Submitted:
20 January 2023
Posted:
23 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterisation
2.3. Ageing Treatments
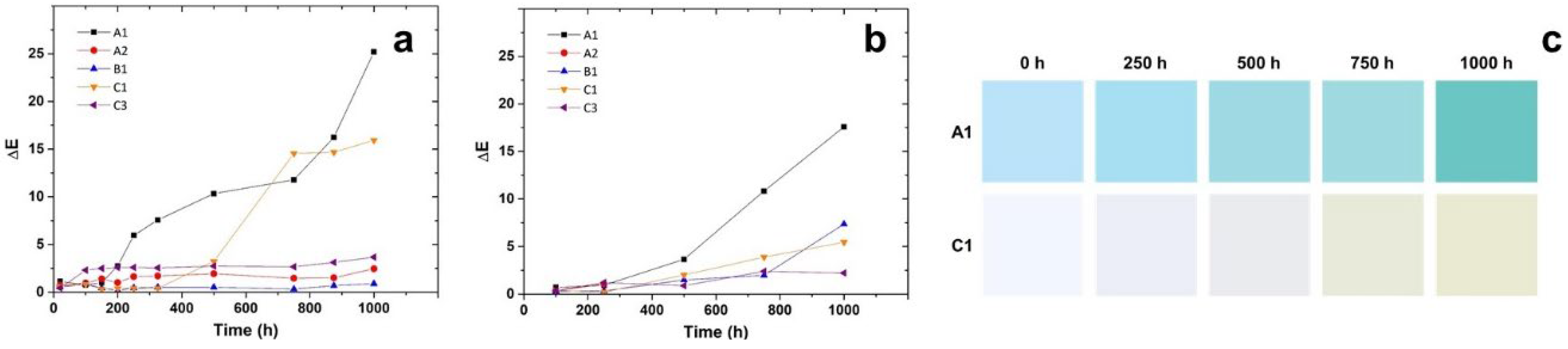
3. Results
3.1. Compositional Analysis
- A1 (spunbond light blue PP);
- A2 (melt-blown PP, identical to B2);
- B1 (spunbond PP, very similar to A3, B3 and C5);
- C1 (spunbond PP, very similar to B1 but with a higher fibre density);
- C2 (PE/PET hot air cotton);
