Methods and Materials
Experimental Design
This study is composed of four parts:
Pilot studies covering the histology of the pancreas, insulin measurement methods, optimization of the glucose tolerance test, and establishment of a protocol for STZ-mediated induction of diabetes in the axolotl.
Investigation of the induction of diabetes and the potential regeneration of β cells in the axolotl by measuring changes in blood glucose levels over time.
Histological examination of the diabetic state on day 22, 35, and 84 of the experiment.
Characterization of systemic effects of the STZ-induced disease.
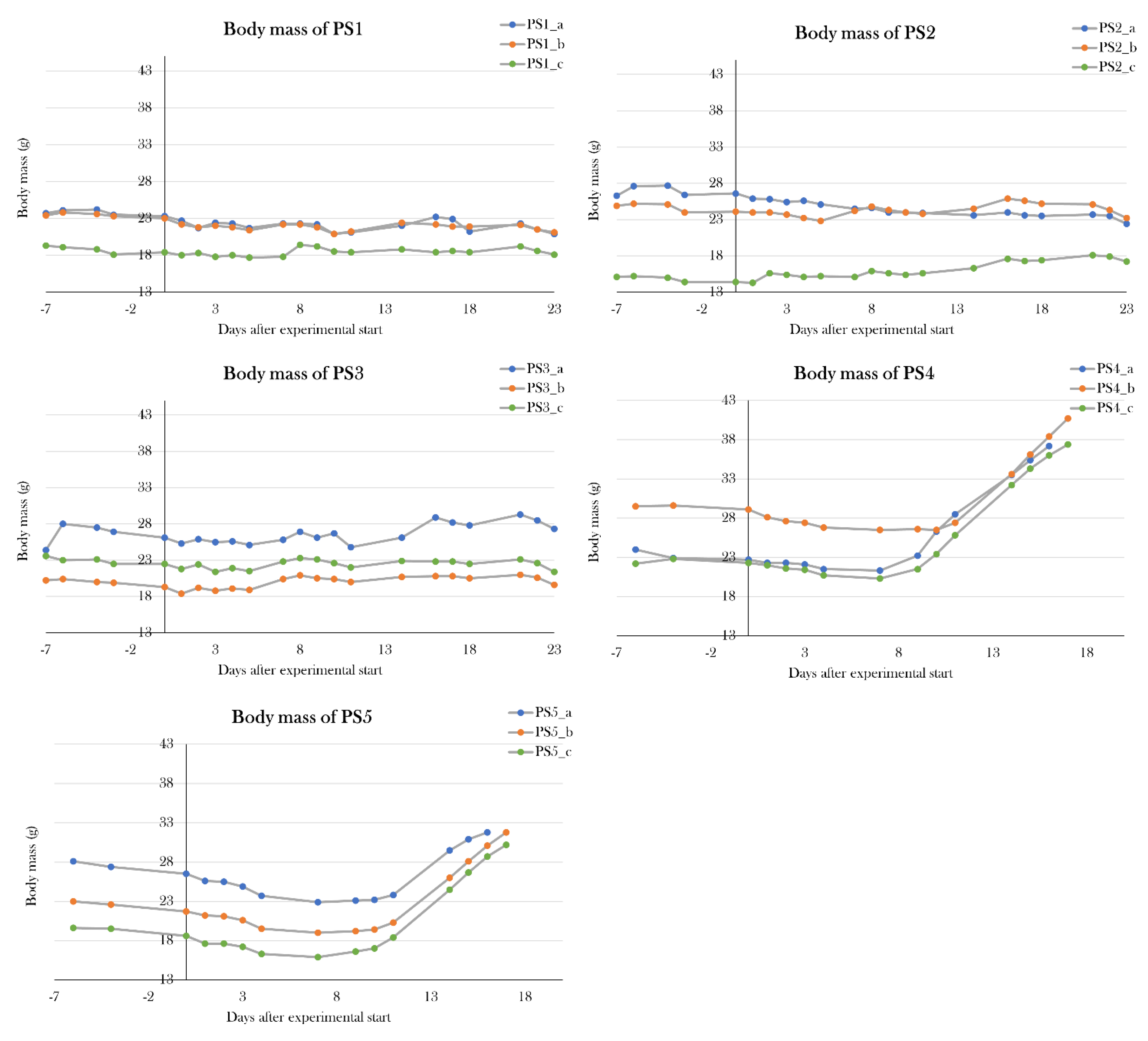
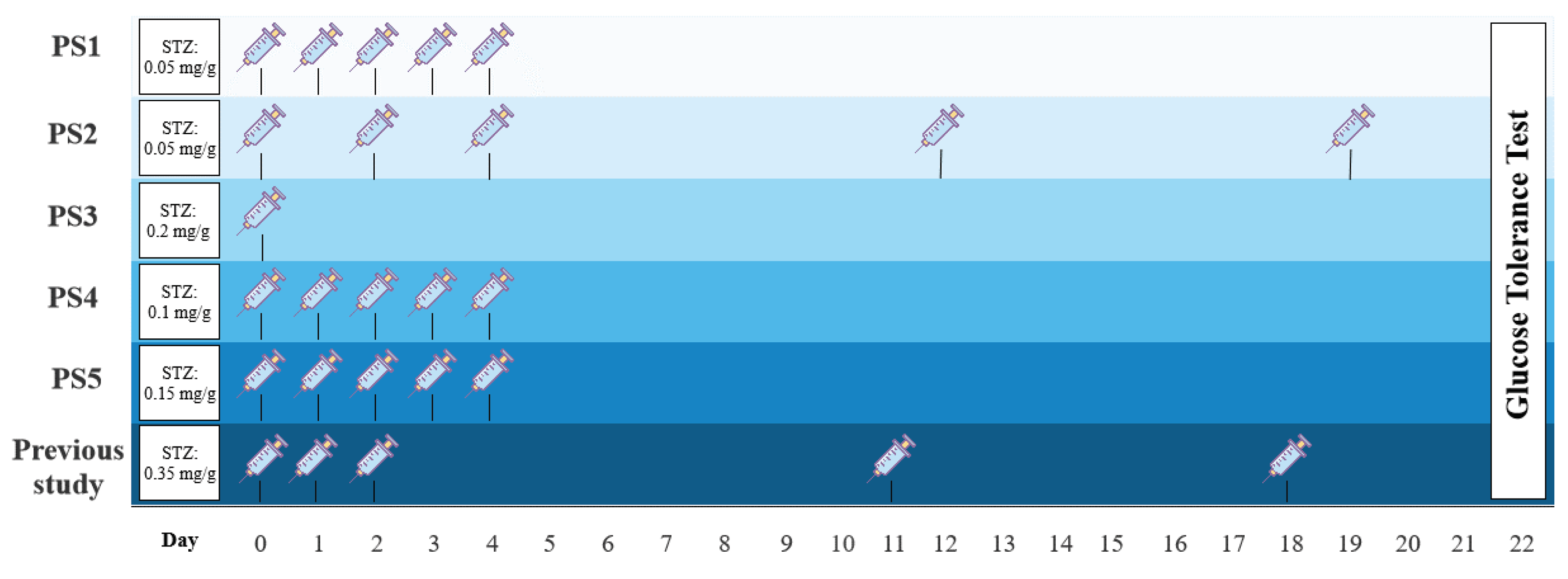
In part I, several pilot studies (PS1-5) were conducted to find the optimal dosage regime of STZ (
Figure 2). Previously, I have examined a protocol inspired by a study in zebrafish[
90], in which 0.35 mg/g body mass of STZ was i.p. (intraperitoneal) injected at day 0, 1, 2, 11, and 18 of the experiment. However, the pilot study resulted in high mortality and premature termination[
91]. In this study, I investigate several dosage regimes inspired by rodent studies to find a treatment protocol that makes the animals diabetic without causing high mortality. Firstly, PS1 was treated with 0.05 mg/g body mass of STZ on 5 consecutive days, group PS2 received 0.05 mg/g body mass of STZ at day 0, 2, 4, 12, and 19, and PS3 was given a single dose of STZ of 0.2 mg/g body mass at day 0. Two additional pilot groups were included, in which PS4 was treated with 0.15 mg/g body mass of STZ, and PS5 was given 0.2 mg/g body mass of STZ, both at 5 consecutive days. The best protocol was decided by evaluation of blood glucose levels using fasting blood glucose levels and a glucose tolerance test.
In part II, the optimal STZ treatment protocol was applied to two groups, one receiving STZ (stz_d84) and one receiving a sham treatment (sham_d84). Stz_d84 was subjected to a glucose tolerance test at day 22 to confirm hyperglycemia caused by the treatment, and again at day 32, 42, 52, and 72 of the experiment to monitor changes in blood glucose levels over time to identify potential regeneration of β cells. Sham_d84 was subjected to the glucose tolerance test at day 22, 42, and 72.
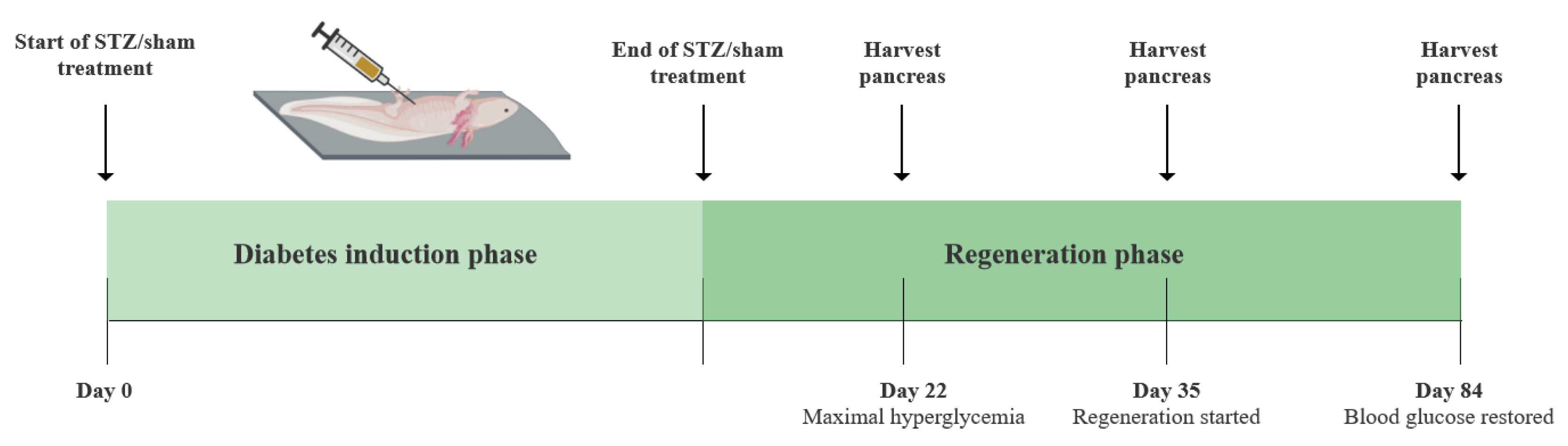
In part III, four additional groups were included – stz_d22 and stz_d35, which received the STZ treatment, and sham_d22 and sham_d35, which received a sham treatment. The diabetic state after the STZ treatment was investigated at three distinct stages: 1. When hyperglycemia peaked (day 22), 2. When the regenerative process was assumed to initiate (day 35), and 3. When β cell function was restored (day 84) (
Figure 3).
In part IV, potential whole-body effects of the STZ-induced diabetes were characterized by examination of changes in body mass, percentage of red blood cells in whole blood, plasma osmolarity, and several plasma and urine parameters.
Table 2.
Data table of the animal groups used in the pilot study and the main study.
Table 2.
Data table of the animal groups used in the pilot study and the main study.
| Group name |
Treatment type |
Mean start body mass (g) |
Mean end body mass (g) |
Mean of start and end length (snout to tail) (cm) |
# animals at the endpoint |
Color* |
Birth date |
| Pilot study groups |
|
| PS1 |
STZ |
21.6±2.8 |
20.0±1.7 |
14.5±0.5 |
3 |
WT: 33.3% Leu: 33.3% Alb: 33.3% |
10 March 2020 |
| PS2 |
STZ |
21.7±6.4 |
20.9±3.3 |
14.8±1.2 |
3 |
WT: 33.3% Leu: 33.3% Alb: 33.3% |
10 March 2020 |
| PS3 |
STZ |
22.6±3.5 |
22.8±4.0 |
15.1±0.6 |
3 |
WT: 66.6% Leu: 33.3% Alb: 0% |
10 March 2020 |
| PS4 |
STZ |
24.7±3.8 |
38.4±2.0 |
14.6±0.4 |
3 |
WT: 33.3% Leu: 66.6% Alb: 0% |
10 March 2020 |
| PS5 |
STZ |
22.3±4.0 |
31.5±1.2 |
14.2±1.1 |
3 |
WT: 66.6% Leu: 33.3% Alb: 0% |
10 March 2020 |
| PS_control |
- |
20.1±3.2 |
- |
14.4±1.0 |
3 |
WT: 33.3% Leu: 33.3% Alb: 33.3% |
10 March 2020 |
| Ins_baseline |
- |
40.3±9.1 |
- |
- |
3 |
WT: 66.6% Leu: 0% Alb: 33.3% |
Mixed |
| Main study groups |
|
| Stz_d22 |
STZ |
29.5±8.1 |
44.4±22.8 |
16.0±1.1 |
6 |
WT: 66.6% Leu: 33.3% Alb: 0% |
Mixed |
| Sham_d22 |
Sham |
30.8±7.6 |
32.3±7.2 |
15.5±0.2 |
4 |
WT: 75% Leu: 25% Alb: 0% |
Mixed |
| Stz_d35 |
STZ |
23.4±3.9 |
32.5±11.5 |
14.8±0.2 |
4 |
WT: 50% Leu: 25% Alb: 25% |
Mixed |
| Sham_d35 |
Sham |
24.5±7.7 |
26.9±7.3 |
15.7±1.4 |
3 |
WT: 33.3% Leu: 66.6% Alb: 0% |
Mixed |
| Stz_d84 |
STZ |
23.2±2.5 |
30.3±4.1 |
16.1±0.6** |
5 |
WT: 0% Leu: 100% Alb: 0% |
10 March 2020 |
| Sham_d84 |
Sham |
22.7±4.5 |
31.3±3.9 |
16.5±0.9** |
6 |
WT: 33.3% Leu: 66.6% Alb: 0% |
10 March 2020 |
Axolotl Husbandry
Axolotls were bought from a local breeder and housed in groups of 1-3 animals. A mixture of wild type, leucistic, and albino salamanders of both sexes were included in the experiments (
Table 2). Animals were housed in containers (39cm x 28cm x 14cm) with tap water and a water depth of 10cm at 19-22
and exposed to 12h light-dark cycles. Water was changed 2-3 times a week, and animals were fed 3 pills 3 times a week.
Animals acclimated to the housing conditions for one week and were fasting during the acclimatization period to achieve the correct dosage of STZ during the treatment period. During the treatment period, animals given STZ were kept under a fume hood to prevent contamination of the environment. Due to lack of space in the fume hood, the study groups were treated in different periods (
Appendix A. – Study schedule). Animals were housed in lower water depth (6-7 cm depth) during the injection period, to minimize the production of STZ waste. For information on the used animals, see
Table 2. The experiments were approved by the Danish National Animal Experiments Inspectorate (protocol# 2020-15-0201-00688).
Anesthesia
Animals were anesthetized by full-body submersion in 200 mg/L benzocaine solution (Sigma-Aldrich) dissolved in 2-3 mL acetone, in 2L water tanks. Animals were anesthetized for 20 min prior to STZ-, EdU-, and glucose injection and all blood collections, and they were anesthetized for a minimum of 30 min before euthanasia. Anesthesia is absorbed via the gills and transdermally, and animals were kept in paper towels soaked in anesthetic water during the procedures to keep them anesthetized. Following the procedures, the animals were moved to their normal housing and regained consciousness within an hour.
STZ Injection
Injections of the STZ/sham treatment were performed under a fume hood and with protective equipment (double nitrile rubber gloves and a back-closure gown). STZ (solid) (EMD Millipore Corp., cat. no: 572201) was kept at -20 before use and was diluted in amphibian Ringer’s solution and mixed well with a pipette. Sham-treated animals received an injection of amphibian Ringer’s solution without STZ. The dosage was adjusted to the body mass of the individual animal. The STZ solution was administered within 30 minutes of preparation. Animals were anesthetized and placed on wet blankets on a hard surface, and the STZ/sham solution was i.p. injected with a BD Micro-Fine 0.5 mL insulin syringe (30G). After injection, the animals were transferred to their normal housing and monitored until normal gill movement. Animals were fasting for 24h prior to STZ injections and a week prior to the first injection.
Glucose Tolerance Test
To monitor changes in blood glucose levels, animals were subjected to a glucose tolerance test. Animals were fasted for three days before each test. 1.75 mg/g body mass of a 30% D(+)-glucose solution (Sigma, cat. no. G8270) was injected into the vena cava after measurement of fasting blood glucose levels. After the injection, animals were placed in the normal living tank until the next blood collection. Blood glucose was measured at several time points over the span of 24h with a FreeStyle Precision Neo glucometer and glucose test strips (Abbott). In the pilot studies, blood glucose was measured 0h, 10min, 4h 40 min, 9h 20 min, 14h, and 24h after glucose injection. In the main studies, blood glucose was measured 0h, 10min, 14h, and 24h after glucose injection. Before euthanasia, blood glucose was measured 0h, 10 min, and 9h 20 min after glucose injection.
Blood Ketone Measurements
Blood ketone was measured before the glucose tolerance test with a FreeStyle Precision Neo glucometer and ketone test strips (Abbott).
