Submitted:
26 January 2023
Posted:
27 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
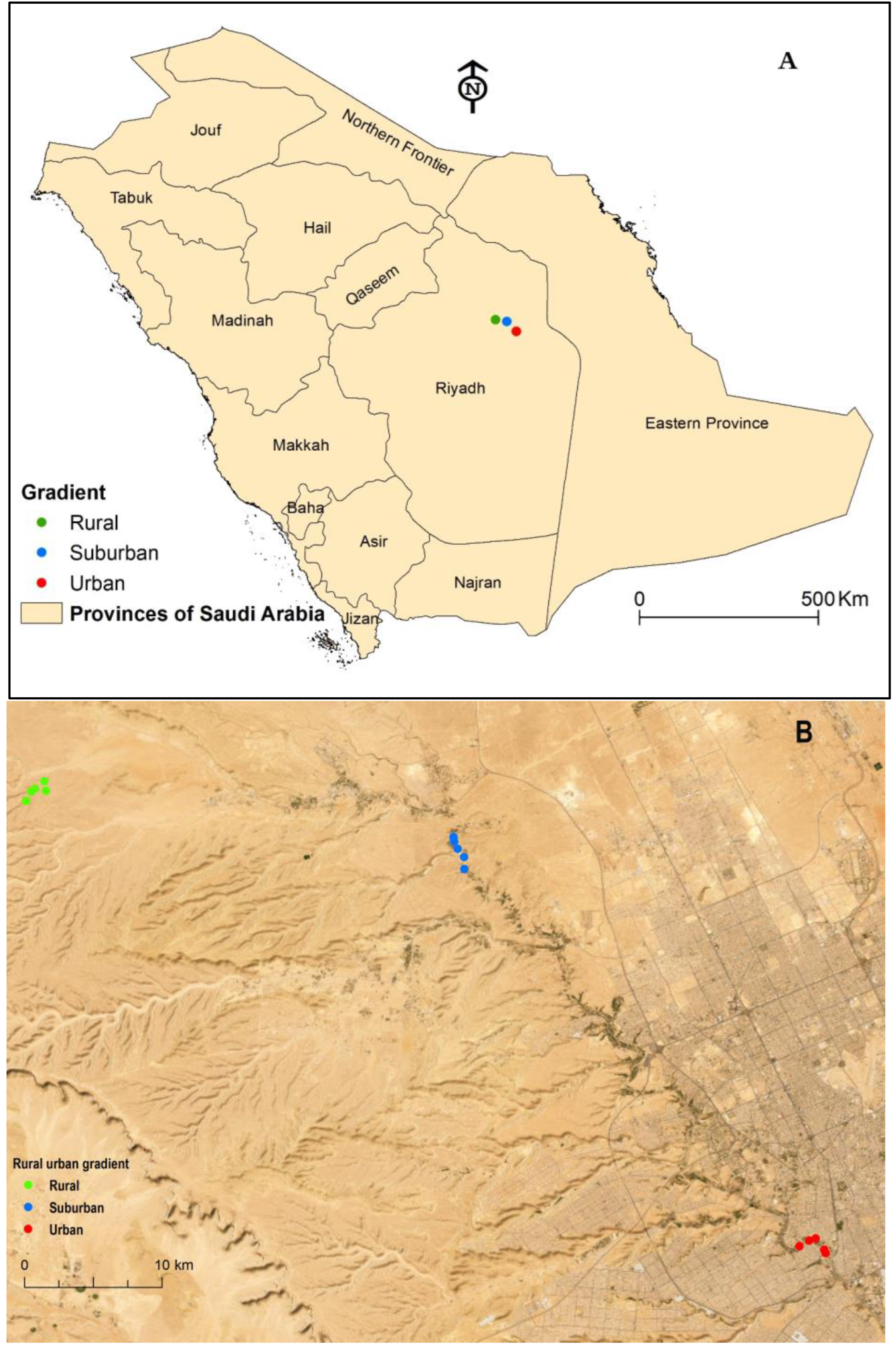
2.1. Study area
2.2. Sampling procedure
2.3. Data analysis
3. Results
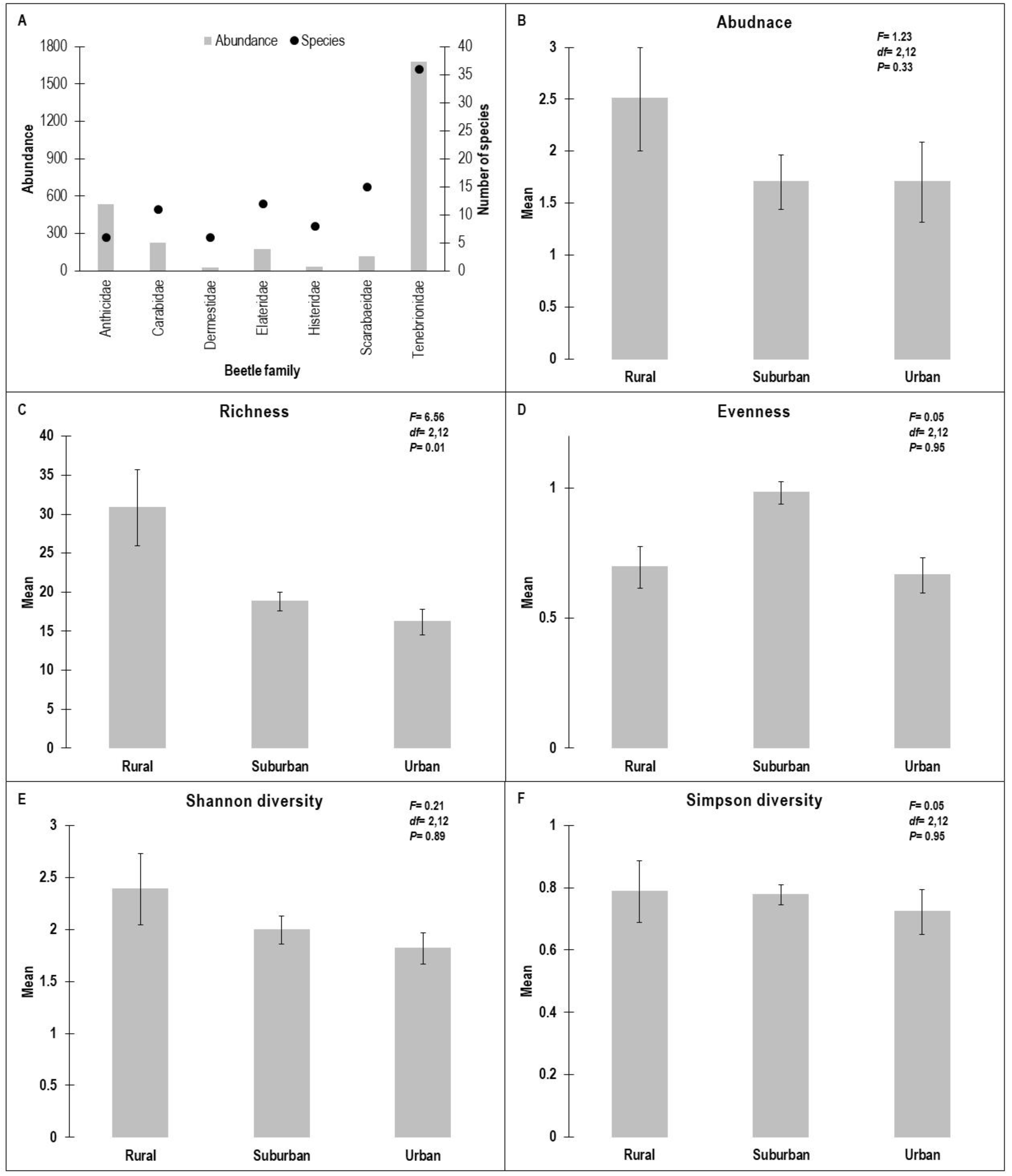
3.1. Beetle diversity
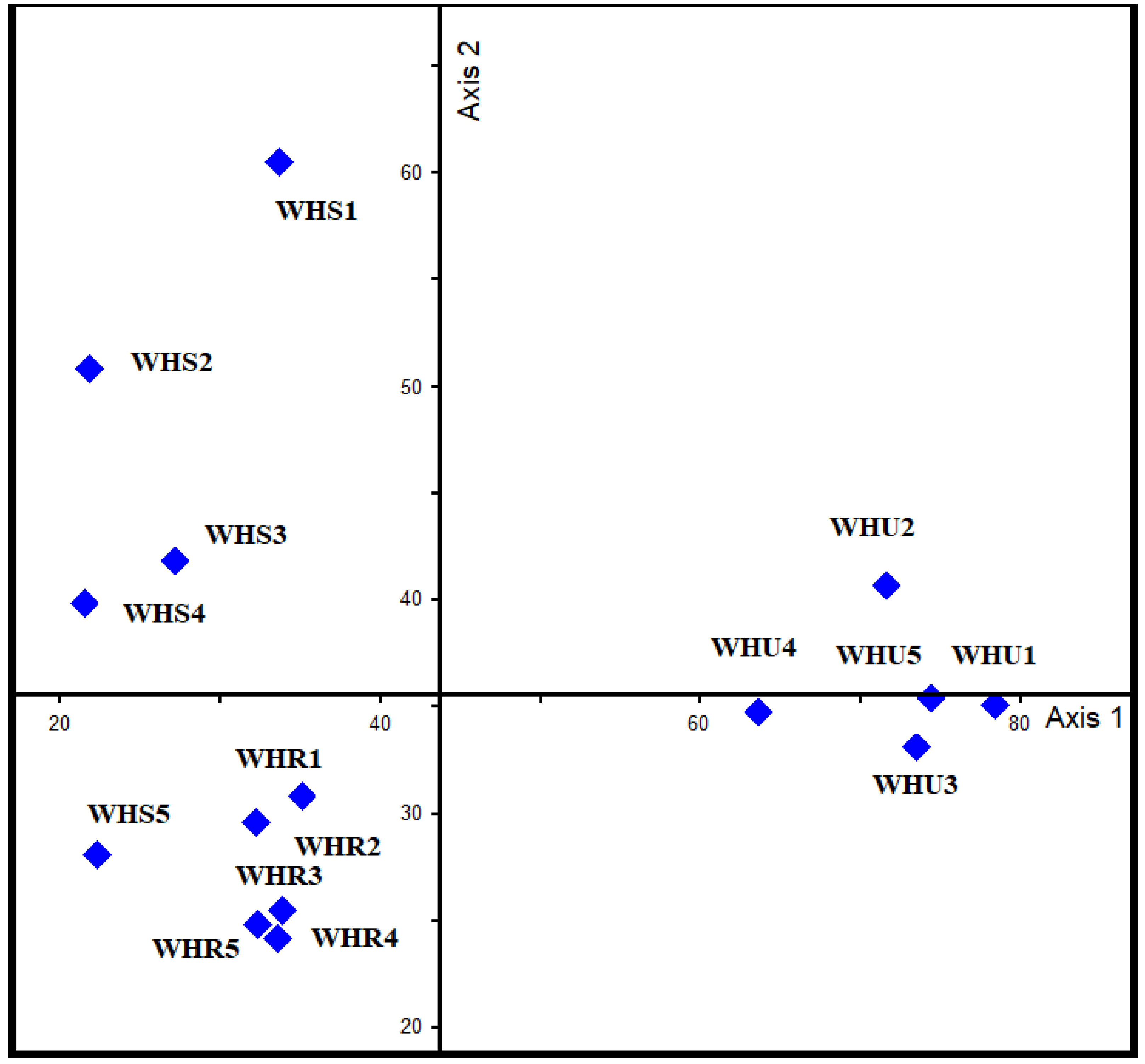
3.2. Species composition
3.3. Beetle indicator species
4. Discussion
4.1. Beetle diversity
4.2. Species composition
4.3. Beetle indicator species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antrop, M. Changing patterns in the urbanized countryside of Western Europe. Landsc. Ecol. 2000, 15, 257-270. [CrossRef]
- Magura, T.; Lövei, G.L. Consequences of Urban Living: Urbanization and Ground Beetles. Curr. Landsc. Ecol. Rep. 2021, 6, 9-21. [CrossRef]
- Chaolin, G. Urbanization. In International Encyclopedia of Human Geography, Kobayashi, A., Ed.; Elsevier: Oxford, 2020; Volume 14, pp. 141-153.
