Submitted:
30 January 2023
Posted:
31 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sampling sites
2.2. Sampling of horse chestnut leaves
2.3. Collection and identification of phytoseiid mites
2.4. Data presentation and statistical analysis
3. Results
3.1. Species composition
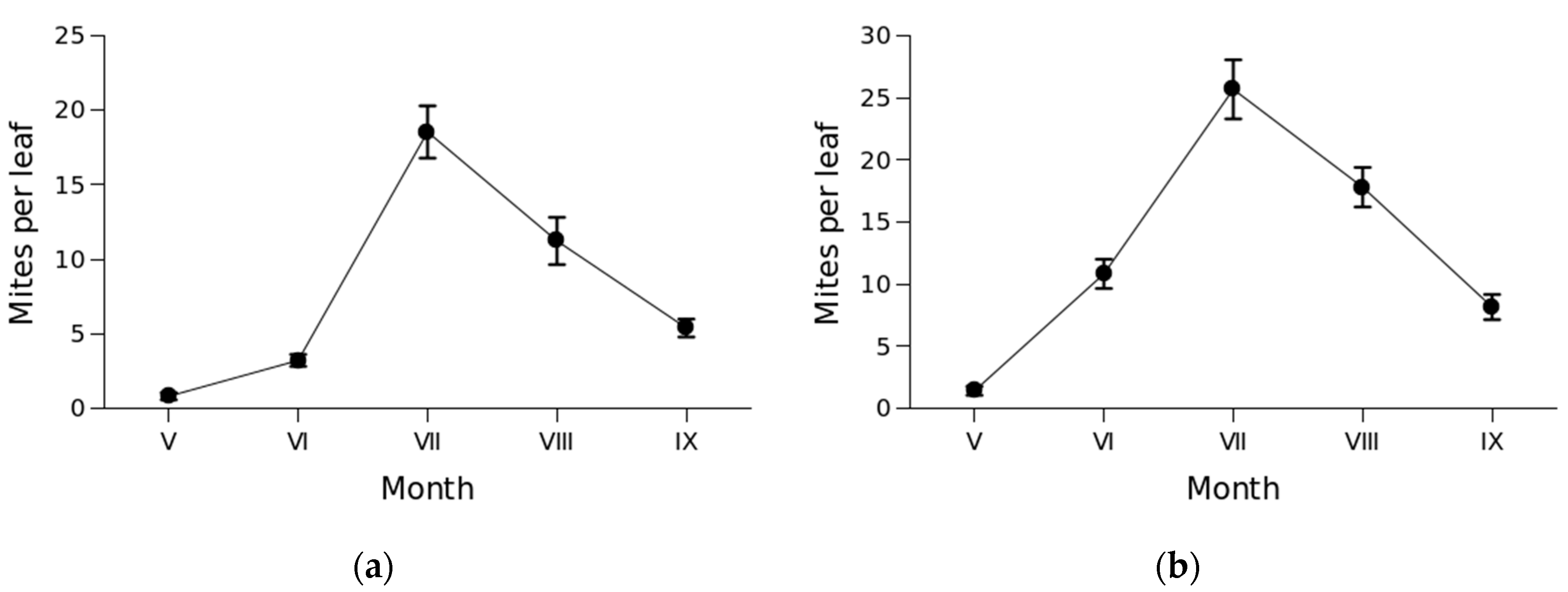
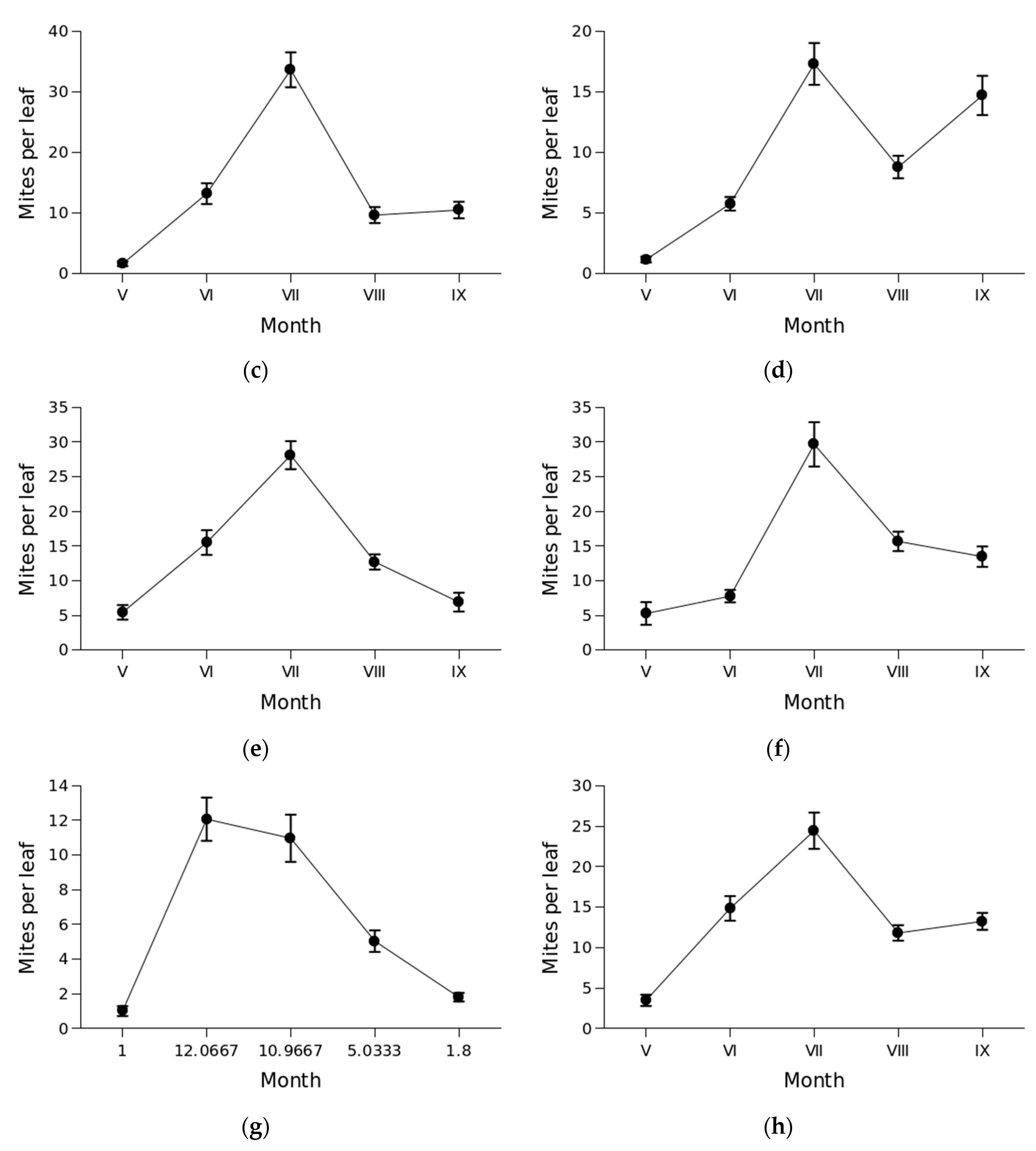
3.2. Mite abundance and seasonal dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, P.A.; Alhamd, O.; Iszkuło, G.; Dering, M.; Mukassabi, T.A. Biological Flora of the British Isles: Aesculus hippocastanum. J. Ecol. 2019, 107, 992–1030. [Google Scholar] [CrossRef]
- Ulbricht, C.; Tiffany, N.; Boon, H.; Ulbricht, C.; Basch, E.; Bent, S.; Barrette, E.P.; Smith, M.; Sollars, D.; Dennehy, C.; et al. Horse Chestnut: A Multidisciplinary Clinical Review. Journal of Herbal Pharmacotherapy 2002, 2, 71–85. [Google Scholar] [CrossRef]
- Somme, L.; Moquet, L.; Quinet, M.; Vanderplanck, M.; Michez, D.; Lognay, G.; Jacquemart, A.-L. Food in a Row: Urban Trees Offer Valuable Floral Resources to Pollinating Insects. Urban Ecosyst 2016, 19, 1149–1161. [Google Scholar] [CrossRef]
- Tuovinen, T.; Rokx, J.A.H. Phytoseiid Mites (Acari: Phytoseiidae) on Apple Trees and in Surrounding Vegetation in Southern Finland. Densities and Species Composition. Exp Appl Acarol 1991, 12, 35–46. [Google Scholar] [CrossRef]
- Tuovinen, T. Influence of Surrounding Trees and Bushes on the Phytoseiid Mite Fauna on Apple Orchard Trees in Finland. Agriculture, Ecosystems & Environment 1994, 50, 39–47. [Google Scholar] [CrossRef]
- Kopačka, M.; Stathakis, T.I.; Broufas, G.; Papadoulis, G.Th.; Zemek, R. Diversity and Abundance of Phytoseiidae (Acari: Mesostigmata) on Horse Chestnut (Aesculus hippocastanum L.) in an Urban Environment: A Comparison between Greece and the Czech Republic. Acarologia 2018, 58, 83–90. [Google Scholar] [CrossRef]
- Kabíček, J.; Řeháková, M. Phytoseiid Mite Community on Aesculus Hippocastanum in the Parks. Acta Fytotechnica et Zootechnica 2004, 7, 114–115. [Google Scholar]