Tissue Sample Harvest
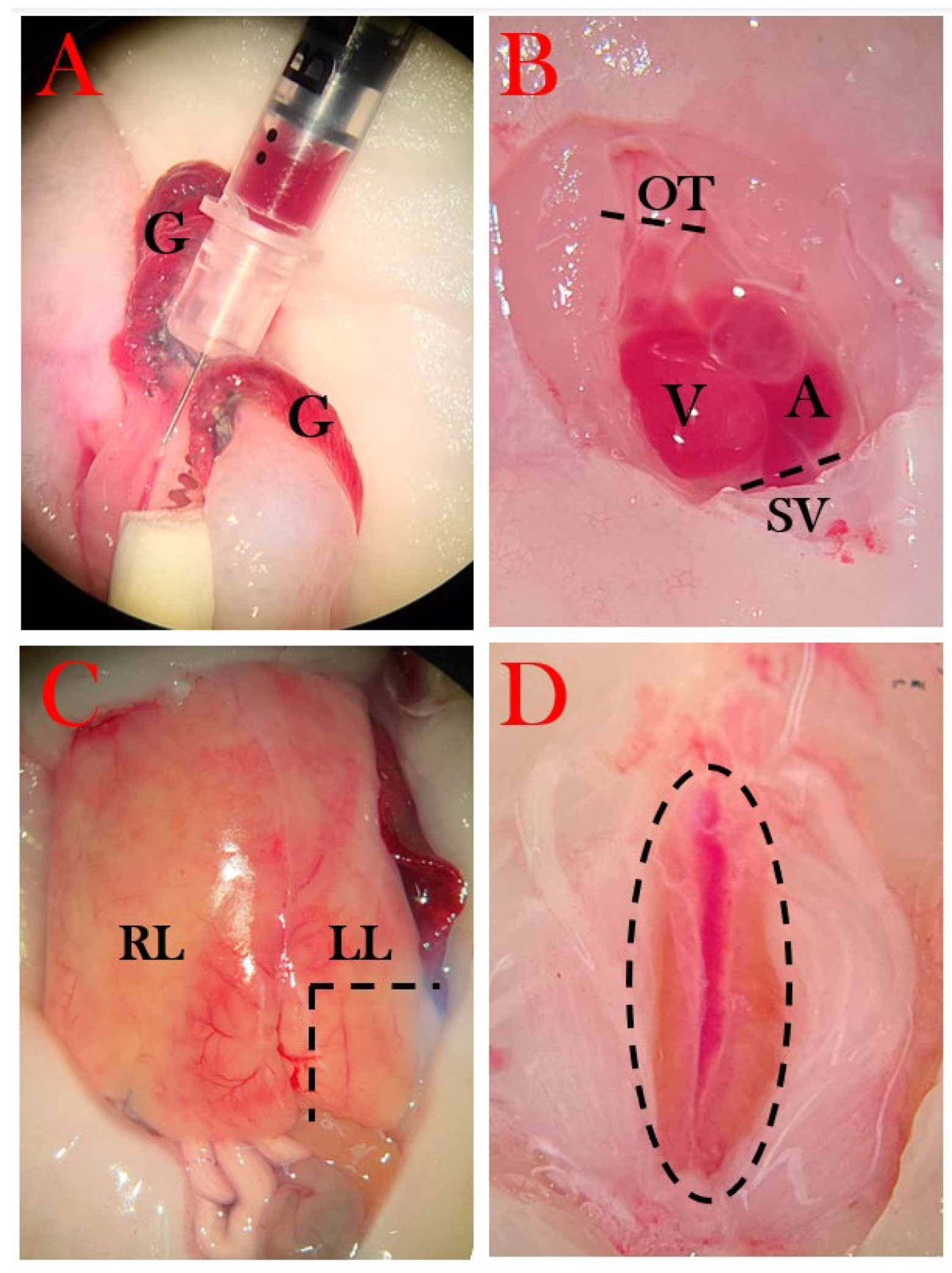
Blood was harvested either from gill arteries or directly from the heart (only at the endpoint) with firstly a heparin-coated 0.5 mL insulin syringe, followed by an EDTA (ethylenediaminetetraacetic acid)-coated syringe (
Figure 4A). Whole blood was transferred to a pre-weighted Eppendorf tube and spun at 6000 rpm for 5 min, and plasma was collected and deposited in a new pre-weighed tube. The tubes were weighed, from which the percentage of red blood cells was calculated, followed by immediate snap-freezing in liquid nitrogen and storage at -80°C.
The thoracic cavity was opened using micro scissors by removing skin, muscle, and the cartilage plates protecting the heart, followed by an incision in the pericardium. The outflow tract was severed from which blood could be collected, followed by severing of other vessels (sinus venosus, among others) to free the heart (
Figure 4B). The heart was moved to a petri dish with amphibian Ringer’s solution, in which it remained pulsating until most blood had exited the heart, and it was transferred to a mold and covered with OCT cryo embedding matrix (CellPath) before snap freezing in liquid nitrogen and stored in -80°C.
Next, the abdominal cavity was opened to expose the bladder. Urine was removed by carefully inserting a syringe while extending the bladder with a tweezer, and collected urine was transferred to a tube and snap-frozen.
A piece of the left liver lobe was excised and transferred to a mold and covered in OCT cryo embedding matrix followed by snap freezing (
Figure 4C).
The pancreas was removed by first separating the liver lobe from the pancreas (
Figure 5B), then severing the duodenum from the stomach (
Figure 5C), and severing pancreas tissue from connecting tissue (
Figure 5D). The pancreas was cleaned by rinsing with amphibian Ringer’s solution through the duodenal opening and by removing blood clots (
Figure 5E). Next, the pancreas was severed at the midpoint, and the half attached to the duodenal part closest to the stomach was separated from the duodenum and transferred to a tube for later insulin measurements (
Figure 5F). The other half was placed in OCT and snap-frozen.
The kidneys were removed by opening to the lower part of the abdominal cavity and first severing the rectum as close to the cloaca as possible, followed by separation of the kidney tissue from the abdominal wall (
Figure 4D). It was transferred to an OCT-embedded mold and snap-frozen.
Lastly, the eyes were removed carefully with micro scissors, and the left eye was OCT-embedded in a mold, while the right eye was transferred to a tube and snap-frozen.
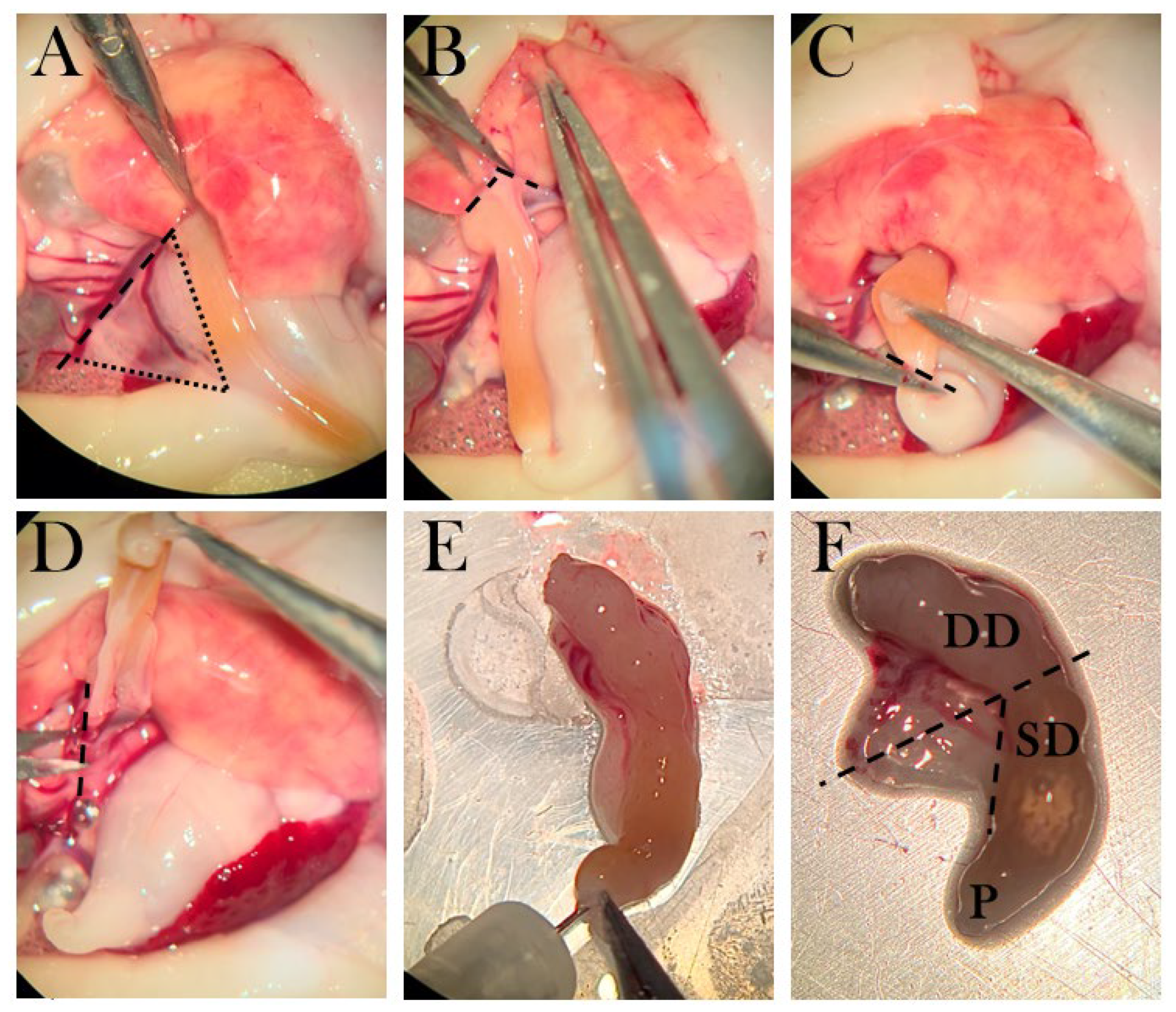
Figure 5.
The dissection method of the pancreas. A) The triangular pancreas, in which dotted lines frame the organ and the striped line shows the incision from connective tissue. B) Pancreas is separated from the liver lobe. C) The duodenum is cut near the pylorus. D) Connective tissue is separated from the pancreas. E) The duodenum is rinsed with amphibian Ringer’s solution. F) The pancreas is connected to a superior duodenal (SD) part near the pylorus (P) and a descending part (DD). Striped lines indicate the incision points, in which the part connected to SD is transferred to a tube for ELISA, and the part connected to DD is transferred to a mold.
Figure 5.
The dissection method of the pancreas. A) The triangular pancreas, in which dotted lines frame the organ and the striped line shows the incision from connective tissue. B) Pancreas is separated from the liver lobe. C) The duodenum is cut near the pylorus. D) Connective tissue is separated from the pancreas. E) The duodenum is rinsed with amphibian Ringer’s solution. F) The pancreas is connected to a superior duodenal (SD) part near the pylorus (P) and a descending part (DD). Striped lines indicate the incision points, in which the part connected to SD is transferred to a tube for ELISA, and the part connected to DD is transferred to a mold.
Osmolarity
Osmolarity was measured on plasma samples from swollen animals using an osmometer (Advanced Instruments, Inc., Model 3320).
Histology and Immunostaining
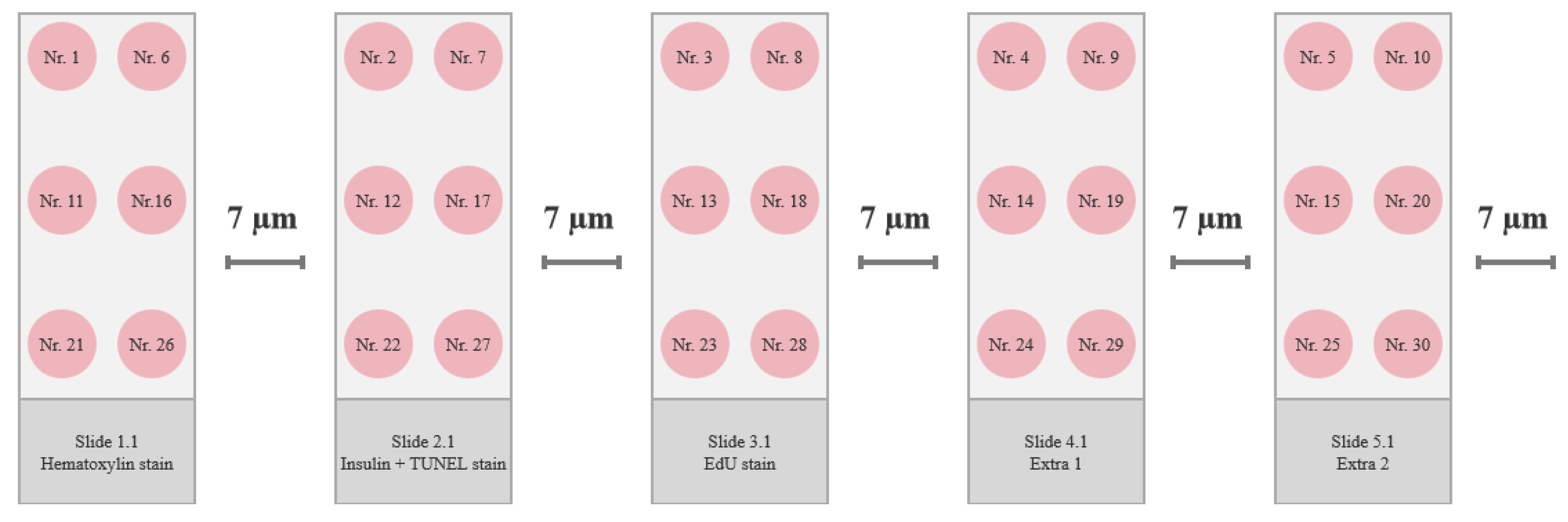
Frozen pancreas tissue was sectioned as follows. A 7 µm section was placed on one slide, and the next 7 µm section was discarded before placing the next 7 µm section on the next slide, creating a series of neighboring sections with 7 µm between each (
Figure 6). There were prepared 2-3 slides for every stain type, and the four best neighboring pancreas sections were chosen using the hematoxylin-stained slides for guidance, and the best sections were used in the immunofluorescence staining procedures. Sections were stored at -80°C.
Hematoxylin Staining
All sections in the hematoxylin slide series were equilibrated to RT (room temperature) for 5-7 min before fixation in methanol for 1 min, followed by washing in demineralized water. Slides were immersed in RT-equilibrated hematoxylin (Sigma-Aldrich, cat. No. GHS316) for 1 min, and slides were washed and dried, before applying Eukitt Quick-hardening mounting medium (Sigma-Aldrich, cat no. 03989) and coverslips.
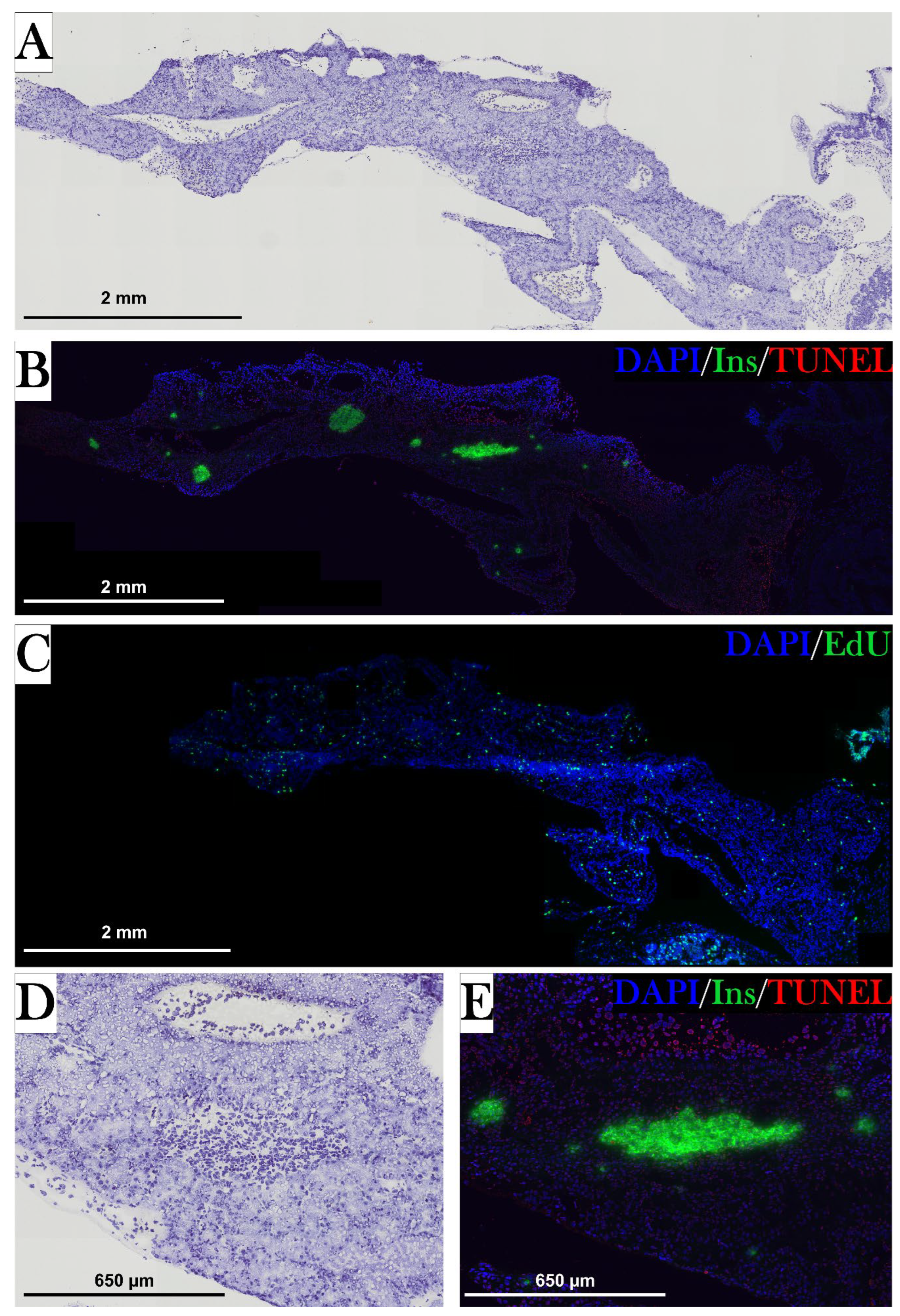
Staining of Apoptotic Cells with a TUNEL Assay in the Pancreas
Four neighboring pancreas sections were chosen to examine apoptotic cells and insulin-positive cells.
To stain apoptotic cells, the Click-iT Plus TUNEL Assay (Invitrogen) was used. All stock solutions were prepared per the manufacturer’s protocol.
Firstly, tissue sections were equilibrated to RT for 5-7 min before fixation in 4% paraformaldehyde (PFA) (Alfa Aesar, cat no. J61899) for 15 min in RT, washed in 70% Phosphate-buffered saline (PBS), and permeabilized using 1:25 proteinase K in PBS solution, followed by incubation for 7 min in RT in a humidity chamber. Slides were then washed in PBS for 2x 5 min and fixated again in 4% PFA for 5 min. The washing step was repeated twice, and slides were rinsed in deionized water before the addition of TdT reaction buffer for 10 min at 37 °C to perform the TdT reaction. The TdT reaction buffer was removed, and 50 µL of TdT reaction mixture (47 µL TdT reaction buffer, 1 µL EdUTP, and 2 µL TdT enzyme) was added to each tissue section, then incubated for 1h at 37 °C in the humidity chamber. The slides were rinsed in deionized water and immersed in permeabilizing solution (3% bovine serum albumin (BSA) and 0.1% Triton X-100 in PBS (Sigma)) for 5 min, followed by 2x 5 min wash in 70% PBS. The Click-iT Plus reaction was performed by adding 50 µL Click-iT Plus TUNEL reaction cocktail (45 µL Click-iT Plus TUNEL Supermix and 5µL 10X Click-iT Plus TUNEL reaction buffer additive) and incubated for 30 min in 37 °C in darkness, before 5 min washing in 3% BSA and 2x 5 min wash in 70% PBS, thus ending the TUNEL stain.
