Submitted:
21 January 2023
Posted:
26 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Human heart
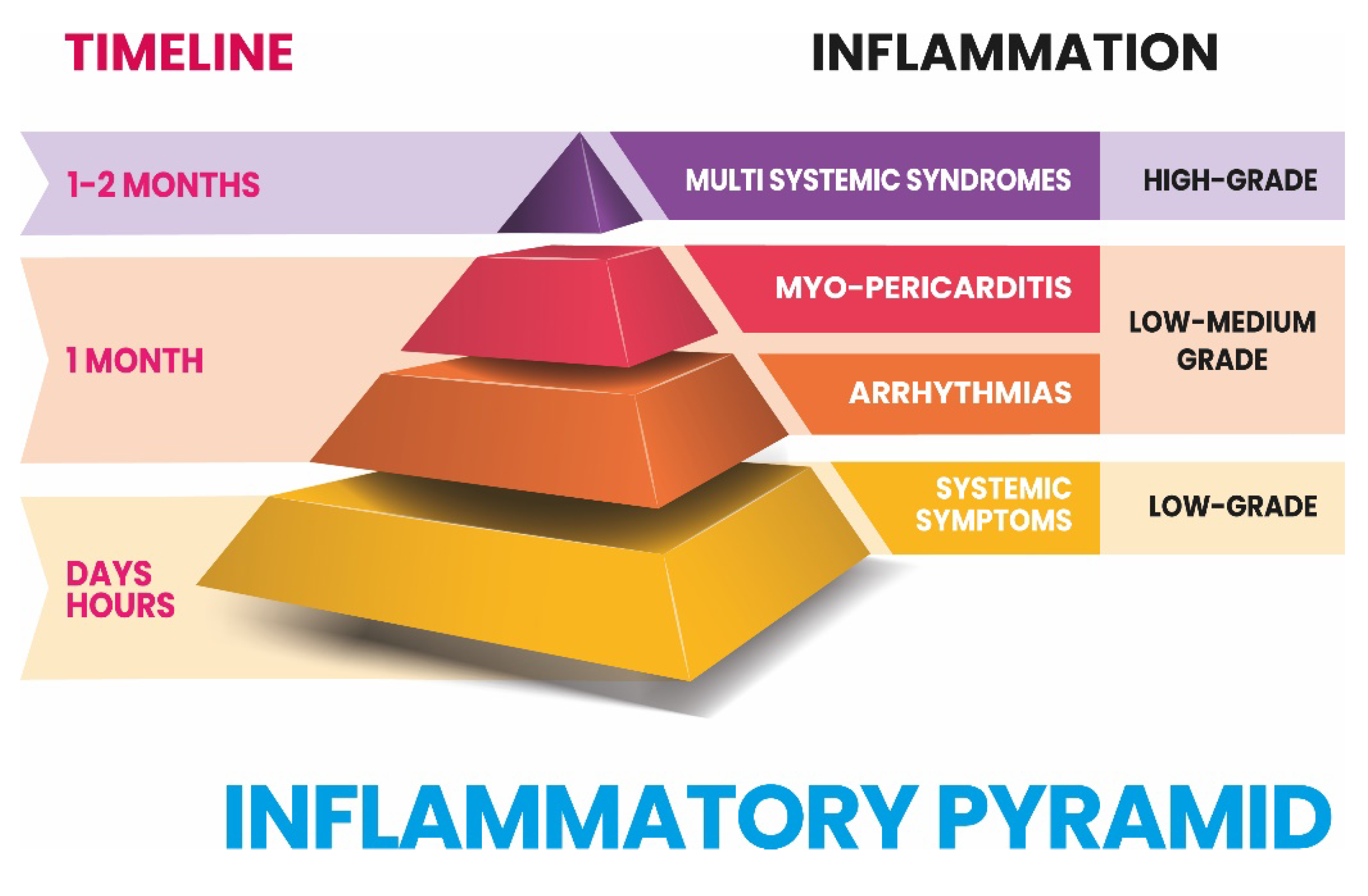
3. Systemic Symptoms
4. Inflammatory cardiac channelopathies and arrhytmias
5. The cardiac Action Potential (AP)
6. The ionic basis of AP
7. Proinflammatory cytokines
8. Myo-pericarditis
8.1. Probable pathogenesis of myocarditis
8.1.1. One or two shots?
8.1.2. Inflammatory infiltration of myocardium
8.1.3. Who opens the gate?
8.1.4. Exosomes
8.1.5. Spike protein induces endothelial cells (ECs) dysfunction
Spike Protein and cardiomyocytes (CM)
8.1.6. Young males: the favorite target.
8.1.7. Other pathological mechanisms triggered by the Spike protein
Renin–Angiotensin–Aldosterone System (RAAS)
Toxicity of lipid nanoparticles (LNPs)
8.1.8. TCD8, TCD3/CD45R0 and Macrophages CD68 in Myo-pericarditis
8.2. Diagnostic Items
8.3. Trans-endothelial migration towards heart tissue
8.4. Immune Black Hole
8.5. Experimental myocarditis
9. Multisystem Inflammatory Syndromes (MIS)
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-Converting Enzyme |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| AEs | Adverse Events |
| ANS | Autonomic Nervous System |
| AP | Action Potential |
| AT1R axis | Angiotensin II/ AT1R axis |
| AT2R axis | Angiotensin II /AT2R axis |
| CECs | Cardiac Endothelial Cells |
| CD | Cluster of Differentiation Molecules |
| CFs | Cardiac Fibroblasts |
| CMR | Cardiac Magnetic Resonance |
| CMs | Cardiomyocytes |
| CoV-2-S1 | S1 Subunit of CoV-2 Spike Protein |
| DCs | Dendritic Cells |
| ECM | Extracellular Matrix |
| ECs | Endothelial cells |
| EMB | Endomyocardial Biopsy |
| HPA axis | Hypothalamic–Pituitary–Adrenal axis |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IP | Inflammatory Pyramid |
| LGE | Late Gadolinium Enhancement |
| LNPs | Lipid Nanoparticles |
| LQT1 | Long QT syndrome type 1 |
| MIS-A | Multisystem-Inflammatory-Syndrome in adult |
| MIS-C | Multisystem-Inflammatory-Syndrome in children |
| MIS-V | Multisystem-Inflammatory-Syndrome following COVID-19 mRNA vaccines |
| MHC | Major Histocompatibility Complex |
| MSCs | Mesenchymal Stem Cells |
| NF-κB | Nuclear Factor- κB |
| RAAS | Renin—Angiotensin—Aldosterone System |
| SAM axis | Sympathetic-Adreno-Medullar axis |
| SRS | Stress Response System |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor-α |
| VCAM-1 | Vascular Cell Adhesion Protein 1 |
References
- Wang, L.I.; Yu, P.; Zhou, B.; Song, J.; Li, Z.; Zhang, M.; Guo, G.; Wang, Y.; Chen, X.; Han, L.; et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat Cell Biol. 2020, 22, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Fuseler, J.W.; Price, R.L.; Borg, T.K.; Baudino, T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007, 293, H1883–H1891. [Google Scholar] [CrossRef] [PubMed]
- Tucker, N.R.; Chaffin, M.; Fleming, S.J.; Hall, A.W.; Parsons, V.A.; Bedi KCJr Akkad, A.D.; Herndon, C.N.; Arduini, A.; Papangeli, I.; et al. Transcriptional and cellular diversity of the human heart. Circulation. 2020, 142, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature. 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Zhou P, Pu WT. Recounting cardiac cellular composition. Circ Res. 2016, 118, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Nag, AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980, 28, 41–61. [Google Scholar] [PubMed]
- Vliegen, H.W.; van der Laarse, A.; Cornelisse, C.J.; Eulderink, F. Myocardial changes in pressure overload-induced left ventricular hypertrophy: a study on tissue composition, polyploidization and multinucleation. Eur Heart J. 1991, 12, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.; Szewczykowska, M.; Jackowska, T.; dos Remedios, C.; et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Yang, Y.; J Lv, S. Jiang, Z. Ma, D. Wang, W. Hu, C. Deng, C. Fan, S. Di, Y. Sun, and W. Yi. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 2016, 7, e2234. [CrossRef]
- Su, Q.; Lv, X.; Sun, Y.; Ye, Z.; Kong, B.; Qin, Z. Role of TLR4/MyD88/NF-κB signaling pathway in coronary microembolization-induced myocardial injury prevented and treated with nicorandil. Biomed Pharmacother. 2018, 106, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Planz, O. Could a Lower Toll-like Receptor (TLR) and NF-κB Activation Due to a Changed Charge Distribution in the Spike Protein Be the Reason for the Lower Pathogenicity of Omicron? Int J Mol Sci. 2022, 23, 5966. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Gehmlich, K.; Denning, C.; Pavlovic, D. Complex Relationship Between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J Am Heart Assoc. 2021, 10, e019338, Epub 2021 Feb 15. PMID: 33586463, PMCID: PMC8174279. [Google Scholar] [CrossRef] [PubMed]
- Li J, Philip JL, Xu X, Theccanat T, Razzaque MA, Akhter SA.β-arrestins regulate human cardiac fibroblast transformation and collagen synthesis in adverse ventricular remodeling. J Mol Cell Cardiol. 2014, 76, 73–83. [CrossRef]
- Ongstad, E.; Kohl, P. Fibroblast-myocyte coupling in the heart: potential relevance for therapeutic interventions. J Mol Cell Cardiol. 2016, 91, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-J.; Ong, J.J.C.; Hwang, C.; Lee, J.J.; Fishbein, M.C.; Czer, L.; Trento, A.; Blanche, C.; Kass, R.M.; Mandel, W.J.; et al. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. J Am Coll Cardiol. 1998, 32, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA.Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol. 2016, 106, 62–69. [CrossRef]
- Eckhouse, S.R.; Spinale, F.G. Changes in the myocardial interstitium and contribution to the progression of heart failure. Heart Fail Clin. 2012, 8, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Song, D.; Dong, J.; Zhu, P.; Liu, J.; Liu, W.; Ma, X.; Zhao, L.; Ling, S. Current Understanding of the Pathophysiology of Myocardial Fibrosis and Its Quantitative Assessment in Heart Failure. Front Physiol. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D., Francis J. M., Myerson S., Selvanayagam J. B., Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J. Am. Coll. Cardiol. 2009, 54, 1407–1424. [CrossRef]