- UnitedNations. World Urbanization Prospects: The 2014 Revision: Highlights in P.D. . Available online: https://population.un.org/wup/publications/files/wup2014-report.pdf (accessed on April, 11).
- McDonnell, M.J.; Pickett, S.T.A.; Groffman, P.; Bohlen, P.; Pouyat, R.V.; Zipperer, W.C.; Parmelee, R.W.; Carreiro, M.M.; Medley, K. Ecosystem processes along an urban-to-rural gradient. Urban Ecosyst. 1997, 1, 21-36. [CrossRef]
- Piano, E.; De Wolf, K.; Bona, F.; Bonte, D.; Bowler, D.E.; Isaia, M.; Lens, L.; Merckx, T.; Mertens, D.; van Kerckvoorde, M.; et al. Urbanization drives community shifts towards thermophilic and dispersive species at local and landscape scales. Glob. Chang. Biol. 2017, 23, 2554-2564. [CrossRef]
- Kwon, T.-S.; Kim, Y.S.; Lee, S.W.; Park, Y.-S. Changes of soil arthropod communities in temperate forests over 10 years (1998–2007). J. Asia-Pacif. Entomol. 2016, 19, 181-189. [CrossRef]
- Parris, K.M. Ecology of Urban Environments, First ed.; Wiley-Blackwell: New York, United States, 2016; p. 240.