Staining of Insulin-Positive Cells in the Pancreas
Following the TUNEL stain, sections were immersed in blocking buffer (PBS with 3% BSA and 5% normal goat serum) for 3h in RT and under gentle shaking. Primary antibody against insulin (Insulin/proinsulin monoclonal antibody (D3E7 (5B6/6), ThermoFisher Scientific, cat no. MA1-83256) was diluted in a 50% blocking buffer in PBS solution (1:75) and added to sections, followed by overnight incubation in a humidity chamber at 4°C. After removal of the primary antibody and 5x 5 min of washing in PBS, goat anti-mouse IgG1 Alexa Fluor 488 secondary antibody (ThermoFisher Scientific, cat no. A-21121) in a 50% blocking buffer in PBS solution (1:500) was applied and incubated in a humidity chamber at RT for 3h in darkness. Slides were again washed 5x 5 min in PBS, air-dried, and DAPI Diamond mounting media (ThermoFischer Scientific, cat no. P36966) was applied. Coverslips were added and cured in RT for at least 24h before imaging. For storage, slide edges were sealed with nail polish, and slides were kept in darkness at 4°C before imaging.
Staining of glut2-Positive Cells in the Pancreas
GLUT2 polyclonal primary antibody (ThermoFisher Scientific, cat no. PA5-77459) was evaluated on control pancreas, liver, and kidney tissue. Slides were fixated for 10 min in PFA, followed by 3x 5 min washing. Staining was evaluated both with and without a permeabilization step (15 min with 0.25% Triton X-100). Slides were washed 2x 5 min and blocked with 3% BSA and 5% goat serum in PBS for 3h. The primary antibody was assessed in three concentrations (1:50, 1:100, and 1:500) in 50% blocking buffer in PBS and incubated for 24h in a humidity chamber at 4°C. After washing 5x 5 min, goat anti-rabbit IgG (H+L) Alexa Fluor 647 secondary antibody (ThermoFisher Scientific, cat no. A32733) was used as secondary antibody and applied to the sections, followed by incubation in a humidity chamber for 3h in RT. Slides were mounted with DAPI Diamond mounting media and covered with coverslips. Slides were stored in darkness at 4°C before imaging.
Staining of Proliferating Cells in the Pancreas
Neighbor sections were stained for proliferating cells using a Click-iT® EdU Imaging Kit (Invitrogen, cat no. C10637), in which 5-ethynyl-2’-deoxyuridine (EdU) is incorporated into DNA during active DNA synthesis.
Injection of EdU Solution
Firstly, EdU was injected into animals 24h before euthanasia. Animals were anesthetized as described previously. EdU was dissolved in Amphibian Ringer’s solution shortly before use. Animals received 0.035 mg/g body mass of EdU solution using body weight measurements from one week before the time of injection to adjust to the edema seen in some animals and to minimize expenses. Animals were placed in wet towels on ice for 1h before returning to normal housing.
Staining of Pancreas Tissue Sections
Slides with tissue sections were fixated in a 4% PFA solution for 15 min and washed in 3% BSA in PBS 2x 5 min and in 70% PBS 2x 5 min, followed by permeabilization in 0.5% Triton X-100 in PBS for 20 min and washed again as mentioned before. For EdU detection, 100 µL Click-iT® reaction cocktail was added to each tissue section and incubated for 30 min in RT in darkness. The Click-iT® was prepared per the manufacturer’s protocol, by mixing 86 µL 1X Click-iT® reaction buffer, 4 µL CuSO4, 0.24 µL Alexa Fluor® azide, and 10 µL reaction buffer additive per tissue section (ingredients were added in this order).
After reaction cocktail incubation, slides were washed as before and nuclear stained by adding 100 µL Hoechst 33342 solution (1:2000 in PBS) and incubated for 30 min in darkness. The nuclear stain was removed, and the slides were washed as before, including a wash in dH2O before mounting with ProLong Diamond Antifade Mountant (ThermoFischer Scientific, cat. no. P36965). Sections were placed in darkness in RT for 24h and then moved to 4 before imaging.
Imaging and Analysis
All stained slides were imaged using a slide scanner (Upright Widefield Fluorescence) Olympus VS120 at Health Bioimaging Core Facility, Aarhus University.
Hematoxylin-stained slides were imaged in 20x magnification, while immunofluorescence-stained slides were imaged in 40x magnification.
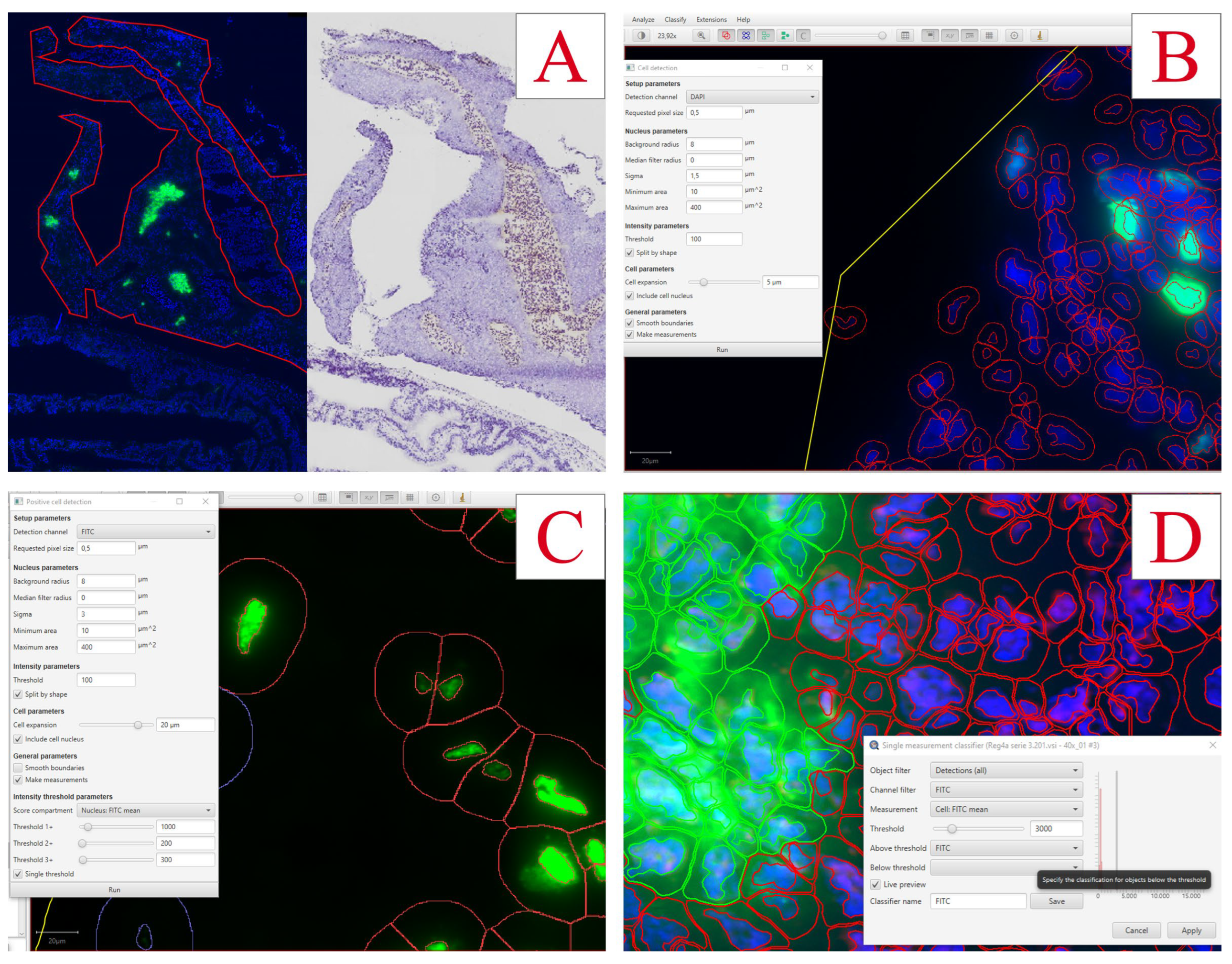
Insulin-positive cells and EdU-positive cells were quantified using QuPath v0.3.2 Software. The pancreas was defined as the region of interest using the QuPath toolset and by comparing it to the hematoxylin stains of neighbor sections (
Figure 7A). The total number of cells was detected using the DAPI channel with the Cell Detection tool accessed by the following commands: Ctrl+L => Cell detection (
Figure 7B). With small adjustments to the nucleus parameters and a threshold of 100, nuclei were detected for all cells in the region. To find a fitting threshold for all channel detections, intensity measurements of all detected cells were evaluated by the following commands: Measure => Show detection measurements.
For EdU-positive cells, FITC-stained nuclei were detected by following commands: Ctrl+L => Positive cell detection (
Figure 7C). The sigma value was set to 3, the threshold to 100, and the tool was set to detect the mean FITC intensity in the nucleus.
For insulin-positive cells, FITC-stained cells were detected by first detecting DAPI-positive cells and then classifying insulin-positive cells with the following commands: Classify => Object classification => Create single measurement classifier (
Figure 7D). The classifier was set to measure FITC mean intensity in the total cell with a threshold of 3000. To detect cells fitting this classification: Classify => Object classification => Load object classifier.
For both EdU-positive cells and insulin-positive cells, the percentage of positive cells compared to the total number of cells was calculated for each section. Four sections per animal were used in the analyses, and a mean percentage of positive cells was found for each animal and used for statistical analysis.
Figure 7.
Analyses of immunofluorescence staining in pancreas using QuPath Software. A) Pancreas tissue is outlined using QuPath tools, and the hematoxylin-stained neighbor section is used as reference. B) The settings for cell detection using the DAPI nuclear stain. C) The settings used for detecting EdU-positive cells. D) The settings used for the detection of insulin-positive cells.
Figure 7.
Analyses of immunofluorescence staining in pancreas using QuPath Software. A) Pancreas tissue is outlined using QuPath tools, and the hematoxylin-stained neighbor section is used as reference. B) The settings for cell detection using the DAPI nuclear stain. C) The settings used for detecting EdU-positive cells. D) The settings used for the detection of insulin-positive cells.
Measurement of Insulin in Plasma and Pancreas Tissue with ELISA
A mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen, cat no. EMINS) was assessed using both plasma samples and excised pancreas tissue to investigate the possibility to quantify the amount of insulin in the tissue. The plasma was collected with EDTA-coated syringes and the pancreas tissue was collected as described in Tissue sample harvest. The kit was used per the manufacturer’s instructions and all reagents were diluted as instructed. To calculate the concentration in the samples, a standard curve was prepared with concentrations ranging from 400-6.25 µIU/mL.
Dilution of Samples
To find the appropriate concentration for both plasma samples and pancreas tissues, several dilutions were evaluated as follows. Two plasma samples from control animals were diluted with a dilution factor of 1, 2, 4, 8, and 20. As for pancreas extract, dilution factors of 2, 4, 8, 20, 40, 100, 140, 200, 300, 400, 600, 750, 1000, 1200, and 2000 were assessed.
The ELISA Procedure
100 µL of samples (dilutions of pancreas supernatant and plasma), blanks, and standards were loaded in duplicates on the insulin-coated 96-well plate, and the plate was incubated at a shaker for 2.5h with a speed of 200 rpm at 20. After incubation, the wells were washed by adding 4x 300 µL of wash buffer, followed by the addition of 100 µL biotin solution and incubation for 1h with the same settings. The washing step was repeated, and 100 µL streptavidin-horseradish peroxidase solution was added to each well and incubated for 45 min. Again, the wells were washed as before, and 100 µL 3,3',5,5'-Tetramethylbenzidine (TMB) substrate was added, and the plate was incubated for 30 min in darkness, followed by the addition of 50 µL stop solution to each well, and the OD450nm was measured. Curve fitting of the standard curve was used to calculate the concentrations of the samples, giving the following equation: .
Measurements of Blood Parameters with Microplate Assays
Blood samples were collected from axolotl gills and heart at the endpoint using a heparin-coated syringe and transferred to an Eppendorf tube as described previously (see Tissue sample harvest).
Albumin Assay
Albumin was measured in the plasma by using the QuantiChrom BCP Albumin Assay Kit (BioAssay Systems, cat no. DIAP-250). Plasma was thawed on ice and 20 µL was used from each animal for the assay. The standard curve was performed using BSA.
20 µL plasma or standard was added to each well in a 96-well plate, followed by adding 200 µL working solution. The plate was incubated for 5 min in RT and OD610nm was measured using a Synergy H1 microplate reader (BioTek).
Albumin concentration was calculated using the equation of the standard curve where x is the concentration of albumin and y is the measured optical density:
Alkaline Phosphatase Activity Assay
Alkaline phosphatase activity was measured using QuantiChrom Alkaline Phosphatase Assay Kit (BioAssay Systems, cat no. DALP-250). Plasma was thawed on ice and 10 µL was collected from each animal for the assay. The working solution was prepared by mixing 200 µL Assay Buffer, 5 µL Mg Acetate (final concentration of 5 mM), and 2 µL pNPP liquid substrate (10 mM) for each well. For concentration calculations, 200 µL distilled water and 200 µL Calibrator were added in duplicates. As for samples, 10 µL plasma was added in singlets, followed by the addition of 190 µL working solution. OD405nm was measured at t=0 and t=4 min on a plate reader.
The alkaline phosphatase activity was calculated with the following equation:
In which t shows minutes between measurements.
Creatinine Assay
Creatinine was measured in plasma samples using QuantiChrom Creatinine Assay Kit (BioAssay Systems, cat no. DICT-500). Plasma was thawed on ice and 10 µL was collected from each animal for the assay. Plasma was diluted in distilled water to a total volume of 30 µL. The standard was diluted by adding 5 µL 50 mg/dL standard stock to 120 µL distilled water, resulting in a concentration of 2 mg/dL, and 30 µL was added in doublets to a 96-well plate. 30 µL diluted plasma was added in singlets to the plate, followed by the addition of 100 µL of a 1:1 solution of Reagent A and Reagent B to each well. OD510nm was measured after 0 min (t=0) and 5 min (t=5) using a Synergy H1 microplate reader (BioTek).
Creatinine concentration was calculated with the following equation:
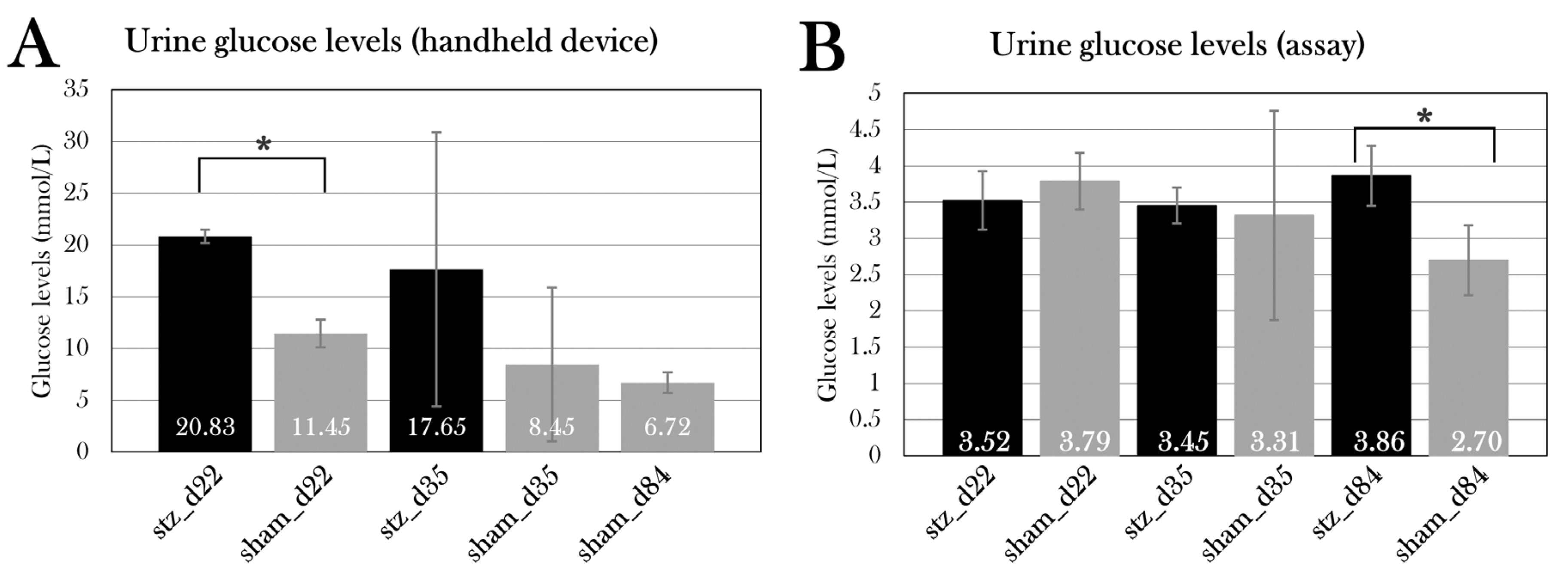
Glucose Measurements of Urine Samples
When extracting urine from the bladder, urine glucose was measured using the FreeStyle Precision Neo glucometer and glucose test strips before snap-freezing.
Glucose was also measured using a glucose assay kit II (Abnova, cat. No. KA0832). A standard curve was prepared by diluting the standard stock, ending with standards of 0, 2, 4, 6, 8, and 10 nmol, generating a curve with the following equation used to calculate the glucose quantity in samples:
Before glucose assaying, urine samples were deproteinized using the Deproteinizing Sample Preparation Kit – TCA (abcam, cat no. ab204708). For protein precipitation, 9 µL cold TCA was added to 60 µL urine sample and incubated on ice for 15 min, followed by centrifugation at 12,000 x g for 5 min. The supernatant was collected, and the TCA in the samples was neutralized by adding 6 µL cold neutralization solution and mixed. Samples were incubated on ice for 5 min, before the initiation of the glucose assay.
