1. Introduction
Wellbeing of any animal population depends on the balance between productivity and survival [1, 2]. In the current world many species of long-distance migrant shorebirds are declining, especially on the East Asian-Australasian Flyway due to illegal hunting and habitat loss that reduce survival of both juvenile and adult birds [3-6]. In spite of the fact that some success is achieved in dealing with these threats, there is still a long way to go for improvement of the situation in dependent bird populations. At the same time possibilities exist to increase productivity of at least some of the most endangered local bird populations to reduce chances of their extinction and hopefully to help their recovery after these stresses.
Headstarting (HS) is a conservation approach that suggests offering an advantage to a population by improving survival of embryos and/or juveniles. A number of successful HS projects are known for reptiles [
7], while practical examples are far less numerous for birds. This approach has been applied to increase productivity of the maleo
Macrocephalon maleo [
7], takahe
Porphyrio mantelli [
8], mangrove finch
Camarhynchus heliobates [
9], as well as of several species of waders, the american oystercatcher
Haematopus palliatus [
10], black-tailed godwit
Limosa limosa [
11], black stilt
Himantopus novaezelandiae [
12], piping plover
Charadrius melodus [
13] and snowy plover
Ch. nivosus [
14]. Conservation activities in these cases ranged from simply eggs being moved to a safe place for parentless natural incubation (maleo) to a one-year-long captive-rearing process after artificial incubation (takahe).
Except improving nests, embryos, and chicks’ survival, in some cases, HS can also help to increase egg production if a replacement clutch is induced by taking the first one for artificial incubation. Another feature of HS, in fact almost never discussed, is damping population productivity drops in years with unfavourable environmental conditions. Sequences of severe conditions on breeding and non-breeding grounds can lead to a population collapse and may require long restoration [
15]. For the species with a sex-skewed survival of chicks, as it is shown in our study below, implementation of HS can equalise sex ratio among first-breeders by elimination of environmental reasons driving this bias, and so to assist faster population growth.
The spoon-billed sandpiper (SbS)
Calidris pygmaea (L., 1758) is a small wader species belonging to the East Asian-Australasian Flyway, which has a restricted breeding range limited to the coast of the Chukchi Sea and Asian side of the Bering Sea [
16] and wintering grounds in South-East Asia [
17]. In 2008, this species was listed as Critically Endangered (IUCN 3.1) in the IUCN Red List due to a sharp population decline experienced during recent decades [18-20]. The current global breeding population of the species is estimated from 120 [
17] to 210–228 pairs [21, 22]. Much international activity is going on recently throughout the East Asian–Australasian Flyway on conservation of SbS and their habitat (e.g., [17, 23-26]), which all together gives a hope for stopping the negative SbS population trend that was estimated to be 26% annual decline in 2000s [
18] although the more recent estimates still suggest the population is declining at 8–10% per year [21, 27, 28]. The breeding population of SbS in the vicinity of Meinypil’gyno Village, Southern Chukotka, Russia, was discovered in 2001 [
29] and remains to be the largest local currently known one within the species breeding range. From at least 80 pairs in 2003 [
18], the number of pairs in the main monitoring area declined to at most 11 in 2012 [
30].
Based on the former studies of another, northern breeding population of SbS [20, 31-33], it is known that birds of this species are long-living monogamous creatures, territorial in the pre-nesting period, and highly site faithful (that means apparent survival of adults to be very close to reality). SbS normally breed at 2-years of age [
34]. As in many other waders, young birds migrate southward mostly separately from adults [
35], and thus, they do not need parents’ guidance for migration or getting back to natal areas, which facilitates aviculture significantly.
In 2011–2012, 20 eggs annually were collected for the ‘SbS Captive Breeding Programme’ implementation at WWT Slimbridge in the United Kingdom. This was done to conserve a breeding population in case the species becomes extinct in the wild. It has been assumed that eggs produced by this captive population would be then transported back to Chukotka, hatched there, and the chicks released. To develop methods for this next step, the ‘SbS Headstarting Programme’ was initiated in 2012 in Meinypil’gyno. Although no eggs have been supplied from the captive population, HS is still implemented yearly to date as a self-sufficient project.
In this paper, we report the HS impact on the local SbS population through 10 years, and discuss possible application of obtained results for conservation actions in relation to declining populations of various species in general.
2. Methods
Intensive studies of the SbS breeding population analysed in this paper were conducted in 2012–2021 in the vicinity of Meinypil’gyno Village (N 62.54°, E 177.05°), Chukotka Autonomous Okrug, northern Russian Far East, although monitoring of the population was conducted since 2003 [
18]. It is a coastal area with tundra vegetation of different kinds developed on several landscape features (coastal spits, coastal plain, moraine hills) clamped between the Bering Sea, mountains of Koryak Highland and two sizable lakes (Pekul’neyskoye and Vaamychgyn lakes). Water from the catchments of these two lakes flows into the sea by two rivers running along the coastal spit into the single river mouth, which in some years is blocked by ice and gravel washed ashore by autumn storms. This causes flooding of bird nests in spring on the floodplains on the shores of the lakes which are among preferable habitats of SbS in the area. The main survey area is of about 40×12 km in size covered by ATVs, boats, and on foot.
2.1. Ringing and Colour Marking
Individual colour-marking of SbS in the population has been implemented since 2012 using combination of a standard metal ring of the Moscow Bird Ringing Centre and a plastic flag 10×5 mm in size with a unique engraving of two alphanumeric characters; both markers were placed on different tibias of a bird with metal ring on right leg and flag on left leg of adult birds and with their opposite position on legs of chicks. For wild birds caught and marked, flags of light green colour were used, while flags of white colour were used for birds raised in captivity (HS). Such colour-marking allowed individual recognition of birds through their life by reading the engraving with help of binoculars, scopes or photography. SbS chicks could be marked right after hatching because precocial waders at hatching have well developed legs, allowing rings of the same diameter for adults (2.8–3.0 mm) to be put on chicks as well.
Adult SbS were caught for marking with help of a walk-in-trap (in few cases with a clap-net) mostly on their nests after the clutch had been taken for HS and replaced by dummy eggs. Some adults were caught during brood rearing period after catching chicks; they were attracted to the trap by play-back of distress calls of chicks from a mobile phone placed into the trap. Adults were sexed preliminarily by their plumage colouration (males are usually slightly brighter) and biometrics [
36]. Sex of a bird can also be estimated in subsequent years by observations of its behaviour in pairs during the prenesting period. When sex of one bird was known we assumed that the partner(s) is (are) of the opposite sex.
Position and colour of a flag provides additional preliminary knowledge about belonging of a bird to a marking cohort. Such individual recognition of SbS was necessary for various purposes: local population monitoring, obtaining demographic parameters, behavioural observations, and learning about local and flyway movements of birds. Information about records of colour-marked SbS was obtained from the flyway due to a network of observers created by the SbS Task Force of the EAAFP.
2.2. Collection of Eggs and Artificial Incubation for HS
The aim of HS is to increase local population productivity by preventing nest predation and chick death, while most of the involved pairs still have an opportunity to breed producing a replacement clutch. It has been performed annually since 2012 based in Meinypil’gyno Village, and only data for birds headstarted up to 2021 have been analysed if not otherwise specified. The latest version of the HS protocol is described below in brief, but has been subject to minor modification to improve the outcome as the project has developed. Detailed description of the HS protocol is a topic for a separate article to be published. So here we presented only details essential for the results understanding. Egg collection and bird handling were performed according to permits issued yearly by the Russian Ministry of Natural Resources and Ecology.
From the arrival of first SbS on 30 May – 6 June (median 2 June, n=14), we were surveying the area for individual territories of SbS males and home ranges of pairs. During these ca. 7 days birds are rather vocally active this makes them easier to locate. Once found, the behaviours were continually monitored. This often led to nest cup scraping registration, where eggs were laid eventually, or to visits of the nest already with eggs.
During the nesting season, we avoided approaching nests without a need for manipulations with eggs or chicks, or confirmation of nest loss to avoid exposing the nests to additional risk of predation. Nest sites were visited usually about once a week, but almost daily during the estimated hatching date. For reference, we have monitored the fate of other wader species nests in the area in the same way. In most cases at least one SbS partner had been individually colour-marked allowing us to know whether we were dealing with a first or replacement clutch. There were several cases of nest loss due to flood or early predation when the first clutch was terminated before clutch completion, resulting in reduced clutch size in another nest cup; we considered these clutches being the first ones. In other few cases pairs were observed early in the season, but their eggs hatched late; the latter were assumed to be replacements without finding first clutches of such pairs.
Collecting fresh eggs reduces risks of nest loss due to predation or flooding (which is the first positive impact of HS), although there is an increased risk of embryo damage during transportation, along with a reduced hatch rate [
37]. Therefore, eggs were collected for HS at the earliest appropriate opportunity (including collecting incomplete clutches, with eggs being replaced by dummies when necessary), taking into consideration the distance of the nest from the incubation facility and the terrain. For that, they were transported to the rearing station in the village using portable incubators (AB Newlife Houbara and AB Newlife Toolbox) running between 35–36°C and ca. 45% relative humidity (RH) running from a 12 V battery. These were transported on foot, by boat or using ATV driving cautiously between 5–10 km/h within a time frame not exceeding 4 hrs. The farthest delivery was 27.0 km (geodesic), mean 11.1±8.5 km (min. 2.2 km, median 10.5 km, n=73, here and below mean values are provided with ± SD if other is not specified).
Upon delivery to the rearing station (
Figure 1a), the eggs were immediately marked and weighed (for individual monitoring through incubation), then measured and candled to ascertain age if not already known. They were then placed in the standard parameter incubator.
Incubation period length estimated for the last egg in a clutch in the northern population was 21.5–23 days (22.43±0.61, n=7, [
32]), while for artificially incubated eggs collected for HS with known laying date it was 20.5–23 days (21.9±0.9, n=11) and 21–22 days (21.6±0.5, n=5) for wild ones.
2.3. Chicks Rearing and Release
After hatching, the chicks were weighed and fitted with a split plastic colour ring on the left or right tarsus for identification purposes before being placed in the brooder, usually for 18–24 hours.
Once ca. 8 days old, chicks were moved to the aviary located on the shore of Pekul’neyskoye Lake about 3km from the village (
Figure 1b). This is a slightly brackish lake with similar vegetation which adult SbS are often found to breed and rear chicks on; a good example of habitat for which they should return to.
Chicks were released around the 25–31 July, the youngest ones 18–30 days old, depending mostly on the breeding schedule of SbS and related egg collection. The age at release turned out to be very important for the subsequent survival – earlier the better [
38]. Post-release monitoring was performed twice a day for around two weeks, or until no more observations had been recorded for a period of at least three days.
Subsequent observations of HS birds came from the flyway (Kamchatka Peninsula in Russia, South Korea, Japan, China and Taiwan, Thailand, Myanmar, Bangladesh) and from the vicinity of Meinypil’gyno once those were back for breeding.
2.4. Calculations
We used the following formulas for calculations of estimation of annual number of fledged chicks per pair: EHS = CHS × HHS × FHS + RHS × Cr × Sw,r × Hw × Fw and Ew = (Cf × Sw,f + (1–Sw,f) × Rw × Cr × Sw,r) × Hw × Fw, where C is average clutch size, H – hatch rate (ratio of the number of eggs hatched to the total number of eggs available), F – fledge rate (ratio of the number of chicks fledged to ones hatched), R – replacement probability, S – nest safety rate (ratio of the number of nests with at least one egg hatched to the number of nests found), indices designate HS – headstarted, w – wild, f – first, r – replacement. To estimate annual output of hatched chicks, fledge rates are not meaningful FHS = Fw =1; for egg production (number of eggs survived to hatching), hatch rates are equal to unity, too. Gain at a certain stage is evaluated as the ratio of HS output parameters (including induced output from replacement clutches) to the relevant natural one.
