1.0. Introduction
Artificial light at night (ALAN), and light emitting diodes (LEDs) in particular, are amongst the numerous anthropogenic pressures that are affecting earth’s myriad life forms, of which humans are only a small part [
1,
2,
3,
4,
5,
6,
7]. While earth has been home to all its different life forms for billions of years, humans emerged as a life form only about 200,000 years ago – a relatively short span in the geologic time scale [
8,
9]. Humans rely on these life forms for their own survival by consuming all kinds of life forms, which are part of larger interconnected ecosystems [
10,
11]. Majority of current research on LEDs has been directed with an egocentric view of investigating the impacts on human health, while impacts on other life forms have been considered within the context of improving productivity for satisfying human needs with respect to livestock and derived products [
1,
12]. Houser [
2] therefore argues that the concept of human-centricity or placing humans first in lighting may come with collateral damage: some lighting designed to support a subset of human needs such as ALAN, causes unintended negative effects on both human and non-human life by disrupting natural ecosystems. Additionally, ALAN tends to negatively disrupt ecosystems that depend on lunar light cycles [
5,
7]. The lunar light cycle refers to the lunar month (29.5 days) and the lunar day (24.8 h) required for the moon to orbit around the earth, and to travel by the same spot on the earth respectively, which give rise to several environmental cycles, such as light levels, tides and geomagnetic fields [
13]. While these disruptions may not have immediate or obvious effects, more serious consequences can be expected with respect to the geologic time scales.
Simply put, lighting schemes that erode biodiversity over time cannot be considered life-centric or even human-centric. The spirit behind human-centric lighting has been best captured by the term ‘integrative’ lighting coined by the CIE/ISO [
14]. While avoiding the hype, integrative lighting captures what lighting aspires to be: good human outcomes driven by good design for visibility, visual amenity and visual comfort [
15]. Integrative lighting solutions support applications for humans by aiming for biologically high potency during the day and low potency during the night [
16]. Houser and Esposito [
17] argue that integrative lighting begins with conceptualizing the project, and continues through prioritizing design goals, architectural design (including daylighting design), specifying lighting equipments, commissioning, and operating lighting systems requiring buy-in from all stakeholders including occupants. Veitch [
18] further argues that integrative lighting is a design-led dynamic principle, where lighting schemes are built from evidence-based design findings that also call for on-going analysis of their impacts via post-occupancy evaluation once put in practice.
The future of earth will be better served if the lighting profession is guided by a vision that extends humane care to the long-term health of the biodiversity and ecosystems that support human health and comfort [
2]. Therefore, it is necessary for lighting professionals to understand the requirements of a wide variety of life forms so as to preserve the biodiversity and ecosystems that support human life. Widening the scope of lighting design to include as many life forms as possible will enable the lighting profession to quickly widen the capabilities of ‘good design.’ In providing generalisations about good design however, integrative lighting has only defined lighting characteristics to meet the visual and non-visual requirements of humans. This leads to the following questions that are central to this paper:
What collective criteria define ‘life-centric’ lighting that cares for as many life forms as possible?
How can these criteria for life-centric lighting simultaneously include the criteria of integrative lighting?
1.1. Background
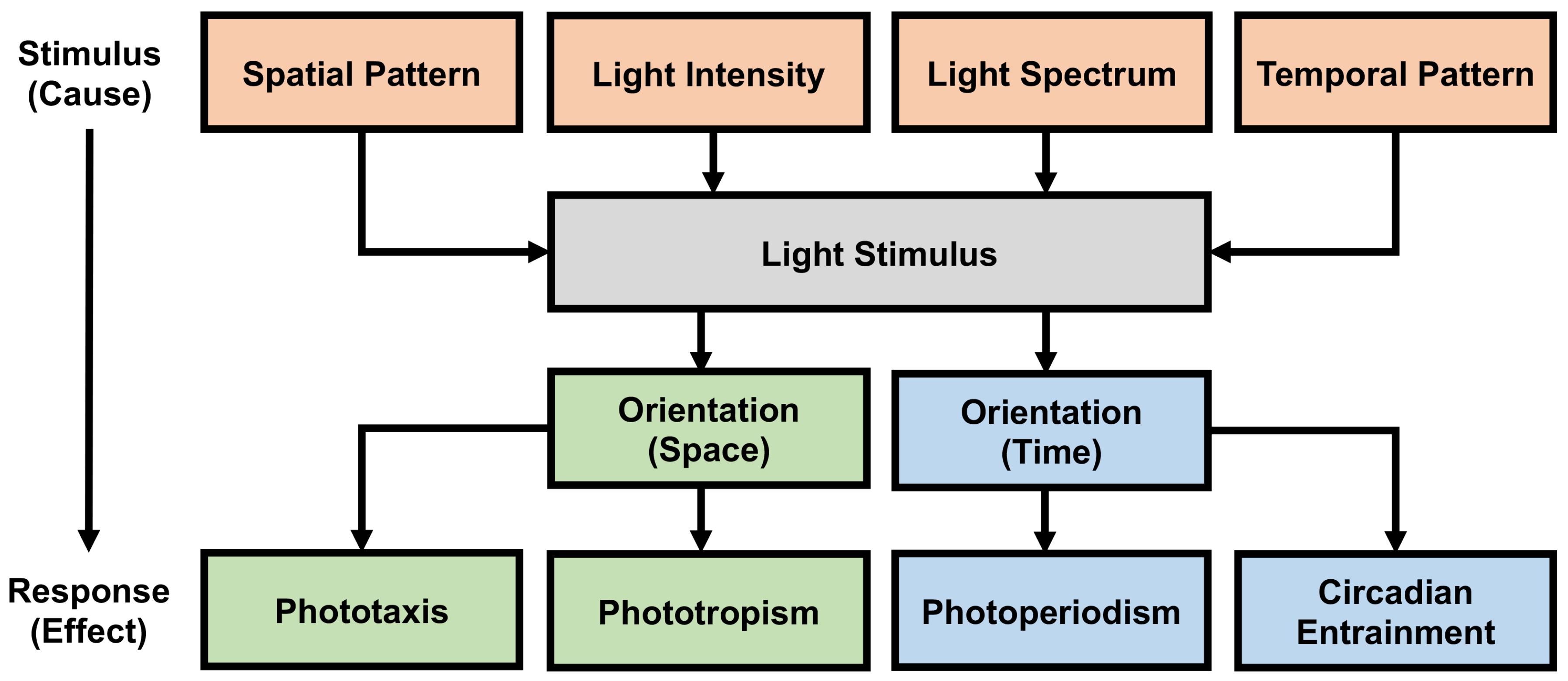
The earth’s daily cycle of dark and light rhythms or photoperiod governs various life-sustaining behaviours of its myriad life forms such as, digestion, evolution, nutrition, protection, reaction, reproduction, restoration, and transformation. Light information allows spatial and temporal orientation for these life forms: spatial orientation, defined as phototaxis in animals and phototropism in plants, are movements in response to the lighting environment; temporal orientation, is the synchronization of the endogenous clocks provided by the circadian system that drive the daily, lunar and annual rhythms of metabolic, physiological and behavioural functions; and ultimately accomplish their essential biological needs [
1,
3]. Circadian clocks are present in virtually all life forms, their ubiquitous character being the persistence of rhythmic activity under constant light (LL) or constant darkness (DD); and the alternation of light and dark during the 24 h light dark (LD) cycle is one of the main environmental input signal for temporal orientation [
1]. The circadian system constitutes the rhythmic input to the clocks, the clocks themselves and their rhythmic outputs, which governs the myriad metabolic, physiological and behavioural processes of these life forms, thereby synchronizing their activities with the natural periodicities [
19,
20,
21,
22,
23].