Urine samples were prepared in a 5:50 dilution in glucose assay buffer, and 50 µL standards and diluted samples were added to a 96-well plate in duplicates before adding 50µL glucose reaction mix (46 µL glucose assay buffer, 2 µL glucose enzyme mix, and 2 µL glucose substrate mix) to each well. The plate was incubated for 30 min in RT in darkness, followed by measurement of the absorbance at 450 nm. Glucose concentration was calculated using the equation of the standard curve to calculate the amount of glucose, from which the concentration was calculated by the following conversion:
Protein Measurement of Urine Samples
Using Pierce Microplate BCA Protein Assay Kit (Thermo Scientific, cat no. 23252), protein was quantified in urine samples. Before the assay, the pH of the urine samples from two animals per group was measured to adjust the protocol for this. All pH levels were 6-7.5, and the reagents were then prepared per the manufacturer’s protocol.
Standard curves were prepared in a serial dilution, ending up with concentrations of 2000, 1500, 1000, 750, 500, 250, and 125 µg/mL. As for blanks, a working reconstitution buffer without reducing agents was used. 9µL of standards, blanks, and samples were loaded in duplicates in a 96-well plate. 4 µL Compatibility Reagent Solution was added to each well, and the plate was shaken for 1 min and incubated for 15 min at 37°C. 260µL Working Reagent was added to each well, followed by shaking for 1 min and incubation for 30 min at 37°C. After cooling in RT for 5 min, OD
562nm was measured immediately. Sample concentrations were calculated by extracting the blank and using equation of the standard curve:
Statistical Analysis
Statistical analyses were performed using Microsoft Excel. Unpaired Student’s T-Test was used to compare the STZ-treated groups with sham-treated groups for each day of euthanasia (day 22, 35, and 84), taking the variance of the respective dataset into account. For comparison of paired blood glucose or ketone measurements for stz_d84 and sham_d84, respectively, a one-way repeated measurements ANOVA test was used to determine changes over time. For comparison of all groups with the same treatment type (STZ or sham), a single factor ANOVA test was used to determine differences. For all tests, a p-value < 0.05 was considered statistically significant, and grouped data is displayed as mean ± standard deviation.
Discussion
In this project, I have investigated the axolotl as a potential regeneration-positive animal model of diabetes. As the axolotl has not been used in studies on diabetes prior to this project, a protocol for inducing diabetes was needed. For this, I have previously tested a protocol, in which 0.35 mg/g body mass of STZ is given five times over the span of 19 days, inspired by a previous study in zebrafish[
90]. This protocol resulted in severe systemic toxicity and high mortality[
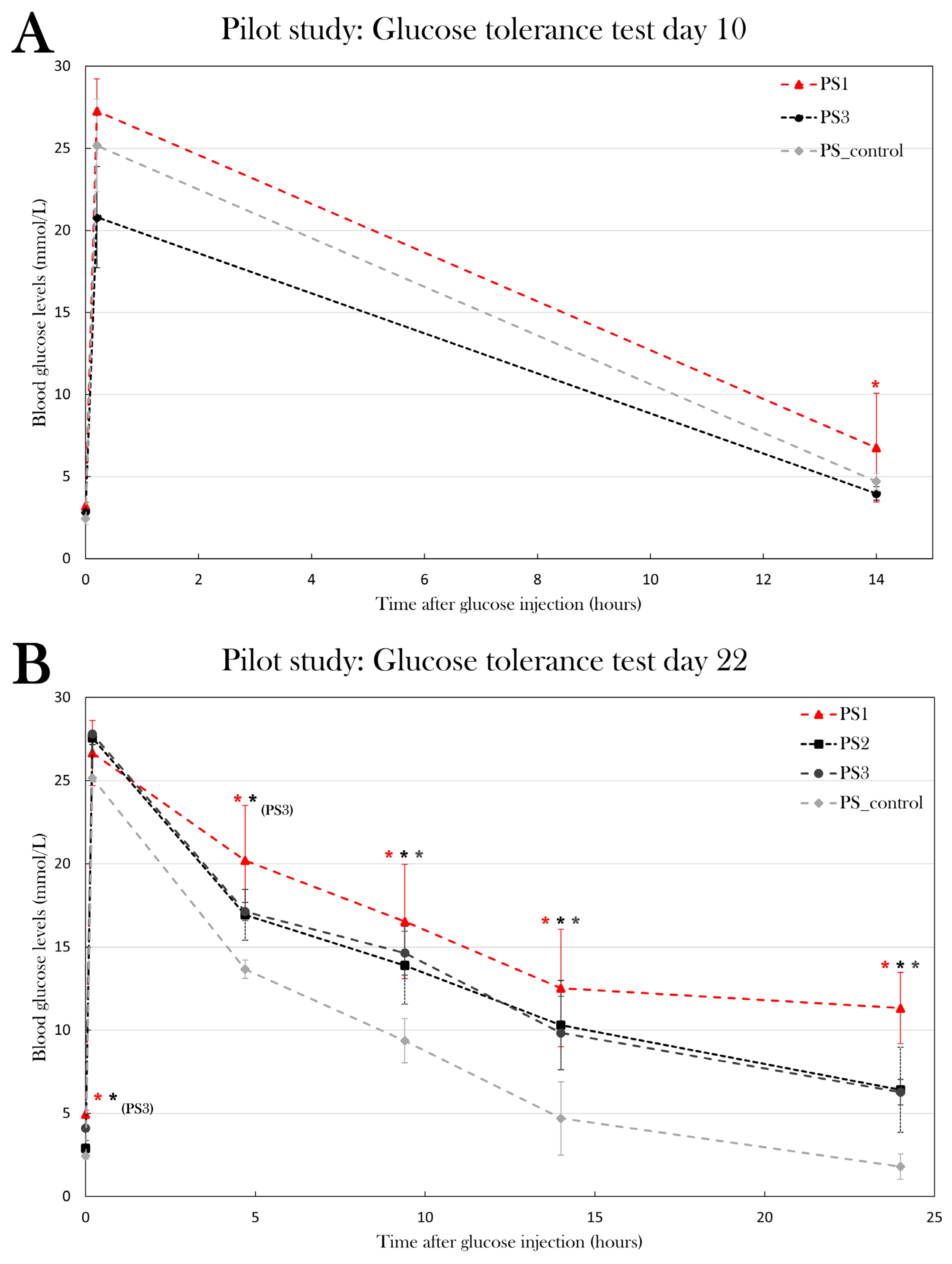
91]. In this study, I have optimized the protocol and assessed five different treatment regimes in five pilot groups, in which the glycemic state of the animals was evaluated using the glucose tolerance test to measure the metabolic activity of glucose. Results showed that especially two treatment protocols made the animals hyperglycemic – PS1, in which 0.05 mg/g body mass was given five consecutive days, and PS3, in which 0.2 mg/g body mass was given once. Higher doses resulted in edema. Even though the protocol used in PS3 is easier to manage, the PS1 protocol was chosen as the most optimal protocol, as this already proved to have a minor effect after 10 days, and because studies in rodents have shown that multiple smaller doses of STZ have a better and less toxic effect than one larger dose[
63]. It is possible that the protocol used on PS2 would make the animals diabetic at a later point in time than day 22, since the STZ injections are spread over a larger period than in PS1 and PS3, but in the interest of time, this was not investigated further, as a fast treatment response is of the most interest in experimental animals.
A method to quantify the amount of insulin was investigated, as such a method could be used to characterize diabetes in the animals. For this, a mouse insulin ELISA assay was evaluated on both plasma samples and pancreas tissue. The amount of insulin in plasma samples proved to be beyond the range of the assay, even when undiluted, indicating that the axolotl has a small level of insulin in the blood. More knowledge of the role of insulin in axolotl metabolism is heavily needed. On the other hand, the assay could detect insulin in pancreas tissue of control animals. To continue this study, I will use the ELISA kit and measure insulin content in the pancreas of the animals used in this project, but another assay with a lower range is needed for plasma measurements.
To characterize if I was able to induce diabetes in the animals, the glucose tolerance test was used, as it is a commonly used diagnostic tool. I have previously adjusted this test for axolotls[
91], as they have a slow metabolic rate compared to rodents and humans, but the test was further optimized in this study, by including additional measurements (after 4h 40 min and 9h 20 min) during the test and adjusting the test to span over 24h instead of 14h, as blood glucose levels have returned to normal after 24h. This process can result in excessive blood loss, even though only small amounts of blood are collected for glucose measurements using a handheld device. The blood collection procedure itself will result in some loss of blood and, as seen when collecting blood samples throughout the test for insulin measurements, excessive blood loss can result in mortality. Thus, blood glucose levels were only measured 0h, 10 min, 14h, and 24h after glucose injection in the main study, as these animals were subjected to the test every tenth day.
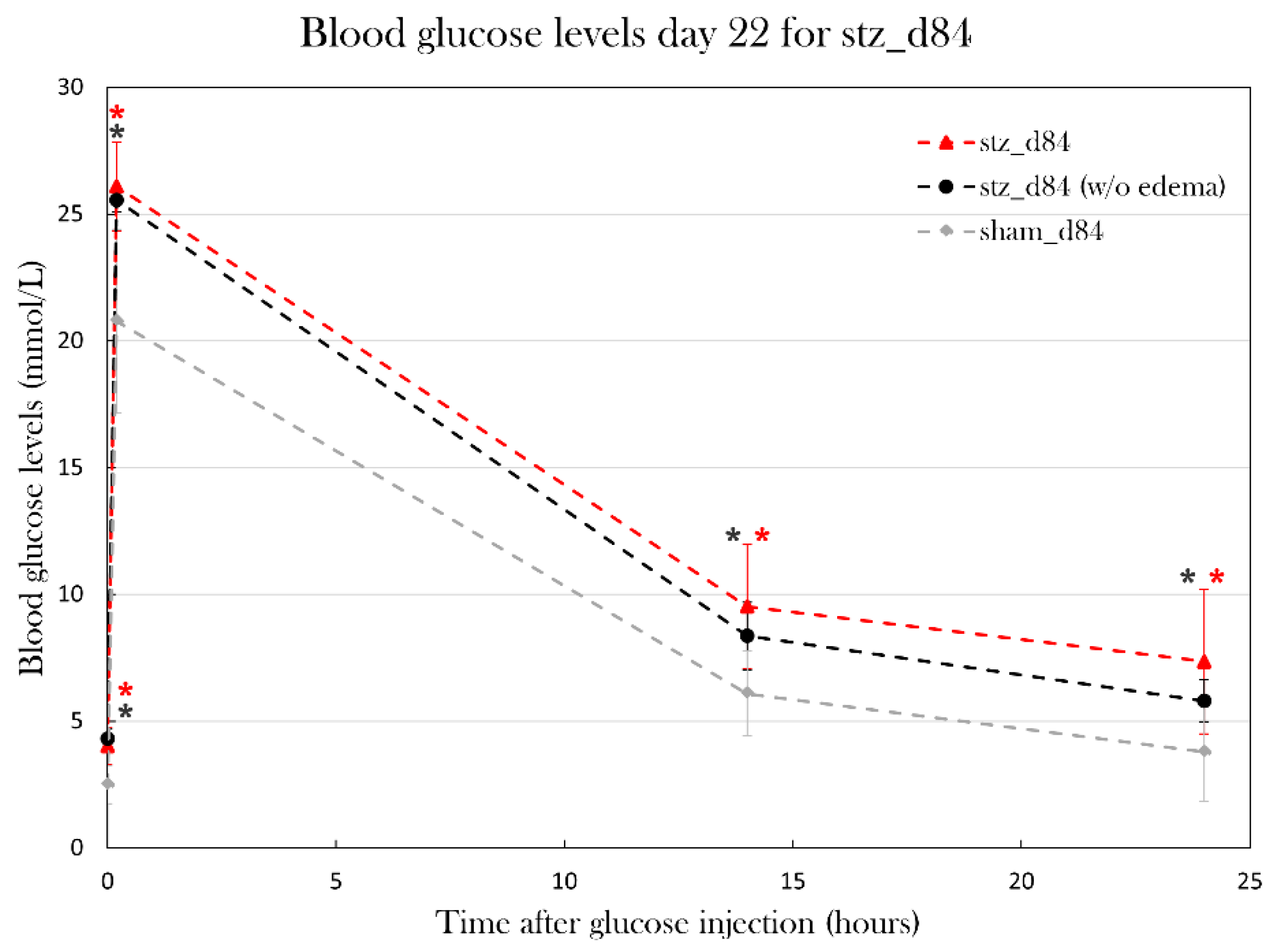
Even though the injected glucose amount was adjusted to the body mass of the individual animal, the blood glucose measurement after 10 min varied between groups and between measurement days, as seen in sham_d84 (
Figure 13B). This difference was not expected since glucose consumption is not believed to be initiated yet with the slow metabolic rate seen in axolotls. It can be speculated if this difference is a result of uneven distribution of glucose in the blood of the body, and it, therefore, does not reflect the true blood glucose content. It could also be a result of difficulty in correct injection, as puncture of the vessel with the syringe could cause spillage of glucose out in the tissue, causing blood glucose levels to be lower. However, a comparison of the glucose tolerance test results of sham_d84 at day 22, 42, and 84 suggests that the blood glucose levels 10 min after glucose injection do not affect blood glucose levels after 14h and 24h. Even though the blood glucose levels after 10 min differed at day 22, 42, and 84, the glucose levels were indifferent after 14h and 24h (
Figure 13B). It suggests that the blood glucose levels 10 min after glucose injection does not affect the glucose consumption in the body and the blood glucose levels afterward.
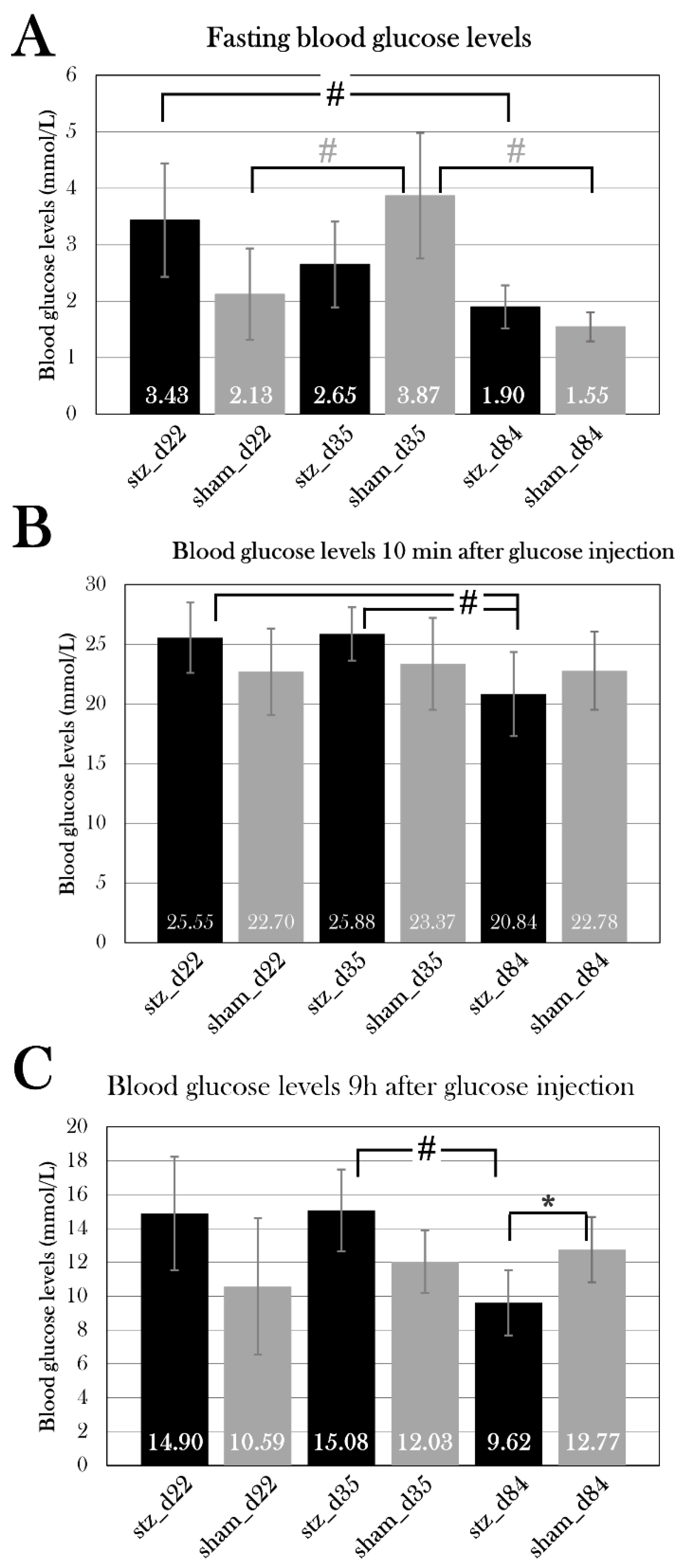
As correct glucose injection is important for the glucose tolerance test as a diagnostic tool, glucose dosage was adjusted to the body mass of the animals. However, some animals experienced massive body mass changes caused by edema, which brings the question if the glucose injection should be adjusted to the exact weight of the animals, or if the mass of fluid should be considered metabolic inactive and thus be subtracted from the used weight. In this study, glucose was given with the assumption that the whole-body mass of the animal reflected the amount of metabolic active tissue, and that glucose also would be distributed evenly in the excessive fluid. As seen in the glucose measurements at day 22 in stz_d84, animals were hyperglycemic even when excluding the animals affected by swelling, and blood glucose levels did not differ particularly between stz_d84 with and without swollen animals, indicating that these assumptions of glucose distribution in the body are correct.
