Submitted:
26 January 2023
Posted:
30 January 2023
You are already at the latest version
Abstract
Keywords:
Introduction
The Extracellular Matrix Macromolecules
Collagens
Other Extracellular Matrix macromolecules
Laminin
Fibronectin
Periostin
Hyaluronic acid
Extracellular Matrix Function
Extracellular Matrix Modifications
Proteolytic Degradation of the Extracellular Matrix
Fibroblasts and Extracellular Matrix Remodeling
Extracellular Matrix Signaling
Extracellular Matrix and Cell Invasion and Metastasis
Extracellular Matrix in Drug Resistance: The Extracellular Matrix shield tumor cells from anti-cancer drugs
Therapeutic strategies targeting the Extracellular Matrix
Conclusions
References
- Bissell, M.J.; Hall, H.G.; Parry, G. How does the extracellular matrix direct gene expression? Journal of theoretical biology 1982, 99, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Bissell, M.J. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2006, 22, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of extracellular matrix in development and cancer progression. International journal of molecular sciences 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Pupa, S.M.; Ménard, S.; Forti, S.; Tagliabue, E. New insights into the role of extracellular matrix during tumor onset and progression. Journal of cellular physiology 2002, 192, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Clause, K.C.; Barker, T.H. Extracellular matrix signaling in morphogenesis and repair. Current opinion in biotechnology 2013, 24, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Vogelsang, M.; Parker, M.I. Wnt/β-catenin and MEK-ERK signaling are required for fibroblast-derived extracellular matrix-mediated endoderm differentiation of embryonic stem cells. Stem Cell Reviews and Reports 2015, 11, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Turnley, T.; Wishart, A.; Rowe, A.; Kallmeyer, K.; Van Vollenstee, F.A.; Thomford, N.E.; Dandara, C.; Chopera, D.; Pepper, M.S. Fibroblast-derived extracellular matrix induces chondrogenic differentiation in human adipose-derived mesenchymal stromal/stem cells in vitro. International journal of molecular sciences 2016, 17, 1259. [Google Scholar] [CrossRef]
- Yin, H.; Wang, J.; Li, H.; Yu, Y.; Wang, X.; Lu, L.; Lv, C.; Chang, B.; Jin, W.; Guo, W.; et al. Extracellular matrix protein-1 secretory isoform promotes ovarian cancer through increasing alternative mRNA splicing and stemness. Nature Communications 2021, 12, 4230. [Google Scholar] [CrossRef]
- Dussoyer, M.; Page, A.; Delolme, F.; Rousselle, P.; Nyström, A.; Moali, C. Comparison of extracellular matrix enrichment protocols for the improved characterization of the skin matrisome by mass spectrometry. J Proteomics 2022, 251, 104397. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Gonzales, N.L.; Beall, J.M.; Pechanec, M.Y. Basic Structure, Physiology, and Biochemistry of Connective Tissues and Extracellular Matrix Collagens. Adv Exp Med Biol 2021, 1348, 5–43. [Google Scholar] [CrossRef]
- Giblin, S.P.; Schwenzer, A.; Midwood, K.S. Alternative splicing controls cell lineage-specific responses to endogenous innate immune triggers within the extracellular matrix. Matrix Biol 2020, 93, 95–114. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, Y.; Li, C.; Mao, H.; Liu, B.; Jiang, X. Modulating Tumor Extracellular Matrix by Simultaneous Inhibition of Two Cancer Cell Receptors. Adv Mater 2021, e2109376. [Google Scholar] [CrossRef]
- Gu, X.; Ge, L.; Ren, B.; Fang, Y.; Li, Y.; Wang, Y.; Xu, H. Glucocorticoids Promote Extracellular Matrix Component Remodeling by Activating YAP in Human Retinal Capillary Endothelial Cells. Front Cell Dev Biol 2021, 9, 738341. [Google Scholar] [CrossRef] [PubMed]
- Laurito, T.L.; França, F.T.; Vieira-Damiani, G.; Pelegati, V.B.; Baratti, M.O.; de Carvalho, H.F.; Cesar, C.L.; de Moraes, A.M.; Cintra, M.L.; Teixeira, F. The texture of collagen in the microenvironments of Merkel cell carcinoma. Medicine (Baltimore) 2021, 100, e27925. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. Taking a Full Snapshot of Cancer Biology: Deciphering the Tumor Microenvironment for Effective Cancer Therapy in the Oncology Clinic. Omics 2020, 24, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Leaner, V.D.; Parker, M.I. Feedback regulation of the α2(1) collagen gene via the Mek-Erk signaling pathway. IUBMB Life 2012, 64, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Jonker, T.; Rowe, A.; Thomford, N.E.; Munro, D.; Dandara, C.; Wonkam, A.; Govender, D.; Calder, B.; Soares, N.C.; et al. The Role of Tumor Microenvironment in Chemoresistance: 3D Extracellular Matrices as Accomplices. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Mazeedi, M.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef]
- Cox, T.R. The matrix in cancer. Nat Rev Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Bissell, M.J.; Hall, H.G.; Parry, G. How does the extracellular matrix direct gene expression? J Theor Biol 1982, 99, 31–68. [Google Scholar] [CrossRef]
- Seo, B.R.; Bhardwaj, P.; Choi, S.; Gonzalez, J.; Eguiluz, R.C.A.; Wang, K.; Mohanan, S.; Morris, P.G.; Du, B.; Zhou, X.K. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Science translational medicine 2015, 7, 301ra130–301ra130. [Google Scholar] [CrossRef]
- Lukashev, M.E.; Werb, Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends in cell biology 1998, 8, 437–441. [Google Scholar] [CrossRef]
- Ghajar, C.M.; Bissell, M.J. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochemistry and cell biology 2008, 130, 1105. [Google Scholar] [CrossRef]
- Dzobo, K.; Vogelsang, M.; Thomford, N.E.; Dandara, C.; Kallmeyer, K.; Pepper, M.S.; Parker, M.I. Wharton’s Jelly-Derived Mesenchymal Stromal Cells and Fibroblast-Derived Extracellular Matrix Synergistically Activate Apoptosis in a p21-Dependent Mechanism in WHCO1 and MDA MB 231 Cancer Cells<i> In Vitro</i>. Stem Cells International 2016, 2016, 4842134. [Google Scholar] [CrossRef]
- Sarrazy, V.; Billet, F.; Micallef, L.; Coulomb, B.; Desmoulière, A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair and Regeneration 2011, 19, s10–s15. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Galassi, C.; Galluzzi, L. Stress responses in stromal cells and tumor homeostasis. Pharmacology & therapeutics 2019, 200, 55–68. [Google Scholar]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.-J. The role of the extracellular matrix in cancer stemness. Frontiers in cell and developmental biology 2019, 7, 86. [Google Scholar] [CrossRef]
- Dzobo, K.; Senthebane, D.A.; Dandara, C. The tumor microenvironment in tumorigenesis and therapy resistance revisited. Cancers 2023, 15, 376. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumor Biology 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Current opinion in cell biology 2010, 22, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Deegan, D.B.; Zimmerman, C.; Skardal, A.; Atala, A.; Shupe, T.D. Stiffness of hyaluronic acid gels containing liver extracellular matrix supports human hepatocyte function and alters cell morphology. Journal of the mechanical behavior of biomedical materials 2016, 55, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Molecular pathways: connecting fibrosis and solid tumor metastasis. Clin Cancer Res 2014, 20, 3637–3643. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef]
- Houghton, A.M. Mechanistic links between COPD and lung cancer. Nat Rev Cancer 2013, 13, 233–245. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Cox, T.R.; Bird, D.; Baker, A.M.; Barker, H.E.; Ho, M.W.; Lang, G.; Erler, J.T. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 2013, 73, 1721–1732. [Google Scholar] [CrossRef]
- Joo, Y.N.; Jin, H.; Eun, S.Y.; Park, S.W.; Chang, K.C.; Kim, H.J. P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget 2014, 5, 9322–9334. [Google Scholar] [CrossRef]
- Choi, S.K.; Kim, H.S.; Jin, T.; Moon, W.K. LOXL4 knockdown enhances tumor growth and lung metastasis through collagen-dependent extracellular matrix changes in triple-negative breast cancer. Oncotarget 2017, 8, 11977–11989. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Hsia, S.M.; Shieh, T.M. Lysyl Oxidase and the Tumor Microenvironment. Int J Mol Sci 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Giussani, M.; Landoni, E.; Merlino, G.; Turdo, F.; Veneroni, S.; Paolini, B.; Cappelletti, V.; Miceli, R.; Orlandi, R.; Triulzi, T. Extracellular matrix proteins as diagnostic markers of breast carcinoma. Journal of cellular physiology 2018, 233, 6280–6290. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biology 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Giussani, M.; Triulzi, T.; Sozzi, G.; Tagliabue, E. Tumor extracellular matrix remodeling: new perspectives as a circulating tool in the diagnosis and prognosis of solid tumors. Cells 2019, 8, 81. [Google Scholar] [CrossRef]
- Yang, J.D.; Nakamura, I.; Roberts, L.R. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. in Seminars in cancer biology. 2011. Elsevier. [CrossRef]
- Jun, J.-I.; Lau, L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nature reviews Drug discovery 2011, 10, 945–963. [Google Scholar] [CrossRef]
- Wood, S.L.; Pernemalm, M.; Crosbie, P.A.; Whetton, A.D. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer treatment reviews 2014, 40, 558–566. [Google Scholar] [CrossRef]
- Zanconato, F.; Battilana, G.; Cordenonsi, M.; Piccolo, S. YAP/TAZ as therapeutic targets in cancer. Current opinion in pharmacology 2016, 29, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: glycans as novel therapeutic targets. Nature Reviews Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduction and Targeted Therapy 2021, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harbor perspectives in biology 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 2010, 11, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumour Biol 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb Perspect Biol 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Gay, S.; Vijanto, J.; Raekallio, J.; Penttinen, R. Collagen types in early phases of wound healing in children. Acta Chirurgica Scandinavica 1978, 144, 205–211. [Google Scholar] [PubMed]
- Nyström, A.; Velati, D.; Mittapalli, V.R.; Fritsch, A.; Kern, J.S.; Bruckner-Tuderman, L. Collagen VII plays a dual role in wound healing. The Journal of clinical investigation 2013, 123, 3498–3509. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery (Oxford) 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Dzobo, K. Matrix-mediated regulation of type 1 collagen synthesis and degradation in cultured fibroblasts. 2009. [Google Scholar]
- Scott, I.; Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays in biochemistry 2012, 52, 113–133. [Google Scholar] [CrossRef]
- Garnero, P.; Borel, O.; Gineyts, E.; Duboeuf, F.; Solberg, H.; Bouxsein, M.L.; Christiansen, C.; Delmas, P.D. Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 2006, 38, 300–309. [Google Scholar] [CrossRef]
- Sottile, J.; Shi, F.; Rublyevska, I.; Chiang, H.-Y.; Lust, J.; Chandler, J. Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. American journal of physiology-Cell Physiology 2007, 293, C1934–C1946. [Google Scholar] [CrossRef]
- Kubow, K.E.; Vukmirovic, R.; Zhe, L.; Klotzsch, E.; Smith, M.L.; Gourdon, D.; Luna, S.; Vogel, V. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nature communications 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Dzobo, K.; Leaner, V.D.; Parker, M.I. Feedback regulation of the alpha2(1) collagen gene via the Mek-Erk signaling pathway. IUBMB Life 2012, 64, 87–98. [Google Scholar] [CrossRef]
- Dzobo, K.; Leaner, V.D.; Parker, M.I. Absence of feedback regulation in the synthesis of COL1A1. Life Sci 2014, 103, 25–33. [Google Scholar] [CrossRef]
- Chelyshev, Y.A.; Kabdesh, I.M.; Mukhamedshina, Y.O. Extracellular Matrix in Neural Plasticity and Regeneration. Cell Mol Neurobiol 2020. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Fibrosis, cancer and the premetastatic niche. Breast Cancer Management 2014, 3, 453–455. [Google Scholar] [CrossRef]
- Hastings, J.F.; Skhinas, J.N.; Fey, D.; Croucher, D.R.; Cox, T.R. The extracellular matrix as a key regulator of intracellular signalling networks. British journal of pharmacology 2019, 176, 82–92. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 2006, 4, 38. [Google Scholar] [CrossRef]