- C3 (spundbond PP with light stabilizers, as C4).
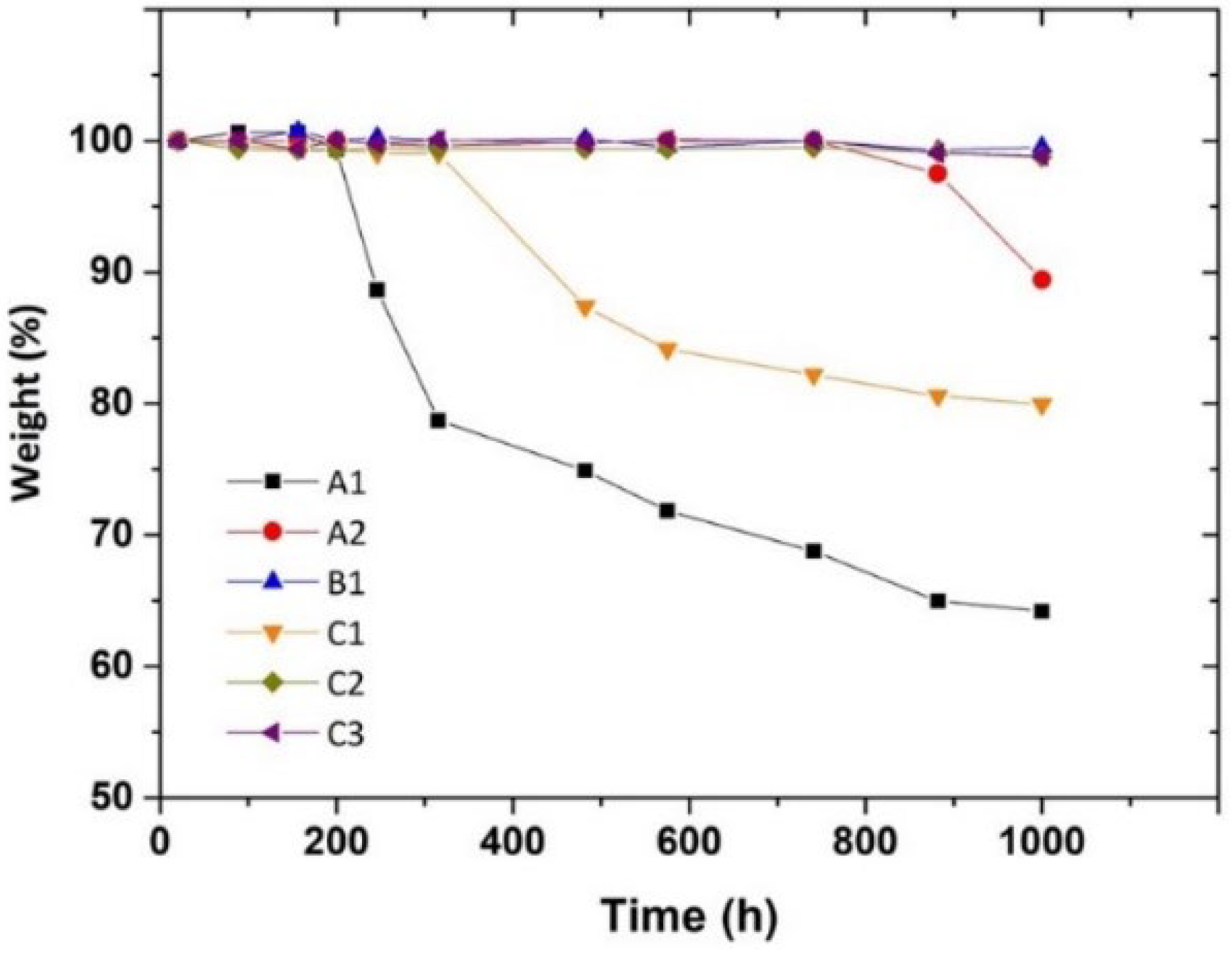
3.2. Accelerated Degradation
3.3. Preliminary Outdoor Ageing and Validation of Artificial Ageing
4. Conclusions
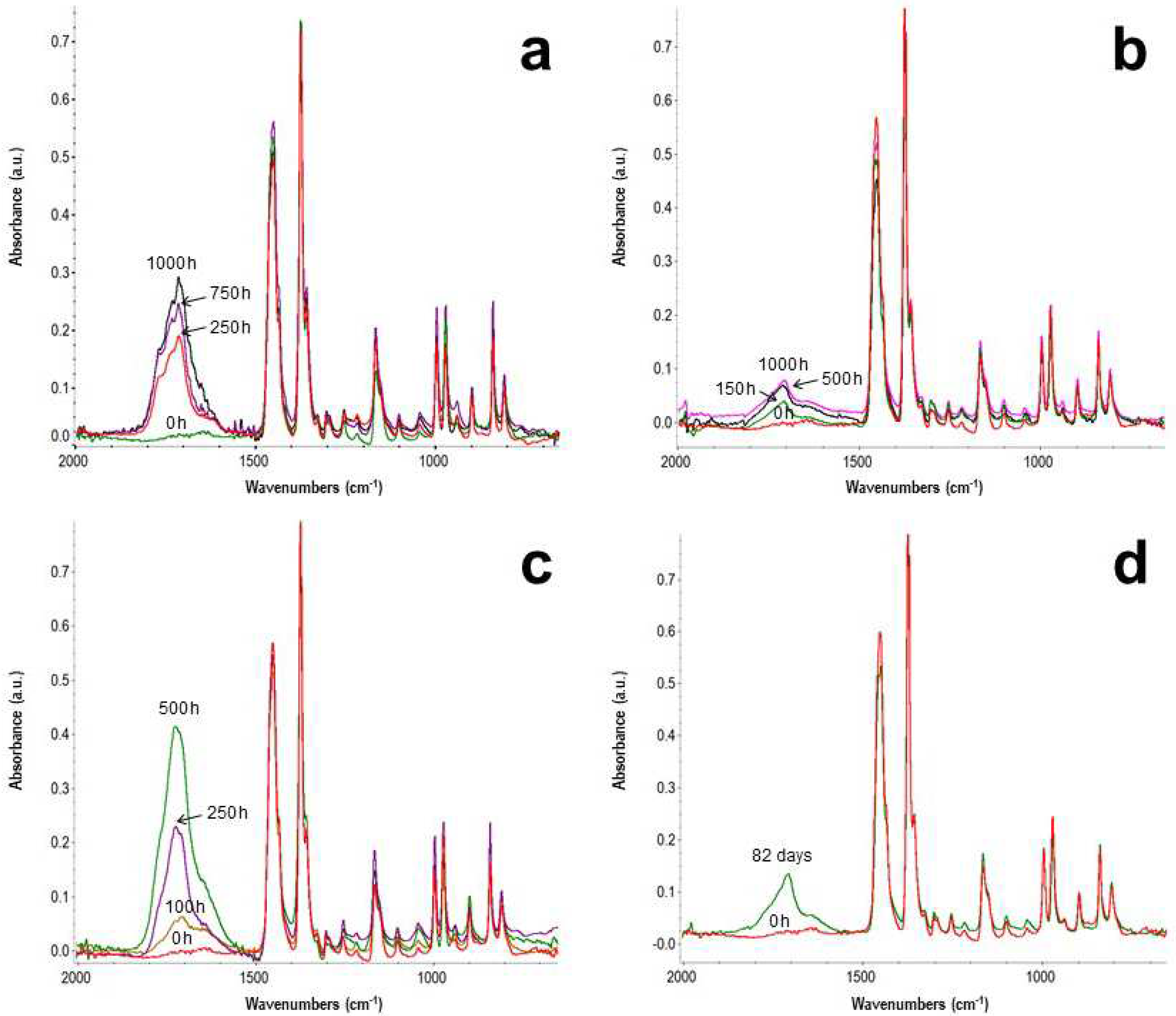
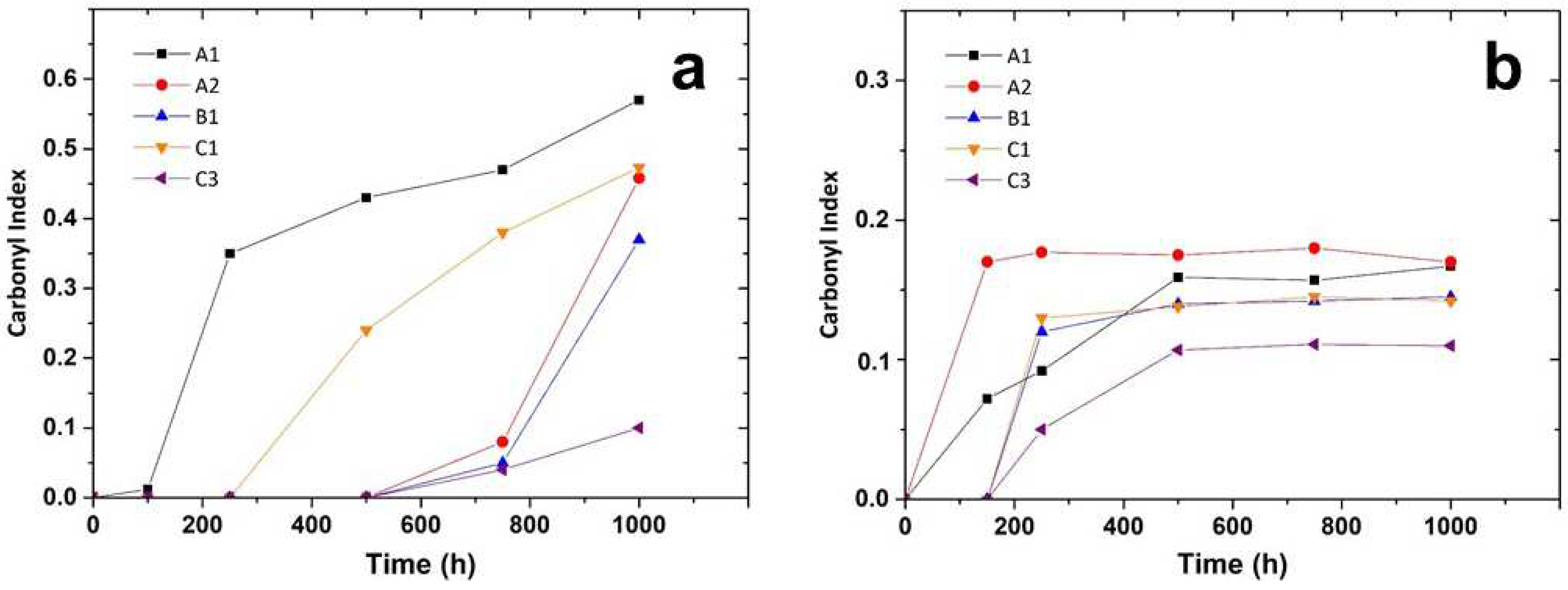
- after the induction period, which is characteristic for each layer, the first chemical sign of the beginning of the oxidation consists in the formation of oxygen-containing groups, mainly hydroperoxides and carboxylic groups and, to minor extent, ketones, esters and lactones. The induction length is possibly related to the combination of two factors: (i) the use of antioxidants in the PP formulation, directly identified, as for C3, or eventually present but not detected; (ii) previous thermal processing that foster further degradation.