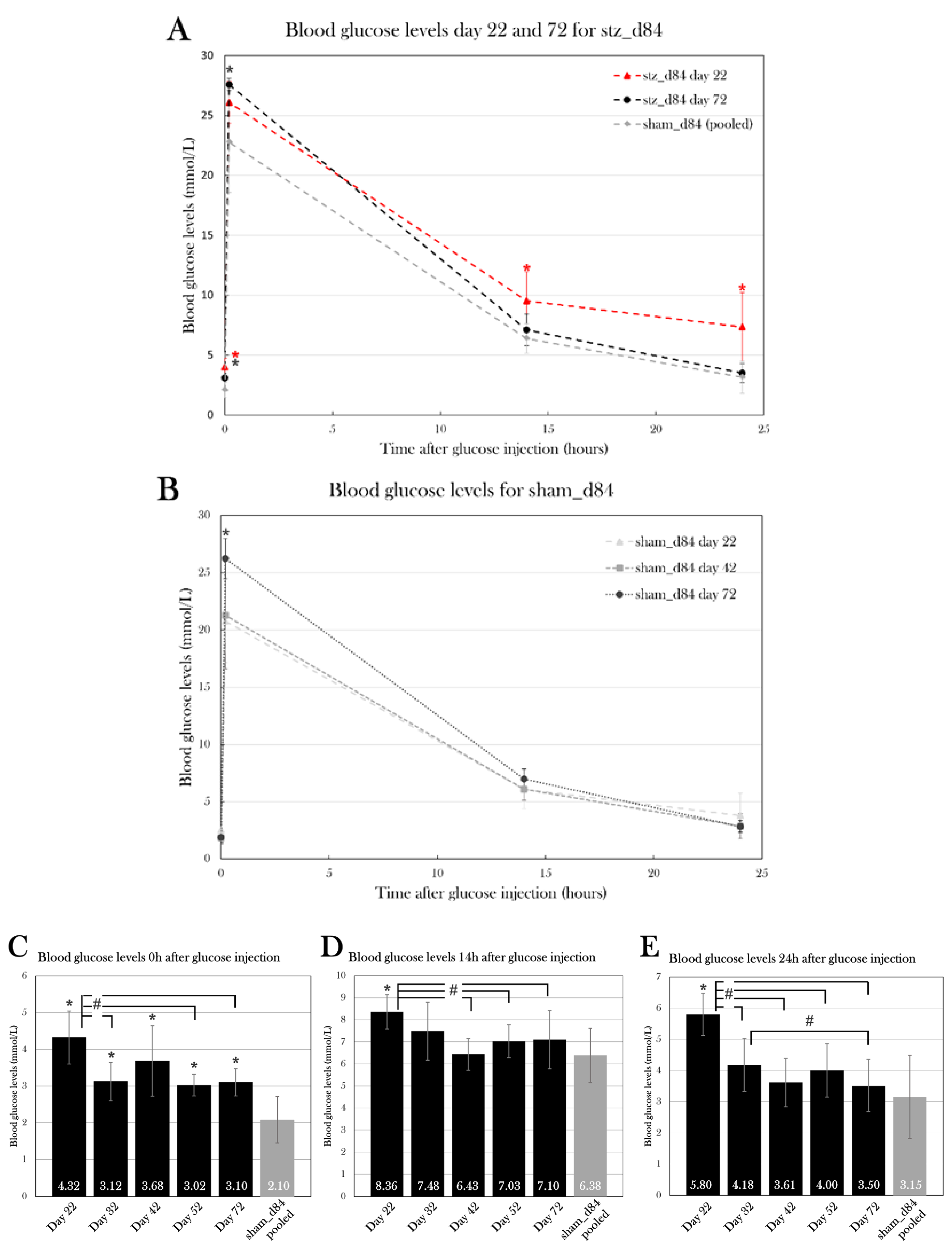
By using the adjusted STZ protocol and glucose tolerance test, the study of blood glucose changes over the span of 72 days was initiated. stz_d84 was hyperglycemic at day 22, like what was seen in the pilot experiment, and the blood glucose values normalized over time, nearly returning to blood glucose values seen in sham_d84 by day 72. The hyperglycemic state seen at day 22 indicates that I was able to induce diabetes in the stz_d84 and that some physiological response was initiated around day 32, as blood glucose levels were already lower at this point compared to day 22, but still significantly higher than blood glucose levels at day 72. Only at day 22 were the blood glucose levels during the glucose tolerance test significantly higher in stz_d84 compared to sham_d84, but fasting blood glucose levels continued to be higher in stz_d84 than in sham_d84 throughout the period. Thus, the animals were able to initiate a response to the sudden rise in blood glucose levels after glucose injection, but the animals were not able to return to the low fasting blood glucose levels. The high fasting blood glucose levels seen in stz_d84 are not considered diabetic blood glucose levels when considering diagnostic values used in humans (7 mmol/L[
1]), but these parameters are not comparable between axolotls and humans, as the metabolic need is different between species. Thus, the fasting blood glucose levels seen in stz_d84 can be considered an indicator of diabetes, as they are significantly different from sham_d84.
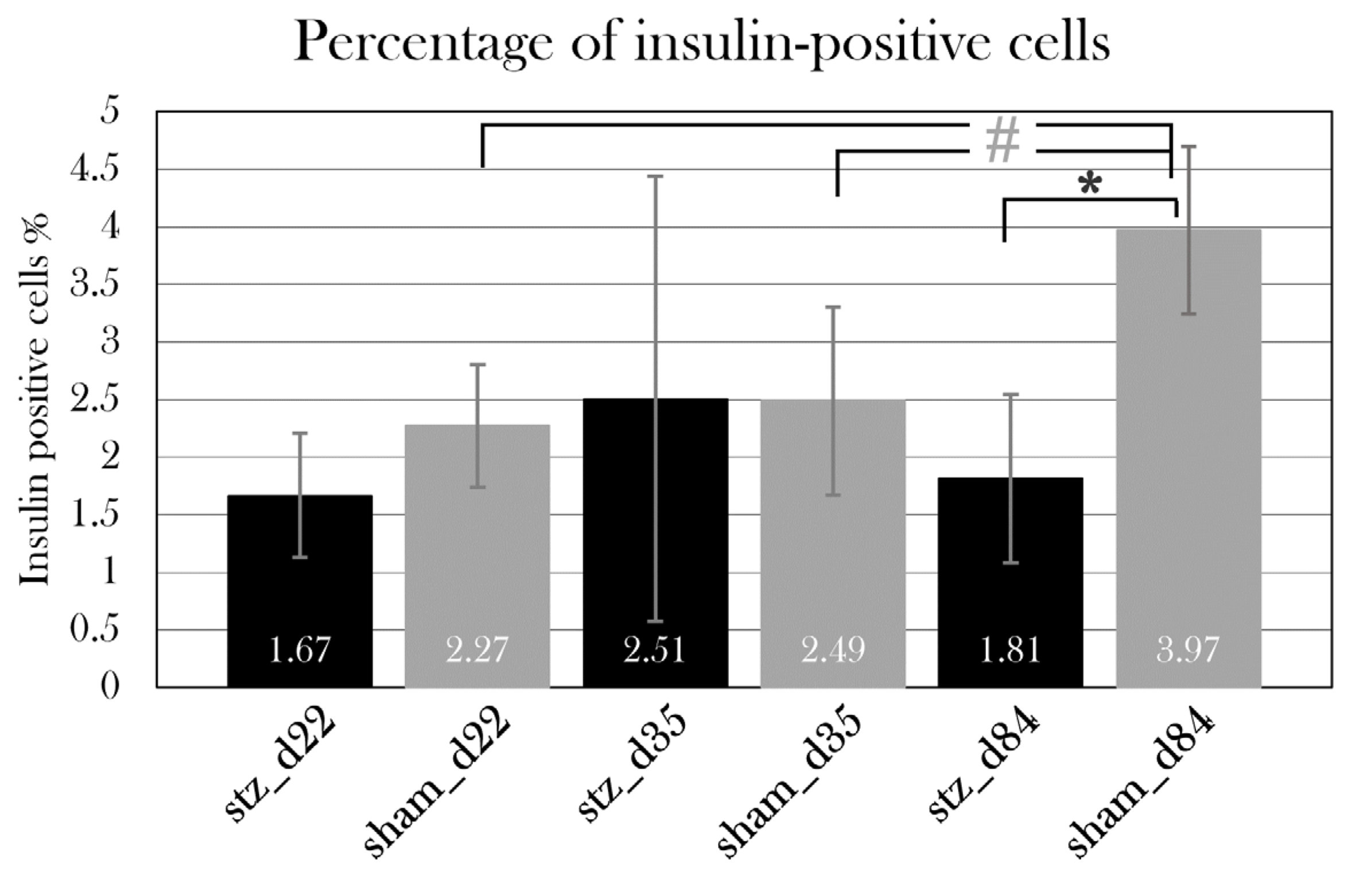
The histological examination of β cell mass further emphasized that diabetes was induced in stz_d84, as stz_d84 showed a reduced β cell mass after the STZ treatment. Interestingly, this reduction in β cell mass was no longer reflected in the blood glucose levels at this point, as fasting blood glucose levels were significantly lower in stz_d84 at day 84 than in sham_d84, indicating that stz_d84 regained full β cell function with only 45.7% of the β cell mass seen in sham_d84. Therefore, the results suggest that diabetes was induced in stz_d84, having a full disease response around day 22, followed by normalization of blood glucose levels and restoration of β cell function at day 84.
stz_d22, sham_d22, stz_d35, and sham_d35 were included in the study, but blood glucose measurements did not indicate that diabetes was induced by the STZ treatment in these animals. Fasting blood glucose levels were not significantly different when comparing STZ-treated and sham-treated groups for each day of euthanasia, and sham_d35 even displayed higher fasting blood glucose levels than stz_d35. The histological examination of β cell mass in the pancreas did not show a difference between the STZ-treated and sham-treated groups, either. These four additional groups comprised animals with great variation in size and age, whereas stz_d84 and sham_d84, including all the animals used in the pilot studies, were animals from the same batch. Therefore, it can be hypothesized that these variations in blood glucose values could be caused by the biological variations of the animals. Therefore, it would be of interest to replicate the study, but with animals from the same batch, to investigate if the variations in age and size of the animals are the cause of the results in this study being inconclusive. This signifies one of the limitations with using axolotls as an experimental animal, as they are not commonly used, and supply in great amounts with limited variations can therefore be a challenge. Another way to adjust to the problem of animal variations would be to increase the sample size, as blood glucose values showed a hyperglycemic trend, and it is possible that this would be statistically significant with a larger sample size.
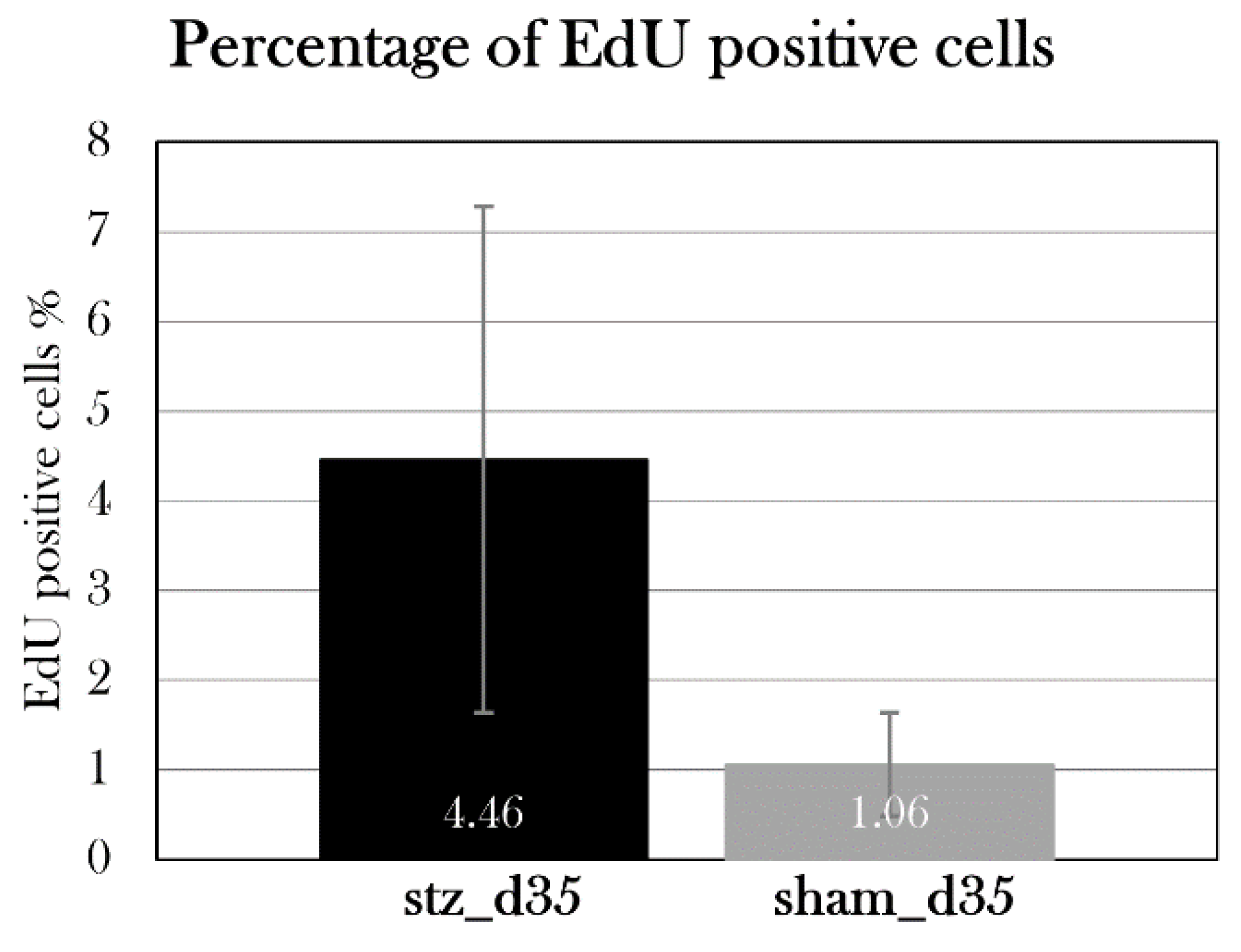
Even though stz_d35 did not display hyperglycemia, stz_d35 and sham_d35 were subjected to an EdU assay to detect eventual proliferation in the pancreas, which would be expected in a regenerative process, but the proliferating cells were scattered throughout the pancreas instead of being centered in the islets of β cells. Furthermore, even though there was a higher percentage of proliferating cells in stz_d35 than sham_d35, this was not statistically significant. This can be explained by the variations between the animals, but as the results indicate that we were not able to induce diabetes in these animals and thus not induce β cell death, we would not expect a proliferating response. Another thing to consider is the dosage of EdU for injection. Contradicting to what I did with the glucose injections, EdU injections were adjusted to the body masses of the animals one week prior to the experimental endpoint, as the animals experienced swelling shortly before the endpoint. As the glucose tolerance test showed that injected glucose was distributed in the whole tissue, unaffected by edema, EdU could have been administered accordingly, but as the EdU assay is expensive, it was adjusted as mentioned, and the staining still resulted in detectable signals in all animals.
Other histological examinations were conducted, including the TUNEL assay, which detects DNA fragmentations and would show if the STZ treatment resulted in β cell death, but this stain resulted in staining of most cells in the pancreas, indicating that the staining process failed and must be optimized for axolotl pancreas tissue. Furthermore, another β cell marker was investigated. Glucose transporter 2 is the transmembrane protein used by glucose, but also by STZ to enter β cells and induce its cytotoxic effects. An antibody was used to detect this protein, but this resulted in unspecific staining. It can be a challenge to find antibodies and kits in general that work in axolotl tissue as there are no species-specific kits on the market. This is yet another limitation of the axolotl as an experimental animal model. In future studies, it would be of interest to include more stains to detect other pancreatic cells, such as staining of glucagon to identify α cells. If the axolotl proves to be able to regenerate the pancreas, it would be of interest to identify the source of new β cells.