- Amatangelo, M.D.; Bassi, D.E.; Klein-Szanto, A.J.; Cukierman, E. Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am J Pathol 2005, 167, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. Journal of cell science 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Hay, E.D. Cell biology of extracellular matrix; Springer Science & Business Media, 2013. [Google Scholar]
- Sonbol, H.S. Extracellular matrix remodeling in human disease. Journal of microscopy and ultrastructure 2018, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Moes, S.; Asgeirsson, D.O.; Halfter, K.; Oertle, P.; Melo Herraiz, E.; Plodinec, M.; Jenoe, P.; Henrich, P.B. Diabetes-related changes in the protein composition and the biomechanical properties of human retinal vascular basement membranes. PLoS One 2017, 12, e0189857. [Google Scholar] [CrossRef]
- Candiello, J.; Balasubramani, M.; Schreiber, E.M.; Cole, G.J.; Mayer, U.; Halfter, W.; Lin, H. Biomechanical properties of native basement membranes. The FEBS journal 2007, 274, 2897–2908. [Google Scholar] [CrossRef]
- Amenta, P.S.; Briggs, K.; Xu, K.; Gamboa, E.; Jukkola, A.F.; Li, D.; Myers, J.C. Type XV collagen in human colonic adenocarcinomas has a different distribution than other basement membrane zone proteins. Hum Pathol 2000, 31, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Amenta, P.S.; Hadad, S.; Lee, M.T.; Barnard, N.; Li, D.; Myers, J.C. Loss of types XV and XIX collagen precedes basement membrane invasion in ductal carcinoma of the female breast. J Pathol 2003, 199, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Tosios, K.; Kapranos, N.; Papanicolaou, S. Loss of basement membrane components laminin and type IV collagen parallels the progression of oral epithelial neoplasia. Histopathology 1998, 33, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Jolly, L.A.; Novitskiy, S.; Owens, P.; Massoll, N.; Cheng, N.; Fang, W.; Moses, H.L.; Franco, A.T. Fibroblast-mediated collagen remodeling within the tumor microenvironment facilitates progression of thyroid cancers driven by BrafV600E and Pten loss. Cancer research 2016, 76, 1804–1813. [Google Scholar] [CrossRef]
- Spivey, K.A.; Chung, I.; Banyard, J.; Adini, I.; Feldman, H.A.; Zetter, B.R. A role for collagen XXIII in cancer cell adhesion, anchorage-independence and metastasis. Oncogene 2012, 31, 2362–2372. [Google Scholar] [CrossRef]
- Madsen, C.D. Pancreatic cancer is suppressed by fibroblast-derived collagen I. Cancer Cell 2021, 39, 451–453. [Google Scholar] [CrossRef]
- Kresse, H.; Schönherr, E. Proteoglycans of the extracellular matrix and growth control. Journal of cellular physiology 2001, 189, 266–274. [Google Scholar] [CrossRef]
- Lee, K.; Loganathan, D.; Merchant, Z.; Linhardt, R. Carbohydrate analysis of glycoproteins A review. Applied biochemistry and biotechnology 1990, 23, 53–80. [Google Scholar] [CrossRef]
- Hughes, R.C. Membrane glycoproteins: a review of structure and function. 2014. [Google Scholar]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Iozzo, R.V. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 1998, 67, 609–652. [Google Scholar] [CrossRef]
- Hardingham, T.E.; Fosang, A.J. Proteoglycans: many forms and many functions. Faseb j 1992, 6, 861–870. [Google Scholar] [CrossRef]
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nature Communications 2018, 9, 2163. [Google Scholar] [CrossRef]
- Schönherr, E.; Hausser, H.-J. Extracellular Matrix and Cytokines: A Functional Unit. Developmental Immunology 2000, 7, 031748. [Google Scholar] [CrossRef]
- Broekelmann, T.J.; Bodmer, N.K.; Mecham, R.P. Identification of the growth factor–binding sequence in the extracellular matrix protein MAGP-1. Journal of Biological Chemistry 2020, 295, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Bohaumilitzky, L.; Huber, A.K.; Stork, E.M.; Wengert, S.; Woelfl, F.; Boehm, H. A Trickster in Disguise: Hyaluronan's Ambivalent Roles in the Matrix. Front Oncol 2017, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- Price, Z.K.; Lokman, N.A.; Ricciardelli, C. Differing roles of hyaluronan molecular weight on cancer cell behavior and chemotherapy resistance. Cancers 2018, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Januchowski, R.; Zawierucha, P.; Ruciński, M.; Nowicki, M.; Zabel, M. Extracellular Matrix Proteins Expression Profiling in Chemoresistant Variants of the A2780 Ovarian Cancer Cell Line. BioMed Research International 2014, 2014, 365867. [Google Scholar] [CrossRef] [PubMed]
- Timpl, R.; Rohde, H.; Robey, P.G.; Rennard, S.I.; Foidart, J.M.; Martin, G.R. Laminin--a glycoprotein from basement membranes. The Journal of biological chemistry 1979, 254, 9933–9937. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, E.; Yurchenco, P.D. Laminins in basement membrane assembly. Cell adhesion & migration 2013, 7, 56–63. [Google Scholar]
- Domogatskaya, A.; Rodin, S.; Tryggvason, K. Functional diversity of laminins. Annual review of cell and developmental biology 2012, 28, 523–553. [Google Scholar] [CrossRef]
- Hohenester, E. Structural biology of laminins. Essays Biochem 2019, 63, 285–295. [Google Scholar] [CrossRef]
- Aumailley, M. The laminin family. Cell Adh Migr 2013, 7, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, S.; Shinto, E.; Tsuda, H.; Ueno, H.; Shikina, A.; Kajiwara, Y.; Yamamoto, J.; Hase, K. Laminin β3 expression as a prognostic factor and a predictive marker of chemoresistance in colorectal cancer. Japanese Journal of Clinical Oncology 2015, 45, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Govaere, O.; Wouters, J.; Petz, M.; Vandewynckel, Y.P.; Van den Eynde, K.; Van den Broeck, A.; Verhulst, S.; Dolle, L.; Gremeaux, L.; Ceulemans, A.; et al. Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. Journal of hepatology 2016, 64, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Hasebe, T.; Oda, T.; Sasaki, S.; Kinoshita, T.; Konishi, M.; Ochiai, T.; Ochiai, A. Cytoplasmic expression of laminin γ2 chain correlates with postoperative hepatic metastasis and poor prognosis in patients with pancreatic ductal adenocarcinoma. Cancer 2002, 94, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Koshikawa, N.; Schenk, S.; Quaranta, V. The LG3 module of laminin-5 harbors a binding site for integrin α3β1 that promotes cell adhesion, spreading, and migration. Journal of Biological Chemistry 2001, 276, 33045–33053. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-C.; Ziober, B.L.; Squillace, R.M.; Kramer, R.H. α7 integrin mediates cell adhesion and migration on specific laminin isoforms. Journal of Biological Chemistry 1996, 271, 25598–25603. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Azzariti, A.; Fransvea, E.; Porcelli, L.; Antonaci, S.; Paradiso, A. Laminin-5 offsets the efficacy of gefitinib (‘Iressa’) in hepatocellular carcinoma cells. British Journal of Cancer 2004, 91, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Tsurutani, J.; West, K.A.; Sayyah, J.; Gills, J.J.; Dennis, P.A. Inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin-mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy. Cancer research 2005, 65, 8423–8432. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. Journal of Cell Science 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Gopal, S.; Veracini, L.; Grall, D.; Butori, C.; Schaub, S.; Audebert, S.; Camoin, L.; Baudelet, E.; Radwanska, A.; Beghelli-de la Forest Divonne, S.; et al. Fibronectin-guided migration of carcinoma collectives. Nature communications 2017, 8, 14105. [Google Scholar] [CrossRef]
- Rintoul, R.C.; Sethi, T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Clinical Science 2002, 102, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, L.A.; Argilagos, R.F.; Emmons, M.; Boulware, D.; Beam, C.A.; Sullivan, D.M.; Dalton, W.S. Cell adhesion to fibronectin (CAM-DR) influences acquired mitoxantrone resistance in U937 cells. Cancer research 2006, 66, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Kosmehl, H.; Berndt, A.; Strassburger, S.; Borsi, L.; Rousselle, P.; Mandel, U.; Hyckel, P.; Zardi, L.; Katenkamp, D. Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. British Journal of Cancer 1999, 81, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, M.; Zardi, L.; Neri, D. Fibronectin as target for tumor therapy. International journal of cancer 2006, 118, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheng, S.; Asa, S.L.; Ezzat, S. The melanoma-associated antigen A3 mediates fibronectin-controlled cancer progression and metastasis. Cancer research 2008, 68, 8104–8112. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.K.; Kim, A.; Kim, M.K.; Choi, J.E.; Kang, S.H.; Lee, S.J. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Human pathology 2013, 44, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ansari, D.; Zhou, Q.; Sasor, A.; Said Hilmersson, K.; Andersson, R. Stromal fibronectin expression in patients with resected pancreatic ductal adenocarcinoma. World journal of surgical oncology 2019, 17, 1–8. [Google Scholar] [CrossRef]
- Thomas, G.; Nyström, M.; Marshall, J. αvβ6 integrin in wound healing and cancer of the oral cavity. Journal of oral pathology & medicine 2006, 35, 1–10. [Google Scholar]
- Singh, P.; Reimer, C.L.; Peters, J.H.; Stepp, M.A.; Hynes, R.O.; Van De Water, L. The spatial and temporal expression patterns of integrin α9β1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. Journal of investigative dermatology 2004, 123, 1176–1181. [Google Scholar] [CrossRef]
- Han, S.; Sidell, N.; Roser-Page, S.; Roman, J. Fibronectin stimulates human lung carcinoma cell growth by inducing cyclooxygenase-2 (COX-2) expression. International Journal of Cancer 2004, 111, 322–331. [Google Scholar] [CrossRef]
- Han, S.; Roman, J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: pro-oncogenic effects mediated by PI3-kinase and NF-κB. Oncogene 2006, 25, 4341–4349. [Google Scholar] [CrossRef]
- Han, S.; Sidell, N.; Roman, J. Fibronectin stimulates human lung carcinoma cell proliferation by suppressing p21 gene expression via signals involving Erk and Rho kinase. Cancer letters 2005, 219, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Weng, D.; Chen, G.; Tao, W.; Zhu, T.; Yang, X.; Meng, L.; Wang, S.; Lu, Y.; Ma, D. Activation of fibronectin/PI-3K/Akt2 leads to chemoresistance to docetaxel by regulating survivin protein expression in ovarian and breast cancer cells. Cancer letters 2008, 261, 108–119. [Google Scholar] [CrossRef]
- Horiuchi, K.; Amizuka, N.; Takeshita, S.; Takamatsu, H.; Katsuura, M.; Ozawa, H.; Toyama, Y.; Bonewald, L.F.; Kudo, A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 1999, 14, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko, T.; Wincewicz, A.; Koda, M.; Domysławska, I.; Sulkowski, S. Role of periostin in esophageal, gastric and colon cancer. Oncology letters 2016, 12, 783–787. [Google Scholar] [CrossRef]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer research 2002, 62, 5358–5364. [Google Scholar]
- Underwood, T.J.; Hayden, A.L.; Derouet, M.; Garcia, E.; Noble, F.; White, M.J.; Thirdborough, S.; Mead, A.; Clemons, N.; Mellone, M.; et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. The Journal of pathology 2015, 235, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tamai, K.; Shibuya, R.; Nakamura, M.; Mochizuki, M.; Yamaguchi, K.; Abe, J.; Takahashi, S.; Sato, I.; Kudo, A. Periostin is a negative prognostic factor and promotes cancer cell proliferation in non-small cell lung cancer. Oncotarget 2018, 9, 31187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Fejzo, M.S.; Anderson, L.; Dering, J.; Ginther, C.; Ramos, L.; Gasson, J.C.; Karlan, B.Y.; Slamon, D.J. Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecologic oncology 2010, 119, 337–344. [Google Scholar] [CrossRef]
- Tumbarello, D.A.; Temple, J.; Brenton, J.D. ß3 integrin modulates transforming growth factor beta induced (TGFBI) function and paclitaxel response in ovarian cancer cells. Molecular cancer 2012, 11, 36–36. [Google Scholar] [CrossRef]
- Sung, P.-L.; Jan, Y.-H.; Lin, S.-C.; Huang, C.-C.; Lin, H.; Wen, K.-C.; Chao, K.-C.; Lai, C.-R.; Wang, P.-H.; Chuang, C.-M.; et al. Periostin in tumor microenvironment is associated with poor prognosis and platinum resistance in epithelial ovarian carcinoma. Oncotarget 2016, 7, 4036–4047. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, L. Role of pancreatic stellate cells and periostin in pancreatic cancer progression. Tumor Biology 2015, 36, 3171–3177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Fejzo, M.S.; Anderson, L.; Dering, J.; Ginther, C.; Ramos, L.; Gasson, J.C.; Karlan, B.Y.; Slamon, D.J. Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecologic oncology 2010, 119, 337–344. [Google Scholar] [CrossRef]
