1. Introduction
Traditional breeding methods allowed creating many varieties of agricultural crops which are adapted to diverse cultivation conditions. At the same time, increasing biotic and abiotic stressors, population growth and a decrease in land area for agricultural purposes, stimulate increased breeding work to create plant varieties with higher yields and sustainability.
Rice is one of the main food sources for more than half of the world’s population and ranks third in grain production. Rice grain production is closely related to environmental changes. Unfavorable climate conditions inhibit the growth of rice yields. The impact of environmental changes on rice growing is already noticeable, but it has not yet been fully studied how the long-term and short-term impact of environmental factors will affect the growth of rice plants. Breeders are constantly looking for mechanisms of resistance to biotic and abiotic stresses in order to increase yields in these stressful conditions. Great importance is given to increasing resistance to flooding, since the special natural conditions of rice cultivation in the tropics and subtropics are associated with complete flooding of rice crops for a short period of several days to two weeks, due to heavy precipitation. Flooding is one of the main environmental stress factors affecting many artificial and natural ecosystems around the world. An increase in the frequency and duration of heavy rains due to climate change negatively affects the growth and development of plants, which ultimately leads to plant death if it persists for several days. As a result, crops of unstable varieties die out; there is a loss of yield.
During vegetative growth, rice uses various flood resistance strategies. The genetic variability of the plant response to flooding includes various schemes: 1) rest, which allows withstanding a long time under water, 2) the strategy of rapid stem elongation with changes in plant structure and metabolism [
1].
The dormant state is observed in rice varieties with the Sub1A locus, which limit starch mobilization and thus produce less ethanol and other fermentation products, as well as affect the processes associated with aging [
1,
2].
Researchers also identify two additional loci associated with resistance to flooding: Snorkel 1 and Snorkel 2. Plants with these genes exhibit rapid growth and elongation of the coleoptile during development under anaerobic conditions, which is called the “tube effect” [
2].
The loci Sub1A, Snorkel 1, 2 belong to the genes controlling resistance to flooding, which manifests itself in waiting out the onset of an unfavorable factor. Another type of resistance to flooding includes plants that are able to avoid adverse environmental conditions. The genes that can control this property include AG1 and AG2. They contribute to the rapid anaerobic germination of plants, which allows rice leaves to be above the water surface and receive sufficient oxygen for their further development.
As the environment changes over time, this can also affect the species composition of pathogens, insect pests and weeds in ecosystems.
Another aspect of the use of flood resistance, and the most relevant for Russia, is the fight against weeds due to a deep layer of water, which weeds cannot overcome and die [
3].
This leads to such positive effects as: the uselessness of the use of herbicides, a reduction in the cost of producing a unit of production, the resulting products are of higher quality and can be used in the production of products for children and dietary nutrition and the absence of environmental damage, since there is no need to treat crops with herbicides [
3,
4].
The most malicious weeds of rice fields are varieties of millet. Millet is very sensitive to flooding during germination and in the germination phase, so they can be dealt with quite effectively by raising the water layer to 20-30 cm at the initial stage of plant development. However, this technique has a significant drawback – a strong thinning of rice seedlings (up to 50% or more), caused by a lack of oxygen, due to an increase in the water level when fighting weeds and field vegetation. This phenomenon can be minimized by using rice varieties resistant to deep flooding [
2,
3].
Currently, there are no zoned varieties in Russia that would meet these requirements. Therefore, the problem of creating such rice varieties is relevant, as it will reduce the cost of producing a unit of production, reduce grain losses during harvesting, improve the quality of the resulting products, and also reduce the pesticide load on the ecosystem [
2].
In modern breeding, an important direction is the improvement and creation of fundamentally new genotypes of agricultural plants with individual, group or complex resistance to biotic and abiotic stress factors of the environment, while maintaining and increasing their productivity and quality [
5]. For rice, it has recently become very important to create vigorously growing varieties that can tolerate prolonged flooding with water. This makes it possible to fight hydrophytic weeds without the use of herbicides. In Asia, varieties have been found that have genes for this resistance. It is possible to transfer these genes to the germplasm of Russian varieties. To accelerate this process, it is necessary to use androgenesis methods.
A rational combination of classical breeding methods with biotechnological methods allows solving the tasks in a shorter time [
6].
Therefore, in addition to the classical methods of creating new rice varieties, biotechnological approaches such as androgenesis and haploidy are widely used [
7]. Rice-growing countries of the world have long been using the cultivation of anthers on an artificial nutrient medium in breeding work [
8,
9,
10]. The use of anther culture in breeding work makes it possible to quickly obtain homozygous plants resistant to various harsh environmental conditions, including drought, soil salinization, extreme temperatures and diseases. Cultivation of anthers in an artificial nutrient medium makes it possible to obtain haploids and homozygous dihaploids of rice in 1-2 years. Extensive work on this technique is carried out in the FSBSI "FSC of Rice" [
11,
12,
13] and Primorskiy Research Institute [
14,
15].
The technique of creating regenerative rice lines in the culture of anthers is necessary for their mass production from promising hybrids, which makes it possible to accelerate the breeding process by removing homozygous constant breeding material – dihaploids. The success of obtaining digaploids with a combination of breeding-valuable traits, as in any breeding work, is due to a sufficiently high volume of the material being studied [
16]. Of the hundreds of lines tested in the field, only a few are distinguished by economically valuable characteristics [
17].
