1. Introduction
Desert sand scorpions (
Paruroctonus utahensis) establish homes in self-dug burrows that they remain loyal to for most of their lives [
1]. These scorpions hunt at night and may travel as far as five meters from their burrows [
1,
2]. Because of dangers posed by predators and poor weather, selection should favor animals with efficient ways to return to their shelters [
3]. It has been speculated that scorpions use such cues as prevailing winds or vision to return home [
1]. However, more recent evidence suggests that scorpions may also use innate behaviors such as learning walks [
4] and path integration [
5] to find their burrows.
Path integration (PI) is the ability for an animal to integrate distance and direction while leaving its home to compute an approximate home-bound vector [
6,
7]. PI has been documented in many animals, including but not limited to ants [
8], bees [
9], and some species of spiders [
10,
11,
12]. Historically PI has been tested using displacement experiments. For example, desert ants displaced after discovering a feeding station ran in a relatively straight vector towards where their home would have been had they not been displaced [
6,
13]. Wolf spiders (
Lycosa tarantula) coaxed from their homes in a rectangular arena and transferred to the center of a circular arena moved at a vector that correlated with where their home would have been had they not been displaced [
11].
The lesser Asian scorpion,
Mesobuthus eupeus, may also be using PI to return to a home location [
5]. Scorpions were maintained in boxes that were placed in a circular arena of sand, and they were monitored as they ventured away on their own [
5]. The scorpions (including those with coverings over their eyes) took non-linear paths to leave the box but relatively direct sigmoid paths when returning to the box [
5].
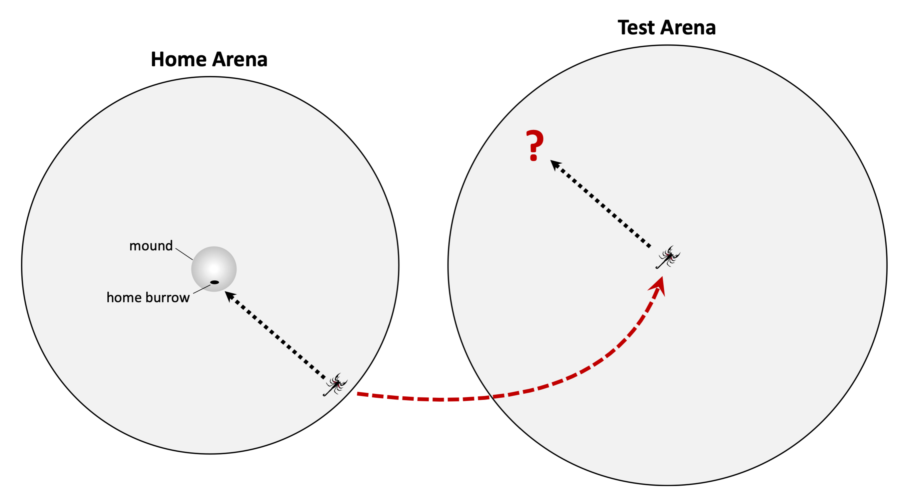
Additional experiments are needed to fully explore PI in scorpions. In this study, we used a displacement protocol modified from that of Ortega-Escobar (2002) to test whether sand scorpions demonstrated behavior consistent with intrinsic PI [
11]. Specifically, we allowed scorpions to venture from self-dug burrows in circular home arenas before physically displacing them to larger featureless arenas where we tracked their subsequent movements. If the scorpions were capable of PI, we expected that their movement vectors after displacement would correspond with the general direction of the burrow before displacement occurred. Conversely, if the post-displacement movement vectors were unrelated to the burrow direction, there would be evidence that PI based on ideothetic information is insufficient for functional navigation in sand scorpions. We found no significant homeward directionality in the scorpion paths after displacement; as such, we speculate that additional reference cues may be necessary to facilitate PI in these animals.
2. Materials and Methods
2.1. Animals, collection details, storage, and maintenance
Our study used 18 adult female desert grassland scorpions (
Paruroctonus utahensis), as females are known to be more loyal to their burrows than their male counterparts [
2]. The scorpions were collected from a sandy area in the Chihuahuan Desert near Monahans, TX. We used UV lights to find the animals at night during a period of the new moon in early March 2022. The animals were maintained in 3.8 L glass jars with 100 ml of sand from their native habitat along with a 3 cm half PVC pipe for shelter. They were misted with water (approximately 2 ml) and fed one small cricket (
Acheta domesticus) once a week. The jars were kept at a temperature of approximately 20
oC on shelves in a room inside the animal laboratory building on the University of Oklahoma campus. The room was windowless and set on a reverse day cycle, with the lights turning on at 20:00 and off at 06:00 to adjust the scorpions’ normal nighttime activity period to times convenient for the researchers. The animals were maintained under the altered light regime for at least a week before testing began.
