1. Introduction
Fractures are common, and healing is a physiological process characterized by the bone's ability to regenerate itself. Bone tissue engineering is a promising approach for secondary bone reconstruction after severe trauma, shifting the use of traditional bone graft substitutes (Bose and Sarkar, 2020).
Bone grafts are used in regions where significant bone tissue loss has occurred to reconstruct the injured bone (Freitas et al., 2008). More than 2.2 million bone graft procedures are performed annually worldwide to repair bone defects in orthopedics, neurosurgery, and dentistry (Giannoudis et al., 2005). The search for new bone substitutes like autologous graft materials has been advancing as alternatives to bone repair (Wickramasinghe et al., 2022) .
Bone regeneration can be stimulated using biomaterials. Interestingly, the use of medicinal plants as bone grafts is highly relevant, as they are free from infecting microorganisms, biocompatible, and easy to apply, in addition to stimulating bone growth. Therefore, medicinal plants should be an alternative for bone grafting and a source of raw material for the confection of new biomaterials prototypes (Ngueguim et al., 2012; Pinheiro Neto et al., 2015; Zhao et al., 2014) .
The species Alternanthera brasiliana and Fridericia platyphylla have the potential to induce bone healing. They have anti-inflammatory, antioxidant, healing, antineoplastic, and antimicrobial properties (Alencar Filho et al., 2020; da Rocha et al., 2017; de Araújo et al., 2021; de Menezes Filho, 2020; Nunes et al., 2020; Samudrala et al., 2015). Therefore, we report a novel study evaluating the effect of bone graft produced from the plant's extract of A. brasiliana and F. platyphylla on osteoregeneration after bone injury induced by radius fracture in rats. The relevance of the work relies on the use of biomaterials with previous ethnopharmacological properties and the potential they might have in bone healing.
2. Methods
2.1. Plants Materials
The leaves of the species A. brasiliana were collected at the Horto Medicinal Berta Lanjes de Morretes of the Federal University of Maranhão (UFMA). The species F. platyphylla was collected in the "Cerrado" of Maranhão (GPS - 7° 19' S 47° 20' 06" W). The crude hydroalcoholic extract was obtained with EtOH:H20 v/v in the proportion of 7:3 (70%). All the processing and chemical analyzes were carried out at the Laboratory of Chemistry of Natural Products (LPQN) and the Analytical Center of the Department of Chemistry at UFMA. The plant was collected in agreement with Brazilian laws concerning the protection of biodiversity (SisGen No. A451DE4)
2.2. Chemical constituents of the extracts
5 mg of A. brasiliana and F. platyphylla extract were solubilized in 1 mL of MeOH: H2O (1:1, v/v). The solution underwent solid phase extraction (SPE) using Phenomenex Strata C18 cartridges (500 mg stationary phase) previously activated with 5 mL of MeOH and equilibrated with 5 mL of MeOH: H2O (1:1, v/v ). Compounds were eluted from the cartridges using 1 mL of MeOH: H2O (1:1, v/v) to a final volume of 5 mL. The samples were then filtered through a 0.22 μm PTFE filter and dried. The dry extracts were diluted to 10 mg/mL in HPLC-grade solvent. Twenty μL aliquots were injected directly into the HPLC-PDA at 254 nm detection. A Shimadzu model HPLC system (Shimadzu Corp., Kyoto, Japan) consisted of a solvent injection module with a binary pump and a PDA detector (SPA-20A). The column used was a Luna 5 μm C18 100 A (150 μm x 4.6 μm). The solvents’ elutions were A (2% acetic acid in water) and B (methanol). Samples were eluted according to the following gradient: 5% to 60% B in 60 min. The flow was 1 mL/min, and the column temperature was 20 °C. The sample injection volume was 20 μL. Data were collected and processed using LC Solution software (Shimadzu).
2.3. Analysis by Liquid Chromatography Coupled with Mass Spectrometry
Crude extracts of A. brasiliana and F. platyphylla were analyzed by LC-MS on an LCQ Fleet mass spectrometer, Thermo Scientific®. The HPLC separation was performed using a Kinetex® C18 100 Å chromatographic column with 5µm pores and dimensions of 4.6 x 100 mm. The mobile phase consisted of water, 0.1% formic acid (A), and acetonitrile plus 0.1% formic acid (B), added to 0.1% formic acid through an exploratory gradient. The exploratory gradient started with B at 10% to 100% in 60 minutes, at a flow rate of 1.0 mL/min. The mass spectra were obtained on the Thermo Scientific® LCQ Fleet mass spectrometer, equipped with a device for direct sample insertion via continuous flow injection analysis (FIA). The sample was electrospray ionized (ESI), and the fragmentations were obtained in multiple stages (MSn) through an ion-trap interface (IT). The negative mode was chosen for the generation and analysis of all spectra. The experimental conditions were: capillary voltage -35 V, spray voltage -5000 V, capillary temperature at 350oC, carrier gas (N2), and flow 60 (arbitrary units). The acquisition range was m/z100-2000, with two or more sweep events performed simultaneously on the spectrum.
2.4. Production of bone grafts
The grafts were obtained through the formulation of 2% chitosan gel associated with 0.5% hydroalcoholic extract of both plants and Tween 80 (polysorbate). Soon after, they were placed in 0.1ml of the ready-made compound to fill the bone fracture of the experimental protocols.
2.5. Animals
Male rats of the species Rattus norvegicus, Wistar lineage, between 90 and 100 days old, weighing around 350g, were used. They were provided by the Central Animal House of the Federal University of Maranhão (UFMA). This project was approved by the Ethics Committee on the Use of Animals of UFMA (Proc. No. 23115.026168/2018-91) following CONCEA standards.
The animals were randomly divided into four experimental groups, with 12 animals in each group: Negative control group (NC) - Chitosan gel; Positive control group (PC) - bovine mineral bone graft (Lumina Bone®, fine powder 0.5); Graft Group - FRID - 0.5% F. platyphylla bone graft; Graft Group – (ABRA) - 0.5% A. brasiliana bone graft. The animals of each group were divided and evaluated at three different times, 30, 60, and 90 days after the fracture induction, with four animals included for each time.
2.6. Fracture Model
The animals were submitted to anesthesia through the association of Ketamine (40mg/kg) and Xylazine (2.5mg/kg) intraperitoneally. A complete, simple, transverse, 0.2 cm diaphyseal fracture was made in the radius using a drill machine. Next, 0.1 ml of the grafts (plant extracts) were immediately placed in the bone fracture (Pinheiro Neto et al., 2015)
In the immediate postoperative period, the animals received a single dose of analgesic (tramadol), antibiotic (penicillin), and anti-inflammatory (meloxican), followed by daily curative. Euthanasia was performed through anesthetic overdose (ketamine, 240 mg/kg and xylazine, 30 mg/kg) to remove and analyze the fractured bone segment.
2.7. Biochemical Parameters of Bone Markers
After 30-, 60- and 90-days post-surgery, blood was collected from the animals' abdominal aorta to measure bone alkaline phosphatase (BSAP) by subtracting BSAP from alkaline phosphatase through the heat denaturation technique using a Labtest® kit (Mccomb et al., 2013).
2.8. Radiographic Evaluation
Radiographs were taken with a 600 mA digital device (Siemens Healthineers®) at 30, 60, and 90 days after surgery in the craniocaudal position to assess the bone healing process. For that, fracture gap closure, the intensity of periosteal reaction, bone callus formation, and bone remodeling were evaluated according to a modified scale (Tawonsawatruk et al., 2014).
2.9. Histological Evaluation
The distal third of the radius was collected and fixed in 10% buffered formalin for 24 hours and decalcified in 10% nitric acid (Franco et al., 2001). Slides were prepared using hematoxylin-eosin (H&E) and Picrosirus Red staining to assess type I collagen at 30, 60, and 90 days. The formation of cartilaginous tissue and bone cells' periosteal reaction (osteoblasts, osteocytes, and osteoclasts) were evaluated semi-quantitatively using scores from 1 to 3. The presence or absence of alveolar cleft bone grafting was assessed qualitatively, between 0 and 1, for all scores.
