Submitted:
25 January 2023
Posted:
28 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Polyacetylenes and Inflammation
2.1. Chronic Inflammation Disease and Cancer
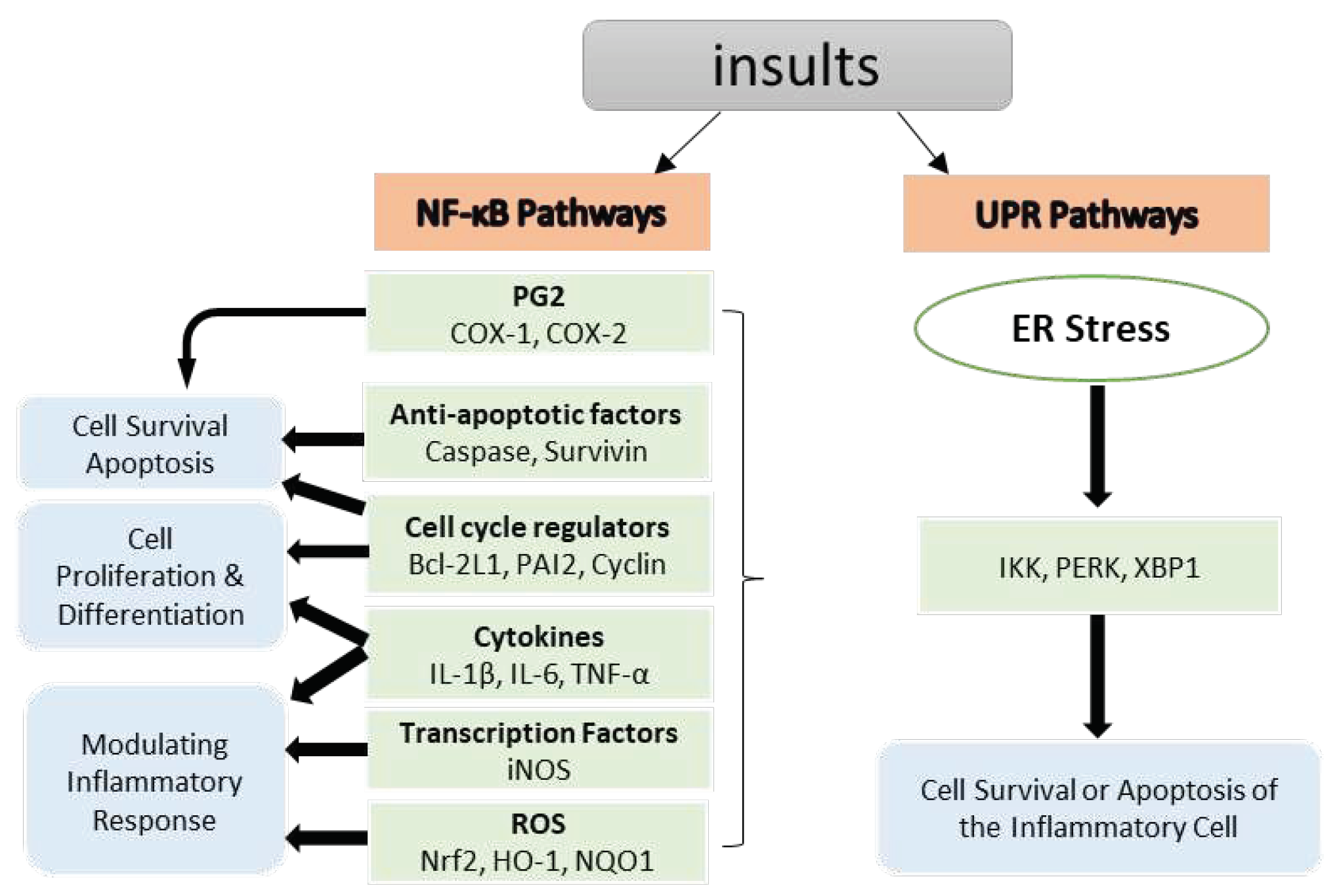
2.1.1. Inhibition of Nuclear Factor kappa B (NF-κB) Pathways
2.2. Oxidative Stress
2.2.1. Inhibition of Nitric oxide synthase (NOS) and Pro-inflammatory Cytokines Pathways
2.2.2. Reactive Oxygen Species (ROS) Pathways
2.3. Unfolded Protein Response (UPR) Pathways
3. Cancer
3.1. In vitro
3.1.1. Anti-Proliferative Activity
3.1.2. Pro-apoptosis Activity
3.1.3. Gut microbiota composition
3.1.4. Other Effects
3.2. In vivo
4. Polyacetylene Toxicology
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X., et al., Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. Postgraduate Medical Journal, 2014. 349.