- Parris, K.M.; Schneider, A. Impacts of Traffic Noise and Traffic Volume on Birds of Roadside Habitats. Ecol. Soc. 2009, 14. [CrossRef]
- Rotholz, E.; Mandelik, Y. Roadside habitats: effects on diversity and composition of plant, arthropod, and small mammal communities. Biodivers. Conserv. 2013, 22, 1017-1031. [CrossRef]
- Small, E.C.; Sadler, J.P.; Telfer, M.G. Carabid beetle assemblages on urban derelict sites in Birmingham, UK. J. Insect Conserv. 2002, 6, 233-246. [CrossRef]
- Tóthmérész, B.; Máthé, I.; Balázs, E.; Magura, T. Responses of carabid beetles to urbanization in Transylvania (Romania). Landsc. Urban Plan. 2011, 101, 330-337. [CrossRef]
- Piano, E.; Bona, F.; Isaia, M. Urbanization drivers differentially affect ground arthropod assemblages in the city of Turin (NW-Italy). Urban Ecosyst. 2020, 23, 617-629. [CrossRef]
- Al-Homaidan, A.A.; Al-Ghanayem, A.A.; Alkhalifa, A.H. Green algae as bioindicators of heavy metal pollution in Wadi Hanifah Stream, Riyadh, Saudi Arabia. Int. j. water resour. 2011, 1, 10-15.
- Asabere, S.B.; Zeppenfeld, T.; Nketia, K.A.; Sauer, D. Urbanization Leads to Increases in pH, Carbonate, and Soil Organic Matter Stocks of Arable Soils of Kumasi, Ghana (West Africa). Front. Environ. Sci. 2018, 6. [CrossRef]
- Kark, S.; Iwaniuk, A.; Schalimtzek, A.; Banker, E. Living in the city: can anyone become an ‘urban exploiter'? J. Biogeogr. 2007, 34, 638-651. [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161-176. [CrossRef]
- Knop, E. Biotic homogenization of three insect groups due to urbanization. Glob. Chang. Biol. 2016, 22, 228-236. [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247-260. [CrossRef]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Nilon, C.H.; Pouyat, R.V.; Zipperer, W.C.; Costanza, R. Urban Ecological Systems: Linking Terrestrial Ecological, Physical, and Socioeconomic Components of Metropolitan Areas. Annu. Rev. Ecol. Syst. 2001, 32, 127-157. [CrossRef]
- New, T.R. Insect conservation and urban environments, First ed.; Springer Cham: Switzerland, 2015; p. 244.
- Niemelä, J.; Kotze, D.J. Carabid beetle assemblages along urban to rural gradients: A review. Landsc. Urban Plan. 2009, 92, 65-71. [CrossRef]
- Bouchard, P.; Grebennikov, V.V.; Smith, A.B.T.; Douglas, H. Biodiversity of Coleoptera. In Insect biodiversity: science and society, Foottit, R.G., Adler, P.H., Eds.; Wiley-Blackwell: West Sussex, UK, 2009; pp. 265-301.
- New, T.R. Beetles in conservation, First ed.; Springer: Dordrecht, Netherlands, 2010; p. 246.
- NCWCD. The national strategy for conservation of biodiversity in the Kingdom of Saudi Arabia. Available online: http://www.cbd.int/doc/world/sa/sa-nbsap-01-en.pdf. (accessed on 11 April 2022).
- Aldryhim, Y.N.; Mills, C.W.; Aldawood, A.S. Ecological distribution and seasonality of darkling beetles (Coleoptera: Tenebrionidae) in the central region of Saudi Arabia. J. Arid Environ. 1992, 23, 415-422. [CrossRef]
- Al-Dawood, A.S.; Alahmed, A.M.; Kheir, S.M.; Hussein, S.M. Population dynamics of sandflies (Diptera: Psychodidae) in Hanifah valley, Riyadh, Saudi Arabia. Pak. J. biol. Sci 2004, 7, 464-467. [CrossRef]
- Abdel-Dayem, M.S.; Sharaf, M.R.; Majer, J.D.; Al-Sadoon, M.K.; Aldawood, A.S.; Aldhafer, H.M.; Orabi, G.M. Ant diversity and composition patterns along the urbanization gradients in an arid city. J. Nat. Hist. 2021, 55, 2521-2547. [CrossRef]
- al-Asad, M.; Yavuz, Y. Wadi Hanifah Wetland. Available online: https://archive.archnet.org/sites/4458/publications/1206 (accessed on 23 Dec 2023).
- Samhouri, W. On Site Review Report: Wadi Hanifa Wetlands. Available online: http://archnet.org (accessed on 23 Dec 2022).
- Ter Braak, C.J. Canonical community ordination. Part I: Basic theory and linear methods. Ecoscience 1994, 1, 127-140. [CrossRef]
- Ter Braak, C.J. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167-1179. [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345-366. [CrossRef]
- Ramalho, C.E.; Hobbs, R.J. Time for a change: dynamic urban ecology. Trends Ecol. Evol. 2012, 27, 179-188. [CrossRef]
- Alhamid, A.A.; Alfayzi, S.A.; Hamadto, M.A. A sustainable water resources management plan for Wadi Hanifa in Saudi Arabia. J. King Saud Univ. Sci. 2007, 19, 209-221. [CrossRef]
- Al-Obaid, S.; Samraoui, B.; Thomas, J.; El-Serehy, H.A.; Alfarhan, A.H.; Schneider, W.; O’connell, M. An overview of wetlands of Saudi Arabia: Values, threats, and perspectives. Ambio 2017, 46, 98-108. [CrossRef]
- Faragalla, A.; Adam, E. Pitfall trapping of tenebrionid and carabid beetles (Coleoptera) in different habitats of the Central Region of Saudi Arabia. J. Appl. Entomol. 1985, 99, 466-471. [CrossRef]
- Abdel-Dayem, M.S.; Orabi, G.M.; Semida, F.M. Assessing the potential role of beetles as bioindicators in south Sinai, Egypt. In Proceedings of the Second International Conference of Entomological Society of Egypt, Cairo, Egypt, 8-11 December 2007.
- Piñero, F.; Tinaut, A.; Aguirre-Segura, A.; Miñano, J.; Lencina, J.; Ortiz-Sánchez, F.; Pérez-López, F. Terrestrial arthropod fauna of arid areas of SE Spain: Diversity, biogeography, and conservation. J. Arid Environ. 2011, 75, 1321-1332. [CrossRef]
- El Surtasi, E.I.; Semida, F.M.; Abdel-Dayem, M.S.; El Bokl, M.M. The threat of urbanization on beetle diversity in New Damietta City, Egypt. Nat. Sci. Sleep. 2012, 10, 15-23.
