1. Introduction
All animal species, including humans, exhibit individual behavioral characteristics that can be inherited. Individual behavioral characteristics that are inherited traits benefit organisms by optimizing their adaptability due to natural selection [
1]. In addition to natural selection, artificial selection allows humans to create populations of animals with given individual characteristics. Therefore, in experimental physiology and pharmacology, selective strains of mice and rats with behavioral features are used, for example, with different levels of motor activity [
2,
3], anxiety [
4,
5,
6] , or learnability [
7,
8] . In addition to genetic mechanisms of inheritance, various epigenetic influences can affect the formation of individual behavioral characteristics in offspring. These can be intrauterine effects of physical [
9,
10], chemical [
11,
12], or stress [
13,
14] factors on a pregnant female. In addition, environmental factors such as physical [
15,
16], chemical [
17,
18], and social [
19,
20,
21] factors as well as emotional stress [
22] can influence the formation of behavioral characteristics, acting during the postnatal and adolescence periods. Additionally, the behavior of adult animals can change under the influence of age [
23]. The aging process has been shown to have a huge impact on the behavior of animals and humans [
24,
25]. Thus, the initial genetic individual behavioral characteristics of an animal, in the course of life, can change significantly due to various epigenetic influences.
Previously, we reported that an epigenetic influence on the formation of the behavior of future offspring was possible at the time of conception [
26] . This influence can come from the male mating with the female, i.e., in addition to the transfer of genetic material, the male can provide non-genetic inheritance of his behavioral characteristics.
The purpose of this work was to study the, so far, unexplored possibility that non-genetic inheritance of animal behavioral characteristics could depend on the state of the parents at the time of conception. At the same time, we studied inheritance in the first generation of high and low motor and exploratory activity in rats.
2. Materials and Methods
Animals
The experiments were carried out on Wistar rats that weighed 210–240 g (males) and 170–200 g (females) and were obtained from the Stolbovaya Animal Clinic (Moscow, Russia). The rats were housed in ventilated Techniplast Green Line 1500U cages with natural corn bedding (“Zolotoy Kot”, Voronezh, Russia), at 4–5 rats in each cage, with free access to water and combined food (3 kcal/g, “Profgryzun”, Moscow, Russia), at a temperature of 21°C, average humidity 20%, and in the presence of lighting (90 lux) from 20:00 to 08:00.
The protocols and procedures for this study were reviewed and approved by the ethics committee of the P.K. Anokhin (permit number 375) and comply with Directive 2010/63/EC.
Determining the Level of Motor Activity