- Kopacka, M.; Zemek, R. Spatial Variability in the Level of Infestation of the Leaves of Horse Chestnut by the Horse Chestnut Leaf Miner, Cameraria ohridella (Lepidoptera: Gracillariidae) and in the Number of Adult Moths and Parasitoids Emerging from Leaf Litter in an Urban Environment. Eur. J. Entomol. 2017, 114, 42–52. [Google Scholar] [CrossRef]
- Jura, S. Určování Stáří Stromů. Silva Bohemica 1, 19–21.
- Zacharda, M.; Pultar, O.; Muška, J. Washing Technique for Monitoring Mites in Apple Orchards. Exp Appl Acarol 1988, 5, 181–183. [Google Scholar] [CrossRef]
- Beglyarov, G.A. Opredelitel chiscnych klescej fitoseiid (Parasitiformes, Phytoseiidae) fauny SSSR. Info Bjull IOBC 1981, 3, 1–45. [Google Scholar]
- Miedema, E. Survey of Phytoseiid Mites (Acari: Phytoseiidae) in Orchards and Surrounding Vegetation of Northwestern Europe, Especially in the Netherlands. Keys, Descriptions and Figures. Netherlands Journal of Plant Pathology 1987, 93, 1–63. [Google Scholar] [CrossRef]
- Chant, D.A.; Shaul, E.Y. A World Review of the Soleiger Species Group in the Genus Typhlodromus Scheuten (Acarina: Phytoseiidae). Can. J. Zool. 1982, 60, 3021–3032. [Google Scholar] [CrossRef]
- Chant, D.A.; Yoshida-Shaul, E. A World Review of the Tiliarum Species Group in the Genus Typhlodromus Scheuten (Acari: Phytoseiidae). Can. J. Zool. 1989, 67, 1006–1046. [Google Scholar] [CrossRef]
- Karg, W. Acari (Acarina), Milben, Parasitiformes (Anactinochaeta), Cohors Gamasina Leach, Raubmilben, Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise, 2nd ed.; VEB Gustav Fischer Verlag: Jena, 1993. [Google Scholar]
- SAS Institute SAS/STAT 14. 3: User’s Guide.; SAS Institute: Cary, NC, USA, 2017. [Google Scholar]
- Adams, J.S. Species Richness: Patterns in the Diversity of Life; Springer-Praxis books in environmental sciences; Springer ; in Association with Praxis: Berlin ; New York : Chichester, UK, 2009; ISBN 978-3-540-74277-7. [Google Scholar]
- Dajoz, R. Introduction to Ecology; Hodder and Stoughton: London, 1977; ISBN 978-0-340-16254-5. [Google Scholar]
- Ragusa di Chiara, S.; Papaioannou-Souliotis, P.; Tsolakis, H.; Tsagkarakou, N. Acari Fitoseidi (Parasitiformes, Phytoseiidae) Della Grecia Associati a Piante Forestali a Diverse Altitudini. Boll. Zool. Agr. Bachicol. 1995, 27, 85–91. [Google Scholar]