2.10. Statistical analysis
The results of serological tests were expressed as mean ± standard error of means (SEM.) and submitted to the Cramer-Von Mises normality test. After, the data were submitted to Analysis of Variance (ANOVA), and Tukey's test compared the means. X-ray and bone histology assessment scores were expressed as median and compared using the Kruskal Wallis and Wilcoxon test. For all tests, the significance level was p < 0.05, using the Statistical Analysis System (SAS) Software.
3. Results
3.1. Phytochemical analysis of Fridericia platyphylla
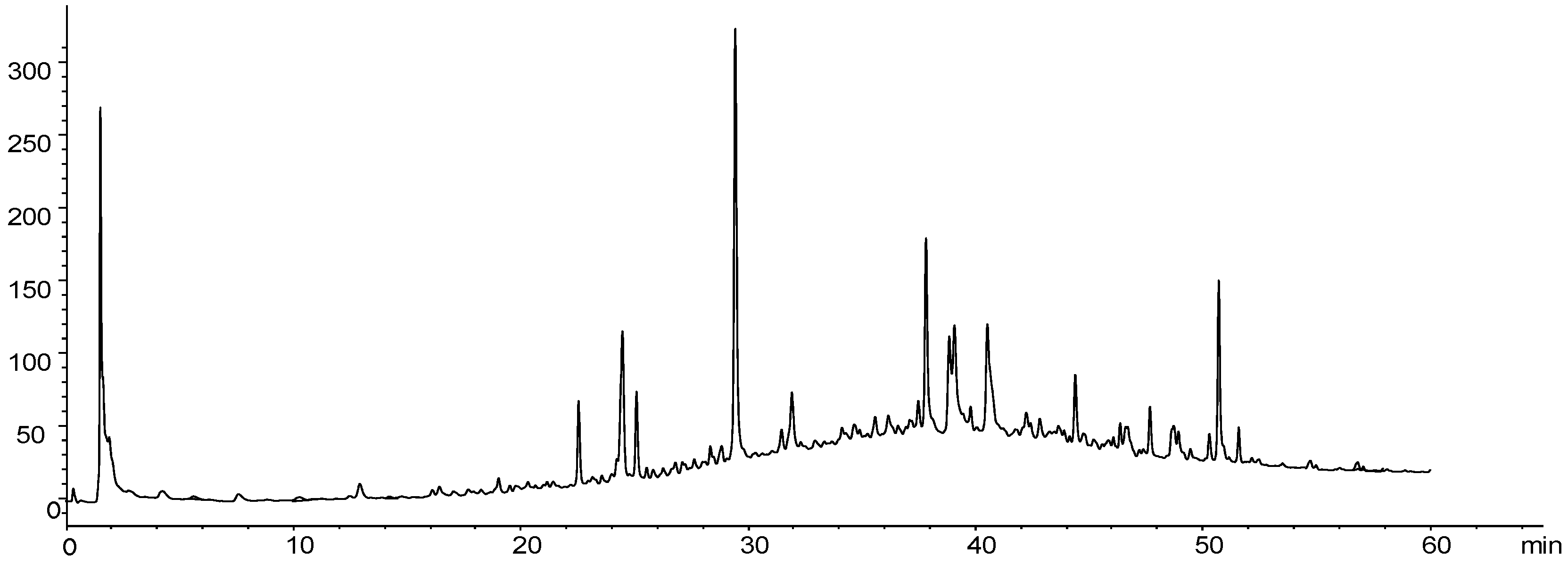
The chromatographic profiles are widely used in the study of plants, and it is possible to compare and suggest classes of compounds present in extracts using diode array detectors, ultraviolet, or mass spectrometry, for example.
Figure 1 presents the chromatographic profile of the hydroethanolic extract in 70% of the leaves of
Fridericia platyphylla; the result shows the presence of high and medium polarity compounds, being rich in phenolic compounds, mainly in flavonoids. Several peaks were observed in the chromatogram, mainly derived from phenolic compounds and, in approximately 25 minutes, the presence of a very extensive area compound. This majority peak in the chromatogram corresponds to the flavonol rutin, confirmed by mass spectrometry (
Figure 1).
The mass of 10 substances identified in the extract of
F. platyphylla leaves is seen in
Table 1. The analysis was performed using a direct flow injection device; the molecules were ionized by electrospray, and the fragmentations were obtained in multiple stages using an ion trap analyzer. Confirming the data obtained from the HPLC-PDA, most substances identified by the mass-charge ratio (m/z) belong to the flavonoid class.
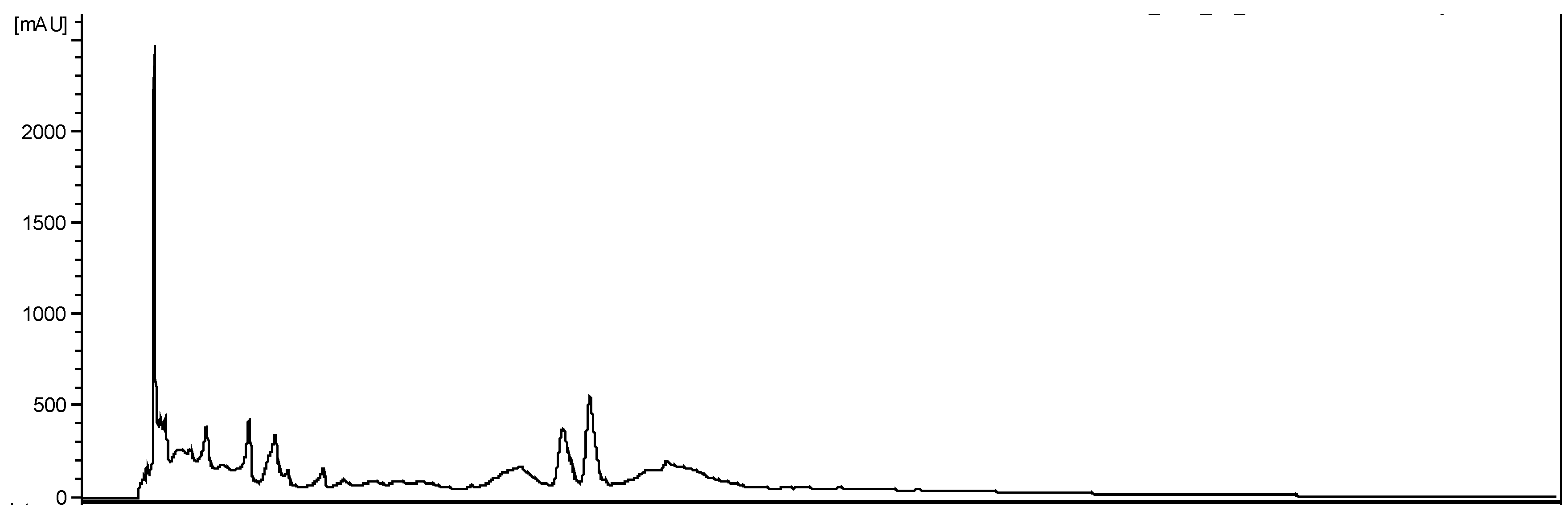
3.2. Phytochemical analysis of Alternanthera brasiliana
The hydroethanolic extract of
A. brasiliana showed high and medium polarity metabolites. The primary class of secondary metabolites was flavonoids (
Figure 3).
The six major substances identified in the extract of
A. brasiliana leaves are seen in
Table 2. The analysis was performed using liquid chromatography coupled to diode array detectors and a mass spectrometer. All compounds identified in the LC-MS analysis were flavonoids. The identification was compared in the literature and by fragmentation.
3.3. Bone-specific alkaline phosphatase (BSAP)
The serum concentrations of BSAP are seen in
Table 3. The ABRA group at 30 days was similar at 60 days but with higher values at 90 days (p<0.05). The FRID group, however, showed no difference between treatment periods, maintaining stable values throughout bone healing. BSAP for PC and NC were similar at 90 days.
3.5. Fracture Area Radiographic Analysis
There was no statistical difference in fracture line between the control groups studied (
Table 4). The FRID and ABRA groups had improved fracture closure lines at 90 days compared to the others, especially the FRID group, seen right after 60 days (p< 0.05;
Figure 4).
The periosteal reaction was similar between control groups (
Figure 4). A statistical difference was observed (p<0.05) for FRID and ABRA as bone healing progressed. The ABRA group, in particular, showed a higher periosteal reaction after 30 days (
Table 4).