2.2. Pilot Studies
We ran several pilot tests to optimize parameters including room configuration, arena shapes, mound structure, scorpion transfer procedures, lighting and camera positions, experimental protocol, etc. Initial tests consisted of a rectangular homing arena and a circular testing arena. The homing arena had a sand mound in the southwest corner of the rectangle and scorpions were given unlimited time to burrow. We observed that most scorpions would burrow within four days of placing them in the homing arena. We also determined that mounds needed to be moistened to provide additional structural support and that added illumination from overhead drop lights induced more burrowing. We played with displacing the animals from the rectangular homing arena to the testing arena using various containers including clear beakers, bottle caps, and cups placed on top of the animal. We also tried to use playing cards, sieves, and perforated cards to slide underneath the animal. We found that a sieve and an opaque cap was the most effective mechanism for transporting the scorpion. We felt it important to allow the home arena sand to sift from below the captured animal to remove residual home arena cues that could affect the animals’ behavior in the testing arena. We also determined that one minute under the cap when placed in the testing arena was sufficient to calm the animal before release. We completed a series of runs with several animals and recorded the testing arena with IR cameras processed through a MATLAB script. While this study helped us refine our protocol, there were problems. One being that the rectangle to circle conversion made it difficult to determine the home position relative to the scorpion. It also required that the radius of our circular arena needed to be larger than the diagonal of the rectangular arena to allow the animal sufficient room to reveal a directional choice before contacting the testing arena wall. At this point, we adjusted our protocol for two reasons: 1) we did not have ready access to sufficiently large circular arenas and 2) larger arenas would make it difficult to accurately track the scorpions via overhead IR cameras.
The next series of pilot studies consisted of four circular arenas: two smaller homing arenas and two larger testing arenas like in our final experiment. We completed a series of trials with methods similar to those currently described in our main experiment, but without the added plastic pizza saver inside the mound (see section 2.3 below). Eight of the 10 animals used in these pilot studies burrowed in the sand mounds within four days. Of these eight animals, three completed at least one usable run with an average of 1.66 runs per animal and a total of six usable runs. We plotted these runs as independent trials according three differently rendered plots (see section 2.5 below) and determined the resultant vector angle that the scorpions crossed the decision line (Figure S1A). We used these vectors to calculate the composite vectors and determine the significance of the runs using Raleigh’s Z test (Figure S1B). We found there was significant directionality in the animals’ raw paths biased toward the southwest corner of the experimental room.