The recruitment to the global breeding population was estimated as a number of chicks annually reaching maturity per pair: GHS = CHS × HHS × FHS × MHS + RHS × Cr × Sw,r × Hw × 0.9 × Mw and Gw = (Cf × Sw,f + (1–Sw,f) × Rw × Cr×Sw,r) × Hw × 0.9 × Mw, where 0.9 is the evaluation of wild chicks survival from hatching to marking (mean 2.6 days old, n=75 in 2013–2016), M – maturity rate calculated as the number of birds observed on breeding grounds or at the age of at least 23 months elsewhere to the number of marked birds in the relevant cohort. Headstarting penetration rate is a ratio of pairs involved in these activities during the breeding season to the total number of pairs in the local population; in our case, it was 50% on average (varied in 40–67% margins).
3. Results
3.1. Nests Fate
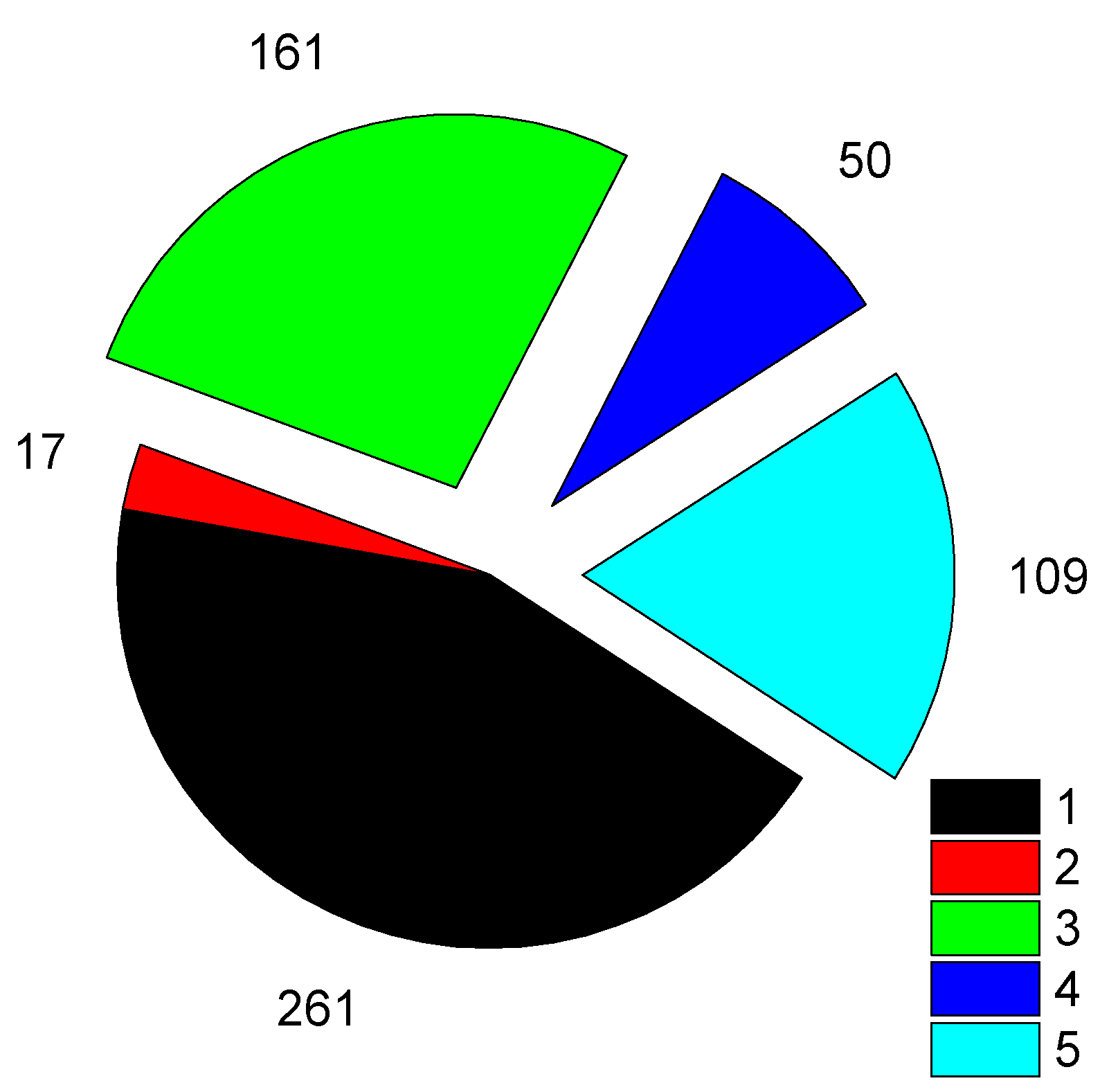
Most of the SbS clutches found in the beginning of a breeding season, in relatively easily accessible sites of the area, were collected for HS. This in total provided slightly less than a half of all eggs found in 2012–2019 (
Figure 2). Since those were early clutches, most of them were the first ones of pairs in a season. First clutches contained on average 3.85±0.04 SE eggs (2–4, n=97). In general, replacement clutches contained fewer eggs – mean 3.13±0.11 SE (1–4, n=52).
Figure 2.
Distribution categories of eggs found in the study area in 2012–2020 (n=598). Eggs collected for HS (n=278): 1 – first clutches, 2 – replacement clutches. Eggs left in nature for incubation by parents (n=320): 3 – first clutches, 4 – natural replacements, 5 – HS induced replacements.
Figure 2.
Distribution categories of eggs found in the study area in 2012–2020 (n=598). Eggs collected for HS (n=278): 1 – first clutches, 2 – replacement clutches. Eggs left in nature for incubation by parents (n=320): 3 – first clutches, 4 – natural replacements, 5 – HS induced replacements.
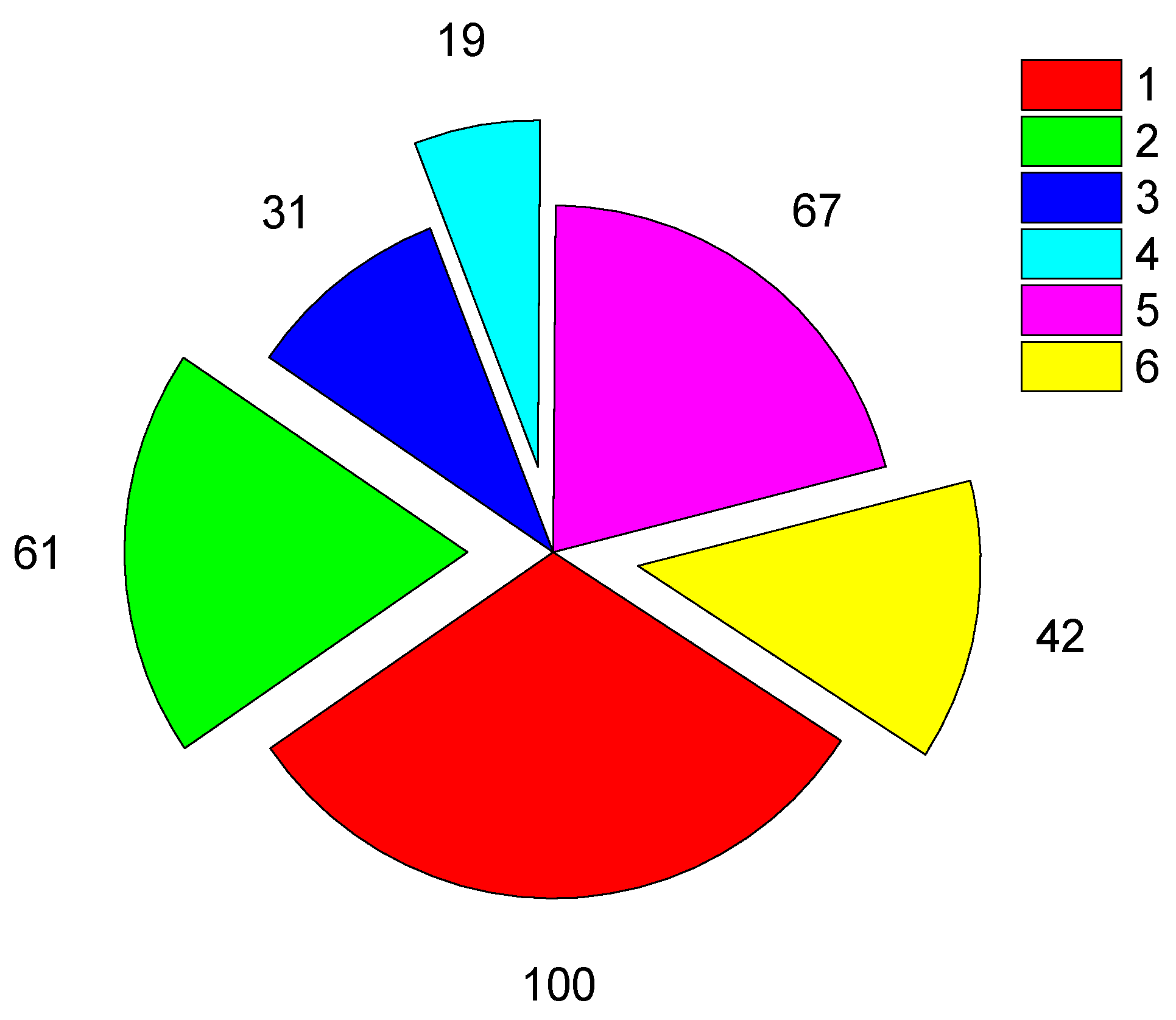
Figure 3.
Distribution fate categories of eggs left in nature after egg collecting for HS (n=320). Eggs survived to hatch (n=198): 1 – first clutches, 3 – replacement clutches of pairs not subjected to egg collecting for HS, 5 – HS induced replacement. Depredated eggs (n=122): 2, 4, 6 (exploded wedges) correspond to 1, 3, 5.
Figure 3.
Distribution fate categories of eggs left in nature after egg collecting for HS (n=320). Eggs survived to hatch (n=198): 1 – first clutches, 3 – replacement clutches of pairs not subjected to egg collecting for HS, 5 – HS induced replacement. Depredated eggs (n=122): 2, 4, 6 (exploded wedges) correspond to 1, 3, 5.
The situation with nest predation is rather different every year (
Table 1), and cannot always be predicted or explained straightforwardly.
Figure 3 shows that 37.9% of eggs of first clutches are lost, and for all replacements this rate is similar (38.4%). It can also be seen that survival of SbS nests is usually slightly lower than generalised values for other local waders (
Table 1). More detail of nest predation of other waders in our study area, revealed with help of nest cameras, is published in [
39].
If a pair lost its clutch due to egg collection for HS, predation or flood within ca. 10 days after the clutch completion (usually, 21 June was a cut-off date), birds were capable of laying a replacement clutch, but we have two documented cases when pairs produced replacement clutches even later, after losing their first clutch after 21 June in late summer seasons 2015 and 2017. Time gap between first and replacement clutch hatching dates of a pair varied from 7.5 to 15.5 days (10.1±2.5, median 9, n=15).
Of 73 clutches collected for HS through the years, 35 replacement clutches or broods were found after first clutches had been taken for HS. Laying of a replacement clutch was impossible due to different reasons (e.g., late collection, collection of a replacement clutch) in 24 cases. That gives 71.4%, which is a minimum replacement probability for pairs involved in HS. To compare, of 22 depredated first clutches not related to HS, 8 were replaced; that gives 36.4% replacement probability regardless of nest predation date. Therefore, HS-induced relaying probability is about twice higher than after natural loss of clutches. That could be explained by early collecting of the eggs when birds are still physiologically ready to produce more eggs.