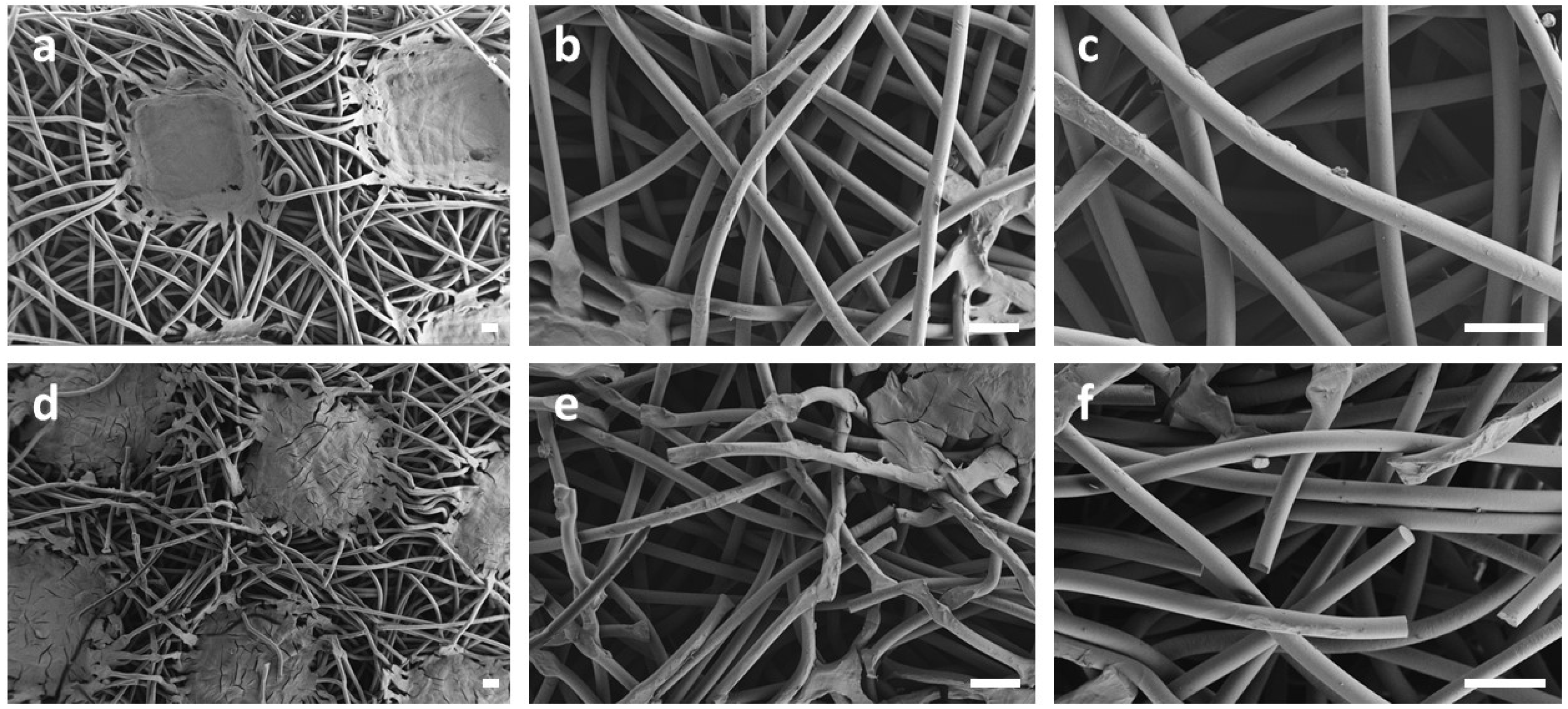
- Their oxidation is limited by diffusion, a phenomenon that depends on temperature, being lesser for the photoageing treatment at 24℃. Yellowing and the Tm drop are early indicators of oxidation, which may be detected before the end of the induction period. Morphology changes also induce a loss of mechanical properties, observable as embrittlement of the fabric fibres, which leads to a complete pulverization of the layer under the stress of the cooling system flow.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
References
- Armentano, I.; Barbanera, M.; Carota, E.; Crognale, S.; Marconi, M.; Rossi, S.; Rubino, G.; Scungio, M.; Taborri, J.; Calabrò, G. Polymer Materials for Respiratory Protection: Processing, End Use, and Testing Methods. ACS Appl. Polym. Mater. 2021, 3, 531–548. [Google Scholar] [CrossRef]
- Spennemann, D.H.R. Covid-19 Face Masks as a Long-Term Source of Microplastics in Recycled Urban Green Waste. Sustain. 2022, 14, 207. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. & Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Plassmann, M.M.; Macleod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Material Weathering, 6th ed.; ChemTec Publishing: Toronto, ON, Canada, 2018. [Google Scholar]
- Lazzari, M.; Reggio, D. What Fate for Plastics in Artworks? An Overview of Their Identification and Degradative Behaviour. Polymers (Basel). 2021, 13, 883. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Turnbull, A. Weathering of Polymers: Mechanisms of Degradation and Stabilization, Testing Strategies and Modelling. J. Mater. Sci. 1994, 29, 584–613. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Wiles, D.M. The Photooxidative Degradation of Polypropylene. Part I. Photooxidation and Photoinitiation Processes. J. Macromol. Sci. Part C 1976, 14, 65–106. [Google Scholar] [CrossRef]