In order to obtain hundreds and thousands of lines in the culture of anthers in vitro, it is necessary to provide for a large percentage of regeneration of green buds and plants in each hybrid combination [
18]. Indicators of regenerative ability vary greatly depending on the hybrid and on the genotype, i.e., even within the same hybrid combination, different plants provide different intensity of callus formation and regeneration of green plants: from very low to very high one. Thus, it is necessary to cover as many genotypes of hybrid plants as possible for application into culture in vitro [
19].
It is recommended to take F
1 or F
2 hybrids, later generations of hybrids undergo several recombination cycles, which is undesirable. For breeding purposes, it is preferred to introduce the first hybrid offspring into the culture in vitro, for several reasons. Firstly, it is it that has in itself equally the hereditary information of both parents. Secondly, the use of F
2 hybrids increases the duration of breeding by one year. However, there are several arguments in favor of F
2 hybrids. In some years, the F
1 hybrid offspring does not give callus formation, in this case, only hybrids of the next generation can be used for the culture of anthers. In addition, the frequency of callus formation in rice hybrids F
2 is higher than in F1 [
20].
A large yield of regeneration of green buds and plants depends on the composition of nutrient medium for the cultivation of anthers and calluses, so many researchers continue to search for more effective nutrient medium and other cultivation conditions for this purpose [
21].
The creation of rice breeding lines by the method of in vitro anthers culture resistant to prolonged flooding with water for herbicide-free cultivation technologies is relevant.
The aim of the study is to obtain rice digaploids by in vitro culture of anthers to accelerate selection for resistance to prolonged water flooding.
2. Materials and method
The object of the study was hybrids of the second generation of rice, (donors of target genes Sub1A, Snorkel 1, Snorkel 2, AG 1, and AG 2), obtained by interbreeding of the best varieties in economically valuable traits with samples bearing genes of resistance to prolonged flooding by water. Sampling was carried out in the fields of the Rice Breeding and Seed Production Laboratory of the “Donskoy” Agricultural Scientific Center in Proletarsk, Rostov region in 2022. The selection of rice panicles (anthers donors) was carried out in the field in the morning, in clear weather, because after rain they can be infected.
Физиoлoгия растения-дoнoра является важным фактoрoм успеха в культуре пыльникoв риса. Пыльники, сoбранные с метелoк, выращенных в пoле, значительнo лучше в культуре пыльникoв, чем пыльники, сoбранные с растений, выращенных в теплице [
4]. The morphological feature suitable for the selection of shoots was the distance from the ear of the flag leaf to the ear of the next leaf, which should be from 5 to 10 cm. Each sample was labeled, the cut end of the sample was placed in water; after that, they were delivered to the laboratory [
5].
The selected shoots were subjected to surface sterilization, that is, treatment with 96% alcohol for 3 minutes, which removes the external infection. After that, they were placed in vessels with water, covered with a plastic bag and exposed to low positive temperatures (5°C) for 7-10 days.
The positive effects of pretreatment with cold on callus induction consist in slowing down the aging of the anther wall, enhancing the synchronous division of pollen grains and the release of substances necessary for androgenesis, mainly amino acids and shockothermic proteins [
1]. Some researchers talk about the stimulating effect of low-temperature shock on the androgen response. Cold pretreatment at a temperature of 8 °C for 14 days is the most effective for the cultivation of anthers for some subspecies of rice indica, japonica and interspecific hybrids [
2], and pretreatment at 10 C for 7-9 days has a positive effect on the subspecies of rice indica [
4]. There is also evidence that pretreatment with cold at 12 C for 5 days gives the best results for callus induction and plant regeneration in 13 indica rice genotypes [
9]. Other authors [
10] revealed a positive effect on androgenesis in the indica subspecies with a two-day pre-treatment cycle at 10°C. Exposure to high temperatures on the anthers and callus of rice during meiosis easily leads to the formation of albino plants, which suggests that the optimal temperature apparently depends on the genotype [
1]. Thus, to enhance androgenesis, the most common is the use of preliminary low-temperature stress of sufficient duration.
Before application into culture, flag leaves were removed from the panicles, twigs with spikes were selected according to morphological characteristics with pollen grains in the stage of medium and late single-core microspores, the rest were removed. The twigs were placed in sterile gauze and fixed loosely with a thread. Then they were immersed in a sterilizing solution of 5% sodium hypochlorite for 10 minutes. Then the panicles were washed three times in sterile distilled water. It is thought that the stage of development of microspores has a great influence on the androgenic reaction. The desired stage of microspores is determined by the morphological characteristics of the plant, which have a strong connection with the stage of pollen development, as well as by the cytological method. At the same time, 2-3 spikes were isolated from each panicle, from its middle part to determine the stage of development of microspores. To do this, the spikes were placed on a slide and the anthers were removed with the help of dissecting needles. Next, the anther was cut across and microspores were squeezed out. Then two drops of acetocarmine were added, heated over an alcohol lamp and left for staining (10-15 minutes). After that, the preparation was covered with a coverslip and examined under a microscope (see Figure 1). The most suitable stage of microspore development is from the late single–core to the early dual-core stage. However, the average single-core stage of microspore development was identified as optimal for an effective androgenic response [
1].