- Aldhafer, H., M., Abdel-Dayem, Mahmoud, S. ; Aldryhim, Y., N. ; Fadl, H., H. ; El-Torkey, A., M.; Elgharbawy, A., A. ; Setyaningrum, H.; . Diversity and composition of ground-dwelling beetle assemblages (Insecta: Coleoptera) in Rawdhat Khorim National Park, Kingdom of Saudi Arabia. J. Arid Environ. 2016, 127, 187-191. [CrossRef]
- Magura, T.; Tóthmérész, B.; Molnár, T. A species-level comparison of occurrence patterns in carabids along an urbanisation gradient. Landsc. Urban Plan. 2008, 86, 134-140. [CrossRef]
- Bennett, A.B.; Gratton, C. Measuring natural pest suppression at different spatial scales affects the importance of local variables. Environ. Entomol. 2012, 41, 1077-1085. [CrossRef]
- Ismail, O.K.; Bartholomew, A. Increased preference of darkling beetles Akis subtricostata Redtenbacher, 1850 and Trachyderma philistina Reiche and Saulcy, 1857 (Coleoptera, Tenebrionidae) for vegetation with increasing temperature. Bull. Iraq Nat. Hist. Mus. 2020, 16, 113-124. [CrossRef]
- Pickett, S.T.; Cadenasso, M.L.; Grove, J.M.; Boone, C.G.; Groffman, P.M.; Irwin, E.; Kaushal, S.S.; Marshall, V.; McGrath, B.P.; Nilon, C.H. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manage. 2011, 92, 331-362. [CrossRef]
- Qiu, J.; Yang, X.; Cao, B.; Chen, Z.; Li, Y. Effects of urbanization on regional extreme-temperature changes in China, 1960–2016. Sustainability 2020, 12, 6560. [CrossRef]
- Foster, C.W.; Kelly, C.; Rainey, J.J.; Holloway, G.J. Effects of urbanisation and landscape heterogeneity mediated by feeding guild and body size in a community of coprophilous beetles. Urban Ecosyst. 2020, 23, 1063-1077. [CrossRef]
- Park, J.-W.; Lee, C.M. Response of Ground Beetle (Coleoptera: Carabidae) Communities to Effect of Urbanization in Southern Osaka: An Analytical Approach Using GIS. Sustainability 2021, 13, 7134. [CrossRef]
- Magura, T.; Horváth, R.; Tóthmérész, B. Effects of urbanization on ground-dwelling spiders in forest patches, in Hungary. Landsc. Ecol. 2010, 25, 621-629. [CrossRef]
- Gaublomme, E.; Hendrickx, F.; Dhuyvetter, H.; Desender, K. The effects of forest patch size and matrix type on changes in carabid beetle assemblages in an urbanized landscape. Biol. Conserv. 2008, 141, 2585-2596. [CrossRef]
- Philpott, S.M.; Cotton, J.; Bichier, P.; Friedrich, R.L.; Moorhead, L.C.; Uno, S.; Valdez, M. Local and landscape drivers of arthropod abundance, richness, and trophic composition in urban habitats. Urban Ecosyst. 2014, 17, 513-532. [CrossRef]
- Vergnes, A.; Pellissier, V.; Lemperiere, G.; Rollard, C.; Clergeau, P. Urban densification causes the decline of ground-dwelling arthropods. Biodivers. Conserv. 2014, 23, 1859-1877. [CrossRef]
- Egerer, M.H.; Arel, C.; Otoshi, M.D.; Quistberg, R.D.; Bichier, P.; Philpott, S.M. Urban arthropods respond variably to changes in landscape context and spatial scale. J. Urban Ecol. 2017, 3, jux001. [CrossRef]
- Chiu, M.-C.; Chou, T.-Y.; Kuo, M.-H. Seasonal patterns of stream macroinvertebrate communities in response to anthropogenic stressors in monsoonal Taiwan. J. Asia-Pacif. Entomol. 2018, 21, 423-429. [CrossRef]
- Eversham, B.C.; Roy, D.B.; Telfer, M.G. Urban, industrial and other manmade sites as analogues of natural habitats for Carabidae. In Proceedings of the Ann. Zool. Fenn., 1996; pp. 149-156.
- Magura, T.; Tóthmérész, B.; Molnár, T. Changes in carabid beetle assemblages along an urbanisation gradient in the city of Debrecen, Hungary. Landsc. Ecol. 2004, 19, 747-759. [CrossRef]
- Zolotarev, M.P.; Belskaya, E.A. Ground-dwelling invertebrates in a large industrial city: Differentiation of recreation and urbanization effects. Contemp. Probl. Ecol. 2015, 8, 83-90. [CrossRef]
- Philpott, S.M.; Albuquerque, S.; Bichier, P.; Cohen, H.; Egerer, M.H.; Kirk, C.; Will, K.W. Local and Landscape Drivers of Carabid Activity, Species Richness, and Traits in Urban Gardens in Coastal California. Insects 2019, 10, 112. [CrossRef]
- Castro, A.V.; Porrini, D.P.; Lupo, S.; Cicchino, A.C. Minimal stories in Southeast Buenos Aires grasslands: carabid beetle biodiversity throughout an urban-rural gradient. Urban Ecosyst. 2020, 23, 331-343. [CrossRef]
- Pywell, R.F.; James, K.L.; Herbert, I.; Meek, W.R.; Carvell, C.; Bell, D.; Sparks, T.H. Determinants of overwintering habitat quality for beetles and spiders on arable farmland. Biol. Conserv. 2005, 123, 79-90. [CrossRef]
- Deichsel, R. Species change in an urban setting—ground and rove beetles (Coleoptera: Carabidae and Staphylinidae) in Berlin. Urban Ecosyst. 2006, 9, 161-178. [CrossRef]
- Elek, Z.; Lövei, G.L. Patterns in ground beetle (Coleoptera: Carabidae) assemblages along an urbanisation gradient in Denmark. Acta Oecol. 2007, 32, 104-111. [CrossRef]
- Do, Y.; Lineman, M. Effects of agricultural abandonment on carabid beetles in paddy fields. Balt. J. Coleopterol. 2012, 12, 65-75.
- Fusco, N.A.; Zhao, A.; Munshi-South, J. Urban forests sustain diverse carrion beetle assemblages in the New York City metropolitan area. PeerJ 2017, 5, e3088. [CrossRef]
- Work, T.T.; Shorthouse, D.P.; Spence, J.R.; Volney, W.J.A.; Langor, D. Stand composition and structure of the boreal mixedwood and epigaeic arthropods of the Ecosystem Management Emulating Natural Disturbance (EMEND) landbase in northwestern Alberta. Can. J. For. Res. 2004, 34, 417-430. [CrossRef]
- Orabi, G.M.; Semida, F.M.; Abdel-Dayem, M.S.; Sharaf, M.R.; Zalat, S.M. Diversity patterns of ants along an elevation gradient at St. Catherine Protectorate, South Sinai, Egypt. Zool. Middle East 2011, 54, 101-112. [CrossRef]
- Liu, J.-L.; Li, F.-R.; Liu, C.-A.; Liu, Q.-J. Influences of shrub vegetation on distribution and diversity of a ground beetle community in a Gobi desert ecosystem. Biodivers. Conserv. 2012, 21, 2601-2619. [CrossRef]
- Kosewska, A.; Nietupski, M.; Damszel, M. Role of urban forests as a source of diversity of carabids (Coleoptera: Carabidae) in urbanised areas. Balt. J. Coleopterol. 2013, 13, 27-39.