- Tammi, M.I.; Day, A.J.; Turley, E.A. Hyaluronan and homeostasis: a balancing act. Journal of Biological Chemistry 2002, 277, 4581–4584. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Kakehi, K.; Kinoshita, M.; Yasueda, S.-i. Hyaluronic acid: separation and biological implications. Journal of Chromatography B 2003, 797, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. The FASEB journal 1992, 6, 2639–2645. [Google Scholar] [CrossRef]
- Kupper, S.; Kłosowska-Chomiczewska, I.; Szumała, P. Collagen and hyaluronic acid hydrogel in water-in-oil microemulsion delivery systems. Carbohydrate polymers 2017, 175, 347–354. [Google Scholar] [CrossRef]
- Pereira, H.; Sousa, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Hyaluronic acid. Osteochondral Tissue Engineering 2018, 137–153. [Google Scholar]
- Henry, C.B.; Duling, B.R. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. American Journal of Physiology-Heart and Circulatory Physiology 1999. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, R.; Lamponi, S.; Borzacchiello, A.; Ambrosio, L.; Fini, M.; Torricelli, P.; Giardino, R. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials 2002, 23, 4503–4513. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. Journal of controlled release 2000, 69, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. Journal of drug targeting 2015, 23, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.S.; Svechkarev, D.; Souchek, J.; Hill, T.K.; Taylor, M.; Natarajan, A.; Mohs, A.M. Impact of structurally modifying hyaluronic acid on CD44 interaction. Journal of Materials Chemistry B 2017, 5, 8183–8192. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): a review. Veterinarni medicina 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Brown, N.H. Extracellular matrix in development: insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb Perspect Biol 2011, 3. [Google Scholar] [CrossRef]
- Senthebane, D.A.; Jonker, T.; Rowe, A.; Thomford, N.E.; Munro, D.; Dandara, C.; Wonkam, A.; Govender, D.; Calder, B.; Soares, N.C. The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. International journal of molecular sciences 2018, 19, 2861. [Google Scholar] [CrossRef]
- Entchev, E.V.; González-Gaitán, M.A. Morphogen gradient formation and vesicular trafficking. Traffic 2002, 3, 98–109. [Google Scholar] [CrossRef]
- Marois, E.; Mahmoud, A.; Eaton, S. The endocytic pathway and formation of the Wingless morphogen gradient. Development 2006, 133, 307–317. [Google Scholar] [CrossRef]
- Uhler, C.; Shivashankar, G.V. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat Rev Mol Cell Biol 2017, 18, 717–727. [Google Scholar] [CrossRef]
- Ilic, D.; Damsky, C.H.; Yamamoto, T. Focal adhesion kinase: at the crossroads of signal transduction. Journal of cell science 1997, 110, 401–407. [Google Scholar] [CrossRef]
- Kai, F.; Laklai, H.; Weaver, V.M. Force Matters: Biomechanical Regulation of Cell Invasion and Migration in Disease. Trends Cell Biol 2016, 26, 486–497. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.-H.; Levchenko, A. Topotaxis: a new mechanism of directed cell migration in topographic ECM gradients. Biophysical journal 2018, 114, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Janson, I.A.; Putnam, A.J. Extracellular matrix elasticity and topography: Material-based cues that affect cell function via conserved mechanisms. Journal of biomedical materials research Part A 2015, 103, 1246–1258. [Google Scholar] [CrossRef]
- Doyle, A.D.; Petrie, R.J.; Kutys, M.L.; Yamada, K.M. Dimensions in cell migration. Current opinion in cell biology 2013, 25, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, R.C.; Silvestre, J.; Gaskins, H.R.; Kenis, P.J.; Leckband, D.E. Cell migration and polarity on microfabricated gradients of extracellular matrix proteins. Langmuir 2006, 22, 4250–4258. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mao, Z.; Tan, H.; Han, L.; Ren, T.; Gao, C. Gradient biomaterials and their influences on cell migration. Interface focus 2012, 2, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Shellard, A.; Mayor, R. All roads lead to directional cell migration. Trends in cell biology 2020, 30, 852–868. [Google Scholar] [CrossRef]
- Palecek, S.P.; Loftus, J.C.; Ginsberg, M.H.; Lauffenburger, D.A.; Horwitz, A.F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 1997, 385, 537–540. [Google Scholar] [CrossRef]
- Hartman, C.D.; Isenberg, B.C.; Chua, S.G.; Wong, J.Y. Extracellular matrix type modulates cell migration on mechanical gradients. Exp Cell Res 2017, 359, 361–366. [Google Scholar] [CrossRef]
- Hartman, C.D.; Isenberg, B.C.; Chua, S.G.; Wong, J.Y. Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proc Natl Acad Sci U S A 2016, 113, 11190–11195. [Google Scholar] [CrossRef]
- Plotnikov, S.V.; Waterman, C.M. Guiding cell migration by tugging. Curr Opin Cell Biol 2013, 25, 619–626. [Google Scholar] [CrossRef]
- Pathak, A.; Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proceedings of the National Academy of Sciences 2012, 109, 10334–10339. [Google Scholar] [CrossRef]
- Charras, G.; Sahai, E. Physical influences of the extracellular environment on cell migration. Nature reviews Molecular cell biology 2014, 15, 813–824. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Pytliak, M.; Vargová, V.; Mechírová, V. Matrix metalloproteinases and their role in oncogenesis: a review. Onkologie 2012, 35, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Rainero, E. Extracellular matrix endocytosis in controlling matrix turnover and beyond: emerging roles in cancer. Biochem Soc Trans 2016, 44, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Hinck, L.; Silberstein, G.B. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res 2005, 7, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Alford, D.; Baeckström, D.; Geyp, M.; Pitha, P.; Taylor-Papadimitriou, J. Integrin-matrix interactions affect the form of the structures developing from human mammary epithelial cells in collagen or fibrin gels. J Cell Sci 1998, 111 Pt 4, 521–532. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Nelson, C.M. Extracellular matrix and cytoskeletal dynamics during branching morphogenesis. Organogenesis 2012, 8, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Larsen, M.; Yamada, K.M. Fibronectin requirement in branching morphogenesis. Nature 2003, 423, 876–881. [Google Scholar] [CrossRef]
- Ortega, N.; Werb, Z. New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci 2002, 115, 4201–4214. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Kouros-Mehr, H.; Lu, P.; Werb, Z. Hormonal and local control of mammary branching morphogenesis. Differentiation 2006, 74, 365–381. [Google Scholar] [CrossRef]
- Yue, B. Biology of the extracellular matrix: an overview. Journal of glaucoma 2014, S20. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: a dynamic view. Developmental biology 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Walma, D.A.C.; Yamada, K.M. The extracellular matrix in development. Development 2020, 147, dev175596. [Google Scholar] [CrossRef]
- Streuli, C.H.; Schmidhauser, C.; Bailey, N.; Yurchenco, P.; Skubitz, A.P.; Roskelley, C.; Bissell, M.J. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol 1995, 129, 591–603. [Google Scholar] [CrossRef]
- Muncie, J.M.; Weaver, V.M. The physical and biochemical properties of the extracellular matrix regulate cell fate. Current topics in developmental biology 2018, 130, 1–37. [Google Scholar] [PubMed]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Dityatev, A.; Schachner, M.; Sonderegger, P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nature Reviews Neuroscience 2010, 11, 735–746. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Disease models & mechanisms 2011, 4, 165–178. [Google Scholar]
- Mongiat, M.; Andreuzzi, E.; Tarticchio, G.; Paulitti, A. Extracellular matrix, a hard player in angiogenesis. International journal of molecular sciences 2016, 17, 1822. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.; Wang, Q.; Cai, L.; Du, W.; Li, X.; Zhou, X.; Xie, J. Extracellular matrix elasticity regulates osteocyte gap junction elongation: involvement of paxillin in intracellular signal transduction. Cellular Physiology and Biochemistry 2018, 51, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-J.; Kang, M.H.; Ooi, Y.H.; Choi, K.R.; Sage, E.H.; Rhee, D.J. Overexpression of SPARC in human trabecular meshwork increases intraocular pressure and alters extracellular matrix. Investigative ophthalmology & visual science 2013, 54, 3309–3319. [Google Scholar]
- Terajima, M.; Taga, Y.; Cabral, W.A.; Liu, Y.; Nagasawa, M.; Sumida, N.; Kayashima, Y.; Chandrasekaran, P.; Han, L.; Maeda, N. Cyclophilin B control of lysine post-translational modifications of skin type I collagen. PLoS genetics 2019, 15, e1008196. [Google Scholar] [CrossRef]
- Reynders, M.; Matsuura, B.S.; Bérouti, M.; Simoneschi, D.; Marzio, A.; Pagano, M.; Trauner, D. PHOTACs enable optical control of protein degradation. Science advances 2020, 6, eaay5064. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Ma, L.; He, Z.; Wang, D.; Liu, Y.; Lin, Q.; Zhang, T.; Gray, N.; Kaniskan, H.Ü. Light-induced control of protein destruction by opto-PROTAC. Science advances 2020, 6, eaay5154. [Google Scholar] [CrossRef]
- Ryan, A.; Liu, J.; Deiters, A. Targeted protein degradation through fast optogenetic activation and its application to the control of cell signaling. Journal of the American Chemical Society 2021, 143, 9222–9229. [Google Scholar] [CrossRef] [PubMed]
- Petrie, R.J.; Gavara, N.; Chadwick, R.S.; Yamada, K.M. Nonpolarized signaling reveals two distinct modes of 3D cell migration. Journal of Cell Biology 2012, 197, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Hayward, M.-K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef] [PubMed]
- Viji Babu, P.K.; Rianna, C.; Mirastschijski, U.; Radmacher, M. Nano-mechanical mapping of interdependent cell and ECM mechanics by AFM force spectroscopy. Scientific reports 2019, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Roycik, M.D.; Fang, X.; Sang, Q.X. A fresh prospect of extracellular matrix hydrolytic enzymes and their substrates. Curr Pharm Des 2009, 15, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Myllyharju, J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol 2003, 22, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, R. Roles of PLODs in Collagen Synthesis and Cancer Progression. Front Cell Dev Biol 2018, 6, 66. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, R. Roles of PLODs in collagen synthesis and cancer progression. Frontiers in cell and developmental biology 2018, 6, 66. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Bajpai, S.; Wong, C.C.; Chaturvedi, P.; Hubbi, M.E.; Wirtz, D.; Semenza, G.L. Procollagen Lysyl Hydroxylase 2 Is Essential for Hypoxia-Induced Breast Cancer MetastasisPLOD2 Is Essential for Hypoxia-Induced Metastasis. Molecular cancer research 2013, 11, 456–466. [Google Scholar] [CrossRef]
- Eddy, A.A. Molecular basis of renal fibrosis. Pediatric nephrology 2000, 15, 290–301. [Google Scholar] [CrossRef]
- Alcolado, R.; Arthur, M.; Iredale, J. Pathogenesis of liver fibrosis. Clinical science (London, England: 1979) 1997, 92, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Libring, S.; Shinde, A.; Chanda, M.K.; Nuru, M.; George, H.; Saleh, A.M.; Abdullah, A.; Kinzer-Ursem, T.L.; Calve, S.; Wendt, M.K. The dynamic relationship of breast cancer cells and fibroblasts in fibronectin accumulation at primary and metastatic tumor sites. Cancers 2020, 12, 1270. [Google Scholar] [CrossRef] [PubMed]
- Comoglio, P.M.; Trusolino, L. Cancer: the matrix is now in control. Nature medicine 2005, 11, 1156–1158. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Greenshields-Watson, A.; Jones, E.; Smart, K.; Lauder, S.N.; Somerville, M.; Milutinovic, S.; Kendrick, H.; Hindley, J.P.; French, R.; et al. Immune Remodeling of the Extracellular Matrix Drives Loss of Cancer Stem Cells and Tumor Rejection. Cancer Immunol Res 2020, 8, 1520–1531. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Wang, C.Y.; Werb, Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol 2015, 44-46, 184–190. [Google Scholar] [CrossRef]