- François-Heude, A.; Richaud, E.; Desnoux, E.; Colin, X. Influence of Temperature, UV-Light Wavelength and Intensity on Polypropylene Photothermal Oxidation. Polym. Degrad. Stab. 2014, 100, 10–20. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Mondal, B.; Sarkar, I.; Sinha, A.; Ray, S.S.; Kwon, Y.N.; Nakamura, K.; Govardhan, K. The Face behind the Covid-19 Mask — A Comprehensive Review. Environ. Technol. Innov. 2022, 28, 102837. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic Waste Release Caused by COVID-19 and Its Fate in the Global Ocean. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2111530118. [Google Scholar] [CrossRef] [PubMed]
- Foffi, R.; Savuto, E.; Stante, M.; Mancini, R.; Gallucci, K. Study of Energy Valorization of Disposable Masks via Thermochemical Processes: Devolatilization Tests and Simulation Approach. Energies 2022, 15, 2103. [Google Scholar] [CrossRef]
- Siwal, S.S.; Chaudhary, G.; Saini, A.K.; Kaur, H.; Saini, V.; Mokhta, S.K.; Chand, R.; Chandel, U.K.; Christie, G.; Thakur, V.K. Key Ingredients and Recycling Strategy of Personal Protective Equipment (PPE): Towards Sustainable Solution for the COVID-19 like Pandemics. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Crespo, C.; Ibarz, G.; Sáenz, C.; Gonzalez, P.; Roche, S. Study of Recycling Potential of FFP2 Face Masks and Characterization of the Plastic Mix-Material Obtained. A Way of Reducing Waste in Times of Covid-19. Waste and Biomass Valorization 2021, 12, 6423–6432. [Google Scholar] [CrossRef]
- Zuri, G.; Oró-Nolla, B.; Torres-Agulló, A.; Karanasiau, A.; Lacorte, S. Migration of Microplastics and Phthalates from Face Masks to Water. Molecules 2022, 27, 6859. [Google Scholar] [CrossRef]
- Dissanayake, J.; Torres-Quiroz, C.; Mahato, J.; Park, J. Facemasks: A Looming Microplastic Crisis. Int. J. Environ. Res. Public Health 2021, 18, 7068. [Google Scholar] [CrossRef] [PubMed]
- Geus, H.G. Developments in Manufacturing Techniques for Technical Nonwovens. In Advances in Technical Nonwovens; Elsevier Inc., 2016; pp. 133–153.