To correct this bias, we removed a large mirror that hung on the wall in the southwest corner of the experimental room. We also minimized extraneous light sources by drilling a hole through the wall and running the camera wires through a light blocking grommet to exclude light from the adjacent storage room where the computers were stationed. We also put the adjacent room on the same reverse night:day cycle as the experimental room to further minimize any extraneous light. In addition, a light blocking curtain was strung between the homing arenas to prevent variations in light levels within the arenas due to light cast from the neighboring arena’s lamps. These actions helped ensure that there was uniformity in the experimental room to avoid any potential visual bias.
2.3. Experimental Room Set-up
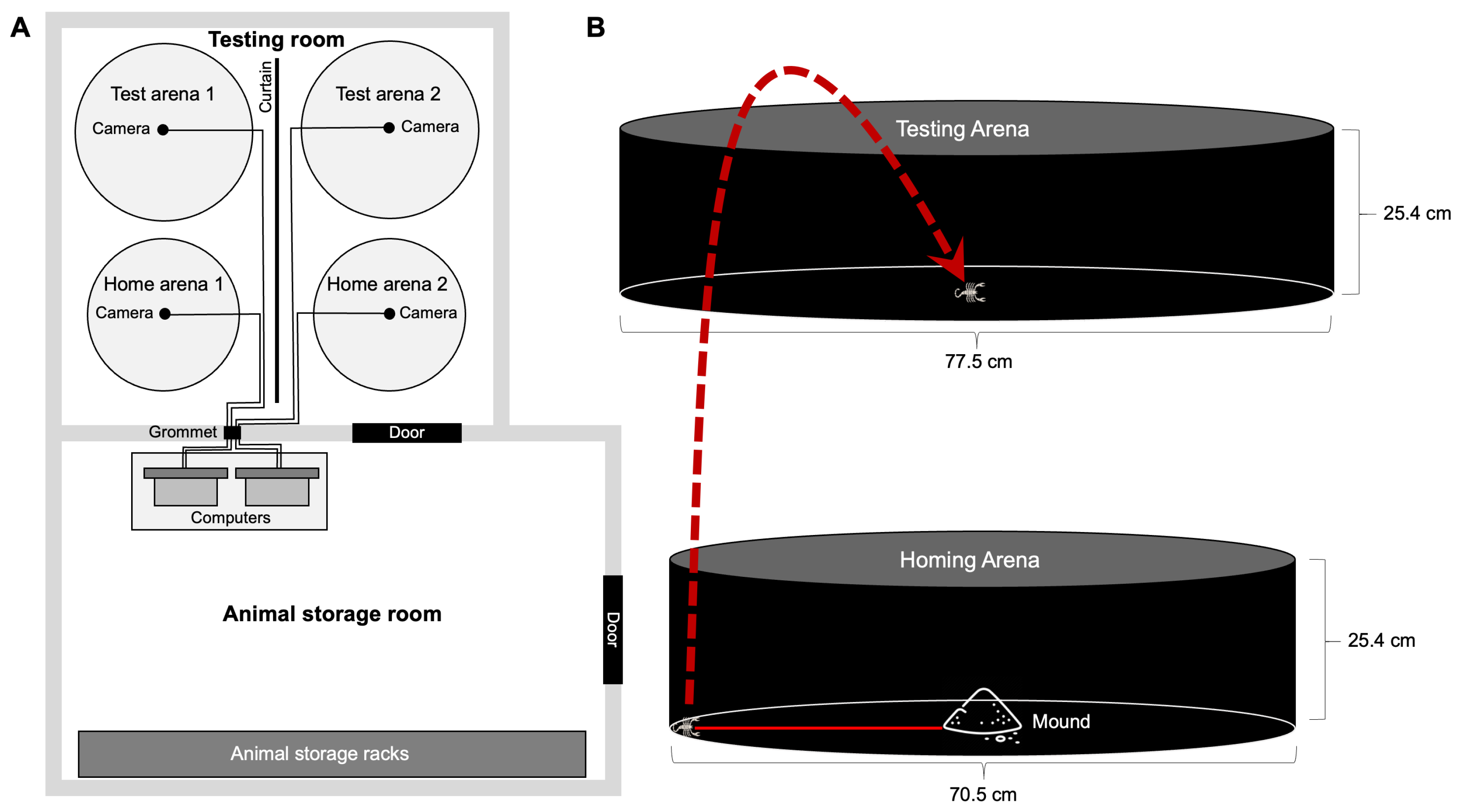
The experimental room (also windowless) was adjacent to the animal storage room and was maintained at the same temperature and light cycle as the animal storage room. The experimental room contained four circular arenas: two smaller homing arenas and two larger testing arenas (
Figure 1). The homing arenas were 70.5 cm in diameter open at the top, with 25.4 cm tall walls (visual angle to top of wall = 34.4
o from arena center). These arenas were constructed from aluminum flashing wrapped around and attached to a circular plywood base. The testing arenas were open-top aluminum water heater drain pans (Camco Manufacturing; 77.5 cm diameter). An additional wall (25.4 cm tall) covered with black felt (25.4 cm tall) was placed around each testing arena (visual angle to top of wall = 33.3
o from arena center). The four arenas sat atop rubber floor mats (Ottomanson multi-purpose 61 × 61 cm exercise tile mat) to stifle any extraneous vibrations that could have disturbed the animals. All arenas were lined with 500 ml of leveled screened native sand. In the center of each homing arena, we poured a mound of sand (90 ml) over a 2.5 cm wide, 1 cm tall three-legged plastic pizza saver, creating an internal cavity the scorpions could use as a retreat. We funneled the sand through a 5 mm hole in the bottom of a plastic yogurt cup (157 ml) to create the mound. One side of the pizza saver was left exposed to entice the scorpions to form a burrow (Figure S2). We sprayed 15 ml of water over the top of the mound to increase its stability.
We built an angle iron support structure to suspend lights and infrared cameras (IR) above the arenas (
Figure 1). The IR cameras were mounted 145 cm above the floor of the testing arenas and 168 cm above the floor of the homing arenas, with one camera monitoring each arena. Each camera was connected to a desktop computer located outside of the experimental room through a small hole in the wall with a spacer to inhibit any extraneous light from entering the experimental room. Two lamps equipped with 75 W light bulbs (Duracell Ultra light bulbs, 1100 lumens) were suspended 165 cm over each homing arena. These lamps were connected to a timing switch which cycled the lights on and off according to the same night:day schedule as the main room lights. Because scorpions are attracted to shadows, a light-blocking curtain was strung between the homing arenas to prevent variations in light levels within the arenas due to light cast from the neighboring arena’s lamps.
2.4. Procedure
Prior to testing, the animals were equipped with 5 mm dome rhinestones to allow the scorpions to be detected by the IR cameras from all positions in the arenas. To apply the rhinestones, each scorpion was placed in a plastic container and restrained using a small plastic sheet that contained a 7 mm hole in its middle. The plastic sheet was maneuvered to center its hole over the animal’s dorsal mesosoma, allowing the rhinestone to be attached with double-sided tape to the scorpion.