- Fonović, M.; Turk, B. Cysteine cathepsins and extracellular matrix degradation. Biochim Biophys Acta 2014, 1840, 2560–2570. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Fibrosis and Cancer: Partners in Crime or Opposing Forces? Trends Cancer 2016, 2, 279–282. [Google Scholar] [CrossRef]
- Filipe, E.C.; Chitty, J.L.; Cox, T.R. Charting the unexplored extracellular matrix in cancer. Int J Exp Pathol 2018, 99, 58–76. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Elife 2021, 10. [Google Scholar] [CrossRef]
- Kench, J.A.; Russell, D.M.; Fadok, V.A.; Young, S.K.; Worthen, G.S.; Jones-Carson, J.; Henson, J.E.; Henson, P.M.; Nemazee, D. Aberrant wound healing and TGF-β production in the autoimmune-prone MRL/+ mouse. Clinical immunology 1999, 92, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bao, L.; Chan, L.S.; DiPietro, L.A.; Chen, L. Aberrant wound healing in an epidermal interleukin-4 transgenic mouse model of atopic dermatitis. PLoS One 2016, 11, e0146451. [Google Scholar] [CrossRef] [PubMed]
- Hertle, M.D.; Kubler, M.-D.; Leigh, I.M.; Watt, F. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. The Journal of clinical investigation 1992, 89, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Stephens, P.; Grenard, P.; Aeschlimann, P.; Langley, M.; Blain, E.; Errington, R.; Kipling, D.; Thomas, D.; Aeschlimann, D. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. Journal of cell science 2004, 117, 3389–3403. [Google Scholar] [CrossRef] [PubMed]
- Chitty, J.L.; Setargew, Y.F.I.; Cox, T.R. Targeting the lysyl oxidases in tumour desmoplasia. Biochem Soc Trans 2019, 47, 1661–1678. [Google Scholar] [CrossRef] [PubMed]
- Ponce, I.; Garrido, N.; Tobar, N.; Melo, F.; Smith, P.C.; Martínez, J. Matrix stiffness modulates metabolic interaction between human stromal and breast cancer cells to stimulate epithelial motility. Metabolites 2021, 11, 432. [Google Scholar] [CrossRef]
- DuFort, C.C.; DelGiorno, K.E.; Hingorani, S.R. Mounting Pressure in the Microenvironment: Fluids, Solids, and Cells in Pancreatic Ductal Adenocarcinoma. Gastroenterology 2016, 150, 1545–1557. [Google Scholar] [CrossRef]
- Scarpellini, A.; Germack, R.; Lortat-Jacob, H.; Muramatsu, T.; Billett, E.; Johnson, T.; Verderio, E.A. Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J Biol Chem 2009, 284, 18411–18423. [Google Scholar] [CrossRef]
- Barsigian, C.; Fellin, F.M.; Jain, A.; Martinez, J. Dissociation of fibrinogen and fibronectin binding from transglutaminase-mediated cross-linking at the hepatocyte surface. J Biol Chem 1988, 263, 14015–14022. [Google Scholar] [CrossRef]
- Cardoso, I.; Stamnaes, J.; Andersen, J.T.; Melino, G.; Iversen, R.; Sollid, L.M. Transglutaminase 2 interactions with extracellular matrix proteins as probed with celiac disease autoantibodies. Febs j 2015, 282, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Akimov, S.S.; Krylov, D.; Fleischman, L.F.; Belkin, A.M. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 2000, 148, 825–838. [Google Scholar] [CrossRef]
- Richter, P.; Junker, K.; Franz, M.; Berndt, A.; Geyer, C.; Gajda, M.; Kosmehl, H.; Berndt, A.; Wunderlich, H. IIICS de novo glycosylated fibronectin as a marker for invasiveness in urothelial carcinoma of the urinary bladder (UBC). Journal of cancer research and clinical oncology 2008, 134, 1059–1065. [Google Scholar] [CrossRef]
- Freire-de-Lima, L.; Gelfenbeyn, K.; Ding, Y.; Mandel, U.; Clausen, H.; Handa, K.; Hakomori, S.-i. Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process. Proceedings of the National Academy of Sciences 2011, 108, 17690–17695. [Google Scholar] [CrossRef] [PubMed]
- SUZUkI, O.; Abe, M.; Hashimoto, Y. Sialylation and glycosylation modulate cell adhesion and invasion to extracellular matrix in human malignant lymphoma: Dependency on integrin and the Rho GTPase family. International Journal of Oncology 2015, 47, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Shyanti, R.K.; Singh, V.; Kale, R.K.; Mishra, J.P.; Singh, R.P. Integrin expression and glycosylation patterns regulate cell-matrix adhesion and alter with breast cancer progression. Biochemical and biophysical research communications 2018, 499, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, R.; Alahari, S.K. Important role of integrins in the cancer biology. Cancer and Metastasis Reviews 2010, 29, 223–237. [Google Scholar] [CrossRef]
- Yalak, G.; Vogel, V. Ectokinases as novel cancer markers and drug targets in cancer therapy. Cancer medicine 2015, 4, 404–414. [Google Scholar] [CrossRef]
- Yalak, G.; Shiu, J.-Y.; Schoen, I.; Mitsi, M.; Vogel, V. Phosphorylated fibronectin enhances cell attachment and upregulates mechanical cell functions. PloS one 2019, 14, e0218893. [Google Scholar] [CrossRef]
- Wolanska, K.I.; Morgan, M.R. Fibronectin remodelling: cell-mediated regulation of the microenvironment; Portland Press Ltd., 2015. [Google Scholar] [CrossRef]
- Soares Da Costa, D.; Reis, R.L.; Pashkuleva, I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annual review of biomedical engineering 2017, 19, 1–26. [Google Scholar] [CrossRef]
- Escobar Galvis, M.L.; Jia, J.; Zhang, X.; Jastrebova, N.; Spillmann, D.; Gottfridsson, E.; Van Kuppevelt, T.H.; Zcharia, E.; Vlodavsky, I.; Lindahl, U. Transgenic or tumor-induced expression of heparanase upregulates sulfation of heparan sulfate. Nature chemical biology 2007, 3, 773–778. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Gross-Cohen, M.; Weissmann, M.; Ilan, N.; Sanderson, R.D. Opposing functions of heparanase-1 and heparanase-2 in cancer progression. Trends in biochemical sciences 2018, 43, 18–31. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nature reviews Molecular cell biology 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Hamacher, S.; Matern, S.; Roeb, E. Extracellular matrix--from basic research to clinical significance. An overview with special consideration of matrix metalloproteinases. Deutsche Medizinische Wochenschrift (1946) 2004, 129, 1976–1980. [Google Scholar] [CrossRef]
- Toth, M.; Fridman, R. Assessment of gelatinases (MMP-2 and MMP-9 by gelatin zymography. In Metastasis research protocols; Springer, 2001; pp. 163–174. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Gao, S.; Zhou, M.; Qi, F.; Ding, N.; Zhang, J.; Li, R.; Wang, J.; Shi, J. Excessive DNA damage mediates ECM degradation via the RBBP8/NOTCH1 pathway in sporadic aortic dissection. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2022, 1868, 166303. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor perspectives in biology 2011, 3, a005058. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G.; Liotta, L.A.; Kleiner Jr, D.E. Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. The FASEB Journal 1993, 7, 1434–1441. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Lim, S.Y.; Kutikhin, A.G.; Gordon-Weeks, A.N. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 2018, 1870, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Cena, J.; Schulz, R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. British journal of pharmacology 2007, 152, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.D.; Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 2004, 16, 558–564. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Siqueira, A.S.; Carvalho, M.R.; Monteiro, A.C.; Freitas, V.M.; Jaeger, R.G.; Pinheiro, J.J. Matrix metalloproteinases, TIMPs and growth factors regulating ameloblastoma behaviour. Histopathology 2010, 57, 128–137. [Google Scholar] [CrossRef]
- Rodríguez, D.; Morrison, C.J.; Overall, C.M. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2010, 1803, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Hamacher, S.; Matern, S.; Roeb, E. [Extracellular matrix -- from basic research to clinical significance. An overview with special consideration of matrix metalloproteinases]. Dtsch Med Wochenschr 2004, 129, 1976–1980. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Ohtsuka, T.; Okada, Y.; Kanai, Y. Stromal metalloproteinases: Crucial contributors to the tumor microenvironment. Pathol Int 2021, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 2020, 11, 5120. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.h.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate functions of matrix metalloproteinases in physiological and pathological conditions. Journal of cellular physiology 2016, 231, 2599–2621. [Google Scholar] [CrossRef]
- Jacob, M.P. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomedicine & pharmacotherapy 2003, 57, 195–202. [Google Scholar]
- Stadlmann, S.; Pollheimer, J.; Moser, P.L.; Raggi, A.; Amberger, A.; Margreiter, R.; Offner, F.A.; Mikuz, G.; Dirnhofer, S.; Moch, H. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur J Cancer 2003, 39, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Åström, P.; Juurikka, K.; Hadler-Olsen, E.S.; Svineng, G.; Cervigne, N.K.; Coletta, R.D.; Risteli, J.; Kauppila, J.H.; Skarp, S.; Kuttner, S.; et al. The interplay of matrix metalloproteinase-8, transforming growth factor-β1 and vascular endothelial growth factor-C cooperatively contributes to the aggressiveness of oral tongue squamous cell carcinoma. Br J Cancer 2017, 117, 1007–1016. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and their inhibitors: potential for the development of new therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef]
- Markland, F.S. Snake venoms and the hemostatic system. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef]
- Rossello, A.; Nuti, E.; Ferrini, S.; Fabbi, M. Targeting ADAM17 Sheddase Activity in Cancer. Curr Drug Targets 2016, 17, 1908–1927. [Google Scholar] [CrossRef]
- Van Goor, H.; Melenhorst, W.B.; Turner, A.J.; Holgate, S.T. Adamalysins in biology and disease. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland 2009, 219, 277–286. [Google Scholar] [CrossRef]
- Killar, L.; White, J.; Black, R.; Peschon, J. Adamalysins: a family of metzincins including TNF-α converting enzyme (TACE). Annals of the New York Academy of Sciences 1999, 878, 442–452. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 2012, 1824, 68–88. [Google Scholar] [CrossRef]
- Conus, S. Cathepsins and their involvement in immune responses. Swiss medical weekly 2010. [Google Scholar] [CrossRef]
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The ins and outs of cathepsins: physiological function and role in disease management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef]
- Ishidoh, K.; Kominami, E. Procathepsin L degrades extracellular matrix proteins in the presence of glycosaminoglycans in vitro. Biochem Biophys Res Commun 1995, 217, 624–631. [Google Scholar] [CrossRef]
- Taleb, S.; Cancello, R.; Clément, K.; Lacasa, D. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology 2006, 147, 4950–4959. [Google Scholar] [CrossRef]
- Nomura, T.; Katunuma, N. Involvement of cathepsins in the invasion, metastasis and proliferation of cancer cells. The journal of medical investigation 2005, 52, 1–9. [Google Scholar] [CrossRef]
- Tan, G.-J.; Peng, Z.-K.; Lu, J.-P.; Tang, F.-Q. Cathepsins mediate tumor metastasis. World journal of biological chemistry 2013, 4, 91. [Google Scholar] [CrossRef]
- Rudzińska, M.; Parodi, A.; Soond, S.M.; Vinarov, A.Z.; Korolev, D.O.; Morozov, A.O.; Daglioglu, C.; Tutar, Y.; Zamyatnin, A.A. The role of cysteine cathepsins in cancer progression and drug resistance. International journal of molecular sciences 2019, 20, 3602. [Google Scholar] [CrossRef]
- Llorens, F.; Thüne, K.; Sikorska, B.; Schmitz, M.; Tahir, W.; Fernández-Borges, N.; Cramm, M.; Gotzmann, N.; Carmona, M.; Streichenberger, N. Altered Ca2+ homeostasis induces Calpain-Cathepsin axis activation in sporadic Creutzfeldt-Jakob disease. Acta neuropathologica communications 2017, 5, 1–20. [Google Scholar] [CrossRef]
- Büth, H.; Buttigieg, P.L.; Ostafe, R.; Rehders, M.; Dannenmann, S.R.; Schaschke, N.; Stark, H.-J.; Boukamp, P.; Brix, K. Cathepsin B is essential for regeneration of scratch-wounded normal human epidermal keratinocytes. European journal of cell biology 2007, 86, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.R.; Keles, S.; Greenspan, D.S. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biology 2007, 26, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Steiglitz, B.M.; Ayala, M.; Narayanan, K.; George, A.; Greenspan, D.S. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. Journal of Biological Chemistry 2004, 279, 980–986. [Google Scholar] [CrossRef]