- Negri, R., Polyacetylenes from terrestrial plants and fungi: recent phytochemical and biological advances. Fitoterapia, 2015. 106: p. 92-109.
- Dawid, C., et al., Bioactive C17-polyacetylenes in carrots (Daucus carota L.): current knowledge and future perspectives. Journal of Agricultural and Food Chemistry, 2015. 63(42): p. 9211-9222.
- Hansen, L. and P.M. Boll, Polyacetylenes in Araliaceae: their chemistry, biosynthesis and biological significance. Phytochemistry, 1986. 25(2): p. 285-293.
- Stefanson, A. and M. Bakovic, Dietary polyacetylene falcarinol upregulated intestinal heme oxygenase-1 and modified plasma cytokine profile in late phase lipopolysaccharide-induced acute inflammation in CB57BL/6 mice. Nutrition Research, 2020. 80: p. 89-105.
- Moore, T., Vitamin A and carotene: The association of vitamin A activity with carotene in the carrot root. Biochemical Journal, 1929. 23(4): p. 803.
- Butnariu, M., Therapeutic Properties of Vegetable. Journal of Bioequivalence & Bioavailability, 2014. 6: p. e55.
- Chaparala, A., et al., Panaxynol, a bioactive component of American ginseng, targets macrophages and suppresses colitis in mice. Oncotarget, 2020. 11(22): p. 2026.
- Cambria, C., S. Sabir, and I.C. Shorter, Ginseng. 2019.
- Hong, H., D. Baatar, and S.G. Hwang, Anticancer activities of ginsenosides, the main active components of ginseng. Evidence-Based Complementary and Alternative Medicine, 2021. 2021.
- Yang, S., et al., Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. Journal of Ethnopharmacology, 2022. 283: p. 114739.
- Mares-Perlman, J.A., et al., The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. The Journal of Nutrition, 2002. 132(3): p. 518S-524S.
- Sommer, A. and K.S. Vyas, A global clinical view on vitamin A and carotenoids. The American Journal of Clinical Nutrition, 2012. 96(5): p. 1204S-1206S.
- Tapiero, H., D.M. Townsend, and K.D. Tew, The role of carotenoids in the prevention of human pathologies. Biomedicine & Pharmacotherapy, 2004. 58(2): p. 100-110.
- Kordiak, J., et al., Role of Beta-Carotene in Lung Cancer Primary Chemoprevention: A Systematic Review with Meta-Analysis and Meta-Regression. Nutrients, 2022. 14(7): p. 1361.
- Brandt, K., et al., Health promoting compounds in vegetables and fruits: A systematic approach for identifying plant components with impact on human health. Trends in Food Science & Technology, 2004. 15(7-8): p. 384-393.
- Kim, Y.-J., et al., Anti-colitic effect of purple carrot on dextran sulfate sodium (DSS)-induced colitis in C57BL/6J Mice. Preventive Nutrition and Food Science, 2018. 23(1): p. 77.
- Metzger, B.T., D.M. Barnes, and J.D. Reed, Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. Journal of Agricultural and Food Chemistry, 2008. 56(10): p. 3554-3560.