- Brutsaert, D.L. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol. Rev. 2003, 83, 59–115. [Google Scholar] [CrossRef]
- Chatterjee, V.; Yang, X.; Ma, Y.; et al. Extracellular vesicles: new players in regulating vascular barrier function. American Journal of Physiology-Heart and Circulatory Physiology 2020, 319, H1181–H1196. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020, 116, 1097–1100, Erratum in: Cardiovasc Res. 2020 Oct 1;116(12):1994. PMID: 32227090; PMCID: PMC7184507. [Google Scholar] [CrossRef]
- Stark, K.; Eckart, A.; Haidari, S.; Tirniceriu, A.; Lorenz, M.; von Brühl, M.-L.; Gärtner, F.; et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2012, 14, 41–51. [Google Scholar] [CrossRef]
- Giannotta, G.; N Giannotta. Vaccines and neuroinflammation. Int J Pub Health Safe 2018, 163, 2.
- Giannotta G and Giannotta, N. Post-vaccination inflammatory syndrome: a new syndrome. Clin Case Rep Rev 2019, 5. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; et al. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Nat. Rev. Cardiol. 2017, 14, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Capecchi PL &Laghi-Pasini, F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur. Heart J. 2017, 38, 1717–1727.
- Lazzerini, P.E.; et al. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J. Am. Heart Assoc. 2018, 7, e010595. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Laghi-Pasini, F.; Boutjdir, M. Cardioimmunology of arrhythmias: the role of autoimmuneand inflammatory cardiacchannelopathies. Nat Rev Immunol 2019, 19, 63–64. [Google Scholar] [CrossRef]
- Pappano, A.J.; Wier, W.G. Excitation: The Cardiac Action Potential. In Cardiovascular physiology, 11th ed.; Elsevier: Philadelphia, 2019; pp. 10–48. [Google Scholar]
- Gutman, G.A.; Chandy, K.G.; Grissmer, S.; et al. International Union of Pharmacology L. III. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005, 57, 473–508. [Google Scholar] [CrossRef] [PubMed]
- Bertaso, F.; Sharpe, C.C.; Hendry, B.M.; James, A.F. Expression of voltage-gated K+ channels in human atrium. Basic Res Cardiol. 2002, 97, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.B. Potassium Channels in the Heart. In Channelopathies in Heart Disease; Dierk, T., Remme, C.A., Eds.; Springer Nature: 2018; chapter 3, pp. 47–75.
- Sanguinetti, M.C.; Jurkiewicz, N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990, 96, 195–215. [Google Scholar] [CrossRef]
- Lundby, A.; Andersen, M.N.; Steffensen, A.B.; et al. In vivo phosphoproteomics analysis reveals the cardiac targets of beta-adrenergic receptor signaling. Sci Signal. 2013, 6, rs11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Curran, M.E.; Splawski, I.; et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996, 12, 17–23. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Priori, S.G.; Spazzolini, C.; et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001, 103, 89–95. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Long QT Syndrome: An Emerging Role for Inflammation and Immunity. Front Cardiovasc Med. 2015, 2, 26. [Google Scholar] [CrossRef]
- Yu, P.S.; Thouta, S.; Claydon, T.W. Modulation of hERG K+ Channel Deactivation by Voltage Sensor Relaxation Frontiers in Pharmacology. Available online: https://www.frontiersin.org/article/10.3389/fphar.2020.00139.
- Aromolaran, A.S.; Srivastava, U.; Alí, A.; et al. Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS One. 2018, 13, e0208321. [Google Scholar] [CrossRef]
- de Boer, T.P., Houtman M. J., Compier M., van der Heyden M. A.The Mammalian K(IR)2.X Inward Rectifier Ion Channel Family: Expression Pattern and Pathophysiology. Acta Physiol. 2010, 199, 243–256. [CrossRef]
- Melnyk, P.; Zhang, L.; Shrier, A.; Nattel, S. Differential distribution of Kir2.1 and Kir2.3 subunits in canine atrium and ventricle. Am J Phys Heart Circ Phys. 2002, 283, H1123–33. [Google Scholar] [CrossRef]
- Hager, N.A.; McAtee, C.K.; Lesko, M.A.; O’Donnell, A.F. Inwardly Rectifying Potassium Channel Kir2.1 and its “Kir-ious” Regulation by Protein Trafficking and Roles in Development and Disease. Front Cell Dev Biol. 2022, 9, 796136. [Google Scholar] [CrossRef] [PubMed]
- Rose, RA.; Backx, PH. Calcium channels in the heart. In Cardiac electrophysiology—from cell to bedside, vol. 6; Zipes, D.P., Jalife, J., Eds.; Elsevier: New York, 2014; pp. 13–22. [Google Scholar]
- Shah, K.; Seeley, S.; Schulz, C.; Fisher, J.; Gururaja Rao, S. Calcium Channels in the Heart: Disease States and Drugs. Cells 2022, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Betzenhauser, M.J.; Pitt, G.S.; Antzelevitch, C. Calcium Channel Mutations in Cardiac Arrhythmia Syndromes. Curr. Mol. Pharmacol. 2015, 8, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Oz, S.; Benmocha, A.; Dascal, N. Regulation of cardiac L-type Ca²⁺ channel CaV1.2 via the β-adrenergic-cAMP-protein kinase A pathway: old dogmas, advances, and new uncertainties. Circ Res. 2013, 113, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Nargeot, J.; Lory, P.; Richard, S. Molecular basis of the diversity of calcium channels in cardiovascular tissues. Eur. Heart J. 1997, 18, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M.; Perez-Reyes, E. Ca channels in cardiac myocytes: Structure and function in Ca influx and intracellular Ca release. Cardiovasc. Res. 1999, 42, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, P.L.; Laghi-Pasini, F.; El-Sherif, N.; Qu, Y.; Boutjdir, M.; Lazzerini, P.E. Autoimmune and inflammatory K+channelopathies in cardiac arrhythmias: Clinical evidence and molecular mechanisms. Heart Rhythm. 2019, 16, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Wang J, Wang H, Zhang Y, Gao H, Nattel S, Wang Z.Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. The Journal of biological chemistry. 2004, 279, 13289–13292.
