1. Introduction
African swine fever virus (ASFV) is a large enveloped double stranded DNA virus. As the only member of the
Asfarviridae family [
1], it can infect domestic pigs and wild boars of all ages causing African swine fever (ASF). ASF is a notifiable disease to the World Organization for Animal Health (WOAH, formerly OIE), and is the number one threat for the global swine industry and a major limitation for global trading [
2]. Since 2007, a virulent ASFV strain (genotype II) has emerged causing mortality rates up to 100% and has spread in Europe, China, South-East Asia and more recently, in Dominican Republic and Haiti, where millions of animals have succumbed to the disease [
3].
Currently, there is no commercial vaccine available to fight the ASF pandemic at a global level. Lack of efficacy of inactivated vaccines and the poor protection afforded so far with recombinant vaccines based on ASFV specific antigens, left live attenuated viruses (LAVs) as the short-medium term choice to develop ASFV vaccines [
4,
5,
6]. The recent launching of the first commercial vaccine against ASFV in Vietnam was based on a recombinant deletion mutant lacking the I177L gene from the Georgia 2007 ASFV isolate [
7] and reflects the high expectation that this technology has opened in the field. Unfortunately, vaccination in some regions of Vietnam was suspended due to unexpected pig deaths (
https://www.reuters.com/world/asia-pacific/vietnam-suspends-african-swine-fever-vaccine-after-pigdeaths-2022-08-24). This was most probably due to defects in vaccination implementation, reopening biosafety concerns about the use of ASF LAVs in the field [
8].
Together with the necessary implementation of standardized protocols for registration and approval of ASFV vaccines, we should continue investing efforts to better understand the mechanisms involved in protection against ASF, aiming to develop the safest and most efficient preventive and therapeutic strategies. Both antibodies [
9] and CD8+ cells [
10] play important roles in protection, together with an appropriate innate immune response [
11]. Recent work performed among others in our own laboratory has confirmed the key importance of Th1-like responses and specific cytotoxic T lymphocytes (CTLs) in protection against ASF. These findings were obtained independently of working with subunit experimental vaccines [
12,
13] or with BA71∆CD2, a cross-protective recombinant live attenuated virus [
14].
In parallel to the current efforts to develop efficient vaccines against ASF, we and others have also demonstrated that the pig immune status and/or their microbiota significantly influences the disease outcome [
15,
16,
17]. In the present study we aimed to evaluate the effects of feeding pigs with spray-dried plasma (SDP) on the protection afforded by the BA71∆CD2 vaccine prototype. SDP derived from porcine (SDPP) or bovine (SDBP) origin are dry functional ingredients that are extensively used in pig starter diets and consistently improve performance, feed efficiency, and animal survival, especially under stressful conditions like pathogen challenge [
18]. SDP contains a diverse mixture of many functional compounds such as immunoglobulins, albumin, growth factors, biologically active peptides, transferrin, amino acids, and other molecules that have biological activity independent of their nutritional value. Although the modes of action of SDP are not completely known, it has been shown to modulate the efficiency of the immune system [
19]. Based on the demonstrated capability of SDPP feeding to accelerate the induction of specific Th1-like responses and to delay experimental ASFV transmission and disease progression [
20] (back-to-back submitted manuscript), the objective of this study was to evaluate the effects of feeding SDPP on the protection afforded by the BA71∆CD2 vaccine prototype.
2. Materials and Methods
Clinical Monitoring
The clinical state of the animals and the end-point criteria was evaluated by scoring the ASF-compatible clinical signs following a previously reported guide [
21] with slight modifications. A score from 0 to 5 according to severity was applied as follows: 0: no clinical signs, 1: mild pyrexia (39.6–40.0 ºC), 2: mild pyrexia (39.6–40.0 ºC) and mild clinical signs (skin, digestive), 3: moderate pyrexia (40.0–40.5 ºC) and mild-moderate clinical signs (distal ear spots, mild limp, lying down, but remaining alert), 4: moderate-high pyrexia (40.5-41ºC) and moderate clinical signs (remains dormant, only stands up when touched, hesitant step, subcutaneous bleeding <10%, diarrhea, mild tremors), and 5: pyrexia higher than 41ºC and moderate-severe clinical signs (generalized subcutaneous bleeding, ataxia, spasticity, clouding, prostration, bloody diarrhea).
Study Design
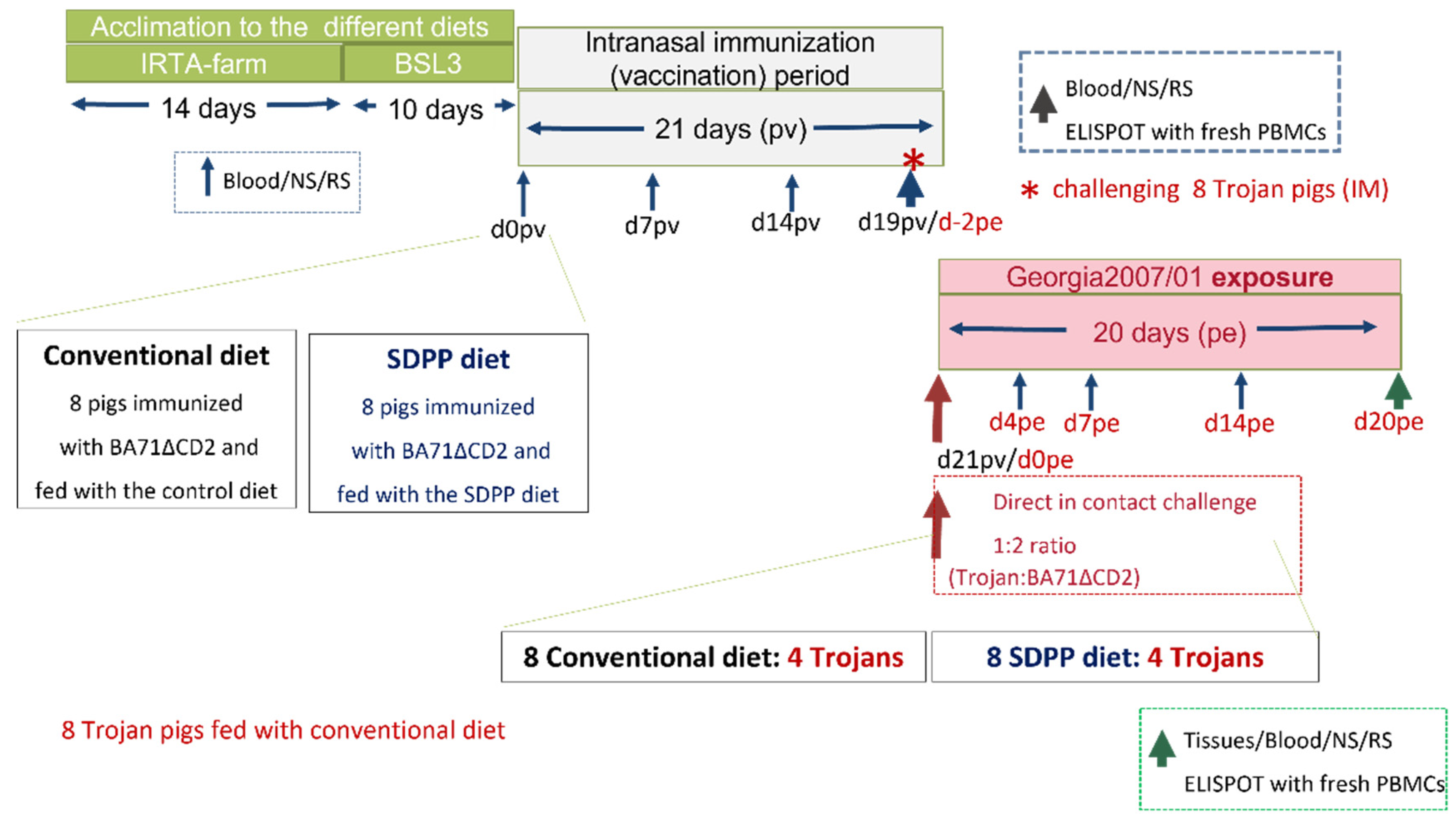
For this study, 24 4-week-old Landrace x Large White male pigs were used. Pigs were randomly divided into two separate groups at IRTA-Monells, animal facility and acclimated to their assigned diet (Table 1), as described by Blazquez et al. [
20].