- Benetou, V., A. Lagiou, and P. Lagiou, Chemoprevention of cancer: Current evidence and future prospects. F1000Research, 2015. 4(F1000 Faculty Rev).
- Wang, M.-W., X. Hao, and K. Chen, Biological screening of natural products and drug innovation in China. Philosophical Transactions of the Royal Society B: Biological Sciences, 2007. 362(1482): p. 1093-1105.
- Singh, N., et al., Inflammation and cancer. Annals of African Medicine, 2019. 18(3): p. 121.
- Prasad, S., B. Sung, and B.B. Aggarwal, Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Preventive Medicine, 2012. 54: p. S29-S37.
- Nasef, N.A., S. Mehta, and L.R. Ferguson, Susceptibility to chronic inflammation: an update. Archives of Toxicology, 2017. 91(3): p. 1131-1141.
- Colotta, F., et al., Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis, 2009. 30(7): p. 1073-1081.
- Raposo, T., et al., Inflammation and cancer: till death tears them apart. The Veterinary Journal, 2015. 205(2): p. 161-174.
- Macarthur, M., G.L. Hold, and E.M. El-Omar, Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2004. 286(4): p. G515-G520.
- De Marzo, A.M., et al., Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. The American journal of pathology, 1999. 155(6): p. 1985-1992.
- Kuper, H., H.O. Adami, and D. Trichopoulos, Infections as a major preventable cause of human cancer. Journal of internal medicine, 2001. 249(S741): p. 61-74.
- Scholl, S., et al., Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. Journal of the National Cancer Institute, 1994. 86(2): p. 120-126.
- Ernst, P.B. and B.D. Gold, The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annual Review of Microbiology, 2000. 54: p. 615.
- Ness, R.B. and C. Cottreau, Possible role of ovarian epithelial inflammation in ovarian cancer. Journal of the National Cancer Institute, 1999. 91(17): p. 1459-1467.