- McMurtry, J.A.; Croft, B.A. Life-Styles of Phytoseiid Mites and Their Roles in Biological Control. Annu. Rev. Entomol. 1997, 42, 291–321. [Google Scholar] [CrossRef]
- Kabíček, J. Broad Leaf Trees as Reservoirs for Phytoseiid Mites (Acari: Phytoseiidae). Plant Prot. Sci. 2003, 39, 65–69. [Google Scholar] [CrossRef]
- Kabíček, J. Intraleaf Distribution of the Phytoseiid Mites (Acari, Phytoseiidae) on Several Species of Wild Broad Leaf Trees. Biologia 2005, 60, 523–528. [Google Scholar]
- Kabíček, J. Linden Trees Are Favourable Host Plants for Phytoseiid Generalists in Urban Environment. BALT FOR 2019, 25. [Google Scholar] [CrossRef]
- Kabíček, J.; Povondrová, K. Phytoseiid Mite Communities on Urban Deciduous Trees. Acta Fytotechnica et Zootechnica 2004, 7, 119–121. [Google Scholar]
- Omeri, I. Phytoseiid Mites (Parasitiformes, Phytoseiidae) on Plants in Trostyanets Dendrological Park (Ukraine). Vestnik Zoologii 2009, 43, e–7. [Google Scholar] [CrossRef]
- Barbar, Z. Occurrence, Population Dynamics and Winter Phenology of Spider Mites and Their Phytoseiid Predators in a Citrus Orchard in Syria. Acarologia 2014, 54, 409–423. [Google Scholar] [CrossRef]
- Grabovska, S.L.; Kolodochka, L.A. Species Complexes of Predatory Phytoseiid Mites (Parasitiformes, Phytoseiidae) in Green Urban Plantations of Uman’ (Ukraine). Vestnik Zoologii 2014, 48, 495–502. [Google Scholar] [CrossRef]
- Vieira de Souza, I.; Argolo, P.S.; Gondim Júnior, M.G.C.; de Moraes, G.J.; Bittencourt, M.A.L.; Oliveira, A.R. Phytoseiid Mites from Tropical Fruit Trees in Bahia State, Brazil (Acari, Phytoseiidae). ZK 2015, 533, 99–131. [Google Scholar] [CrossRef]
- Grabovska, S.L.; Mykolaiko, I.I. Кліщі Рoдини Phytoseiidae (Acari, Parasitiformes) в Урбанізoваних Рoслинних Насадженнях. Ukr J Ecol 2017, 7, 216–222. [Google Scholar] [CrossRef]
- Grabovska, S.L.; Mykolaiko, I.I.; Mykolaiko, V.P. Осoбливoсті Структури Кoмплексів Фітoсеїдних Кліщів в Рoслинних Асoціаціях Міст. Ukr J Ecol 2017, 7, 179–186. [Google Scholar] [CrossRef]