Headstarting improves egg production per pair (number of eggs survived to hatching) by 82% – from natural 2.84 to HS-related 5.16 (see Methods). Thus, using averaged figures from
Table 2, 4.25 vs. 2.53 chicks are hatched per pair. Of 278 eggs collected for HS, 6 appeared infertile, and 52 embryos died at different stages of incubation. The sample size for hatching rate estimations in nature is comparatively low since it is harder to track without exposing birds to additional risk associated with frequent visits to nests. This could partially explain slightly higher artificial incubation success in some years.
3.2. Fate of Chicks
In general, we do not know the sex of either embryos in the SbS eggs collected for HS or chicks subsequently hatched and released from those eggs. From 20 SbS eggs taken to The Wildfowl and Wetlands Trust, Slimbridge, UK in 2012 for captive breeding, 9♂ and 8♀ hatched; of those, 4♂ and 5♀ were still alive by the end of 2019.
Fledging rate in HS is 92.9%, but we are unable to evaluate this parameter in nature. Our optimistic expert evaluation is 62.5% based on both local broods’ fate tracking and subsequent records of birds marked as chicks. Application of these estimates to the numbers of chicks hatched per a pair involved in HS (including replacements) and in nature gives 3.63 and 1.58 fledged chicks per pair, respectively. For HS 2012–2022, 12 chicks died during rearing due to unpreventable or untreatable issues (genetic defects, prolonged hatch, infection, etc.). For 3 chicks the leg flags were removed before release (infected lesions at the heel joint or severe leg flicking). And at least 3 released are known to die before migrating (infect or predation).
Numbers of flagged chicks are presented in
Table 3.
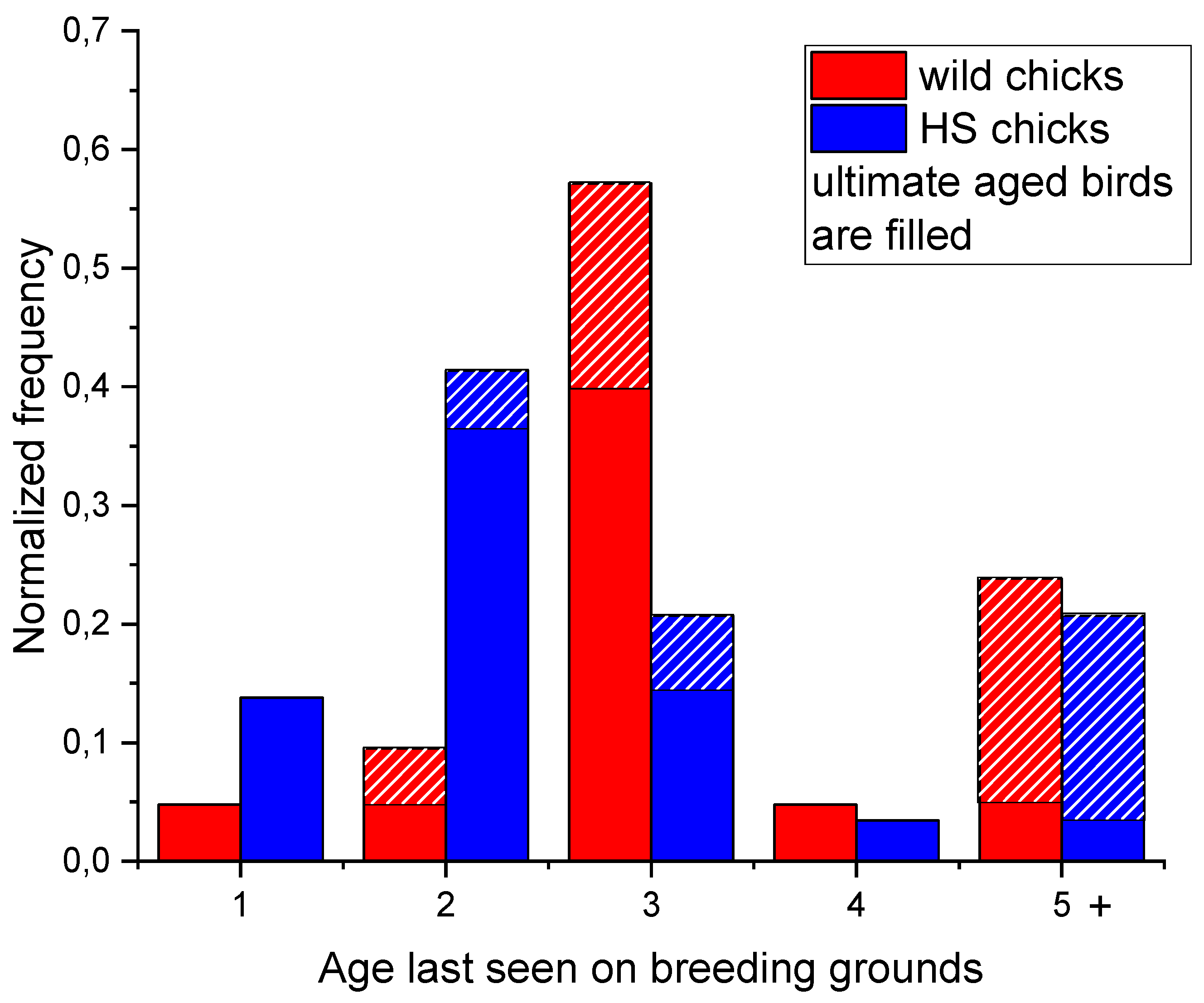
Figure 4 shows the age at last observation of HS and wild chicks. It can be seen that 76.8% of HS chicks have never been observed either on the flyway or returned back to the natal area, which is a slightly higher rate than 73.8% of wild chicks. However, in comparison with HS birds the wild cohort has a higher percentage of birds not seen after the age of 1 year – 30.2% vs. 27.1% of relevant juveniles ever observed.
By observations both in our study area and on the flyway, the expected recruitment of chicks from our study area to the global breeding population calculated by reaching maturity (see Methods) is higher for HS chicks (0.681) vs. wild ones (0.42 birds) per pair per year which is due to higher primary output of pairs involved in HS. For successful first-breeders contribution to the local population 0.416 and 0.3 birds per pair per year, respectively.
By the end of 2022, 29 HS (15
♂, 11
♀, and 3 birds of unknown sex) and 22 wild chicks (16
♂, 5
♀, and 1 birds of unknown sex) have ever come back later to the natal area (
Figure 5). Return rates in these groups are almost equal – ca. 14%. For 7 out of 29 returned HS birds, no breeding or at least successful mating was recorded – those are the 3 unsexed birds, 2
♂ and 2
♀ whose sex was assumed according to their behaviour and interaction with other birds, and 6
♂ staying solitary during their first breeding season. So, of HS birds, there were 12
♂ and 9
♀ breeding. Among breeding birds marked as wild chicks, sex ratio was quite different to HS: 12
♂:4
♀. Nearly same sex ratios in cohorts were observed each year considered separately. Noteworthy, among the breeding birds marked as wild chicks, 3 originated from HS-induced replacement clutches.
Of returned HS birds, 4 (15%) were yearlings. Yearling
♂ ‘
white 0C’ bred successfully having two clutches in one season: first one taken for HS, and replacement one resulted in 2 chicks (one fledged), we should note his mate was also a HS first-time breeder. For other yearlings (1
♀ and 2 unsexed birds) we do not have observations indicating their breeding. None of all these HS yearlings has ever been seen later again. As to returned wild chicks, 3 (15%) were yearlings: 2
♂ were breeding successfully for several years (one breeding from the very first summer, which is the very first documented case of such kind for SbS males). Such return rates of yearlings are unexpectedly high according to our previous knowledge from Northern Chukotka [
33]
Of 24 HS birds breeding in their natal area, only 7 bred more than once. One of such males (‘
L pink’) made breeding attempts during 4 successive years till 2019, but we are unaware of any success of the bird (all nests were predated). Only four HS males and two female were breeding up to the age of 5 years. Of the birds marked as wild chicks, all ever bred did that more than once (if possible). There is 1
♂ breeding at the age of 9 years and 1
♀ – at the age of 7. Noteworthy, while almost breeding birds marked as wild chicks started doing this being 2-y old at most, of HS birds, 1
♂ and 2
♀ started breeding being 3-y old and 2
♂ – 4-y old [
34].
There is a dramatic difference in apparent survival of the birds after their first arrival to the breeding grounds. While birds marked as wild chicks were back in 89.5% (n=19) cases, only 44.4% (n=27) of HS birds made it for the second time. In subsequent years, apparent survival was similar to the adult birds: 75% (n=20) and 70% (n=10), respectively.
For the birds marked as chicks that were observed on the flyway at the age of at least 23 months and never seen in Meinypil’gyno vicinity again in subsequent years, we assume that they dispersed from the natal area. There were 7 HS and 5 ‘wild’ such birds, so post-natal dispersal rates from the Meinypil’gyno area are 20.5% and 20.0%, respectively. Thus, the post-natal site fidelity (philopatry) can be evaluated as ca. 80% among young birds that reached maturity. Apparent maturity evaluated as breeding attempt or reaching 23 months age has been achieved by 16.0% HS and 16.7% wild chicks marked in 2012–2018, or 57.9% (n=57) and 56.0% (n=50), respectively, of the birds marked within the same timeframe and ever seen alive by the end of 2022 after first departure from the natal area.
Successful pairing was observed in 68.5% (HS, n=35) and 81.3% (marked as wild chicks, n=32) cases calculated for bird×years present in the study area. Egg safety was slightly higher for the pairs containing a HS partner compared to those with a partner marked as a wild chick: 46.8% (n=47 eggs) vs. 40% (n=55 eggs). Similar differences were found for hatch rates: 81.8% (n=6 nests) vs. 75% (n=10 nests), respectively. To calculate fledge rates (only for males since they are responsible for broods at this stage), we have only 2 very successful traceable cases for each cohort, therefore calculations are meaningless.
Data on post-natal dispersal are presented in
Table 4. For HS chicks the distance should be evaluated from the aviary, since that area has to be imprinted as natal one. We suggest that median values are more appropriate for consideration in this case since the distance distribution is different to normal. For both sexes, HS birds have nearly half lower post-natal dispersal than wild ones. It is worth noting that the large Pekul’neyskoye Lake restricts the NE sector from the aviary, which means that less land is available for the birds within the same range compared to many other SbS territories.
4. Discussion
It can be expected that chicks of various bird species raised in captivity by humans have reduced abilities for independent living in nature, which is especially possible in long-distance migrants experiencing variable environmental conditions in different parts of the species distribution range during the annual cycle. Our study allows, for the first time, estimation of survival, return rate, productivity, and several natural history characteristics for chicks of a long-distance migrant wader species, the SbS, raised in semi-natural conditions in comparison with chicks raised by parents in nature.
4.1. Survival of Chicks
High probability of replacement clutches early in a breeding season shows that egg collection for HS does not cause significant pressure on the local SbS population, where only about a half of pairs is engaged in HS especially if we take into consideration that not likely all replacement clutches were found before their loss due to predation. It is found in this study that overall return rates and maturity ages are similar in the two categories of SbS chicks under consideration. Despite this finding, we cannot be sure that there is no difference in HS and wild chick survival because the survival rate of HS chicks is considered for fledglings, while wild chicks were marked mainly during the first days of their lives, and not more than 62.5% of those would fledge. Therefore, to get equal return rates for the two groups of chicks, mortality of wild chicks presumably should be compensated by higher survival rate after fledging.