- Vadon-Le Goff, S.; Hulmes, D.J.; Moali, C. BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling. Matrix Biol 2015, 44-46, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Greenspan, D.S. Developmental roles of the BMP1/TLD metalloproteinases. Birth Defects Res C Embryo Today 2006, 78, 47–68. [Google Scholar] [CrossRef]
- Vadon-Le Goff, S.; Hulmes, D.J.; Moali, C. BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling. Matrix Biology 2015, 44, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Malecaze, F.; Massoudi, D.; Fournié, P.; Tricoire, C.; Cassagne, M.; Malbouyres, M.; Hulmes, D.J.; Moali, C.; Galiacy, S.D. Upregulation of bone morphogenetic protein-1/mammalian tolloid and procollagen C-proteinase enhancer-1 in corneal scarring. Investigative Ophthalmology & Visual Science 2014, 55, 6712–6721. [Google Scholar]
- Muir, A.M.; Massoudi, D.; Nguyen, N.; Keene, D.R.; Lee, S.-J.; Birk, D.E.; Davidson, J.M.; Marinkovich, M.P.; Greenspan, D.S. BMP1-like proteinases are essential to the structure and wound healing of skin. Matrix Biology 2016, 56, 114–131. [Google Scholar] [CrossRef]
- Stern, R. Hyaluronidases in cancer biology. Semin Cancer Biol 2008, 18, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tolg, C.; Turley, E. Dissecting the Dual Nature of Hyaluronan in the Tumor Microenvironment. Front Immunol 2019, 10, 947. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yamamoto, H.; Tobisawa, Y.; Irie, F. TMEM2: A missing link in hyaluronan catabolism identified? Matrix Biol 2019, 78-79, 139–146. [Google Scholar] [CrossRef]
- Tammi, M.I.; Oikari, S.; Pasonen-Seppänen, S.; Rilla, K.; Auvinen, P.; Tammi, R.H. Activated hyaluronan metabolism in the tumor matrix - Causes and consequences. Matrix Biol 2019, 78-79, 147–164. [Google Scholar] [CrossRef]
- Stern, R. Hyaluronan metabolism: a major paradox in cancer biology. Pathologie Biologie 2005, 53, 372–382. [Google Scholar] [CrossRef]
- Girish, K.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life sciences 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: an information-rich system. European journal of cell biology 2006, 85, 699–715. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in Tissue Injury and Repair. Annual Review of Cell and Developmental Biology 2007, 23, 435–461. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiological reviews 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix biology 2002, 21, 25–29. [Google Scholar] [CrossRef]
- Heldin, P.; Basu, K.; Olofsson, B.; Porsch, H.; Kozlova, I.; Kahata, K. Deregulation of hyaluronan synthesis, degradation and binding promotes breast cancer. The Journal of Biochemistry 2013, 154, 395–408. [Google Scholar] [CrossRef]
- Bame, K.J. Heparanases: endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology 2001, 11, 91R–98R. [Google Scholar] [CrossRef]
- Zcharia, E.; Zilka, R.; Yaar, A.; Yacoby-Zeevi, O.; Zetser, A.; Metzger, S.; Sarid, R.; Naggi, A.; Casu, B.; Ilan, N. Heparanase accelerates wound angiogenesis and wound healing in mouse and rat models. The FASEB journal 2005, 19, 211–221. [Google Scholar] [CrossRef]
- Crispel, Y.; Ghanem, S.; Attias, J.; Kogan, I.; Brenner, B.; Nadir, Y. Involvement of the heparanase procoagulant domain in bleeding and wound healing. Journal of Thrombosis and Haemostasis 2017, 15, 1463–1472. [Google Scholar] [CrossRef]
- Sanderson, R.D.; Elkin, M.; Rapraeger, A.C.; Ilan, N.; Vlodavsky, I. Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy. Febs j 2017, 284, 42–55. [Google Scholar] [CrossRef]
- Khanna, M.; Parish, C.R. Heparanase: Historical Aspects and Future Perspectives. Adv Exp Med Biol 2020, 1221, 71–96. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Wang, C.-Y.; Werb, Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix biology 2015, 44, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biology 2015, 44, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular research 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circulation research 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.A.; Leaper, D.J. Profiles of matrix metalloproteinases and their tissue inhibitors in intraperitoneal drainage fluid: relationship to wound healing. Wound Repair and Regeneration 2003, 11, 268–274. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle) 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Breznik, B.; Mitrović, A.; Lah, T.T.; Kos, J. Cystatins in cancer progression: More than just cathepsin inhibitors. Biochimie 2019, 166, 233–250. [Google Scholar] [CrossRef]
- van Gent, D.; Sharp, P.; Morgan, K.; Kalsheker, N. Serpins: structure, function and molecular evolution. The international journal of biochemistry & cell biology 2003, 35, 1536–1547. [Google Scholar]
- Rau, J.; Beaulieu, L.; Huntington, J.; CHURCH, F.C. Serpins in thrombosis, hemostasis and fibrinolysis. Journal of thrombosis and haemostasis 2007, 5, 102–115. [Google Scholar] [CrossRef]
- Carrell, R.W.; Evans, D.L.; Stein, P.E. Mobile reactive centre of serpins and the control of thrombosis. Nature 1991, 353, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol 2010, 10, 712–723. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Vallet, S.D. Proteases decode the extracellular matrix cryptome. Biochimie 2016, 122, 300–313. [Google Scholar] [CrossRef]
- Lee, J.H.; Isayeva, T.; Larson, M.R.; Sawant, A.; Cha, H.R.; Chanda, D.; Chesnokov, I.N.; Ponnazhagan, S. Endostatin: A novel inhibitor of androgen receptor function in prostate cancer. Proc Natl Acad Sci U S A 2015, 112, 1392–1397. [Google Scholar] [CrossRef]
- Magnon, C.; Galaup, A.; Mullan, B.; Rouffiac, V.; Bouquet, C.; Bidart, J.M.; Griscelli, F.; Opolon, P.; Perricaudet, M. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with alphavbeta3 and alphavbeta5 integrins. Cancer Res 2005, 65, 4353–4361. [Google Scholar] [CrossRef]
- Wang, S.; Lu, X.A.; Liu, P.; Fu, Y.; Jia, L.; Zhan, S.; Luo, Y. Endostatin has ATPase activity, which mediates its antiangiogenic and antitumor activities. Mol Cancer Ther 2015, 14, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Colorado, P.C.; Torre, A.; Kamphaus, G.; Maeshima, Y.; Hopfer, H.; Takahashi, K.; Volk, R.; Zamborsky, E.D.; Herman, S.; Sarkar, P.K.; et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res 2000, 60, 2520–2526. [Google Scholar] [PubMed]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361. [Google Scholar] [CrossRef]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The double edge sword of fibrosis in cancer. Transl Res 2019, 209, 55–67. [Google Scholar] [CrossRef]
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The evolving relationship of wound healing and tumor stroma. JCI insight 2018, 3. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: wounds that do not heal—a historical perspective with a focus on the fundamental roles of increased vascular permeability and clotting. in Seminars in thrombosis and hemostasis. 2019. Thieme Medical Publishers. [CrossRef]
- Dzobo, K. Taking a Full Snapshot of Cancer Biology: Deciphering the Tumor Microenvironment for Effective Cancer Therapy in the Oncology Clinic. Omics 2020. [Google Scholar] [CrossRef]
- Dzobo, K.; Dandara, C. Architecture of Cancer-Associated Fibroblasts in Tumor Microenvironment: Mapping Their Origins, Heterogeneity, and Role in Cancer Therapy Resistance. Omics 2020, 24, 314–339. [Google Scholar] [CrossRef]
- Dzobo, K.; Dandara, C. Broadening Drug Design and Targets to Tumor Microenvironment? Cancer-Associated Fibroblast Marker Expression in Cancers and Relevance for Survival Outcomes. Omics 2020, 24, 340–351. [Google Scholar] [CrossRef]
- Bochet, L.; Lehuédé, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res 2013, 73, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Rana, S.; Zöller, M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia 2013, 15, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.P.; Spary, L.K.; Sanders, A.J.; Chowdhury, R.; Jiang, W.G.; Steadman, R.; Wymant, J.; Jones, A.T.; Kynaston, H.; Mason, M.D.; et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 2015, 34, 290–302. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Yao, H.H.; Zhu, Z.Q.; Ning, Z.L.; Huang, Q. Stromal Myofibroblasts Are Associated with Poor Prognosis in Solid Cancers: A Meta-Analysis of Published Studies. PLoS One 2016, 11, e0159947. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Ogawa, T.; Zhang, X.; Hanamura, N.; Kashikura, Y.; Takamura, M.; Yoneda, M.; Shiraishi, T. Role of stromal myofibroblasts in invasive breast cancer: stromal expression of alpha-smooth muscle actin correlates with worse clinical outcome. Breast Cancer 2012, 19, 170–176. [Google Scholar] [CrossRef]
- Tsujino, T.; Seshimo, I.; Yamamoto, H.; Ngan, C.Y.; Ezumi, K.; Takemasa, I.; Ikeda, M.; Sekimoto, M.; Matsuura, N.; Monden, M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res 2007, 13, 2082–2090. [Google Scholar] [CrossRef]
- Liu, C.; Mak, M. Fibroblast-mediated uncaging of cancer cells and dynamic evolution of the physical microenvironment. Sci Rep 2022, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017, 214, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov 2019, 9, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Öhlund, D.; Rickelt, S.; Lidström, T.; Huang, Y.; Hao, L.; Zhao, R.T.; Franklin, O.; Bhatia, S.N.; Tuveson, D.A.; et al. Cancer Cell–Derived Matrisome Proteins Promote Metastasis in Pancreatic Ductal Adenocarcinoma. Cancer Research 2020, 80, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Chua, M.L.K.; Yeong, J.P.S.; Tan, S.J.; Lim, W.-T.; Lim, C.T. Pan-cancer analysis connects tumor matrisome to immune response. NPJ precision oncology 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Rafaeva, M.; Erler, J.T. Framing cancer progression: influence of the organ-and tumour-specific matrisome. The FEBS journal 2020, 287, 1454–1477. [Google Scholar] [CrossRef]
- Le, C.P.; Nowell, C.J.; Kim-Fuchs, C.; Botteri, E.; Hiller, J.G.; Ismail, H.; Pimentel, M.A.; Chai, M.G.; Karnezis, T.; Rotmensz, N.; et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun 2016, 7, 10634. [Google Scholar] [CrossRef]
- Tacconi, C.; Correale, C.; Gandelli, A.; Spinelli, A.; Dejana, E.; D'Alessio, S.; Danese, S. Vascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion. Gastroenterology 2015, 148, 1438–1451. [Google Scholar] [CrossRef]
- Le, C.P.; Karnezis, T.; Achen, M.G.; Stacker, S.A.; Sloan, E.K. Lymphovascular and neural regulation of metastasis: shared tumour signalling pathways and novel therapeutic approaches. Best Pract Res Clin Anaesthesiol 2013, 27, 409–425. [Google Scholar] [CrossRef]
- Nagaraja, A.S.; Dood, R.L.; Armaiz-Pena, G.; Kang, Y.; Wu, S.Y.; Allen, J.K.; Jennings, N.B.; Mangala, L.S.; Pradeep, S.; Lyons, Y.; et al. Adrenergic-mediated increases in INHBA drive CAF phenotype and collagens. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Insua-Rodríguez, J.; Pein, M.; Hongu, T.; Meier, J.; Descot, A.; Lowy, C.M.; De Braekeleer, E.; Sinn, H.P.; Spaich, S.; Sütterlin, M.; et al. Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis. EMBO Mol Med 2018, 10. [Google Scholar] [CrossRef]
- Afasizheva, A.; Devine, A.; Tillman, H.; Fung, K.L.; Vieira, W.D.; Blehm, B.H.; Kotobuki, Y.; Busby, B.; Chen, E.I.; Tanner, K. Mitogen-activated protein kinase signaling causes malignant melanoma cells to differentially alter extracellular matrix biosynthesis to promote cell survival. BMC Cancer 2016, 16, 186. [Google Scholar] [CrossRef]
- Steins, A.; van Mackelenbergh, M.G.; van der Zalm, A.P.; Klaassen, R.; Serrels, B.; Goris, S.G.; Kocher, H.M.; Waasdorp, C.; de Jong, J.H.; Tekin, C.; et al. High-grade mesenchymal pancreatic ductal adenocarcinoma drives stromal deactivation through CSF-1. EMBO Rep 2020, 21, e48780. [Google Scholar] [CrossRef]
- Vera, R.E.; Fernandez-Zapico, M.E. Stromal deactivation by CSF1: a new feature of the aggressive pancreatic cancer microenvironment. EMBO Rep 2020, 21, e50468. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, S.; Zeng, S.; Shen, H. The critical roles of activated stellate cells-mediated paracrine signaling, metabolism and onco-immunology in pancreatic ductal adenocarcinoma. Mol Cancer 2018, 17, 62. [Google Scholar] [CrossRef]
- Wei, L.; Ye, H.; Li, G.; Lu, Y.; Zhou, Q.; Zheng, S.; Lin, Q.; Liu, Y.; Li, Z.; Chen, R. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis 2018, 9, 1065. [Google Scholar] [CrossRef] [PubMed]
- Palumbo Jr, A.; Ferreira, L.B.; de Souza, P.A.R.; de Oliveira, F.L.; Pontes, B.; Viana, N.B.; Machado, D.E.; Palmero, C.Y.; Alves, L.M.; Gimba, E.R. Extracellular matrix secreted by reactive stroma is a main inducer of pro-tumorigenic features on LNCaP prostate cancer cells. Cancer letters 2012, 321, 55–64. [Google Scholar] [CrossRef]