- Schäfer, K. Melt Spinning: Technology. In Polypropylene. Polymer Science and Technology Series Vol. 2; Springer, Dordrecht, 1999.
- Richaud, E.; Farcas, F.; Divet, L.; Paul Benneton, J. Accelerated Ageing of Polypropylene Geotextiles, the Effect of Temperature, Oxygen Pressure and Aqueous Media on Fibers—Methodological Aspects. Geotext. Geomembranes 2008, 26, 71–81. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M. Fundamentals of Polymer Degradation and Stabilization; Springer Science & Business Media, 1992.
- Polymer Durability; Degradation, Stabilization, and Lifetime Prediction; Clough, R., Billingham, N., Gillen, K., Eds.; ACS advances in chemistry series 249. American Chemical Society: Washington, 1996.
- Hoff, A.; Jacobsson, S. Thermal Oxidation of Polypropylene in the Temperature Range of 120–280 °C. J. Appl. Polym. Sci. 1984, 29, 465–480. [Google Scholar] [CrossRef]
- Iring, M.; Tüdős, F. Thermal Oxidation of Polyethylene and Polypropylene: Effects of Chemical Structure and Reaction Conditions on the Oxidation Process. Prog. Polym. Sci. 1990, 15, 217–262. [Google Scholar] [CrossRef]
- François-Heude, A.; Richaud, E.; Leprovost, J.; Heninger, M.; Mestdagh, H.; Desnoux, E.; Colin, X. Real-Time Quantitative Analysis of Volatile Products Generated during Solid-State Polypropylene Thermal Oxidation. Polym. Test. 2013, 32, 907–917. [Google Scholar] [CrossRef]
- Philippart, J.L.; Posada, F.; Gardette, J.L. Mass Spectroscopy Analysis of Volatile Photoproducts in Photooxidation of Polypropylene. Polym. Degrad. Stab. 1995, 49, 285–290. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, Y.; Yang, J.; Kong, M.; Yang, H.; Zhao, J.; Li, G. Outdoor and Accelerated Laboratory Weathering of Polypropylene: A Comparison and Correlation Study. Polym. Degrad. Stab. 2015, 112, 145–159. [Google Scholar] [CrossRef]
- Allen, N.S.; Chirinos-Padron, A.; Henman, T.J. Photoinitiated Oxidation of Polypropylene: A Review. Prog. Org. Coatings 1985, 13, 97–122. [Google Scholar] [CrossRef]
- Lacoste, J.; Vaillant, D.; Carlsson, D.J. Gamma-, Photo-, and Thermally-Initiated Oxidation of Isotactic Polypropylene. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 715–722. [Google Scholar] [CrossRef]
- Grause, G.; Chien, M.F.; Inoue, C. Changes during the Weathering of Polyolefins. Polym. Degrad. Stab. 2020, 181, 109364. [Google Scholar] [CrossRef]
- Celina, M.C.; Quintana, A. Oxygen Diffusivity and Permeation through Polymers at Elevated Temperature. Polymer (Guildf) 2018, 150, 326–342. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Baiamonte, M.; Santangelo, S.; Scaffaro, R.; Mistretta, M.C. Influence of Different Environments and Temperatures on the Photooxidation Behaviour of the Polypropylene. Polymers (Basel) 2022, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Gardette, M.; Perthue, A.; Gardette, J.L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and Thermal-Oxidation of Polyethylene: Comparison of Mechanisms and Influence of Unsaturation Content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- Bolland, J.L.; Gee, G. Kinetic Studies in the Chemistry of Rubber and Related Materials. II. The Kinetics of Oxidation of Unconjugated Olefins. Trans. Faraday Soc. 1946, 42, 236–243. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Hodgson, J.L.; Coote, M.L. Revising the Mechanism of Polymer Autooxidation. Org. Biomol. Chem. 2011, 9, 480–490. [Google Scholar] [CrossRef]
- Morlat, S.; Mailhot, B.; Gonzalez, D.; Gardette, J.L. Photo-Oxidation of Polypropylene/Montmorillonite Nanocomposites. 1. Influence of Nanoclay and Compatibilizing Agent. Chem. Mater. 2004, 16, 377–383. [Google Scholar] [CrossRef]
- Luo, M.R.; Cui, G.; Rigg, B. The Development of the CIE 2000 Colour-Difference Formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Ion, R.-M.; Nuta, A.; Sorescu, A.-A.; Iancu, L. Photochemical Degradation Processes of Painting Materials from Cultural Heritage. In Photochemistry and Photophysics - Fundamentals to Applications; InTech, 2018.