First, 53 male and 62 female rats, at the age of 2 months, were placed in individual experimental chambers (Phenomaster, TSE, Germany), where horizontal locomotor activity was automatically measured for 60 min over intervals of 10 min. The experimental chambers were identical to the “home” cages in which the rats were housed in. The experiments were carried out from 11:00 to 15:00 in the absence of light. All females during the measurement of motor activity were in a state of diestrus, determined by a vaginal smear.
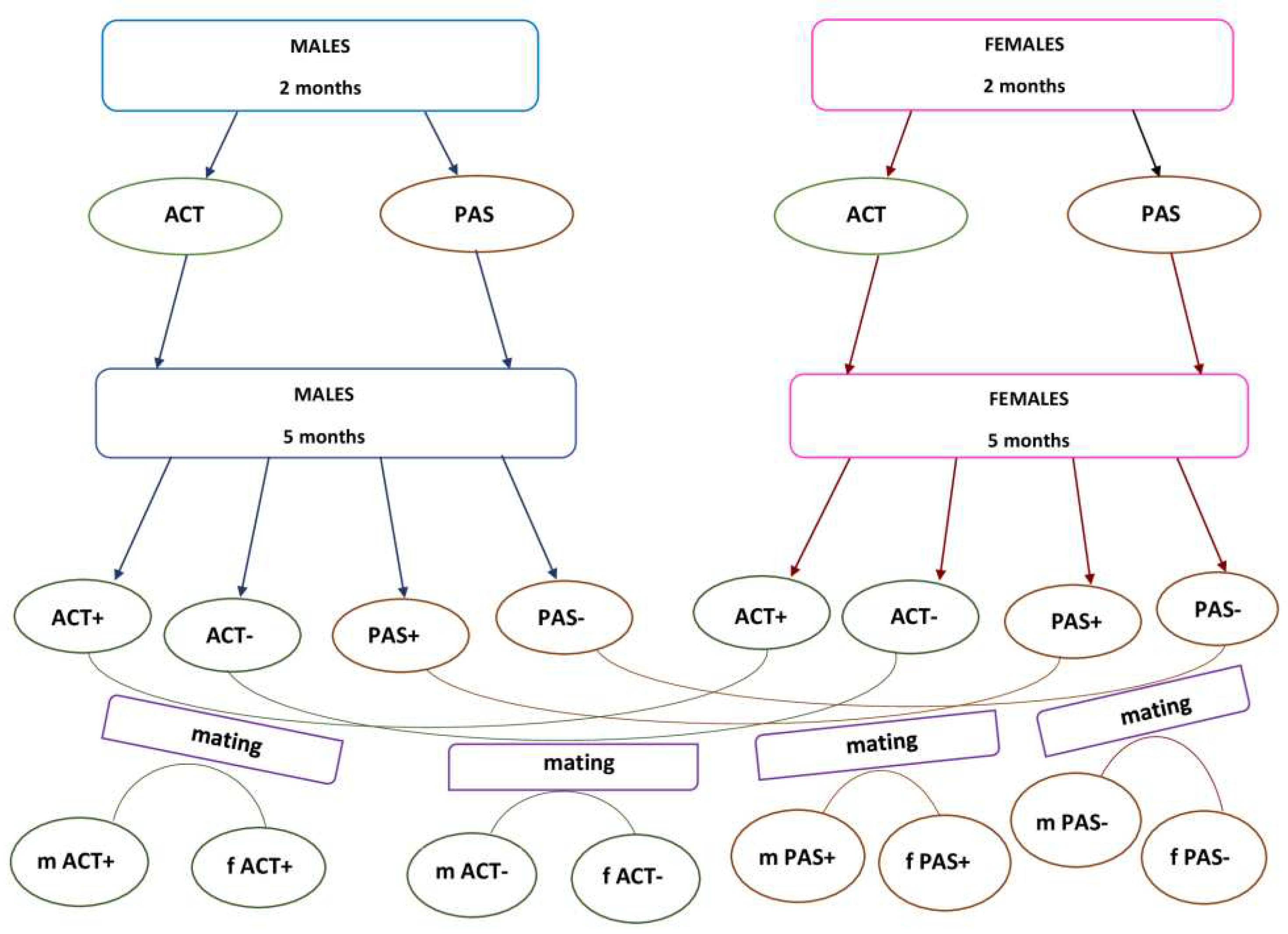
Based on these results, two groups of males and two groups of females were formed with 20 animals in each group. The ACT groups of male and female rats had the highest horizontal motor activity. They also had the highest motor activity in the center of the cage, and more rearings. The PAS groups of male and female rats had the lowest horizontal motor activity, low motor activity in the center of the cage, and a small number of rearings.
At the age of 5 months, ACT and PAS animals were returned to the Phenomaster experimental chambers, where the level of motor activity and the number of rearings were examined for 1 hour. After repeated testing, four groups of animals were identified. Group 1 (ACT+) included rats from the ACT group, which at the age of 5 months increased their activity as compared with their activity at the age of 2 months. Group 2 (ACT–) included rats from the ACT group, which significantly decreased their activity at the age of 5 months as compared with their activity at the age of 2 months. Group 3 (PAS+) included rats from the PAS group, which at the age of 5 months increased their activity as compared with their activity at the age of 2 months. Group 4 (PAS–) included rats from the PAS group, which decreased their activity at the age of 5 months as compared with their activity at the age of 2 months. The level of general motor activity, motor activity in the center of the cage, and the number of rearings of animals in each group are shown in
Table 1.
Immediately upon reaching the state of oestrus, females after the study of motor activity at the age of 5 months were mated with males. The following pairings were carried out:
Mating 3 ACT+ females with 2 ACT+ males resulted in offspring of 17 males mACT+ and 15 females fACT+.
Mating 4 ACT– females with 2 ACT– males resulted in offspring of 24 males mACT– and 15 females fACT–.
Mating 4 PAS+ females with 2 PAS+ males resulted in offspring of 15 males mPAS+ and 11 females fPAS+.
Mating 3 PAS– females with 2 PAS– males resulted in offspring of 17 males mPAS– and 15 female fPAS–.
Figure 1.
Scheme of the experiment.
Figure 1.
Scheme of the experiment.
Statistics. Statistics were performed using the statistical software Statistica 10.0. The data obtained in each experimental subgroup was assessed for normality using the Shapiro–Wilk and Kolmogorov–Smirnov tests. If the test showed the absence of a normal distribution, the calculations were carried out using the Kruskal–Wallis nonparametric test with a post hoc Newman–Keuls test. Differences were considered to be significant at p < 0.05.