- Stojnić, B.; Mladenović, K.; Marčić, D. Spider Mites and Predatory Mites (Acari: Tetranychidae, Phytoseiidae) on Stone Fruit Trees ( Prunus Spp.) in Serbia. International Journal of Acarology 2018, 44, 322–329. [Google Scholar] [CrossRef]
- Ripka, G. Checklist of the Phytoseiidae of Hungary (Acari: Mesostigmata). Folia Entomologica Hungarica 2006, 67, 229–260. [Google Scholar]
- Ripka, G. New Data to the Knowledge on the Phytoseiid Fauna in Hungary (Acari: Mesostigmata). Acta Phytopathologica et Entomologica Hungarica 1998, 33, 395–405. [Google Scholar]
- Komlovszky, S.Z.I.; Jenser, G. The frequent occurrence ofthe predatory mites Amblyseius finlandicus Oudemans and Phytoseius plumifer. Növényvédelem 1987, 23, 193–201. [Google Scholar]
- Gyenis, K.; Pénzes, B.; Hegyi, T. Phytophagous and predatory mites on the horse chestnut tree. Növényvédelem 2005, 41, 143–148. [Google Scholar]
- Ripka, G.; de Lillo, E. New Data to the Knowledge on the Eriophyoid Fauna in Hungary (Acari: Eriophyoidea). Folia Entomologica Hungarica 1997, 58, 147–157. [Google Scholar]
- Karban, R.; English-Loeb, G.; Walker, M.A.; Thaler, J. Abundance of Phytoseiid Mites on Vitis Species: Effects of Leaf Hairs, Domatia, Prey Abundance and Plant Phylogeny. Exp Appl Acarol 1995, 19, 189–197. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Haratym, W. Leaf Micromorphology of Aesculus Hippocastanum L. and Damage Caused by Leaf-Mining Larvae of Cameraria Ohridella Deschka and Dimić. Acta Agrobot 2012, 65, 25–34. [Google Scholar] [CrossRef]
- Muhammad, S.; Wuyts, K.; Samson, R. Atmospheric Net Particle Accumulation on 96 Plant Species with Contrasting Morphological and Anatomical Leaf Characteristics in a Common Garden Experiment. Atmospheric Environment 2019, 202, 328–344. [Google Scholar] [CrossRef]
- Overmeer, W.P.J.; Zon, A.Q. The Preference of Amblyseius Potentillae (Garman) (Acarina: Phytoseiidae) for Certain Plant Substrates.; Griffiths, D., Bowman, C., Eds.; Ellis Horwood Limited, Chichester: Chichester, 1984; pp. 591–596. [Google Scholar]