Almost all HS chicks come from first clutches, while a significant part of chicks marked in the wild are from replacements. This late hatching from replacement clutches can also influence survival of chicks and juveniles, which is shown for SbS at Belyaka Spit on the arctic coast of the species breeding range in 1986–1988 [
32]. Of 50 birds marked as wild chicks in 2013–2021 and ever observed after first departure from the natal area, 16 originated from replacement clutches. That gives 25% resighting probability for birds coming from first clutches (n=104) and 41.5% for those coming from replacements (n=41). Thus, this difference in survival rates is opposite to that on the arctic coast at least in some years. If replacement clutches are considered separately in terms of natural and HS-induced, the resighting probabilities are similar: 38.5% (n=13) and 42.9% (n=28), respectively.
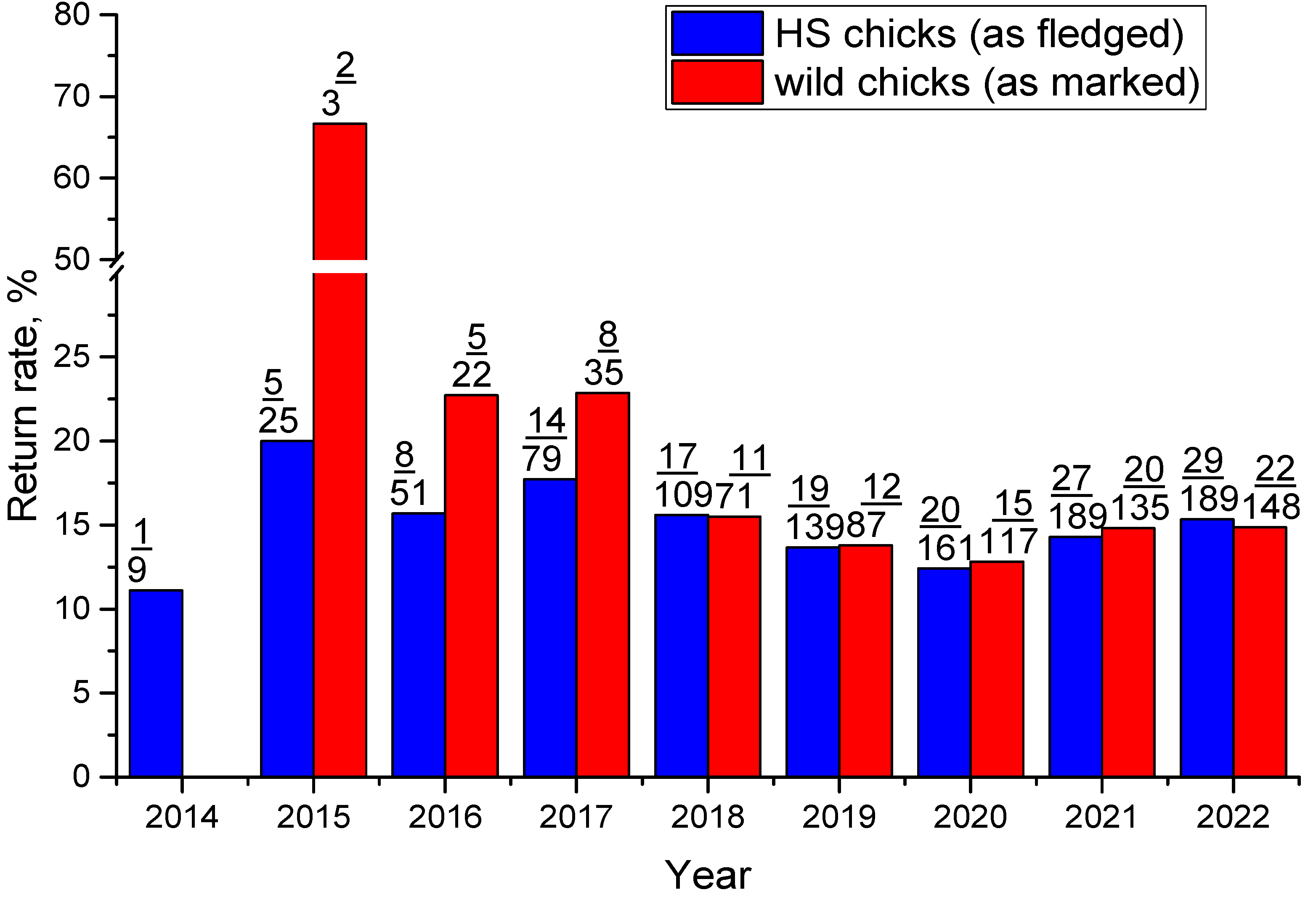
It can be seen in
Figure 6, that greater fraction (even not normalised by fledging rate) of the wild marked birds normally return to the breeding grounds. It can also be seen that survival has decreased significantly for both cohorts marked in 2017 and 2018. In 2020, for the first time, we had no unmarked birds in the core monitoring area. That means no new recruits came due to post-natal dispersal from other populations or the local chicks remained unmarked (not caught, missed broods, etc.). While adults' return rate was still about the long-term average. Not considering life experience, the main differences between juveniles’ and adults’ life outside the breeding grounds are: 1) earlier first southward migration; 2) staying for 2
nd calendar year summer on the Yellow sea coast outside the breeding grounds [
40]. Recent transformations in the latter area [41, 42] could be the key to this drop in juveniles' survival. Since the median age of the first observation is 6 months, the fraction of birds who reached maturity among ever observed is similar to the adults’ apparent survival of 68.8% (see below) in our study area. Similarity of this fraction in HS and ‘wild’ cohorts and slight advantage of wild-marked chicks in apparent maturity rate not normalised by fledging rate (0.625) indicate increased mortality of HS juveniles before the first observation.
However, long-term survival rate is even more obvious from the maximum age analysis for birds observed on the breeding grounds (
Figure 7) where more than a half of HS returned chicks were recorded only in one breeding season! Regardless, it is obvious that chicks marked wild have higher long-term survival rate. Also it appears that, except lower survival, HS birds are less able to form a breeding pair (
Figure 5). However, there have been three successful pairs consisting of both HS first-time breeders; moreover, one male was a yearling and his father was also a HS bird. Low apparent survival of yearlings coming to breeding grounds and their lower pairing abilities suggest this phenomenon does not lead to the population productivity increase.
4.2. Sex Issues in Chicks
We found that sex ratio in returned birds was not the same among those marked as HS and wild chicks. Annual sex ratios in the local breeding population of SbS never differed significantly from being equal, although territorial solitary males were always present in small numbers. If females have significantly wider post-natal dispersal than males, then it is likely that the same effect should be characteristic for HS birds too. It is also possible that the aviary is an attraction point for HS birds, and being situated in the centre of the monitoring team activities better completeness of subsequent discovery of birds of this cohort could happen (this is unlikely in our opinion). Unlike the HS birds, among chicks hatched and marked in the wild, only four females were recorded later breeding in the study area, and it is interesting that two of them hatched in 2015, and other two in 2018. This could evidence a significant influence of some unknown conditions in some seasons on chicks’ survival and thus on the subsequent sex bias.
Since HS birds have lower post-natal dispersal in comparison with those marked as wild chicks, we would suggest that this finding evidences a good choice of the aviary site. This also suggests a potentially increased risk of inbreeding. However, in the only case when HS male and female siblings returned to the natal area they settled 27 km apart.
Adult SbS males have shown 2.3% lower apparent survival rate than females (even though females are less site faithful and more cryptic so could be easier missed in surveys). But males in our study area are outnumbering females, similarly to the other local populations of monogamous waders. To match this balance at initial parity in embryos sex, high mortality of females should take place prior to breeding: among embryos, chicks, juveniles, and sub-adults.
It is reasonable to consider sex ratio in embryos to be close to parity in waders (e.g., [
43]), our data on SbS embryos also confirm this. Since for HS females survival from fledging to arrival to natal area appears similar to HS males, it is logical to suggest, in wild birds most females are lost prior to fledging. We were unable to get direct evidence for this. So we searched for similar issues in literature.
Liker et al. [
44] found that species with male-biased care usually have male-biased adult sex ratio. The ratio can also be biased already at hatching as it is shown for the mountain plover
Charadrius montanus ([
45], but just within 10% of sex ratio difference. Heg et al. [
43] did not find any sexual difference in survival of chicks during the hatch-to-fledge period in eurasian oystercatshers
Haematopus ostralegus. Some higher hatch-to-fledge survival was shown for males of mountain plover chicks: 54.8% vs. 47.2% in females [
45]. But Saunders & Cuthbert [
46] found significant male-biased survival during pre-fledging in
Ch. melodus. While Eberhart-Phillips et al. [47, 48] state that survival rate of juveniles (fledging to first breeding) mainly defines sex ratio in breeding populations of the polygamous snowy plover
Ch. nivosus being also male biased. For
L. limosa, male-biased chicks’ survival was explained by larger females failure to grow fast enough in poor habitats [
49]. Hallgrimsson et al. [
50] also found female juveniles were in a poorer condition than male in
C. maritima. Significant difference in sex-related survival of chicks was found in Kentish plover
Charadrius alexandrinus ([
51]; although it appears to be too strong.
Even though HS female chicks also had some slower growth rate, our data indicate that they survive at least no worse than males during captive rearing, but in nature this could be the reason for higher mortality [
52]. Significant part of chicks marked in the wild come from replacement clutches. This late hatching can also affect slower growing female chicks’ survival. Slower growth rate of females after nearly equal initial size of chicks seems to be the most probable of the reasons.
Just few data could be found on sex ratio in juvenile calidrids based on capturing those during their first migration. For great knot
C. tenurirostris, at migration stopover in Kamchatka, Russia, average sex ratio (identified genetically) was found to be also male biased (2:1, n=300) (A. Ivanov, pers.comm). Hallgrimsson et al. [
50] found no significant deviation of sex rate from parity in
C. maritima chicks, but it was male biased in juvenile birds (1.56:1). While for dunlin
C. alpina in Poland, it was female biased (1:1.33, n=56) [
53]. However, in the latter study, sex was identified by dissection of the birds died accidentally at banding sites that might affect the sample composition, and actually it evidences there are more females among dead birds. There are some more papers with sex ratios evaluations in waders, but mainly obtained for the birds on wintering grounds where authors suggest bias could be caused by sex-specific spatial distribution over habitats. However, Summers et al. (2013) still found an apparent male-bias in first-year
C. maritima and
Limosa lapponica and suggested increased juvenile females mortality to be responsible for the bias in breeding population.
4.3. Impact on the Local and Global Population
We should state there is no evidence of long-term HS affecting birds’ productivity or breeding behaviour: there are males and females whose first clutches are taken (or predated), but they keep fledging chicks from replacement clutches staying at the same territories for more than 5 years, and in most cases disappearing birds are never seen again anywhere.