3. Results
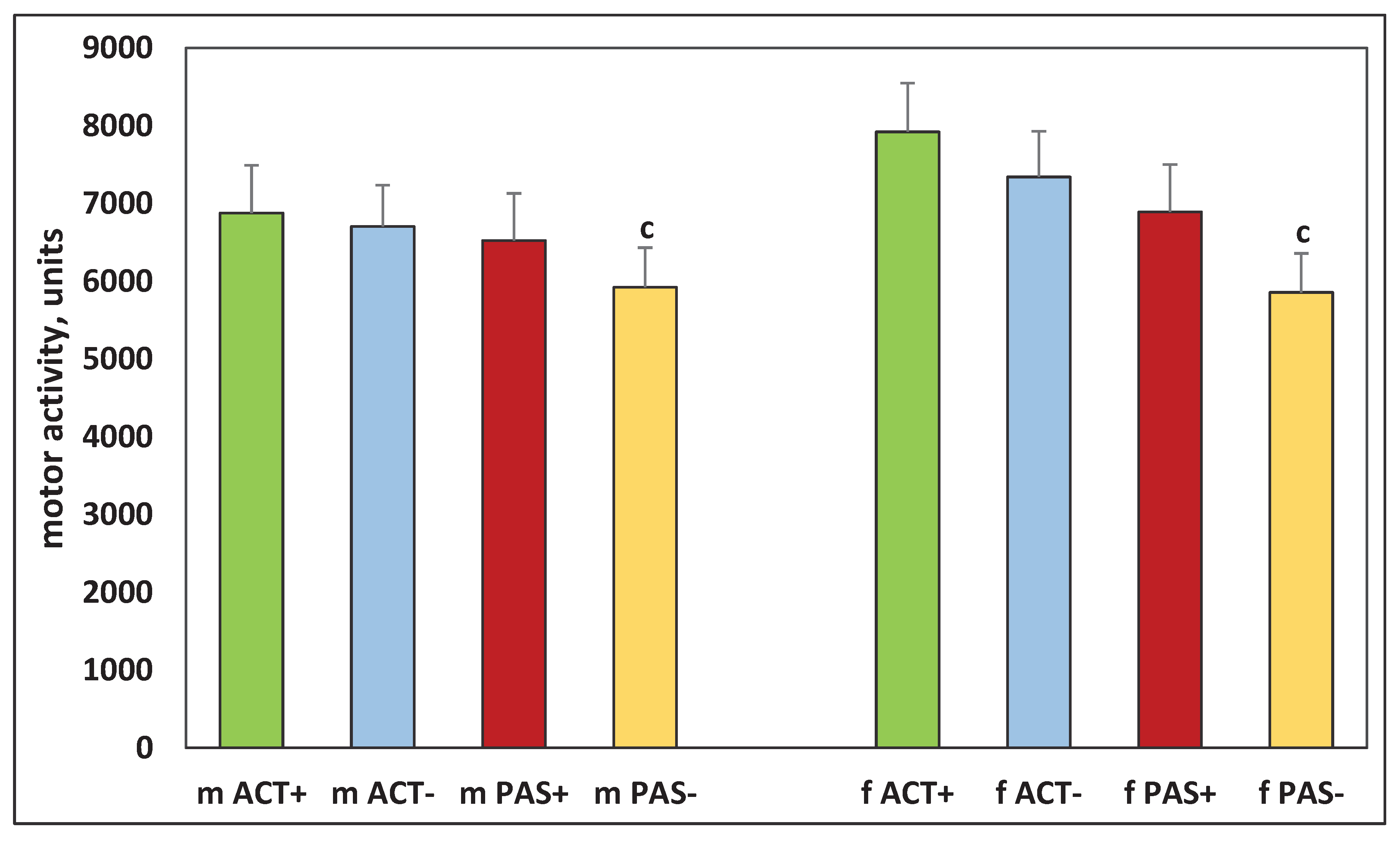
It was found that there were significant differences in the behavior of the offspring resulting from the mating of animals that had different levels of activity. Thus, both males and females born from highly active males and females which only increased their activity by five months of life (ACT+) had significantly higher motor activity, which was observed in the first 10 minutes (H (1,
N = 34) =18.24421,
p = 0.00002 for males), (H (1,
N = 30) =10.92001,
p = 0.0010 for females) (
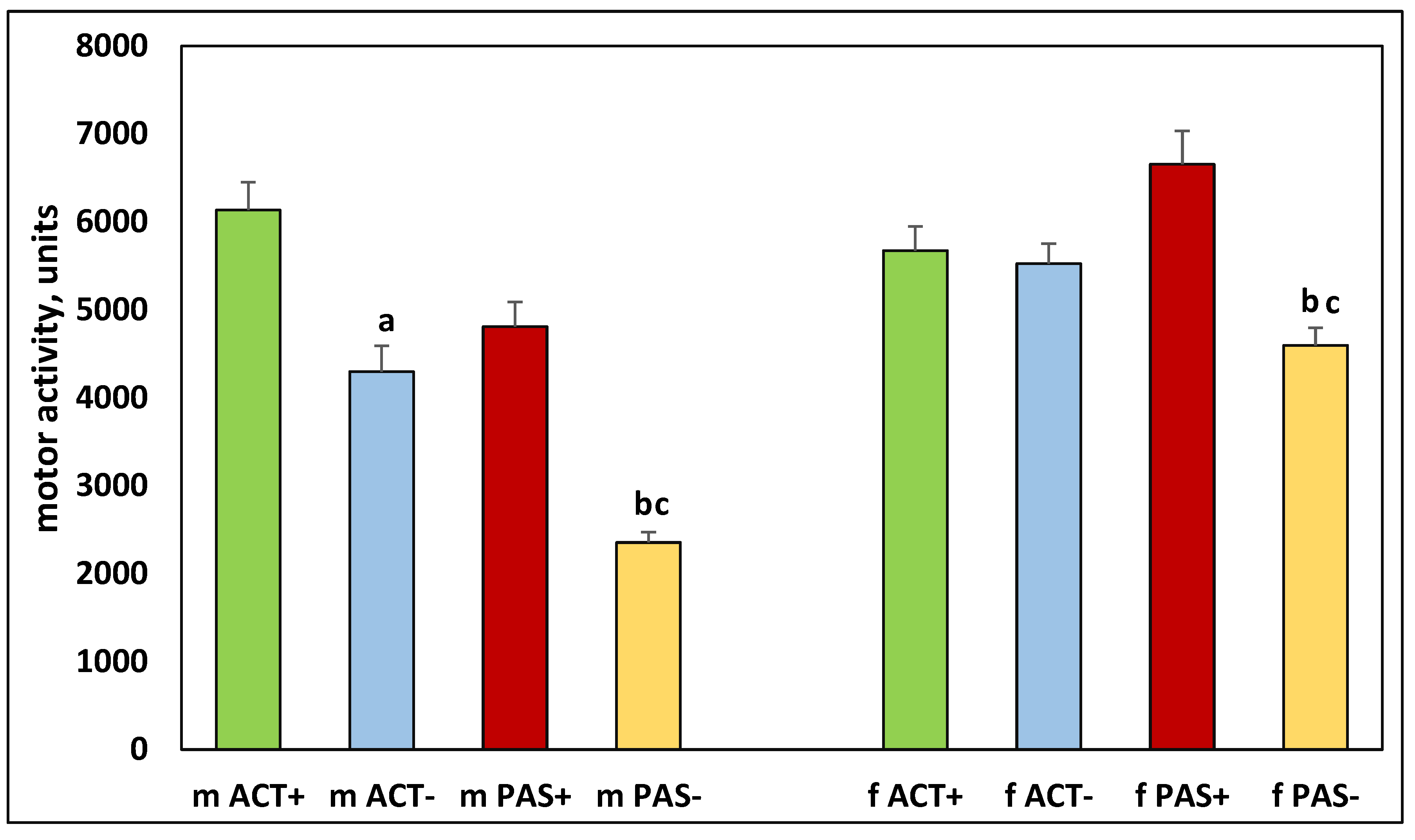
Figure 2), in the next 20–60 minutes (H (1,
N = 34) = 6.187675,
p = 0.0129 for males), (H (1,
N = 30) =15.54319,