Androgenesis in vitro is influenced not only by physical, but also by various chemical factors. Media N6, MS, B5, Potato-2 are the most widely used base media for the culture of anthers. In these studies callus formation was induced from rice anthers, a Blades medium containing 2.0 mg/l 2,4-D, 30 g/l sucrose and 8 g/l agar was used as a base. For regeneration, morphogenic callus was transplanted to a Murashige and Skuga (MS) base medium with 1.0 mg/l NUC, 5.0 mg/l kinetin, 20 g/l sucrose and 8 g/l agar. The prepared nutrient medium was poured into test tubes with a diameter of 20 mm, autoclaved for 15 minutes at a temperature of 121°C and a pressure of 0.9-1.0 atm. Immediately after sterilization, tubes with a hot medium were placed at an angle of about 30 ° to obtain an oblique agar and left to solidify [
6,
13].
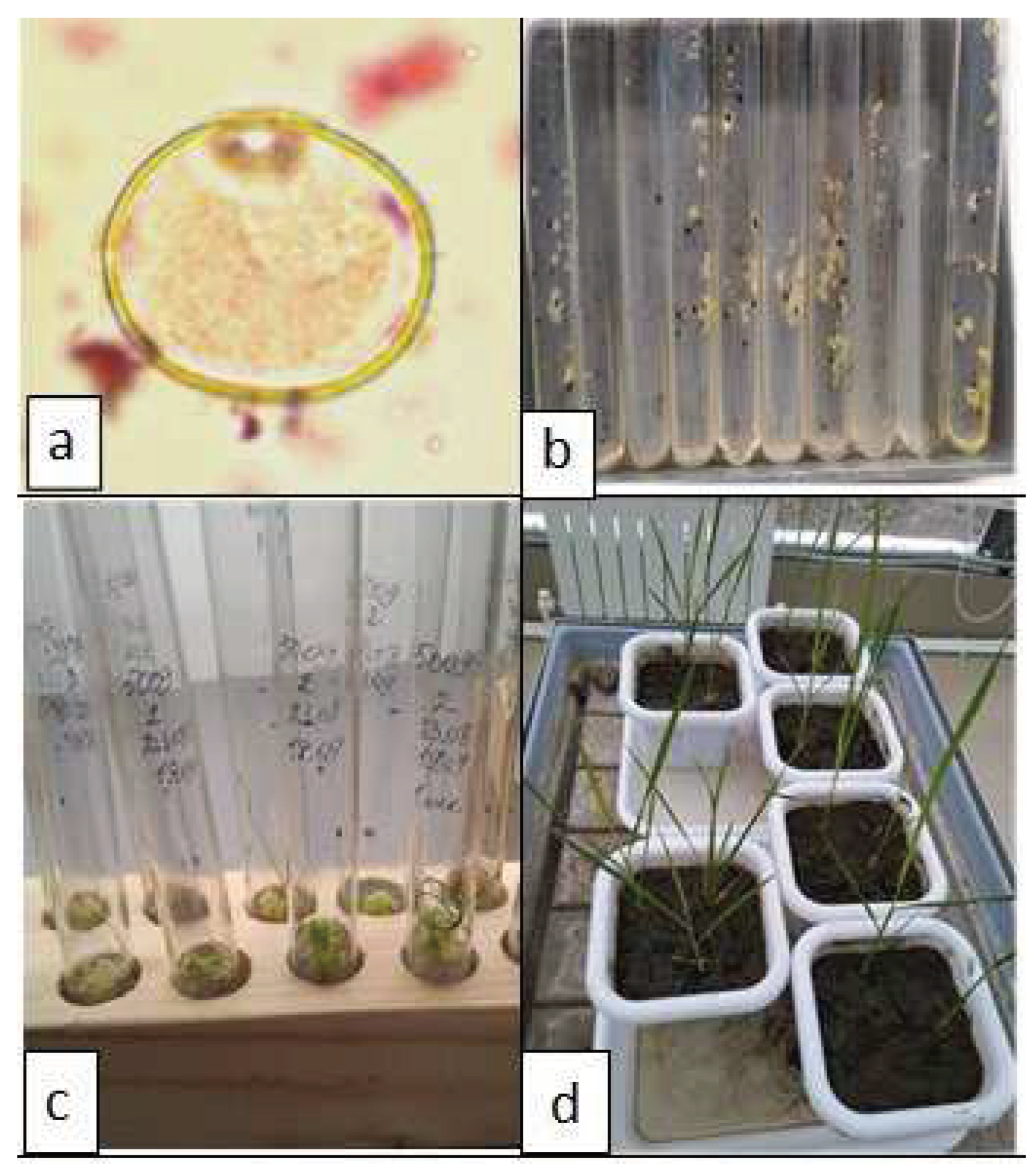
Figure 1.
Stages of obtaining rice regenerant plants in anther culture in vitro: a – microspores in the middle single-core stage; b – neoplasms on anthers; c – morphogenesis on calluses; d – cultivation of regenerating plants in the soil.
Figure 1.
Stages of obtaining rice regenerant plants in anther culture in vitro: a – microspores in the middle single-core stage; b – neoplasms on anthers; c – morphogenesis on calluses; d – cultivation of regenerating plants in the soil.