- Fernandez-Velasco M, Ruiz-Hurtado G, Hurtado O, Moro MA, Delgado C.TNF-alpha downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. American journal of physiology Heart and circulatory physiology. 2007, 293, H238–45. [CrossRef] [PubMed]
- Kawada, H.; Niwano, S.; Niwano, H.; Yumoto, Y.; Wakisaka, Y.; Yuge, M.; et al. Tumor necrosis factor-alpha downregulates the voltage gated outward K+ current in cultured neonatal rat cardiomyocytes: a possible cause of electrical remodeling in diseased hearts. Circulation journal: official journal of the Japanese Circulation Society. 2006, 70, 605–609. [Google Scholar] [CrossRef]
- Li YH, Rozanski GJ.Effects of human recombinant interleukin-1 on electrical properties of guinea pig ventricular cells. Cardiovascular research. 1993, 27, 525–530. [CrossRef]
- Petkova-Kirova, P.S.; Gursoy, E.; Mehdi, H.; McTiernan, C.F.; London, B.; Salama, G. Electrical remodeling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-alpha. American journal of physiology Heart and circulatory physiology. 2006, 290, H2098–107. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, G.; Alarcon, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; et al. Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice. Nature communications. 2016, 7, 13344. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, N.; Mathieu, S.; Fiset, C. Interleukin-1beta reduces L-type Ca2+current through protein kinase C activation in mouse heart. J Biol Chem. 2014, 289, 21896–21908. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, S.E.; et al. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1561–H1567. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.A.; Parsons, G.T.; Gering, S.K.; Meier, A.R.; Hutchinson, I.V.; Robicsek, A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA. 2021, 326, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Alberto Cordero,D.C., Escribano David, Quintanilla Maria Amparo, López-Ayala José Maria, Berbel Patricio Pérez, Bertomeu-González Vicente. Myocarditis after RNA-based vaccines for coronavirus. Int. J. Cardiol. 2022, 353, 131–134. [PubMed]
- Moroni, F.; Mbualungu, J.; Abbate, A. Myocarditis after RNA-based COVID-19 vaccines: Where do we stand? Int J Cardiol. 2022, 356, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Gavin, J.; Madanchi, N.; Kim, C.; Shah, P.R.; Klein, K.; et al. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int. J. Cardiol. 2021, 340, 119–121. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.Y. Myocarditis after Covid-19 mRNA vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef]
- Lim Y, Kim MC, Kim KH, Jeong I-S, Cho YS, Choi YD and Lee JE. Case Report: Acute Fulminant Myocarditis and Cardiogenic Shock After Messenger RNA Coronavirus Disease 2019 Vaccination Requiring Extracorporeal Cardiopulmonary Resuscitation. Front. Cardiovasc. Med. 2021, 8, 758996. [Google Scholar] [CrossRef]
- Le Vu, S.; Bertrand, M.; Jabagi, M.J.; et al. Age and sex-specific risks of myocarditis and pericarditis following COVID-19 messenger RNA vaccines. Nat Commun 2022, 13, 3633. [Google Scholar] [CrossRef] [PubMed]
- Massari M, SpilaAlegiani S, Morciano C, Spuri M, Marchione P, Felicetti P, Belleudi V, Poggi FR, Lazzeretti M, Ercolanoni M, Clagnan E, Bovo E, Trifirò G, Moretti U, Monaco G, Leoni O, Da Cas R, Petronzelli F, Tartaglia L, Mores N, Zanoni G, Rossi P, Samez S, Zappetti C, Marra AR, Menniti Ippolito F; TheShinISS-Vax|COVIDSurveillance Group. Postmarketing active surveillance of myocarditis and pericarditis following vaccination with COVID-19 mRNA vaccines in persons aged 12 to 39 years in Italy: A multi-database, self-controlled case series study. PLoS Med. 2022, 19, e1004056. [Google Scholar] [CrossRef]
- Hui-Lee Wong, Mao Hu, Cindy Ke Zhou, Patricia C Lloyd, Kandace L Amend, Daniel C Beachler, Alex Secora, Cheryl N McMahill-Walraven, Yun Lu, Yue Wu, Rachel P Ogilvie, Christian Reich, Djeneba Audrey Djibo, Zhiruo Wan, John D Seeger, Sandia Akhtar, Yixin Jiao, Yoganand Chillarige, Rose Do, John Hornberger, Joyce Obidi, Richard Forshee, Azadeh Shoaibi, Steven A Anderson. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet 2022, 399, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Husby A, and Kober L. COVID-19 mRNA vaccination and myocarditis or pericarditis. Comment. Vol 399. Available online: www.thelancet.com (accessed on 11 June 2022).
- EMA. Available online: www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_it.pdf (accessed on 2 August 2022).
- EMA. Available online: www.ema.europa.eu/en/documents/product-information/Spikevax-previously-covid-19-vaccine-moderna-epar-product-information_it.pdf (accessed on 2 August 2022).
- Bachmann, M.F.; Beerli, R.R.; Agnellini, P.; Wolint, P.; Schwarz, K.; Oxenius, A. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol. 2006, 36, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Karrer, U.; Sierro, S.; Wagner, M.; Oxenius, A.; Hengel, H.; Koszinowski, U.H.; Phillips, R.E.; Klenerman, P. Memory inflation: Continuous accumulation of antiviral CD8+T cells over time. J. Immunol. 2003, 170, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Karrer, U.; Wagner, M.; Sierro, S.; Oxenius, A.; Hengel, H.; Dumrese, T.; Freigang, S.; et al. Expansion of protective CD8 (+) T-cell responses driven by recombinant cytomegaloviruses. J. Virol. 2004, 78, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Y.; Zhao, Y.; Lung, D.C.; Ye, Z.; Song, W.; Liu, F.F.; Cai, J.P.; Wong, W.M.; Yip, C.C.; Chan, J.F.; To, K.K.; Sridhar, S.; Hung, I.F.; Chu, H.; Kok, K.H.; Jin, D.Y.; Zhang, A.J.; Yuen, K.Y. Intravenous Injection of Coronavirus Disease 2019 (COVID-19) mRNA Vaccine Can Induce Acute Myopericarditis in Mouse Model. Clin Infect Dis. 2022, 74, 1933–1950, Erratum in: Clin Infect Dis. 2021, 73, 2372–2373. PMID: 34406358, PMCID: PMC8436386. [Google Scholar] [CrossRef]
- Won,T., Gilotra, N. A., Wood, M. K., Hughes, D. M., Talor, M. V., Lovell, J., Milstone, A. M., Steenbergen, C., &Čiháková, D. Increased Interleukin 18-Dependent Immune Responses Are Associated With Myopericarditis After COVID-19 mRNA Vaccination. Frontiers in immunology 2022, 13, 851620. [CrossRef] [PubMed]
- Dai-Jen Lee, Fei Du, Shih-Wei Chen, Manando Nakasaki, Isha Rana, Vincent F. S. Shih, Alexander Hoffmann, Colin Jamora. Regulation and Function of the Caspase-1 in an Inflammatory Microenvironment. Journal of Investigative Dermatology 2015, 135, 2012–2020. [Google Scholar] [CrossRef]
- Oka, A.; Sudo, Y.; Miyoshi, T.; Ozaki, M.; Kimura, Y.; Takagi, W.; Ugawa, S.; Okada, T.; Nosaka, K.; Doi, M. Fulminant myocarditis after the second dose of COVID-19 mRNA vaccination. Clin Case Rep. 2022, 10, e05378. [Google Scholar] [CrossRef]
- Kazama, S.; Okumura, T.; Kimura, Y.; Ito, R.; Araki, T.; Mizutani, T.; Oishi, H.; Kuwayama, T.; Hiraiwa, H.; Kondo, T.; Morimoto, R.; Saeki, T.; Murohara, T. Biopsy-Proven Fulminant Myocarditis Requiring Mechanical Circulatory Support Following COVID-19 mRNA Vaccination. CJC Open. 2022, 4, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Kiblboeck, D.; Klingel, K.; Genger, M.; Traxler, S.; Braunsteiner, N.; Steinwender, C.; Kellermair, J. Myocarditis following mRNA COVID-19 vaccination: call for endomyocardial biopsy. ESC Heart Fail. 2022, 9, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T., Sugano, Y., Yokokawa, T., Nagai, T., Matsuyama, T.-a., Ohta-Ogo, K., Ikeda, Y., Ishibashi-Ueda, H., Nakatani, T., Ohte, N., Yasuda, S. and Anzai, T. Clinical impact of the presence of macrophages in endomyocardial biopsies of patients with dilated cardiomyopathy. Eur J Heart Fail 2017, 19, 490–498. [CrossRef]