- Barcus, C.E.; Holt, E.C.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Dense collagen-I matrices enhance pro-tumorigenic estrogen-prolactin crosstalk in MCF-7 and T47D breast cancer cells. PloS one 2015, 10, e0116891. [Google Scholar] [CrossRef]
- Brown, Y.; Hua, S.; Tanwar, P.S. Extracellular matrix-mediated regulation of cancer stem cells and chemoresistance. The international journal of biochemistry & cell biology 2019, 109, 90–104. [Google Scholar]
- Yeldag, G.; Rice, A.; del Río Hernández, A. Chemoresistance and the self-maintaining tumor microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef]
- Pietilä, E.A.; Gonzalez-Molina, J.; Moyano-Galceran, L.; Jamalzadeh, S.; Zhang, K.; Lehtinen, L.; Turunen, S.P.; Martins, T.A.; Gultekin, O.; Lamminen, T. Co-evolution of matrisome and adaptive adhesion dynamics drives ovarian cancer chemoresistance. Nature Communications 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Keeratichamroen, S.; Lirdprapamongkol, K.; Svasti, J. Mechanism of ECM-induced dormancy and chemoresistance in A549 human lung carcinoma cells. Oncology reports 2018, 39, 1765–1774. [Google Scholar] [CrossRef]
- Mocanu, M.M.; Fazekas, Z.; Petrás, M.; Nagy, P.; Sebestyén, Z.; Isola, J.; Tímár, J.; Park, J.W.; Vereb, G.; Szöllosi, J. Associations of ErbB2, beta1-integrin and lipid rafts on Herceptin (Trastuzumab) resistant and sensitive tumor cell lines. Cancer Lett 2005, 227, 201–212. [Google Scholar] [CrossRef]
- Guo, W.; Pylayeva, Y.; Pepe, A.; Yoshioka, T.; Muller, W.J.; Inghirami, G.; Giancotti, F.G. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 2006, 126, 489–502. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Sleiman, M.; Moriarty, T.; Herrick, W.G.; Peyton, S.R. Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials 2014, 35, 5749–5759. [Google Scholar] [CrossRef]
- Keely, P.J. Mechanisms by which the extracellular matrix and integrin signaling act to regulate the switch between tumor suppression and tumor promotion. Journal of mammary gland biology and neoplasia 2011, 16, 205–219. [Google Scholar] [CrossRef]
- Weigelt, B.; Lo, A.T.; Park, C.C.; Gray, J.W.; Bissell, M.J. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat 2010, 122, 35–43. [Google Scholar] [CrossRef]
- Dzobo, K. Integrins Within the Tumor Microenvironment: Biological Functions, Importance for Molecular Targeting, and Cancer Therapeutics Innovation. Omics 2021, 25, 417–430. [Google Scholar] [CrossRef]
- Dzobo, K.; Vogelsang, M.; Parker, M.I. Wnt/β-Catenin and MEK-ERK Signaling are Required for Fibroblast-Derived Extracellular Matrix-Mediated Endoderm Differentiation of Embryonic Stem Cells. Stem Cell Rev Rep 2015, 11, 761–773. [Google Scholar] [CrossRef]
- Guerrero, P.A.; McCarty, J.H. Integrins in Vascular Development and Pathology. Adv Pharmacol 2018, 81, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.W.; Jacquemet, G. Cell matrix adhesion in cell migration. Essays Biochem 2019, 63, 535–551. [Google Scholar] [CrossRef]
- Zhang, Y.; Reif, G.; Wallace, D.P. Extracellular matrix, integrins, and focal adhesion signaling in polycystic kidney disease. Cell Signal 2020, 72, 109646. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Ivaska, J. Every step of the way: integrins in cancer progression and metastasis. Nature Reviews Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Samaržija, I.; Dekanić, A.; Humphries, J.D.; Paradžik, M.; Stojanović, N.; Humphries, M.J.; Ambriović-Ristov, A. Integrin crosstalk contributes to the complexity of signalling and unpredictable cancer cell fates. Cancers 2020, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Varner, J.A.; Emerson, D.A.; Juliano, R.L. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Molecular biology of the cell 1995, 6, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Stoeltzing, O.; Liu, W.; Reinmuth, N.; Fan, F.; Parry, G.C.; Parikh, A.A.; McCarty, M.F.; Bucana, C.D.; Mazar, A.P.; Ellis, L.M. Inhibition of integrin α5β1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. International journal of cancer 2003, 104, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Varner, J. Integrins: roles in cancer development and as treatment targets. British journal of cancer 2004, 90, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Varner, J.A.; Cheresh, D.A. Integrins and cancer. Current opinion in cell biology 1996, 8, 724–730. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. αV integrins in angiogenesis and cancer. Cold Spring Harbor perspectives in medicine 2011, 1, a006478. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. Journal of Biological Chemistry 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Truong, H.; Danen, E.H. Integrin switching modulates adhesion dynamics and cell migration. Cell adhesion & migration 2009, 3, 179–181. [Google Scholar]
- Zuidema, A.; Wang, W.; Sonnenberg, A. Crosstalk between cell adhesion complexes in regulation of mechanotransduction. Bioessays 2020, 42, 2000119. [Google Scholar] [CrossRef]
- Kerrisk, M.E.; Cingolani, L.A.; Koleske, A.J. ECM receptors in neuronal structure, synaptic plasticity, and behavior. Progress in brain research 2014, 214, 101–131. [Google Scholar]
- Boraschi-Diaz, I.; Wang, J.; Mort, J.S.; Komarova, S.V. Collagen type I as a ligand for receptor-mediated signaling. Frontiers in Physics 2017, 5, 12. [Google Scholar] [CrossRef]
- Takai, K.; Drain, A.P.; Lawson, D.A.; Littlepage, L.E.; Karpuj, M.; Kessenbrock, K.; Le, A.; Inoue, K.; Weaver, V.M.; Werb, Z. Discoidin domain receptor 1 (DDR1) ablation promotes tissue fibrosis and hypoxia to induce aggressive basal-like breast cancers. Genes & development 2018, 32, 244–257. [Google Scholar]
- Gonzalez, M.E.; Martin, E.E.; Anwar, T.; Arellano-Garcia, C.; Medhora, N.; Lama, A.; Chen, Y.-C.; Tanager, K.S.; Yoon, E.; Kidwell, K.M. Mesenchymal stem cell-induced DDR2 mediates stromal-breast cancer interactions and metastasis growth. Cell reports 2017, 18, 1215–1228. [Google Scholar] [CrossRef]
- Bayer, S.V.; Grither, W.R.; Brenot, A.; Hwang, P.Y.; Barcus, C.E.; Ernst, M.; Pence, P.; Walter, C.; Pathak, A.; Longmore, G.D. DDR2 controls breast tumor stiffness and metastasis by regulating integrin mediated mechanotransduction in CAFs. Elife 2019, 8, e45508. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-C.; Yen, H.-R.; Chen, C.-H.; Chu, Y.-H.; Song, Y.-C.; Tseng, T.-J.; Liu, C.-H. CHPF promotes malignancy of breast cancer cells by modifying syndecan-4 and the tumor microenvironment. American journal of cancer research 2021, 11, 812. [Google Scholar] [PubMed]
- Leblanc, R.; Sahay, D.; Houssin, A.; Machuca-Gayet, I.; Peyruchaud, O. Autotaxin-β interaction with the cell surface via syndecan-4 impacts on cancer cell proliferation and metastasis. Oncotarget 2018, 9, 33170. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, T.; Li, R.; Sy, M.-S. Mechanisms regulating the binding activity of CD44 to hyaluronic acid. Frontiers in Bioscience-Landmark 1998, 3, 631–636. [Google Scholar]
- Naor, D. interaction between hyaluronic acid and its receptors (CD44, RHAMM) regulates the activity of inflammation and cancer. 2016, Frontiers Media SA. p. 39. [CrossRef]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan–CD44 interactions as potential targets for cancer therapy. The FEBS journal 2011, 278, 1429–1443. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan-CD44 interactions in cancer: paradoxes and possibilities. Clinical Cancer Research 2009, 15, 7462–7468. [Google Scholar] [CrossRef]
- Jalkanen, S.; Jalkanen, M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. The Journal of cell biology 1992, 116, 817–825. [Google Scholar] [CrossRef]
- Dzobo, K.; Sinkala, M. Cancer Stem Cell Marker CD44 Plays Multiple Key Roles in Human Cancers: Immune Suppression/Evasion, Drug Resistance, Epithelial–Mesenchymal Transition, and Metastasis. OMICS: A Journal of Integrative Biology 2021, 25, 313–332. [Google Scholar] [CrossRef]
- Thapa, R.; Wilson, G.D. The importance of CD44 as a stem cell biomarker and therapeutic target in cancer. Stem cells international 2016, 2016. [Google Scholar] [CrossRef]
- Hirata, K.; Suzuki, H.; Imaeda, H.; Matsuzaki, J.; Tsugawa, H.; Nagano, O.; Asakura, K.; Saya, H.; Hibi, T. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. British journal of cancer 2013, 109, 379–386. [Google Scholar] [CrossRef]
- Palapattu, G.S.; Wu, C.; Silvers, C.R.; Martin, H.B.; Williams, K.; Salamone, L.; Bushnell, T.; Huang, L.S.; Yang, Q.; Huang, J. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. The Prostate 2009, 69, 787–798. [Google Scholar] [CrossRef]
- Yae, T.; Tsuchihashi, K.; Ishimoto, T.; Motohara, T.; Yoshikawa, M.; Yoshida, G.J.; Wada, T.; Masuko, T.; Mogushi, K.; Tanaka, H. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nature communications 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.L.-H.; Fiscus, R.R.; Tung, J.W.; Tin, V.P.-C.; Cheng, L.C.; Sihoe, A.D.-L.; Fink, L.M.; Ma, Y.; Wong, M.P. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PloS one 2010, 5, e14062. [Google Scholar] [CrossRef]
- Smith, L.R.; Cho, S.; Discher, D.E. Stem cell differentiation is regulated by extracellular matrix mechanics. Physiology 2018, 33, 16–25. [Google Scholar] [CrossRef]
- Hoffmann, E.J.; Ponik, S.M. Biomechanical contributions to macrophage activation in the tumor microenvironment. Frontiers in Oncology 2020, 10, 787. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Charrier, E.E.; Pogoda, K.; Wells, R.G.; Janmey, P.A. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nature communications 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Elosegui-Artola, A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Current Opinion in Cell Biology 2021, 72, 10–18. [Google Scholar] [CrossRef]
- Tschumperlin, D.J.; Lagares, D. Mechano-therapeutics: targeting mechanical signaling in fibrosis and tumor stroma. Pharmacology & therapeutics 2020, 212, 107575. [Google Scholar]
- Pratt, S.J.; Lee, R.M.; Martin, S.S. The mechanical microenvironment in breast cancer. Cancers 2020, 12, 1452. [Google Scholar] [CrossRef]
- Nazemi, M.; Rainero, E. Cross-talk between the tumor microenvironment, extracellular matrix, and cell metabolism in cancer. Frontiers in Oncology 2020, 10, 239. [Google Scholar] [CrossRef]
- Pizzo, A.M.; Kokini, K.; Vaughn, L.C.; Waisner, B.Z.; Voytik-Harbin, S.L. Extracellular matrix (ECM) microstructural composition regulates local cell-ECM biomechanics and fundamental fibroblast behavior: a multidimensional perspective. Journal of applied physiology 2005, 98, 1909–1921. [Google Scholar] [CrossRef]
- Wala, J.; Das, S. Mapping of biomechanical properties of cell lines on altered matrix stiffness using atomic force microscopy. Biomechanics and Modeling in Mechanobiology 2020, 19, 1523–1536. [Google Scholar] [CrossRef]
- Souza, S.T.; Agra, L.C.; Santos, C.E.; Barreto, E.; Hickmann, J.M.; Fonseca, E.J. Macrophage adhesion on fibronectin evokes an increase in the elastic property of the cell membrane and cytoskeleton: an atomic force microscopy study. European Biophysics Journal 2014, 43, 573–579. [Google Scholar] [CrossRef]
- Park, J.S.; Burckhardt, C.J.; Lazcano, R.; Solis, L.M.; Isogai, T.; Li, L.; Chen, C.S.; Gao, B.; Minna, J.D.; Bachoo, R. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature 2020, 578, 621–626. [Google Scholar] [CrossRef]
- Vitillo, L.; Kimber, S.J. Integrin and FAK regulation of human pluripotent stem cells. Current stem cell reports 2017, 3, 358–365. [Google Scholar] [CrossRef]
- Dedhar, S. Cell–substrate interactions and signaling through ILK. Current opinion in cell biology 2000, 12, 250–256. [Google Scholar] [CrossRef]
- Schlie-Wolter, S.; Ngezahayo, A.; Chichkov, B.N. The selective role of ECM components on cell adhesion, morphology, proliferation and communication in vitro. Experimental cell research 2013, 319, 1553–1561. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Rada, M.; Heiserman, J.P.; Cha, J.; Sage, J.; Zhou, B.; Yang, W.; Hu, Y.; Korgaonkar, C.; Hanos, C.T. Inhibition of collagen XI alpha 1-induced fatty acid oxidation triggers apoptotic cell death in cisplatin-resistant ovarian cancer. Cell death & disease 2020, 11, 1–12. [Google Scholar]