p = 0.0004 for females) (
Figure 3), in the center of the cell (H (1,
N = 34) = 5.241954,
p = 0.022 for males), (H (1,
N = 30) = 8.963092,
p = 0.0113 for females) (
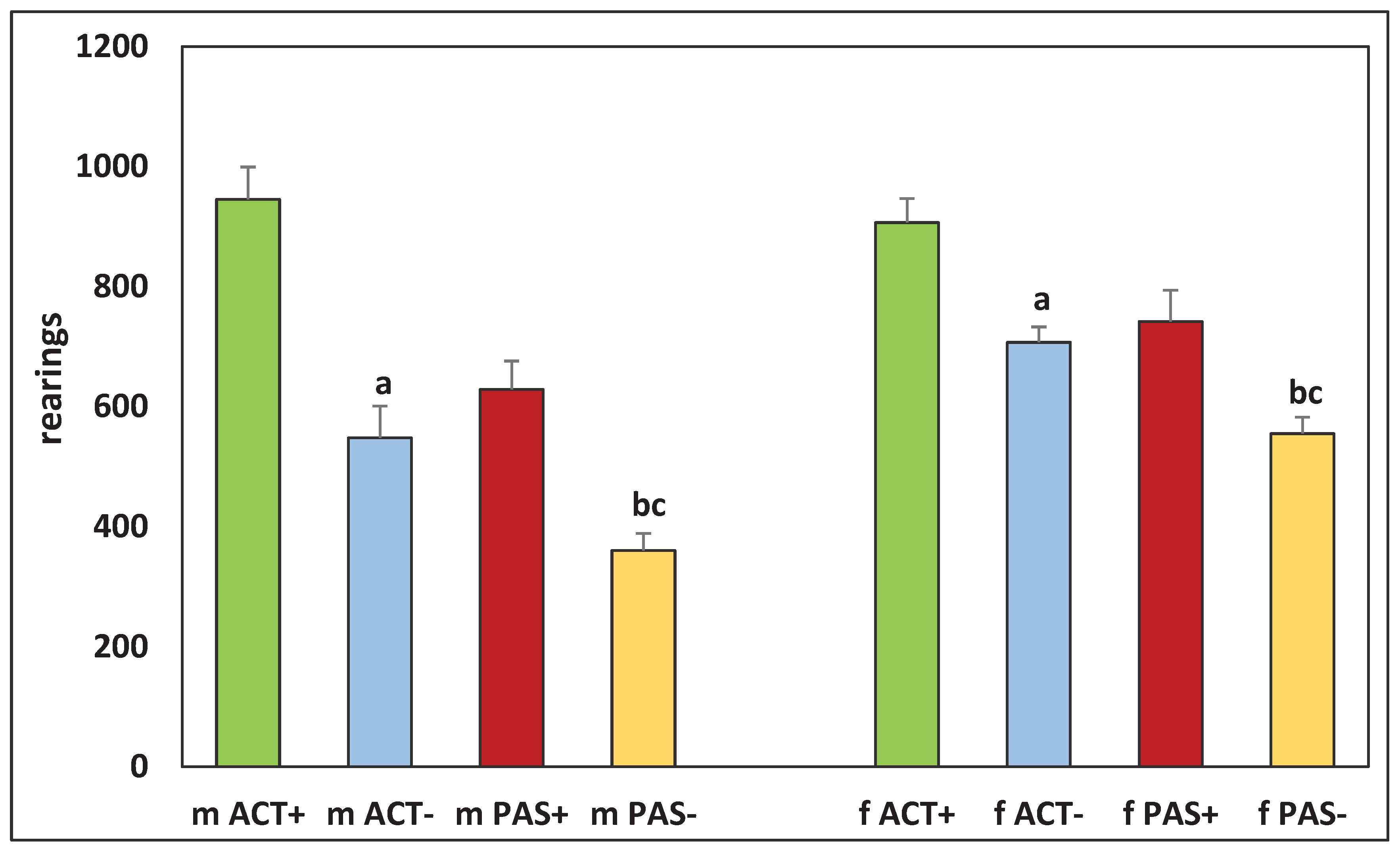
Figure 4) and more rearings (H (1,
N = 34) = 7.943444,
p = 0.0048 for males) and (H (1,
N = 30) = 21.01156,
p = 0.00001 for females) (
Figure 5) as compared with low-active rats, which decreased their activity even more by the age of five months (PAS–).
In the offspring whose parents were five-month-old rats that changed their motor activity as compared with two-month-old ones, differences in behavior were observed. Thus, there were significant differences in all parameters between groups of males mACT+ and mACT– (H (1, N = 41) = 12.29345,
p = 0.005). At the same time, in females fACT + and fACT–, there were no significant differences in all indicators, except for the number of rearings (H (1, N = 30) = 6.125567,
p = 0.0133) (Figures 2, 3, 4, and 5). There were also significant differences between the offspring of PAS+ and PAS– animals, both in males (H (1, N = 32) = 7.37458,
p = 0.0047) and in females (H (1, N = 26) = 11.47563,
p = 0.0007). There were no significant differences in motor activity in the period from 20 to 60 minutes of measurement between groups of offspring of five-month-old rats that changed their activity (
Figure 3).
Figure 2.