Circadian entrainment is a stable relationship between this rhythmic activity and an external environmental input signal; circadian phase shift refers to a change in the timing of circadian rhythms, where a phase-advance will move the sleep and wake-up time to earlier in the day, and a phase-delay will move the sleep and wake-up time to later in the day [
17]. Additionally, the ability of these life forms to measure environmental day length to ascertain the time of year is defined as photoperiodism [
24].
1.2. Aim and Objectives
Many wonder if it is even possible to create lighting designs catering to the complex needs of all life forms on earth. Therefore, this paper aims to provide a design process for an inclusive lighting design that embodies the spirit of ‘life-centric’ lighting by including as many life forms as possible, with a current scope that outlines the early stages of the design process. The design process itself is based on a proposed five-step process [
17] that organizes information gathering and decision-making processes for human-centric lighting with the effective prioritization of design goals. Published lighting guides [
25,
26,
27] meant to prevent and reduce light pollution through the proper application of quality outdoor electric lighting, along with other studies [
1,
28,
29,
30,
31,
32] proposing ways to mitigate the anthropogenic changes caused by ALAN with a more reasonable use of available technology such as carefully restricting and directing lighting to more essential areas and hours for avoiding wasteful radiation, as well as selecting spectral emissions for reducing impacts on circadian clocks are used as references. However, the paper objectivises to go a step further by drawing inspiration from the definition of integrative lighting and proposing an associated term for comprehensively capturing the true aspiration of life-centric lighting: good human and non-human outcomes for all life-sustaining behaviours on earth driven by good design for phototaxis, phototropism, photoperiodism and circadian entrainment. Given the myriad different life forms, the current scope focuses on the life-sustaining behaviours of animals (amphibians, birds, fishes, insects, mammals and reptiles), humans and plants. These can be further classified under diurnal (day-active) and nocturnal (night-active) life forms based on their natural periodicities [
33].
2.0. Simplifying Life-Centric Lighting Design
This section presents simplified concepts of life-centric lighting for supporting conversational understanding of the most relevant lighting design considerations.
2.1. Identifying the Light/Dark Stimulus Responses of Life Forms
The two key responses demonstrated by almost all life forms towards any light/dark stimulus, and considered in the current scope are identified as spatial (phototaxis and phototropism) and temporal (photoperiodism and circadian entrainment) responses. The four manupulable characteristics of lighting variables in the design of a light/dark stimulus include: light intensity and spectrum, and spatial and temporal patterns; at any given space and time, the light intensity and spectrum can be adjusted to alter the biological potency of a light/dark stimulus [
17,
34,
35,
36,
37,
38,
39]. Light intensity refers to the quantity of light in radiometric or photometric units, light spectrum refers to the spectral power distribution (SPD) that governs color qualities, spatial pattern refers to the spatial distribution of light in the three-dimensional light field, and temporal pattern refers to the timing and duration of exposure to a light stimulus [
17]. Spatial pattern supports phototaxis and phototropism both positively and negatively i.e. spatial response of moving towards or away from the light stimulus [
40,
41]. Temporal pattern supports temporal responses for telling time by observing the natural daily LD pattern [
42].
The complexity in life-centric lighting design arises from the fact that the same light/dark stimulus may be beneficial to one life form or at one time of the day, but detrimental to another life form or at another time of the day. For example, high-intensity light in the morning and during the day will support biological activities of diurnal life forms i.e. life forms that are active during the day, relatively less active in the evening and sleep at night, whereas the same high-intensity light in the evening may delay the onset of sleep and be detrimental to these life forms [
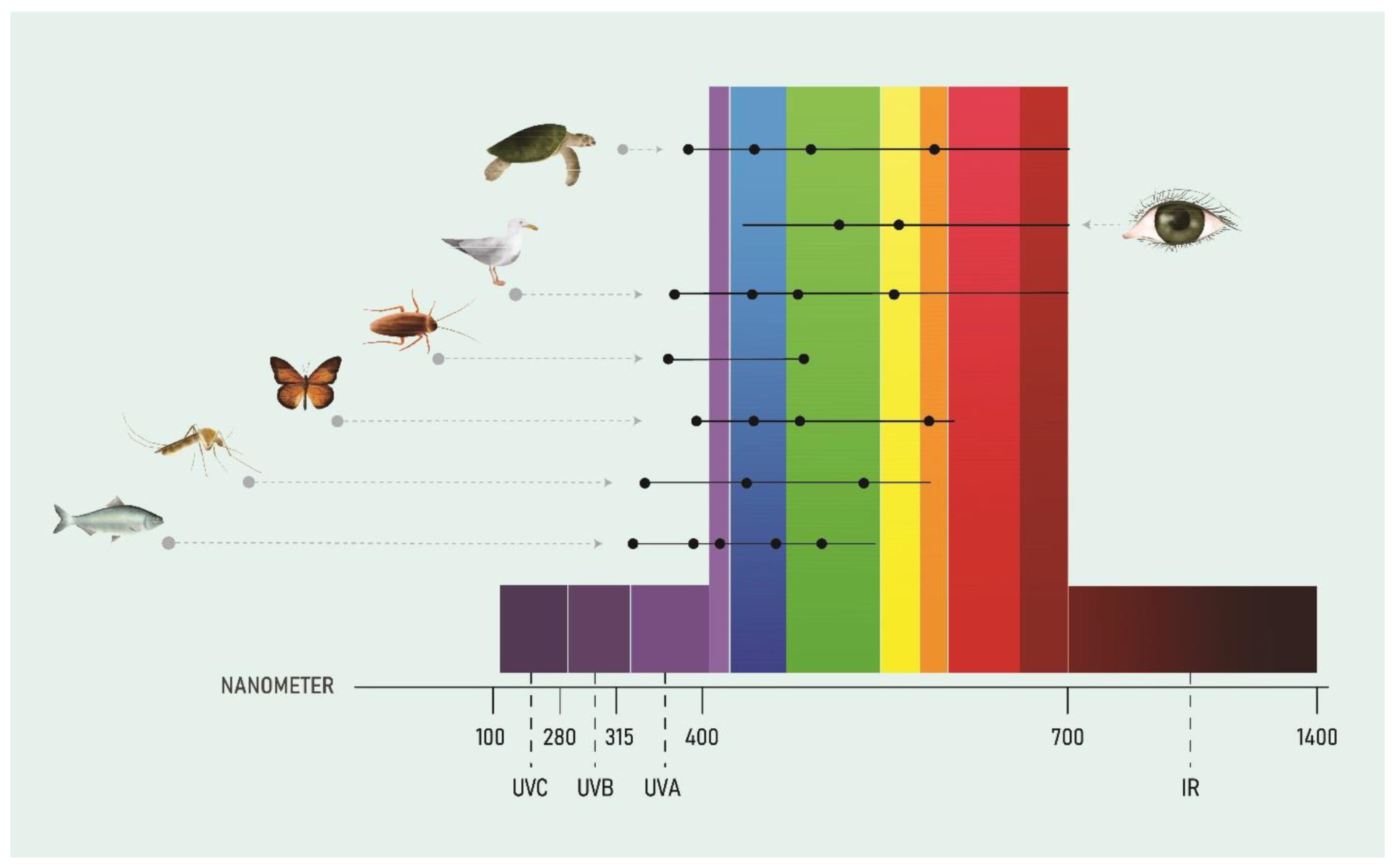
33]. Phototaxis for most animals is often triggered by blue light detection, however it can also extend beyond this spectrum [
43]. Literature reveals that phototaxis may cover the entire light spectrum, from UV up to near-infrared in certain Cyanobacteria [
44,