- Belskaya, E.; Zolotarev, M.; Zinovyev, E. Carabidae assemblages in pine forests with different recreation regimes within and outside a megalopolis. Urban Ecosyst. 2020, 23, 27-38. [CrossRef]
- Niemelä, J.; Haila, Y.; Halme, E.; Lahti, T.; Pajunen, T.; Punttila, P. The distribution of carabid beetles in fragments of old coniferous taiga and adjacent managed forest. Ann. Zool. Fenn. 1988, 25, 107-119.
- Nietupski, M. Assemblages of epigeic Carabidae [Col.] in a peatbog nature reserve situated in an urban area. Pol. J. Nat. Sci. 2008, 23, 611-623. [CrossRef]
- Seibold, S.; Bässler, C.; Baldrian, P.; Reinhard, L.; Thorn, S.; Ulyshen, M.D.; Weiß, I.; Müller, J. Dead-wood addition promotes non-saproxylic epigeal arthropods but effects are mediated by canopy openness. Biol. Conserv. 2016, 204, 181-188. [CrossRef]
- Ouchtati, N.; Brandmayr, P.; Saouache, Y. A comparative analysis of the community of carabid beetles associated with two native xerophytic shrub species (Atriplex halimus L. and Artemisia herba alba L.) in the semi-arid zone of Algeria. Afr. Entomol. 2020, 28, 164-174, 111. [CrossRef]
- Ariza, G.M.; Jácome, J.; Esquivel, H.E.; Kotze, D.J. Early successional dynamics of ground beetles (Coleoptera, Carabidae) in the tropical dry forest ecosystem in Colombia. ZooKeys 2021, 1044, 877-906. [CrossRef]
- Langor, D.; Pohl, G.; Hammond, H. A coarse-filter approach to conserving arthropod biodiversity in Canadian forests. Arthropods of Canadian Forests Newsletter 2006, 2, 9-13.
- Bergeron, C.; Spence, J.; Volney, J. Landscape patterns of species-level association between ground-beetles and overstory trees in boreal forests of western Canada (Coleoptera, Carabidae). ZooKeys 2011, 147, 577-600. [CrossRef]
- Sanderson, R.A.; Rushton, S.P.; Cherrill, A.; Byrne, J.P. Soil, Vegetation and Space: An Analysis of Their Effects on the Invertebrate Communities of a Moorland in North-East England. J. Appl. Ecol. 1995, 32, 506-518, doi:doi.org/10.2307/2404648.
- Ings, T.; Hartley, S. The effect of habitat structure on carabid communities during the regeneration of a native Scottish forest. For. Ecol. Manage. 1999, 119, 123-136. [CrossRef]
- Niemelä, J.K.; Spence, J.R. Distribution of forest dwelling carabids (Coleoptera): spatial scale and the concept of communities. Ecography 1994, 17, 166-175. [CrossRef]
- Koivula, M.; Punttila, P.; Haila, Y.; Niemelä, J. Leaf Litter and the Small-Scale Distribution of Carabid Beetles (Coleoptera, Carabidae) in the Boreal Forest. Ecography 1999, 22, 424-435. [CrossRef]
- Hopp, P.W.; Ottermanns, R.; Caron, E.; Meyer, S.; ROß-NICKOLL, M. Recovery of litter inhabiting beetle assemblages during forest regeneration in the Atlantic forest of Southern Brazil. Insect. Syst. Divers. 2010, 3, 103-113. [CrossRef]
- Guillemain, M.; Loreau, M.; Daufresne, T. Relationships beetween the regional distribution of carabid beetles (Coleoptera, Carabidae) and the abundance of their potential prey. Acta Oecol. 1997, 18, 465-483. [CrossRef]
- Molnár, T.; Magura, T.; Tóthmérész, B.; Elek, Z. Ground beetles (Carabidae) and edge effect in oak-hornbeam forest and grassland transects. Eur. J. Soil Biol. 2001, 37, 297-300. [CrossRef]
- Fuller, R.J.; Oliver, T.H.; Leather, S.R. Forest management effects on carabid beetle communities in coniferous and broadleaved forests: implications for conservation. Insect. Syst. Divers. 2008, 1, 242-252. [CrossRef]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623-1627. [CrossRef]
- Lal, R.; Augustin, B.J. Carbon Sequestration in Urban Ecosystems. In Proceedings of the Springer Netherlands, 2012.
- Hemerik, L.; Brussaard, L. Diversity of soil macro-invertebrates in grasslands under restoration succession. Eur. J. Soil Biol. 2002, 38, 145-150. [CrossRef]
- Magdoff, F.; Weil, R.R. Soil organic matter in sustainable agriculture, First ed.; CRC press: Boca Raton, Florida, USA, 2004; p. 412.