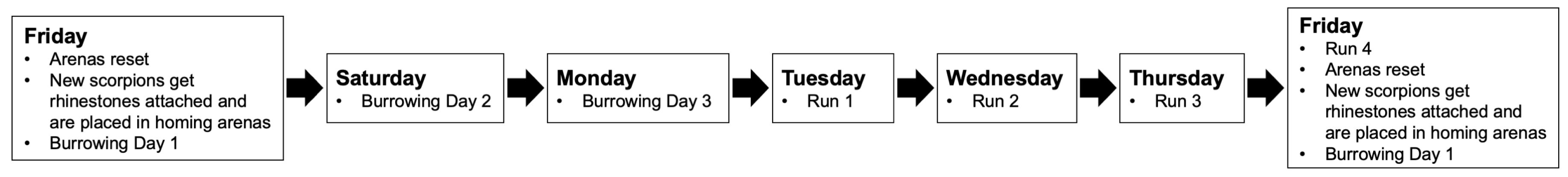
Each Friday for nine weeks, one test scorpion was moved from its home jar to each of the two homing arenas and allowed four days to establish a burrow in the central mound (
Figure 2). On the following Tuesday, we used a dim red light attached to a headlamp to check for signs of burrowing activity in the central mound. If a scorpion did not borrow over the course of four days, it was removed from the experiment and not used in displacement tests. Due to low burrowing activity, at week five we began placing the animals in the homing arena facing the center mound, a strategy that seemed to increase burrowing activity. Scorpions that showed burrowing activity were checked periodically on Tuesdays, Wednesdays, Thursdays, and Fridays; if it was found along the wall, we initiated a test run.
To initiate a test run, we activated the IR cameras over both the homing and testing arenas for a 40-minute recording session. This allowed us to video the entirety of the experiment without suffering from a lapse in recording. The scorpion was first videoed undisturbed for two minutes to record its initial location in the homing arena. We used the red headlamp to determine the animal’s position on the wall relative to the central home burrow and the direction the animal was facing on screen. To capture the scorpion for displacement to the testing arena, we simultaneously covered it with an opaque plastic cap (4.5 cm diameter) and slid a fine mesh sieve (8x12 cm piece of TORIS window screen with 1.6 mm mesh holes) under it. The scorpion remained under the cap for two additional minutes and was then lifted by the sieve and suspended for 30 seconds to allow the sand to sift out. The contained scorpion was then displaced to the center of the testing arena. After another minute, the cap was removed, the direction the animal was facing on the screen was noted, and the IR camera over the testing arena monitored the subsequent movements of the scorpion until the 40 min recording session expired. After the run was complete, the animal was returned to its homing arena. Between each run, the sand in the testing arena was stirred and leveled.
Test runs occurred over four days (Tuesday through Friday), with a maximum of one run per day. Test runs were only initiated for scorpions that were found along the wall through the day; a single trial therefore consists of the one to four test runs taken by one scorpion. After four days, each scorpion was returned to its jar and placed back in the animal storage room. Between trials, the mounds in the homing arenas were removed, and the sand of both the homing and testing arenas was thoroughly stirred and re-leveled. The homing arenas were reset on Fridays with new mounds and new scorpions.
2.5. Video recording, data processing, and calculations
To quantify the scorpion’s paths, we first established a decision line within the testing arena. Because the homing arena’s diameter is 7 cm smaller than that of the testing area, we established a fictive decision line spaced 3.5 cm inside the wall of the testing arena. We then used a MATLAB script to review the recordings taken of each scorpion’s run. The run was considered complete once the scorpion crossed the fictive decision line. A run was omitted from the data set if the scorpion did not cross the decision line within 30 minutes of its release in the testing arena. For the complete trials we used a MATLAB script to analyze the video taken of the testing arena at one frame per second so we could hand plot the scorpion’s movements. We also noted the location that the scorpion crossed the fictive decision line which correlates to a circular angle.