45] or just part of the spectrum such as UV to green in fruit fly larvae [
46]; UV/blue in the citrus psylla [
47]; near infrared in zebrafish larvae [
48]; infrared in rattlesnakes [
49]; yellow/orange in some seabirds [
50]; and green in bats [
51].
Figure 1 illustrates the differential abilities of animals and humans to perceive the different light spectrums. Phototropism in fungi and plants is triggered by both red and UV/blue light, while in flowering plants it is triggered predominantly by blue light [
52,
53,
54].
In animals, light/darkness is the main input to the clocks with the strong involvement of short and middle wavelengths in synchronization and entrainment: in vertebrates, the effective wavelengths are comprised between 420-500 nm with the highest efficiency being obtained between 450-480 nm [
37,
55]; in mammals, melanopsin from the intrinsically photosensitive retinal ganglion cells (ipRGCs) of the retina is the corresponding spectral response [
56]. In the pineal organ of fish, frogs and lizards, phase advances or delays in the circadian rhythm can be induced by the colour opponent mechanisms or opposing effects of wavelengths depending on light intensity and spectral composition [
57]; such effects have also been observed in cave-dwelling bats (blue vs. green), wild rabbits (blue vs. yellow), and mice (UV vs. yellow) [
1]. In insects such as flies, one set of photoreceptors (cryptochromes) sensitive to blue wavelengths (470 nm) are involved both in light capture and molecular function of the clock [
58], while another set of photoreceptors (rhodopsins) are implicated in entrainment to green, red and yellow wavelengths [
59]. In terrestrial higher plants, one set of photopigments (phytochromes) mediate the effects of red and infrared wavelengths (700-750 nm), while another set of photopigments (cryptochromes) mediate the effects of blue light [
60,
61]. In microalgae, the clock is reset by a wide range of wavelengths: violet, blue/green and red [
62,
63]. Finally, in fungi the light/dark entrainment of the clock is mediated by proteins sensitive to blue wavelengths [
64].
Table 1 lists the light/dark stimulus responses of various life forms based on the four characteristics of lighting variables.
2.2. Classifying the Life-Sustaining Behaviours within Lighted Environments
The behaviour of all life forms is defined as coordinated responses such as actions or inactions to an internal or external stimulus [
65,
66]; where light/darkness is considered as the only external stimulus within the current scope. All the life-sustaining behaviours such as, digestion, evolution, nutrition, protection, reaction, reproduction, restoration, and transformation considered in the current scope with respect to the spatial and temporal responses are classified under spatial [
67,
68] and temporal [
4,
69] orientation. Masking by light/dark stimulus is an immediate response that overrides the different life forms’ endogenous clocks thereby playing an important role in shaping daily patterns of activity [
70,
71,
72]. Diurnal and nocturnal life forms generally have inverted masking responses to light/dark stimuli; the masking effect of light typically increases activity in diurnal life forms (positive masking) and decreases it in nocturnal life forms (negative masking), while the effect of darkness is vice-versa [
71,
72,
73,
74,
75,
76,
77].
Table 2 lists the masking effects of darkness and light on the life-sustaining behaviours of life forms.
Changes in light intensity leads to considerable changes in the spatial orientation patterns. For example, exposure to high intensity tends to reverse the natural lunar-guided foraging patterns, which decrease during brighter moonlight in naturally lit conditions and increase when exposed to high intensity ALAN [
5]; similarly, slight increase or decrease in light intensity leads to considerable changes in leaf morphology [
78], and compared to high intensity light, reduced light intensity can increase the net nutrition rate of plants [
79]. Spatial heterogeneity in light intensity is also recommended to support the life-sustaining behaviours of certain life forms [
67,
80]. Additionally, sleep or restoration patterns of life forms vary greatly. For example, 1.9 h for the giraffe to 19.9 h for the brown bat [
81,
82]. The life-sustaining behavioural cycles of these life forms are also dependent upon other characteristics of the circadian clocks such as genetic determination where each life form has its proper period close to 24 h; synchronization by other factors, for example, rainfalls, lunar cycles, food intake, tides in addition to the LD cycle; lengthening or shortening of the period with light intensity under LL; induction of phase advances or phase delays by light sequences applied at different times under DD [
1].