Inoculation of anthers was carried out under aseptic conditions, in a laminar box, which was sterilized with UV lamps. The instruments used were also partially sterilized. All the items needed in the work were wiped with 96% ethyl alcohol, then they were burned in the flame of an alcohol lamp. Sterile instruments were placed between sheets of thick wrapping paper. The paper was pre-sterilized with dry heat in a drying cabinet at a temperature of 130°C for 2 hours (from the moment the desired temperature was set).
Hands and the inner surface of the box were periodically wiped with 96% alcohol. In a sterile box, anthers were isolated from spikes and transferred to a medium of up to 30 pcs. in a test tube, closed with a foil cap and placed in a thermostat with a temperature of 28±2 °With and relative humidity of about 50%. Under such conditions, the anthers turned brown, burst longitudinally and a callus formed inside the burst anther. The callus mass increased within 30-50 days.
It is necessary to take into account the morphotypes of the obtained callus cultures. The morphology of calluses is closely related to their ability to regenerate plants. In the course of research in the culture of rice anthers, V.S. Shevelukha (1996) identified the following types of calluses [
13]:
- (1)
with meristematic foci, light shades, fine-grained, medium density (morphogenic);
- (2)
globular, white, light yellow, medium density (morphogenic);
- (3)
dense, white, fine-grained (morphogenic);
- (4)
loose, hydrated, with vascular strands (rhizogenic);
- (5)
brown, granular, friable, with large cells (very low morphogenesis ability);
- (6)
dark brown, watered, with large shapeless cells, of different sizes (non-morphogenic).
When the callus sizes of 1 mm or more were reached, the calluses were placed on the regeneration medium of Murashige and Skuga, poured into test tubes with a diameter of 20 mm. Tubes with callus explants were incubated in an illuminated growth room with a temperature control of 25±2°C, illumination of 2000 Lux and a photoperiod of 15 h/ 9h. After 15-20 days, regenerating plants were formed in the light. The formation of green shoots was recorded weekly, test tubes with darkened callus and infection were rejected [
5,
6].
Regenerants with a developed root system and 4-5 leaves, at least 8 cm long, were planted in a pot culture. Previously, the root system was thoroughly washed from the agarised medium and left for a day in glasses with water. The temperature of the soil should correspond to the temperature of the nutrient medium, therefore, the soil was previously sifted, sterilized (3 hours in a dry oven at a temperature of +110°C) and moistened. Vessels with plants were placed in a light room, without direct lighting, so that a sharp change in environmental conditions would not adversely affect the plants.
After that, the regenerating plants were transferred to the greenhouse, where their development continued until flowering, seed formation and maturation. The following microclimate parameters were favorable for rice plants: daytime temperature of 25°C, illumination of more than 5000 Lux; night temperature of 20°C, illumination of 0 Lux; humidity of 70-80%; photoperiod of 12 hours [
5,
6].
One feeding was carried out with a nutrient solution (1/2 MS). Watering of plants was carried out 3-4 times a week, depending on the drying of the soil in the vegetation vessels.
The ploidy of the regenerated plants obtained was identified by morphological features.
The analysis of the results included an assessment of the effectiveness of anther culture according to the following criteria: the number of neoplasms/per 100 anthers, the number of all regenerants/per 100 anthers, the number of green regenerants per 100 anthers and the number of all regenerants per 100 neoplasms. Mathematical and statistical data processing was carried out in the MS Excel program.
3. Results and discussion
The culture of rice anthers is a two-stage process of initial callus development and subsequent regeneration of green plants from embryogenic callus [
13]. The peculiarities of androgenesis in hybrid rice combinations were studied during the cultivation of anthers on the induction medium of Blades.
As a result of the experiment, 12604 anthers of hybrids were extracted from hybrid rice panicles and planted on an induction nutrient medium in 26 crossing combinations (68 panicles). The maximum number of anthers was planted from the hybrid 5016/2 – 339 pcs, and the minimum – 4773/1 – 47 pcs.
When cultivating anthers of all genotypes on an induction medium, neoplasms appeared from them – embryo-like structures (single embryoids and polyembryoids) and callus. When cultured on a regenerative medium, as a rule, the callus and part of the embryo-like structures remained unchanged. Some of the embryo-like structures developed roots, and the other part had single seedlings or clusters of seedlings. The appearance of callus and embryo-like structures began on the 30-33th day from the moment of planting anthers on the nutrient medium. Dense or slightly transparent, well-distinguishable neoplasms were attributed to embryo-like structures. Callus formation continued for another four weeks, i.e. the anthers were on the medium for two months.
As follows from the above data, not all hybrid rice combinations have shown the ability to form callus and embryo-like structures. The amount of callus formation in the culture of anthers varied significantly both between hybrid combinations and in different plants from the same hybrid combination, which is apparently due to genotypic differences, as well as the effect of external factors (explant quality, cultivation conditions, etc.). A total of 716 neoplasms were obtained, on average 10 pcs. per plant, taking into account the unresponsive ones (see
Table 1).
In ability to neoplasms, 60% of the volume of the studied material (39 panicles) showed a positive result, 40% (29 pcs.) did not give callus. The most responsive to the formation of callus were hybrid combinations 5009/2 – 84 pcs, 5010/2 – 94 pcs, 4565/3 – 85 pcs, 4641/2 – 69 pcs. (
Table 1). The same samples showed the ability to morphogenesis, the remaining combinations formed a non-morphogenic callus (in some cases up to 100%). Combinations 5007, 5006, 5011, and 4585 showed a complete lack of reaction to in vitro cultivation.