- Angus, T. Stock, Nicholas Collins, Gordon K. Smyth, Yifang Hu, Jacinta A. Hansen, Damian B. D’Silva, Hamdi A. Jama, X, Andrew M. Lew, Thomas Gebhardt, Catriona A. McLean, and Ian P. Wicks. The Selective Expansion and Targeted Accumulation of Bone Marrow–Derived Macrophages Drive Cardiac Vasculitis. The Journal of Immunology 2019, 202, 3282–3296. [Google Scholar]
- Betjes, M.G.; Haks, M.C.; Tuk, C.W.; et al. Monoclonal antibody EBM11 (anti-CD68) discriminates between dendritic cells and macrophages after short-term culture. Immunobiology 2010, 183, 79–87. [Google Scholar] [CrossRef]
- Kim, Y.; Nurakhayev, S.; Nurkesh, A.; Zharkinbekov, Z.; Saparov, A. Macrophage Polarization in Cardiac Tissue Repair Following Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 2715. [Google Scholar] [CrossRef]
- Fleisher, TA.; Shearer, WT.; Schrieder HW., Jr; Frew, AJ.; Weyand, CM. Appendix 1. Selected CD Molecules and Their Characteristics. In Clinical Immunology, 5nd ed.; Editor Rich RR., Eds.; Elsevier Limited. 2019, pp. 1311–1315.
- Conigliaro, A.; Corrado, C.; Fontana, S.; Alessandro, R. Exosomebasicmechanisms. In Exosomes A Clinical Compendium. Eds.; Academic Press, Elsevier. 2020, Chapter 1, pp 1-21.
- Gao, W., Liu, H., Yuan, J., Wu, C., Huang, D., Ma, Y., Zhu, J., Ma, L., Guo, J., Shi, H., Zou, Y. and Ge, J. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway. J. Cell. Mol. Med. 2016, 20, 2318–2327. [CrossRef]
- Pitt, J.M.; André, F.; Amigorena, S.; et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016, 126, 1224–1232. [Google Scholar] [CrossRef]
- Varikuti, S.; Kumar, J.B.; Holcomb, E.A.; et al. The role of vascular endothelium and exosomes in human protozoan parasitic diseases. Vessel Plus 2020, 4, 28. [Google Scholar] [CrossRef]
- Wan, A.; Rodrigues, B. Endothelial cell-cardiomyocyte crosstalk in diabetic cardiomyopathy. Cardiovasc. Res. 2016, 111, 172–183. [Google Scholar] [CrossRef]
- Pizzirani, C.; Ferrari, D.; Chiozzi, P.; Adinolfi, E.; Sandona, D.; et al. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 2007, 109, 3856–3864. [Google Scholar] [CrossRef]
- Gulinelli, S.; Salaro, E.; Vuerich, M.; Bozzato, D.; Pizzirani, C.; et al. IL-18 associates to microvesicles shed from human macrophages by a LPS/TLR-4 independent mechanism in response to P2X receptor stimulation. Eur J Immunol 2012, 42, 3334–3345. [Google Scholar] [CrossRef] [PubMed]
- Cossett, i.C.; Iraci, N.; Mercer, T.R.; Leonardi, T.; Alpi, E.; et al. Extracellular vesicles from neural stem cells transfer IFN-gamma via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell 2014, 56, 193–204. [Google Scholar] [CrossRef]
- Zhang, H.G.; Liu, C.; Su, K.; Yu, S.; Zhang, L.; et al. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol 2006, 176, 7385–7393. [Google Scholar] [CrossRef]
- Perez, P.S.; Romaniuk, M.A.; Duette, G.A.; Zhao, Z.; Huang, Y.; et al. Extracellular vesicles and chronic inflammation during HIV infection. J Extracell Vesicles 2019, 8, 1687275. [Google Scholar] [CrossRef]
- Chen, Z.; Larregina, A.T.; Morelli, A.E. Impact of extracellular vesicles on innate immunity. CurrOpinOrganTransplant 2019, 24, 670–678. [Google Scholar] [CrossRef]
- Robles, J.P.; Zamora, M.; Martinez, G.; et al. The Spike protein of SARS-CoV-2 induces endothelial inflammation through integrin, α.5.β.1.; NF-κB signaling. J. Biol. Chem. 2022, 298, 101695. [Google Scholar] [CrossRef]
- Negron, S.G.; Kessinger, C.W.; Xu, B.; et al. Selectively expressing SARS-CoV-2 Spike protein S1 subunit in cardiomyocytes induces cardiac hypertrophy in mice. bioRxiv preprint. [CrossRef]
- Yang, Y.K.; Lv, S.; Jiang, Z.; Ma, D.; Wang, W.; Hu, C.; Deng, C.; Fan, S.; Di, Y.; Sun, W.Y. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 2016, 7, e2234. [Google Scholar] [CrossRef]
- Katare, P.B.; Nizami, H.L.; Paramesha, B.; et al. Activation of toll like receptor 4 (TLR4) promotes cardiomyocyte apoptosis through SIRT2 dependent p53 deacetylation. Sci Rep 2020, 10, 19232. [Google Scholar] [CrossRef] [PubMed]
- Baumeier, C.; Aleshcheva, G.; Harms, D.; Gross, U.; Hamm, C.; Assmus, B.; Westenfeld, R.; Kelm, M.; Rammos, S.; Wenzel, P.; Münzel, T.; Elsässer, A.; Gailani, M.; Perings, C.; Bourakkadi, A.; Flesch, M.; Kempf, T.; Bauersachs, J.; Escher, F.; Schultheiss, H.-P. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int. J. Mol. Sci. 2022, 23, 6940. [Google Scholar] [CrossRef] [PubMed]
- Shirato K, Kizaki T. SARS-CoV-2 Spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon. 2021, 7, e06187. [Google Scholar] [CrossRef]
- Aboudounya MM, Heads RJ. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediators Inflamm. 2021, 2021, 8874339. [Google Scholar] [CrossRef]
- Larson, R, Ham, M. Stress and “storm and stress” in early adolescence: the relationship of negative events with dysphoric affect. Dev Psychol. 1993, 29, 130–140. [Google Scholar] [CrossRef]
- Seidman, E.; Allen, L.; Aber, J.L.; Mitchell, C.; Feinman, J. The impact of school transitions in early adolescence on the self-system and perceived social context of poor urban youth. Child Dev. 1994, 65, 507–522. [Google Scholar] [CrossRef]
- Romeo, R.D. The Teenage Brain: The Stress Response and the Adolescent Brain. Curr Dir Psychol Sci. 2013, 22, 140–145. [Google Scholar] [CrossRef]
- Schroeder, A.; Notaras, M.; Du, X.; Hill, R.A. On the Developmental Timing of Stress: Delineating Sex-Specific Effects of Stress across Development on Adult Behavior. Brain Sci. 2018, 8, 121. [Google Scholar] [CrossRef]
- Glier, S.; Campbell, A.; Corr, R.; Pelletier-Baldelli, A.; Yefimov, M.; Guerra, C.; Scott, K.; Murphy, L.; Bizzell, J.; Belger, A. Coordination of autonomic and endocrine stress responses to the Trier Social Stress Test in adolescence. Psychophysiology 2022, e14056. [Google Scholar] [CrossRef]
- Engert, V.; Vogel, S.; Efanov, S.I.; Duchesne, A.; Corbo, V.; Ali, N.; Pruessner, J.C. Investigation into the cross-correlation of salivary cortisol and alpha-amylase responses to psychological stress. Psychoneuroendocrinology 2011, 36, 1294–1302. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; Umeoka, E.H.L. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A., Hamer, M., and Chida, Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007, 21, 901–912. [CrossRef] [PubMed]
- Musumeci, V.; Baroni, S.; Cardillo, C.; Zappacosta, B.; Zuppi, C.; Tutinelli, F.; Folli, G. Cardiovascular reactivity, plasma markers of endothelial and platelet activity and plasma renin activity after mental stress in normals and hypertensives. J Hypertens 1987, (Suppl. S5), s1–s4. [Google Scholar]
- Spieker, L.E.; Hürlimann, D.; Ruschitzka, F.; Corti, R.; Enseleit, F.; Shaw, S.; Hayoz, D.; Deanfield, J.E.; Lüscher, T.F.; Noll, G. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002, 105, 2817–2820. [Google Scholar] [CrossRef] [PubMed]
- Geir Stene-Larsen, John A. Ask, Karen B. Helle, Resch Fin. Activation of cardiac beta2adrenoceptors in the human heart. American Journal of Cardiology. 1986, 57, PF7–F10. [CrossRef]
- Han, W.; Wang, Z.; Nattel, S. Am J Physiol Heart Circ Physiol.2001, 280, H1075–H1080.