Figure 2 provides an overview of the stimulus-response relationship between light/darkness and life forms, while schematically subdividing the life-sustaining behaviours in terms of spatial and temporal orientation.
2.3. Quantifying Life-Centric Lighting Design in Terms of Biological Potency
As there are no globally recognised standard methods for quantifying life-centric lighting design [
26,
83], integrative lighting design is used as a reference source given some of the commonalities between humans and other life forms. For example, although the perception of light by animals is different from humans, both use vision as a critical cue for orientation [
84]. Similar to integrative lighting design which has quantified light as a non-visual stimulus based on the spectral response of the various photoreceptors as well as nocturnal suppression of the hormone melatonin [
17], the proposed quantification of the biological potency of light for life-centric lighting design will be based on the masking effect of a stimulus. Optimal (neither positive nor negative) masking effect is the desired result when the life forms do not demonstrate any unnatural phototaxis, phototropism, photoperiodism and circadian entrainment other than their natural periodicities due to the light/dark stimulus. For this to happen, the life forms should not be exposed to any unnatural light/dark stimulus in terms of light intensity and spectrum, and spatial and temporal patterns.
Table 3 provides a simple guide for recording and/or assessing any unnatural behaviours and masking effects that can be caused by any unnatural exposure to the different lighting variables.
Optimal masking effect is possible when the four characteristics of the lighting variables closely match the natural solar light cycles during the day and natural lunar light cycles during the night. However, current understanding of biological rhythms and clocks is largely restricted to the solar light cycles [
85]. As the variable brightness of the night sky affects animals, humans as well as plants [
7], night-time lighted environments should especially be in sync with the lunar light cycles [
86].
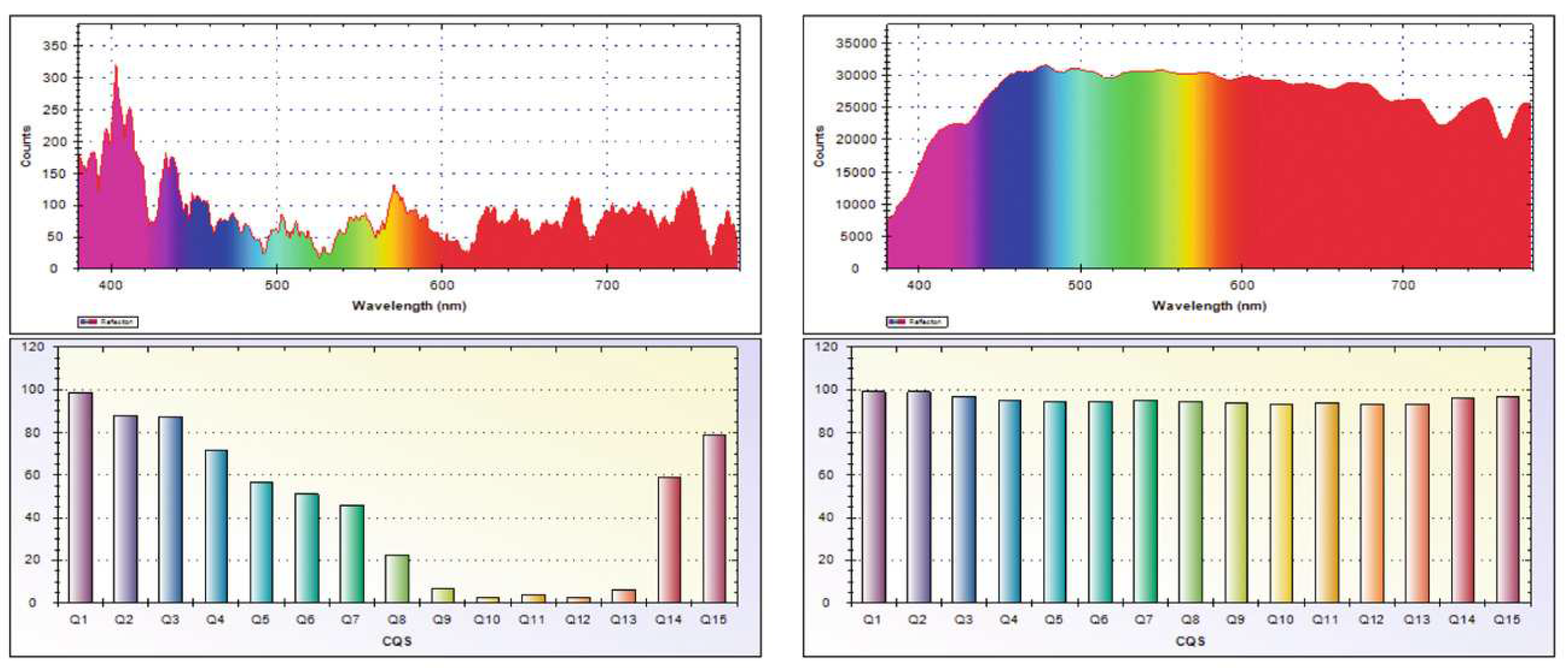
Figure 3 illustrates the spectrometer readings for natural moonlight and sunlight, with average readings (illuminance, blue-peak, green-peak and red-peak levels) for moonlight (2.24 lx, 409.67 nm, 545.78 nm, 678 nm) and sunlight (85282.96 lx, 480 nm, 542 nm, 604.5 nm) in the year 2017.
3.0. Objectifying Life-Centric Lighting Design
This section offers a basic guidance framework from existing knowledge for addressing the basic outcomes of spatial and temporal orientation through lighting design decisions.
The first step is to establish the type of lighting application, its primary activities and occurrences, and its desired operational goals. For example, outdoor park environments are typified by their intent of providing restful and safe environments. Operational goals will likely include safe navigation for humans, without disturbing the animals and plants. These activities should be adequately supported by life-centric lighting strategies. Design criteria should be prioritised based on a thorough understanding of the desired outcomes so as to facilitate rational design decisions when it is not possible to simultaneously achieve all the outcomes [
17]. For example, in an outdoor park, temporary disturbance of restoration cycles for diurnal life forms may be acceptable if it increases the speed and efficacy with which humans can safely navigate through the space (e.g., pathway lights while being necessary for humans, can be considered very bright when viewed from the perspective of animals).