- Bröcker, E., et al., Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. International Journal of Cancer, 1988. 41(4): p. 562-567.
- Kundu, J.K. and Y.-J. Surh, Inflammation: gearing the journey to cancer. Mutation Research/Reviews in Mutation Research, 2008. 659(1-2): p. 15-30.
- Coussens, L.M. and Z. Werb, Inflammation and cancer. Nature, 2002. 420(6917): p. 860-867.
- Multhoff, G., M. Molls, and J. Radons, Chronic inflammation in cancer development. Frontiers in immunology, 2012. 2: p. 98.
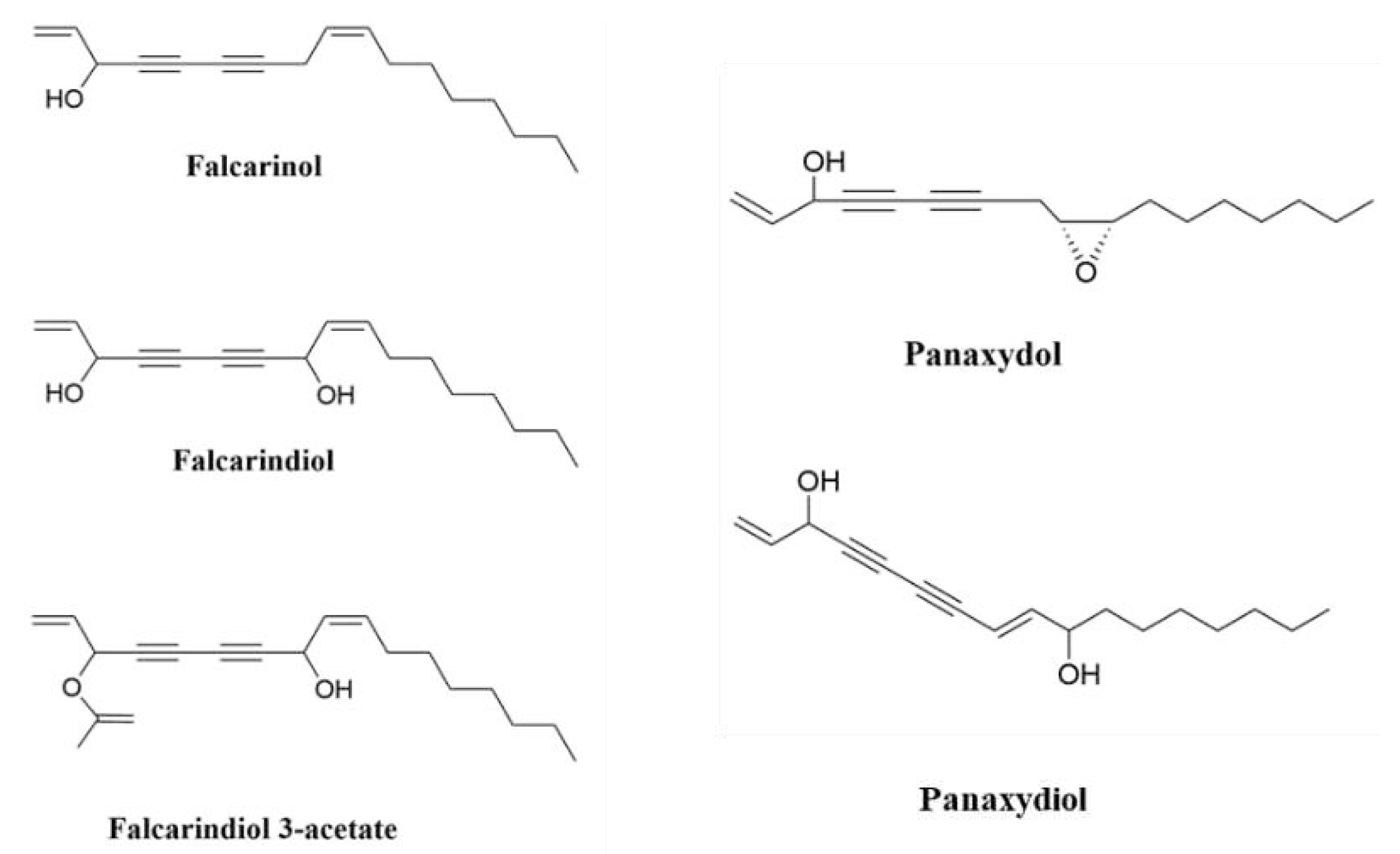
- Christensen, L.P., Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development. Molecules, 2020. 25(11): p. 2568.
- Spooner, R. and Ö. Yilmaz, The role of reactive-oxygen-species in microbial persistence and inflammation. International journal of molecular sciences, 2011. 12(1): p. 334-352.
- Kauppinen, A., et al., Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cellular signalling, 2013. 25(10): p. 1939-1948.
- Crusz, S.M. and F.R. Balkwill, Inflammation and cancer: advances and new agents. Nature Reviews Clinical Ocology, 2015. 12(10): p. 584-596.
- Song, W., et al., Translational significance for tumor metastasis of tumor-associated macrophages and epithelial–mesenchymal transition. Frontiers in Immunology, 2017. 8: p. 1106.
- Kwon, H.-J., et al., Stepwise phosphorylation of p65 promotes NF-κB activation and NK cell responses during target cell recognition. Nature Communications, 2016. 7(1): p. 1-15.
- Hayden, M.S. and S. Ghosh, Shared principles in NF-κB signaling. Cell, 2008. 132(3): p. 344-362.
- Sau, A., et al., Persistent activation of NF-κB in BRCA1-deficient mammary progenitors drives aberrant proliferation and accumulation of DNA damage. Cell Stem Cell, 2016. 19(1): p. 52-65.