- Brock, C.; Oberholzer, H.R.; Franko, U. Soil organic matter balance as a practical tool for environmental impact assessment and management support in arable farming. Eur. J. Soil Sci. 2017, 68, 951-952. [CrossRef]
- Lundholm, J.T.; Marlin, A. Habitat origins and microhabitat preferences of urban plant species. Urban Ecosyst. 2006, 9, 139-159. [CrossRef]
- Sadler, J.; Small, E.C.; Fiszpan, H.A.; Telfer, M.; Niemela, J. Investigating environmental variation and landscape characteristics of an urban-rural gradient using woodland carabid assemblages. J. Biogeogr. 2006, 33, 1126-1138. [CrossRef]
- Gagne, S.A.; Fahrig, L. Do birds and beetles show similar responses to urbanization? Ecol. Appl. 2011, 21, 2297-2312. [CrossRef]
- Hartley, J.D.; Koivula, J.M.; Spence, R.J.; Pelletier, R.; Ball, E.G. Effects of urbanization on ground beetle assemblages (Coleoptera, Carabidae) of grassland habitats in western Canada. Ecography 2007, 30, 673-684. [CrossRef]
| Environmental variable | Rural | Suburban | Urban |
|---|---|---|---|
| Elevation range (m) | 800‒820 | 690-710 | 570-590 |
| Average Buildings (area in km2) | 0 | 0.0218 | 0.2564 |
| Average Road & Asphalt (length in km) | 0 | 3.728 | 11.06 |
| Average Pavement (length in km) | 0 | 1.094 | 2.242 |
| Average pH | 8.618 | 8.562 | 8.416 |
| Average EC (µS/cm) | 159.10 | 493.42 | 1196.30 |
| Average Soil Organic Carbon (SOC) | 0.383 | 0.311 | 0.883 |
| Average Soil Organic Matter (SOM) | 0.659 | 0.534 | 1.521 |
| Average clay | 24 | 21.5 | 23.5 |
| Average silt | 14.5 | 10.5 | 14.5 |
| Average sand | 61.5 | 68 | 62 |
| Texture | Sandy Clay Loam |
Sandy Clay Loam |