Motor activity in the first 10 minutes of measurement in the offspring of ACT+, ACT–, PAS+, and PAS– rats. a p < 0.05 between ACT+ and ACT–, b p < 0.05 between PAS+ and PAS-, c p < 0.05 between ACT+ and PAS–. Values are presented as M ± SEM.
Figure 2.
Motor activity in the first 10 minutes of measurement in the offspring of ACT+, ACT–, PAS+, and PAS– rats. a p < 0.05 between ACT+ and ACT–, b p < 0.05 between PAS+ and PAS-, c p < 0.05 between ACT+ and PAS–. Values are presented as M ± SEM.
Figure 3.
Motor activity from 20 to 60 minutes of measurement in the offspring of ACT+, AC–, PAS+ and PAS– rats. The designations are the same as in
Figure 2.
Figure 3.
Motor activity from 20 to 60 minutes of measurement in the offspring of ACT+, AC–, PAS+ and PAS– rats. The designations are the same as in
Figure 2.
Figure 4.
Motor activity in the center of the cage in the offspring of ACT+
ACT–, PAS+, and PAS– rats. The designations are the same as in
Figure 2.
Figure 4.
Motor activity in the center of the cage in the offspring of ACT+
ACT–, PAS+, and PAS– rats. The designations are the same as in
Figure 2.
Figure 5.
The number of rearings in the offspring of ACT+, ACT–, PAS+, and PAS– rats. The designations are the same as in
Figure 2.
Figure 5.
The number of rearings in the offspring of ACT+, ACT–, PAS+, and PAS– rats. The designations are the same as in
Figure 2.
4. Discussion
The results of the experiment on the mating of rats with high (ACT+) and low (PAS–) motor and exploratory activity, determined at the age of both two and five months, showed that the offspring obtained from these parents also differed in their activity levels. This was in agreement with previously described results by [
5,
27] and was solely attributed to genetic inheritance. As a rule, general motor activity is inherited. It is known that, when an animal is placed in a new cage, initially, increased motor activity is observed associated with examination of the space for potentially dangerous or beneficial factors [
28] . An analysis of motor activity at various time intervals after an animal is placed in an experimental chamber shows that, in the first 10 minutes, increased activity occurs, which decreases by 20 minutes. In offspring from ACT+ and PAS– parents, differences were observed both in general locomotor activity and in the level of exploratory activity, defined as the intensity of movement in the first 10 minutes, the intensity of movement in the center, and the number of rearings.
As we showed earlier [
23], in adult rats from two months to five months of age, significant changes in the level of motor activity and anxiety-like behavior can occur. This allowed us, through observation, to separate the genetic and non-genetic inheritance of the features of these forms of behavior. From the same group of highly active rats at the age of two months, animals were isolated in which the activity increased even more by the age of five months and animals in which it decreased to the level of passive animals. If we assume that the genetic characteristics of these animals have not changed, then the differences we obtained in the level of behavior of the offspring from animals of these groups are not related to genetic inheritance. We found significant differences in the severity of exploratory activity between the offspring of
ACT+ and ACT– rats. Moreover, these differences were observed only in males, but not in females. Differences between the offspring of PAS+ and PAS– rats were observed in both males and females. The motor activity of animals in the period from 20 minutes after the start of measurements did not differ between groups. Thus, it can be considered that the inheritance of individual characteristics of general motor activity is carried out mainly due to genetic factors, while differences in the level of exploratory activity, apparently, are formed due to non-genetic influences from parents during mating.
It remains to be unclear what these non-genetic effects might be. There are several possible explanations that require further investigation. First, the formation of offspring behavior can be influenced by the action of certain pheromones. Thus, it has been found that the presentation of the male pheromone before mating enhanced the development of mammary glands in females during pregnancy, which was reflected in the development of the mammary glands in the postpartum period during lactation. It has been shown that in the offspring of females treated with male pheromone, there was an increase in the expression of polysialyltransferases in the brain. At an older age, such offspring showed a greater ability for spatial learning [
29] .
Secondly, the effect of maternal education is not excluded, apparently, more active females raise more active offspring despite genetics; however, the data on this subject are contradictory. For example, it has been shown that neither exploratory nor play behavior, nor the level of anxiety in rats significantly depend on maternal influence [
30,
31]. In addition, cross fostering had no effect on genetically determined behavioral changes in the open field and elevated plus maze tests. [
32]. However, to confirm the presence or absence of maternal influence on the formation of exploratory behavior, additional experiments are required.