At 30 days FRID group had higher scores, and at 60 and 90 days, the bone callus scores remained elevated for both the FRID and ABRA groups (p<0.05,
Table 4). At 90 days, a higher rate of bone remodeling was observed for the FRID and ABRA groups (
Figure 4).
The data from the upper table can be visually observed by radiographic evaluation in
Figure 4 below.
3.6. Histological evaluation of bone healing at different times
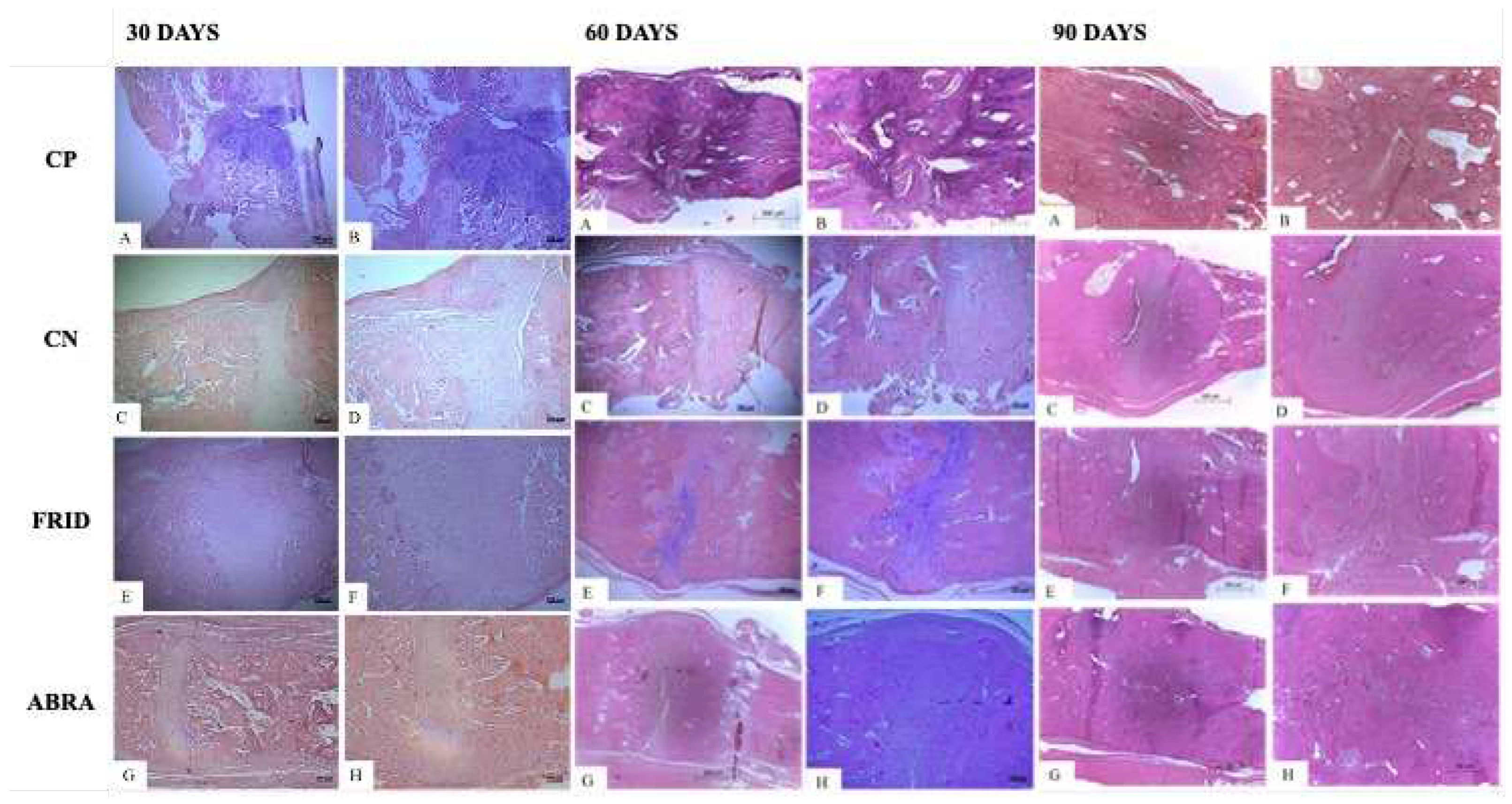
A predominance of alveolar cleft bone grafting for all groups was seen after 30 days, except for the ABRA graft, where a complete coaptation of the fracture line was observed (
Figure 5). Immature bone with cartilage was seen for all treatments, confirmed by a large number of hypertrophied chondrocytes. The exception was the ABRA group, with a lower amount of cartilage tissue and the presence of matrix osteoid instead of immature tissue (
Table 5).
All groups had similar tissue architecture with endochondral ossification and osteoblasts in higher numbers at 60 days (
Figure 5). The PC group had lower scores, presenting disorganized tissue healing. Invasion of mature bone tissue was observed, replacing the cartilaginous callus and a conspicuous endochondral healing pattern in the FRID and ABRA groups (
Figure 5). In addition, higher numbers of active osteoblasts for FRID and ABRA were observed (
Table 5).
At 90 days, mature bone tissue and bone remodeling with a higher number of osteoclasts were observed, mainly in the FRID, ABRA, and PC grafts (
Figure 5). Later, a mature osteoid matrix replaced the immature bone callus. On the other hand, the NC control group delayed the onset of endochondral healing and the appearance of cartilaginous cells and active osteoblasts (
Table 5).
Histological parameters for 30, 60 and 90 days after grafting are shown in
Figure 5.
3.7. Collagen Type Analysis
It was possible to estimate the level of bone maturation in the selected periods (30, 60, and 90 days). The yellow-to-orange and reddish birefringence pattern differentiated collagen type I from collagen type III, the last as greenish yellow. In the initial periods (30 days) of bone deposition, the percentage of reddish-yellow fibers detected was similar for all treatments, except for FRID, where a significant reduction in the percentage of type I collagen was observed (
Figure 6).
At 60 days, however, an increase in reddish-yellow birefringence was observed, indicating increased mature bone collagen (type I), except in the PC group (
Figure 6). Finally, at 90 days, the NC group had more immature collagen (greenish-yellow color.) In contrast, the other groups showed elevated type I collagen in the fracture foci (
Figure 6).
The photomicrograph of collagen deposition at 30, 60 and 90 days can be seen in
Figure 6.
4. Discussion
We developed the first study with biomaterials produced from crude hydroalcoholic extracts of Fridericia platyphylla (FRID) and Alternanthera brasiliana (ABRA) to induce healing in experimental fractures in rats. The extract of both leaves is rich in phenolic substances, predominantly flavonoids. After 90 days of graft insertion, we saw an increase in bone-specific alkaline phosphatase (BSAP) on the newly formed bone matrix, indicating the presence of active osteoblasts. Additionally, mature bone tissue and bone remodeling were present after 90 days for both plant extracts. An increase in osteoclasts and the reduction of immature bone callus replaced by mature osteoid matrix, together with the conspicuous presence of type I collagen, are strong indicators of successful bone regeneration using these plant extracts.
F. Platyphylla is rich in phenolic substances, and rutin is the most abundant secondary metabolite. Other studies highlighted the presence of flavonoids and the anti-inflammatory and antioxidant effects of rutin in this plant (da Rocha et al., 2017; de Menezes Filho, 2020). Therefore, rutin may play an essential in anti-inflammatory and antioxidant effects and bone graft regeneration (Macêdo et al., 2017). For A. brasiliana, phenolic compounds, including flavonols, are a rich source of natural bioactive compounds and antioxidant activity (Deladino et al., 2017). Flavonoids are known to affect bone metabolism directly, decrease bone resorption by osteoclasts, promote pro-osteoblastic cell differentiation and mineralization, and increase alkaline phosphatase activity (Souza et al., 2020; Welch and Hardcastle, 2014). Thus, the conspicuous presence of flavonoids in F. platyphylla and A. brasiliana grafts may have contributed to the bone regeneration process observed here.