Sandy Clay Loam |
| Average bar ground percentage | 34 | 26 | 20 |
| Average plant cover percentage | 66 | 74 | 79 |
| Average litter cover percentage | 1.36 | 0.5 | 11 |
| Average litter depth | 0.31 | 0.05 | 0.01 |
| Average log percentage | 7.94 | 32.54 | 28.08 |
| Eigenvalue | F | P | Weighted correlation matrix | ||||
|---|---|---|---|---|---|---|---|
| Axis 1 | Axis 2 | Total | Axis 1 | Axis 2 | |||
| Urbanization level | 0.638 | 0.356 | 2.169 | ||||
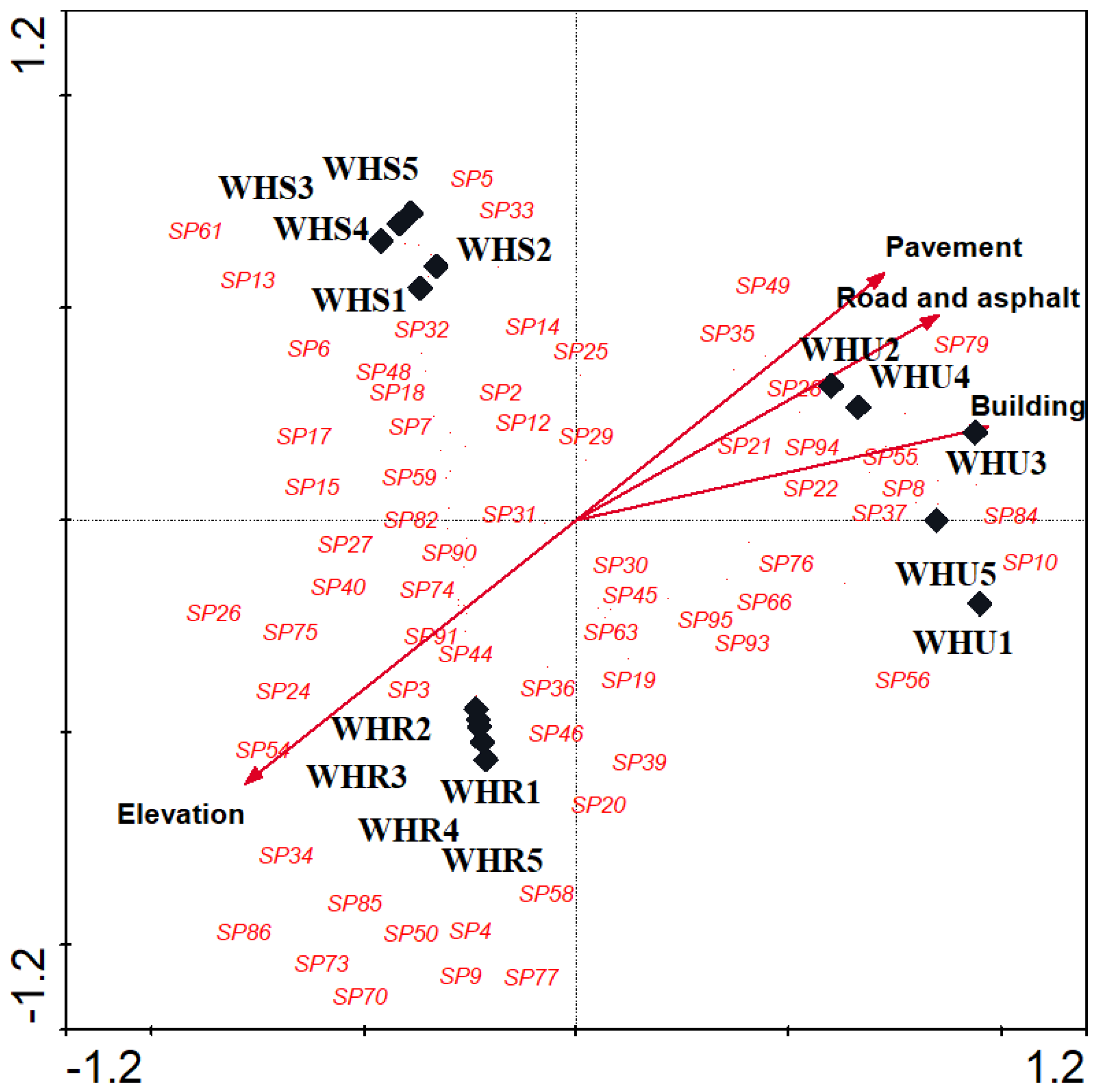
| Buildings | 5.18 | 0.0020 | 0.9589 | 0.2135 | |||
| Pavements | 1.21 | 0.270 | 0.7182 | 0.5657 | |||
| Roads and asphalt | 0.62 | 0.884 | 0.8437 | 0.4683 | |||
| Elevation | 3.75 | 0.0020 | -0.7707 | -0.6028 | |||
| Vegetation cover | 0.459 | 0.314 | 2.169 | ||||
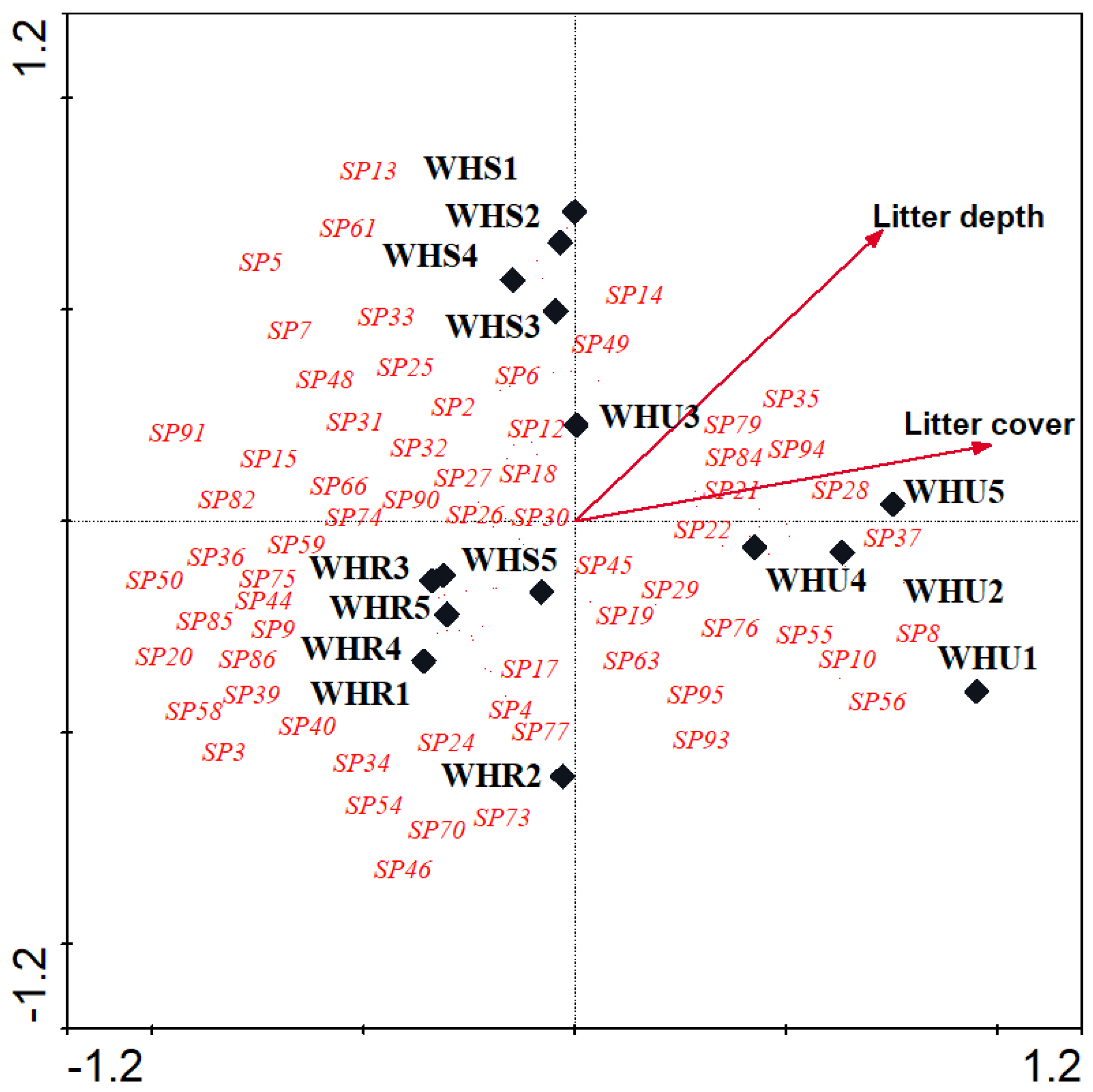
| Litter cover | 3.45 | 0.0020 | 0.8676 | 0.1654 | |||
| Litter depth | 2.74 | 0.0020 | 0.6417 | 0.6300 | |||
| Flora | 0.649 | 0.366 | 2.195 | ||||
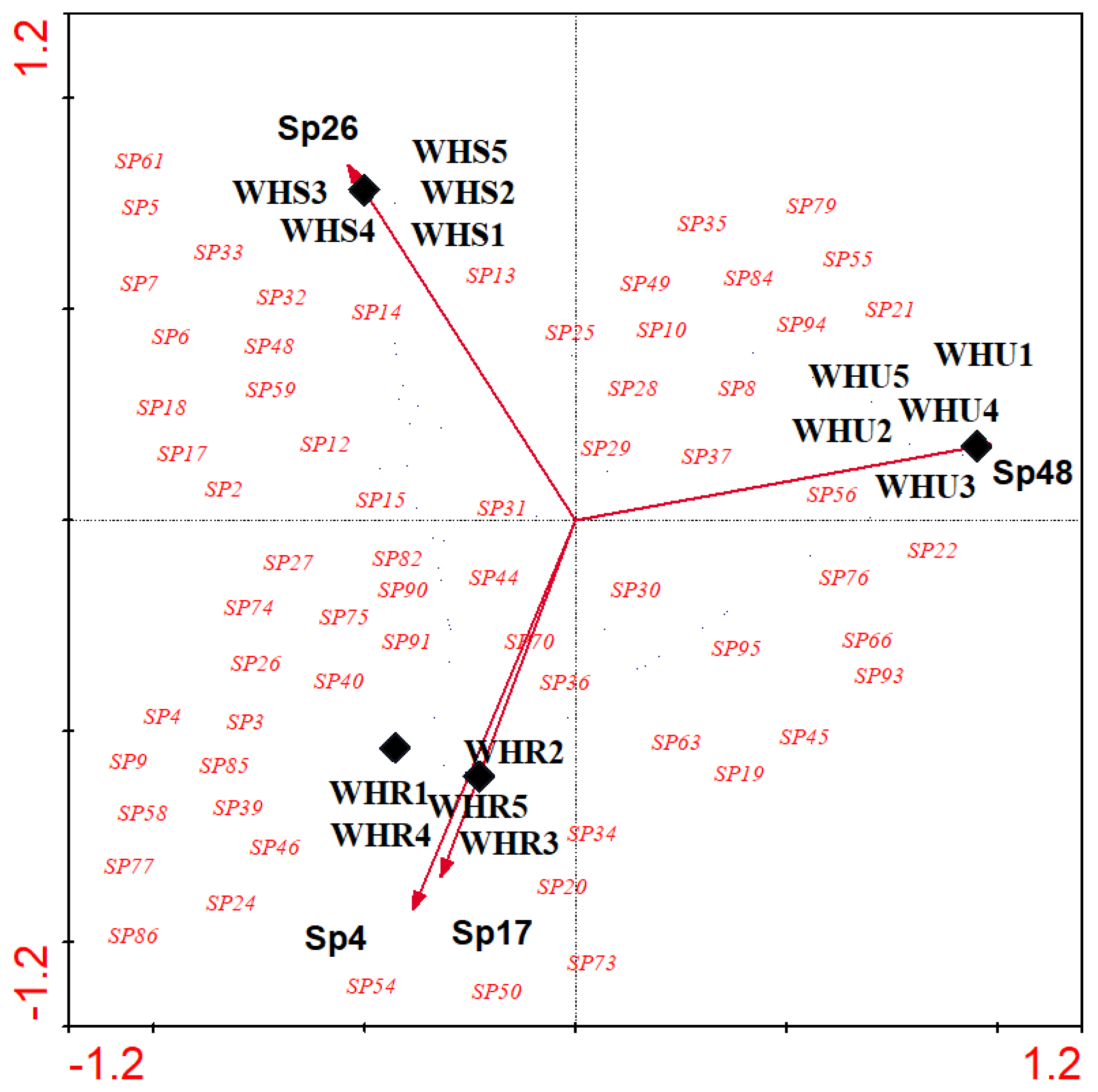
| Alhagi graecorum | 3.327 | 0.01 | -0.5362 | 0.8231 | |||
| Heliotropium currasavicum | 5.33 | 0.0020 | 0.9770 | 0.1765 | |||
| Launaea capitata | 3.80 | 0.0020 | -0.3841 | -0.9034 | |||
| Lycium shawii | 1.88 | 0.0260 | -0.3173 | -0.8271 | |||
| Soil chemical properties | 0.568 | 0.385 | 2.195 | ||||
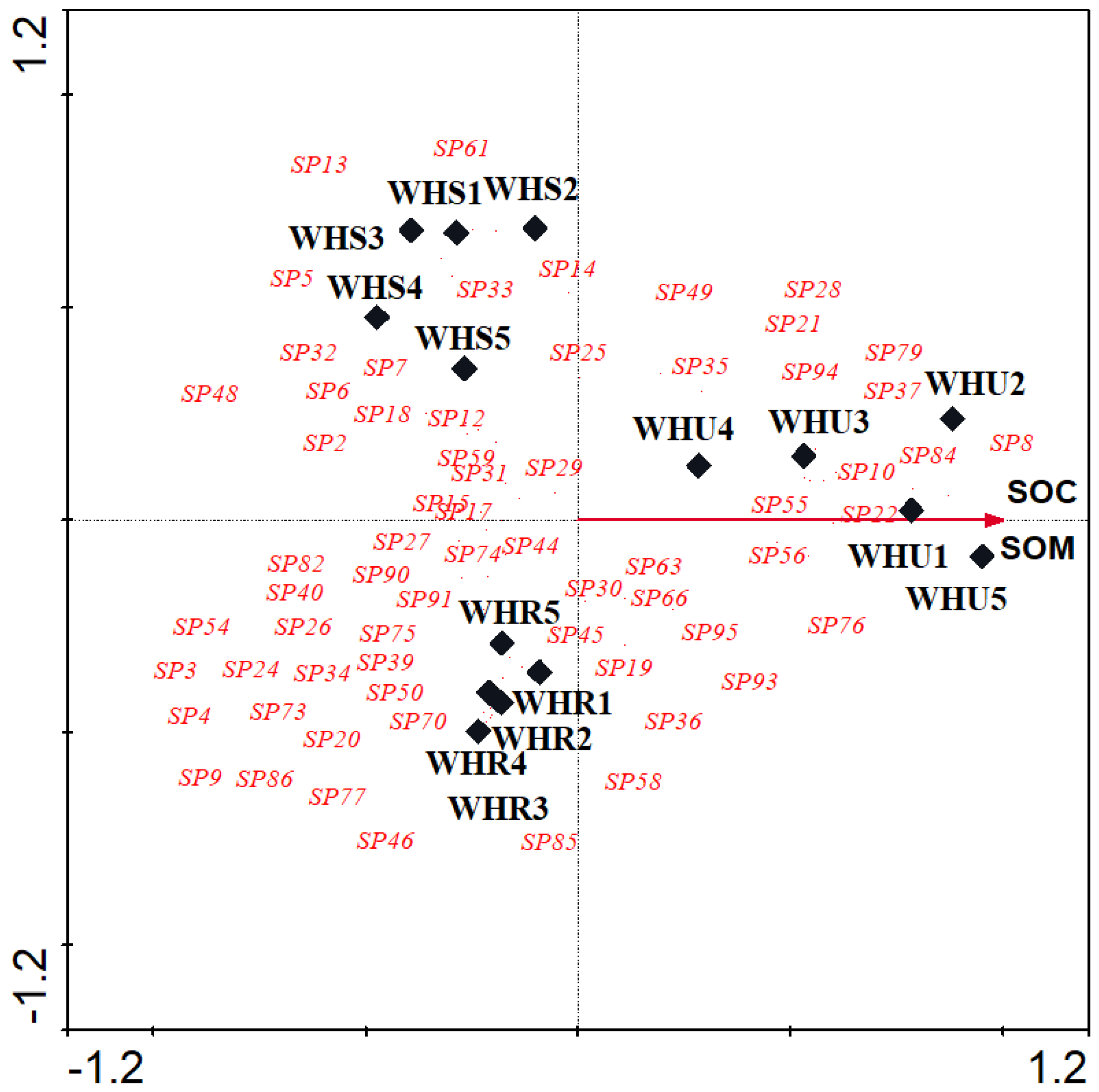
| Soil Organic Carbon (SOC) | 4.53 | 0.0020 | 0.9420 | 0.0000 | |||
| Soil Organic Matter (SOM) | 0.67 | 0.80 | 0.9415 | -0.0007 | |||
| Family | Species | Gradient | IV | P |
|---|---|---|---|---|
| Tenebrionidae | Adesmia stoeckleini Koch, 1940 | 0 | 100 | 0.001 |