The second step is to determine whether diurnal, nocturnal, or both types of life forms are involved in the application. Diurnal life forms have behavioural cycles and lighting needs that are generally synchronous with the day-night cycle, which may conflict with the behavioural cycles and lighting needs of nocturnal life forms that are generally asynchronous with the day-night cycle. Diurnal life forms require light with high biological potency during the day, which progressively lowers in biological potency with the approach of the night. Nocturnal life forms on the other hand require minimal outcomes associated with an asynchronous life cycle and night-time light exposure. Specialized design solutions may be required for applications involving both diurnal and nocturnal life forms, which include advanced lighting controls (e.g., preset controls for controlling intensity or spectrum, scenes or zones) and/or architectural interventions (e.g., obtrusive light blocked with barriers, zonal spaces created with temporary or permanent partitions) that go beyond traditional architectural and spatial relationships [
17]. Advanced lighting controls allow the ability to provide lighted environments that can be as close to the natural environments as possible by matching the natural solar light cycles during the day and lunar light cycles during the night. For example, façade lighting should be provided with an advanced control system that would either dim or completely turn off the lighting (e.g., especially during the migration periods of local birds in the region).
The third step is to determine if the application includes life forms that will be in their restoration periods e.g. sleep. If yes, determine if these life forms will be diurnal, nocturnal, or both. As restoration is promoted by darkness, and spaces where life forms are undergoing restoration should be as dark as possible while providing enough light for the safe navigation of humans [
87]. Restoration for diurnal life forms primarily occurs in the evening and throughout the night, which may require blockage of intrusive light through window treatments. Restoration for nocturnal life forms primarily occurs during day-time hours, which almost certainly requires necessary window treatments. For example, outdoor park environments are likely to have a combination of needs from both diurnal and nocturnal life forms. The separate and distinct needs of these life forms change throughout the day, and the interplay between conflicting needs by different life forms demands a considered understanding along with careful examination and prioritization of trade-offs. The timing, duration and spatial distribution of light can be customised to cause optimal masking effects for as many life forms as possible, while meeting all the minimum requirements for visibility. For example, adaptive lighting controls for street lighting applications should be programmed to brighten when sensing activity and dim or completely switch off while not (e.g., to support the restorative cycles of the surrounding trees while meeting the desired requirements of safety and visibility for passers-by).
The fourth step is to be informed by industry guidelines that are striving to bridge the gap between scientific understanding and design applications. The principles for Best Practice Lighting Design [
26] as well as Responsible Outdoor Lighting At Night [
25] provide guidelines that focus on protecting natural darkness through good quality lighting design and management for the benefit of all life forms. The principles generally describe natural darkness as a starting point and using light only for specific purposes by considering its impact on the surrounding area. The direction of light should be carefully aimed to target only the intended area or object, and shielded by pointing downward, keeping close to the ground and not spilling beyond where it is needed. The intensity of light should be the lowest required with mindful consideration for dark-colored non-reflective surfaces. Adaptive light controls should be used to manage light color, intensity and timing that ensures appropriate light is available when it is needed, dimmed when possible, and turned off when not needed. Finally, the amount of shorter wavelength (blue-violet-UV) light should be limited wherever possible by using warmer colored lights.
The fifth and final step is to establish design criteria and numerical design targets, after defining the application characteristics and operational goals, establishing the life forms’ behavioral cycles, determining the life forms’ restoration requirements, and reviewing the published guidelines. While it is not possible to control any of the external and pertinent factors (e.g., climate), there is some amount of control over the light/dark stimulus received by the life forms in the designed spaces. Good outcomes can be achieved by incorporating the masking effects table for providing customized light with appropriate qualities (e.g., intensity, spectrum, spatial pattern, controllability) to support the spatial and temporal orientation at the right time of day. Additionally, following the natural solar light cycles during the day and lunar light cycles during the night will help in achieveing lighted environments that are as close to the natural environments for each of the life forms present in the space.
4.0. Declassifying Life-Centric Lighting Design
This section proposes a term for comprehensively capturing the spirit of life-centric lighting design while being semantically appropriate for this aspiration.
4.1. Arguing for the Term ‘Biodynamic’
‘Biodynamic lighting’ is presented as a seemingly appropriate term for capturing the spirit of life-centric design given its collective meaning and origins. Biodynamic is rooted in the work of Austrian philosopher and scientist Rudolf Steiner, who founded an alternative form of agriculture that would ‘heal the earth’ by integrating scientific understanding with recognition of the spirit in nature; it is an ecological, ethical and holistic approach towards cultivation and nutrition that looks to the cosmos before planting and harvesting crops, thereby providing a deeper connection with the farm and its place in nature [
88,
89]. The term ‘biodynamic’ is derived from the Greek words ‘bios’ meaning life and ‘dynamics’ meaning energy: collectively meaning working with the energies that create and maintain life [
90]. The key reason for proposing this term to capture the true aspiration of life-centric lighting is the different parallels that can be drawn between the biodynamic calendar (e.g. utilizing the lunar calendar along with the solar calendar based on the positioning of the moon and the sun respectively) [
91]. Paying attention to the phases of the moon is essential as science has already proven lunar influence on water in the form of tides, which enables an easy extension to all other life forms that are mainly composed of water [
92]. Biodynamic methods can be applied anywhere with thoughtful adaptation to climate, culture, landscape, and scale [
93]. However, the scientific community remains sceptical about these methods – which are argued to be developed through mysticism instead of scientific methodology – as they cannot be tested and validated due to the non-existence of evidence to prove improvement in plant or soil quality [
91].
4.2. Exploring Other ‘Bio-Inspired’ Terms
The term ‘bio-inspired design’ is a generally accepted umbrella term for categorising design and engineering approaches that use biology as a resource for solutions. Biological regulation allows all life forms to modulate their own constitutive dynamics in response to the effects of internal and external stimuli [
94]. Within the family of bio-inspired design there are other terms such as biomimetic, biomorphic, biophilic and bioutilised designs. Biomimetic design [
95,
96] emphasises on studying and emulating specific functional strategies created by the regenerative solutions of living systems. Biophilic design [
97,
98,
99] emphasises on increasing human connectivity to the natural environment through the use of direct or indirect nature along with space and place conditions. Biomorphic design [
100] refers to designs that visually resemble elements from life forms, which are not necessarily better performing or sustainable given their lack of adherence to biological principles. Bioutilised design on the other hand refers to the use of biological materials or life forms in designs either as natural materials or solution to a problem. However, none of these terms are able to comprehensively capture the spirit of life-centric lighting design as much as the term biodynamic design.