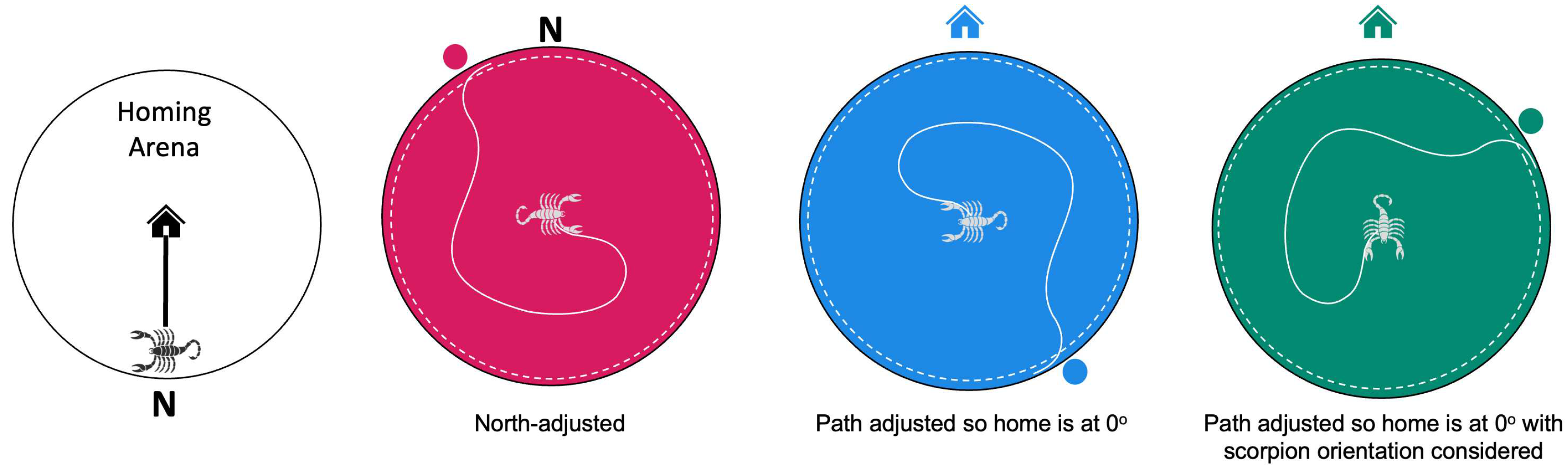
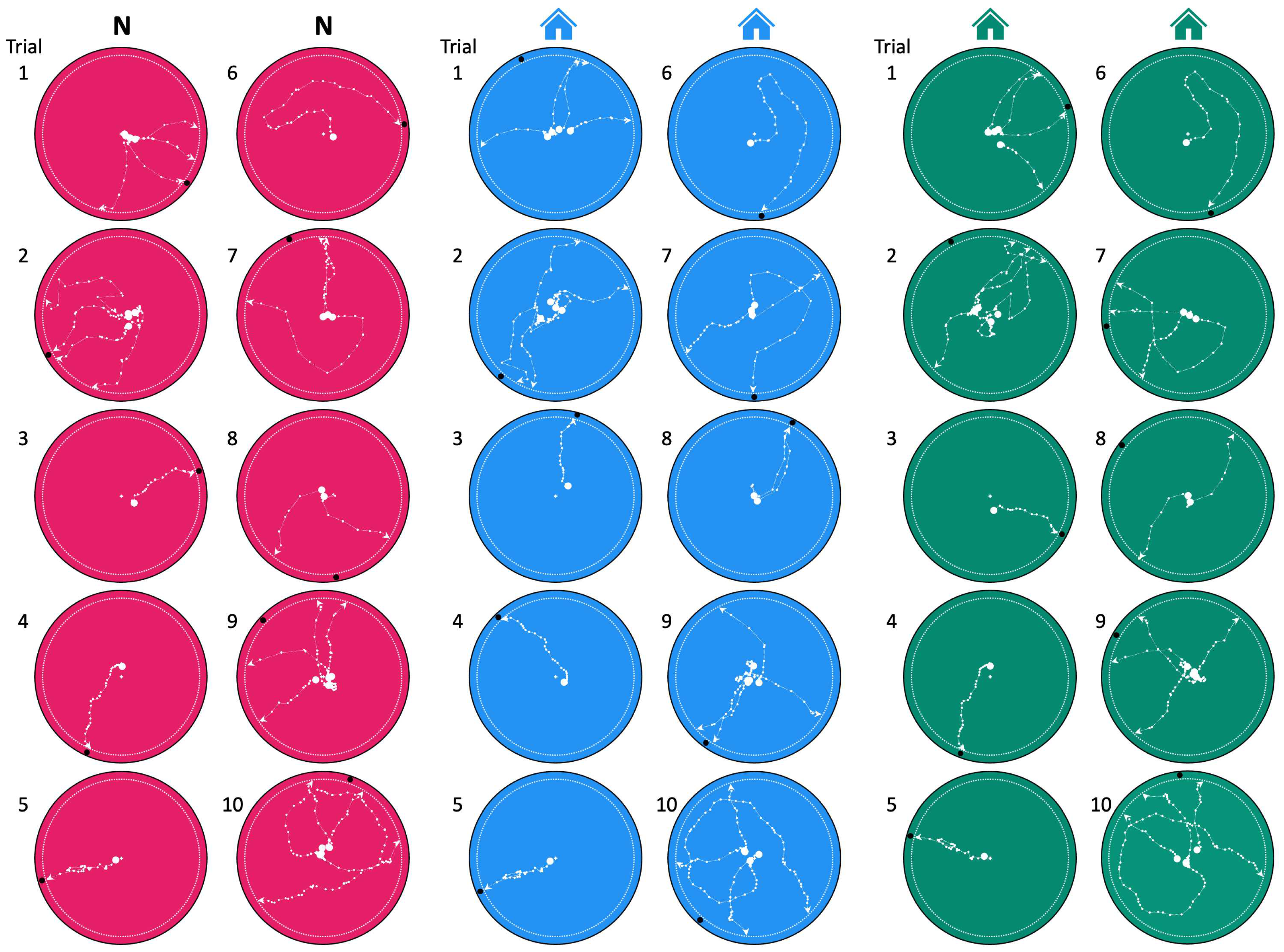
We entered the circular angle for each animal’s runs into a MATLAB circular statistics script to find the averaged resultant vector for each trial. The resultant vectors were calculated from plots rendered three different ways (
Figure 3). One plot displayed the raw path the scorpion took relative to true North. The second plot displayed the path the scorpion took relative to the position of its home burrow prior to displacement. The final plot displayed the path the scorpion took relative to its home burrow after being further adjusted based on the difference between the direction the scorpion was facing when released in the testing arena compared to how it was facing when captured in the homing arena. The averaged resultant vectors were calculated for all trials and displayed all three ways. We used Raleigh’s Z test to determine the significance of the vectors (P < 0.05 alpha level).