Eight pigs were fed a diet supplemented with 8% SDPP (AP920 produced by APC Europe S.L.U.-Granollers, Spain) and 16 animals were fed a conventional diet with 10.09% soy protein concentrate replacing SDPP. Fourteen days later animals were moved to IRTA-CReSA BSL-3 animal facility, fed their originally assigned diet, and distributed in two separate rooms (Rooms 1 & 2) divided in half with fences. After 10 days acclimation, eight of the sixteen pigs from the Room 1 fed the conventional diet and the eight pigs from Room 2 fed the SDPP diet were intranasally immunized (1 mL per nostril) with a dose of 10
5 PFU of BA71ΔCD2 ASFV. Each group continued to be fed their respective diet until the end of the study. Nineteen days after vaccination (d19pv), each one of the non-immunized animals (two groups of four pigs fed with the conventional diet), were intramuscularly infected with 1 mL of a lethal dose (10
3 GECs/ml) of the pandemic Georgia 2007/1 ASFV strain [
22]) and were kept in the different area separated by the fences in each room. Two days later, fences were removed, allowing the exposure of vaccinated pigs with “trojan” pigs previously infected with Georgia 2007/01 (day 0 post-exposure, d0pe) with a final 1:2 ratio of trojan to vaccinated pigs. This proportion was considered optimal for transmission of the virus by direct contact [
14]. All trojans pigs had to be sacrificed between 3- and 7-days post-infection. The study ended at d20pe, 41 days after initiating the vaccination (
Figure 1).
Throughout the experimental period, pigs were fed ad libitum and observed for clinical signs and rectal temperature daily. Blood samples (10 mL tubes with EDTA), and nasal and rectal swabs were taken at: d0pv (before intranasal vaccination), d7pv, d14pv and d21pv (d0pe, the first day trojan and vaccinated pigs were in contact), d4pe, d7pe, d14pe and d20pe (d41pv). At necropsy, lesions were registered and samples of spleen, tonsil, and gastro-hepatic, submaxillary, and retropharyngeal lymph nodes were frozen and kept at -75 ºC until use. Once thawed, all samples were simultaneously analyzed by real time PCR (qPCR) to detect ASFV virus loads as described below [
23].
ELISPOT analysis was conducted using fresh peripheral blood mononuclear cells (PBMCs) from blood obtained with EDTA at d21pv (d0pe) and at d9pe and d20pe.
Laboratory Analyses
DNA extraction was done using Indimag Pathogen Kit (Indical Biosciences, Leipzig, Germany). Viremia was determined by qPCR analysis using the primers described by Fernández-Pinero et al. [
23], and the probe ASF-VP72P1 described in the current OIE ASF chapter (Terrestrial Manual OIE, Section 3.9, Chapter 3.9.1 African Swine Fever Virus pages 1-18) with the following modification in the thermoprofile made by the Spanish National Reference Laboratory for ASF: 10 min at 95ºC, 5 cycles 1 min at 95ºC + 30 sec at 60ºC, 40 cycles 10 min at 95ºC + 30 sec at 60ºC with fluorescence acquisition in the FAM channel at the end of each PCR cycle. According to these amplification settings, results were considered as positive when Ct ≤ 30, inconclusive Ct between 30 to 35 and negative when Ct >35.
The differential detection of BA71ΔCD2 was performed using a recently described probe-based SYBR Green qPCR (Applied Biosystems Path-ID qPCR Master Mix), targeting the LacI reporter gene, only present in the genome of the BA71ΔCD2 vaccine virus [
14]. The detection limit of this qPCR was 20 copies/reaction, with a Ct value of 34.93, standardized using serial dilutions of a plasmid encoding the LacI gene.
Seroconversion was determined by ELISA (INgezim PPA COMPAC, INGENASA; Madrid, Spain). Nasal and rectal swabs were analyzed for the presence of ASFV viral genome using the procedures previously mentioned. Once the nasal and rectal swabs arrived at the laboratory, the end of the swab was cut and placed in a tube with 1 mL of PBS. Tubes were stored at -75 ºC until DNA extraction and analysis by qPCR.
For each tissue sample, 0.1 g of tissue was diluted 1:10 and homogenized using sterile PBS and TyssueLyser II (Qiagen, Hilden, Germany), DNA extraction and qPCR were done as detailed above.
Plasma samples obtained from pigs were stored at -75 ºC until use. Once thawed, levels of IFNα, IFNγ, IL-1β, IL-10, IL-12/IL-23p40, IL-4, IL-6, IL-8, and TNFα in plasma were quantified using the Luminex xMAP technology following the manufacturer’s instructions (ProcartaPlex Porcine Cytokine & Chemokine Panel 1; ThermoFisher Scientific). Concentrations of each cytokine were calculated using the xPONENT software (Luminex). Levels of TGFβ and IL-17α were quantified by ELISA (KingFisher Biotech [DIY0730S-003] and Invitrogen [CHC1683Kit], respectively), following manufacturer’s instructions.
PBMCs were purified from EDTA blood samples by density-gradient centrifugation with Histopaque 1077 (Sigma-Aldrich, Misouri, USA). To quantify by ELISPOT assay the number of IFNγ secreting cells, fresh PBMC were stimulated for 16 hours with BA71∆CD2 (vaccine prototype) and/or Georgia 2007/01 (pandemic challenge virus) at a multiplicity of infection (MOI) of 0.2. Commercial mAbs (Porcine IFNγ P2G10 and biotin P2C11, BD Biosciences Pharmingen, California, USA) at 5 µg/mL were used, as previously described [
24] (Díaz & Mateu 2005). Plates were revealed using HRP-conjugated Streptavidin (Life Technologies, California, USA) and TMB substrate (MABTECH, Stockholm, Sweden), and spots were counted under a magnifying glass.
Statistical Analysis
Data were analyzed as a completely randomized design using the GLM procedures of SAS (SAS Inst., Inc., Cary, NC). An analysis of variance was conducted to detect differences among treatments. The independent variable was treatment. Dependent variables were body temperatures, blood, nasal and rectal swab and tissue Ct values. The LSMEANS procedure was used to calculate the mean values by treatment. If treatment effects were detected, least squares means were separated using the PDIFF option in SAS. Pig was considered the experimental unit. Means are considered significantly different if P < 0.05 while trends are reported as P = 0.05 to 0.10.
3. Results
In the group fed the conventional diet, one animal died before starting the vaccination period. Therefore, this group started with 7 animals instead of 8. In addition, another animal (#396) died in this group on d31pv due to acute meningitis. In the SDPP group, one animal (#391) was euthanized on d21pv to balance both groups to 7 animals during the exposure period. Another animal (#376) died on d35pv due to intestinal prolapse. Both groups finished at d41pv with 6 pigs.