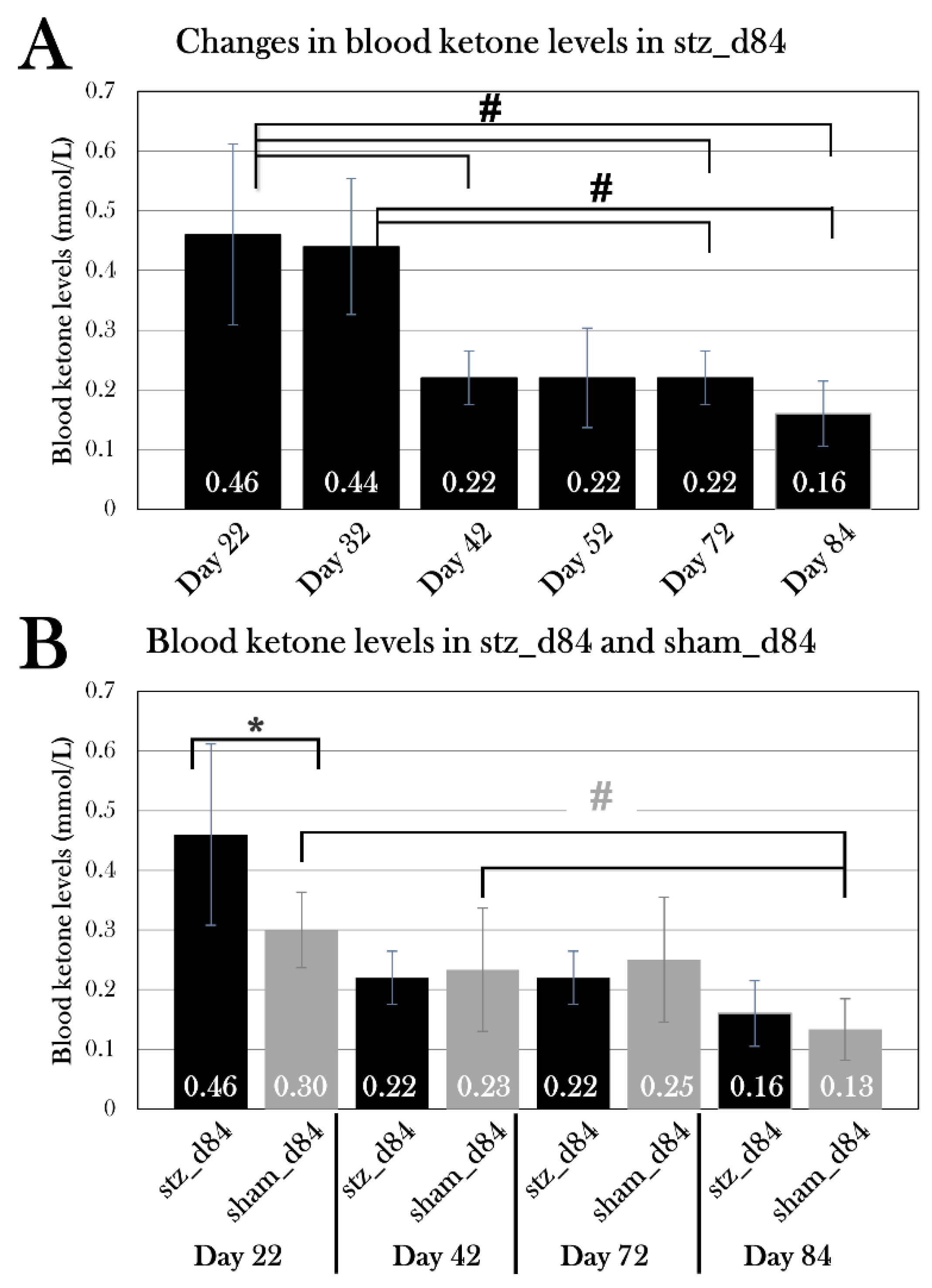
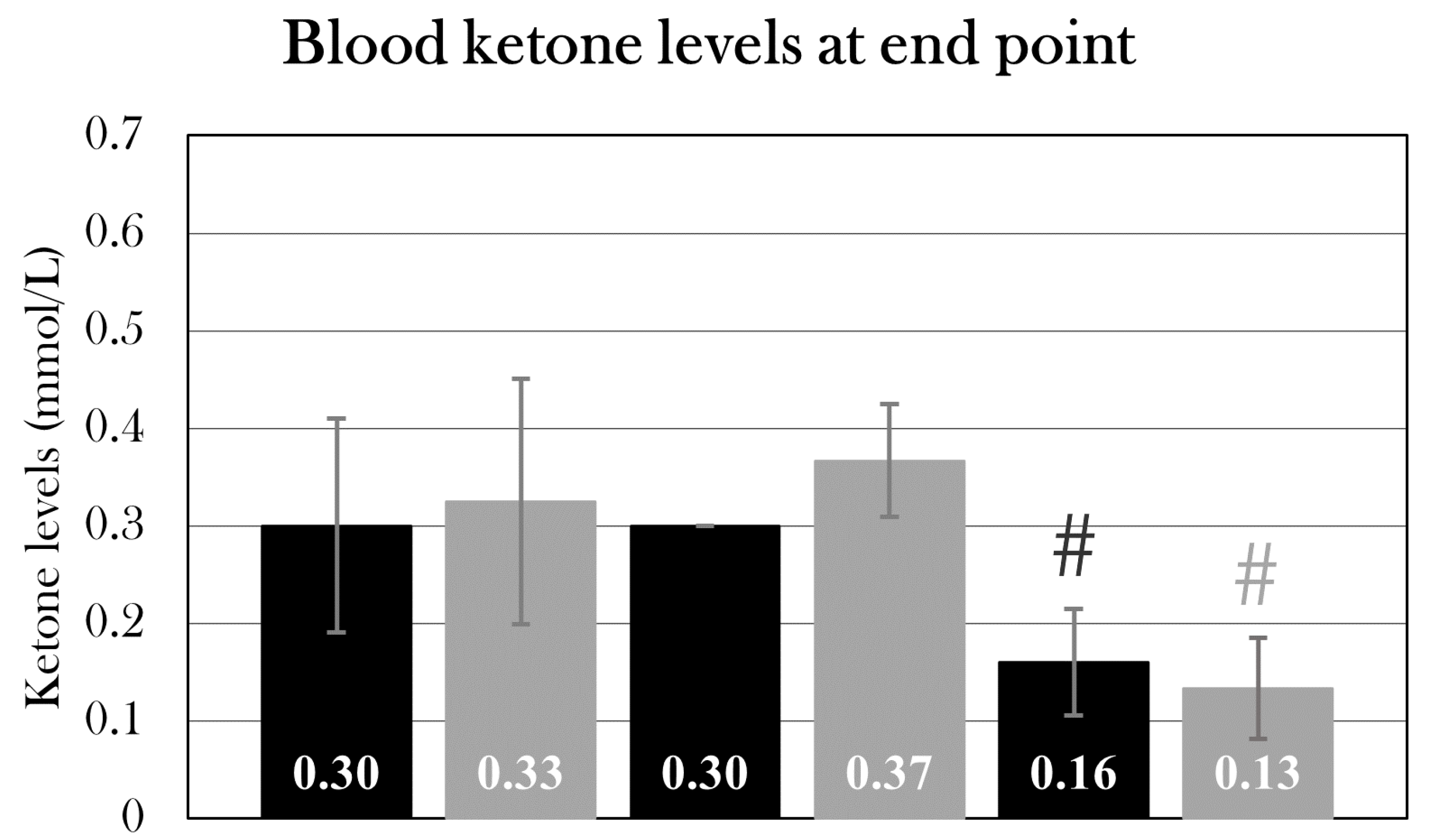
Blood ketone levels were also measured in all the groups at the endpoint, but there was no difference between the ketone values at day 22, 35, and 84 in the STZ-treated or sham-treated groups. However, the endpoint blood ketone levels were significantly lower in both stz_d84 and sham_d84 compared to the other groups of their treatment type, but this is not an effect of the STZ treatment. This can be explained again by inter-group variations, as stz_d84 and sham_d84 are from the same batch, whereas the other groups consist of animals of different ages and origins.
The continued measurements of blood ketone levels of stz_d84 and sham_d84 from day 22 to day 84 showed an initial increase of ketone bodies after the STZ treatment, which decreased over time, similar to what was observed in the glucose measurements. As with blood glucose levels, diagnostic values of blood ketone levels used in patients, with levels over 0.5 mmol/L seen in diabetic patients[
95], cannot be used in axolotls, as their metabolic rate and their primary diet differ from humans. Though, stz_d84 displayed blood ketone levels of 0.5 mmol/L at day 22, and such high levels were not seen in any of the other groups. This suggests that there was a need for another metabolic fuel, like if glucose was not available.
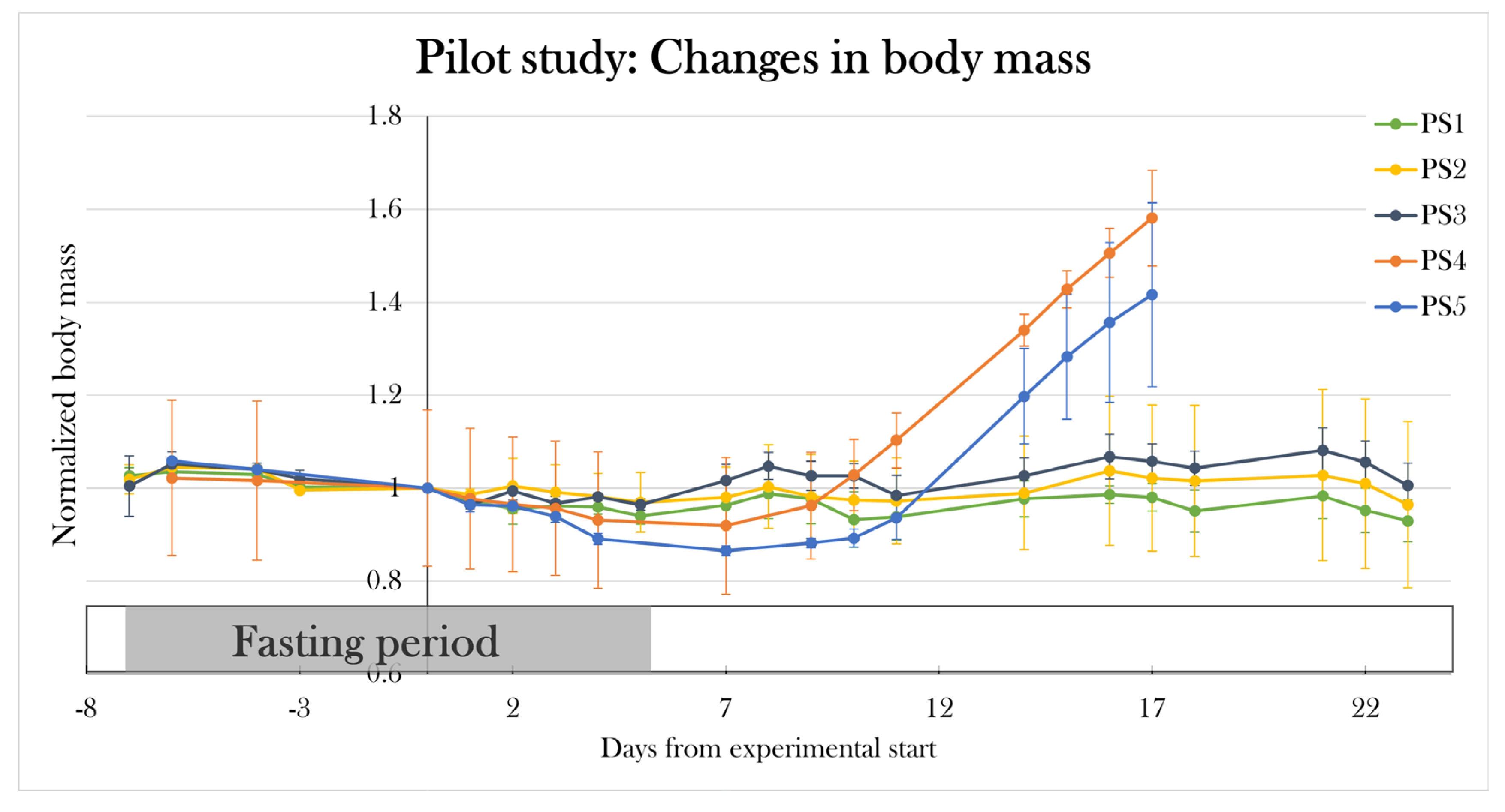
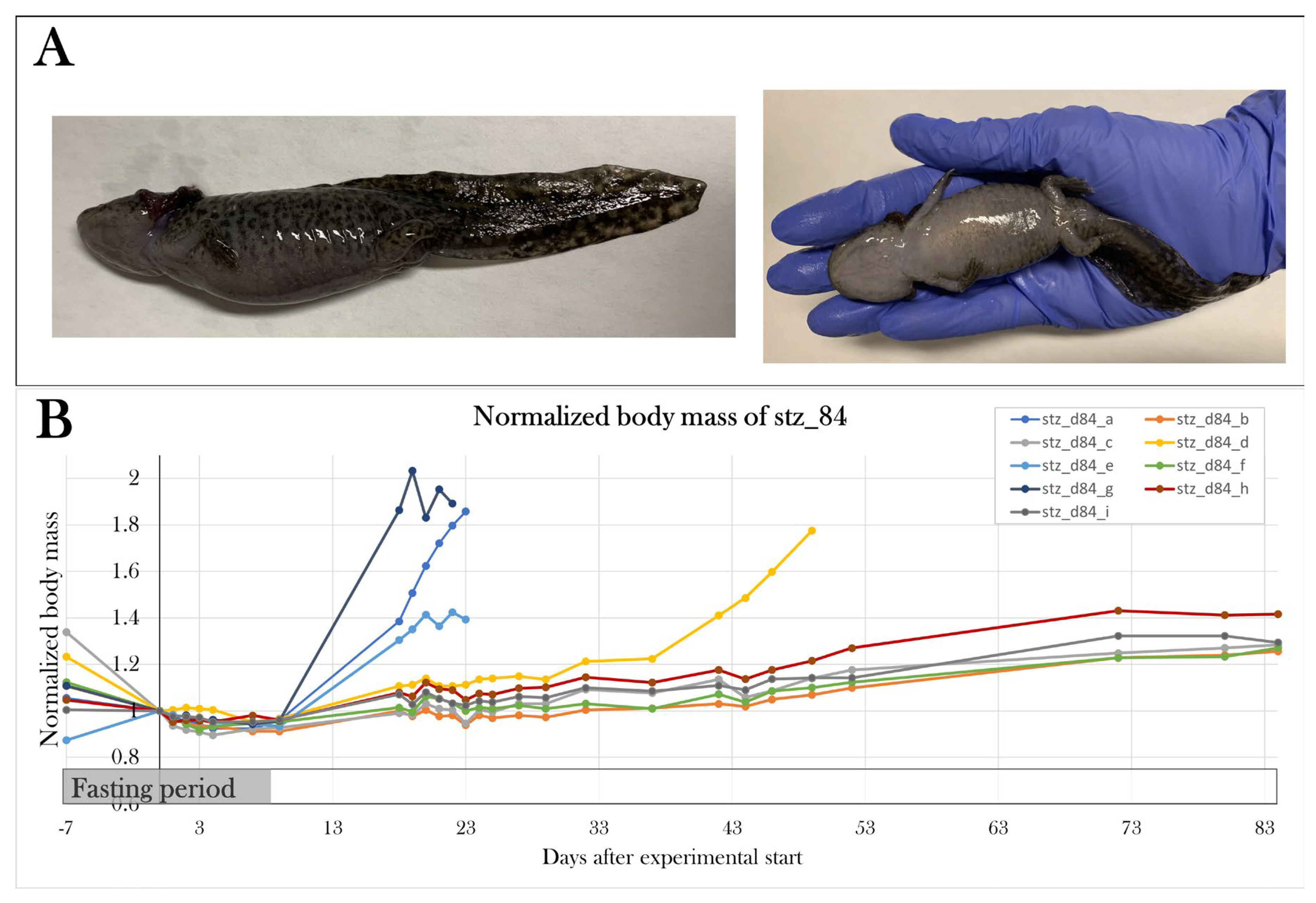
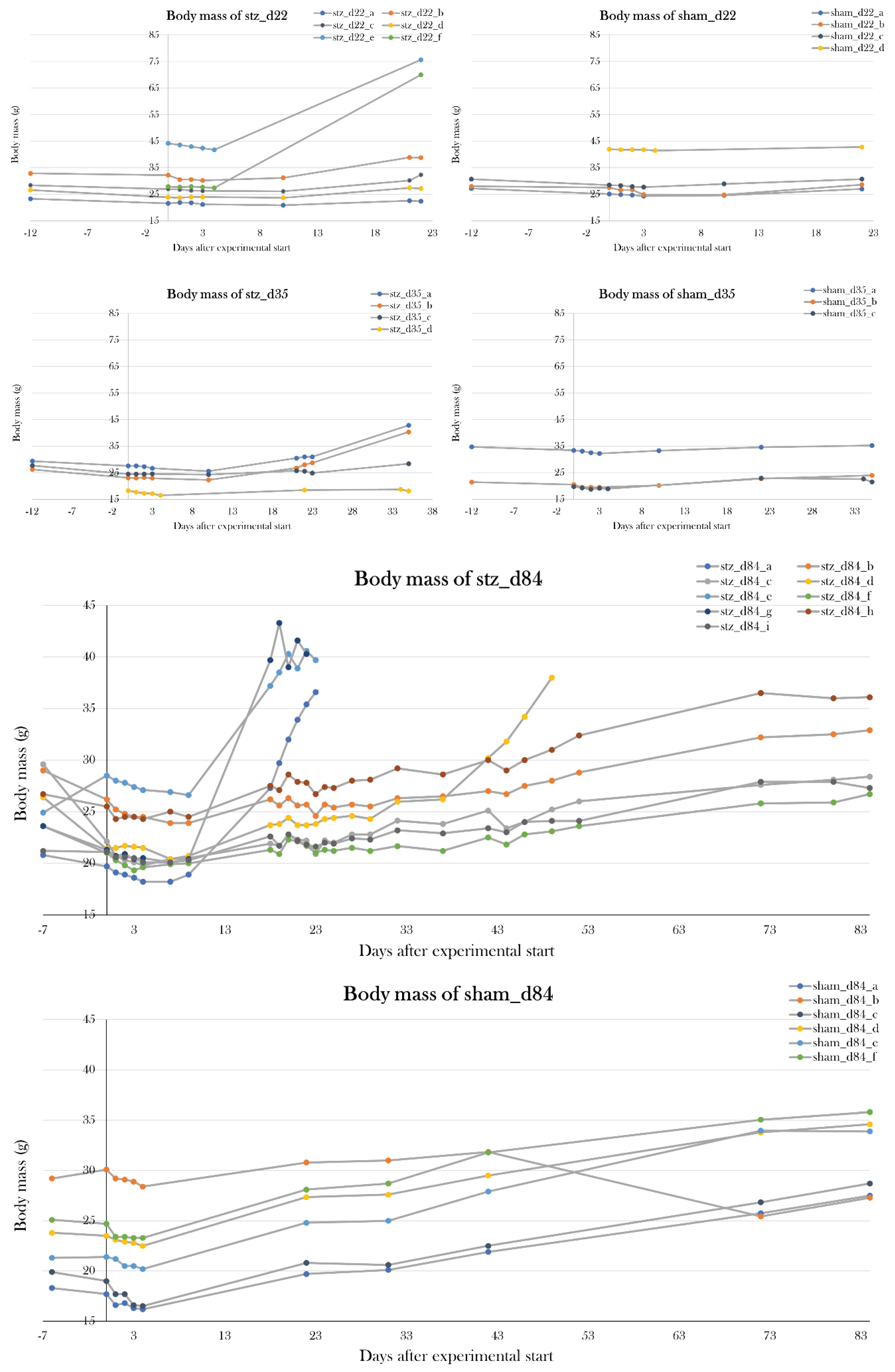
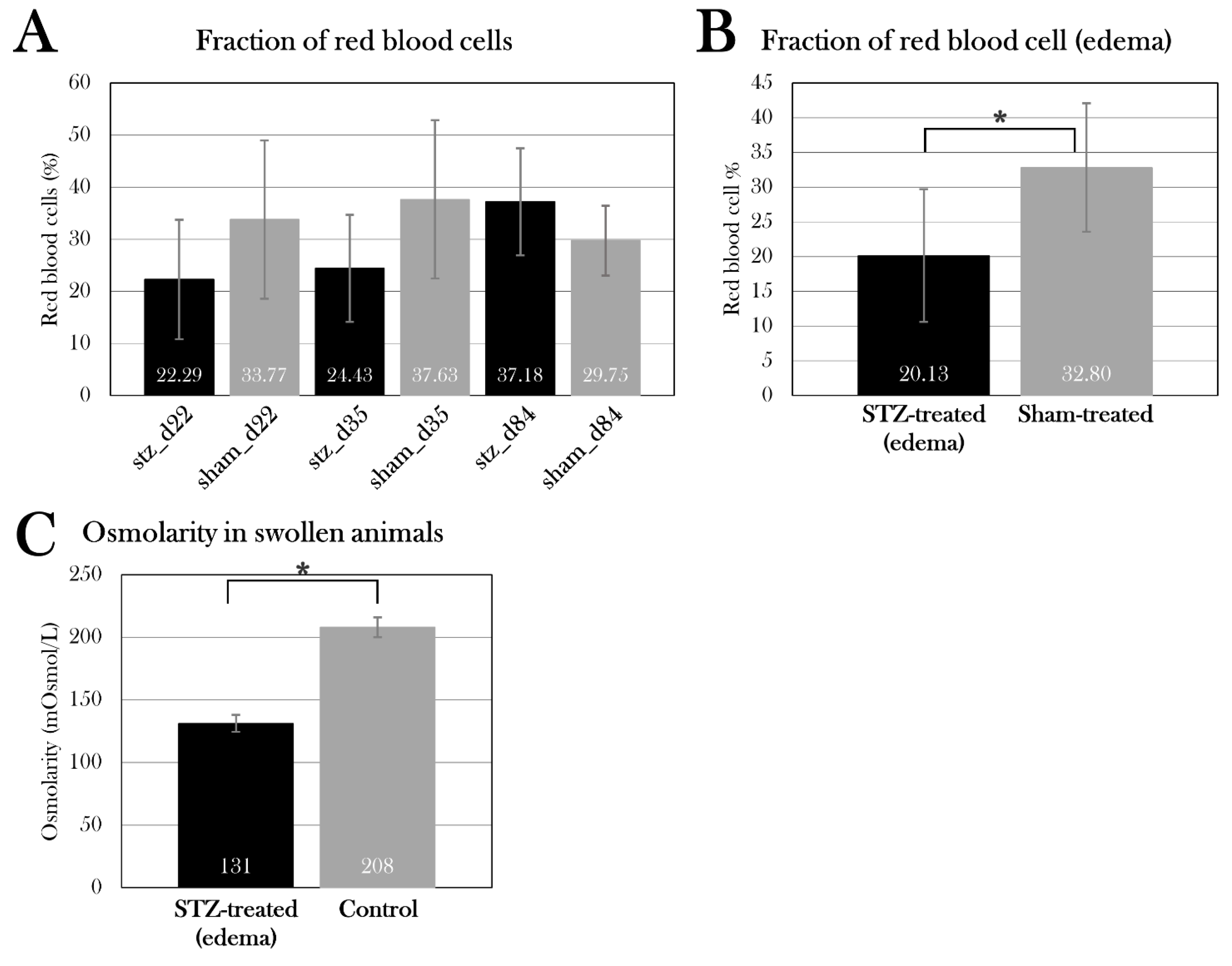
Previously, we have seen an increase in body mass caused by edema in STZ-treated axolotls[
91], but this effect was not seen in the pilot studies. However, all STZ-treated groups in the main study were affected by an increase in body mass caused by edema (
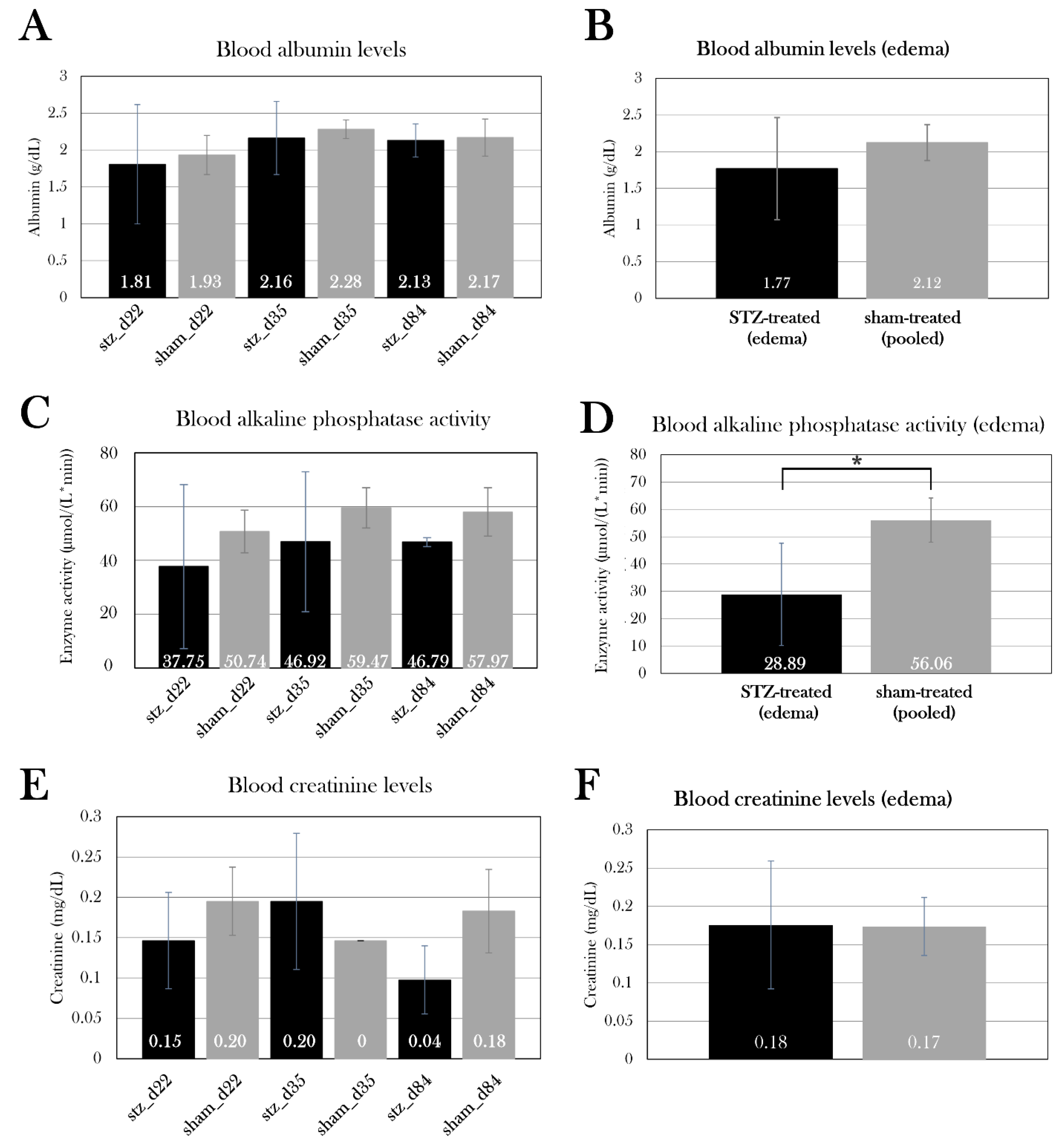
Appendix B. – Body mass of experimental animals). The excessive fluid was distributed in the whole body, both in the abdomen, pericardium, and blood, which was emphasized by a lowered osmolarity and fraction of red blood cells in edema-affected animals. To investigate the cause of edema, kidney and liver function were studied by measuring albumin concentration, creatinine concentration, and alkaline phosphatase activity in blood. There was no significant difference between the STZ-treated groups compared to the sham-treated groups, but by pooling the swollen animals in stz_d22 and stz_d35, it showed that the edema-affected animals had decreased blood alkaline phosphatase activity. Normally, liver damage is characterized by elevation of alkaline phosphatase activity in blood, and lower levels of alkaline phosphatase are less common, but this is seen in anemia, among other diseases[
96]. As swollen animals suffer from a reduced fraction of red blood cells in the blood, this could be the cause of the lowered alkaline phosphatase activity. More likely, it is an indicator of an imbalance in hematocrit values rather than liver and kidney functions.
Liver and kidney tissue have been harvested from all animals, and it will be investigated in future studies if there are any histological changes in the tissue which could indicate dysfunction, especially in the kidneys. The kidneys have a key role in maintaining the hydromineral balance in amphibians, by removing excess water from the body and minimizing ion loss[
97], and a study in larval
Ambystoma punctatum showed that the removal of the kidneys led to an expansion of the pericardial and abdominal cavities, caused by edema[
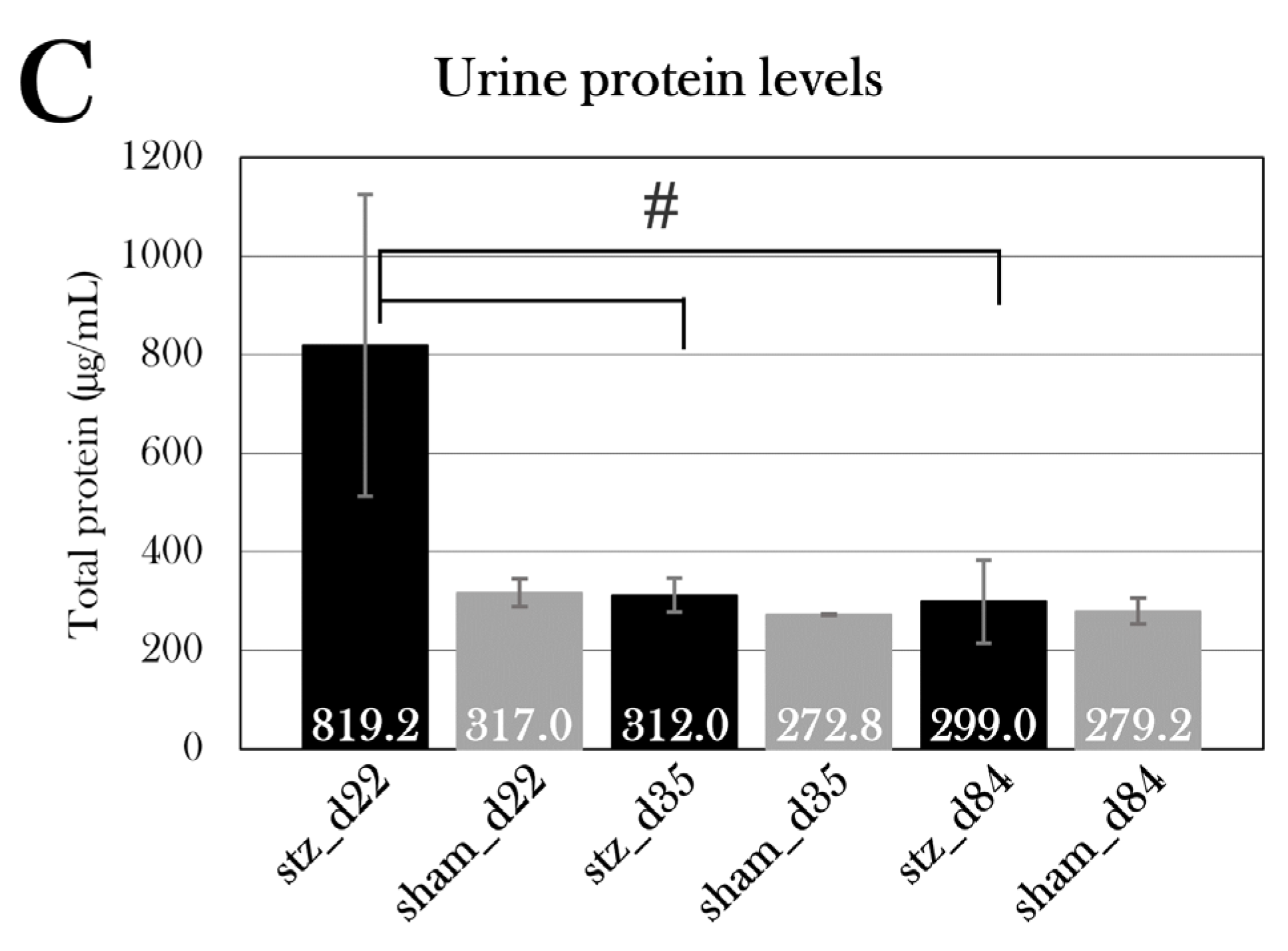
98]. Urine glucose and protein levels were measured, which also can reflect renal function, but urine was not obtained from all animals suffering from severe edema due to puncture of the bladder. Yet, stz_d22 displayed a high level of protein in the urine, but this was not statistically significant. Also, stz_d84 had a higher level of glucose in the urine than sham_d84, which could indicate renal dysfunction, but it could also be a result of the animals’ inability to reabsorb all the glucose due to the reduced β cell mass and, thus, a higher need for excretion of excessive glucose. The kidneys must be further investigated, and in future studies, the function of the kidneys could be examined by measuring the glomerular filtration rate.
Other than the kidneys, the lymph system is important for the body-fluid balance in amphibians. In amphibians, fluid is collected by small lymph capillaries and transported to subcutaneous lymph sacs, in which it is redistributed and passed along by the pumping of the lymph hearts[
99]. These lymph hearts are composed of skeletal muscle and connective tissue[
99]. Edema could be caused by dysfunction of the lymph system, specifically the lymph hearts. Glucose is an important fuel for muscle tissue, and without knowing which glucose transporter handles the uptake of glucose in the muscle tissue of the lymph hearts, it is possible that STZ can enter these cells and cause cell death. This must be investigated in future studies.
Even though it is still unknown what caused edema in some of the animals, a method to drain the animals of excessive fluid could be investigated to avoid loss of animals. However, there is a risk that drainage would be a short-term solution, and that the animals would swell up again shortly after drainage. Another solution could be to house the animals in isosmotic water (Amphibian Ringer’s solution), as our group previously has prevented edema in long-term anesthetic animals by adjusting the osmolarity of the housing water.
Other than the liver and kidneys, the heart and eyes were harvested from the animals to characterize the effect of diabetes on these organs in future studies. Using the liver, kidney, and heart, glycogen storage would be investigated, as diabetes leads to a decrease in glycogen content in the liver, but an increase in glycogen in the heart and kidney[