- Castejón, M.L.; Tiemblo, P.; Gómez-Elvira, J.M. Photo-Oxidation of Thick Isotactic Polypropylene Films. II. Evolution of the Low Temperature Relaxations and of the Melting Endotherm along the Kinetic Stages. Polym. Degrad. Stab. 2000, 71, 99–111. [Google Scholar] [CrossRef]
- Paukkeri, R.; Lehtinen, A. Thermal Behaviour of Polypropylene Fractions: 1. Influence of Tacticity and Molecular Weight on Crystallization and Melting Behaviour. Polymer (Guildf) 1993, 34, 4075–4082. [Google Scholar] [CrossRef]
- Olivares, N.; Tiemblo, P.; Gómez-Elvira, J.M. Physicochemical Processes along the Early Stages of the Thermal Degradation of Isotactic Polypropylene I. Evolution of the γ Relaxation under Oxidative Conditions. Polym. Degrad. Stab. 1999, 65, 297–302. [Google Scholar] [CrossRef]
- Lacey, D.J.; Dudler, V. Chemiluminescence from Polypropylene. Part 1: Imaging Thermal Oxidation of Unstabilised Film. Polym. Degrad. Stab. 1996, 51, 101–108. [Google Scholar] [CrossRef]
- Fayolle, B.; Audouin, L.; Verdu, J. Oxidation Induced Embrittlement in Polypropylene — a Tensile Testing Study. Polym. Degrad. Stab. 2000, 70, 333–340. [Google Scholar] [CrossRef]
- Rabello, M.S.; White, J.R. Crystallization and Melting Behaviour of Photodegraded Polypropylene — I. Chemi-Crystallization. Polymer (Guildf) 1997, 38, 6379–6387. [Google Scholar] [CrossRef]
| Selected items | Layer Code | Composition |
|---|---|---|
| Surgical mask A | A1 | spunbond PP with blue dye |
| A2 | melt-blown PP | |
| A3 | spunbond PP | |
| Surgical mask B | B1 | spunbond PP |
| B2 | melt-blown PP | |
| B3 | spunbond PP | |
| Filtering respirator C | C1 | spunbond PP |
| C2 | PE/PET hot air cotton | |
| C3 | spunbond PP with light stabilizer | |
| C4 | spunbond PP with light stabilizer | |
| C5 | spunbond PP |
| Layer | |||||
|---|---|---|---|---|---|
| Time (h) | A1 | A2 | B1 | C1 | C3 |
| 0 | 165 | 158 | 164 | 164 | 156 |
| 100 | 163 | 156 | 165 | - | - |
| 250 | 141 | 157 | 165 | 165 | 154 |
| 500 | 140 | 156 | 165 | 141 | 154 |
| 1000 | 137 | 154 | 163 | 139 | 153 |
| Layer | |||||
|---|---|---|---|---|---|
| Time (h) | A1 | A2 | B1 | C1 | C3 |
| 0 | 165 | 158 | 164 | 164 | 156 |
| 100 | 162 | 151 | 160 | 160 | 156 |
| 250 | 157 | 147 | 159 | 158 | 154 |
| 500 | 159 | 147 | 157 | 160 | 154 |
| 1000 | 158 | - 1 | 158 | 160 | 154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