| Tenebrionidae | Blaps kollari kollari Seidlitz, 1896 | 0 | 66.7 | 0.01 |
| Tenebrionidae | Scleropatroides sp. | 0 | 66.7 | 0,02 |
| Tenebrionidae | Oxycara saudarabica Kaszab, 1979 | 0 | 72.3 | 0.007 |
| Carabidae | Mesolestes quadriguttatus (Mateu, 1979) | 0 | 66.7 | 0.02 |
| Carabidae | Eremolestes sulcatus (Chaudoir, 1876) | 0 | 66.7 | 0.02 |
| Tenebrionidae | Akis spinosa (Linnaeus, 1764) | 0 | 66.7 | 0.016 |
| Tenebrionidae | Pimelia thomasi thomasi Blair, 1931 | 1 | 75 | 0.01 |
| Tenebrionidae | Gonocephalum soricinum (Reiche & Saulcy, 1857) | 1 | 73.3 | 0.01 |
| Tenebrionidae | Tentyrina deserta deserta Kaszab, 1981 | 1 | 54.5 | 0.045 |
| Scarabaeidae | Rhyssemus saudi Pittion, 1984 | 1 | 63.8 | 0.035 |
| Tenebrionidae | Zophosis punctata Brullé , 1832 | 1 | 72.5 | 0.007 |
| Dermestidae | Anthrenus malkini Mroczkowski, 1980 | 1 | 50 | 0,05 |
| Tenebrionidae | Gonocephalum besnardi Kaszab, 1982 | 2 | 60 | 0.02 |
| Tenebrionidae | Sclerum orientalis (Fabricius, 1775) | 2 | 80 | 0,006 |
| Anthicidae | Anthelephila caeruleipennis (La Ferté-Sénectère, 1847) | 2 | 85.7 | 0.003 |
| Anthicidae | Anthicus crinitus LaFerté-Sénectère, 1849 | 2 | 66.7 | 0.011 |
| Scarabaeidae | Maladera insanabilis (Brenske, 1894) | 2 | 61 | 0.03 |
| Anthicidae | Endomia lefebvrei (LaFerté-Sénectère, 1849) | 2 | 100 | 0.001 |
| Scarabaeidae | Pentodon algerinus (Füessly, 1778) | 2 | 60 | 0.03 |
| Carabidae | Microlestes infuscatus (Motschulsky, 1859) | 2 | 60 | 0.03 |
| Tenebrionidae | Gonocephalum prolixum (Erichson, 1843) | 2 | 60 | 0.02 |
| Tenebrionidae | Gonocephalum rusticum (Olivier, 1811) | 2 | 70 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