- Wu, C.T.; Nattel, S. Triggering of cardiac arrhythmic events in long QT syndrome: lessons from funny bunnies. J Physiol. 2012, 590, 1311–1312. [Google Scholar] [CrossRef]
- McCarty, R., Horwatt, K., &Konarska, M. Chronic stress and sympathetic-adrenal medullary responsiveness. Social science & medicine 1988, 26, 333–341.
- Konarska, M., Stewart, R. E., & McCarty, R. Sensitization of sympathetic-adrenal medullary responses to a novel stressor in chronically stressed laboratory rats. Physiology&behavior 1989, 46, 129–135.
- Nocentini, A.; Palladino, B.E.; Menesini, E. Adolescents’ Stress Reactions in Response to COVID-19 Pandemic at the Peak of the Outbreak in Italy. Clinical Psychological Science. 2021, 9, 507–514. [Google Scholar] [CrossRef]
- Giannotta, G.; Giannotta, N. mRNA COVID-19 Vaccines and Long-Lived Plasma Cells: A Complicated Relationship. Vaccines 2021, 9, 1503. [Google Scholar] [CrossRef]
- Huang, C.J.; Webb, H.E.; Zourdos, M.C.; Acevedo, E.O. Cardiovascular reactivity, stress, and physical activity. Front Physiol. 2013, 4, 314. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Suzuki YJ, Gychka SG. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines. Vaccines (Basel). 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J., Nikolaienko S.I., Dibrova V.A., Dibrova Y.V., Vasylyk V.M., Novikov M.Y., Shults N.V., Gychka S.G. SARS-CoV-2 Spike protein-mediated cell signaling in lung vascular cells. Vascul. Pharmacol. 2020; 106823. [CrossRef]
- Patra T., Meyer K., Geerling L., Isbell T.S., Hoft D.F., Brien J., Pinto A.K., Ray R.B., Ray R. SARS-CoV-2 Spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoSPathog. 2020, 16, e1009128. [CrossRef]
- Ghazi, L.; Drawz, P. Advances in understanding the renin-angiotensin-aldosterone system (RAAS) in blood pressure control and recent pivotal trials of RAAS blockade in heart failure and diabetic nephropathy. F1000Research 2017, 6, F1000. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, N.E.; Barakat, A.G.; Reilkoff, R.; Bezdicek, T.; Schacker, T.; Chipman, J.G.; Tignanelli, C.J.; Puskarich, M.A. Understanding the renin-angiotensin-aldosterone-SARS-CoV axis: a comprehensive review. Eur Respir J. 2020, 56, 2000912. [Google Scholar] [CrossRef]
- Dandona, P.; Dhindsa, S.; Ghanim, H.; et al. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens 2007, 21, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Johnson, S.C.; Villarin, J.J.; Chin, M.T.; Nieves-Cintrón, M.; Chen, T.; Marcinek, D.J.; Dorn, G.W.; Kang, Y.J.; Prolla, T.A.; et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Gαq overexpression-induced heart failure. Circ. Res. 2011, 108, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.L.; Boyle, E.; Jefferson, S.R.; Heslop, C.R.A.; Mohan, P.; Mohanraj, G.G.J.; Sidow, H.A.; Tan, R.C.P.; Hill, S.J.; Woolard, J. Role of the Renin–Angiotensin–Aldosterone and Kinin–Kallikrein Systems in the Cardiovascular Complications of COVID-19 and Long COVID. International Journal of Molecular Sciences. 2021, 22, 8255. [Google Scholar] [CrossRef]
- Cong, H.; Li, X.; Ma, L.; Jiang, H.; Mao, Y.; Xu, M. Angiotensin II receptor type 1 is upregulated in atrial tissue of patients with rheumatic valvular disease with atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2010, 140, 298–304. [Google Scholar] [CrossRef]
- Chan, Y.C.; Leung, P.S. Angiotensin II type 1 receptor-dependent nuclear factor-kappaB activation-mediated proinflammatory actions in a rat model of obstructive acute pancreatitis. J Pharmacol Exp Ther 2007, 323, 10–18. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, J.J.; Chang, N.C.; Chen, C.H.; Liu, J.C.; Chen, T.H.; Jeng, C.J.; Chao, H.H.; Cheng, T.H. Role of reactive oxygen species-sensitive extracellular signal-regulated kinase pathway in angiotensin II-induced endothelin-1 gene expression in vascular endothelial cells. J Vasc Res 2004, 41, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Wei, X.B.; Sun, R.; Cai, Y.W.; Lou, H.Y.; Wang, J.W.; Chen, A.F.; Zhang, X.M. Angiotensin II stimulates intercellular adhesion molecule-1 via an AT1 receptor/nuclear factor-kappaB pathway in brain microvascular endothelial cells. Life Sci 2006, 78, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.E.; Gonzalez, W.; Nicoletti, A.; Savoie, F.; Arnal, J.F.; Michel, J.B. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol 2000, 20, 645–651. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Lorenzo, O.; Ruperez, M.; Suzuki, Y.; Egido, J. Angiotensin II activates nuclear transcription factor-kappaB in aorta of normal rats and in vascular smooth muscle cells of AT1 knockout mice. Nephrol Dial Transplant 2001, 16, S27–S33. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ruiz-Ortega, M.; Lorenzo, O.; Ruperez, M.; Esteban, V.; Egido, J. Inflammation and angiotensin II. Int J Biochem Cell Biol 2003, 35, 881–900. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Frisbee, J.C.; Laher, I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am J Physiol Heart Circ Physiol. 2015, 308, H1476–H1498. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Hongtao Lv, Shubiao Zhang, Bing Wang, Shaohui Cui, Jie Yan. Toxicity of cationic lipids and cationic polymers in gene delivery. Journal of Controlled Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021, 24, 103479. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. Three decades of messenger RNA vaccine development. Nano Today. Comprehensive review on mRNA vaccines. 2019, 28, 1–17. [Google Scholar] [CrossRef]
- Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano. 2020, 14, 12522–12537. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.M. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine. 2014, 10, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Legat, A.; Adam, E.; Steuve, J.; Gatot, J.S.; Vandenbranden, M.; Ulianov, L.; Lonez, C.; Ruysschaert, J.M.; Muraille, E. DiC14-amidine cationic liposomes stimulate myeloid dendritic cells through toll-like receptor 4. Eur J Immunol. 2008, 38, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.M. Cationic lipids activate intracellular signaling pathways. Adv Drug Deliv Rev. 2012, 64, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Igyártó, B.Z.; Jacobsen, S.; Ndeupen, S. Future considerations for the mRNA-lipid nanoparticle vaccine platform. CurrOpinVirol. 2021, 48, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gómez Román, V.R., Murray J.C., Weiner L.M. Antibody Fc: Linking Adaptive and Innate Immunity.2013. Antibody-Dependent Cellular Cytotoxicity (ADCC).
