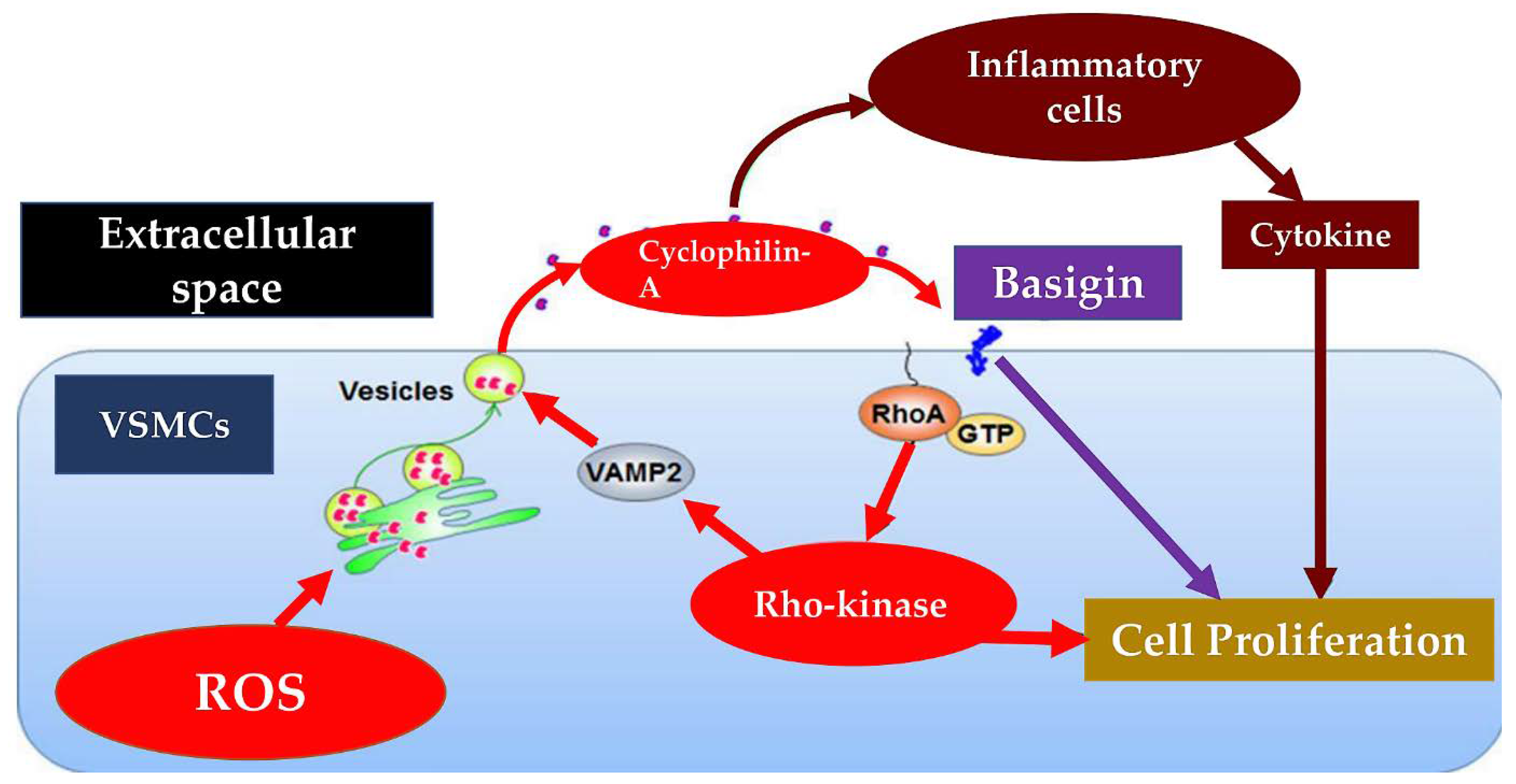
| Satoh et al (2008) Antioxid Redox Signal [28] |
Animal Model Single Center (Japan) |
CyPA knockout mice vs Wild-type mice vs VSMC-Tg mice |
To evaluate contribute of CyPA to vascular remodeling | CyPA regulate inflammatory cell accumulation, flow-mediated vascular remodeling and intima formation. |
| Satoh et al (2014) Circ Res [29] |
Animal Model Single Center (Japan) |
CyPA (±) mice Vs Bsg (±) mice |
To determine the role of CyPA/Bsg signaling in the development of PH. | Increased CyPA levels in patients with PH. Cell proliferation reduced in Bsg (±) compared with Bsg (+/+) VSMCs. |
| Xe et al.(2017) Arterioscler Thromb Vasc Biol [30] |
Human/Animal Model Single Center (USA) |
CyPA (±) mice Vs Human pulmonary EC |
To evaluated the role of extracellular CypA in PH. To compare effects of acetylated CypA (AcK-CypA) and CypA on EC dysfunction. | EC-derived CypA (especially AcK-CypA) favor PH due to apoptosis, inflammation, and oxidative stress |
| Rosa et al (2022) Int J Mol Sci. [32] |
Animal/Human Model Prospective Multicenter (GermanyUK,) |
CyPA (±) mice Vs Human pulmonary EC |
Whether K82 and K125 acetylation is required for release of CyPA from platelets | Acetylation of CyPA no major protein modification in platelets. CyPA acetylation is not required |
| Meloche et al (2014) Circulation [39] |
Prospective Single Center (Canada) |
Human PH EC Vs Healthy tissues/cells |
To study PAH-PASMCs increasing during activation of poly (ADP-ribose) polymerase-1 (PARP-1) | PH development related to DNA damage/PARP-1 signaling pathway |
| Meloche et al (2015) Am J Physiol Cell Physiol [40] |
Prospective Single Center (Canada) |
Human PH EC Vs Healthy tissues/cells |
Wether miR-223 downregulation triggers PARP-1 overexpression | Downregulation of miR-223 in PH |
| Archer et al (2010) Circulation [41] |
Animal/ Human Model Single center (USA) |
FHR (PH rat) Vs *Sprague-Dawley rat PH pts |
To study expression of SOD2 and its correlation with PH | Epigenetic SOD2 deficiency induce PH due to impairing redox signaling and creating a proliferative, apoptosis-resistant PASMC |
| Yamaji-Kegan et al (2014) Am J Physiol Lung Cell Mol Physiol [44] |
AnimalModel Single Center (USA) |
IL-4/STAT6 KO mice Vs wild-type (WT) mice |
To evaluate how HIMF lead to lung inflammation and vascular remodeling | IL-4 signaling exert substantial role in HIMF-induced lung inflammation and vascular remodeling. |
| Johns et al (2016) Arterioscler Thromb Vasc Biol [45] |
Animal Model Single Center (USA) |
HIF-1α(+/-) mice Vs wild-type (HIF-1α (+/+) vs Human resistin-like molecule-β |
To evaluate hypoxia-inducible factor-1 (HIF-1) is a critical downstream signal mediator of HIMF during PH development. | HIMF can induce HIF-1, vascular endothelial growth factor-A, and interleukin-6. Mediators for hypoxic inflammation and PH pathophysiology. |
| Lin et al (2018) Arterioscler Thromb Vasc Biol [46] |
Animal/Human Model Single Center (USA) |
HIMF KO mice Vs Human RELM-β |
To investigated the immunomodulatory properties of HIMF signaling in PH pathogenesis | In HIMF-induced PH, HMGB1-RAGE mediates EC-smooth muscle cell crosstalk |
| First Author/Year Ref | Type of Study | Cohort | Aims | Finding |