The increase in BSAP was observed right after 30 days for ABRA. It may indicate the presence of active osteoblasts on the surface of the newly formed bone matrix, initiating the healing process via osteoclast stimulation, followed by active bone remodeling (Kikuta and Ishii, 2018). BSAP is in the plasma membrane of the osteoblasts and is secreted when the bone matrix is active, indicating bone formation rate (Lim et al., 2015). The enzyme controls mineralization inhibitors, increases the local concentration of phosphate ions, and acts as an ion transporter, improving bone callus and matrix formation (Golub and Boesze-Battaglia, 2007) .
4.1. Radiographic analysis
Callus and bone remodeling were observed in the ABRA and FRID grafts at 90 days. The reduced volume of calluses and the remodeling process indicate bone calluses' maturity, advanced healing, and restoration of pre-fracture properties. (Pinheiro Neto et al., 2015) observed similar findings on radius bone fractures in rabbits with an aqueous graft of Chenopodium ambrosioides. Acceleration of fracture healing, fracture line absence at 90 days, and bone remodeling are all indicators of complete bone healing. Plant material inducing the recovery of femoral fractures in rats was seen in other studies (Bigham-Sadegh et al., 2018; Estai et al., 2011; Florence et al., 2017).
4.2. Histological Analysis
ABRA graft showed no fracture cleft, the development of osteoid matrix, and cartilaginous callus formation at 30 days. These results align with BSAP increase to initiate the healing process and the cartilaginous callus formation. After, the callus progressed and successfully mineralized at 90 days, as evidenced by our results. According to (Carano and Filvaroff, 2003), the soft bone callus is formed after three weeks, followed by a decrease in osteoblastic activity. Later, the presence of a cartilaginous callus characterizes the first stage of bone regeneration. The ethanolic extract of Sambucus Williamsii notably accelerated fracture healing through an increase in serum alkaline phosphatase and osteoid matrix formation in the callus fracture (Yang et al., 2016).
At 60 days, the absence of fracture cleft and endochondral healing were remarkable, especially for the FRID and ABRA experimental groups. In addition, the FRID and ABRA grafts used here showed mature bone tissue, osteoclasts, and consequent bone remodeling at 90 days. Other plants are known for bone-regeneration potential. At 30 days Messina hemostopper, for example, proved to be an effective graft, increasing cell proliferation and bone formation (Aydin et al., 2022).The graft of R. communis was used to fill defects in the calvaria of rabbits. Bone regeneration and local production of cartilaginous tissue were evident (Souza et al., 2020).
The plant extracts demonstrated the significant presence of type I collagen. Type I collagen is the primary substance secreted by osteoblasts, a cell responsible for synthesizing organic bone matrix together with fibers associated with hydroxyapatite to give resistance to bone callus (Ziebart et al., 2018). Usually, in the early stages of fracture healing in rats, type III collagen predominates. However, with trabecular bone, a large amount of type I collagen is secreted (Maynes, 2012), as observed here.
The plants extract here showed promising results in osteoregeneration. Studies involving fractionation of the extracts should elucidate and deepen the knowledge of the mechanisms of action of these extracts in bone tissue healing.
5. Conclusions
The study demonstrated that the grafts of Fridericia Platyphylla (FRID) and Alternanthera Brasiliana (ABRA) produced early bone neoformation, improving bone repair. There was an increase in BSAP, involved in bone tissue regeneration process, confirmed by radiographic images and histology. The results suggest new perspectives for using these species as a graft, as they are biocompatible and stimulate cells, enzymes, and proteins, which are critical for bone healing.
6. Patents
The research resulted in an innovation product whose filing number is BR 10 2019 016399 2. This was submitted to the national patent agency INPI (National Institute of Industrial Property).
Author Contributions
CSM: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Validation; Visualization; Writing original draft; Review & editing. VFPN: Methodology; Validation; Visualization; Review & editing. JCSS: Methodology; Validation; Review & editing. JRSJ: Methodology; statistical analysis. CQRO: Conceptualization; Data curation; Formal analysis; Methodology; Validation; Review & editing. ECSP: Methodology; Review & editing. TKRS: Methodology; Review & editing. IRDC: Methodology; Validation; Review & editing. ACRB: Conceptualization; Methodology; Review & editing. FHEA: Methodology; Review & editing. MORB: Supervision; Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Validation; Visualization; Review & editing.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics and Research Committee of the Federal University of Maranhão (n° 23115.026168/2018-91, October 16, 2020).
Acknowledgments
We want to thank CAPES – Brazil for a doctorate scholarship to CSM.
Conflict ff Interest
There are no conflicts of interest to disclose.
References
- Alencar Filho, J.M.T. de, Teixeira, H.A.P., Sampaio, P.A., Pereira, E.C.V., Amariz, I.A. e., Rolim Neto, P.J., Rolim, L.A., Araújo, E.C. da C., 2020. Phytochemical analysis in Alternanthera brasiliana by LC-MS/MS and GC-MS. Nat Prod Res 34, 429–433. [CrossRef]
- Aydin, P., Akdeniz, S., Akcay, E., 2022. Histologic Evaluation of the Effect of Mecsina Hemostopper on Bone Regeneration for Critical-Size Defects. Int J Oral Maxillofac Implants 37, 771–777. [CrossRef]
- Bigham-Sadegh, A., Karimi, I., Hoseini, F., Oryan, A., Sharifi, S., Pakzad, A., 2018. Effects of honey and hydroxyapatite on bone healing in rats. Trauma Mon 23, e56119–e56120. [CrossRef]
- Bose, S., Sarkar, N., 2020. Natural Medicinal Compounds in Bone Tissue Engineering. Trends Biotechnol 38, 404–417. [CrossRef]
- Carano, R.A.D., Filvaroff, E.H., 2003. Angiogenesis and bone repair. Drug Discov Today. [CrossRef]
- da Rocha, C.Q., de-Faria, F.M., Marcourt, L., Ebrahimi, S.N., Kitano, B.T., Ghilardi, A.F., Luiz Ferreira, A., de Almeida, A.C.A., Dunder, R.J., Souza-Brito, A.R.M., Hamburger, M., Vilegas, W., Queiroz, E.F., Wolfender, J.L., 2017. Gastroprotective effects of hydroethanolic root extract of Arrabidaea brachypoda: Evidences of cytoprotection and isolation of unusual glycosylated polyphenols. Phytochemistry 135, 93–105. [CrossRef]
- de Araújo, A.D., de Barros Pimentel, M. do C., Santos, C. da S., da Silva, R.A., Cadena, P.G., da Silva, N.H., Gusmão, N.B., Sleifer, B.A., da Silva, M. da P.C., Henriques, A.T., 2021. Aqueous extract of fresh leaves from Alternanthera brasiliana (L.) Kuntze: chemical evaluation and antimycobacterial and anticandidal activities. Advances in Traditional Medicine 21, 1–11. [CrossRef]
- de Menezes Filho, A., 2020. Avaliação química, antifúngica e antioxidante do óleo essencial da flor de Fridericia platyphylla (Cham.) LG Lohmann. Scientia Naturalis 2.