- EMA Assessment report: Comirnaty 19 February 2021. Committee for Medicinal Products for Human Use (CHMP). Metabolism of the two novel LNP-excipients ALC-0315 and ALC-0159, pages 47-48. Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf (accessed on 21 July 2022).
- Saadati, F.; Cammarone, S.; Ciufolini, M.A. A Route to Lipid ALC-0315, a Key Component of a COVID-19 mRNA Vaccine. Chemistry. 2022, e202200906. [Google Scholar] [CrossRef] [PubMed]
- Soudani, N.; Troudi, A.; Bouaziz, H.; Ben Amara, I.; Boudawara, T.; Zeghal, N. Cardioprotective effects of selenium on chromium (VI)-induced toxicity in female rats. Ecotoxicol Environ Saf. 2011, 74, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, J.; Gao, H.; Yang, X.; Fu, Y.; Peng, Y.; Xia, Y.; Zhou, D. Hexavalent Chromium Causes Apoptosis and Autophagy by Inducing Mitochondrial Dysfunction and Oxidative Stress in Broiler Cardiomyocytes. Biol Trace Elem Res. 2022, 200, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M.; Lugli, E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013, 43, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Hamann, D.; Baars, P.A.; Rep, M.H.; Hooibrink, B.; Kerkhof-Garde, S.R.; Klein, M.R.; van Lier, R.A. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997, 186, 1407–1418. [Google Scholar] [CrossRef]
- Akbar, A.N.; Terry, L.; Timms, A.; Beverley, P.C.; Janossy, G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J. Immunol. 1988, 140, 2171–2178. [Google Scholar] [CrossRef]
- Merkenschlager, M.; Terry, L.; Edwards, R.; Beverley, P.C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1, implications for differential CD45 expression in T cell memory formation. Eur. J. Immunol. 1988, 18, 1653–1661. [Google Scholar] [CrossRef]
- Wiedle, G.; Dunon, D.; Imhof, B.A. Current concepts in lymphocyte homing and recirculation. Crit Rev Clin Lab Sci 2001, 38, 1–31. [Google Scholar] [CrossRef]
- Wirth, T.C.; Martin, M.D.; Starbeck-Miller, G.; Harty, J.T.; Badovinac, V.P. Secondary CD8+ T-cell responses are controlled by systemic inflammation. Eur J Immunol. 2011, 41, 1321–1333. [Google Scholar] [CrossRef]
- Martin, M.D.; Badovinac, V.P. Defining Memory CD8 T Cell. Front Immunol. 2018, 9, 2692. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Kubin N, Manfred Richter, Bedriye Sen-Hild, Hakan Akintürk, Markus Schönburg, Thomas Kubin, Ayse Cetinkaya. Macrophages represent the major pool of IL-7Rα expressing cells in patients with myocarditis. Cytokine 2020, 130, 155053. [Google Scholar] [CrossRef]
- Fox SE, Lacey Falgout, Richard S. Vander Heide. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovascular Pathology 2021, 54, 107361. [Google Scholar] [CrossRef]
- Bracamonte-Baran, W.; Čiháková, D. Cardiac Autoimmunity: Myocarditis. Adv Exp Med Biol. 2017, 1003, 187–221. [Google Scholar] [CrossRef]
- Law YM, Lal AK, Chen S, Čiháková D, Cooper LT Jr, Deshpande S, Godown J, Grosse-Wortmann L, Robinson JD, Towbin JA; American Heart Association Pediatric Heart Failure and Transplantation Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Stroke Council. Diagnosis and Management of Myocarditis in Children: A Scientific Statement From the American Heart Association. Circulation. 2021, 144, e123–e135, Epub 2021 Jul 7. Erratum in: Circulation. 2021 Aug 10;144(6):e149. PMID: 34229446. [CrossRef] [PubMed]
- Grabie, N.; 1 Michael, W. Delfs,1 Jason R. Westrich,1 Victoria A. Love,1 George Stavrakis,2 Ferhaan Ahmad,3 Christine E. Seidman,3 Jonathan G. Seidman,3 and Andrew H. Lichtman1,2. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J. Clin. Invest. 2003, 111, 671–680. [CrossRef]
- Hayward SL, NormaBautista-Lopez, KunimasaSuzuki, AlexeyAtrazhev, PeterDickie, John F. Elliott. CD4 T Cells Play Major Effector Role and CD8 T Cells Initiating Role in Spontaneous Autoimmune Myocarditis of HLA-DQ8 Transgenic IAb Knockout Nonobese Diabetic Mice. The Journal of Immunology 2006, 176, 7715–7725. [Google Scholar] [CrossRef] [PubMed]
- Wenzel P, Sabrina Kopp, Sebastian Göbel, Thomas Jansen, Martin Geyer, Felix Hahn, Karl-Friedrich Kreitner, Felicitas Escher, Heinz-Peter Schultheiss, Thomas Münzel, Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovascular Research 2020, 116, 1661–1663. [CrossRef]
- Hu, G.; Wang, S. Tumor-infiltrating CD45RO+Memory T Lymphocytes Predict Favorable Clinical Outcome in Solid Tumors. Sci Rep 2017, 7, 10376. Vojdani, A., Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [CrossRef]
- Blanton RM, Carrillo-Salinas FJ, Alcaide P. T-cell recruitment to the heart: friendly guests or unwelcome visitors? Am J Physiol Heart Circ Physiol. 2019, 317, H124–H140. [CrossRef]
- Bajpai, G.; Schneider, C.; Wong, N.; Bredemeyer, A.; Hulsmans, M.; Nahrendorf, M.; Epelman, S.; Kreisel, D.; Liu, Y.; Itoh, A.; Shankar, T.S.; Selzman, C.H.; Drakos, S.G.; Lavine, K.J. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018, 24, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- von Andrian, U.H., Mackay, C. R.,T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000, 343, 1020–1034. [CrossRef]
- Harty, J.T.; Badovinac, V.P. Shaping and reshaping CD8+T-cell memory. NatRevImmunol. 2008, 8, 107–119. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J. Heterogeneity and cell-fate decisions in effector and memory CD8+T cell differentiation during viral infection. Immunity. 2007, 27, 393–405. [Google Scholar] [CrossRef]
- Surh, C.D.; et al. Homeostasis of memory T cells. Immunol Rev. 2006, 211, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Błyszczuk, P. Myocarditis in Humans and in Experimental Animal Models. Front Cardiovasc Med. 2019, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Havari, E.; Pinto, S.; Gottumukkala, R.V.; Cornivelli, L.; Raddassi, K.; et al. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest. 2011, 121, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.A.; Rowley, A.H. Current Insights Into the Pathophysiology of Multisystem Inflammatory Syndrome in Children. Curr Pediatr Rep. 2021, 9, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; EBRose SMHorwitz et, a.l. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Riphagen S, XGomez, CGonzalez-Martinez, NWilkinson, PTheocharis Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [CrossRef] [PubMed]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.P.; Top, K.A.; Karatzios, C.; Hilmers, D.C.; Tapia, L.I.; Moceri, P.; Giovannini-Chami, L.; Wood, N.; Chandler, R.E.; Klein, N.P.; et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021, 39, 3037–3049. [Google Scholar] [PubMed]