- Even-Ram, S.; Yamada, K.M. Cell migration in 3D matrix. Current opinion in cell biology 2005, 17, 524–532. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the hallmarks of metastasis. Cancer research 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G.; Lehembre, F. Distinct mechanisms of tumor invasion and metastasis. Trends in molecular medicine 2007, 13, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Gangnon, R.E.; Sprague, B.L.; Van Gemert, L.; Hampton, J.M.; Eliceiri, K.W.; Bredfeldt, J.S.; Liu, Y.; Surachaicharn, N.; Newcomb, P.A. Collagen Alignment as a Predictor of Recurrence after Ductal Carcinoma In SituCollagen Alignment and Recurrence of DCIS. Cancer Epidemiology, Biomarkers & Prevention 2018, 27, 138–145. [Google Scholar]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. The American journal of pathology 2011, 178, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Drifka, C.R.; Loeffler, A.G.; Mathewson, K.; Keikhosravi, A.; Eickhoff, J.C.; Liu, Y.; Weber, S.M.; Kao, W.J.; Eliceiri, K.W. Highly aligned stromal collagen is a negative prognostic factor following pancreatic ductal adenocarcinoma resection. Oncotarget 2016, 7, 76197. [Google Scholar] [CrossRef]
- Yang, N.; Friedl, A. Syndecan-1-induced ECM fiber alignment requires integrin αvβ3 and syndecan-1 ectodomain and heparan sulfate chains. PloS one 2016, 11, e0150132. [Google Scholar] [CrossRef]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. Journal of Cell Biology 2017, 216, 3799–3816. [Google Scholar] [CrossRef]
- Ao, M.; Brewer, B.M.; Yang, L.; Franco Coronel, O.E.; Hayward, S.W.; Webb, D.J.; Li, D. Stretching fibroblasts remodels fibronectin and alters cancer cell migration. Scientific reports 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Feinberg, T.Y.; Zheng, H.; Liu, R.; Wicha, M.S.; Yu, S.M.; Weiss, S.J. Divergent matrix-remodeling strategies distinguish developmental from neoplastic mammary epithelial cell invasion programs. Developmental cell 2018, 47, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. Journal of enzyme inhibition and medicinal chemistry 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Werb, Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends in cell biology 2001, 11, S37–S43. [Google Scholar] [CrossRef]
- Elia, I.; Rossi, M.; Stegen, S.; Broekaert, D.; Doglioni, G.; van Gorsel, M.; Boon, R.; Escalona-Noguero, C.; Torrekens, S.; Verfaillie, C. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature 2019, 568, 117–121. [Google Scholar] [CrossRef]
- Ge, J.; Cui, H.; Xie, N.; Banerjee, S.; Guo, S.; Dubey, S.; Barnes, S.; Liu, G. Glutaminolysis promotes collagen translation and stability via α-ketoglutarate–mediated mTOR activation and proline hydroxylation. American journal of respiratory cell and molecular biology 2018, 58, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, D.S.; Maheswaran, S.; Haber, D.A. A conduit to metastasis: circulating tumor cell biology. Genes & development 2017, 31, 1827–1840. [Google Scholar]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y. Pre-metastatic niches: organ-specific homes for metastases. Nature Reviews Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Gao, Y.; Bado, I.; Wang, H.; Zhang, W.; Rosen, J.M.; Zhang, X.H.-F. Metastasis organotropism: redefining the congenial soil. Developmental cell 2019, 49, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Taylor, M.L.; Gutschner, T.; Pradeep, S.; Cho, M.S.; Sheng, J.; Lyons, Y.M.; Nagaraja, A.S.; Dood, R.L.; Wen, Y. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nature communications 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Yu, M.; Ting, D.T.; Stott, S.L.; Wittner, B.S.; Ozsolak, F.; Paul, S.; Ciciliano, J.C.; Smas, M.E.; Winokur, D.; Gilman, A.J. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 2012, 487, 510–513. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C.; Riethdorf, S. Cancer micrometastases. Nature reviews Clinical oncology 2009, 6, 339–351. [Google Scholar] [CrossRef]
- Goddard, E.T.; Bozic, I.; Riddell, S.R.; Ghajar, C.M. Dormant tumour cells, their niches and the influence of immunity. Nature cell biology 2018, 20, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Yeh, A.C.; Ramaswamy, S. Mechanisms of cancer cell dormancy—another hallmark of cancer? Cancer research 2015, 75, 5014–5022. [Google Scholar] [CrossRef]
- Boire, A.; Coffelt, S.B.; Quezada, S.A.; Vander Heiden, M.G.; Weeraratna, A.T. Tumour dormancy and reawakening: Opportunities and challenges. Trends in Cancer 2019, 5, 762–765. [Google Scholar] [CrossRef]
- Phan, T.G.; Croucher, P.I. The dormant cancer cell life cycle. Nature Reviews Cancer 2020, 20, 398–411. [Google Scholar] [CrossRef]
- Gay, L.J.; Malanchi, I. The sleeping ugly: tumour microenvironment's act to make or break the spell of dormancy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 2017, 1868, 231–238. [Google Scholar] [CrossRef]
- Ghajar, C.M.; Peinado, H.; Mori, H.; Matei, I.R.; Evason, K.J.; Brazier, H.; Almeida, D.; Koller, A.; Hajjar, K.A.; Stainier, D.Y. The perivascular niche regulates breast tumour dormancy. Nature cell biology 2013, 15, 807–817. [Google Scholar] [CrossRef]
- Mancini, M.L.; Sonis, S.T. Mechanisms of cellular fibrosis associated with cancer regimen-related toxicities. Frontiers in pharmacology 2014, 5, 51. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Fibrosis and cancer: partners in crime or opposing forces? Trends in cancer 2016, 2, 279–282. [Google Scholar] [CrossRef]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The double edge sword of fibrosis in cancer. Translational Research 2019, 209, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Molecular pathways: connecting fibrosis and solid tumor metastasis. Clinical Cancer Research 2014, 20, 3637–3643. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Narbutis, M.; Kumar, S.; Park, A.; Viswakarma, N.; Dorman, M.J.; Kamath, S.D.; Grippo, P.J.; Fishel, M.L.; Hwang, R.F. Long-Term Gemcitabine Treatment Reshapes the Pancreatic Tumor Microenvironment and Sensitizes Murine Carcinoma to Combination ImmunotherapyGemcitabine Primes Pancreatic Cancer for Immunotherapy. Cancer research 2020, 80, 3101–3115. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.J.; Sharma, A.; Vuong, D.-V.; Erler, J.T.; Pruschy, M.; Broggini-Tenzer, A. Ionizing radiation induces tumor cell lysyl oxidase secretion. BMC cancer 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.M.; Walsh, L.A. Candidate prognostic markers in breast cancer: focus on extracellular proteases and their inhibitors. Breast Cancer: Targets and Therapy 2014, 6, 81. [Google Scholar]
- Kanayama, H.o.; Yokota, K.y.; Kurokawa, Y.; Murakami, Y.; Nishitani, M.; Kagawa, S. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer: Interdisciplinary International Journal of the American Cancer Society 1998, 82, 1359–1366. [Google Scholar] [CrossRef]
- Bergamaschi, A.; Tagliabue, E.; Sørlie, T.; Naume, B.; Triulzi, T.; Orlandi, R.; Russnes, H.; Nesland, J.; Tammi, R.; Auvinen, P. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. The Journal of pathology 2008, 214, 357–367. [Google Scholar] [CrossRef]
- Valdembri, D.; Serini, G. The roles of integrins in cancer. Faculty reviews 2021, 10. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Bordeleau, F.; Mason, B.N.; Lollis, E.M.; Mazzola, M.; Zanotelli, M.R.; Somasegar, S.; Califano, J.P.; Montague, C.; LaValley, D.J.; Huynh, J.; et al. Matrix stiffening promotes a tumor vasculature phenotype. Proceedings of the National Academy of Sciences of the United States of America 2017, 114, 492–497. [Google Scholar] [CrossRef]
- Hui, L.; Zhang, J.; Ding, X.; Guo, X.; Jiang, X. Matrix stiffness regulates the proliferation, stemness and chemoresistance of laryngeal squamous cancer cells. Int J Oncol 2017. [Google Scholar] [CrossRef]
- McLane, J.S.; Ligon, L.A. Stiffened Extracellular Matrix and Signaling from Stromal Fibroblasts via Osteoprotegerin Regulate Tumor Cell Invasion in a 3-D Tumor in Situ Model. Cancer Microenviron 2016. [Google Scholar] [CrossRef]
- Tadeo, I.; Berbegall, A.P.; Navarro, S.; Castel, V.; Noguera, R. A stiff extracellular matrix is associated with malignancy in peripheral neuroblastic tumors. Pediatr Blood Cancer 2017. [Google Scholar] [CrossRef]
- Qin, X.; Lv, X.; Li, P.; Yang, R.; Xia, Q.; Chen, Y.; Peng, Y.; Li, L.; Li, S.; Li, T. Matrix stiffness modulates ILK-mediated YAP activation to control the drug resistance of breast cancer cells. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2020, 1866, 165625. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Ge, H.; Ghadban, T.; Reeh, M.; Güngör, C. The Extracellular Matrix: A Key Accomplice of Cancer Stem Cell Migration, Metastasis Formation, and Drug Resistance in PDAC. Cancers 2022, 14, 3998. [Google Scholar] [CrossRef]
- Grantab, R.H.; Tannock, I.F. Penetration of anticancer drugs through tumour tissue as a function of cellular packing density and interstitial fluid pressure and its modification by bortezomib. BMC cancer 2012, 12, 214. [Google Scholar] [CrossRef]
- Harisi, R.; Jeney, A. Extracellular matrix as target for antitumor therapy. Onco Targets Ther 2015, 8, 1387–1398. [Google Scholar] [CrossRef]
- Holle, A.W.; Young, J.L.; Spatz, J.P. In vitro cancer cell-ECM interactions inform in vivo cancer treatment. Advanced drug delivery reviews 2016, 97, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnight, T.R.; et al. Tissue mechanics promote IDH1-dependent HIF1alpha-tenascin C feedback to regulate glioblastoma aggression. Nat Cell Biol 2016, 18, 1336–1345. [Google Scholar] [CrossRef]
- Mittal, V.; El Rayes, T.; Narula, N.; McGraw, T.E.; Altorki, N.K.; Barcellos-Hoff, M.H. The Microenvironment of Lung Cancer and Therapeutic Implications. Adv Exp Med Biol 2016, 890, 75–110. [Google Scholar] [CrossRef] [PubMed]
- Mumenthaler, S.M.; Foo, J.; Choi, N.C.; Heise, N.; Leder, K.; Agus, D.B.; Pao, W.; Michor, F.; Mallick, P. The Impact of Microenvironmental Heterogeneity on the Evolution of Drug Resistance in Cancer Cells. Cancer Inform 2015, 14, 19–31. [Google Scholar] [CrossRef]
- Nieponice, A.; McGrath, K.; Qureshi, I.; Beckman, E.J.; Luketich, J.D.; Gilbert, T.W.; Badylak, S.F. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc 2009, 69, 289–296. [Google Scholar] [CrossRef]
- Barcus, C.E.; O'Leary, K.A.; Brockman, J.L.; Rugowski, D.E.; Liu, Y.; Garcia, N.; Yu, M.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast cancer research : BCR 2017, 19, 9. [Google Scholar] [CrossRef]
- Brechbuhl, H.M.; Finlay-Schultz, J.; Yamamoto, T.; Gillen, A.; Cittelly, D.M.; Tan, A.C.; Sams, S.B.; Pillai, M.; Elias, A.; Robinson, W.A.; et al. Fibroblast subtypes regulate responsiveness of luminal breast cancer to estrogen. Clinical cancer research : an official journal of the American Association for Cancer Research 2016. [Google Scholar] [CrossRef]
- Cui, L.; Tse, K.; Zahedi, P.; Harding, S.M.; Zafarana, G.; Jaffray, D.A.; Bristow, R.G.; Allen, C. Hypoxia and cellular localization influence the radiosensitizing effect of gold nanoparticles (AuNPs) in breast cancer cells. Radiat Res 2014, 182, 475–488. [Google Scholar] [CrossRef]
- Cun, X.; Ruan, S.; Chen, J.; Zhang, L.; Li, J.; He, Q.; Gao, H. A dual strategy to improve the penetration and treatment of breast cancer by combining shrinking nanoparticles with collagen depletion by losartan. Acta Biomater 2016, 31, 186–196. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Sung, S.Y.; Hsieh, C.L.; Wu, D.; Chung, L.W.; Johnstone, P.A. Tumor microenvironment promotes cancer progression, metastasis, and therapeutic resistance. Curr Probl Cancer 2007, 31, 36–100. [Google Scholar] [CrossRef]
- Whatcott, C.J.; Han, H.; Posner, R.G.; Hostetter, G.; Von Hoff, D.D. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov 2011, 1, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Mbeunkui, F.; Johann, D.J. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer chemotherapy and pharmacology 2009, 63, 571–582. [Google Scholar] [CrossRef]
- Xiao, Q.; Ge, G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer microenvironment 2012, 5, 261–273. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Q.; Ma, H.; Li, L.; Liu, J.; Feng, Y.; Fang, Z.; Wu, J.; Han, X.; Zhang, J. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proceedings of the National Academy of Sciences 2010, 107, 18892–18897. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays in biochemistry 2019, 63, 349–364. [Google Scholar]