Undoubtedly, our study has certain limitations. One limitation is the relatively small number of litters (3 or 4). However, the high significance of the data obtained and a large number of animals in the groups provide grounds to consider our conclusions as relatively reasonable.
It is possible that the phenomenon we discovered is important for maintaining a certain level of exploratory activity in animals of specific populations. It counteracts natural selection, which would lead to a constant increase in the activity of animals.
5. Conclusions
Individual characteristics of general motor activity are due to genetically inherited factors, while differences in the level of exploratory activity, apparently, are formed due to non-genetic influences from parents during mating.
Author Contributions
Conceptualization: SKS; Methodology: SKS; Investigation: NGB, GAN; Visualization: NGB; Supervision: SKS; Writing—original draft: SKS; Writing—review & editing: SKS, NGB, GAN.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocols and procedures for this study were ethically reviewed and approved by the Animal Care and Use Committee of the P.K. Anokhin Research Institute of Normal Physiology (Permission number 375) and conform to Directive 2010/63/EU.
Data Availability Statement
References
- Roff DA, Heibo E, Vøllestad LA (2006) The importance of growth and mortality costs in the evolution of the optimal life history. J Evol Biol. 19:1920-1930. [CrossRef] [PubMed]
- Garland TJ, Kelly SA (2006) Phenotypic plasticity and experimental evolution. J Exp Biol. 209:2344-2361. [CrossRef]
- Smyers ME, Bachir KZ, Britton SL, Koch LG, Novak CM (2015) Physically active rats lose more weight during calorie restriction. Physiol Behav. 139:303-313. [CrossRef]
- Ramos A, Correia EC, Izídio GS, and Brüske GR (2003) Genetic selection of two new rat lines displaying different levels of anxiety-related behaviors. Behav Genet. 33:657-668. [CrossRef]
- Ohl F (2005) Animal models of anxiety. Handb Exp Pharmacol 169:35–69. [CrossRef]
- Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmanna M et al (2007) Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci Biobehav Rev 31:89–102. [CrossRef]
- Giorgi O, Piras G, Corda MG (2007) The psychogenetically selected Roman high- and low-avoidance rat lines: a model to study the individual vulnerability to drug addiction. Neurosci Biobehav Rev 31:148-163. [CrossRef]
- Ohta R, Kojima K (2019) Hatano rats selectively bred for high- and low-avoidance learning: an overview. Exp Anim 68:127-136. [CrossRef]
- Zhang Y, Li Z, Gao Y, Zhang C (2015) Effects of fetal microwave radiation exposure on offspring behavior in mice. J Radiat Res 56:261-268. [CrossRef]
- Azimzadeh M, Jelodar G (2020) Prenatal and early postnatal exposure to radiofrequency waves (900 MHz) adversely affects passive avoidance learning and memory. Toxicol Ind Health 36:1024-1030. [CrossRef]
- Rouzer SK, Cole JM, Johnson JM, Varlinskaya EI, Diaz MR (2017) Moderate Maternal Alcohol Exposure on Gestational Day 12 Impacts Anxiety-Like Behavior in Offspring. Front Behav Neurosci 11:183. [CrossRef]
- Dong T, Guan Q, Hu W, Zhang M, Zhang Y, Chen M et al (2020) Prenatal exposure to glufosinate ammonium disturbs gut microbiome and induces behavioral abnormalities in mice. J Hazard Mater 389:122152. [CrossRef]
- Babenko O, Kovalchuk I, Metz GA (2015) Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev 48:70-91. [CrossRef]
- Bock J, Wainstock T, Braun K, Segal M (2015) Stress in Utero: Prenatal Programming of Brain Plasticity and Cognition. Biol Psychiatry 78:315-326. [CrossRef]
- Stasinopoulou M, Fragopoulou AF, Stamatakis A, Mantziaras G, Skouroliakou K, Papassideri IS et al (2016) Effects of pre- and postnatal exposure to 1880-1900MHz DECT base radiation on development in the rat. Reprod Toxicol 65:248-262. [CrossRef]
- Zhang JP, Zhang KY, Guo L, Chen QL, Gao P, Wang T et al (2017) Effects of 1.8 GHz Radiofrequency Fields on the Emotional Behavior and Spatial Memory of Adolescent Mice. Int J Environ Res Public Health 14:1344. [CrossRef]