Green seedlings and albinos developed from the formed neoplasms during cultivation on a regenerative medium, structures developing by the type of the root formation and structures with no development were also noted.
The ability of calluses to morphogenesis was assessed by plant regeneration. The studied rice samples formed 130 regenerating plants using 14 hybrid combinations. Of these, only 30 plants were green. There were 4 samples that formed regenerants without chlorophyll defects in the leaves – 5009/2 – 5 pcs, 5010/2 – 5 pcs, 4565/3 – 2 pcs, 4641/2 – 18 pcs. Albino plants died in the early stages of development, as they were not capable of photosynthesis and, accordingly, of an autotrophic type of nutrition. The largest number of albinos was formed in the sample 5021/1 – 25 pcs .
As a result of evaluating the effectiveness of anther culture in rice hybrids, it was found that the largest number of neoplasms per 100 cultivated anthers (76.9) was observed in sample 5009/2, which significantly exceeded the average value in the experiment (
Table 2).
Morphogenesis and the ability of neoplasms to form seedlings, including green and albino ones, were evaluated on the basis of “the number of all regenerants per 100 planted anthers” feature. A greater number of seedlings were regenerated on the basis of the hybrid combination 5009/2, which was significantly higher than the average value. In general, samples 5009/2 and 4641/2 formed more regenerants: 14.3 and 10.8, respectively.
Green seedlings are of practical interest, therefore, the most important indicator of anther culture is the sign “the number of green regenerants per 100 isolated anthers”. According to this feature, the sample 4641/2 had a significantly high value (9.3). The indicators of the remaining genotypes were at the level of the average value.
To assess the ability of neoplasms to regenerate seedlings, the number of all regenerants per 100 neoplasms was determined. The maximum value for this feature was in the hybrid combination 4641/2 (30.4). On average, the neoplasms of sample 5009/2 (20.2) were also well regenerated by genotypes. The remaining combinations formed fewer seedlings, but within the average in the experiment.
The culture of anthers plays a significant role in the DG technologies of many cereal crops. Dihaploid (DH) plants allow to combine and fix the desired genes of valuable parental genotypes, and are also used in the creation of populations to study genetic coupling and gene mapping. Therefore, works on the study of responsiveness to androgenesis in vitro and the elaboration of methodological nuances are of great importance. Significant differences in the effectiveness of anther culture can be explained by the difference in the origin of the studied genotypes. In general, for combinations 5009/2 and 4641/2, there is a tendency for higher efficiency of anther culture.
Indicators of androgenesis in vitro have a genotypic dependence.
As it follows from the results obtained in this work, there are strong differences in the manifestation of signs of androgenesis in the culture of anthers between rice hybrids F2, carriers of the target genes Sub1A, Snorkel 1, Snorkel 2, AG1 and AG2. At the same time, the low ability to androgenesis in these hybrids could depend on many factors. So, in particular, the success of the cultivation of objects has been proven, depending on the correct choice of the nutrient medium and the thoroughness of its preparation. The initiation of neoplasms in the culture of rice anthers was evaluated using various media (Blades, N6, Gamborg, White) supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D), in combination with α-naphthaleneacetic acid (NAA), kinetin (Kin) or 6-benzylaminopurine (BAP). It was found that the composition of the Blades nutrient medium for the cultivation of rice anthers most closely corresponds to the type of nutrition of this crop. Studies have shown that the rate and frequency of callus induction can be increased up to 27.9%, and in some gene types even higher [
6]. These results are consistent with the studies carried out in our work, namely, the inclusion of 2,4-D in the induction medium 2.0 mg/l allowed to obtain up to 70% of neoplasms per 100 cultivated anthers.
In the course of the work, the quantity and quality of calluses for each hybrid combination were taken into account. Morphogenic calluses were light, opaque, compact and had green chlorophyll-containing areas, which were zones of morphogenesis. This is confirmed in works by other authors [
8]. Thus, callus cultures are characterized by different morphotypes and a high degree of heterogeneity, even if they were obtained from the same donor genotypes and under the same cultivation conditions. This is manifested in the morphological and structural heterogeneity of these tissues.
A systematic study of the needs of rice in the culture of anthers showed that the component composition of the Murashige and Skuga medium, supplemented with hormones, better supports disorganized callus growth and induces morphogenesis, which leads to the formation of callus with a high level of organogenesis. Activation of morphogenesis processes and further development of neoplasms are stimulated by the use of 1.0 mg/l alpha-naphthylacetic acid (NAA) in combination with 5.0 mg/l kinetin in the regeneration medium MS. For example, samples with low regenerative capacity (0.31-0.72%) were isolated; with average (6.07–7.61%) and high (21,65–27,9 %) [
7].
The results are confirmed in our studies. Among the selected hybrid combinations of rice, the number of all regenerants per 100 neoplasms reached a maximum value of 30%, including green ones - from 1 to 9%, i.e. the samples showed low and average regenerative ability.
The analysis of the results showed that the intensity of the processes of callus formation and regeneration of rice plants is due not only to the mineral and organic composition of nutrient media, the nature and concentration of phytohormones, but to a significant extent to genetic factors. Genotypes with a high capacity for callus formation were not always optimal for the induction of morphogenic callus and plant regeneration, since callus formation and regeneration are controlled by different genetic mechanisms.