- Salazar, L., et al., Fibroblast growth factor receptor 3 interacts with and activates TGFβ-activated kinase 1 tyrosine phosphorylation and NFκB signaling in multiple myeloma and bladder cancer. PLoS One, 2014. 9(1): p. e86470.
- Burstein, E. and E.R. Fearon, Colitis and cancer: a tale of inflammatory cells and their cytokines. The Journal of Clinical Investigation, 2008. 118(2): p. 464-467.
- Sun, S.-C., The non-canonical NF-κB pathway in immunity and inflammation. Nature Reviews Immunology, 2017. 17(9): p. 545-558.
- Zhao, Y., et al., Study on the antidepressant effect of panaxynol through the IκB-α/NF-κB signaling pathway to inhibit the excessive activation of BV-2 microglia. Biomedicine & Pharmacotherapy, 2021. 138: p. 111387.
- Chen, T., et al., The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. International immunopharmacology, 2015. 25(1): p. 55-64.
- Kang, H., et al., Protective effect of the methanol extract from Cryptotaenia japonica Hassk. against lipopolysaccharide-induced inflammation in vitro and in vivo. BMC Complementary and Alternative Medicine, 2012. 12(1): p. 1-7.
- Shiao, Y.-J., et al., Falcarindiol impairs the expression of inducible nitric oxide synthase by abrogating the activation of IKK and JAK in rat primary astrocytes. British journal of pharmacology, 2005. 144(1): p. 42.
- Kobaek-Larsen, M., et al., Dietary polyacetylenic oxylipins falcarinol and falcarindiol prevent inflammation and colorectal neoplastic transformation: A mechanistic and dose-response study in a rat model. Nutrients, 2019. 11(9): p. 2223.
- Kim, H.N., et al., Heracleum moellendorffii roots inhibit the production of pro-inflammatory mediators through the inhibition of NF-κB and MAPK signaling, and activation of ROS/Nrf2/HO-1 signaling in LPS-stimulated RAW264. 7 cells. BMC complementary and alternative medicine, 2019. 19(1): p. 1-10.
- Yao, C. and S. Narumiya, Prostaglandin-cytokine crosstalk in chronic inflammation. British Journal of Pharmacology, 2019. 176(3): p. 337-354.
- Lee, S., et al., Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways. Journal of Inflammation, 2011. 8(1): p. 1-9.
- Nagaraju, G.P. and B.F. El-Rayes, Cyclooxygenase-2 in gastrointestinal malignancies. Cancer, 2019. 125(8): p. 1221-1227.
- Ghosh, N., et al., COX-2 as a target for cancer chemotherapy. Pharmacological Reports, 2010. 62(2): p. 233-244.
- Agrawal, U., et al., Overexpression of COX2 indicates poor survival in urothelial bladder cancer. Annals of Diagnostic Pathology, 2018. 34: p. 50-55.
- Harris, R.E., B.C. Casto, and Z.M. Harris, Cyclooxygenase-2 and the inflammogenesis of breast cancer. World Journal of Clinical Oncology, 2014. 5(4): p. 677.
- Petkova, D., et al., Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respiratory Medicine, 2004. 98(2): p. 164-172.
- Yip-Schneider, M.T., et al., Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis, 2000. 21(2): p. 139-146.
- Saba, N.F., et al., Role of Cyclooxygenase-2 in Tumor Progression and Survival of Head and Neck Squamous Cell CarcinomaRole of COX-2 in Head and Neck Cancer. Cancer Prevention Research, 2009. 2(9): p. 823-829.
- Masferrer, J.L., et al., Antiangiogenic and Antitumor Activities of cyclooxygenase-2 inhibitors. Cancer Research, 2000. 60(5): p. 1306-1311.
- Shi, G., et al., Upregulation of cyclooxygenase-2 is associated with activation of the alternative nuclear factor kappa B signaling pathway in colonic adenocarcinoma. Am J Transl Res, 2015. 7(9): p. 1612-20.