- O’Dowd, D.J.; Willson, M.F. Leaf Domatia and Mites on Australasian Plants: Ecological and Evolutionary Implications. Biological Journal of the Linnean Society 1989, 37, 191–236. [Google Scholar] [CrossRef]
- Walter, D.E.; O’Dowd, D.J. Leaves with Domatia Have More Mites. Ecology 1992, 73, 1514–1518. [Google Scholar] [CrossRef]
- Kreiter, S.; Tixier, M.-S.; Croft, B.A.; Auger, P.; Barret, D. Plants and Leaf Characteristics Influencing the Predaceous Mite Kampimodromus Aberrans (Acari: Phytoseiidae) in Habitats Surrounding Vineyards. Environ Entomol 2002, 31, 648–660. [Google Scholar] [CrossRef]
- Matos, C.H.C.; Pallini, A.; Chaves, F.F.; Schoereder, J.H.; Janssen, A. Do Domatia Mediate Mutualistic Interactions between Coffee Plants and Predatory Mites? Entomologia Experimentalis et Applicata 2006, 118, 185–192. [Google Scholar] [CrossRef]
- Loughner, R.; Goldman, K.; Loeb, G.; Nyrop, J. Influence of Leaf Trichomes on Predatory Mite (Typhlodromus Pyri) Abundance in Grape Varieties. Exp Appl Acarol 2008, 45, 111–122. [Google Scholar] [CrossRef]
- Loughner, R.; Wentworth, K.; Loeb, G.; Nyrop, J. Leaf Trichomes Influence Predatory Mite Densities through Dispersal Behavior. Entomologia Experimentalis et Applicata 2010, 134, 78–88. [Google Scholar] [CrossRef]
- Loughner, R.; Wentworth, K.; Loeb, G.; Nyrop, J. Influence of Leaf Trichomes on Predatory Mite Density and Distribution in Plant Assemblages and Implications for Biological Control. Biological Control 2010, 54, 255–262. [Google Scholar] [CrossRef]
- O’Connell, D.M.; Lee, W.G.; Monks, A.; Dickinson, K.J.M. Does Microhabitat Structure Affect Foliar Mite Assemblages? Ecological Entomology 2010, 35, 317–328. [Google Scholar] [CrossRef]
- Schmidt, R.A. Leaf Structures Affect Predatory Mites (Acari: Phytoseiidae) and Biological Control: A Review. Exp Appl Acarol 2014, 62, 1–17. [Google Scholar] [CrossRef]