5.0. Conclusions
Biodynamically lighted environments should ideally follow the natural solar light cycles during the day and lunar light cycles during the night in order to support the spatial and temporal orientation of diurnal as well as nocturnal life forms. Providing light of high biologically potency during the day and low biological potency at night is probably the simplest guidance to support the life-sustaining behaviors of both diurnal and nocturnal life forms. However, lighting design solutions for hosting both diurnal and nocturnal life forms also demand a complete consideration of trade-offs. A framework – that characterizes the lighting application, determines the likely behavioral cycle(s) of life forms, determines the restoration needs of these life forms, reviews published guidelines to develop goals and design criteria that support human and non-human outcomes – is proposed to guide the lighting design process. The framework provides a collective set of criteria that define ‘life-centric’ lighting by simultaneously including certain key criteria for integrative lighting, which can then be used to establish design criteria that will guide the design decision-making process. The implementation of this process will hopefully facilitate the realization of biodynamic lighting solutions that will support good human and non-human outcomes.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Falcón, J.; Torriglia, A.; Attia, D.; Viénot, F.; Gronfier, C.; Behar-Cohen, F.; Martinsons, C.; Hicks, D. Exposure to Artificial Light at Night and the Consequences for Flora, Fauna, and Ecosystems. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Houser, K.W. Ethics and Fallacies of Human-Centric Lighting and Artificial Light at Night. LEUKOS 2021, 17, 319–320. [Google Scholar] [CrossRef]
- Gaston, K.J.; Visser, M.E.; Hölker, F. The biological impacts of artificial light at night: the research challenge. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140133. [Google Scholar] [CrossRef]
- Raap, T.; Pinxten, R.; Eens, M. Light pollution disrupts sleep in free-living animals. Sci. Rep. 2015, 5, 13557. [Google Scholar] [CrossRef] [PubMed]
- Tidau, S.; Whittle, J.; Jenkins, S.R.; Davies, T.W. Artificial light at night reverses monthly foraging pattern under simulated moonlight. Biol. Lett. 2022, 18, 20220110. [Google Scholar] [CrossRef] [PubMed]
- Kyba, C.C.M.; Kuester, T.; Sánchez de Miguel, A.; Baugh, K.; Jechow, A.; Hölker, F.; Bennie, J.; Elvidge, C.D.; Gaston, K.J.; Guanter, L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2022, 3, e1701528. [Google Scholar] [CrossRef] [PubMed]
- Krieg, J. Influence of moon and clouds on night illumination in two different spectral ranges. Sci. Rep. 2021, 11, 20642. [Google Scholar] [CrossRef] [PubMed]
-
A Geologic Time Scale 2004; Gradstein, F.M., Ogg, J.G., Smith, A.G., Eds.; Cambridge University Press: Cambridge, UK, 2005; ISBN 9780511536045. [Google Scholar]
- Gradstein, F.M.; Ogg, J.G.; Smith, A.G.; Bleeker, W.; Lourens, L.J. A new Geologic Time Scale, with special reference to Precambrian and Neogene. Episodes 2004, 27, 83–100. [Google Scholar] [CrossRef]
- Mindell, D.P. Science; 2009; pp. 1562–1563. [Google Scholar]
- Malmstrom, C.M. Ecologists Study the Interactions of Organisms and Their Environment. Nat. Educ. Knowl. 2010, 3, 88. [Google Scholar]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Dominoni, D.; de la Iglesia, H.; Levy, O.; Herzog, E.D.; Dayan, T.; Helfrich-Forster, C. Chronobiology by moonlight. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123088. [Google Scholar] [CrossRef] [PubMed]
-
CIE Position Statement on Non-Visual Effects of Light: Recommending Proper Light and the Proper Time, 2nd ed.; Commission Internationale de l’Eclairage [CIE]: Vienna, AUSTRIA, 2019.
- Houser, K.W.; Boyce, P.R.; Zeitzer, J.M.; Herf, M. Human-centric lighting: Myth, magic or metaphor? Light. Res. Technol. 2020, 53, 97–118. [Google Scholar] [CrossRef]
- Vetter, C.; Pattison, P.M.; Houser, K.; Herf, M.; Phillips, A.J.K.; Wright, K.P.; Skene, D.J.; Brainard, G.C.; Boivin, D.B.; Glickman, G. A Review of Human Physiological Responses to Light: Implications for the Development of Integrative Lighting Solutions. LEUKOS 2021, 1–28. [Google Scholar] [CrossRef]
- Houser, K.W.; Esposito, T. Human-Centric Lighting: Foundational Considerations and a Five-Step Design Process. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J. Wellness and Integrative Lighting. The Light Review [Internet]. 2019. Available online: https://www.thelightreviewonline.com/wellness-and-integrative-lighting/.
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Melatonin: The chemical expression of darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J.; Besseau, L.; Sauzet, S.; Boeuf, G. Melatonin effects on the hypothalamo-pituitary axis in fish. Trends Endocrinol. Metab. 2007, 18, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J.; Migaud, H.; Muñoz-Cueto, J.A.; Carrillo, M. Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Bloch, G.; Hazan, E.; Rafaeli, A. Circadian rhythms and endocrine functions in adult insects. J. Insect Physiol. 2013, 59, 56–69. [Google Scholar] [CrossRef]
- Walton, J.C.; Weil, Z.M.; Nelson, R.J. Influence of photoperiod on hormones, behavior, and immune function. Front. Neuroendocrinol. 2011, 32, 303–319. [Google Scholar] [CrossRef]
- Hartley, R.; Liebel, B. Five Principles for Responsible Outdoor Lighting.