- Deladino, L., Alvarez, I., de Ancos, B., Sánchez-Moreno, C., Molina-García, A.D., Schneider Teixeira, A., 2017. Betalains and phenolic compounds of leaves and stems of Alternanthera brasiliana and Alternanthera tenella. Food Research International 97, 240–249. [CrossRef]
- Estai, M.A., Suhaimi, F.H., Das, S., Fadzilah, F.M., Alhabshi, S.M.I., Shuid, A.N., Soelaiman, I.N., 2011. Piper sarmentosum enhances fracture healing in ovariectomized osteoporotic rats: A radiological study. Clinics 66, 865–872. [CrossRef]
- Florence, N.T., Huguette, S.T.S., Hubert, D.J., Raceline, G.K., Desire, D.D.P., Pierre, K., Theophile, D., 2017. Aqueous extract of Peperomia pellucida (L.) HBK accelerates fracture healing in Wistar rats. BMC Complement Altern Med 17, 188–190. [CrossRef]
- Franco, K.L., Borges, A.P.B., Vilória, M.I.V., Fernandes, E.S., Fehlberg, A.F., 2001. Pure synthetic hydroxyapatite, collagen associated synthetic hydroxyapatite and liposome associated synthetic hydroxyapatite as a bone substitute for defects in bone healing of dogs: transmitted light microscopy osteointegration aspects. Arq Bras Med Vet Zootec 53, 1–7. [CrossRef]
- Freitas, S.H. de, Dória, R.G.S., Mendonça, F. de S., Evêncio, J., Camargo, L.M. de, 2008. Aspecto radiológico de heteroenxerto ósseo cortical fragmentado na reparação de falhas ósseas em coelhos. Revista Brasileira de Ciência Veterinária 15, 107–110. [CrossRef]
- Giannoudis, P. v., Dinopoulos, H., Tsiridis, E., 2005. Bone substitutes: an update. Injury. [CrossRef]
- Golub, E.E., Boesze-Battaglia, K., 2007. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. [CrossRef]
- Kikuta, J., Ishii, M., 2018. Bone imaging: Osteoclast and osteoblast dynamics, in: Methods in Molecular Biology. pp. 1–9. [CrossRef]
- Lim, E.K., Keem, J.O., Yun, H.S., Jung, J., Chung, B.H., 2015. Smart nanoprobes for the detection of alkaline phosphatase activity during osteoblast differentiation. Chemical Communications 51, 547–558. [CrossRef]
- Macêdo, I.S. v., Cunha, K.G., Alves, A.T. v., Martins, R.M., Simões, M.O.S., 2017. Atividade antioxidante da rutina: uma revisão. BioFarm 13.
- Maynes, R., 2012. Structure and Function of Collagen Types, Structure and Function of Collagen Types. Elsevier. [CrossRef]
- Mccomb, R.B., Bowers Jr, G.N., Posen, S., 2013. Alkaline phosphatase. Springer Science & Business Media, 2013. Springer Science & Business Media.
- Ngueguim, F.T., Khan, M.P., Donfack, J.H., Siddiqui, J.A., Tewari, D., Nagar, G.K., Tiwari, S.C., Theophile, D., Maurya, R., Chattopadhyay, N., 2012. Evaluation of Cameroonian plants towards experimental bone regeneration. J Ethnopharmacol 141, 331–337. [CrossRef]
- Nunes, H.L., Tuttis, K., Serpeloni, J.M., Nascimento, J.R. do, da Rocha, C.Q., Silva, V.A.O., Lengert, A. van H., Reis, R.M., de Syllos Cólus, I.M., 2020. Characterization of the in vitro cytotoxic effects of brachydins isolated from Fridericia platyphylla in a prostate cancer cell line. Journal of Toxicology and Environmental Health - Part A: Current Issues 83, 547–558. [CrossRef]
- Pinheiro Neto, V.F., Ribeiro, R.M., Morais, C.S., Vieira, D.A., Guerra, P.C., Abreu-Silva, A.L., Silva Junior, J.R., Borges, M.O.R., Borges, A.C.R., 2015. Chenopodium ambroisioides in the repair of fractures in rabbits. International Journal of Pharmacology 11, 732–737. [CrossRef]
- Samudrala, P., Augustine, B., Kasala, E., Bodduluru, L., Barua, C., Lahkar, M., 2015. Evaluation of antitumor activity and antioxidant status of Alternanthera brasiliana against Ehrlich ascites carcinoma in Swiss albino mice. Pharmacognosy Res 7, 66–70. [CrossRef]
- Souza, J.M., Tuin, S.A., Robinson, A.G., de Souza, J.G.O., Bianchini, M.A., Miguez, P.A., 2020. Effect of flavonoid supplementation on alveolar bone healing-a randomized pilot trial. Dent J (Basel) 8, 86–86. [CrossRef]
- Tawonsawatruk, T., Hamilton, D.F., Simpson, A.H.R.W., 2014. Validation of the use of radiographic fracture-healing scores in a small animal model. Journal of Orthopaedic Research 32. [CrossRef]
- Welch, A.A., Hardcastle, A.C., 2014. The effects of flavonoids on bone. Curr Osteoporos Rep. [CrossRef]
- Wickramasinghe, M.L., Dias, G.J., Premadasa, K.M.G.P., 2022. A novel classification of bone graft materials. J Biomed Mater Res B Appl Biomater 110, 1724–1749. [CrossRef]
- Yang, B., Lin, X., Tan, J., She, X., Liu, Y., Kuang, H., 2016. Root bark of Sambucus Williamsii Hance promotes rat femoral fracture healing by the BMP-2/Runx2 signaling pathway. J Ethnopharmacol 191, 107–114. [CrossRef]
- Zhao, S., Baik, O.D., Choi, Y.J., Kim, S.M., 2014. Pretreatments for the Efficient Extraction of Bioactive Compounds from Plant-Based Biomaterials. Crit Rev Food Sci Nutr 54, 1283–1297. [CrossRef]
- Ziebart, J., Fan, S., Schulze, C., Kämmerer, P.W., Bader, R., Jonitz-Heincke, A., 2018. Effects of interfacial micromotions on vitality and differentiation of human osteoblasts. Bone Joint Res 7, 187–195. [CrossRef]
Figure 1.
HPLC-PDA (254 nm) chromatogram for the hydroalcoholic extract of Fridericia platyphylla leaves.
Figure 1.
HPLC-PDA (254 nm) chromatogram for the hydroalcoholic extract of Fridericia platyphylla leaves.
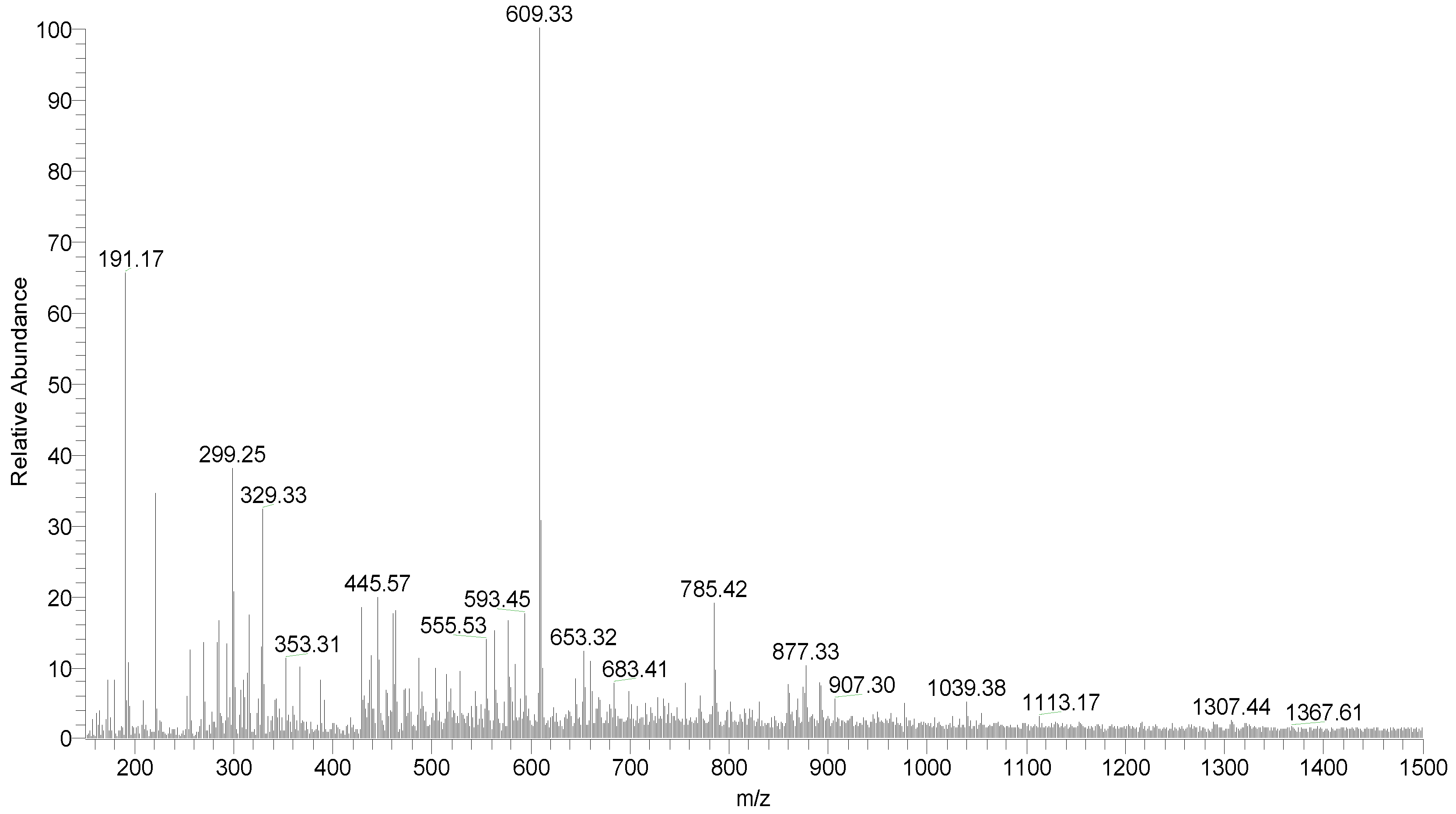
Figure 2.