All pigs intranasally vaccinated with 10
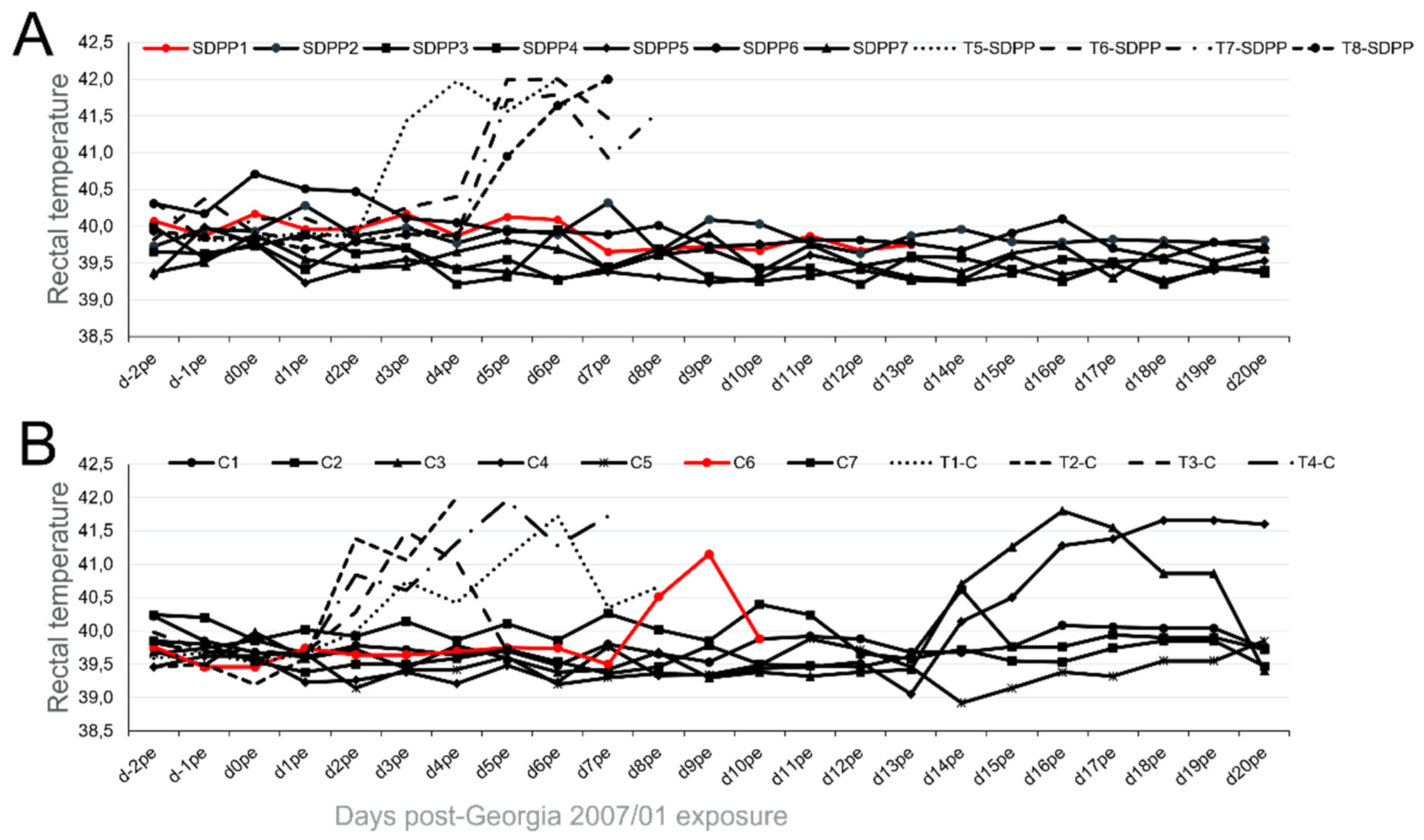
5 PFU of BA71∆CD2 survived the direct-contact challenge with pigs infected with Georgia 2007/01 independently of their assigned diet. However, differences were observed between treatment groups including clinical signs and viral load in blood, excretions, and tissues after exposure to the ASFV infected trojan pigs. Interestingly, no fever was recorded at any time after Georgia 2007/01 challenge in any of the pigs from the SDPP group (
Figure 2A;
Supplementary Table S1).
A proportion of pigs vaccinated with 10
5 PFU intranasally and fed the conventional diet (C3 and C4), showed a peak of fever starting at d14pe (
Figure 2B), with no other signs of ASF infection observed.
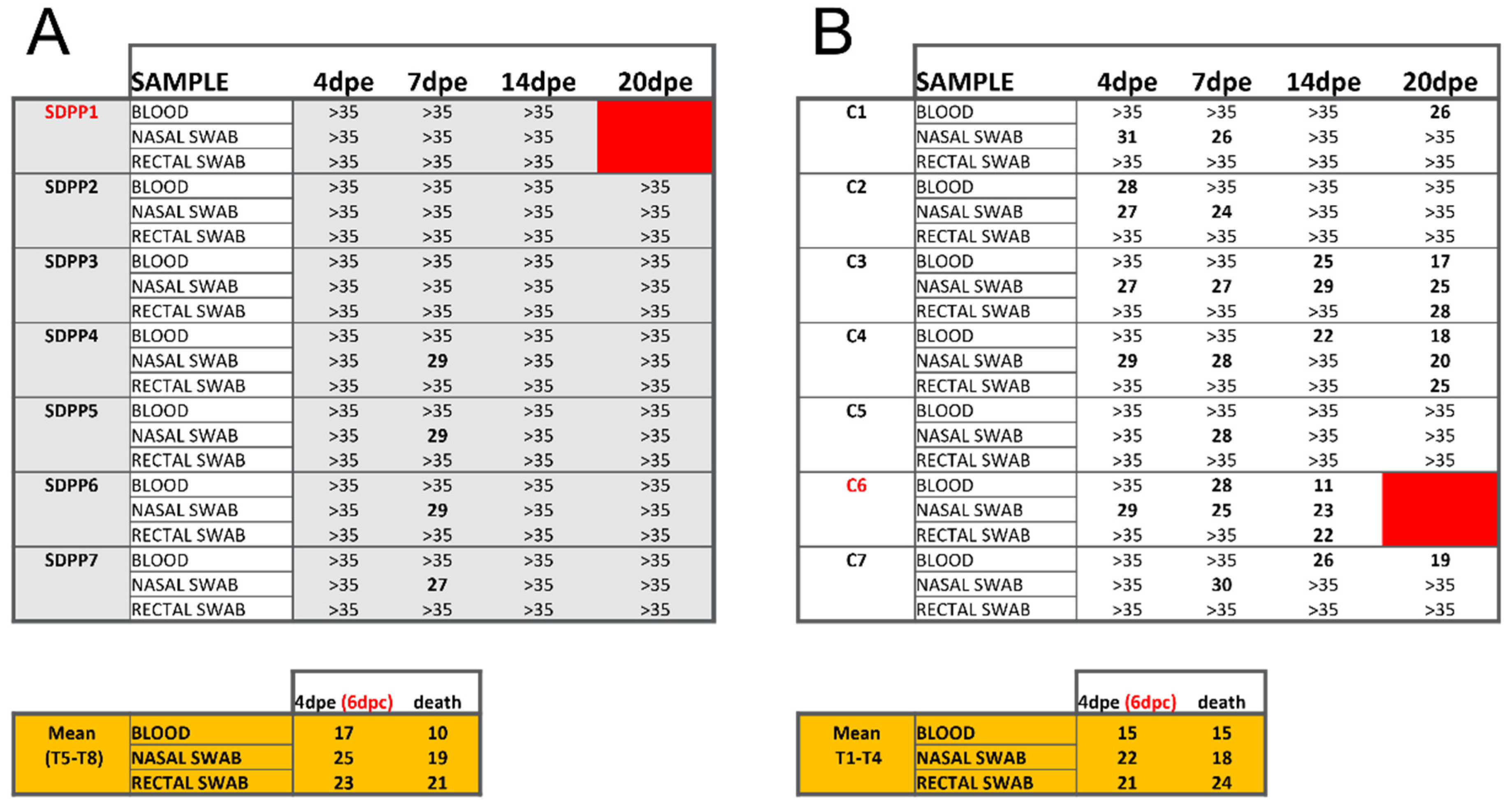
In addition to elevated rectal temperature, pigs #C3 and #C4 were the only ones exhibiting significant virus loads in both their blood and nasal swabs at the end of the experiment (
Figure 3B;
Supplementary Table S2).