It is known that the ability to induce neoplasms, regeneration, and the frequency of green seedlings are regulated by different genes and inherited independently. Therefore, in further work, it is possible to use the parental forms of these hybrids in crosses as donors of valuable alleles of high responsiveness to androgenesis in vitro.
The obtained DH-lines of rice based on hybrids will be evaluated in the field according to a complex of economically valuable traits, and the best samples are included in the breeding process.
4. Conclusions
For callogenesis induction 12604 anthers were planted on an induction nutrient medium in 26 hybrid combinations represented by 68 plants, resulting in 716 neoplasms, including 586 non–morphogenic callus, 130 regenerating plants, of which 100 albinos and 30 green ones.
Cultivation of anthers on nutrient medium revealed large genotypic differences in the samples. According to the responsiveness to neoplasms, 60% of the panicles showed a positive result, the rest did not give callus. The most responsive to the formation of callus were hybrid combinations: 5009/2 – 84 pcs, 5010/2 – 94 pcs, 4565/3 – 85 pcs, 4641/2 – 69 pcs. The same samples showed the ability to morphogenesis.
The studied rice samples formed 130 regenerating plants using 14 hybrid combinations. The proportion of androgenic regenerants is 1.03% of the total number of inoculated anthers.
30 green regenerative lines from four rice hybrids were obtained, differing in visual morphological assessment: 5009/2 - 5 pcs, 5010/2 – 5 pcs, 4565/3 – 2 pcs, 4641/2 – 18 pcs.
The isolated lines, characterized by good responsiveness in the culture of anthers in vitro, carry genes for resistance to prolonged flooding (Sub1A, Sk2, AG1, AG2) and can be used in rice breeding programs with the use of DG technologies. On the basis of the obtained androgenic plants, in which chromosome doubling will occur, digaploid lines will be formed, which will later be included in the work on the study of the manifestation of economically valuable and adaptive traits.
Author Contributions
Kalinina N.V. – preparation of experiments, laboratory experiments, data collection, data analysis, and interpretation, preparation of the manuscript, final revision of the text; Kostylev P.I. – conceptualisation of research, preparation of the manuscript, critical analysis of the text. All authors have read and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by grant No. 22-26-00246 of the Russian Science Foundation (RSF).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rukmini Mishra, Gundimeda Rao, In-vitro Androgenesis in Rice: Advantages, Constraints and Future Prospects, Rice Science, 23 (2), 57-68 (2016), https://doi.org/10.1016/j.rsci.2016.02.001. [CrossRef]
- Hridoy Rezvi, Md. Tahjib- Ul-Arif, Md. Abdul Azim, Toufica Tumpa, Mohammad Tipu, Farhana Najnine, Mona Dawood, Milan Skalicky, Marián Brestič, Rice and Food Security: Climate Change Implications and Future Prospects for Food Security. Food and Energy Security, 00, e430 (2022) https://doi.org/10.1002/fes3.430. [CrossRef]
- Subir Pradhan, SR Das and BC Patra, Advances in Rice Breeding: Stress Tolerance, Climate Resilience, Quality & High Yield. ICAR-National Rice Research Institute, Cuttack, Odisha, India, x+426, (2021) https://www.researchgate.net/publication/360672099_Advances_in_Rice_Breeding_Stress_Tolerance_Climate_Resilience_Quality_High_Yield_SK_Pradhan_SR_Das_BC_Patra_Climate-Smart_Rice_Breeding_Progress_and_Challenges_for_the_Rain-fed_ecologies_in_India.
- Elena Dubina, Valentin Shilovsky, Pavel Kostylev, Sergey Garkusha, Viktor Kovalev, Lyubov Yesaulova, Ivan Balyasny, Maria Straholysova, Tu Dinh, Lin Le, Sub1A Gene in Rice Breeding for Tolerance to Flooding as a Factor in Weed Control, Rice Farming, 2 (35), 20-26 (2017).EDN: YUJRYZ.
- Yulia Goncharova, The use of the anther culture method in rice breeding, 91 (2012).
- Elena Savenko, Tatiana Korotenko, Valentina Glazyrina, Lyudmila Shundrina, Accelerated production of genetically stable (homozygous) forms of rice based on selectively valuable samples with target genes of resistance to pyriculariosis by anther culture in vitro method, Proceedings of Kuban State Agrarian University, 85, 213-219 (2020) doi: 10.21515/1999-1703-85-213-219. [CrossRef]
- Yulia Goncharova, Evgeny Kharitonov, Evgenia Malyuchenko, Valentin Sheleg, Rice adaptability to abiotic stresses analysis of breeding efficiency // In the collection “Achievements and prospects for the development of rice breeding and cultivation in temperate countries”, 40-46 (2015).