Full-scan mass spectrum of hydroalcoholic extract of Fridericia platyphylla leaves evaluated in negative ion mode.
Figure 2.
Full-scan mass spectrum of hydroalcoholic extract of Fridericia platyphylla leaves evaluated in negative ion mode.
Figure 3.
HPLC-PDA (254 nm) chromatogram for the hydroalcoholic extract of A. brasiliana leaves.
Figure 3.
HPLC-PDA (254 nm) chromatogram for the hydroalcoholic extract of A. brasiliana leaves.
Figure 4.
Line 1: X-ray of rats grafted with chitosan gel (negative control, NC), bovine bone powder (positive control, PC), F. platyphylla graft (FRID), and A. brasiliana graft (ABRA) at 30 days. (a) and (b) – Evident fracture of the alveolar cleft with bone axis deviation, minimal periosteal reaction. (c) and (d) – Presence of fracture line with periosteal reaction and initiation of bone callus formation. Line 2: X-ray at 60 days. (a) – Presence of fracture line, minimal periosteal reaction, and beginning of bone callus formation. (b) - Deviation of the bone axis with fracture line (c) - Minimal fracture line, mild periosteal reaction, bone callus, and bone remodeling process. (d) – Fracture line present, absence of periosteal reaction, bone callus formation, and initial bone remodeling. Line 3: X-ray at 90 days. (a) –Fracture line, periosteal reaction, the beginning of bone callus formation, and bone remodeling. (b) – Absence of fracture line, absence of periosteal reaction, the beginning of bone callus formation, and bone remodeling. (c) – Absence of fracture line, absence of periosteal reaction, bone callus, and bone remodeling process present. (d) – Fracture line absent, absence of periosteal reaction, bone callus and bone remodeling process present.
Figure 4.
Line 1: X-ray of rats grafted with chitosan gel (negative control, NC), bovine bone powder (positive control, PC), F. platyphylla graft (FRID), and A. brasiliana graft (ABRA) at 30 days. (a) and (b) – Evident fracture of the alveolar cleft with bone axis deviation, minimal periosteal reaction. (c) and (d) – Presence of fracture line with periosteal reaction and initiation of bone callus formation. Line 2: X-ray at 60 days. (a) – Presence of fracture line, minimal periosteal reaction, and beginning of bone callus formation. (b) - Deviation of the bone axis with fracture line (c) - Minimal fracture line, mild periosteal reaction, bone callus, and bone remodeling process. (d) – Fracture line present, absence of periosteal reaction, bone callus formation, and initial bone remodeling. Line 3: X-ray at 90 days. (a) –Fracture line, periosteal reaction, the beginning of bone callus formation, and bone remodeling. (b) – Absence of fracture line, absence of periosteal reaction, the beginning of bone callus formation, and bone remodeling. (c) – Absence of fracture line, absence of periosteal reaction, bone callus, and bone remodeling process present. (d) – Fracture line absent, absence of periosteal reaction, bone callus and bone remodeling process present.
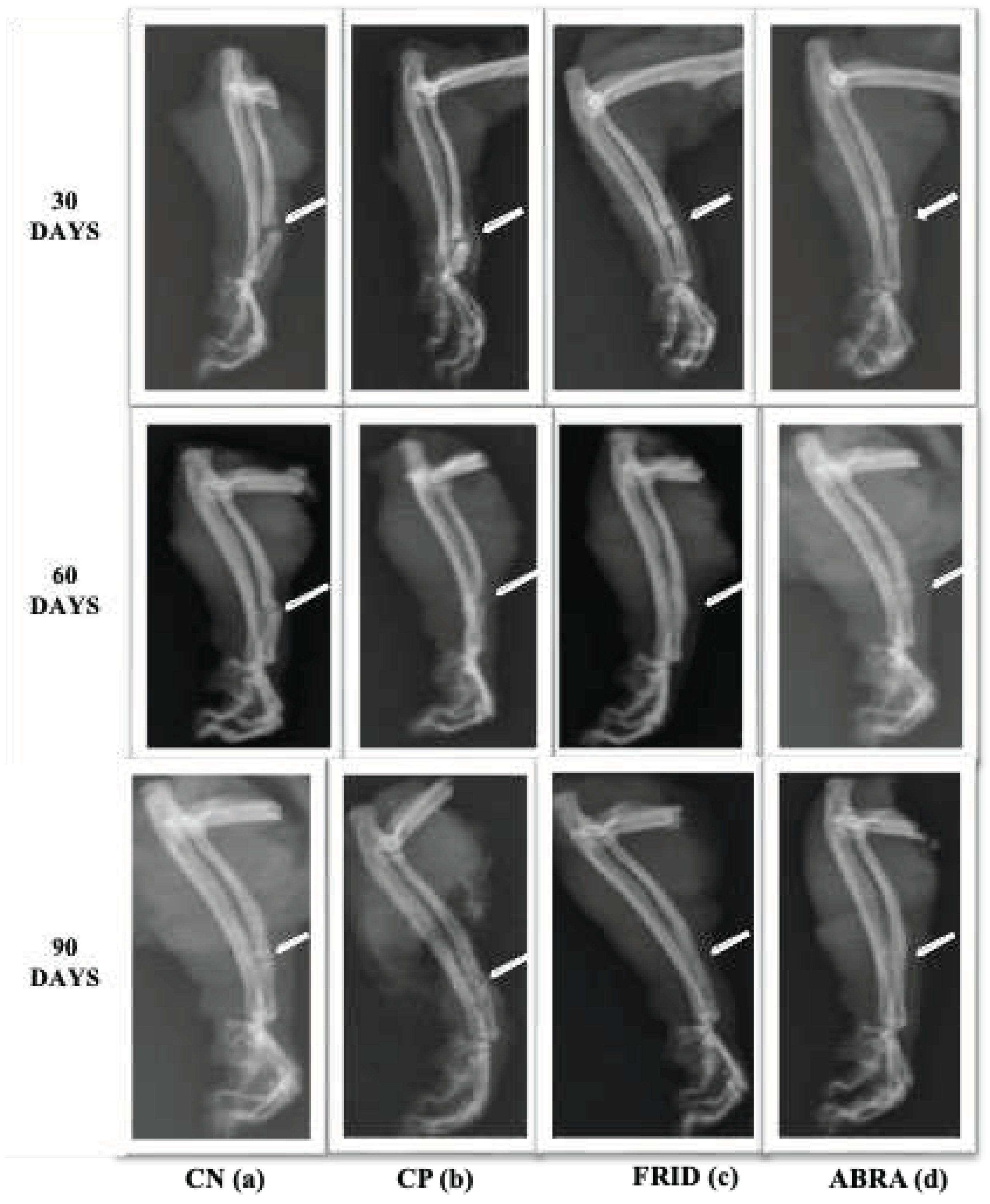
Figure 5.
Photomicrograph of the healing process at the end of 30, 60 and 90 days in rats grafted within rats grafted with: A and B: bovine bone powder (positive control, PC); C and D: chitosan gel (negative control, NC); E and F: F. platyphylla graft (FRID) and G and H: A. brasiliana graft (ABRA). H&E staining (5x and 10x). For 30 days: A to F – Presence of fracture alveolar cleft (a) and cartilaginous tissue (b), with massive presence of hypertrophied chondrocytes characteristic of the initial healing phase (b). G and H – Absence of alveolar cleft (a), smaller amount of cartilaginous tissue (b), presence of early osteoid matrix (c). For 60 days: A and B – Disorganized bone healing process (a). C and D – Presence of cartilaginous tissue. C to H – Endochondral healing pattern (b), Invasion of mature bone tissue (c). For 90 days: A, B, E, F, G, and H: Invasion of mature bone matrix and tissue remodeling. C and D: Late endochondral healing and moderate presence of cartilaginous tissue.