Pigs fed the SDPP containing diet did not exhibit fever, neither become viremic and virus was not detected in rectal swabs at any time post-Georgia 2007/01 exposure (
Figure 3A;
Supplementary Table S2)).
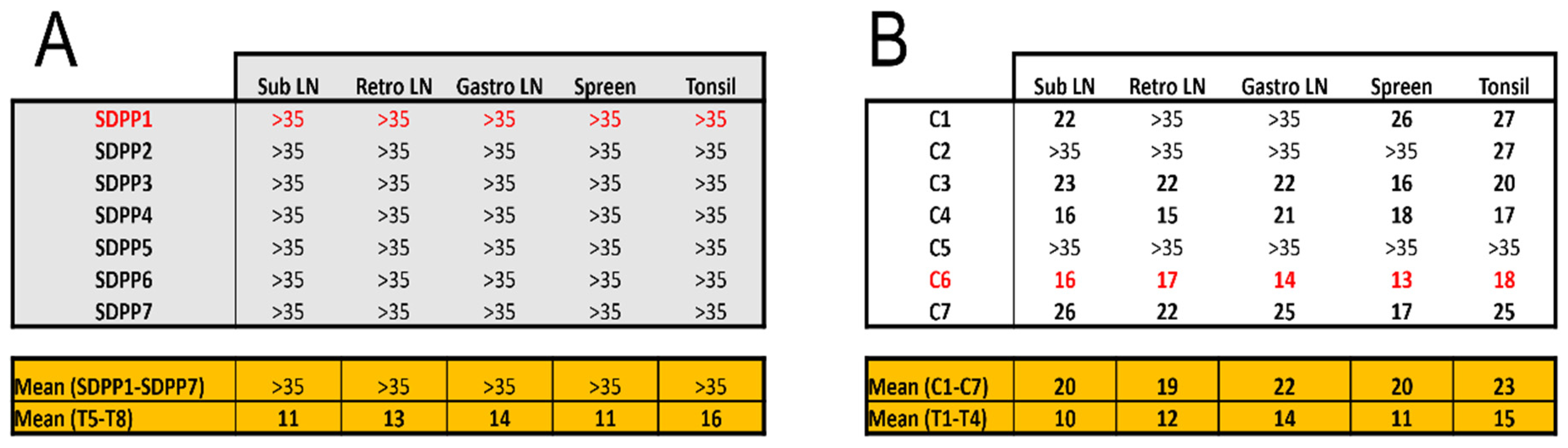
In addition, the vaccine provided sterilizing protection in pigs fed the SDPP containing diet as no virus was detected tissue samples at the end of the study (
Figure 4A). In contrast, virus was present in most of the pigs from the conventional diet group at least in one organ at d20pe (
Figure 4B;
Supplementary Table S3).
Without exception, the average Ct values found in vaccinated pigs, both in fluids during the exposure period and in postmortem tissues, were much higher than those found in Trojans pigs succumbing to the ASFV challenge (see bottom panels in
Figure 3 and
Figure 4;
Supplementary Tables S2 and S3) confirming solid protection afforded by BA71∆CD2. Finally, in all cases, the only virus detectable after Georgia 2007/01 exposure was the pandemic virus, with no detectable traces of the BA71∆CD2 vaccine prototype.
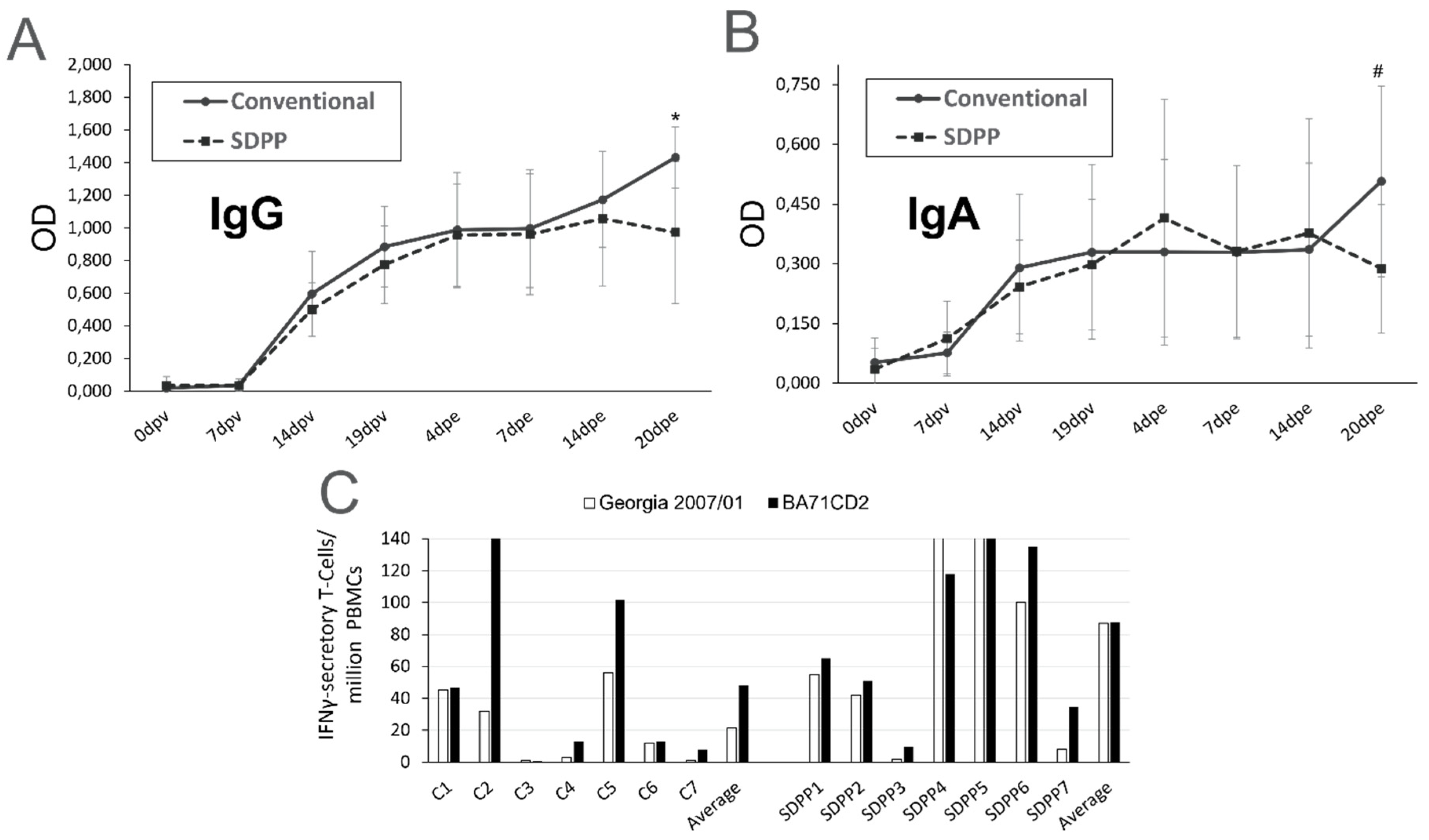
No treatment differences were observed (P>0.1) in the kinetics of induction of ASFV-specific IgG and IgA in sera between either treatment group of pigs before and after challenge, except for d20pe (d40pv) in which the average values for IgG and IgA in conventional pigs was higher (P<0.039 for IgG and P<0.092 for IgA) (
Figure 5A,B;
Supplementary Tables S4 and S5).