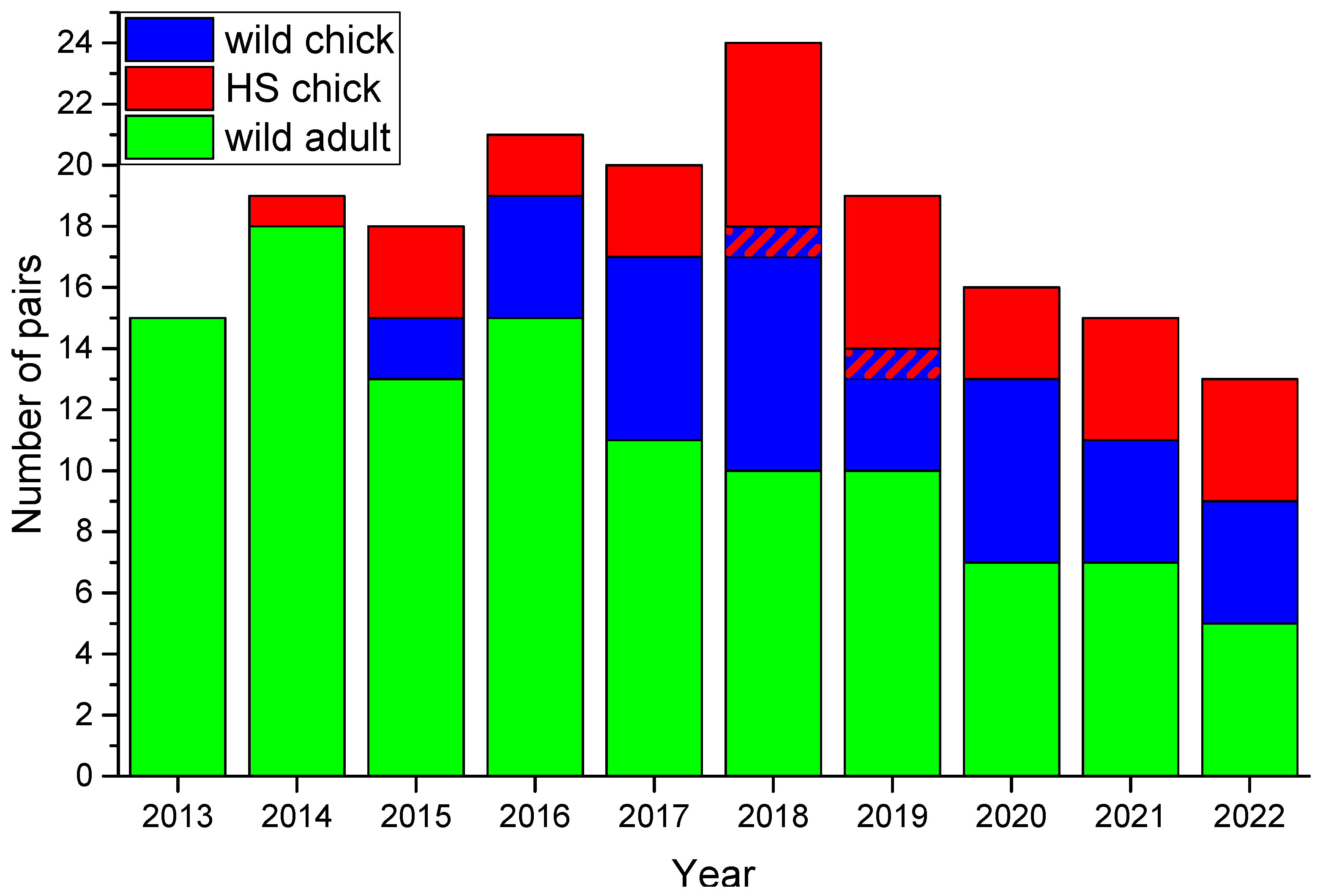
Figure 8 shows that birds marked locally as chicks since 2012 were present in over half of pairs in the local population by 2020. A slight increase in the number of breeding pairs was observed initially, but has changed to decline in recent years rolling back to 2013 numbers when no impact of HS on the population took place. Number of the birds marked as adults is decreasing expectedly. While reducing the return rate of juveniles and their rather short life expectancy (
Figure 7) do not compensate for it anymore. To keep the population sustainable with the current survival rate of birds, increased productivity is needed (
Table 5). There are no natural mechanisms for that, and only artificial efforts, such as HS, could help at least to decelerate the species extinction unless conservation activities outside of the breeding grounds will not improve the survival of birds.
The condition for the local population being self-sustainable is B/(1-q) =1 (sum of the infinite geometric series), where B – successful first-breeder output per breeding partner, q – annual adults’ return rate. It can be simplified to B+q=1. In our study area, q mean values were different (p<0.001) for adult males (0.728, n=575) and females (0.751, n=556), or 0.739 on average. For sustainability (zero-balance in a population), it should be 0.792 at full HS penetration into the population (all available pairs involved), or 0.85 without intervention at the current natural productivity. If we assume that immigration from other populations is equal to the emigration rate we evaluated for our birds (B should be divided by post-natal fidelity equal to 80%), than it can be expected that the target return rates change from 0.723 (and can be further improved if better HS pairing success is shown) to 0.8 in natural conditions. Even full HS penetration does not make the local population sustainable. However, if other local populations are stressed, interchange flows would not be balanced.
We can conclude that at full HS penetration to the local breeding population, it is able to just to decelerate annual decline to –4.4% against –14.0% without it. Based on maturity of recruits (
G/2 taken instead of
B) and adults’ survival rates, optimistic evaluation of population trend is +8.2% of annual rise for HS and –8.6% of annual decline without it. Therefore, there is some chance to make the local population nearly sustainable or even increasing at HS implementation, but definitely no chance without it. Our local population comprises ca. 10% of the global one (Green et. al. 2021), and currently only about a half of it subject to HS activities. This cannot affect the global population decline of -8.1% evaluated in [
21] using other methods, and our evaluation of mature birds trend (same to reference work) matches it perfectly.
Noteworthy, breeding sites of only ca. 50–60 pairs were known worldwide, which comprises about a quarter of the current breeding population estimate [
21]. So in addition to all logistic issues in such remote and unpopulated areas, expansion of HS activities is limited by lack of sites where at least 10 breeding pairs could be found within a reasonable distance from each other and from facilities required for HS activities.
4.4. Comparison to the northern SbS Population
For the SbS population on Belyaka Spit at the arctic coast of Chukotka [
32], 36.4–66.7% (mean 50%) of nests survived annually to hatching (mean 46.9% success accounted for n=311 eggs found), hatchability of eggs was slightly higher than in our study area: 91.5–95.2% annually (mean 93.6%, n=173 eggs survived to hatching). Hatch-to-fledge rate was evaluated as at least 55%. Noteworthy, loss of a whole brood was not a rare event there. Despite rather high replacement probabilities (59% for clutches lost early, which is rather similar to our HS induced value, and 24% on average), input of re-laying into productivity was rather low. On average, 0.57 fledged chicks per pair was a result for first clutches compared to 0.08 for replacements. This could be explained by higher predators’ activity later in the season on both sites and harsh weather conditions on the arctic coast. In our study area in Southern Chukotka, it can be assumed that among first clutches, primarily less secretively behaving pairs were revealed and their eggs collected for HS; that could also affect the reduced success of their replacement clutches.
Thus, for northern populations, HS gain could be significantly improved if first clutches are taken for artificial incubation, and then substituted with re-laid eggs. So egg production is increased, and birds can rear first clutch chicks in more favourable conditions while aviculturists provide extra-care to replacement ones when it is especially needed later in the season.
In total, this northern SbS population got 0.31–0.95 (mean 0.58) fledged chicks per pair annually [
32]. Almost the same figures were obtained for the population in Southern Chukotka in 2003–2007: 0.61±0.14 SE, range 0.32–0.93 [
18]. Similarly, low breeding success happened in our study area in some years. Three two-year old males returned to the arctic natal area (5.76%, n=52), and one unmarked breeding yearling female was also caught. This result is half less than average in our study area, but is similar to recent years’, which might indicate the start of similar fast decline. Therefore, despite the much smaller sample size for the northern population, among first-breeders both the fraction of yearlings and sex ratio matched our findings in the southern population. Combined with the apparent survival of adults of 0.657 reported for Belyaka Spit in 1986–1988, an even stronger decline trend than we currently have in our southern study area can be assumed. Additional evidence to this is a two times larger local population size estimate performed there in 1974 [20, 54]. However, to some extent, this difference could come from methods of counting birds there. Similar apparent survival of adults in both areas 30 years ago and currently also means it can hardly be improved without HS.
4.5. Comparison to Other Headstarted Species
Data comparison between projects is not easy because of differences in methods and situations between HS activities and species natural histories. Thus, not a complete clutch like in case of SbS, but only one egg of two was collected for the takahe project [
8]. For piping plover [
13] and snowy plover [
14], abandoned eggs, and for the latter species also chicks were taken. To improve safety of clutches of american oystercatchers, anchoring of artificial eggs was used (mainly against flood), which increased probability of nest survival to hatching from 7–30% in a control group to 71–74% in HS ones [
10]. For mangrove finches, only 27.7% of nests in nature were successful, therefore egg collection considerably improved egg survival with help of HS [
9].
Artificial hatch rate is not often higher than natural one. Only maleo could be an exception with 41–78% success of parentless natural incubation, and 91% rate was reached in Bronx Zoo [
7]. In case with SbS, artificial incubation success was ca. 10% less on average or equal to natural in some years. For piping plovers [
13], hatch rates were 85% vs. 59% in nature resulting in ca. 30% advantage of HS. Artificial hatching success for american oystercatchers was 62% and 84% in 2 subsequent years [
10], and no data available on success in nature for comparison. For magrove finches, the artificial hatching rate was 88.2% and unlikely to be improved considerably [
9]. Black-tailed godwits artificial hatching rate was 79.3% that comprised 90.7% of eggs viable at collection [
11].
The highest possible fledging rate is one of the main goals for HS projects since captive rearing is usually the most demanding stage for resources. In the case of SbS, HS gain was ca. 1.5-fold compared to natural fledging, and could hardly be improved over 92.9%. For piping plovers [
13], the gain was similar with 77% HS fledging rate compared to natural 50%, but this could still be improved. For maleo, the protocol target was 40% in terms of reaching 30 days age [
7]. For takahe, 90% survival up to 1-year age has been reported with 1 to 4-fold improvement of natural rate in different years [
8]. All artificially hatched mangrove finches were fledged, that was 2.9 higher rate than in nature [
9]. For black-tailed godwits, the fledge rate was 91.3% [
11]. Noteworthy, since captive rearing part was not implemented for american oystercatchers (hatching eggs were returned to the nests), mean fledged chicks output was lower for HS compared to natural – 0.27 vs. 0.35 chicks per pair despite higher safety of clutches [
10]. This means that without captive rearing and giving an opportunity for parents to re-nest, HS can have significantly reduced gain or just be useless.
For released takahe, 14% were resighted 4–9 years after release with no obvious difference in post-release mortality in comparison with birds raised naturally; 8 females and 2 males (sex parity at release) are known to have bred [
8]. No significant difference was found in captive and wild reared snowy plovers [
14]: apparent survival in the age of 1 year (ca. 36%) and of 2+ years (ca. 69%). In both cohorts of snowy plovers, ca. 36% of birds were recruited to the local breeding population the next year after release. Captive reared snowy plovers had just slightly lower hatching and fledging rates than in naturally raised chicks, and these males fledged fewer offspring in the subsequent breeding seasons that could evidence their lower adaptation capability. For piping plovers, 3-fold lower resighting rates (8–9%) for captive reared birds were reported compared to wild reared ones; however, there could be ecological reasons for that, not just lower survival rate [
14]. In black-tailed godwits, 26.6% of yearlings came back to the release site next year [
11], and 30.7% of the returnees attempted to breed. Results achieved for SbS show no significant difference in return rates of the HS and wild cohorts of chicks, but evidenced lower HS birds pairing capabilities. Compared to other species, values for SbS could be expected to be lower, and they are so accordingly, since this species is a long-distance migrant (especially considering their small size), and the majority of young SbS normally return to their natal areas at the age of 2 years only.
4.6. Suggestions for Headstarting Improvement
Our evaluations show that despite headstarting has a positive effect on the population and gives the only chance to reduce its decline, its gain is reduced over the life cycle of the birds. The most critical periods are survival to the first and second arrivals to the breeding grounds. The findings of [
38] should be implemented and further investigated for the earliest age at release since that could double the apparent survival of captive reared birds to first breeding. Reduced survival after first arrival to the breeding grounds could be caused by the numerous threats wild reared birds are more resistant to.
Lower survival of HS birds could be caused by their increased tameness to the presence of humans, which has always been stressed by observers at wintering grounds. This could lead to increased mortality at active hunting or impact from other human-induced activities like leaving open fishnets. This negative effect can be partially reduced by human and predator avoidance training at rearing and just after release of HS birds. As well as nets used as the rearing pen walls could be made less net-like for the birds did not get an experience of safe net impacts.