- Pendoley, K.; Bell, C.; Surman, C.; Choi, J. National Light Pollution Guidelines for Wildlife including Marine Turtles, Seabirds and Migratory Shorebirds, 1st ed.; Commonwealth of Australia, 2020. [Google Scholar]
- Zielinska-Dabkowska, K.M.; Hartley, R. ROLAN Manifesto for lighting professionals. arc Mag. 2022, 102. [Google Scholar]
- Gaston, K.; Davies, T.; Bennie, J.; Hopkins, J. Reducing the ecological consequences of night-time light pollution: options and developments. J. Appl. Ecol. 2012, 49, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Dabkowska, K.M.; Bobkowska, K. Rethinking Sustainable Cities at Night: Paradigm Shifts in Urban Design and City Lighting. Sustainability 2022, 14. [Google Scholar] [CrossRef]
- Durmus, D.; Tengelin, M.N.; Jägerbrand, A. Investigating the methods and health outcomes of research studies on light pollution and human physiology and behaviour: a systematic review. In Proceedings of the 2021 Joint Conference - 11th International Conference on Energy Efficiency in Domestic Appliances and Lighting & 17th International Symposium on the Science and Technology of Lighting (EEDAL/LS:17); 2022; pp. 1–6. [Google Scholar]
- Dugar, A.M.; Slade, J. Dovetailing darkness and light for designing sustainable outdoor spaces. Int. J. Sustain. Light. 2021, 23, 88–99. [Google Scholar]
- Stone, T. The Value of Darkness: A Moral Framework for Urban Nighttime Lighting. Sci. Eng. Ethics 2018, 24, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Smale, L.; Nunez, A.A. Nocturnal/Diurnal. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2009; ISBN 978-3-540-29678-2. [Google Scholar]
- Figueiro, M. Nonvisual Lighting Effects and Their Impact on Health and Well-Being. In Encyclopedia of Color Science and Technology; Shamey, R., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2019; ISBN 978-3-642-27851-8. [Google Scholar]
- Brainard, G.C.; Rollag, M.D.; Hanifin, J.P. Photic Regulation of Melatonin in Humans: Ocular and Neural Signal Transduction. Biol. Rhythm. 1997, 12, 537–546. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Rea, M.S. The Effects of Red and Blue Lights on Circadian Variations in Cortisol, Alpha Amylase, and Melatonin. Int. J. Endocrinol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Prayag, A.S.; Münch, M.; Aeschbach, D.; Chellappa, S.L.; Gronfier, C. Light Modulation of Human Clocks, Wake, and Sleep. Clocks & Sleep 2019, 1, 193–208. [Google Scholar] [CrossRef]
- Frøland Steindal, I.A.; Whitmore, D. Circadian Clocks in Fish—What Have We Learned so far? Biology (Basel). 2019, 8. [Google Scholar] [CrossRef]
- Osorio, D.; Vorobyev, M. Photoreceptor sectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B Biol. Sci. 2005, 272, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, J.; Barreira, A.; Chioccioli, M.; Polin, M.; Tuval, I. Phototaxis beyond turning: persistent accumulation and response acclimation of the microalga Chlamydomonas reinhardtii. Sci. Rep. 2017, 7, 3447. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.; Ye, K.; Li, Q.; Jianyu, Z.; Xing, H.; Wei, P.; Sun, J.; Ciucci, F.; Jacky, W.; et al. Positive/Negative Phototropism: Controllable Molecular Actuators with Different Bending Behavior. CCS Chem. 2020. [Google Scholar] [CrossRef]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between Clocks: Daily Temporal Patterns of Human Chronotypes. J. Biol. Rhythms 2003, 18, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Randel, N.; Jékely, G. Phototaxis and the origin of visual eyes. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150042. [Google Scholar] [CrossRef]
- Chau, R.M.W.; Bhaya, D.; Huang, K.C. Emergent Phototactic Responses of Cyanobacteria under Complex Light Regimes. MBio 2017, 8, e02330–16. [Google Scholar] [CrossRef]
- Wilde, A.; Mullineaux, C.W. Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol. Rev. 2017, 41, 900–922. [Google Scholar] [CrossRef]
- Humberg, T.-H.; Sprecher, S.G. Age- and Wavelength-Dependency of Drosophila Larval Phototaxis and Behavioral Responses to Natural Lighting Conditions. Front. Behav. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Paris, T.M.; Allan, S.A.; Udell, B.J.; Stansly, P.A. Wavelength and Polarization Affect Phototaxis of the Asian Citrus Psyllid. Insects 2017, 8. [Google Scholar] [CrossRef]
- Hartmann, S.; Vogt, R.; Kunze, J.; Rauschert, A.; Kuhnert, K.-D.; Wanzenböck, J.; Lamatsch, D.K.; Witte, K. Zebrafish larvae show negative phototaxis to near-infrared light. PLoS One 2018, 13, e0207264. [Google Scholar] [CrossRef]
- Newman, E.A.; Hartline, P.H. Integration of Visual and Infrared Information in Bimodal Neurons in the Rattlesnake Optic Tectum. Science (80-. ). 1981, 213, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.R. Seabird vision : spectral sensitivity and light-attraction behavior, Madison, WI, USA, 1986; 180.
- Voigt, C.C.; Roeleke, M.; Marggraf, L.; Pētersons, G.; Voigt-Heucke, S.L. Migratory bats respond to artificial green light with positive phototaxis. PLoS One 2017, 12, e0177748. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Szarzynska, B.; Fankhauser, C. Phototropism: at the crossroads of light-signaling pathways. Trends Plant Sci. 2013, 18, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.; Christie, J.M. Plant Phototropic Growth. Curr. Biol. 2015, 25, R384–R389. [Google Scholar] [CrossRef]
- Schumacher, J. How light affects the life of Botrytis. Fungal Genet. Biol. 2017, 106, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.C.R.; Moraes, M.N.C.M.; Poletini, M.O.; Lima, L.H.R.G.; Castrucci, A.M.L. From Blue Light to Clock Genes in Zebrafish ZEM-2S Cells. PLoS One 2014, 9, e106252. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science (80-. ). 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M.; Lucas, R.J.; Brown, T.M. Chromatic clocks: Color opponency in non-image-forming visual function. Neurosci. Biobehav. Rev. 2017, 78, 24–33. [Google Scholar] [CrossRef]
- Saunders, D.S. Insect photoperiodism: seeing the light. Physiol. Entomol. 2012, 37, 207–218. [Google Scholar] [CrossRef]
- Tomioka, K.; Matsumoto, A. A comparative view of insect circadian clock systems. Cell. Mol. Life Sci. 2010, 67, 1397–1406. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light Signal Transduction in Higher Plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.R. Plant Circadian Rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef]