|---|---|---|---|---|
| Loader et al (2015) Arterioscler Thromb Vasc Biol [50] |
Human Study level meta-analysis |
525 healthy pts 540 cardiometabolic pts |
To compare acute hyperglycemia in EF and VSMF | In healthy and diseased subjects macrovascular but not microvascular endothelial dysfunction during acute hyperglycemia were revealed |
| Lespagnol et al (2020) Front Endocrinol. [51] |
Human Study level meta-analysis |
21 study | To evaluate early EF and VSMF alteration in type 1 diabetes. | In children and adults VSM dysfunction with type 1 diabetes is demonstrated. Endothelial dysfunction s more pronounced in large than small vessels. |
| Horton et al. (2022) J Physiol [52] |
Human RCT |
Healthy young adults 6 males 7 females |
To compared macrovascular and microvascular functional responses to euglycemia and hyperglycaemia | Unlike meal-promoted acute hyperglycaemia 4 h of intravenous glucose-induced hyperglycaemia enhances brachial artery flow-mediated dilatation evokes cardiac and skeletal muscle microvascular function without impairing aortic stiffness. |
| Loader et al (2017) Arterioscler Thromb Vasc Biol [53] |
Human RCT |
Healthy young adults (12 males) 600 mL (20 oz.) of water Vs SSB |
To compare EF and VSMF. | SSB infusion mediates endothelial dysfunction with increasing oxidative stress and decreasing NO bioavailability after SSB infusion. |
| Tabit et al (2013) Circulation [54] |
Human Prospective comparative |
40 diabetics type 2 Vs 36 nondiabetic controls |
To study activity of PKCβ nuclear factor κB and reduced No | Altered eNOS activation, reduced insulin action and increased inflammatory activation. Increased PKCβ activity in endothelial insulin resistance. |
| Farb et al (2016) Vasc Med [55] |
Human Visceral adipose tissue arterioles |
43 obeses pts | To investigate the role of WNT5A-JNK leading to insulin-mediated vasodilator responses | Up-regulation WNT5A-JNK signaling and impaired endothelial eNOS activation |
| Walther et al (2015) Arterioscler Thromb Vasc Biol [56] |
Human RCT |
53 pts MetS without T2D Vs 25 pts T2D Vs 40 pts healthy |
To compare EF and VSMF. To measure plasma glucose, insulin and inflammatory markers | MetS was associated with endothelial-dependent and endothelial-independent dysfunction, affecting both the macro- and the microvascular systems. |
| Bretón-Romero et al (2016) Arterioscler Thromb Vasc Biol [57] |
Human Prospective comparative |
42 pts T2D Vs 43 pts healthy |
To evaluate whether increased activation of Wnt5a-JNK signaling contributes to impaired EF. To determine eNOS activation and NO production | Wnt5a-induced impairment of eNOS activation and NO that was reversed by Wnt5a and JNK inhibition. Noncanonical Wnt5a signaling and JNK activity contribute to vascular insulin resistance and endothelial dysfunction. |
| Cho et al (2018) Arterioscler Thromb Vasc Biol [58] |
Human/Animal model | Sprague-Dawley rat Vs Human EC |
To investigate whether SFRP5 could restore WNT5A-induced endothelial dysfunction in vitro and ex vivo. | Compensatory action of SFRP5 against atherosclerosis under conditions of metabolic dysfunction. SFRPS restored WNT5A-induced reduction of NO production via eNOS |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