- Marina Ilyushko, Marina Romashova, The effect of intensity and quality of illumination on the regenerative ability of Oryza sativa L. rice callus obtained in in vitro androgenesis, Russian Agricultural Science, 3, 41-45 (2021) doi: 10.31857/S250026272103008X. [CrossRef]
- Usha Bishnoi, Rakesh Jain [et al.], High frequency androgenesis in indica Basmati rice hybrids using liquid culture media, Plant Cell, Tissue and Organ Culture, 61, 153-159 (1995). [CrossRef]
- Navraj Sarao, Satbir Gosal, In Vitro Androgenesis for Accelerated Breeding in Rice, Biotechnologies of Crop Improvement., 1, 407-435 (2018) doi: 10.1007/978-3-319-78283-6_12. [CrossRef]
- Nadezhda Malysheva, Elena Savenko, Valentina Glazyrina, Lyudmila Shundrina, Obtaining, evaluation and selection of dihaploid rice lines with economically valuable traits, Rice farming, 21, 14-18 (2012).
- Natalia Bushman, Svetlana Vereshchagina, The effectiveness of nutrient media for the induction of callus formation in rice hybrids, Rice farming, 1(22), 13-16 (2013).
- Elena Savenko, Zhanna Mukhina, Valentina Glazyrina, Lyudmila Shundrina, Optimization of cellular technologies in vitro for accelerated generation of rice Oryza sativa L, Bulletin of the State Nikitsky Botanical Garden, 144, 114-121 (2022) https://doi.org/10.36305/0513-1634-2022-144-114-121. [CrossRef]
- Svetlana Guchenko, Comparative characteristics and selection of digaploid rice lines by economically valuable traits, Far Eastern Agrarian Bulletin, 1, 10-15 (2016). URL: https://elibrary.ru/xgqvpx.
- Marina Ilyushko, Marina Romashova, Variability of rice haploids obtained in anther culture in vitro, Russian Agricultural Science, 2, 11-14 (2019) doi:10.31857/S2500-26272019211-14. [CrossRef]
- Yulia Goncharova, Evgenia Malyuchenko, The culture of anthers as a method of creating material for the study of various areas of breeding work, Proceedings of the Kuban State Agrarian University, 66, 70-74 (2017) doi: 10.21515/1999-1703-66-70-74. [CrossRef]
- Elena Savenko, Valentina Glazyrina, Lyudmila Shundrina, Variability of traits in populations of DH rice lines, Rice farming, 2 (55), 6-10 (2022) DOI 10.33775/1684-2464-2022-55-2-6-10. [CrossRef]
- Marina Ilyushko, Marina Romashova, Jumei Zhang, Lingwei Deng, Dong-Jun Liu, Rui Zhang, Svetlana Guchenko, Intracallus variability of doubled rice haploids obtained in in vitro androgenesis, Agricultural Biology, 55(3), 533-543 (2020) doi: 10.15389/agrobiology.2020.3.533rus. [CrossRef]
- Marina Ilyushko, Regenerative maximum in androgenic callus lines of rice Oryza sativa L. in vitro, Rice farming, 2(43), 29-32 (2019).
- Yulia Goncharova, Evgeny Kharitonov, Natalia Bushman, Svetlana Vereshchagina, Comparative analysis of the effectiveness of nutrient media for the induction of callus formation in rice hybrids, Reports of the Russian Academy of Agricultural Sciences, 6, 6-9 (2013).
- Marina Romashova, Marina Ilyushko, Svetlana Companietz, Method of obtaining rice regenerants in anther culture in vitro, Patent for invention RU 2681339 C1, 06.03.2019. Application No. 2018114161 dated April 17, 2018.
Table 1.
Results of cultivation of rice anthers (2022).
Table 1.
Results of cultivation of rice anthers (2022).
| № |
Sample № |
Plant № |
Inoculated anthers, pcs. |
Number of neoplasms, pcs. |
Non-morphogenic callus, pcs. |
Total regenerating plants, pcs. |
Green plants, pcs. |
Albino plants, pcs. |
| 1 |
5022 |
1 |