Another reason lower survival could occur is that less viable chicks are still reared to fledging, but later congenital diseases and other health issues (including in-house infections and reproductive hormones activated issues) affect their long-term survival. Effect of HS on genetic diversity in the population needs a special investigation [55, 56]. Pairs (or partners in long-term) whose nests are easier to find mainly due to proximity to the avicultural station get an advantage in their offspring fraction in the population. Ideally, such partners should be checked for unfavourable allele presence to reduce their spread in the population.
The revealed positive effect of HS on the population of SbS can be increased if sex ratio is changed in favour of females among released birds. There is no simple way to implement that. For example, in case embryos sexed [57, 58], females could be taken for HS, and males redistributed between nests. Some impact on females to stimulate them to produce more female embryos appears even less realistic. At the moment, the most realistic way is to select females among chicks just hatched in incubators for their raising in captivity and thus reducing their mortality to fledging. Males hatched from artificially incubated eggs could be returned to nature while wild female chicks taken instead for captive rearing. This suggests extra work for finding suitably aged broods and quick sexing of chicks hatched in captivity.
Headstarting applied to the Arctic coast populations of SbS where survival from replacement clutches is very low due to harsh weather conditions could be more beneficial than for the southern ones where it was performed.
5. Conclusions
Implementation of HS for 10 years has shown that it helps to sustain or even slightly increase the local population of the critically endangered Spoon-billed Sandpiper. This was provided by a significant increase of egg production per pair due to stimulation of replacement clutches and high hatch-to-fledge survival rate ensured by careful captive rearing resulting in at least 2.1-fold overall gain in the number of fledged chicks. The negative results were in reduced survival from fledging to recruiting in natal area and pairing capabilities in the HS birds, still bringing 1.4 more successful first-time breeders to the population. However, 1.9-fold lower survival of HS first-breeders eliminates the initial advantage, so must be dealt with. The additional unexpected positive result was equal sex ratio among returned birds compared to over 3-fold male biased among wild chicks marked during the same period, and for breeding females recruiting, is still not overweighed by the combination of all negative effects.
Based on recruitment rate of marked chicks into the local breeding population as well as on annual return of recruits, it is shown that the local population potentially can be sustained with implementation of HS, whether it can grow depends mainly on juveniles return rate that has dropped significantly in recent years. Without HS, only decline is expected. Headstarting gain can be improved by these data driven changes of protocols. Assuming that the local SbS population in Meinypil’gyno area comprises ca. 10% of the global one [
21], even achieving maximum productivity at its current scale, HS is unable to redirect the negative global trend, but its scaling up (number of eggs collected) could be helpful. Further improvement of SbS survival on the flyway is still the key to prevent SbS extinction. HS is able to provide extra time for other activities which are able to increase survival of SbS on the flyway.
Activities related to HS have significantly improved understanding of SbS ecology, and ensured species safety at least in the study area. HS also has a significant social effect due to involvement of increasing numbers of people both in the local communities in Chukotka and from many countries on the flyway into searching for marked SbS and learning about waders, raising awareness about ecological problems on the East Asian-Australasian Flyway, thus, making need for conservation actions on the flyway (not for SbS only) more obvious and sensible.
Author Contributions
Conceptualisation, E.Y.L., E.E.S.; methodology, E.Y.L., R.A.D., N.Y.Y., I.A.S., J.P.C., P.S.T., N.S.J., E.E.S.; validation, N.A.C., R.E.G.; formal analysis, E.Y.L., P.S.T., N.A.C., R.E.G.; investigation, E.Y.L., R.A.D., N.N.Y., I.A.S., J.P.C., P.S.T., N.S.J., N.A.C., R.E.G.; resources, E.Y.L., N.S.J., N.A.C., E.G.L., E.E.S.; data curation, E.Y.L., J.P.C., P.S.T., N.A.C.; writing—original draft preparation, E.Y.L., J.P.C., P.S.T.; writing—review and editing, E.Y.L., R.A.D., J.P.C., P.S.T., N.A.C., R.E.G., E.E.S.; visualisation, E.Y.L., P.S.T.; supervision, E.E.S.; project administration, N.S.J., N.N.Y., E.G.L.; funding acquisition, N.S.J., N.A.C., E.G.L, E.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work has been funded from multiple sources over the years. Wildfowl & Wetlands Trust (WWT), Royal Society for the Protection of Birds (RSPB), and Mangrove Conservation Fund (MCF) were the major sponsors. Work of E.G.L. was supported by the Russian Science Foundation (grant No. 22-17-00168).
Acknowledgments
Authors are grateful to the non-authoring aviculturists and their assistants: Yuriy Bragin, Elizabeth Brown, Elizabeth Mackley, Richard Hesketh, Richard Smith, Nicola Hiscock, Anastasia Yukusheva, Yuriy Lobachev, Dmitriy Moseykin. Baz Hughes, Rebecca Lee, Christoph Zockler and Spoon-billed Sandpiper Task Force have provided invaluable organisational support. Numerous reporters of colour-marked SbS observations all over the flyway made these evaluations possible in principle. As well as over a dozen field assistant helped to locate SbS and find nests in our research area. We thank the Animal Welfare and Ethics Committee of the Wildfowl and Wetlands Trust for scrutiny of the head-starting procedures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Payevsky, V. A. Demography of birds. Nauka: Leningrad, 1985.
- Lack, D. The natural regulation of animal numbers. Clarendon Press.: Oxford, 1954; p 343.
- Studds, C. E.; Kendall, B. E.; Murray, N. J.; Wilson, H. B.; Rogers, D. I.; Clemens, R. S.; Gosbell, K.; Hassell, C. J.; Jessop, R.; Melville, D. S.; Milton, D. A.; Minton, C. D. T.; Possingham, H. P.; Riegen, A. C.; Straw, P.; Woehler, E. J.; Fuller, R. A. Rapid population decline in migratory shorebirds relying on Yellow Sea tidal mudflats as stopover sites. Nature Communications 2017, 8 (1), 14895. [CrossRef]
- Piersma, T.; Lok, T.; Chen, Y.; Hassell, C. J.; Yang, H.-Y.; Boyle, A.; Slaymaker, M.; Chan, Y.-C.; Melville, D. S.; Zhang, Z.-W.; Ma, Z. Simultaneous declines in summer survival of three shorebird species signals a flyway at risk. Journal of Applied Ecology 2016, 53 (2), 479-490. [CrossRef]
- Murray, N. J.; Marra, P. P.; Fuller, R. A.; Clemens, R. S.; Dhanjal-Adams, K.; Gosbell, K. B.; Hassell, C. J.; Iwamura, T.; Melville, D.; Minton, C. D. T.; Riegen, A. C.; Rogers, D. I.; Woehler, E. J.; Studds, C. E. The large-scale drivers of population declines in a long-distance migratory shorebird. Ecography 2018, 41 (6), 867-876. [CrossRef]
- Melville, D. S.; Chen, Y.; Ma, Z. Shorebirds along the Yellow Sea coast of China face an uncertain future—a review of threats. Emu - Austral Ornithology 2016, 116 (2), 100-110. [CrossRef]
- Thomas, P.; Boyer, D. M.; Oehler, D. A.; Silver, S.; Perrotti, L. Headstarting as a Conservation Strategy for Threatened and Endangered Species. In Scientific Foundations of Zoos and Aquariums: Their Role in Conservation and Research, Kaufman, A. B.; Bashaw, M. J.; Maple, T. L., Eds. Cambridge University Press: Cambridge, 2019; pp 91-111. [CrossRef]
- Maxwell, J. M.; Jamieson, I. G. Survival and Recruitment of Captive-Reared and Wild-Reared Takahe in Fiordland, New Zealand. Conservation Biology 1997, 11 (3), 683-691. [CrossRef]
- Cunninghame, F.; Switzer, R.; Parks, B.; Young, G.; Carrion, A.; Medranda, P.; Sevilla, C. Conserving the critically endangered mangrove finch: Head-starting to increase population size; Puerto Ayora, 2015.
- Collins, S. A.; Sanders, F. J.; Jodice, P. G. R. Assessing conservation tools for an at-risk shorebird: Feasibility of headstarting for American Oystercatchers Haematopus palliatus. Bird Conservation International 2016, 26 (4), 451-465. [CrossRef]
- Hiscock, N.; Brand, E.; Calvo-Carrasco, D.; Donaldson, L.; R., L. Headstarting the black-tailed godwit Limosa limosa limosa at key breeding sites for the UK population: 2018 Annual Report; Wildfowl & Wetlands Trust: Slimbridge, 2018.
- van Heezik, Y.; Maloney, R. F.; Seddon, P. J. Movements of translocated captive-bred and released Critically Endangered kaki (black stilts) Himantopus novaezelandiae and the value of long-term post-release monitoring. Oryx 2009, 43 (4), 639-647. [CrossRef]
- Powell, A. N.; Cuthbert, F. J.; Wemmer, L. C.; Doolittle, A. W.; Feirer, S. T. Captive-rearing piping plovers: Developing techniques to augment wild populations. Zoo Biology 1997, 16 (6), 461-477. [CrossRef]
- Neuman, K. K.; Stenzel, L. E.; Warriner, J. C.; Page, G. W.; Erbes, J. L.; Eyster, C. R.; Miller, E.; Henkel, L. A. Success of captive-rearing for a threatened shorebird. Endangered Species Research 2013, 22 (1), 85-94. [CrossRef]
- Boyd, H.; Piersma, T. Changing balance between survival and recruitment explains population trends in Red Knots Calidris canutus islandica wintering in Britain, 1969-1995. Ardea 2001, 89 (2), 301-317.
- Lappo, E. G.; Tomkovich, P. S.; Syroechkovskiy, E. E. Atlas of breeding waders in the Russian Arctic. Publishing House “UF Ofsetnaya Pechat”: Moscow, 2012; p 448.