100]. Furthermore, the eyes were collected to study the effect on the retina by measuring the thickness of the retinal layers, inspired by a study in hyperglycemic zebrafish which showed a reduction in the retinal layers[
101].
In future studies, variations between the animals should be minimized. Variations in animals have also shown to be a liability in rodent studies of diabetes, as the effect of STZ is affected by these factors[
63]. Optimally, the animals should be of the same age, from the same batch, and preferably be leucistic or albino, as most of the edema-affected animals were of wild type and edema formation therefore could be avoided. One limitation of the axolotl as an animal model is, as mentioned, the difficulty in animal supply, especially when minimizing the variations in animals.
Another limitation in this study is the systemic effect of the STZ treatment causing edema formation in over half of the animals. It is not uncommon that STZ induces toxicity and mortality in animals[
63], but it should be avoided, if possible. In rats, a STZ dose of 0.06 mg/g causes hyperglycemia, but a dose of 0.075 mg/g results in mortality[
63]. This illustrates the narrow range of optimal STZ-induced diabetic response, which also was seen in the pilot studies where five STZ doses of 0.05 mg/g caused hyperglycemia, but five STZ doses of 0.1 mg/g caused mortality. Thus, I still believe the used STZ protocol in this study is the most optimal for axolotls, and the mortality of the animals must be limited by other factors, such as by housing them in isosmotic water, or by not using wild type animals.
As I was unable to induce diabetes in all animals, the regenerative capacity in the pancreas of the axolotl was not possible to investigate. Therefore, further studies will focus on fully establishing diabetes in the animals and thereafter investigate regeneration with a study design like this one, but with the beforementioned adjustments to minimize animal variations and edema formation.
Conclusions
This study aimed to investigate the axolotl as a novel animal model in diabetes research and to examine their potential regeneration of β cells.
For this, a method to induce diabetes in the axolotl was necessary, and a pilot study consisting of five groups receiving five different STZ treatments was conducted, showing that five consecutive STZ doses of 0.05 mg/g made the animals hyperglycemic at day 22, whereas five consecutive STZ doses of 0.1 mg/g or more resulted in edema formation and mortality. Thus, the most optimal method to induce hyperglycemia and, thus, diabetes in the animals was by five consecutive STZ doses of 0.05 mg/g.
Using this protocol, two groups were included in the main study – one receiving the STZ treatment, the other a sham treatment. The development of hyperglycemia was studied by subjecting the animals to glucose tolerance tests, assessing changes in blood glucose levels from day 22 to day 84 of the experiment. The blood glucose measurements showed that the STZ treatment resulted in severe hyperglycemia at day 22, but the animals regained glycemic control, and the blood glucose levels normalized by day 84. By histological examination of the pancreas, it showed that the STZ-treated animals had a reduced β cell mass at day 84, even though blood glucose levels were normalized. All evidence suggests that diabetes was induced in this group of STZ-treated animals (stz_d84).
With the established protocol, four groups were included to study the STZ-induced ablation of β cells and the potential regeneration of these. For this, STZ-treated and sham-treated animals were euthanized on two different days – day 22, as the previous study showed severe hyperglycemia at this point, and day 35, as blood glucose levels normalized between day 22 and day 42 in the previous study, indicating potential regeneration occurring at this point. However, neither the blood glucose measurements nor the histological examinations indicated diabetic disease, suggesting that the treatment did not have an effect on these animals, or that the great variation between the animals used in these groups (e.g., age and size) affected the measured parameters. Consequently, it was not possible to examine potential regeneration in this study, and a new study with animals from the same batch will be conducted in the future.
The systemic effects of the STZ treatment were investigated by measuring different blood and urine parameters. Over half of the STZ-treated animals experienced edema formation, resulting in a massive gain of body mass and a need for euthanasia in some animals. The edema-affected animals showed a reduced osmolarity and fraction of red blood cells in blood. Blood measurements had no indication of edema was caused by kidney or liver dysfunction, as blood creatinine and albumin levels were normal in STZ-treated animals, both with and without the swollen animals. Blood alkaline phosphatase activity was reduced in swollen animals, but this is more likely a result of the reduced hematocrit values. As for the urine parameters, it was not possible to separate the values of swollen animals from the rest of the STZ-treated animals, as the urinary bladder was punctured in many of the swollen animals. However, urine protein levels were higher in STZ-treated animals at day 22, but not significantly. Furthermore, urine glucose levels were measured, showing a higher excretion of glucose in one STZ-treated group (stz_d84). If this is caused by kidney dysfunction or the diabetic effect on these animals is difficult to determine.
Figure 1.