- Bogdan, C., M. Röllinghoff, and A. Diefenbach, The role of nitric oxide in innate immunity. Immunological Reviews, 2000. 173: p. 17-26.
- Ekmekcioglu, S., E.A. Grimm, and J. Roszik, Targeting iNOS to increase efficacy of immunotherapies. Human Vaccines & Immunotherapeutics, 2017. 13(5): p. 1105-1108.
- Hausel, P., et al., Src-mediated phosphorylation regulates subcellular distribution and activity of human inducible nitric oxide synthase. Oncogene, 2006. 25(2): p. 198-206.
- Pautz, A., et al., Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide, 2010. 23(2): p. 75-93.
- Ichikawa, T., et al., American ginseng preferentially suppresses STAT/iNOS signaling in activated macrophages. Journal of Ethnopharmacology, 2009. 125(1): p. 145-150.
- Qu, C., et al., Identifying panaxynol, a natural activator of nuclear factor erythroid-2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage-induced cardiomyocyte hypertrophy. Journal of Ethnopharmacology, 2015. 168: p. 326-336.
- Ďuračková, Z., Some current insights into oxidative stress. Physiological research, 2010. 59(4).
- Scialo, F. and A. Sanz, Coenzyme Q redox signalling and longevity. Free Radical Biology and Medicine, 2021. 164: p. 187-205.
- Kim, J.Y., et al., Panaxydol induces apoptosis through an increased intracellular calcium level, activation of JNK and p38 MAPK and NADPH oxidase-dependent generation of reactive oxygen species. Apoptosis, 2011. 16(4): p. 347-358.
- Young, J.F., et al., The polyacetylenes falcarinol and falcarindiol affect stress responses in myotube cultures in a biphasic manner. Dose-Response, 2008. 6(3): p. dose-response. 08-008. Young.
- Ohnuma, T., et al., Activation of the Nrf2/ARE pathway via S-alkylation of cysteine 151 in the chemopreventive agent-sensor Keap1 protein by falcarindiol, a conjugated diacetylene compound. Toxicology and Applied Pharmacology, 2010. 244(1): p. 27-36.
- Chiang, S.K., S.E. Chen, and L.C. Chang, A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int J Mol Sci, 2018. 20(1).
- Stefanson, A.L. and M. Bakovic, Falcarinol is a potent inducer of heme oxygenase-1 and was more effective than sulforaphane in attenuating intestinal inflammation at diet-achievable doses. Oxidative Medicine and Cellular Longevity, 2018. 2018.
- Sano, R. and J.C. Reed, ER stress-induced cell death mechanisms. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2013. 1833(12): p. 3460-3470.
- Li, Y., et al., The role and therapeutic implication of endoplasmic reticulum stress in inflammatory cancer transformation. American Journal of Cancer Research, 2022. 12(5): p. 2277-2292.
- Jin, H., et al., The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death & Disease, 2012. 3(8): p. e376-e376.
- Kim, H.S., et al., Panaxydol, a component of P anax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. International Journal of Cancer, 2016. 138(6): p. 1432-1441.
- Andersen, C.B., et al., Falcarindiol Purified From Carrots Leads to Elevated Levels of Lipid Droplets and Upregulation of Peroxisome Proliferator-Activated Receptor-γ Gene Expression in Cellular Models. Frontiers in Pharmacology, 2020. 11: p. 565524.
- Cheung, S.S., et al., Devil’s Club falcarinol-type polyacetylenes inhibit pancreatic cancer cell proliferation. Nutrition and Cancer, 2019. 71(2): p. 301-311.
- Xie, Q. and C. Wang, Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014–2021). Phytochemistry, 2022: p. 113288.
- Zaini, R., et al., Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 2012. 12(6): p. 640-652.
- Zidorn, C., et al., Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. Journal of Agricultural and Food Chemistry, 2005. 53(7): p. 2518-2523.
- Bernart, M.W., et al., Cytotoxic falcarinol oxylipins from Dendropanax arboreus. Journal of Natural Products, 1996. 59(8): p. 748-753.