- Barba, P.; Loughner, R.; Wentworth, K.; Nyrop, J.P.; Loeb, G.M.; Reisch, B.I. A QTL Associated with Leaf Trichome Traits Has a Major Influence on the Abundance of the Predatory Mite Typhlodromus Pyri in a Hybrid Grapevine Population. Hortic Res 2019, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Nyrop, J.; English-Loeb, G. Leaf Pubescence Mediates the Abundance of Non-Prey Food and the Density of the Predatory Mite Typhlodromus Pyri. Experimental and Applied Acarology 2003, 29, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Kugler, H. Blütenökologie; Gustav Fischer Verlag: Stuttgart, Germany, 1970. [Google Scholar]
- Goleva, I.; Zebitz, C.P.W. Suitability of Different Pollen as Alternative Food for the Predatory Mite Amblyseius Swirskii (Acari, Phytoseiidae). Exp Appl Acarol 2013, 61, 259–283. [Google Scholar] [CrossRef] [PubMed]
- Goleva, I.; Rubio Cadena, E.C.; Ranabhat, N.B.; Beckereit, C.; Zebitz, C.P.W. Dietary Effects on Body Weight of Predatory Mites (Acari, Phytoseiidae). Exp Appl Acarol 2015, 66, 541–553. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Tietze, M.; Michońska, M. Ecological Features of the Flowers of Aesculus Hippocastanum L. and Characteristics of Aesculus L. Pollen Seasons under the Conditions of Central-Eastern Poland. Acta Agrobot 2012, 65, 61–68. [Google Scholar] [CrossRef]
- Pekas, A.; Wäckers, F.L. Multiple Resource Supplements Synergistically Enhance Predatory Mite Populations. Oecologia 2017, 184, 479–484. [Google Scholar] [CrossRef]
- Pozzebon, A.; Loeb, G.M.; Duso, C. Role of Supplemental Foods and Habitat Structural Complexity in Persistence and Coexistence of Generalist Predatory Mites. Scientific Reports 2015, 5. [Google Scholar] [CrossRef] [PubMed]
| Local name | Geographical coordinates of geometric centre |
A. hippocastanum age in years (mean ± SE) 1 |
|---|---|---|
| City centre | 48.9744136N, 14.4770594E | 74.99 ± 1.86 |
| Šumava and Máj estate | 48.9841631N, 14.4407714E | 39.02 ± 3.19 |
| Vltava estate | 48.9962106N, 14.4519647E | 41.79 ± 3.49 |
| Třebotovice and Kaliště village | 48.9612606N, 14.5651828E | 17. 82 ± 6.86 |
| Rožnov estate | 48.9601436N, 14.4763944E | 70.49 ± 4.43 |
| Pražské předměstí estate | 48.9860569N, 14.4674681E | 49.74 ± 2.75 |
| Stromovka Park | 48.9670208N, 14.4551158E | 43.53 ± 4.13 |
| Nádražní Street | 48.9779942N, 14.4866511E | 77.74 ± 3.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