3. Results
3.1. Summary of runs and trials
Of the 18 animals used in this study, 10 (55 percent) successfully burrowed in the time allotted. Trials were compiled of all animals that formed burrows and completed at least one successful run (n=10). Each of the 10 animals completed from 1 to 4 runs (2.5 + 0.43, mean + SE). We observed considerable variation in movement during the animals’ runs. Some animals made meandering paths before crossing the decision line while others had relatively direct paths. No matter the path, all animals walked with short movements punctuated by brief pauses and an overall velocity of approximately 3-5 cm per second. Using the hand plotted frames, we determined the time it took for each animal to move at least one body length after the cap was lifted off it in the testing arena. The animals that completed runs took 62.2 + 23.9 s (mean + SE) to move after release and 34.3 + 6.1 s (mean + SE) to cross the decision line after their first movement.
3.2. Path analysis
We plotted each of the animals’ runs based on the three categories of adjustment (
Figure 3) to visualize movement patterns (
Figure 4). For trials that had more than one run, the patterns showed that the animals crossed the decision line at different circular angles for each run.
Figure 4 also shows the averaged resultant vectors of the animals’ runs for each of the trials and for each of the adjustment categories.
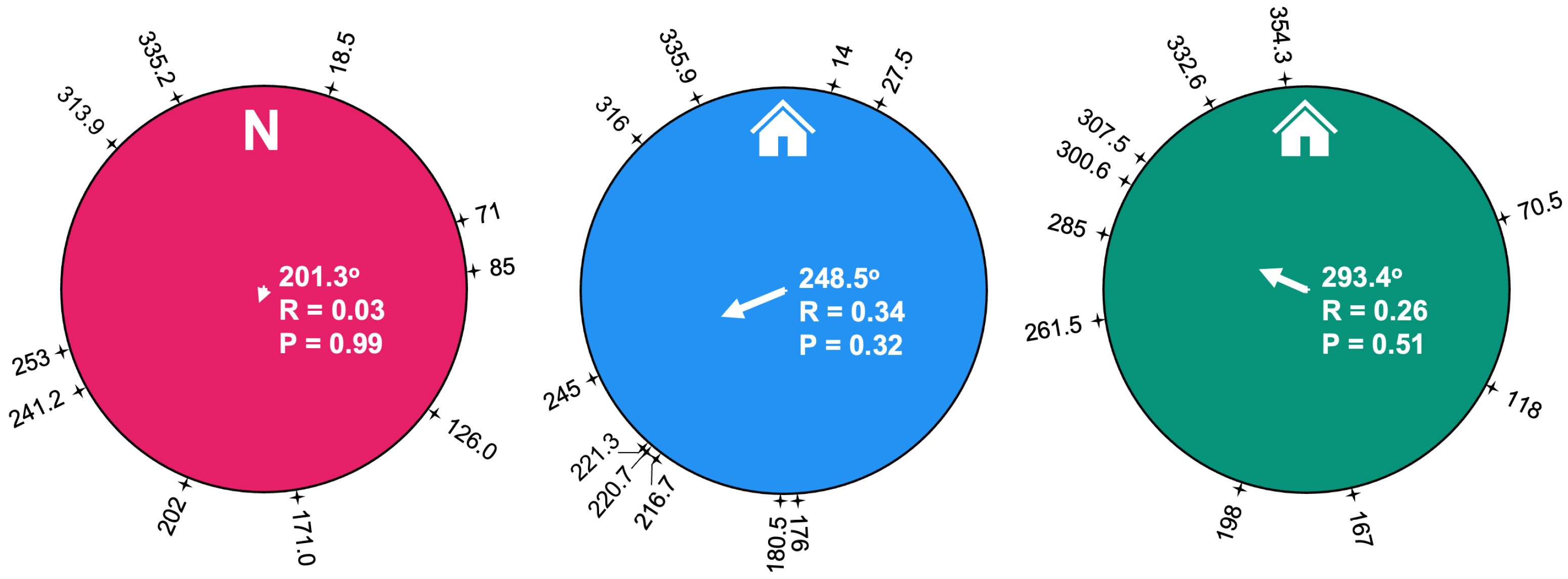
The resultant vectors for each trial were used to calculate the composite resultant vector for the three adjustment categories (
Figure 5). The points on the perimeter of the circles in
Figure 5 indicate the average circular angle that the scorpion crossed the decision line for each trial, and the central arrows depict the overall composite vector based on Rayleigh’s calculation. The plots show wide scattering of the trial vectors and no statistically significant directionality for any of the adjustment categories (P > 0.05; Rayleigh’s Z test).