Figure 5.
Photomicrograph of the healing process at the end of 30, 60 and 90 days in rats grafted within rats grafted with: A and B: bovine bone powder (positive control, PC); C and D: chitosan gel (negative control, NC); E and F: F. platyphylla graft (FRID) and G and H: A. brasiliana graft (ABRA). H&E staining (5x and 10x). For 30 days: A to F – Presence of fracture alveolar cleft (a) and cartilaginous tissue (b), with massive presence of hypertrophied chondrocytes characteristic of the initial healing phase (b). G and H – Absence of alveolar cleft (a), smaller amount of cartilaginous tissue (b), presence of early osteoid matrix (c). For 60 days: A and B – Disorganized bone healing process (a). C and D – Presence of cartilaginous tissue. C to H – Endochondral healing pattern (b), Invasion of mature bone tissue (c). For 90 days: A, B, E, F, G, and H: Invasion of mature bone matrix and tissue remodeling. C and D: Late endochondral healing and moderate presence of cartilaginous tissue.
Figure 6.
Photomicrograph of collagen deposition at the end of 30, 60 and 90 days in grafted rats: (A - B): bovine bone powder (positive control, PC), (C - D): chitosan gel (negative control, NC), (E – F): F. platyphylla graft (FRID) and (G -H): A. brasiliana graft (ABRA). For 30 days: No dark-field imaging, left side dark-field imaging. B, D and H – Increased yellow-orange birefringence at the fracture focus, characteristic of type I collagen, with small greenish areas. F – Marked presence of greenish-yellow birefringence, characteristic of type III collagen. Picrosirius Red staining (2.5x). For 60 days: No dark-field imaging, left side dark-field imaging. D, F and H – Increased yellow-orange birefringence at the fracture focus, characteristic of type I collagen, with small greenish areas. B – Marked presence of greenish-yellow birefringence, characteristic of type III collagen. Picrosirius Red staining (2.5x). For 90 days: No dark-field imaging, left side dark-field imaging. B and D: Presence of greenish yellow birefringence in higher quantity, characteristic of type III collagen. F: Presence of yellow-orange birefringence, characteristic of type I collagen, and small greenish-yellow areas. H: Presence of yellow-orange birefringence, characteristic of type I collagen.
Figure 6.
Photomicrograph of collagen deposition at the end of 30, 60 and 90 days in grafted rats: (A - B): bovine bone powder (positive control, PC), (C - D): chitosan gel (negative control, NC), (E – F): F. platyphylla graft (FRID) and (G -H): A. brasiliana graft (ABRA). For 30 days: No dark-field imaging, left side dark-field imaging. B, D and H – Increased yellow-orange birefringence at the fracture focus, characteristic of type I collagen, with small greenish areas. F – Marked presence of greenish-yellow birefringence, characteristic of type III collagen. Picrosirius Red staining (2.5x). For 60 days: No dark-field imaging, left side dark-field imaging. D, F and H – Increased yellow-orange birefringence at the fracture focus, characteristic of type I collagen, with small greenish areas. B – Marked presence of greenish-yellow birefringence, characteristic of type III collagen. Picrosirius Red staining (2.5x). For 90 days: No dark-field imaging, left side dark-field imaging. B and D: Presence of greenish yellow birefringence in higher quantity, characteristic of type III collagen. F: Presence of yellow-orange birefringence, characteristic of type I collagen, and small greenish-yellow areas. H: Presence of yellow-orange birefringence, characteristic of type I collagen.
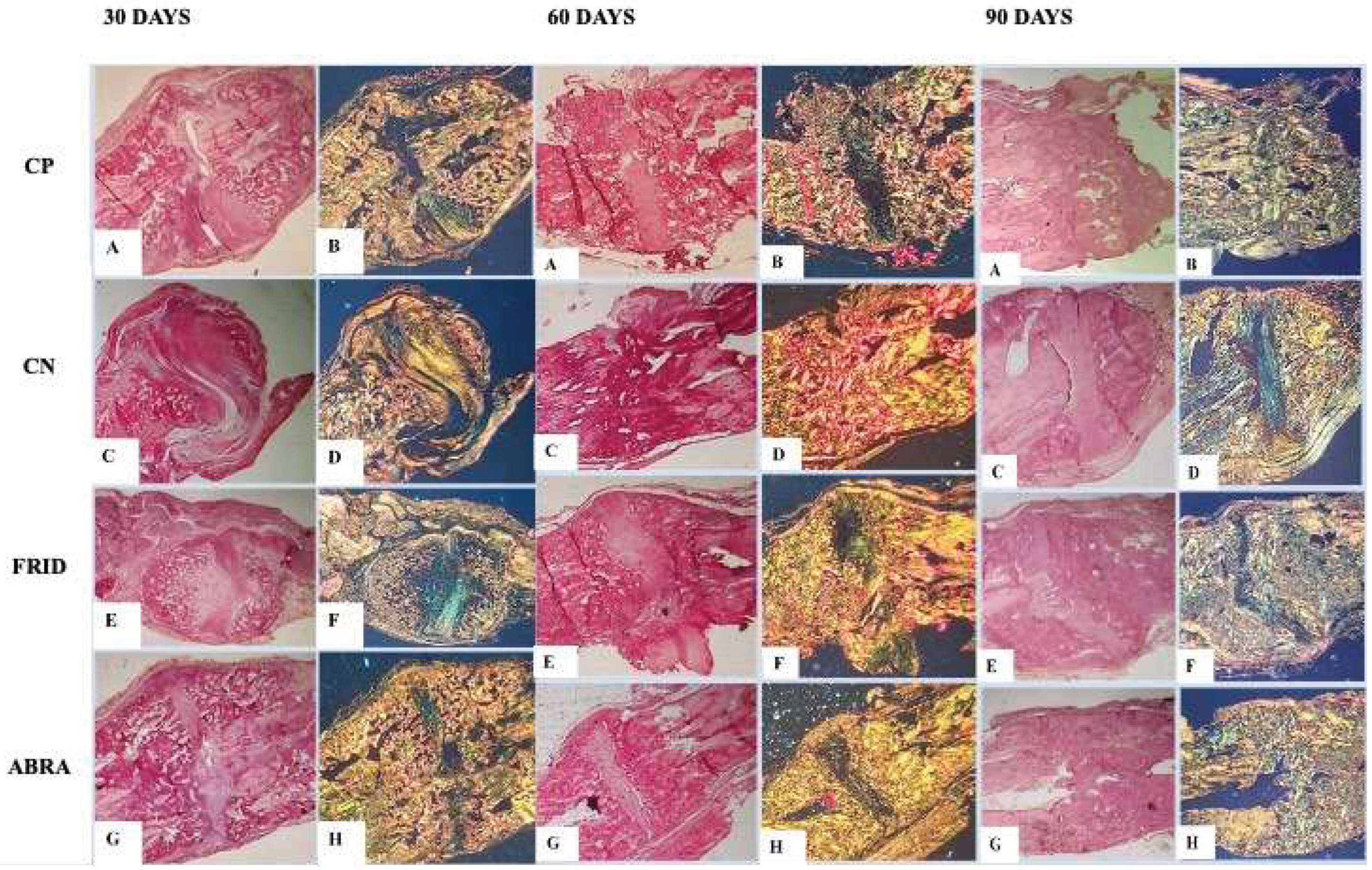
Table 1.
Identified substances from the principal fragments of the hydroalcoholic extract of F. platyphylla leaves obtained by LC-ESI-IT/MS and FIA-ESI-IT/MS. [M H]- = deprotonated molecule e MSn = multiple stages.
Table 1.