- Sapienza, C. and J.-P. Issa, Diet, nutrition, and cancer epigenetics. Annual Review of Nutrition, 2016. 36: p. 665-681.
- Le, H.T., et al., Panaxynol, a natural Hsp90 inhibitor, effectively targets both lung cancer stem and non-stem cells. Cancer Letters, 2018. 412: p. 297-307.
- Chatterjee, S. and T.F. Burns, Targeting heat shock proteins in cancer: a promising therapeutic approach. International journal of molecular sciences, 2017. 18(9): p. 1978.
- Neckers, L. and P. Workman, Hsp90 molecular chaperone inhibitors: are we there yet? Clinical Cancer Research, 2012. 18(1): p. 64-76.
- Nair, A., M.A. Morsy, and S. Jacob, Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Development Research, 2018. 79(8): p. 373-382.
- Kobaek-Larsen, M., et al., Effect of the dietary polyacetylenes falcarinol and falcarindiol on the gut microbiota composition in a rat model of colorectal cancer. BMC Research Notes, 2018. 11(1): p. 1-6.
- Hansen, S.L., S. Purup, and L.P. Christensen, Bioactivity of falcarinol and the influenceof processing and storage on its content in carrots (Daucus carota L). Journal of the Science of Food and Agriculture, 2003. 83(10): p. 1010-1017.
- Purup, S., E. Larsen, and L.P. Christensen, Differential effects of falcarinol and related aliphatic C17-polyacetylenes on intestinal cell proliferation. Journal of Agricultural and Food Chemistry, 2009. 57(18): p. 8290-8296.
- Young, J.F., et al., Biphasic effect of falcarinol on CaCo-2 cell proliferation, DNA damage, and apoptosis. Journal of Agricultural and Food Chemistry, 2007. 55(3): p. 618-623.
- Dolatpanah, M., et al., Falcarindiol attenuates cisplatin-induced nephrotoxicity through the modulation of NF-kB and Nrf2 signaling pathways in mice. 2022.
- Tan, K.W., et al., Dietary polyacetylenes of the falcarinol type are inhibitors of breast cancer resistance protein (BCRP/ABCG2). European Journal of Pharmacology, 2014. 723: p. 346-352.
- Deding, U., et al., Carrot intake and risk of colorectal cancer: A prospective cohort study of 57,053 Danes. Nutrients, 2020. 12(2): p. 332.
- Almqbel, M., C. Seal, and K. Brandt, Effects of carrot powder intake after weaning on tumours in APCMin mice. Proceedings of the Nutrition Society, 2017. 76(OCE4).
- Kobæk-Larsen, M., et al., Inhibitory effects of feeding with carrots or (−)-falcarinol on development of azoxymethane-induced preneoplastic lesions in the rat colon. Journal of Agricultural and Food Chemistry, 2005. 53(5): p. 1823-1827.
- Kobaek-Larsen, M., et al., Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food & Function, 2017. 8(3): p. 964-974.
- Crosby, D. and N. Aharonson, The structure of carotatoxin, a natural toxicant from carrot. Tetrahedron, 1967. 23(1): p. 465-472.
- Uwai, K., et al., Exploring the structural basis of neurotoxicity in C17-polyacetylenes isolated from water hemlock. Journal of Medicinal Chemistry, 2000. 43(23): p. 4508-4515.
- Czepa, A. and T. Hofmann, Structural and sensory characterization of compounds contributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree. Journal of Agricultural and Food Chemistry, 2003. 51(13): p. 3865-3873.
- Downs, C., et al., A hemlock water dropwort curry: a case of multiple poisoning. Emerg Med J, 2002. 19(5): p. 472-3.
- Christensen, L., Bioactivity of Polyacetylenes in food plants: Bioactive Foods in Promoting Health (Chapter 20). 2010, Academic Press, .
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