Similarly, treatment differences were not found (P>0.1) between the number of ASFV specific IFNγ-secreting T-cells present at d0pe and d20pe (data not shown.
Supplementary Table S6). However, by d9pe the number of specific T-cells secreting IFNγ upon ASFV stimulation was numerically higher for the vaccine virus (BA71∆CD2) and a trend to be higher (P = 0.07) for the Georgia 2007/01 pandemic ASFV strain in pigs fed the SDPP diet than the conventional diet (
Figure 5C).
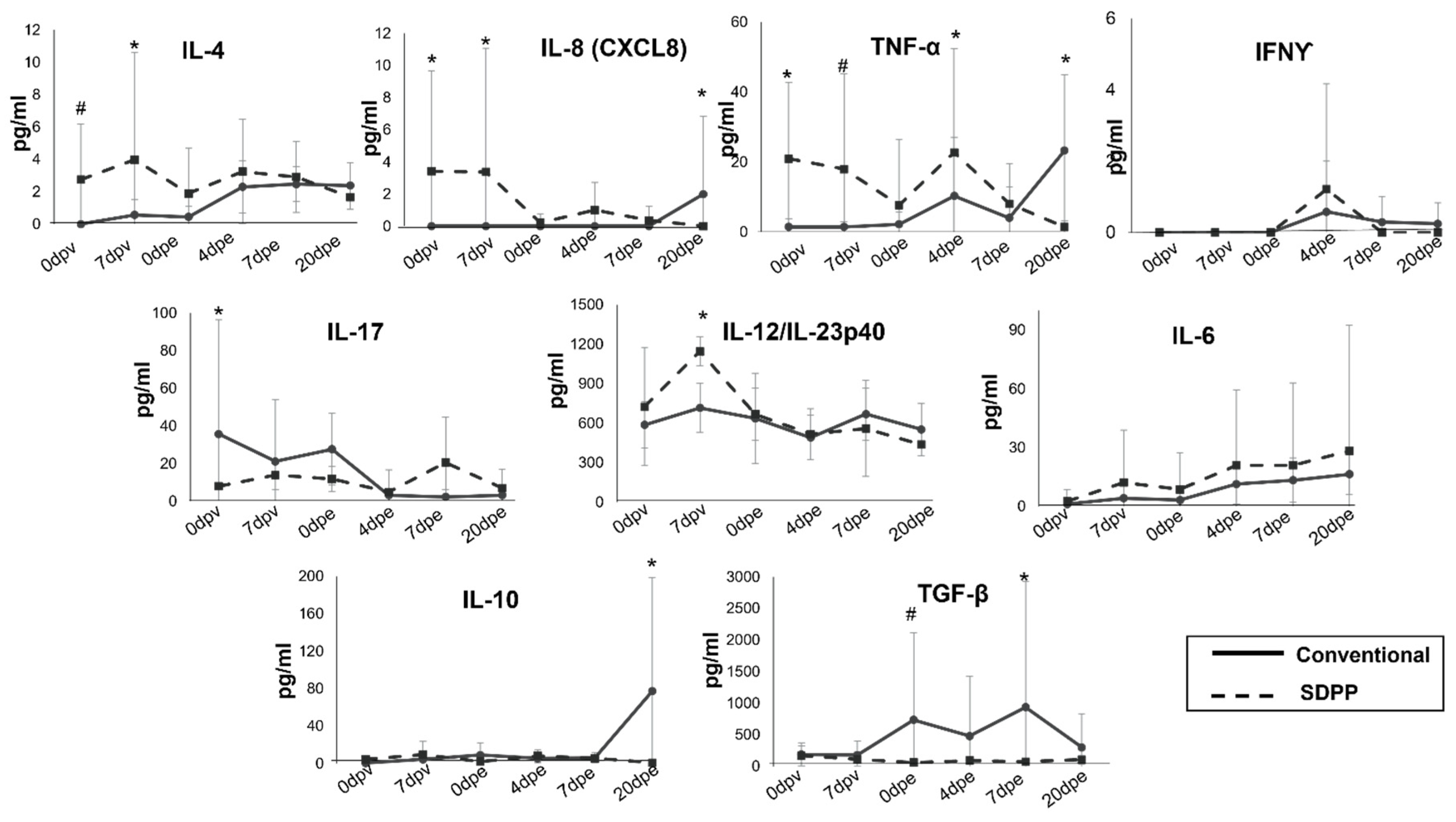
Finally, the immunomodulatory influence of the SDPP containing diet was evident after 24 days of feeding, even before starting the vaccination. Indeed, pigs fed with SDPP showed a modest, but detectable amount in serum of both pro-inflammatory (IL-8 and TNFα) and anti-inflammatory cytokines (IL-4) at vaccination time (
Figure 6).
Interestingly, the levels of the pro-inflammatory cytokine IL-17 before vaccination were lower in the SDPP group than in pigs fed with conventional diet and, as expected, the levels of cytokines in serum varied along the experiment. Furthermore, the Th1 cytokine IL-12/IL-23p40 increased in the SDPP group by 7 dpv (
Figure 6), coinciding with the elevated levels of ASFV-specific T cells observed by ELISPOT (
Figure 5C). Levels of the two cytokines IFNγ and TNFα, also recently identified as markers of vaccine-induced ASFV-specific Th1 response [
14], showed a peak at 4dpe significantly higher in animals fed with SDPP. Despite TGFβ seemed to show some significant differences, they mostly corresponded to the outlier value observed for one pig in the conventional group (#C4), coinciding with an animal showing fever and low Ct values. Finally, IL-8, TNFα and IL-10 peaked at the end of the study in the group of pigs fed the conventional diet, coinciding with the detection of ASFV replication and fever (
Supplementary Table S7).
4. Discussion
Due to the wide expansion of ASFV around the world leading to a negative impact on pig health as well as huge economic consequences for pig producers, research on new treatments and vaccines that could help to mitigate the negative repercussions related with this virus has been intensified during the last years. Since inactivated and subunit vaccine formulations have failed to protect pigs against the pandemic ASFV, research efforts have focused on either natural or recombinant LAVs as the only short-medium term strategy to obtain highly efficient ASF vaccines [
4,
25]. Several recombinant LAV candidates have been described so far in the literature, capable to induce solid protection against experimental challenge with the pandemic genotype II ASFV strains [
5,
6].