- Zockler, C.; Beresford, A. E.; Bunting, G.; Chowdhury, S. U.; Clark, N. A.; Fu, V. W. K.; Htin Hla, T.; Morozov, V. V.; Syroechkovskiy, E. E.; Kashiwagi, M.; Lappo, E. G.; Tong, M.; Long, T. L.; Yu, Y.-T.; Huettmann, F.; Akasofu, H. K.; Tomida, H.; Buchanan, G. M. The winter distribution of the Spoon-billed Sandpiper Calidris pygmaeus. Bird Conservation International 2016, 26 (4), 476-489. [CrossRef]
- Zockler, C.; Syroechkovskiy, E. E.; Atkinson, P. W. Rapid and continued population decline in the Spoon-billed Sandpiper Eurynorhynchus pygmeus indicates imminent extinction unless conservation action is taken. Bird Conservation International 2010, 20 (2), 95-111. [CrossRef]
- Tomkovich, P. S.; Syroechkovski, J. E. E.; Lappo, E. G.; Zöckler, C. First indications of a sharp population decline in the globally threatened Spoon-billed Sandpiper Eurynorhynchus pygmeus. Bird Conservation International 2002, 12 (1), 1-18. [CrossRef]
- Syroechkovski, E. E.; Tomkovich, P. S.; Kashiwagi, M.; Taldenkov, I. A.; Buzin, V. A.; Lappo, E. G.; Zöckler, C. Population decline in the spoon-billed sandpiper (Eurynorhynchus pygmeus) in northern Chukotka based on monitoring on breeding grounds. Biology Bulletin 2010, 37 (9), 941-951. [CrossRef]
- Green, R. E.; Syriechkovsliy, E. E.; Anderson, G. Q. A.; Chang, Q.; Chowdhury, S. U.; Clark, J. A.; Foysal, M.; Gerasimov, Y.; Hughes, B.; Kelly, C.; Lappo, E.; Lee, R.; Leung, K. K. S.; Li, J.; Loktionov, E. Y.; Melville, D. S.; Pillips, J.; Tomkovich, P. S.; Weston, E.; Weston, J.; Yakushev, N.; Clark, N. A. New estimated of the size and trend of the world population of the Spoon-billed Sandpiper using three independent statistical models. Wader Study 2021, 128 (1), 22-35. [CrossRef]
- Clark, N. A.; Anderson, G. Q. A.; Li, J.; Syroechkovskiy, E. E.; Tomkovich, P. S.; Zöckler, C.; Lee, R.; Green, R. E. First formal estimate of the world population of the Critically Endangered spoon-billed sandpiper Calidris pygmaea. Oryx 2018, 52 (1), 137-146. [CrossRef]
- Peng, H.-B.; Anderson, G. Q. A.; Chang, Q.; Choi, C.-Y.; Chowdhury, S. U.; Clark, N. A.; Gan, X.; Hearn, R. D.; Li, J.; Lappo, E. G.; Liu, W.; Ma, Z.; Melville, D. S.; Phillips, J. F.; Syroechkovskiy, E. E.; Tong, M.; Wang, S.; Zhang, L. I. N.; ZÖCkler, C. The intertidal wetlands of southern Jiangsu Province, China – globally important for Spoon-billed Sandpipers and other threatened waterbirds, but facing multiple serious threats. Bird Conservation International 2017, 27 (3), 305-322. [CrossRef]
- Menxiu, T.; Lin, Z.; Li, J.; Zöckler, C.; Clark, N. A. The critical importance of the Rudong mudflats, Jiangsu Province, China in the annual cycle of the Spoon-billed Sandpiper Calidris pygmeus. Wader Study Group Bull. 2012, 119 (3), 208-211.
- Clark, N. A.; Pain, D.; Green, R. E. Saving the Spoon-billed Sandpiper: an update on the conservation programme. British Birds 2014, 107, 467-475.
- Chang, Q.; Anderson, G. Q. A.; Brides, K.; Clark, J. A.; Clark, N. A.; Hearn, R.; Leung, K.; Melville, D. S.; Weston, E.; Weston, J.; Green, R. E. A high proportion of the world population of the Spoon-billed Sandpiper occurs at Tiaozini, China, during the post-breeding moult. Wader Study 2019, 126 (1), 35-42. [CrossRef]
- Zöckler, C.; Chowdhury, S. U.; Sun, L.; Qing, C.; Aung, P. P.; Clements, J.; Klokov, K.; Lappo, E. G.; Syroechkovskiy, E. The Spoon-billed Sandpiper Calidris pygmaea conservation project in 2019 and 2020: population trends continue to be negative. BirdingASIA 2020, 33, 51-56.
- Chowdhury, S. U.; Foysal, M.; Green, R. E. Accelerating decline of an important wintering population of the critically endangered Spoon-billed Sandpiper Calidris pygmaea at Sonadia Island, Bangladesh. Journal of Ornithology 2022, 163 (4), 891-901. [CrossRef]
- Syroechkovskiy, E. E.; Lappo, E. G. Information from regions: Chukotka. Information Materials of the Working Group on Waders 2002, 15 (17-18).
- Tomkovich, P.; Syroechkovskiy, E.; Yakushev, N.; Loktionov, E.; Digby, R.; Shepelev, I. Spoon-billed Sandpipers in Meinypilgyno: update of the 2016 breeding season Spoon-billed Sandpipers Task Force News Bull. 2016, 16 (13-15).
- Tomkovich, P. S. Maximum life longevity of some waders in Chukotka. Information Materials of the Working Group on Waders 2003, 16 (55-56).
- Tomkovich, P. S. Breeding biology and breeding success of the Spoon-billed Sandpiper Eurynorhynchus pygmeus. Russ. J. Ornithol. 1995, 4 (3), 77–91.
- Tomkovich, P. S. Spatial structure of the Spoon-billed Sandpiper (Eurynorhynchus pygmeus) population at the breeding grounds. In Modern Ornithology Kurochkin, E. N., Ed. Nauka: Moscow, 1994; pp 130-148.
- Tomkovich, P. S.; Loktionov, E. Y. Age of first breeding of Spoon-billed Sandpipers Calidris pygmaea. Wader Study 2021, 128 (1), 96-98. [CrossRef]
- Tomkovich, P. S. Migration of the Spoon-billed Sandpiper Eurynorhynchus pygmeus in the Far East of the Russian Federation. Stilt 1992, 21 (29-33).
- Tomkovich, P. S. External morphology of the Spoon-billed Sandpiper (Eurynorhynchus pygmeus) at Chukotski Peninsula. Ornithologia (Moscow) 1991, 25 (135-144).
- Cook, M. I.; Beissinger, S. R.; Toranzos, G. A.; Arendt, W. J. Incubation reduces microbial growth on eggshells and the opportunity for trans-shell infection. Ecology Letters 2005, 8 (5), 532-537. [CrossRef]
- Clements, J.; Loktionov, E. Y.; Yakushev, N.; Clark, N. A.; Digby, R.; Jarrett, N.; Shepelev, I.; Tomkovich, P. S.; Green, R. E. Effect of age at release on the post-release survival of head-started Spoon-billed Sandpipers. Wader Study 2022, 129 (2), 100-104. [CrossRef]
- Tomkovich, P. S.; Loktionov, E. Y.; Syroechkovskiy, E. E. Predators of wader nests in Southern Chukotka, Russia, as leant with camera traps. In Actual issues of wader studies in Northern Eurasia. Proceedings of the XI International Scientific and Practical Conference, Minsk, 2019; pp 5-11.
- Li, J.; Lin, Z.; N., M. Spoon-billed Sandpiper in Jiangsu Province, China and Korea in spring and summer. Spoon-billed Sandpiper Task Force News Bull. 2016, 16, 22–23.
- Moores, N.; Rogers, D. I.; Rogers, K.; Hansbro, P. M. Reclamation of tidal flats and shorebird declines in Saemangeum and elsewhere in the Republic of Korea. Emu 2016, 116 (2), 136-146. [CrossRef]
- Ma, Z.; Melville, D. S.; Liu, J.; Chen, Y.; Yang, H.; Ren, W.; Zhang, Z.; Piersma, T.; Li, B. Rethinking China's new great wall. Science 2014, 346 (6212), 912-914. [CrossRef]
- Heg, D.; Dingemanse, N. J.; Lessells, C. M.; Mateman, A. C. Parental Correlates of Offspring Sex Ratio in Eurasian Oystercatchers. The Auk 2000, 117 (4), 980-986. [CrossRef]
- Liker, A.; Freckleton, R. P.; Székely, T. The evolution of sex roles in birds is related to adult sex ratio. Nature Communications 2013, 4 (1), 1587. [CrossRef]
- Riordan, M. M.; Lukacs, P. M.; Huyvaert, K. P.; Dreitz, V. J. Sex ratios of Mountain Plovers from egg production to fledging. Avian Conservation and Ecology 2015, 10 (2), 3. [CrossRef]
- Saunders, S. P.; Cuthbert, F. J. Chick mortality leads to male-biased sex ratios in endangered Great Lakes Piping Plovers. Journal of Field Ornithology 2015, 86 (2), 103-114. [CrossRef]
- Eberhart-Phillips, L. J.; Küpper, C.; Carmona-Isunza, M. C.; Vincze, O.; Zefania, S.; Cruz-López, M.; Kosztolányi, A.; Miller, T. E. X.; Barta, Z.; Cuthill, I. C.; Burke, T.; Székely, T.; Hoffman, J. I.; Krüger, O. Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nature Communications 2018, 9 (1), 1651. [CrossRef]
- Eberhart-Phillips, L. J.; Küpper, C.; Miller, T. E. X.; Cruz-López, M.; Maher, K. H.; dos Remedios, N.; Stoffel, M. A.; Hoffman, J. I.; Krüger, O.; Székely, T. Sex-specific early survival drives adult sex ratio bias in snowy plovers and impacts mating system and population growth. Proceedings of the National Academy of Sciences 2017, 114 (27), E5474-E5481. [CrossRef]
- Loonstra, A. H. J.; Verhoeven, M. A.; Senner, N. R.; Hooijmeijer, J. C. E. W.; Piersma, T.; Kentie, R. Natal habitat and sex-specific survival rates result in a male-biased adult sex ratio. Behavioral Ecology 2019, 30 (3), 843-851. [CrossRef]
- Hallgrimsson, G. T.; Palsson, S.; Summers, R. W.; Benediktsson, G. Ö. Sex ratio and sexual size dimorphism in Purple Sandpiper Calidris maritima chicks. Bird Study 2011, 58 (1), 44-49. [CrossRef]
- Székely, T.; Cuthill, I. C.; Yezerinac, S.; Griffiths, R.; Kis, J. Brood sex ratio in the Kentish plover. Behavioral Ecology 2004, 15 (1), 58-62. [CrossRef]
- Clark, J. A. Selective mortality of waders during severe weather. Bird Study 2009, 56 (1), 96-102. [CrossRef]
- Meissner, W. Sex determination of juvenile Dunlins migrating through the Polish Baltic region. Journal of Field Ornithology 2005, 76 (4), 368-372. [CrossRef]
- Tomkovich, P. S.; Soloviev, M. Y. Numbers of the Spoon-billed Sandpiper Eurynorhynchus pygmeus at the north of Kolyuchinskaya Gulf, Chukotka, and count methods for the species on breeding grounds. Russ. J. Ornithol. 2000, (99), 3-10.
- Rönkä, N.; Pakanen, V.-M.; Pauliny, A.; Thomson, R. L.; Nuotio, K.; Pehlak, H.; Thorup, O.; Lehikoinen, P.; Rönkä, A.; Blomqvist, D.; Koivula, K.; Kvist, L. Genetic differentiation in an endangered and strongly philopatric, migrant shorebird. BMC Ecology and Evolution 2021, 21 (1), 125. [CrossRef]
- Valdebenito, J. O.; Maher, K. H.; Zachár, G.; Huang, Q.; Zhang, Z.; Young, L. J.; Székely, T.; Que, P.; Liu, Y.; Urrutia, A. O. Sex differences in immune gene expression in the brain of a small shorebird. Immunogenetics 2022, 74 (5), 487-496. [CrossRef]
- Galli, R.; Preusse, G.; Schnabel, C.; Bartels, T.; Cramer, K.; Krautwald-Junghanns, M.-E.; Koch, E.; Steiner, G. Sexing of chicken eggs by fluorescence and Raman spectroscopy through the shell membrane. PLOS ONE 2018, 132 (2), e0192554. [CrossRef]
- Dutton, C. J.; Tieber, A. A Modified Protocol for Sex Identification of in Ovo Avian Embryos and Its Application as a Management TOOL for Endangered Species Conservation Programs. Jornal of Zoo and Wildlife Medicine 2001, 32 (2), 176-180. https://www.jstor.org/stable/20096095.
Figure 1.
Incubator set up at the rearing station (a) and the aviary (b).
Figure 1.
Incubator set up at the rearing station (a) and the aviary (b).