4. Discussion
Some have speculated that internal (idiothetic) mechanisms, such as information gathered by proprioceptors near leg joints [
10] or by mechanoreceptors on the pectines [
4,
14,
15], could aid the homeward return of scorpions via PI (Ortega-Escobar et al. 2023 accepted). Proprioception has been shown to be important for PI in other arachnids. For example, in the banana leaf spider (
Cupiennius salei), displacement experiments showed that vision-occluded animals used lyriform organs on their legs to estimate the distance and angle moved from a prey item [
16]. The funnel web spider (
Agelena labyrinthica) uses both proprioceptors and the plane of polarized light to return home [
17,
18,
19,
20], and proprioception has been shown to contribute distance information to angular information gathered via vision in the wolf spider,
Lycosa tarantula [
21]. However, our study found no significant mean direction of displaced scorpions towards a fictive home burrow when visual information was minimized.
Animals use a variety of external (allothetic) reference mechanisms to aid in PI. Various invertebrates are known to use magnetoreception, celestial positions, landmark orientations, and prevailing winds to return home [
7]. Insects such as ants and bees, as well as some arachnids, use polarized light in navigation. Ants can use both polarized light (e-vectors) and light wavelength information for navigation, even in the absence of direct sunlight [
22]. More closely related to scorpions, the wolf spider (
Lycosa tarantula) uses its anterior medial eyes to navigate using polarized light patterns [
23]. In the absence of polarized light, the spider uses its anterior medial eyes to track outbound rotational information to determine and update its angular position for return trips to its burrow [
12].
Some aspects of the experimental procedure may have affected the "normal" behavior of scorpions. For example, the lab setting and circular test arenas may not properly simulate the natural environment of P. utahensis, which lives in an arid desert climate. Additionally, the abrupt relocation of the scorpions from the homing arena to the testing arena may have erased any path integration information previously stored by the animal.
Future investigations on PI in scorpions are needed to determine if scorpions use allothetic information to integrate their homebound paths. Magnetoreception seems unlikely since the animals in our study had full access to the Earth’s magnetic field. Scorpions have been shown to be behaviorally responsive to polarized light [
24,
25], Their median eyes contain birefringent lenses that alter polarized light into a cross-like pattern [
7,
26] (Ortega-Escobar et al. accepted) that could project intensity differences to the underlying retina cells that relay directional information to higher brain centers. We suggest that future tests should add cues such as polarized light and/or distant light sources while also using animals with their eyes intact or covered. As a first step, repeating our displacement experiment outdoors would introduce polarized light relative to the moon or the setting sun, which could provide scorpions a global reference that may be necessary for deducing direction of movement for PI.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Pilot study movements and patterns, Figure S2: Pizza saver aid in burrowing.
Author Contributions
Conceptualization, A.M. and D.G.; methodology, A.M. and D.G.; software, D.G.; formal analysis, A.M. and D.G.; investigation, A.M.; resources, D.G.; data curation, D.G.; writing—original draft preparation, A.M.; writing—review and editing, D.G.; visualization, A.M. and D.G.; supervision, D.G.; project administration, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Mariëlle Hoefnagels for her valuable edits; Gail Goodson, and Sandra Doan for helping us maintain our animals; George Martin and Liz Cooley for helping with supplies and rigging; Claire Curry for help with our manuscript; the other members of our scorpion lab for their suggestions and support; and Ronnie Miller of the Seely-Smith Ranch for allowing us to collect scorpions on their property. Thanks also go to James Thompson and Eli Bridge for taking the time to read and review this work. We also thank the University of Oklahoma Department of Biology and the University of Oklahoma Honors College undergraduate research program for support and funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Polis, G.A. Ecology. In The biology of scorpions; Stanford University Press, 1990; pp. 247–293. [Google Scholar]
- Polis, G.A.; McReynolds, C.N.; Ford, R.G. Home range geometry of the desert scorpion Paruroctonus mesaensis. Oecologia 1985, 67, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Heinze, S.; Narendra, A.; Cheung, A. Principles of insect path integration. Current Biology 2018, 28, R1043–R1058. [Google Scholar] [CrossRef] [PubMed]