Identified substances from the principal fragments of the hydroalcoholic extract of F. platyphylla leaves obtained by LC-ESI-IT/MS and FIA-ESI-IT/MS. [M H]- = deprotonated molecule e MSn = multiple stages.
| Identified substances |
Molecular Formula (Molecular Weight) |
[M H]-
|
MSn
|
| Apigenin |
C15H10O5 (270)
|
269 |
151 |
| Luteolin |
C15H10O6 (286)
|
285 |
267; 243 |
| Hispiduline |
C16H12O6 (300)
|
299 |
284 |
| Cirsiliol |
C17H14O7 (330)
|
329 |
314 |
| 7-methoxypigenin-6-C-hexose |
C22H22O10 (446)
|
445 |
401; 269 |
| 7-methoxyluteolin-6-C-hexose |
C22H22O11 (462)
|
461 |
443 ; 371; 341; 313; 298 |
| Isoquercitrin |
C21H20O12 (464)
|
463 |
445; 301 |
| Apigenin-6-C-hexose, 8-C-hexose |
C27H30O15 (594)
|
593 |
575; 503; 473; 383; 485 |
| Rutin |
C27H30O16 (610)
|
609 |
447; 301 |
| Arabidoside |
C37H40O19 (786) |
785 |
609; 301
|
Table 2.
Substances identified in the hydroalcoholic extract of A. brasiliana leaves obtained by LC-ESI-IT/MS. [M H]- = deprotonated molecule e MSn = multiple stages
Table 2.
Substances identified in the hydroalcoholic extract of A. brasiliana leaves obtained by LC-ESI-IT/MS. [M H]- = deprotonated molecule e MSn = multiple stages
| Identified substances |
Molecular Formula (Molecular Weight) |
[M H]-
|
MSn
|
| Luteolin-8-C-rhamnosylglucoside |
C27H30O15 (594)
|
593 |
461;431;285 |
| 2''-O-rhamnosylvitexin |
C27H30O14 (578)
|
577 |
432; 414;341; 282 |
| Rutin |
C27H32O16 (610)
|
609 |
300;270 |
| 2''-O-rhamnosyl-6-C-glucosyl methylluteolin |
C28H32O15 (608)
|
607 |
487;462;299 |
| 2''-O-rhamnosilswertisine |
C28H32O14 (592)
|
591 |
471; 325 |
| Vitexin |
C21H20O10 (432)
|
431 |
341; 311; |
Table 3.
Bone-specific alkaline phosphatase (BSAP) values in rats grafted with bovine bone mineral powder (positive control, PC), chitosan gel (negative control, NC), A. brasiliana graft (ABRA), and graft of F. platyphylla (FRID) at 30, 60 and 90 days after treatments.
Table 3.
Bone-specific alkaline phosphatase (BSAP) values in rats grafted with bovine bone mineral powder (positive control, PC), chitosan gel (negative control, NC), A. brasiliana graft (ABRA), and graft of F. platyphylla (FRID) at 30, 60 and 90 days after treatments.
| Variable |
Treatment |
Time (Days) |
p-value |
| 30 |
60 |
90 |
| Bone-specific alkaline phosphatase (BSAP) |
PC |
187.7a±79.4 |
73.2b±19.3 |
241.9a±0 |
0.007 |
| NC |
166.2a±49.1 |
49.7b±16.6 |
192.5a±28.5 |
0.01 |
| FRID |
202.7±35.1 |
143.7±38.3 |
256.2±30.3 |
0.1 |
| ABRA |
107.7ab±28.4 |
39.7a±16.8 |
171.5b±25.5 |
0.04 |
| p-value |
0.27 |
0.18 |
0.30 |
|
Table 4.
Statistical analysis of radiological parameters established as scores for the healing process using bone grafts (FRID and ABRA) and control groups (PC and NC) at 30, 60, and 90 days after bone fracture. Modified from Tawonsawatruk et al., 2014) .
Table 4.
Statistical analysis of radiological parameters established as scores for the healing process using bone grafts (FRID and ABRA) and control groups (PC and NC) at 30, 60, and 90 days after bone fracture. Modified from Tawonsawatruk et al., 2014) .
| Variable |
GROUPS |
TIME |
p-value |
| 30 |
60 |
90 |
| FRACTURE LINE |
P C |
1 |
0.5 |
1 |
0.11 |
| NC |
1 |
1 |
0 |
0.36 |
| FRID |
1a
|
0.5 ab
|
0 b
|
0.02 |
| ABRA |
1 a
|
1 a
|
0 b
|
0.004 |
| p-value |
1 |
0.17 |
0.06 |
|
| PERIOSTEAL REACTION |
P C |
2 |
0.5 |
0 |
0.23 |
| NC |
0.5 |
1 |
0 |
0.73 |
| FRID |
1 a
|
0.5 ab
|
0 b
|
0.02 |
| ABRA |
2 a
|
0 b
|
0 b
|
0.01 |
| p-value |
0.13 |
0.82 |
0.06 |
|
| PRESENCE OF BONE CALLUS |
P C |
1 A
|
2.5 A
|
1.5 |
0.08 |
| NC |
0.5 A
|
1.5 B |
2.5 |
0.08 |
| FRID |
0.5 Aa
|
2 Ab
|
2.5 b
|
0.04 |
| ABRA |
2.5 Bab
|
2 Aa
|
3 b
|
0.02 |
| p-value |
0.04 |
0.02 |
0.06 |
|
| BONE REMODELING |
P C |
0 a
|
2 Ab
|
1 Ab
|
0.03 |
| NC |
0 a
|
1 Ba
|
3 Bb
|
0.04 |
| FRID |
0 a
|
2.5 Ab
|
3 Ab
|
0.01 |
| ABRA |
0 a
|
2 Ab
|
3 Ab
|
0.01 |
| p-value |
1 |
0.04 |
0.01 |
|
Table 5.
Statistical analysis of the histological parameters established as scores for the healing process of bone grafts (FRID and ABRA) and control groups (PC and NC) at 30, 60, and 90 days after bone fracture.
Table 5.
Statistical analysis of the histological parameters established as scores for the healing process of bone grafts (FRID and ABRA) and control groups (PC and NC) at 30, 60, and 90 days after bone fracture.
| Variable |
Treatment |
Time (Days) |
| 30 |
60 |
90 |
Alveolar cleft bone grafting
|
PC |
1.33 A±0.58 |
0.33 A±0.2 |
0.67 A±0.1 |
| NC |
1 Aa±0 |
1.33 Ba±0.3 |
0 Bb±0 |
| ABRA |
0.65 Ba±0.2 |
0.5 Aab±0.2 |
0 Bb±0 |
| FRID |
0.25 C±0.5 |
0.75 A±0.5 |
0.75 B±0.5 |
Osteoblast
|
PC |
1 Aa±0 |
1 Aa±0 |
1.6 Ab±0 |
| NC |
0 Ba±0 |
1 Ab±0 |
1.17 Ab±0.1 |
| ABRA |
2 C±0 |
2 B±0 |
2.25 B±0.3 |
| FRID |
2.25c ±0.2 |
2 B±0 |
2.5 B±0.2 |
Osteocyte
|
PC |
0 Aa±0 |
2 Ab±1.15 |
1.33 Ab±0.58 |
| NC |
0 Aa±0 |
1 Bb±0 |
1 Ab±0 |
| ABRA |
2 B±0.82 |
2 A±0.82 |
2.25 B±0.5 |
| FRID |
1.75Ba±0.5 |
1Bab±0 |
3 Bc±0 |
| Osteoclast |
PC |
0 a±0 |
1.13Ab±0.58 |
1.47Ab±0.58 |
| NC |
0 a±0 |
1 Ab±0.58 |
1.27 Ab±0,.58 |
| ABRA |
0 a±0 |
2 Bb±0 |
2.25 Bb±0.5 |
| FRID |
0 a±0 |
1.8 Bb±0.5 |
2.75 Bc±0.5 |
| Cartilage |
PC |
3 Aa±0 |
1.23Ab±0.15 |
1.73Ab±0.38 |
| |
NC |
3 Aa±0 |
2Bb±0 |
1.93 Ab±0.15 |
| ABRA |
1.5 Ba±0.58 |
1 Aa±0 |
0.25 Bb±0.1 |
| FRID |
2 Ba±0 |
1 Ab±0.22 |
0.85 Bb±0.2 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).