- Seewaldt, V. ECM stiffness paves the way for tumor cells. Nature medicine 2014, 20, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Chong, S.G.; Upagupta, C.; Yanagihara, T.; Saito, T.; Shimbori, C.; Bellaye, P.-S.; Nishioka, Y.; Kolb, M.R. Fibrotic extracellular matrix induces release of extracellular vesicles with pro-fibrotic miRNA from fibrocytes. Thorax 2021, 76, 895–906. [Google Scholar] [CrossRef]
- Netti, P.A.; Baxter, L.T.; Boucher, Y.; Skalak, R.; Jain, R.K. Time-dependent behavior of interstitial fluid pressure in solid tumors: implications for drug delivery. Cancer research 1995, 55, 5451–5458. [Google Scholar]
- Morin, P.J. Drug resistance and the microenvironment: nature and nurture. Drug Resist Updat 2003, 6, 169–172. [Google Scholar] [CrossRef]
- Gouarderes, S.; Mingotaud, A.-F.; Vicendo, P.; Gibot, L. Vascular and extracellular matrix remodeling by physical approaches to improve drug delivery at the tumor site. Expert opinion on drug delivery 2020, 17, 1703–1726. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends in cancer 2018, 4, 292–319. [Google Scholar] [CrossRef]
- Sercu, S.; Zhang, L.; Merregaert, e.J. The extracellular matrix protein 1: its molecular interaction and implication in tumor progression. Cancer investigation 2008, 26, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, A.; Khan, L.; Bensler, N.P.; Bose, P.; De Carvalho, D.D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nature communications 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Troup, S.; Njue, C.; Kliewer, E.V.; Parisien, M.; Roskelley, C.; Chakravarti, S.; Roughley, P.J.; Murphy, L.C.; Watson, P.H. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clinical cancer research 2003, 9, 207–214. [Google Scholar] [PubMed]
- Biaoxue, R.; Xiguang, C.; Hua, L.; Hui, M.; Shuanying, Y.; Wei, Z.; Wenli, S.; Jie, D. Decreased expression of decorin and p57 (KIP2) correlates with poor survival and lymphatic metastasis in lung cancer patients. The International journal of biological markers 2011, 26, 9–21. [Google Scholar] [CrossRef]
- Matsumine, A.; Shintani, K.; Kusuzaki, K.; Matsubara, T.; Satonaka, H.; Wakabayashi, T.; Iino, T.; Uchida, A. Expression of decorin, a small leucine-rich proteoglycan, as a prognostic factor in soft tissue tumors. Journal of surgical oncology 2007, 96, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Järveläinen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacological reviews 2009, 61, 198–223. [Google Scholar] [CrossRef]
- Erkan, M.; Michalski, C.W.; Rieder, S.; Reiser–Erkan, C.; Abiatari, I.; Kolb, A.; Giese, N.A.; Esposito, I.; Friess, H.; Kleeff, J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clinical gastroenterology and hepatology 2008, 6, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Eble, J.A. Hold on or cut? Integrin-and MMP-mediated cell–matrix interactions in the tumor microenvironment. International Journal of Molecular Sciences 2020, 22, 238. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Frontiers in molecular biosciences 2020, 6, 160. [Google Scholar] [CrossRef]
- Hanker, A.B.; Estrada, M.V.; Bianchini, G.; Moore, P.D.; Zhao, J.; Cheng, F.; Koch, J.P.; Gianni, L.; Tyson, D.R.; Sánchez, V. Extracellular Matrix/Integrin Signaling Promotes Resistance to Combined Inhibition of HER2 and PI3K in HER2+ Breast CancerECM Promotes Resistance to HER2/PI3K Inhibition. Cancer research 2017, 77, 3280–3292. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N. HALO 202: randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. Journal of Clinical Oncology 2018, 36, 359–366. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; McDonough, S.L.; Philip, P.A.; Hingorani, S.R.; Lacy, J.; Kortmansky, J.S.; Thumar, J.; Chiorean, E.G.; Shields, A.F.; Behl, D. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. Journal of Clinical Oncology 2019, 37, 1062. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, B.L. MMP inhibition in prostate cancer. Annals of the New York Academy of Sciences 1999, 878, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Turpeenniemi-Hujanen, T. Gelatinases (MMP-2 and-9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie 2005, 87, 287–297. [Google Scholar] [CrossRef]
- Gomes, L.R.; Terra, L.F.; Wailemann, R.A.; Labriola, L.; Sogayar, M.C. TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC cancer 2012, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Matter, H.; Schudok, M. Recent advances in the design of matrix metalloprotease inhibitors. Current Opinion in Drug Discovery & Development 2004, 7, 513–535. [Google Scholar]
- Nuti, E.; Tuccinardi, T.; Rossello, A. Matrix metalloproteinase inhibitors: new challenges in the era of post broad-spectrum inhibitors. Current pharmaceutical design 2007, 13, 2087–2100. [Google Scholar] [CrossRef]
- Mannello, F.; Tonti, G.; Papa, S. Matrix metalloproteinase inhibitors as anticancer therapeutics. Current cancer drug targets 2005, 5, 285–298. [Google Scholar] [CrossRef]
- Radisky, E.S.; Raeeszadeh-Sarmazdeh, M.; Radisky, D.C. Therapeutic potential of matrix metalloproteinase inhibition in breast cancer. Journal of cellular biochemistry 2017, 118, 3531–3548. [Google Scholar] [CrossRef] [PubMed]
- Devel, L.; Czarny, B.; Beau, F.; Georgiadis, D.; Stura, E.; Dive, V. Third generation of matrix metalloprotease inhibitors: Gain in selectivity by targeting the depth of the S1′ cavity. Biochimie 2010, 92, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: a molecular basis for pharmacological inhibition strategies. Cancer and Metastasis Reviews 2015, 34, 643–690. [Google Scholar] [CrossRef] [PubMed]
- Grant, S. Cotargeting survival signaling pathways in cancer. The Journal of clinical investigation 2008, 118, 3003–3006. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, J.-Y.; Wei, W.-Z.; Wu, G.S. Activation of the Akt survival pathway contributes to TRAIL resistance in cancer cells. PloS one 2010, 5, e10226. [Google Scholar] [CrossRef]
- Roy-Luzarraga, M.; Hodivala-Dilke, K. Molecular Pathways: Endothelial Cell FAK—A Target for Cancer TreatmentEndothelial Cell FAK in Cancer Treatment. Clinical Cancer Research 2016, 22, 3718–3724. [Google Scholar] [CrossRef]
- McCormick, F. Small-molecule inhibitors of cell signaling. Current Opinion in Biotechnology 2000, 11, 593–597. [Google Scholar] [CrossRef]
- Morris, J.C.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Reiss, M.; Hsu, F.J.; Berzofsky, J.A.; Lawrence, D.P. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PloS one 2014, 9, e90353. [Google Scholar] [CrossRef]
- Lin, R.; Yi, S.; Gong, L.; Liu, W.; Wang, P.; Liu, N.; Zhao, L.; Wang, P. Inhibition of TGF-β signaling with halofuginone can enhance the antitumor effect of irradiation in Lewis lung cancer. OncoTargets and therapy 2015, 8, 3549. [Google Scholar] [CrossRef]
- Juárez, P.; Mohammad, K.S.; Yin, J.J.; Fournier, P.G.; McKenna, R.C.; Davis, H.W.; Peng, X.H.; Niewolna, M.; Javelaud, D.; Chirgwin, J.M. Halofuginone Inhibits the Establishment and Progression of Melanoma Bone MetastasesHalofuginone Decreases Melanoma Bone Metastases. Cancer research 2012, 72, 6247–6256. [Google Scholar] [CrossRef]
- Miyazono, K. Transforming growth factor-β signaling in epithelial-mesenchymal transition and progression of cancer. Proceedings of the Japan Academy, Series B 2009, 85, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Shaty, M.H.; Arif, I.S.; Al-Ezzi, M.I.; Hanna, D.B. Metformin attenuate fibrosis in both acute and chronic doxorubicin cardiotoxicity in rabbits. Journal of Pharmaceutical Sciences and Research 2018, 10, 1559–1565. [Google Scholar]
- Lampi, M.C.; Reinhart-King, C.A. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Science translational medicine 2018, 10, eaao0475. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Roberts, A.B.; Wakefield, L.M.; Assoian, R.K. Transforming growth factor-β: biological function and chemical structure. Science 1986, 233, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Krane, S.M. Collagenases and collagen degradation. Journal of Investigative Dermatology 1982, 79, 83–86. [Google Scholar] [CrossRef]
- McKee, T.D.; Grandi, P.; Mok, W.; Alexandrakis, G.; Insin, N.; Zimmer, J.P.; Bawendi, M.G.; Boucher, Y.; Breakefield, X.O.; Jain, R.K. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer research 2006, 66, 2509–2513. [Google Scholar] [CrossRef] [PubMed]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nature Reviews Immunology 2004, 4, 617–629. [Google Scholar] [CrossRef]
- Khokha, R.; Murthy, A.; Weiss, A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nature Reviews Immunology 2013, 13, 649–665. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nature reviews Molecular cell biology 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer—trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef]
- Castellani, P.; Viale, G.; Dorcaratto, A.; Nicolo, G.; Kaczmarek, J.; Querze, G.; Zardi, L. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. International journal of cancer 1994, 59, 612–618. [Google Scholar] [CrossRef]
- Glukhova, M.A.; Frid, M.G.; Shekhonin, B.V.; Balabanov, Y.V.; Koteliansky, V.E. Expression of fibronectin variants in vascular and visceral smooth muscle cells in development. Developmental biology 1990, 141, 193–202. [Google Scholar] [CrossRef]
- Han, Z.; Lu, Z.-R. Targeting fibronectin for cancer imaging and therapy. Journal of Materials Chemistry B 2017, 5, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Carnemolla, B.; Leprini, A.; Allemanni, G.; Saginati, M.; Zardi, L. The inclusion of the type III repeat ED-B in the fibronectin molecule generates conformational modifications that unmask a cryptic sequence. Journal of Biological Chemistry 1992, 267, 24689–24692. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.-M.; Lan, Y.; Lauder, S.; Zhang, J.; Brunkhorst, B.; Qin, G.; Verma, R.; Courtenay-Luck, N.; Gillies, S.D. huBC1-IL12, an immunocytokine which targets EDB-containing oncofetal fibronectin in tumors and tumor vasculature, shows potent anti-tumor activity in human tumor models. Cancer Immunology, Immunotherapy 2007, 56, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Kim, S.; Lee, I.-h.; Park, J.; Yu, M.; Lee, J.; Kim, J.-I.; Jon, S. Aptide-conjugated liposome targeting tumor-associated fibronectin for glioma therapy. Journal of Materials Chemistry B 2013, 1, 4723–4726. [Google Scholar] [CrossRef]
- Lokman, N.A.; Price, Z.K.; Hawkins, E.K.; Macpherson, A.M.; Oehler, M.K.; Ricciardelli, C. 4-Methylumbelliferone inhibits cancer stem cell activation and overcomes chemoresistance in ovarian cancer. Cancers 2019, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Kohli, A.G.; Kivimäe, S.; Tiffany, M.R.; Szoka, F.C. Improving the distribution of Doxil® in the tumor matrix by depletion of tumor hyaluronan. Journal of controlled release 2014, 191, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; Tempero, M.; Corrie, P.G. HALO-109–301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future oncology 2018, 14, 13–22. [Google Scholar] [CrossRef]
- Schnittert, J.; Bansal, R.; Storm, G.; Prakash, J. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Advanced drug delivery reviews 2018, 129, 37–53. [Google Scholar] [CrossRef]
- Gutheil, J.C.; Campbell, T.N.; Pierce, P.R.; Watkins, J.D.; Huse, W.D.; Bodkin, D.J.; Cheresh, D.A. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin ανβ3. Clinical Cancer Research 2000, 6, 3056–3061. [Google Scholar] [PubMed]
- Raguse, J.-D.; Gath, H.J.; Bier, J.; Riess, H.; Oettle, H. Cilengitide (EMD 121974) arrests the growth of a heavily pretreated highly vascularised head and neck tumour. Oral oncology 2004, 40, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Rechenmacher, F.; Kessler, H. Cilengitide: the first anti-angiogenic small molecule drug candidate. Design, synthesis and clinical evaluation. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2010, 10, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Tijink, B.M.; Buter, J.; De Bree, R.; Giaccone, G.; Lang, M.S.; Staab, A.; Leemans, C.R.; Van Dongen, G.A. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clinical Cancer Research 2006, 12, 6064–6072. [Google Scholar] [CrossRef]
- Riechelmann, H.; Sauter, A.; Golze, W.; Hanft, G.; Schroen, C.; Hoermann, K.; Erhardt, T.; Gronau, S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral oncology 2008, 44, 823–829. [Google Scholar] [CrossRef]
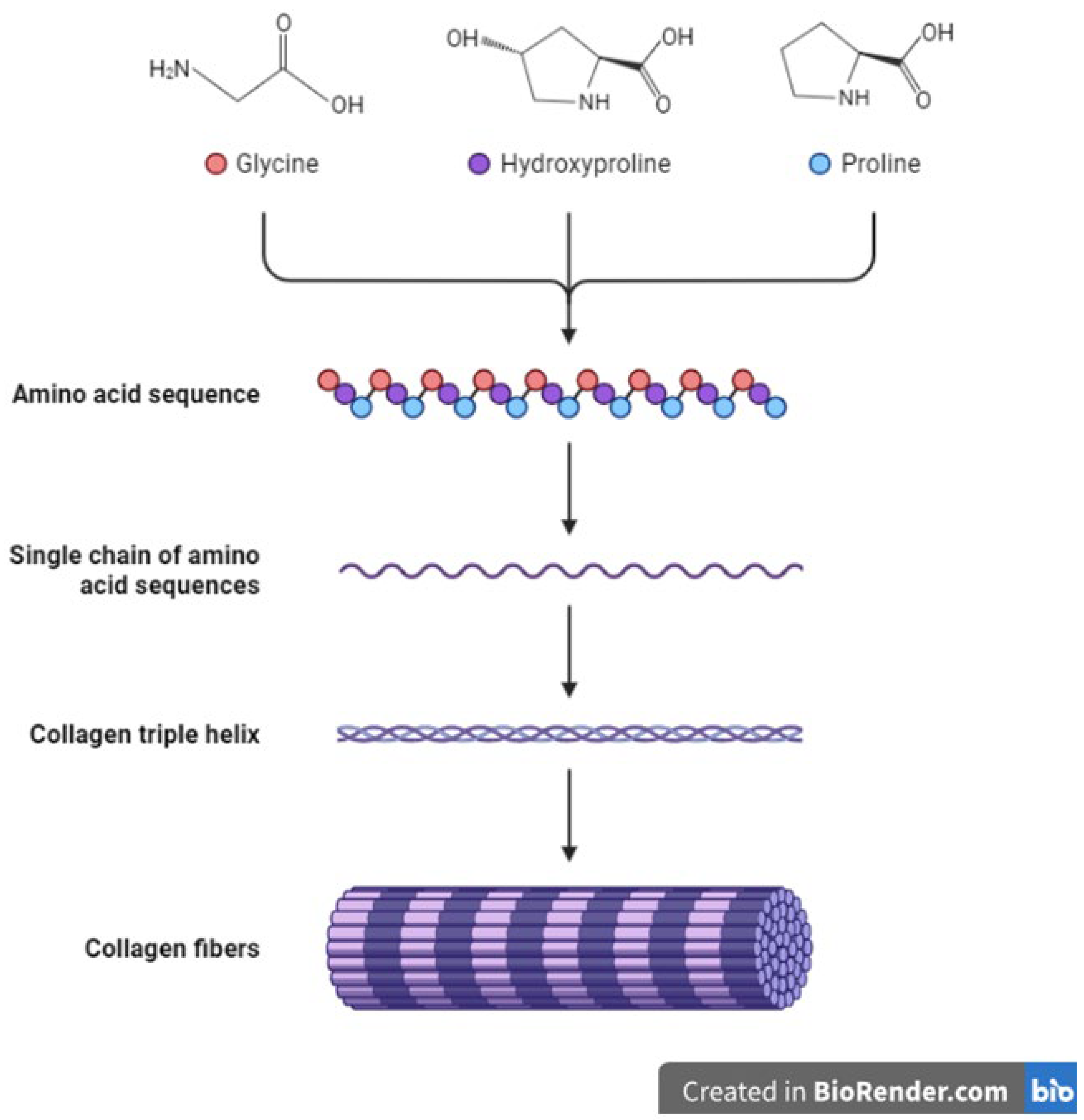

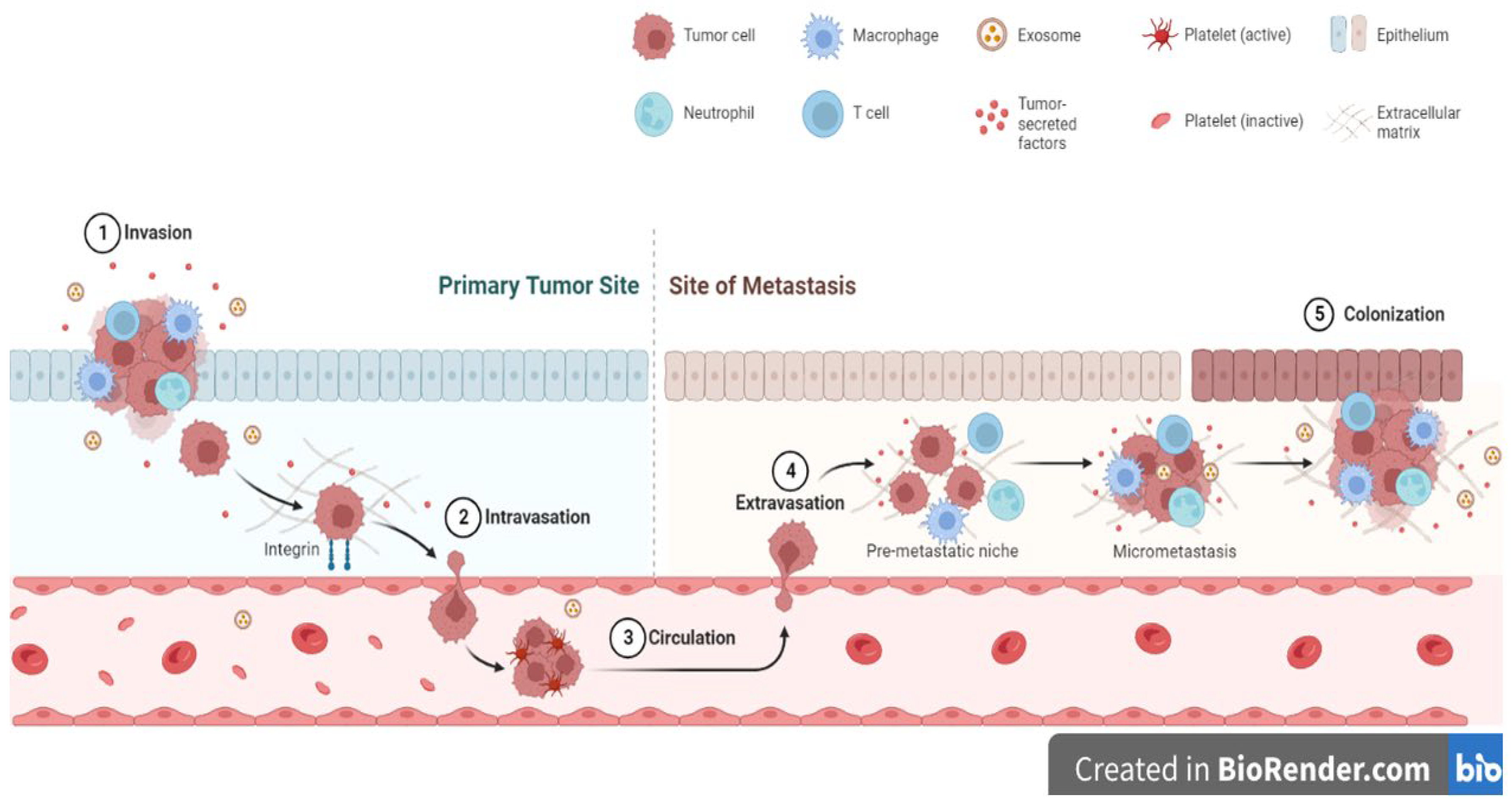
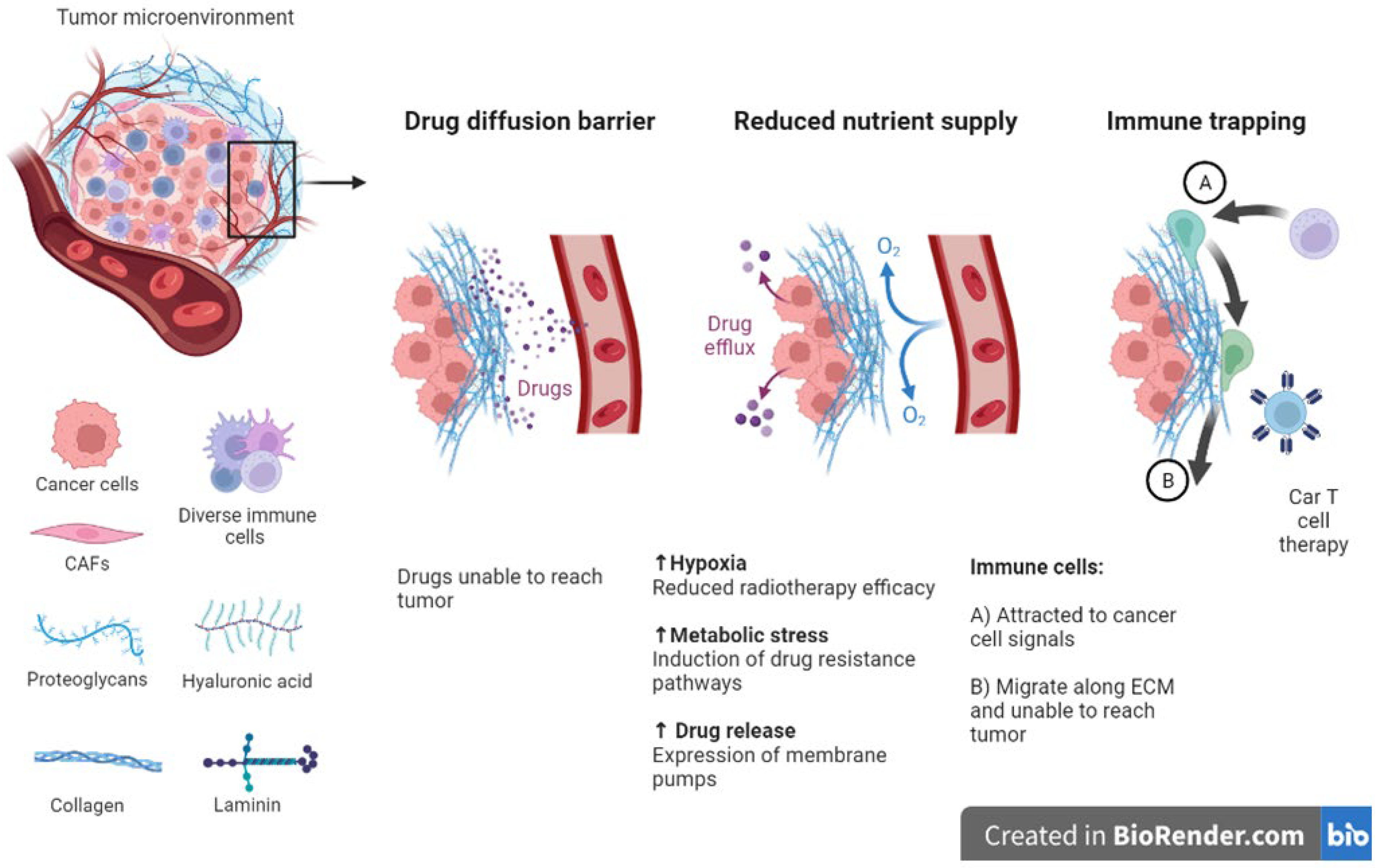
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