- Patel, P.; DeCuir, J.; Abrams, J.; Campbell, A.P.; Godfred-Cato, S.; Belay, E.D. Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults: A Systematic Review. JAMA Netw Open. 2021, 4, e2126456. [Google Scholar] [CrossRef]
- Santilli, V.; Manno, E.C.; Giancotta, C.; Rossetti, C.; Cotugno, N.; Amodio, D.; Rotulo, G.A.; Deodati, A.; Bianchi, R.; Lucignani, G.; Longo, D.; Valeriani, M.; Palma, P. Two Pediatric Cases of Multisystem Inflammatory Syndrome with Overlapping Neurological Involvement Following SARS-CoV-2 Vaccination and Unknown SARS-CoV2 Infection: The Importance of Pre-Vaccination History. Vaccines 2022, 10, 1136. [Google Scholar] [CrossRef]
- Ouldali, N.; Bagheri, H.; Salvo, F.; et al. Hyper inflammatory syndrome following COVID-19 mRNAvaccine in children: A national post-authorization pharmacovigilance study. The Lancet Regional Health- Europe 2022, 17, 100393. [Google Scholar] [CrossRef] [PubMed]
- Karatzios, C.; Scuccimarri, R.; Chédeville, G.; Basfar, W.; Bullard, J.; Stein, D.R. Multisystem Inflammatory Syndrome Following SARS-CoV-2 Vaccination in Two Children. Pediatrics. 2022. [Google Scholar] [CrossRef] [PubMed]
- Nune, A.; Iyengar, K.P.; Goddard, C. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V). BMJ Case Reports CP, 2021; 14, e243888. [Google Scholar]
- Miyashita, Y.; Yoshida, T.; Takagi, Y.; et al. Circulating extracellular vesicle microRNAs associated with adverse reactions, proinflammatory cytokine, and antibody production after COVID-19 vaccination. npj Vaccines 2022, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; Gumeni, S.; Trougakos, I.P.; Dimopoulos, M.A.; Felber, B.K.; Pavlakis, G.N. Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef] [PubMed]
- Kumar N, Zuo Y, Yalavarthi S, Hunker KL, Knight JS, Kanthi Y, Obi AT, Ganesh SK. SARS-CoV-2 Spike Protein S1-Mediated Endothelial Injury and Pro-Inflammatory State Is Amplified by Dihydrotestosterone and Prevented by Mineralocorticoid Antagonism. Viruses. 2021, 13, 2209. [Google Scholar] [CrossRef] [PubMed]
- Barcena, M.L.; Jeuthe, S.; Niehues, M.H.; Pozdniakova, S.; Haritonow, N.; Kühl, A.A.; Messroghli, D.R.; Regitz-Zagrosek, V. Sex-Specific Differences of the Inflammatory State in Experimental Autoimmune Myocarditis. Front Immunol. 2021, 12, 686384. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Petri, M.A.; Coronado, M.J.; Cooper, L.T. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol. 2012, 8, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, R.; Donoiu, I.; Mirea, O.; Bălşeanu, T.A. Testosterone, cardiomyopathies, and heart failure: a narrative review. Asian J Androl. 2021, 23, 348–356. [Google Scholar] [CrossRef]
- Girón-González, J.A.; Moral, F.J.; Elvira, J.; García-Gil, D.; Guerrero, F.; Gavilán, I.; Escobar, L. Consistent production of a higher TH1, TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol. 2000, 143, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Onyimba, J.A.; Coronado, M.J.; Garton, A.E.; Kim, J.B.; Bucek, A.; et al. The innate immune response to coxsackievirus B3 predicts progression to cardiovascular disease and heart failure in male mice. Biol Sex Differ. 2011, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Di Florio, D.N.; Sin, J.; Coronado, M.J.; Atwal, P.S.; Fairweather, D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020, 31, 101482. [Google Scholar] [CrossRef]
- Dinarello, C.A. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J. Endotoxin. Res. 2004, 10, 201–222. [Google Scholar]
- Dinarello, C.A. Cytokines as endogenous pyrogens. J. Infect. Dis. 1999, 179, S294–S304. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.M.; Porter, K.; Karlsson, E.; Schultz-Cherry, S. Proinflammatory cytokine responses correspond with subjective side effects after influenza virus vaccination. Vaccine 2015, 33, 3360–3366. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H. Circulating extracellular vesicles carry immune regulatory miRNAs and regulate vaccine efficacy and local inflammatory response after vaccination. Front. Immunol. 2021, 12, 685344. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Nakao, N.; Ishiuchi, N.; Fukui, T.; Katsuya, N.; Fukumoto, W.; Oka, H.; Yoshikawa, N.; Nagao, T.; Namera, A.; Kakimoto, N.; Oue, N.; Awai, K.; Yoshimoto, K.; Nagao, M. Four cases of cytokine storm after COVID-19 vaccination: Case report. Front Immunol. 2022, 13, 967226. [Google Scholar] [CrossRef] [PubMed]
- Flego D, Cesaroni S, Romiti GF, Corica B, Marrapodi R, Scafa N, Maiorca F, Lombardi L, Pallucci D, Pulcinelli F, Raparelli V, Visentini M, Cangemi R, Piconese S, Alvaro D, Polimeni A, Basili S, Stefanini L; Vax-SPEED-IT Study Group. Platelet and immune signature associated with a rapid response to the BNT162b2 mRNA COVID-19 vaccine. J Thromb Haemost. 2022, 20, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Theodorou, A.; Bakola, E.; Chondrogianni, M.; et al. Covid-vaccine-fear-induced paroxysmal atrial fibrillation causing multiple acute arterial infarctions: a case report. Ther Adv Neurol Disord. 2022, 15, 17562864221094714. [Google Scholar] [CrossRef]
- Esposito, S.; Caminiti, C.; Giordano, R.; Argentiero, A.; Ramundo, G.; Principi, N. Myocarditis Following COVID-19 Vaccine Use: Can It Play a Role for Conditioning Immunization Schedules? Front Immunol. 2022, 13, 915580. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulos, G.; Figliozzi, S.; Sanguineti, F.; Aquaro, G.D.; di Bella, G.; Stamatelopoulos, K.; Chiribiri, A.; Garot, J.; Masci, P.G.; Ismail, T.F. Prognostic Impact of Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance in Myocarditis: A Systematic Review and Meta-Analysis. Circ Cardiovasc Imaging. 2021, 14, e011492. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.E.; Shay, D.K.; Su, J.R.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA. 2022, 327, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; Asleh, R.; Amir, O.; Meir, K.; Cohen, D.; Dichtiar, R.; Novick, D.; Hershkovitz, Y.; Dagan, R.; Leitersdorf, I.; Ben-Ami, R.; Miskin, I.; Saliba, W.; Muhsen, K.; Levi, Y.; Green, M.S.; Keinan-Boker, L.; Alroy-Preis, S. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Buchan, S.A.; Seo, C.Y.; Johnson, C.; et al. Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Netw Open. 2022, 5, e2218505. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, E.S.; Oster, M.E.; Klein, N.P. Myocarditis or Pericarditis Following mRNA COVID-19 Vaccination. JAMA Netw Open. 2022, 5, e2218512. [Google Scholar] [CrossRef]
- Rohr, S. Myofibroblasts in diseased hearts: New players in cardic arrhythmias? Available online: https://www.heartrhythmjournal.com/article/S1547-5271(09)00230-6/fulltext.