The detection of illegally introduced genotypes I and II attenuated ASFV vaccines in Chinese pig farms [
2,
26] confirms the need for caution when delivering ASF LAVs to the field without the appropriate supervision from regulatory agencies. This led to renewed efforts to standardize protocols for registration and approval of the most efficient and safest vaccines [
27]. At the same time, it is important to continue searching for methods to improve LAV prototypes and to develop efficient subunit vaccines for the future.
In the present experiment we extended previous work using BA71ΔCD2, a recombinant vaccine prototype, capable to protect in a dose dependent manner against experimental challenge with homologous and heterologous viruses, including the genotype II pandemic virus [
28,
29]. For comparative studies, in the present work an intranasal dose of 10
5 PFU of BA71ΔCD2, one logarithm below the optimal dose previously reported [
14], was administered to pigs fed a conventional diet with or without SDPP. As expected for the dose and route used, all pigs survived exposure to the trojan pigs infected with Georgia 2007/01, independently of the dietary treatment. However, significant differences were observed between treatment groups. Tissues and fecal samples from all pigs consuming the SDPP containing diet were negative for ASFV genome detection (at levels below our detection methods) at all sampling times following exposure to the trojan pigs. In contrast with this apparent sterilizing protection, many pigs fed the conventional diet showed detectable virus in one or more samples post-exposure to the trojan pigs. It is important to notice that the Georgia 2007/01 virus titers found in fluids and tissues of some vaccinated pigs fed the conventional diet were higher than expected, at least compared with those previously found using lower and higher vaccine doses than the one here tested [
14]. We believe that this might be due to a sub-optimal health status of the animals from origin, which might negatively affect ASFV vaccination and transmission, as it has been postulated [
14,
17]. In fact, and as described in the results section, we had three ASF-unrelated deaths, one of them even before starting the vaccination despite treating them with antibiotics. Independently of this reality, the virus titers found in vaccinated pigs were always below those found in the trojan pigs succumbing to Georgia 2007/01, confirming the solid protection afforded by the vaccine, even in adverse conditions. Therefore, the current data suggest that dietary SDPP improved vaccine efficiency and demonstrates that dietary SDPP could have a direct benefit for ASF vaccination.
Treatment differences, except at the end of the study, were not observed in the serum levels of anti-ASFV IgG and IgA antibodies. Further studies are needed to better understand the kinetics of induced immunoglobulin isotypes as well as the local immune responses induced at the site of immunization and ASFV entry (nasal mucosa) and other mucosal tissues to distinguish any changes in the antibody induction due to feeding diets with SDPP [
30,
31].
Conversely to the antibody kinetics, an increase of specific IFNγ secretory T-cells was observed in vaccinated pigs, detectable by d9pe to the trojan pigs. The fact that virus-specific T-cells recognize both the BA71∆CD2 vaccine and the pandemic virus, confirms the induction of T-cells capable to recognize genotypes I and II, currently circulating in China [
2]. These results and the corresponding peak of serum TNFα early after Georgia 2007/01 exposure of SDPP fed pigs is consistent with the relevance of vaccine induced IFNγ+TNFα+ polyfunctional memory Th1-cells in ASFV protection [
14] and in delayed transmission of ASFV in SDPP fed pigs [
20]. Also in this line, the peak of the Th1 cytokine IL12 [
32] in plasma at 7dpv also suggest the SDPP-driven enhancement of the vaccine-induced Th1 response. Altogether, these results indicate that the addition of SDPP in diet somehow enhances the vaccine-induced ASFV-specific cellular responses. Further studies focused on mucosal immunity and its interplay with systemic immune responses will be required to better characterize the mechanisms behind this observation.
Of particular interest are the differences observed for several immune mediators in the serum of vaccinated pigs consuming the SDPP diet compared to that of pigs fed the conventional diet, even before immunization. Feeding SDPP resulted in elevated levels of pro- and anti-inflammatory cytokines in their plasma, confirming the tight regulation of the immune responses previously reported for SDPP fed animals [
33,
34]. These results also confirm the relevance of the health and immune status of the animals in the responses observed after ASFV vaccination [
15,
16,
17], and open new avenues for dietary intervention.
Interestingly, the sequential increase of IL-17 (from d0pv to d21pv) in vaccinated pigs fed the conventional diet may indicate a tighter control of Th17 and T-regulatory cells favoring the optimal expansion of Th1-like responses [
35]. On this regard, a recently published study associated the presence of regulatory T cells with the lack of long-term memory responses induced by ASF LAVs [
36].
Dietary SDPP was shown to improve vaccine efficiency in a commercial trial where pigs were vaccinated with a commercial vaccination program [
37]. Finally, the increase of IL-10, IL-8 and TNFα at the end of the study in pigs fed the conventional diet is consistent with the presence of Georgia 2007/01 at this time point in the animals from this group. Of course, the theoretical dysregulation of these cytokines is much lower than previously described at a late time post infection in naïve pigs showing acute ASF with an uncontrolled cytokine storm due to the massive ASFV presence [
38,
39,
40,
41].
SDPP is a functional feed ingredient that has been demonstrated to systemically modulate the immune system. In some studies, the effects exerted by SDPP were characterized to promote both Th1-like response and cytokine induction. Díaz et al. [
42] reported a significant reduction in interstitial pneumonia and faster virus clearance in pigs infected with porcine reproductive and respiratory syndrome virus and consuming a SDPP containing diet. The authors concluded that pigs fed SDPP were able to mount a more robust immune response and were able to clear the virus more efficiently. The increase in cytokine expression (IFNγ and IL-1) in lungs of pigs receiving SDPP in their feed, points to a Th1 enhancement. Markowska-Daniel and Pejsak [
43] reported that the administration of SDPP through both water and feed significantly increased the percentage of CD8+ T cells especially in smaller pigs. Elevated CD8+ T-cells has been correlated to reduced ASFV infection [
10]. Thus, dietary SDPP may enhance the immune response of pigs vaccinated with BA71∆CD2 contributing to sterilizing immunity observed in the present experiment.
5. Conclusions
In summary, under the conditions of this study, the addition of SDPP in feed improved the ASFV vaccine prototype efficacy. Pigs fed the diet with SDPP showed lower virus load in nasal secretion and absence of virus in blood and feces after exposure to trojan pigs infected with Georgia 2007/01 compared to those fed the conventional diet. Furthermore, no virus was detected in any organ tissue of the pigs fed the SDPP diet at the time of sacrifice (d20pe). This suggests that dietary SDPP can be used strategically as a health management tool to enhance vaccine efficiency. However, it is important to point out that the current work involved a limited number of animals under controlled conditions. These results should be verified and extended in further studies with higher number of animals under field conditions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Table S1: Rectal temperature throughout the duration of the study; Table S2: Individual PCR results (Ct values) by day in blood, nasal and rectal swab samples; Table S3: Individual PCR results (Ct values) on different tissues at the time of sacrifice; Table S4: individual IgG values (OD) on serum by day; Table S5: Individual IgA values (OD) on serum by day; Table S6: Elispot results at different days during the study; Table S7: Individual cytokines results at different days during the study.