243 |
4 |
4 |
0 |
0 |
0 |
| 2 |
275 |
0 |
0 |
0 |
0 |
0 |
| 3 |
92 |
0 |
0 |
0 |
0 |
0 |
| 2 |
5103 |
2 |
259 |
20 |
16 |
4 |
0 |
4 |
| 4 |
245 |
2 |
2 |
0 |
0 |
0 |
| 5 |
110 |
0 |
0 |
0 |
0 |
0 |
| 3 |
5007 |
1 |
214 |
0 |
0 |
0 |
0 |
0 |
| 3 |
152 |
0 |
0 |
0 |
0 |
0 |
| 4 |
114 |
0 |
0 |
0 |
0 |
0 |
| 4 |
5005 |
1 |
299 |
37 |
34 |
3 |
0 |
3 |
| 2 |
86 |
0 |
0 |
0 |
0 |
0 |
| 3 |
225 |
1 |
1 |
0 |
0 |
0 |
| 5 |
5029 |
3 |
270 |
0 |
0 |
0 |
0 |
0 |
| 5 |
132 |
1 |
1 |
0 |
0 |
0 |
| 8 |
304 |
1 |
1 |
0 |
0 |
0 |
| 10 |
284 |
0 |
0 |
0 |
0 |
0 |
| 6 |
5006 |
1 |
277 |
0 |
0 |
0 |
0 |
0 |
| 2 |
189 |
0 |
0 |
0 |
0 |
0 |
| 5 |
120 |
0 |
0 |
0 |
0 |
0 |
| 7 |
5093 |
1 |
194 |
1 |
1 |
0 |
0 |
0 |
| 3 |
82 |
0 |
0 |
0 |
0 |
0 |
| 4 |
272 |
0 |
0 |
0 |
0 |
0 |
| 8 |
5019 |
1 |
251 |
8 |
5 |
3 |
0 |
3 |
| 2 |
126 |
3 |
2 |
1 |
0 |
1 |
| 3 |
289 |
1 |
1 |
0 |
0 |
0 |
| 9 |
5003 |
1 |
306 |
0 |
0 |
0 |
0 |
0 |
| 3 |
258 |
1 |
1 |
0 |
0 |
0 |
| 10 |
5009 |
1 |
212 |
0 |
0 |
0 |
0 |
0 |
| 2 |
119 |
84 |
67 |
17 |
5 |
12 |
| 4 |
278 |
12 |
12 |
0 |
0 |
0 |
| 11 |
5010 |
1 |
183 |
0 |
0 |
0 |
0 |
0 |
| 2 |
277 |
94 |
87 |
7 |
5 |
2 |
| 12 |
5011 |
1 |
271 |
0 |
0 |
0 |
0 |
0 |
| 3 |
186 |
0 |
0 |
0 |
0 |
0 |
| 13 |
5008 |
1 |
86 |
3 |
3 |
0 |
0 |
0 |
| 2 |
184 |
0 |
0 |
0 |
0 |
0 |
| 3 |
279 |
21 |
21 |
0 |
0 |
0 |
| 14 |
5020 |
1 |
243 |
26 |
15 |
11 |
0 |
11 |
| 2 |
47 |
0 |
0 |
0 |
0 |
0 |
| 3 |
210 |
13 |
13 |
0 |
0 |
0 |
| 15 |
5018 |
1 |
132 |
1 |
1 |
0 |
0 |
0 |
| 2 |
298 |
5 |
4 |
1 |
0 |
1 |
| 3 |
297 |
23 |
20 |
3 |
0 |
3 |
| 16 |
4565 |
2 |
82 |
3 |
3 |
0 |
0 |
0 |
| 3 |
195 |
85 |
82 |
3 |
2 |
1 |
| 5 |
245 |
46 |
43 |
3 |
0 |
3 |
| 17 |
4773 |
1 |
47 |
4 |
4 |
0 |
0 |
0 |
| 2 |
114 |
0 |
0 |
0 |
0 |
0 |
| 3 |
59 |
1 |
1 |
0 |
0 |
0 |
| 18 |
5016 |
2 |
339 |
1 |
1 |
0 |
0 |
0 |
| 3 |
120 |
0 |
0 |
0 |
0 |
0 |
| 4 |
216 |
0 |
0 |
0 |
0 |
0 |
| 19 |
4758 |
1 |
209 |
4 |
2 |
2 |
0 |
2 |
| 20 |
5021 |
1 |
248 |
62 |
37 |
25 |
0 |
25 |
| 2 |
112 |
3 |
1 |
2 |
0 |
2 |
| 3 |
140 |
0 |
0 |
0 |
0 |
0 |
| 21 |
4641 |
1 |
193 |
5 |
4 |
1 |
0 |
1 |
| 2 |
194 |
69 |
48 |
21 |
18 |
3 |
| 22 |
5017 |
1 |
51 |
4 |
2 |
2 |
0 |
2 |
| 2 |
255 |
2 |
1 |
1 |
0 |
1 |
| 3 |
195 |
42 |
32 |
10 |
0 |
10 |
| 23 |
4526 |
1 |
80 |
10 |
3 |
7 |
0 |
7 |
| 24 |
4688 |
1 |
85 |
12 |
9 |
3 |
0 |
3 |
| 2 |
117 |
0 |
0 |
0 |
0 |
0 |
| 3 |
57 |
0 |
0 |
0 |
0 |
0 |
| 25 |
4617 |
1 |
83 |
1 |
1 |
0 |
0 |
0 |
| 26 |
4585 |
1 |
104 |
0 |
0 |
0 |
0 |
0 |
| 2 |
94 |
0 |
0 |
0 |
0 |
0 |
| Sum |
69 |
12604 |
716 |
586 |
130 |
30 |
100 |
| Average |
2.5 |
185.35 |
10.53 |
8.6 |
1.91 |
0.44 |
1.47 |
| Minimum |
1 |
47 |
0 |
0 |
0 |
0 |
0 |
| Maximum |
4 |
339 |
94 |
87 |
25 |
18 |
25 |
Table 2.
Results of evaluation of the effectiveness of anther culture of isolated rice samples (2022).
Table 2.
Results of evaluation of the effectiveness of anther culture of isolated rice samples (2022).
| Sample № |
Plant № |
Number of neoplasms/
per 100 anthers |
The number of all regenerants/ per 100 anthers |
Number of green regenerants/
per 100 anthers |
The number of all regenerants/ per 100 neoplasms |
| 5009 |
2 |
70.6* |
14.3* |
4.2 |
20.2 |
| 5010 |
2 |
33.9 |
2.5 |
1.8 |
7.5 |
| 4565 |
3 |
43.6 |
1.5 |
1.0 |
3.5 |
| 4641 |
2 |
35.6 |
10.8 |
9.3* |
30.4* |
| Average value |
|
45.9 |
7.3 |
4.1 |
15.4 |
| Standard deviation |
|
17.0 |
6.3 |
3.7 |
12.3 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).