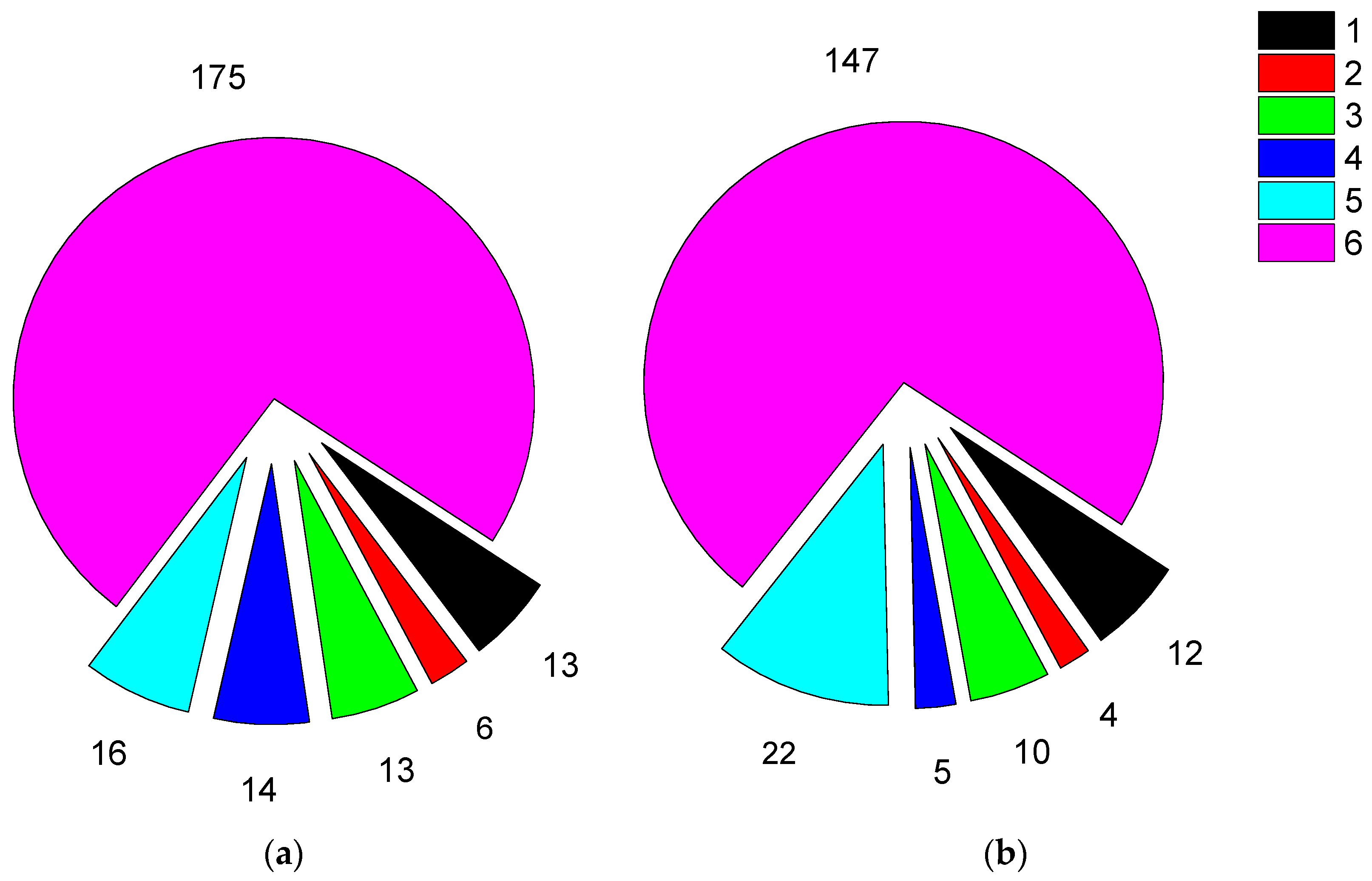
Figure 4.
Maximum ages at last observation of marked birds: a – HS (n=237) and b – wild (n=200) chicks: 1 – 1–6 months, 2 – 6–12 months, 3 – 1–2 years, 4 – 2–3 years, 5 – over 3 years, 6 – never seen.
Figure 4.
Maximum ages at last observation of marked birds: a – HS (n=237) and b – wild (n=200) chicks: 1 – 1–6 months, 2 – 6–12 months, 3 – 1–2 years, 4 – 2–3 years, 5 – over 3 years, 6 – never seen.
Figure 5.
Fate of birds marked in 2012–2020 by the end of 2022 as: a – HS (n=206) and b – wild (n=168) chicks based on known observations: 1 – bred in the natal area, 2 – observed there without an evidence of successful breeding attempt, 3 – apparently reached maturity, 4 – ever seen outside the natal area, 5 – never observed.
Figure 5.
Fate of birds marked in 2012–2020 by the end of 2022 as: a – HS (n=206) and b – wild (n=168) chicks based on known observations: 1 – bred in the natal area, 2 – observed there without an evidence of successful breeding attempt, 3 – apparently reached maturity, 4 – ever seen outside the natal area, 5 – never observed.
Figure 6.
Return rate of headstarted and wild chicks (the upper number indicates the cumulative number of chicks returned by the year, the lower value shows the number of birds in the age of 2+ years that potentially could return).
Figure 6.
Return rate of headstarted and wild chicks (the upper number indicates the cumulative number of chicks returned by the year, the lower value shows the number of birds in the age of 2+ years that potentially could return).
Figure 7.
Maximum age at last observation on breeding grounds of HS and wild SbS chicks. Hatched parts of the columns refer to the birds of ultimate age, i.e. they could not be older at the time of analysis.
Figure 7.
Maximum age at last observation on breeding grounds of HS and wild SbS chicks. Hatched parts of the columns refer to the birds of ultimate age, i.e. they could not be older at the time of analysis.
Figure 8.
Composition of breeding pairs in Meinypil’gyno area. Two-colour hatching shows pairs consisting of partners marked both as wild and HS chicks. Wild partners were marked as adults or remained unmarked.
Figure 8.
Composition of breeding pairs in Meinypil’gyno area. Two-colour hatching shows pairs consisting of partners marked both as wild and HS chicks. Wild partners were marked as adults or remained unmarked.
Table 1.
Portions of wader nests under observation survived to hatching near Meinypil’gyno in 2013–2022.
Table 1.
Portions of wader nests under observation survived to hatching near Meinypil’gyno in 2013–2022.
Year
Species |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
Total |
Spoon-billed
Sandpiper Calidris pygmaea
|
66.7%
(n=6) |
62.5%
(n=8) |
50%
(n=10) |
50%
(n=12) |
50%
(n=12) |
52.9%
(n=17) |
41.7%
(n=12) |
41.7%
(n=12) |
55.6%
(n=9) |
57.1%
(n=7) |
51.4%
(n=105) |
| Total for other various locally breeding waders |
74.0%
(n=46) |
64.0%
(n=44) |
54.5%
(n=55) |
65.4%
(n=52) |
46.0%
(n=50) |
68.6%
(n=51) |
51.1%
(n=47) |
49.1%
(n=57) |
61.0%
(n=59) |
68.6%
(n=51) |
60.0%
(n=512) |
Table 2.
Proportion of SbS eggs hatched in 2011–2020 at artificial incubation and in nature.
Table 2.
Proportion of SbS eggs hatched in 2011–2020 at artificial incubation and in nature.
| Year |
2011* |
2012* |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
Total |
| Incubator |
80.0%
n=25 |
75.7%
n=37 |
87.5%
n=24 |
87.1%
n=31 |
87.9%
n=33 |
86.1%
n=36 |
83.3%
n=36 |
71.4%
n=35 |
68.4%
n=38 |
90.0%
n=30 |
74.4%
n=39 |
no |
80.5%
n=364 |
| Nature |
100%
n=4 |
100%
n=4 |
85.7%
n=7 |
85.7%
n=21 |
84.6%
n=13 |
92.9%
n=14 |
72.7%
n=11 |
91.7%
n=24 |
94.7%
n=19 |
94.4%
n=18 |
78.9%
n=19 |
86.7%
n=15 |
88.2%
n=169 |
| Total |
82.8%
n=29 |
78.0%
n=41 |
87.1%
n=31 |
86.5%
n=52 |
87.0%
n=46 |
88.0%
n=50 |
80.9%
n=47 |
79.7%
n=59 |
77.2%
n=57 |
91.7%
n=48 |
75.9%
n=57 |
86.7%
n=15 |
82.9%
n=533 |
Table 3.
Numbers of SbS chicks marked in 2012–2021 and returned to the natal area in subsequent year(s).
Table 3.
Numbers of SbS chicks marked in 2012–2021 and returned to the natal area in subsequent year(s).
| Year |
Headstarting |
Wild Chicks Marked |
Birds Returned (HS/wild) |
| Eggs Coll. |
Chicks Hatch. |
Young Released |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
| 2012 |
11 |
9 |
9 |
5 |
1/0 |
1/0 |
0/0 |
0/0 |
0/0 |
0/0 |
0/0 |
0/0 |
0/0 |
| 2013 |
24 |
21 |
18 |
3 |
0/0 |
3/1 |
2/1 |
0/1 |
0/1 |
0/0 |
0/1 |
0/1 |
0/1 |
| 2014 |
31 |
27 |
26 |
19 |
– |
1/1 |
2/4 |
2/3 |
2/1 |
2/0 |
0/0 |
0/0 |
0/0 |
| 2015 |
33 |
29 |
28 |
12 |
– |
– |
1/0 |
4/3 |
1/3 |
1/1 |
0/1 |
1/1 |
0/1 |
| 2016 |
35 |
31 |
30 |
38 |
– |
– |
– |
2/1 |
3/4 |
2/4 |
2/3 |
1/3 |
1/4 |
| 2017 |
38* |
30 |
30 |
16 |
– |
– |
– |
– |
0/0 |
1/0 |
1/0 |
2/0 |
2/7 |
| 2018 |
35 |
25 |
22 |
30 |
– |
– |
– |
– |
– |
0/1 |
0/3 |
1/3 |
0/8 |
| 2019 |
38 |
26 |
23 |
26 |
– |
– |
– |
– |
– |
– |
0/0 |
5/4 |
2/6 |
| 2020 |
30 |
27 |
22 |
20 |
– |
– |
– |
– |
– |
– |
– |
0/0 |
2/7 |
| 2021 |
39 |
29 |
28 |
18 |
– |
– |
– |
– |
– |
– |
– |
– |
0/11 |
| 2022 |
0 |
0 |
0 |
13 |
– |
– |
– |
– |
– |
– |
– |
– |
0/8 |
| Total |
314 |
254 |
236 |
200 |
1/0 |
5/2 |
5/5 |
8/8 |
6/9 |
6/6 |
3/8 |
10/12 |
7/22 |
Table 4.
Distances from origin to the first own nest (post-natal dispersal), in m.
Table 4.
Distances from origin to the first own nest (post-natal dispersal), in m.
| |
Wild* |
HS from Aviary |
| |
♂ |
♀ |
♂+♀
|
♂ |
♀ |
♂+♀
|
| n |
11 |
4 |
15 |
10 |
9 |
19 |
| mean |
9251 |
5720 |
8309 |
5191 |
8833 |
6916 |
| s.d. |
10866 |
4671 |
9572 |
7960 |
10646 |
9249 |
| median |
4370 |
6002 |
4370 |
1781 |
3180 |
2502 |
| min |
42 |
146 |
42 |
650 |
763 |
650 |
| max |
30594 |
10730 |
30594 |
26216 |
26846 |
26846 |
Table 5.
Evaluation of HS gain at different stages per a pair in the population under study.
Table 5.
Evaluation of HS gain at different stages per a pair in the population under study.
| |
Eggs
Production |
Chicks Hatched |
Chicks Fledged |
Mature Birds |
Recruits in Natal Area |
Successful First-Breeders |
Successful Second- Breeders |
| HS |
5.15 |
4.29 |
3.67 |
0.681 |
0.496 |
0.416 |
0.299 |
| Nature |
3.08 |
2.73 |
1.71 |
0.42 |
0.32 |
0.3 |
0.282 |
| Gain |
1.68 |
1.57 |
2.14 |
1.62 |
1.55 |
1.39 |
1.06 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).