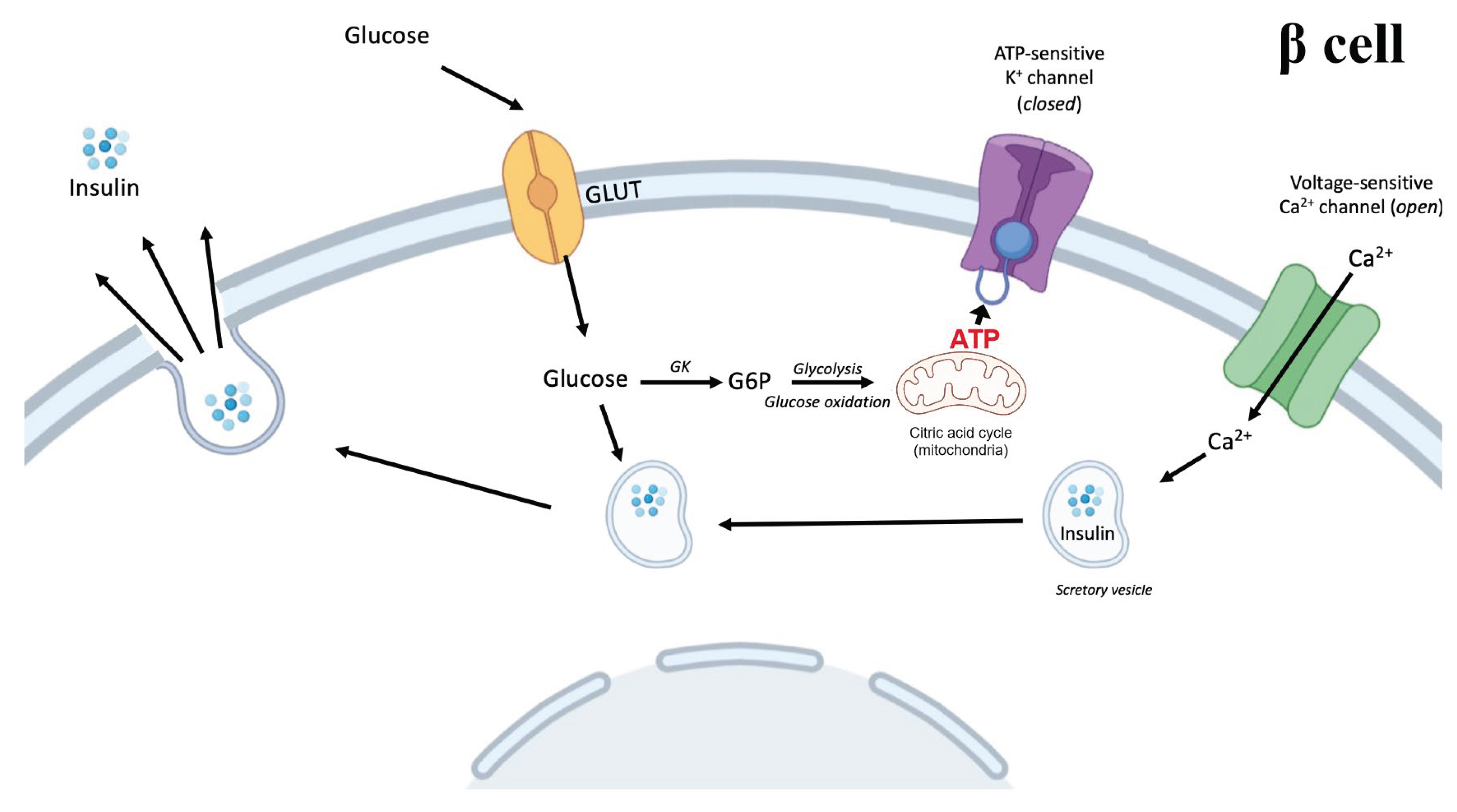
Increased circulating glucose after a meal enters β cells and enters the metabolic pathways. Through glycolysis and mitochondrial oxidation, the intracellular ATP/ADP (adenosine diphosphate) ratio increases and triggers the closing of ATP-sensitive potassium channels. This depolarizes the plasma membrane and opens voltage-sensitive calcium channels. The influx of calcium triggers exocytosis of insulin, which now can enter circulation and have its effect on target tissue such as liver, muscle, and adipose tissue. Elements of the figure are from BioRender.com.
Figure 1.
Increased circulating glucose after a meal enters β cells and enters the metabolic pathways. Through glycolysis and mitochondrial oxidation, the intracellular ATP/ADP (adenosine diphosphate) ratio increases and triggers the closing of ATP-sensitive potassium channels. This depolarizes the plasma membrane and opens voltage-sensitive calcium channels. The influx of calcium triggers exocytosis of insulin, which now can enter circulation and have its effect on target tissue such as liver, muscle, and adipose tissue. Elements of the figure are from BioRender.com.
Figure 2.
Study design of the five pilot studies (PS1-5) investigated in this thesis and a previous pilot study in axolotls performed in our group. Syringes indicate the time of STZ injection over a period of 22 days. Elements of the figure are from BioRender.com.
Figure 2.
Study design of the five pilot studies (PS1-5) investigated in this thesis and a previous pilot study in axolotls performed in our group. Syringes indicate the time of STZ injection over a period of 22 days. Elements of the figure are from BioRender.com.
Figure 3.
Schematic illustration of the study design of part III. Elements of the figure are from BioRender.com.
Figure 3.
Schematic illustration of the study design of part III. Elements of the figure are from BioRender.com.
Figure 4.
Collection of the blood, heart, liver, and kidneys. The incisions are marked with a striped line. A) Blood is collected with a syringe from the gill (G) artery vessel. B) The heart of the axolotl with the outflow tract (OT), ventricle (V), atria (A), and sinus venosus (SV). C) The liver with the right lobe (RL) and left lobe (LL). D) The axolotl kidneys.
Figure 4.
Collection of the blood, heart, liver, and kidneys. The incisions are marked with a striped line. A) Blood is collected with a syringe from the gill (G) artery vessel. B) The heart of the axolotl with the outflow tract (OT), ventricle (V), atria (A), and sinus venosus (SV). C) The liver with the right lobe (RL) and left lobe (LL). D) The axolotl kidneys.
Figure 6.
Serial cryosectioning of pancreatic tissue. The pancreas was sectioned for five different staining methods – hematoxylin, insulin/TUNEL stain, EdU stain, and two extra slides, in case of other stains were needed. Tissue was sectioned and distributed on the slides as numbered. A 7 µm section was placed on one slide, and the next 7 µm section was discarded before placing the next 7 µm section on the next slide, creating a series of neighboring sections with 7 µm between each. 2-3 slides were prepared for each staining method.
Figure 6.
Serial cryosectioning of pancreatic tissue. The pancreas was sectioned for five different staining methods – hematoxylin, insulin/TUNEL stain, EdU stain, and two extra slides, in case of other stains were needed. Tissue was sectioned and distributed on the slides as numbered. A 7 µm section was placed on one slide, and the next 7 µm section was discarded before placing the next 7 µm section on the next slide, creating a series of neighboring sections with 7 µm between each. 2-3 slides were prepared for each staining method.
Figure 9.
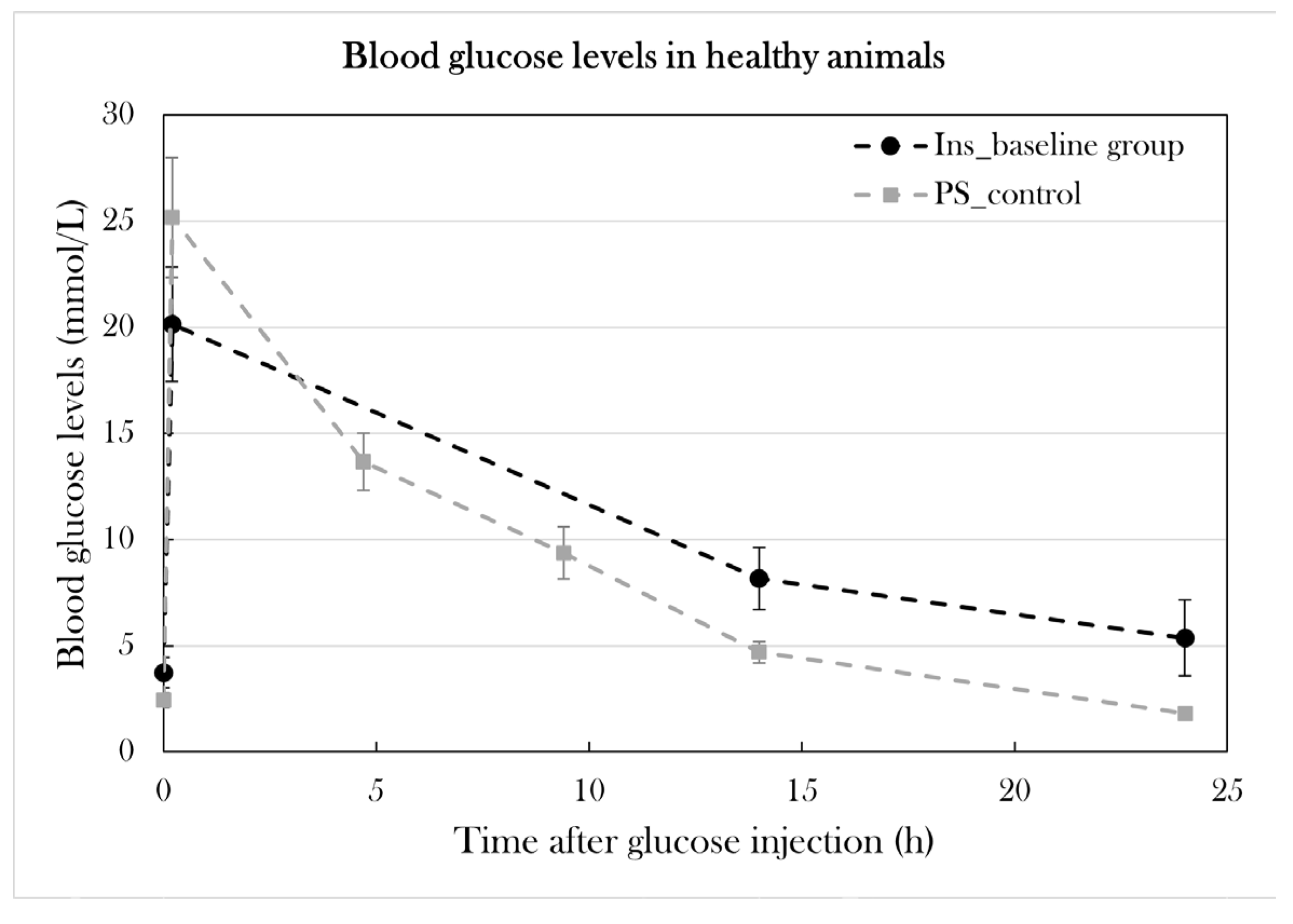
Blood glucose levels in Ins_baseline and PS_control. Plotted data show mean group values ± SD. Blood glucose levels were measured 0h, 10 min, 4h 40 min, 9h 20 min, 14h, and 24h after glucose injection in PS_control to show glucose consumption in healthy animals. To examine the relationship between glucose and insulin, blood was collected in larger quantities (30-40 µL) for 0h, 14h, and 24h after glucose injection in Ins_baseline. Blood collection in this quantity did not cause mortality or decreased glucose consumption significantly when comparing fasting blood glucose levels and blood glucose levels 24h after glucose injection (p=0.12).
Figure 9.
Blood glucose levels in Ins_baseline and PS_control. Plotted data show mean group values ± SD. Blood glucose levels were measured 0h, 10 min, 4h 40 min, 9h 20 min, 14h, and 24h after glucose injection in PS_control to show glucose consumption in healthy animals. To examine the relationship between glucose and insulin, blood was collected in larger quantities (30-40 µL) for 0h, 14h, and 24h after glucose injection in Ins_baseline. Blood collection in this quantity did not cause mortality or decreased glucose consumption significantly when comparing fasting blood glucose levels and blood glucose levels 24h after glucose injection (p=0.12).
Figure 10.
Blood glucose levels in the glucose tolerance test at day 10 and day 22 of the experiment in the pilot study. Plotted data show mean group values ± SD. An asterisk (*) indicates significant differences between a group compared to PS_control determined with a Student’s T-test (*p=0.05). A) PS1 and PS3 was subjected to the glucose tolerance test at day 10 and compared to PS_control. B) PS1, PS2, and PS3 was subjected to the glucose tolerance test and compared to PS_control.
Figure 10.
Blood glucose levels in the glucose tolerance test at day 10 and day 22 of the experiment in the pilot study. Plotted data show mean group values ± SD. An asterisk (*) indicates significant differences between a group compared to PS_control determined with a Student’s T-test (*p=0.05). A) PS1 and PS3 was subjected to the glucose tolerance test at day 10 and compared to PS_control. B) PS1, PS2, and PS3 was subjected to the glucose tolerance test and compared to PS_control.
Figure 14.
Blood ketone levels was measured over a period from day 22 to day 84 in stz_d84 and sham_d84. Plotted data show mean group values ± SD. An octothorpe (#) indicates significant differences between the blood ketone levels of stz_d84 or sham_d84 measured at different days determined with an ANOVA test (#p=0.05). An asterisk (*) indicates significant differences determined with a Student’s T-test (*p=0.05). A) Blood ketone levels of stz_d84 at day 22, 32, 42, 52, 72, and 84. The blood ketone levels decreased significantly from day 22 to day 84. B) Blood ketone levels of stz_d84 (black) and sham_d84 (grey) on day 22, 42, 72, and 84. Blood ketone levels were significantly higher at day 22 in stz_d84 compared to sham_d84.
Figure 14.
Blood ketone levels was measured over a period from day 22 to day 84 in stz_d84 and sham_d84. Plotted data show mean group values ± SD. An octothorpe (#) indicates significant differences between the blood ketone levels of stz_d84 or sham_d84 measured at different days determined with an ANOVA test (#p=0.05). An asterisk (*) indicates significant differences determined with a Student’s T-test (*p=0.05). A) Blood ketone levels of stz_d84 at day 22, 32, 42, 52, 72, and 84. The blood ketone levels decreased significantly from day 22 to day 84. B) Blood ketone levels of stz_d84 (black) and sham_d84 (grey) on day 22, 42, 72, and 84. Blood ketone levels were significantly higher at day 22 in stz_d84 compared to sham_d84.
Figure 16.
Blood ketone levels of the STZ-treated and sham-treated groups at the endpoint. Plotted data show mean group values ± SD. An octothorpe (#) indicates significant differences between the blood ketone levels of either STZ-treated or sham-treated groups determined with an ANOVA test and a pairwise T-Test(#p=0.05). There was no difference between the STZ-treated and sham-treated groups at day 22, 35, or 84, respectively, but both stz_d84 and sham_d84 displayed lower blood ketone levels when comparing the STZ-treated groups and sham-treated groups separately.
Figure 16.
Blood ketone levels of the STZ-treated and sham-treated groups at the endpoint. Plotted data show mean group values ± SD. An octothorpe (#) indicates significant differences between the blood ketone levels of either STZ-treated or sham-treated groups determined with an ANOVA test and a pairwise T-Test(#p=0.05). There was no difference between the STZ-treated and sham-treated groups at day 22, 35, or 84, respectively, but both stz_d84 and sham_d84 displayed lower blood ketone levels when comparing the STZ-treated groups and sham-treated groups separately.
Figure 18.
Percentage of proliferating cells in the pancreas of stz_d35 and sham_d35. For each animal, four pancreas sections were EdU-stained, and the percentage of EdU-positive cells was calculated, and the mean for all section was used as the value for the animal. Plotted data show mean group values ± SD (p=0.05). There was no significant difference between the groups.
Figure 18.
Percentage of proliferating cells in the pancreas of stz_d35 and sham_d35. For each animal, four pancreas sections were EdU-stained, and the percentage of EdU-positive cells was calculated, and the mean for all section was used as the value for the animal. Plotted data show mean group values ± SD (p=0.05). There was no significant difference between the groups.
Figure 21.
The effect of STZ on the fraction of red blood cells and osmolarity in blood. Plotted data show mean group values ± SD. An asterisk (*) indicates significant differences determined with a Student’s T-test (*p=0.05). A) The fraction of red blood cells in blood did not differ between the STZ-treated and sham-treated groups. B) The fraction of red blood cells was significantly lower in STZ-treated animals affected by edema compared to all sham-treated animals. C) The osmolarity was measured in STZ-treated animals affected by edema, and they were hypoosmolar when comparing to the blood osmolarity measured in healthy axolotls (control).
Figure 21.
The effect of STZ on the fraction of red blood cells and osmolarity in blood. Plotted data show mean group values ± SD. An asterisk (*) indicates significant differences determined with a Student’s T-test (*p=0.05). A) The fraction of red blood cells in blood did not differ between the STZ-treated and sham-treated groups. B) The fraction of red blood cells was significantly lower in STZ-treated animals affected by edema compared to all sham-treated animals. C) The osmolarity was measured in STZ-treated animals affected by edema, and they were hypoosmolar when comparing to the blood osmolarity measured in healthy axolotls (control).
Figure 22.
Albumin, creatinine, and alkaline phosphatase activity in the blood of STZ-treated and sham-treated groups. Plotted data show mean group values ± SD. An asterisk (*) indicates significant differences determined with a Student’s T-test (*p=0.05). A) Albumin levels (A), creatinine levels (C), and alkaline phosphatase activity (E) were measured in the STZ-treated groups and compared to the sham-treated groups. There was no difference between the groups. STZ-treated animals affected by edema did not differ from sham-treated animals in blood albumin levels (B) or blood creatinine levels (E), but they had lower alkaline phosphatase activity (D), which could be caused by the lowered fraction of red blood cells in the blood of these animals.
Figure 22.
Albumin, creatinine, and alkaline phosphatase activity in the blood of STZ-treated and sham-treated groups. Plotted data show mean group values ± SD. An asterisk (*) indicates significant differences determined with a Student’s T-test (*p=0.05). A) Albumin levels (A), creatinine levels (C), and alkaline phosphatase activity (E) were measured in the STZ-treated groups and compared to the sham-treated groups. There was no difference between the groups. STZ-treated animals affected by edema did not differ from sham-treated animals in blood albumin levels (B) or blood creatinine levels (E), but they had lower alkaline phosphatase activity (D), which could be caused by the lowered fraction of red blood cells in the blood of these animals.
Figure 24.
Urine protein levels in all the groups. Plotted data show mean group values ± SD. An octothorpe (#) indicates significant differences between either STZ-treated (black) or sham-treated (grey) groups determined with an ANOVA test (#p=0.05). Comparison of the STZ-treated group to the respective sham-treated group for each day showed no statistically significant difference in urine protein levels, even though stz_d22 showed higher urine protein levels than sham_d22. A one factor ANOVA test showed a significant difference between stz_d22 and stz_d35 and stz_d84.
Figure 24.
Urine protein levels in all the groups. Plotted data show mean group values ± SD. An octothorpe (#) indicates significant differences between either STZ-treated (black) or sham-treated (grey) groups determined with an ANOVA test (#p=0.05). Comparison of the STZ-treated group to the respective sham-treated group for each day showed no statistically significant difference in urine protein levels, even though stz_d22 showed higher urine protein levels than sham_d22. A one factor ANOVA test showed a significant difference between stz_d22 and stz_d35 and stz_d84.
Table 1.
Key physiological similarities and differences between humans, mice, zebrafish, and amphibians.
Table 1.
Key physiological similarities and differences between humans, mice, zebrafish, and amphibians.
| |
Adult humans |
Mice |
Zebrafish |
Amphibian |
| β cells are the predominant endocrine cell type in the pancreas |
Yes |
Yes |
Yes |
Yes |
| β cells mass necessary for normal function |
>40-60% |
>10-30% |
Near
total* |
? |
| Loss of substantial β cells mass results in hyperglycemia |
Yes |
Yes |
Yes |
Yes |
| Capable of substantial regeneration of pancreatic tissue |
No |
Limited |
Yes |
Yes |
| Glucose transporter 2 is the primary glucose transporter in β cells |
No |
Yes |
Yes |
Unknown |
| Primary diet |
Omnivore |
Herbivore |
Omnivore |
Carnivore |