- Niwa, Y.; Matsuo, T.; Onai, K.; Kato, D.; Tachikawa, M.; Ishiura, M. Phase-resetting mechanism of the circadian clock in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. 2013, 110, 13666–13671. [Google Scholar] [CrossRef] [PubMed]
- Ryo, M.; Matsuo, T.; Yamashino, T.; Ichinose, M.; Sugita, M.; Aoki, S. Diversity of plant circadian clocks: Insights from studies of Chlamydomonas reinhardtii and Physcomitrella patens. Plant Signal. Behav. 2016, 11, e1116661. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, U.; Thakkar, N.; Das, P.; Pal Bhadra, M. Evolution of circadian rhythms: from bacteria to human. Sleep Med. 2017, 35, 49–61. [Google Scholar] [CrossRef]
- Levitis, D.A.; Lidicker, W.Z.; Freund, G. Behavioural biologists do not agree on what constitutes behaviour. Anim. Behav. 2009, 78, 103–110. [Google Scholar] [CrossRef]
- Gomez-Marin, A.; Ghazanfar, A.A. The Life of Behavior. Neuron 2019, 104, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Juricic, E.; Tran, E. Changes in vigilance and foraging behaviour with light intensity and their effects on food intake and predator detection in house finches. Anim. Behav. 2007, 74, 1381–1390. [Google Scholar] [CrossRef]
- Kelly, N.; Vaštakaitė-Kairienė, V.; Runkle, E.S. Chapter 18 - Indoor lighting effects on plant nutritional compounds. In Plant Factory Basics, Applications and Advances; Kozai, T., Niu, G., Eds.; Academic Press, 2022; pp. 329–349. ISBN 978-0-323-85152-7. [Google Scholar]
- McDaniel, S.; Ostertag, R. Strategic light manipulation as a restoration strategy to reduce alien grasses and encourage native regeneration in Hawaiian mesic forests. Appl. Veg. Sci. 2010, 13, 280–290. [Google Scholar] [CrossRef]
- Aschoff, J. Exogenous and Endogenous Components in Circadian Rhythms. Cold Spring Harb. Symp. Quant. Biol. 1960, 25, 11–28. [Google Scholar] [CrossRef]
- Shuboni, D.D.; Cramm, S.; Yan, L.; Nunez, A.A.; Smale, L. Acute Behavioral Responses to Light and Darkness in Nocturnal Mus musculus and Diurnal Arvicanthis niloticus. J. Biol. Rhythms 2012, 27, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Driessche, T. Vanden; Guisset, J.-L.; Gaspar, T.; Kevers, C.; Koukkari, W.L. Masking in Plants. Chronobiol. Int. 1989, 6, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J.; von Goetz, C. Masking of circadian activity rhythms in hamsters by darkness. J. Comp. Physiol. A 1988, 162, 559–562. [Google Scholar] [CrossRef]
- Aschoff, J.; von Goetz, C. Masking of Circadian Activity Rhythms in Canaries by Light and Dark. J. Biol. Rhythms 1989, 4, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Redlin, U.; Hattar, S.; Mrosovsky, N. The circadian Clock mutant mouse: impaired masking response to light. J. Comp. Physiol. A 2005, 191, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Redlin, U.; Mrosovsky, N. Masking of locomotor activity in hamsters. J. Comp. Physiol. A 1999, 184, 429–437. [Google Scholar] [CrossRef]
- Mrosovsky, N.; Thompson, S. Negative and positive masking responses to light in retinal degenerate slow (rds/rds) mice during aging. Vision Res. 2008, 48, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Raza, M.A.; Li, Z.; Chen, Y.; Khalid, M.H. Bin; Du, J.; Liu, W.; Wu, X.; Song, C.; Yu, L.; et al. The Influence of Light Intensity and Leaf Movement on Photosynthesis Characteristics and Carbon Balance of Soybean. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, G.; Du, S.; Wu, H.; Fu, R.; Yu, X. Light Intensity Influence on Growth and Photosynthetic Characteristics of Horsfieldia hainanensis. Front. Ecol. Evol. 2021, 9. [Google Scholar] [CrossRef]
- Wang, P.; Lei, J.-P.; Li, M.-H.; Yu, F.-H. Spatial Heterogeneity in Light Supply Affects Intraspecific Competition of a Stoloniferous Clonal Plant. PLoS One 2012, 7, e39105. [Google Scholar] [CrossRef]
- Campbell, S.S.; Tobler, I. Animal sleep: A review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 1984, 8, 269–300. [Google Scholar] [CrossRef] [PubMed]
- Chudler, E.; Kuwana, E.; Phillips, M.; Murray, M. How Much Do Animals Sleep?
- Barentine, J.C. Methods for Assessment and Monitoring of Light Pollution around Ecologically Sensitive Sites. J. Imaging 2019, 5. [Google Scholar] [CrossRef] [PubMed]
-
Ecological Consequences of Artificial Night Lighting; Rich, C., Longcore, T., Eds.; Island Press: Washington DC, USA, 2005; ISBN 9781559631297. [Google Scholar]
- Tessmar-Raible, K.; Raible, F.; Arboleda, E. Another place, another timer: Marine species and the rhythms of life. Bioessays 2011, 33, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Breitler, J.-C.; Djerrab, D.; Leran, S.; Toniutti, L.; Guittin, C.; Severac, D.; Pratlong, M.; Dereeper, A.; Etienne, H.; Bertrand, B. Full moonlight-induced circadian clock entrainment in Coffea arabica. BMC Plant Biol. 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; O’Hagan, J.B.; Peirson, S.N.; et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLOS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef] [PubMed]
- Paull, J. Biodynamic Agriculture: The Journey from Koberwitz to the World, 1924-1938. J. Org. Syst. 2011, 6, 27–41. [Google Scholar]
- Organic Farming: Biodynamic Farming.
-
Biodynamic Agriculture; Pathak, R.K., Ram, R.A., Eds.; Central Institute for Subtropical Horticulture: Lucknow, INDIA, 2003. [Google Scholar]
- Chhabra, E. Biodynamic farming is on the rise but how effective is this alternative agricultural practice? Guard. Sustain. Bus. 2017.
- Biodynamic Agriculture.
- Biodynamic Principles and Practices.
- Bich, L.; Mossio, M.; Ruiz-Mirazo, K.; Moreno, A. Biological regulation: controlling the system from within. Biol. Philos. 2016, 31, 237–265. [Google Scholar] [CrossRef]
- Benyus, J.M. Biomimicry: Innovation Inspired by Nature; 1st ed.; William Morrow: New York, NY, USA, 1997; ISBN 0688136915. [Google Scholar]
- What Is Biomimicry?
-
Biophilic Design: The Theory, Science and Practice of Bringing Buildings to Life; Kellert, S.R., Heerwagen, J., Mador, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-16334-4. [Google Scholar]
- Kellert, S.R. Nature by Design: The Practice of Biophilic Design; Yale University Press: New Haven, USA, 2018; ISBN 978-0300214536. [Google Scholar]
- Kellert, S.R.; Calabrese, E.F. >The Practice of Biophilic Design; 2015. [Google Scholar]
- Fikriarini, A.; Ishomuddin, M. Biomorphic architecture approach in building form based on environmental concern. J. Teknol. 2016, 78, 97–202. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).