- Gaffin, D.D.; Muñoz, M.G.; Hoefnagels, M.H. Evidence of learning walks related to scorpion home burrow navigation. Journal of Experimental Biology 2022, 225, jeb243947. [Google Scholar] [CrossRef]
- Prévost, E.D.; Stemme, T. Non-visual homing and the current status of navigation in scorpions. Animal Cognition 2020, 23, 1215–1234. [Google Scholar] [CrossRef] [PubMed]
- Wehner, R. Arthropods. In Animal Homing (ed. F. Papi); Chapman & Hall: London, 1992; pp. 45–144. [Google Scholar]
- Gaffin, D.D.; Curry, C.M. Arachnid navigation – a review of classic and emerging models. Journal of Arachnology 2020, 48, 1–25, Publisher: American Arachnological Society. [Google Scholar] [CrossRef]
- Müller, M.; Wehner, R. Path integration in desert ants, Cataglyphis fortis. Proceedings of the National Academy of Sciences 1988, 85, 5287–5290, Publisher: Proceedings of the National Academy of Sciences. [Google Scholar] [CrossRef]
- Kirchner, W.H.; Braun, U. Dancing honey bees Indicate the location of food sources using path integration rather than cognitive maps. Animal Behaviour 1994, 48, 1437–1441. [Google Scholar] [CrossRef]
- Seyfarth, E.A.; Barth, F.G. Compound slit sense organs on the spider leg: mechanoreceptors involved in kinesthetic orientation. Journal of comparative physiology 1972, 78, 176–191. [Google Scholar] [CrossRef]
- Ortega-Escobar, J. Evidence that the wolf-spider Lycosa tarentula (Araneae, Lycosidae) needs visual input for path integration. The Journal of Arachnology 2002, 30, 481–486. [Google Scholar] [CrossRef]
- Ortega-Escobar, J. Role of the anterior lateral eyes of the wolf spider Lycosa tarentula (Araneae, Lycosidae) during path integration. The Journal of Arachnology 2006, 34, 51–61. [Google Scholar] [CrossRef]
- Wehner, R.; Srinivasan, M.V. Searching behaviour of desert ants, genus Cataglyphis (Formicidae, Hymenoptera). Journal of comparative physiology 1981, 142, 315–338. [Google Scholar] [CrossRef]
- Gaffin, D.D.; Brayfield, B.P. Exploring the chemo-textural familiarity hypothesis for scorpion navigation. Journal of Arachnology 2017, 45, 265–270. [Google Scholar] [CrossRef]
- Musaelian, A.; Gaffin, D.D. High-throughput simulations indicate feasibility of navigation by familiarity with a local sensor such as scorpion pectines. bioRxiv, 2020. [Google Scholar] [CrossRef]
- Barth, F.G.; Seyfarth, E.A. Cupiennius salei Keys. (Araneae) in the highlands of central Guatemala. Journal of Arachnology 1979, 7, 255–263. [Google Scholar]
- Bartels, M. Sinnesphysiologische und psychologische Untersuchungen an der Trichterspinne Agelena labyrinthica(Cl.). Zeitschrift für vergleichende Physiologie 1929, 10, 527–593. [Google Scholar] [CrossRef]
- Görner, P. Über die Koppelung der optischen und kinästhetischen Orientierung bei den Trichterspinnen Agelena labyrinthica (Clerck) und Agelena gracilens C. L. Koch. Zeitschrift für vergleichende Physiologie 1966, 53, 253–276. [Google Scholar] [CrossRef]
- Görner, P. Homing behavior of funnel web spiders (Agelenidae) by means of web-related cues. Naturwissenschaften 1988, 75, 209–211. [Google Scholar] [CrossRef]
- Moller, P. Die systematischen Abweichungen bei der optischen Richtungsorientierung der Trichterspinne Agelena labyrinthica. Zeitschrift für vergleichende Physiologie 1970, 66, 78–106. [Google Scholar] [CrossRef]
- Reyes-Alcubilla, C.; Ruiz, M.A.; Ortega-Escobar, J. Homing in the wolf spider Lycosa tarantula (Araneae, Lycosidae): the role of active locomotion and visual landmarks. Naturwissenschaften 2009, 96, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Duelli, P.; Wehner, R. The spectral sensitivity of polarized light orientation in Cataglyphis bicolor (Formicidae, Hymenoptera). Journal of Comparative Physiology A 1973, 86, 37–53. [Google Scholar] [CrossRef]
- Ortega-Escobar, J.; Muñoz-Cuevas, A. Anterior median eyes of </i>Lycosa tarentula</i> (Araneae, Lycosidae) detect polarized light: behavioral experiments and electroretinographic analysis. The Journal of Arachnology 1999, 27, 663–671. [Google Scholar]
- Brownell, P. Sensory ecology and orientational behaviors. In Scorpion Biology and Research.; Oxford University Press, 2001; pp. 159–183.
- Horváth, G.; Varjú, D. Polarization sensitivity in spiders and scorpions. In Polarized Light in Animal Vision: Polarization Patterns in Nature; Horváth, G., Varjú, D., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2004; pp. 243–246. [Google Scholar] [CrossRef]
- Locket, A. Eyes and vision. In Scorpion Biology and Research; Brownell, P.H.; Polis, G.A., Eds.; Oxford University Press, 2001; pp. 79–106.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).