- Spear LP (2018) Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci 19:197-214. [CrossRef]
- Laviolette SR (2021) Molecular and neuronal mechanisms underlying the effects of adolescent nicotine exposure on anxiety and mood disorders. Neuropharmacology 184:108411. [CrossRef]
- Branchi I (2009) The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev 33:551-559. [CrossRef]
- Kappeler L, Meaney MJ (2010) Epigenetics and parental effects. Bioessays 32:818-827. [CrossRef]
- Sparling JE, Baker SL, Bielajew C (2018) Effects of combined pre- and post-natal enrichment on anxiety-like, social, and cognitive behaviours in juvenile and adult rat offspring. Behav Brain Res 353:40-50. [CrossRef]
- Hollis F, Isgor C, Kabbaj M (2013) The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience 249:232-241. [CrossRef]
- Sudakov SK, Alekseeva EV, Nazarova GA, Bashkatova VG (2021) Age-Related Individual Behavioural Characteristics of Adult Wistar Rats. Animals 11:2282. . [CrossRef]
- Wallace JE, Krauter EE, Campbell BA (1980) Motor and reflexive behavior in the aging rat. J Gerontol 35:364-370. [CrossRef]
- Altun M, Bergman E, Edström E, Johnson H Ulfhake B (2007) Behavioral impairments of the aging rat. Physiol Behav 92:911-923. [CrossRef]
- Sudakov SK, Bogdanova NG, Nazarova GA (2022) Cross-generational impact of epigenetic male influence on physical activity in rat. Biology. 2022; 11(11):1606. [CrossRef]
- Stead JD, Clinton SM, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H (2006) Selective Breeding for Divergence in Novelty-seeking Traits: Heritability and Enrichment in Spontaneous Anxiety-related Behaviors. Behavioral Genetics 36:697–712. [CrossRef]
- Kõiv K, Matrov D, Uusen T, Harro J (2021) Effect of Neuropeptide S Administration on Ultrasonic Vocalizations and Behaviour in Rats with Low vs. High Exploratory Activity. Pharmaceuticals (Basel) 14:524. [CrossRef]
- Koyama S, Soini HA, Wager-Miller J, Alley WR, Pizzo MJ, Rodda C. et al (2015) Cross-generational impact of a male murine pheromone 2-sec-butyl-4,5-dihydrothiazole in female mice. Proc Biol Sci 282:1811. [CrossRef]
- Clinton SM, Bedrosian TA, Abraham AD, Watson SJ, Akil H (2010) Neural and environmental factors impacting maternal behavior differences in high- versus low-novelty-seeking rats. Horm Behav 57:463-473. [CrossRef]
- Siviy SM, Eck SR, McDowell LS, Soroka J (2017) Effects of cross-fostering on play and anxiety in juvenile Fischer 344 and Lewis rats. Physiol Behav 169:147-154. [CrossRef]
- Schroeder M, Weller A (2010) Anxiety-like behavior and locomotion in CCK1 knockout rats as a function of strain, sex and early maternal environment. Behav Brain Res 211:198-207. [CrossRef]
Table 1.
Activity of rats of different experimental groups at the age of 2 and 5 months.
Table 1.
Activity of rats of different experimental groups at the age of 2 and 5 months.
| |
General motor activity
(units)
|
Motor activity in the first 10 minutes
(units) |
Motor activity in the center of the cage
(units) |
Number of rearings
|
2
months |
5
months |
2
months |
5
months |
2
months |
5 months |
2
months |
5 months |
Males
|
ACT+ (n=2) |
7592±716 |
11026±850 |
2110±96 |
2725±127 |
4235±248 |
5855±297 |
935±54
|
938±77 |
|
ACT- (n=2) |
10962±731 |
6640±615 |
3416±205 |
1935±121 |
4297±293 |
2647±215 |
548±53 |
435±61 |
|
PAS+ (n=2) |
3199±287 |
8110±640 |
1426±156 |
1897±154 |
1809±170 |
3420±148 |
629±47 |
675±39 |
|
PAS- (n=2) |
6477
±
511
|
4580
±
529
|
1977±122 |
1180±138 |
2355±116
|
1285±170
|
360±28 |
273±23
|
| Females |
ACT+ (n=3) |
7893±576 |
10364±758 |
2547±178
|
2874±157 |
4137±216 |
5684±277 |
907±40 |
1108±97 |
|
ACT- (n=4) |
13328±775 |
7001±861 |
3790±184 |
2420±218 |
5525±226 |
2971±128 |
707±26 |
586±55 |
|
PAS+ (n=4) |
2998±385 |
7644±743 |
1404±120 |
2296±212 |
1857±179 |
3120±124 |
342±47 |
525±33 |
|
PAS- (n=3) |
6673±589 |
4081±651 |
2013±77 |
1535±177 |
4597±198 |
1357±120 |
555±27 |
260±27 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).