Author Contributions
Conceptualization, BG, EB, FR, J. Polo, J. Pujols, and JS; methodology, C-Y C, EB, FR, JA, J. Pujols, JS, L B-C, and RR; software, FR, LP, JC, J. Polo and J. Pujols; validation, FR, JA, J. Polo, J. Pujols, JS; formal analysis, FR, JA, LP, JC, J. Pujols, J. Polo and LB-C; investigation, C-Y C, FR, JA, J. Pujols, JS and L B-C; resources, J. Pujols and FR; data curation, EB, FR, J. Polo and J. Pujols—original draft preparation, EB, FR, JA, J. Polo, J. Pujols, JS and L B-C; writing—review and editing, FR, JA, J. Polo, JS and L B-C; visualization, FR and J. Polo.; supervision, FR and J. Polo.; project administration, J. Pujols; funding acquisition, J. Polo. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this study was provided by APC Europe, S.L.U., Granollers, Spain and APC LLc, Ankeny, US. Both companies manufacture animal blood products for animal consumption. The companies provided support in the form of salaries for authors EB, JC and J. Polo retrospectively, but none of these companies had any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. We also acknowledge support of the Spanish Ministry of Science and Innovation (grant reference PID2019-107616RB-I00).
Institutional Review Board Statement
The study was approved by the committee of ethics and welfare “Comitè d’Experimentació Animal de la Generalitat de Catalunya” with the protocol approval number CEA-OH/11387/1.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data from this study is provided in the manuscript and supplementary tables.
Acknowledgments
We thank the technicians and PhD students of IRTA-CReSA helping in the experimental setup.
Conflicts of Interest
The authors have read the journal’s policy and the authors of this manuscript have the following competing interests: EB and J. Polo are employed by APC Europe, S.L.U. Granollers, Spain. JC and J. Polo are employed by APC LLC, Ankney, US. Both companies manufacture and sells spray-dried animal plasma; BG is employed by Huvepharma, Sofia, Bulgaria, a company that develop and commercialize vaccines for animal health. However, the companies had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. C-Y C, FR, JA, L B-C, J. Pujols, JS, RR and LP declare no conflict of interest. This does not alter the authors’ adherence to all journal policies on sharing data and materials.
References
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M.; Ictv report consortium. ICTV virus taxonomy profile: Asfarviridae. J Gen Virol. 2018, 99(5), 613-614. [CrossRef]
- Ito, S.; Bosch, J.; Martínez-Avilés, M.; Sánchez-Vizcaíno, J.M. The evolution of African swine fever in China: a global threat? Front Vet Sci. 2022, 0, 248. [CrossRef]
- WOAH - African swine fever: OIE - World Organisation for Animal Health. https://www.woah.org/en/disease/african-swine-fever#ui-id-2.
- Arias, M.; de la Torre, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A.; Martins, C.; Parkhouse, R.M.; Revilla, Y.; Rodriguez, F.A.J.; Sanchez-Vizcaino. Approaches and perspectives for development of African swine fever virus vaccines. Vaccines (Basel) 2017, 5(4), 35. [CrossRef]
- Bosch-Camós, L.; López, E.; Rodriguez, F. African swine fever vaccines: a promising work still in progress. Porcine Health Manag. 2020, 2, 6-17. [CrossRef]
- Gladue, D.P.; Borca, M.V. Recombinant ASF live attenuated virus strains as experimental vaccine candidates. Viruses, 2022, 14(5), 878. [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J Virol. 2020, 17,94(7), e02017-19. [CrossRef]
- Gavier-Widén, D.; Ståhl, K.; Dixon, L. No hasty solutions for African swine fever. Science, 2020,367(6478), 622-624. [CrossRef]
- Onisk, D.V.; Borca, M.V.; Kutish, G.; Kramer, E.; Irusta, P.; Rock, D.L. Passively transferred African swine fever antibodies protect against lethal infection. Virology 1994, 198 (1), 350-354. [CrossRef]
- Oura, C.A.L.; Denyer, M.S.; Takamatsu, H.; Parkhouse, R.M.E. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J Gen Virol. 2005, 86, 2445–2450. [CrossRef]
- Takamatsu, H.H.; Denyer, M.S.; Lacasta, A.; Stirling, C.M.; Argilaguer, J.M.; Netherton, C.L.; Oura, C.A.; Martins, C.; Rodríguez, F. Cellular immunity in ASFV responses. Virus Res. 2013, 173 (1), 110-121. [CrossRef]
- Bosch-Camós, L.; López, E.; Collado, J.; Navas, M.J.; Blanco-Fuertes, M.; Pina-Pedrero, S.; Accensi, F.; Salas, M.L.; Mundt, E.; Nikolin, V.; Rodríguez, F. M448R and MGF505-7R: two African swine fever virus antigens commonly recognized by ASFV-specific T-cells and with protective potential. Vaccines (Basel), 2021a,9(5), :508. [CrossRef]
- Bosch-Camós, L.; López, E.; Navas, M.J.; Pina-Pedrero, S.; Accensi, F.; Correa-Fiz, F.; Park, C.; Carrascal, M.; Domínguez, J.; Salas, M.L.; Nikolin, V.; Collado, J.; Rodríguez, F. Identification of promiscuous African swine fever virus T-cell determinants using a multiple technical approach. Vaccines (Basel), 2021b, 9(1), 29. [CrossRef]
- Bosch-Camós,L.; Alonso, U.; Esteve-Codina, A.; Chang, C-Y.; Martín-Mur, B.; Accensi, F.; Muñoz, M.; Navas, M.J.; Dabad, M.; Vidal, E.; Pina-Pedrero, S.; Pleguezuelos, P.; Caratù, G.; Salas, M.L.; Liu, L.; Bataklieva, S.; Gavrilov, B.; Rodríguez, F.; Argilaguet, J. Cross-protection against African swine fever virus upon intranasal vaccination is associated with an adaptive-innate immune crosstalk. Plos Pathog. 2022, 18(11), e1010931. [CrossRef]
- Lacasta, A.; Ballester, M.; Monteagudo, P.L.; Rodríguez, J.M.; Salas, M.L.; Accensi, F.; Pina-Pedrero, S.; Bensaid, A.; Argilaguet, J.; López-Soria, S.; Hutet, E.; le Potier, M.F.; Rodríguez, F. Expression library immunization can confer protection against lethal challenge with African swine fever virus. J Virol. 2014, 88, 13322–13332. [CrossRef]
- Zhang, J.; Rodríguez, F.; Navas, M.J.; Costa-Hurtado, M.; Almagro, V.; Bosch-Camós, L.; López, E.; Cuadrado, R.; Accensi, F.; Pina-Pedrero, S.; Martínez, J.; Correa-Fiz, F. Fecal microbiota transplantation from warthog to pig confirms the influence of the gut microbiota on African swine fever susceptibility. Sci Rep. 2020, 10, 17605. [CrossRef]
- Radulovic, E.; Mehinagic, K.; Wüthrich, T.; Hilty, M.; Posthaus, H.; Summerfield, A.; Ruggli, N.; Benarafa, C. The baseline immunological and hygienic status of pigs impact disease severity of African swine fever. Plos Pathog. 2022, 18(8), e1010522. [CrossRef]
- Torrallardona, D. Spray dried animal plasma as an alternative to antibiotics in weanling pigs. Asian-Australasian J Anim Sci. 2010, 23, 131–48. [CrossRef]
- Pérez-Bosque, A.; Polo, J.; Torrallardona, D. Spray dried plasma as an alternative to antibiotic in piglet feeds, mode of action and biosafety. Porcine Health Manag. 2016, 2, 16. [CrossRef]
- Blázquez, E.; Pujols, J.; Rodríguez, F.; Segalés, J.; Rosell, R.; Campbell, J.; Polo, J. Feeding spray-dried porcine plasma to pigs reduces African swine fever load in infected pigs and delays virus transmission. Study 1. Vaccines (under review).