- Su, J.R.; McNeil, M.M.; Welsh, K.J.; Marquez, P.L.; Ng, C.; Yan, M.; Cano, M.V. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021, 39, 839–845. [Google Scholar] [CrossRef]
- Kuntz J, Crane B, Weinmann S, Naleway AL; Vaccine Safety Datalink Investigator Team. Myocarditis and pericarditis are rare following live viral vaccinations in adults. Vaccine. 2018, 36, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Perez, Y.; Levy, E.R.; Joshi, A.Y.; et al. Myocarditis Following COVID-19 mRNA Vaccine: A Case Series and Incidence Rate Determination [published online ahead of print, 2021 Nov 3]. Clin Infect Dis. 2021, ciab926. [Google Scholar] [CrossRef]
- Das, B.B.; Kohli, U.; Ramachandran, P.; Nguyen, H.H.; Greil, G.; Hussain, T.; Tandon, A.; Kane, C.; Avula, S.; Duru, C.; Hede, S.; Sharma, K.; Chowdhury, D.; Patel, S.; Mercer, C.; Chaudhuri, N.R.; Patel, B.; Ang, J.Y.; Asmar, B.; Sanchez, J.; Khan, D. Myopericarditis after messenger RNA Coronavirus Disease 2019 Vaccination in Adolescents 12 to 18 Years of Age. J Pediatr. 2021, 238, 26–32.e1. [Google Scholar] [CrossRef]
- Nygaard, U.; Holm, M.; Bohnstedt, C.; Chai, Q.; Schmidt, L.S.; Hartling, U.B.; Petersen, J.J.H.; Thaarup, J.; Bjerre, J.; Vejlstrup, N.G.; Juul, K.; Stensballe, L.G. Population-based Incidence of Myopericarditis After COVID-19 Vaccination in Danish Adolescents. Pediatr Infect Dis J. 2022, 41, e25–e28. [Google Scholar] [CrossRef] [PubMed]
- Fox SE, Falgout L, Vander Heide RS. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc Pathol. 2021, 54, 107361. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Eichhorn, C.; Bière, L.; et al. Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis. J Cardiovasc Magn Reson 2019, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Grun, S.; Schumm, J.; Greulich, S.; et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012, 59, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Schumm, J.; Greulich, S.; Wagner, A.; et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson. 2014, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Sano, M.; Suwa, K.; et al. Distribution of late gadolinium enhancement in various types of cardiomyopathies: Significance in differential diagnosis, clinical features and prognosis. World J Cardiol. 2014, 6, 585–601. [Google Scholar] [CrossRef]
- Schauer J, MD1, Sujatha Buddhe, MD, MS1, Avanti Gulhane, MD, DNB, FSCMR2, Eyal Sagiv, MD, PhD1, Matthew Studer, MD1, Jessica Colyer, MD, MBA1, Sathish Mallenahalli Chikkabyrappa, MD1, Yuk Law, MD1, and Michael A. Portman, MD. Persistent Cardiac Magnetic Resonance Imaging Findings in a Cohort of Adolescents with Post-Coronavirus Disease 2019 mRNA Vaccine Myopericarditis. J Pediatr 2022, 245, 233–237. [Google Scholar] [CrossRef]
- Abu Mouch, S.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Berar Yanay, N. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021, 39, 3790–3793. [Google Scholar] [CrossRef]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; et al. Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 6, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Kracalik I, Oster ME, Broder KR, Cortese MM, Glover M, Shields K, Creech CB, Romanson B, Novosad S, Soslow J, Walter EB, Marquez P, Dendy JM, Woo J, Valderrama AL, Ramirez-Cardenas A, Assefa A, Campbell MJ, Su JR, Magill SS, Shay DK, Shimabukuro TT, Basavaraju SV; Myocarditis Outcomes After mRNA COVID-19 Vaccination Investigators and the CDC COVID-19 Response Team. Outcomes at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination in adolescents and young adults in the USA: a follow-up surveillance study. Lancet Child Adolesc Health. 2022, 6, 788–798, Erratum in: Lancet Child Adolesc Health. 2022 Dec;6(12):e28. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Domke, L.M.; Hartmann, L.; et al. Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination. Clin Res Cardiol 2022. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Yu, S.N.; Chang, S.H.; Ahn, Y.H.; Jeon, M.H. Multisystem Inflammatory Syndrome in an Adult after COVID-19 Vaccination: a Case Report and Literature Review. J Korean Med Sci. 2021, 36, e312. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.J.; Kim, T.; Lim, J.K.; Kim, Y.A.; Park, S.E. Multisystem Inflammatory Syndrome in Children Following COVID-19 Vaccine (Pfizer-BioNTech BNT162b2): A Case Report. Ann Clin Case Rep. 2022, 7, 2199. [Google Scholar]
- Zhang, J.; Hao, Y.; Ou, W.; et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J Transl Med 2020, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Peart Akindele, N.; Kouo, T.; Karaba, A.H.; et al. Distinct Cytokine and Chemokine Dysregulation in Hospitalized Children With Acute Coronavirus Disease 2019 and Multisystem Inflammatory Syndrome With Similar Levels of Nasopharyngeal Severe Acute Respiratory Syndrome Coronavirus 2 Shedding. J Infect Dis. 2021, 224, 606–615. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; Cheng, C.A.; Burgess, E.; Edlow, A.G.; Chou, J.; Dionne, A.; Balaguru, D.; Lahoud-Rahme, M.; Arditi, M.; Julg, B.; Randolph, A.G.; Alter, G.; Fasano, A.; Walt, D.R. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023. [Google Scholar] [CrossRef] [PubMed]
| Channel | Current | Current type | Ion | AP phase | Interference |
|---|---|---|---|---|---|
| Kv 4.3 | Ito | Outward | K+ | 1 | IL-1β, TNF-α |
| L-type | ICa,L | Inward | Ca2+ | 2 | IL-1β, IL-6 |
| Kv 11.1 hERG | IKr | Outward | K+ | 3 | IL-6, TNF-α |
| Kv 7.1 | IKs | Outward | K+ | 3 | TNF-α |
| Kir 2.1/2.3 | IK1 | Inward | K+ | 4 |
| Potential effects after COVID-19 mRNA vaccination. | Ref. |
|---|---|
| Endothelial dysfunctions produced by the Spike protein. | [97] |
| Actions of the Spike protein on the Angiotensin II / AT1 axis. | [127,128,129,130,131,132,133,134,135,136,137,138] |
| Excessive Th1-type immune responses. | [199] |
| Persistence of the Spike protein in circulation for a prolonged period of time. | [190] |
| Prolonged immune and inflammatory response against the Spike protein. | [189,190] |
| Strong pro-inflammatory activity of LNPs. | [141,142,143,144,145,146,147,148] |
| Spike protein alone can easily reach the myocardium. | [98,101] |
| Spike protein was found in Cardiomyocytes (CMs). | [101] |
| Different levels of expression of pro-inflammatory cytokines over time. | [189,198] |
| Circulating CoV-2-S1 is a TLR4-recognizable alarmin that may harm the CMs by triggering their innate immune responses. | [98] |
| TLR4 initiates the expression of several pro-inflammatory genes, cell surface molecules, and chemokines through the MyD88-dependent pathway, which exacerbates the damage to myocardium. | [99] |
| Activation of TLR4 and the TLR4 / NFκB axis in cardiomyocytes by the Spike protein. | [11] |
| Unmitigated TLR4 activation may lead to increased risk for cardiac inflammation. | [100] |
| CoV-2-S1 activates TLR4 signaling to induce pro-inflammatory responses in murine and human macrophages. | [102] |
| Diffuse myocardial macrophages infiltration in the patient biopsy sample, suggest an increased level of IL-18 produced by monocytes and macrophages in the heart with COVID-19 vaccine-related myo-pericarditis. | [76] |
| In an inflammatory microenvironment, caspase-1 is regulated by NF-κB, and this enzyme facilitates the conversion of pro-IL-18 in IL-18. | [77] |
| Lymphocytic infiltration with predominant immunostaining for CD8 and CD68-positive cells (macrophages) is present in myocarditis following COVID-19 mRNA vaccines. | [79] |
| Vaccinated mice showed signs of myocarditis 2 days after injection of the second dose of BNT162b2 vaccine. | [75] |
| Free spike antigen was detected in the blood of adolescents and young adults who developed post-mRNA vaccine myocarditis. | [235] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