- Galindo-Cardiel, I.; Ballester, M.; Solanes, D.; Nofrarías, M.; López-Soria, S.; Argilaguet, J.M.; Lacasta, A.; Accensi, F.; Rodríguez, F.; Segalés, J. Standardization of pathological investigations in the framework of experimental ASFV infections. Virus Res. 2013, 173, 180–190. [CrossRef]
- Chapman, D.A.G.; Darby, A.C.; da Silva, M.; Upton, C.; Radford, A.D.; Dixon, L.K. Genomic analysis of highly virulent Georgia 2007/1 isolate of African swine fever virus. Emerg Infect Dis. 2011, 17, 599–605. [CrossRef]
- Fernández-Pinero, J.; Gallardo, C.; Elizalde, M.; Robles, A.; Go, C. Molecular diagnosis of African swine fever by a new real-time PCR using universal probe library. Transbound Emerg Dis. 2013, 60, 48–58. [CrossRef]
- Díaz, I.; Mateu, E. Use of ELISPOT and ELISA to evaluate IFN-gamma, IL-10 and IL-4 responses in conventional pigs. Vet Immunol Immunopathol. 2005, 15, 106(1-2), 107-12. [CrossRef]
- European Comission, 2017. https://food.ec.europa.eu/system/files/2017-02/cff_animal_vet-progs_asf_blue-print-road-map.pdf.
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; Wang, W.; Li, F.; Liu, R.; Sun, J.; Tian, Z.; Xia, W.; Guan, Y.; He, X.; Zhu, Y.; Zhao, D.; Bu, Z. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg Microbes Infect. 2021, 10, 2183–2193. [CrossRef]
- Brake, D.A. African swine fever modified live vaccine candidates: transitioning from discovery to product development through harmonized standards and guidelines. Viruses 2022, 14, 2619. [CrossRef]
- Monteagudo, P.L.; Lacasta, A.; Lopez, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; Bustos, M.J.; Rodriguez, J.M.; Gallei, A.; Nikolin, V.; Salas, M.L.; Rodriguez, F. BA71ΔCD2: a new recombinant live attenuated African swine fever virus with cross-protective capabilities. J Virol. 2017, 91, e01058-17. [CrossRef]
- Lopez, E.; van Heerden, J.; Bosch-Camós, L.; Accensi, F.; Navas, M.J.; López-Monteagudo, P.; Argilaguet, J.; Gallardo, C.; Pina-Pedrero, S.; Salas, M.L.; Salt, J.; Rodriguez, F. Live attenuated African swine fever viruses as ideal tools to dissect the mechanisms involved in cross-protection. Viruses 2020, 12(12), 1474. [CrossRef]
- Arunachalam, P.S.; Charles, T.P.; Joag, V.; Bollimpelli, V.S.; Scott, M.K.D.; Wimmers, F.; Burton, S.L.; Labranche, C.C.; Petitdemange, C.; Gangadhara, S.; Styles, T.M.; Quarnstrom, C.F.; Walter, K.A.; Ketas, T.J.; Legere, T.; Jagadeesh Reddy, P.B.; Kasturi, S.P.; Tsai, A.; Yeung, B.Z.; Gupta, S.; Tomai, M.; Vasilakos, J.; Shaw, G.M.; Kang, C.Y.; Moore, J.P.; Subramaniam, S.; Khatri, P.; Montefiori, D.; Kozlowski, P.A.; Derdeyn, C.A.; Hunter, E.; Masopust, D.; Amara, R.R.; Pulendran, B. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat Med. 2020, 26(6), 932-940. [CrossRef]
- Oh, J.E.; Iijima, N.; Song, E.; Lu, P.; Klein, J.; Jiang, R.; Kleinstein, S.H.; Iwasaki, A. Migrant memory B cells secrete luminal antibody in the vagina. Nature 2019, 571, 122–126. [CrossRef]
- Hsieh, C.S.; Macatonia, S.E.; Tripp, C.S.; Wolf, S.F.; O’Garra, A.; Murphy, K.M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 1993, 260(5107), 547-9. [CrossRef]
- Touchette, K.J.; Carroll, JA.; Allee, G.L.; Matteri, R.L.; Dyer, C.J.; Beausang, L.A.; Zannelli, M.E. Effect of spray-dried plasma and lipopolysaccharide exposure on weaned pigs: I. Effects on the immune axis of weaned pigs. J Anim Sci. 2002, 80(2), 494-501. [CrossRef] [PubMed]
- Frank, J.W.; Carroll, J.A.; Allee, G.L.; Zannelli, M.E. The effects of thermal environment and spray-dried plasma on the acute-phase response of pigs challenged with lipopolysaccharide. J Anim Sci. 2003, 81(5), 1166-1176. [CrossRef]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017, 9(6), a022236. [CrossRef]
- Sánchez-Cordón, P.J.; Jabbar, T.; Chapman, D.; Dixon, L.K.; Montoya, M. Absence of long-term protection in domestic pigs immunized with attenuated African swine fever virus isolate OURT88/3 or BeninΔMGF correlates with increased levels of regulatory T cells and interleukin-10. J Virol. 2020, 94(14), e00350-20. [CrossRef]
- Pujols, J.; Segalés, J.; Polo, J.; Rodríguez, C.; Campbell, J.; Crenshaw, J. Influence of spray dried porcine plasma in starter diets associated with a conventional vaccination program on wean to finish performance. Porc Health Manag 2016, 2, 4. [CrossRef]
- Herrera-Uribe, J.; Jiménez-Marín, Á.; Lacasta, A.; Monteagudo, P.L.; Pina-Pedrero, S.; Rodríguez, F.; Moreno, A.; Garrido, J.J. Comparative proteomic analysis reveals different responses in porcine lymph nodes to virulent and attenuated homologous African swine fever virus strains. Vet Res 2018, 49, 90. [CrossRef]
- Salguero, F.J.; Ruiz-Villamor, E.; Bautista, M.J.; Sánchez-Cordón, P.J.; Carrasco, L.; Gómez-Villamandos, J.C. Changes in macrophages in spleen and lymph nodes during acute African swine fever: expression of cytokines. Vet Immunol Immunopathol. 2002, 90(1-2), 11-22. [CrossRef]
- Salguero, F.J.; Sánchez-Cordón, P.J.; Núñez, A.; Fernández de Marco, M.; Gómez-Villamandos, J.C. Proinflammatory cytokines induce lymphocyte apoptosis in acute African swine fever infection. J Comp Pathol. 2005, 132(4), 289-302. [CrossRef]
- Gómez-Villamandos, J.C.; Bautista, M.J.; Sánchez-Cordón, P.J.; Carrasco, L. Pathology of African swine fever: the role of monocyte-macrophage. Virus Res. 2013, 173(1), 140-9. [CrossRef]
- Díaz, I.; Lorca, C.; Galindo, I.; Campbell, J.; Barranco, L.; Kuzemtseva, L.; Rodríguez-Gómez, I.-M.; Crenshaw, J.; Russell, L.; Polo, J.; Pujols, J. Potential positive effect of commercial spray-dried porcine plasma on pigs challenged with PRRS virus. Proceedings of 21st international pig veterinary society (IPVS), Vancouver, Canada, 2010. Pp. 560.
- Markowska-Daniel, I.; Pejsak, Z. Immunological and production parameters in pigs fed spray-dried animal plasma. Bull Vet Inst Pulawy. 2006, 50. https://www.semanticscholar.org/paper/IMMUNOLOGICAL-AND-PRODUCTION-PARAMETERS-IN-PIGS-FED-Markowska-Daniel-Pejsak/56d66722cd8bb6148a42cdafbc1544b2793f241d.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).