Introduction
Bone is a dynamic tissue, uniquely capable of self-renewing through the process of bone remodeling which involves the sequential and coupled activities of bone-forming osteoblasts (OBs) and bone-resorbing osteoclasts (OCs) [
1]. OBs and OCs in turn are regulated by osteocytes, the most abundant bone cells that originate from matrix-entrapped OBs [
2,
3]. Osteocytes integrate hormonal and mechanical signals to regulate bone homeostasis. As endocrine cells, osteocytes secrete several key factors including sclerostin (Sost), Dickkopf-1 (DKK1), and osteoprotegerin (Opg) which regulate OB and OC functions [
4,
5,
6]. However, the molecular mechanisms by which osteocytes regulate bone remodeling are not fully understood.
The Ca
2+/ calmodulin (CaM)-dependent protein kinase (CaMK) signaling cascade, initiated by transient increases in intracellular Ca
2+, involves multifunctional serine/threonine protein kinases CaMKK1 and CaMKK2, and their canonical substrates CaMKI and CaMKIV [
7]. CaMKK2 additionally phosphorylates and activates adenosine monophosphate activated protein kinase (AMPK) to coordinate cellular and organismal energy balance [
8,
9,
10,
11]. Consequently, inhibition or loss of CaMKK2 in mice protects from diet-induced obesity and insulin resistance [
10,
11]. CaMKK2 also coordinates inflammatory responses in macrophages and chondrocytes [
12,
13]. In the skeleton, CaMKK2 plays cell-intrinsic roles in OBs and OCs. Mice lacking CaMKK2 globally (
Camkk2-/-) possess more OBs, fewer OCs and increased bone mass compared to wild-type (WT) mice [
14]. Blocking CaMKK2 activity using its selective pharmacological inhibitor STO-609 reverses age-associated decline in trabecular and cortical bone mass and strength, prevents ovariectomy-induced bone loss, and enhances bone fracture healing [
15,
16,
17].
Camkk2-/- bone marrow-derived progenitors yield higher numbers of alkaline phosphatase-positive OBs
in vitro than WT, in part via activation of cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA), and fewer OCs through the downregulation of cyclic adenosine monophosphate (cAMP) response element binding protein (pCREB) – nuclear factor of activated T cells, cytoplasmic (NFATc1) signaling, indicating cell intrinsic roles for CaMKK2 in OBs and OCs [
14]. However, the specific role(s) of CaMKK2 in osteocytes remain unknown.
In this study, we tested the hypothesis that osteocyte-derived CaMKK2 plays an important role in the regulation of bone growth and maintenance using in vivo and in vitro approaches. Our findings reveal a cell-intrinsic role for CaMKK2 in osteocytes in the regulation of OCs in a sex-dependent manner through a novel paracrine mechanism involving secreted calpastatin.
Materials and Methods
Mice: All animal procedures were performed with prior approval from Indiana University School of Medicine Institutional Animal Care and Use Committee (IACUC) and the Department of Defense Animal Care and Use Review Office (ACURO). All experiments were performed in compliance with NIH guidelines on the use and care of laboratory and experimental animals. All animals were housed in the Indiana University School of Medicine Laboratory Animal Resource Center (LARC, Indianapolis, IN, USA) under a 12-hour (h) light, 12-h dark cycle. Food and water were provided ad libitum. All mice generated in this study were derived of C57BL/6J background.
Dentin matrix protein (
Dmp1)-8kb-
Cre+ mice [
18,
19] were provided by Dr. Teresita Bellido and
Camkk2flox/flox mice have been described previously [
11]. We first generated
Dmp1-Cre-Camkk2flox+/- by breeding the
Dmp1-Cre and
Camkk2fl/fl mice. Then we crossed the heterozygous mice to generate
Dmp1-Cre::Camkk2+/+ (
Control) mice and
Dmp1-Cre::Camkk2fl/fl (Camkk2OCY) mice (
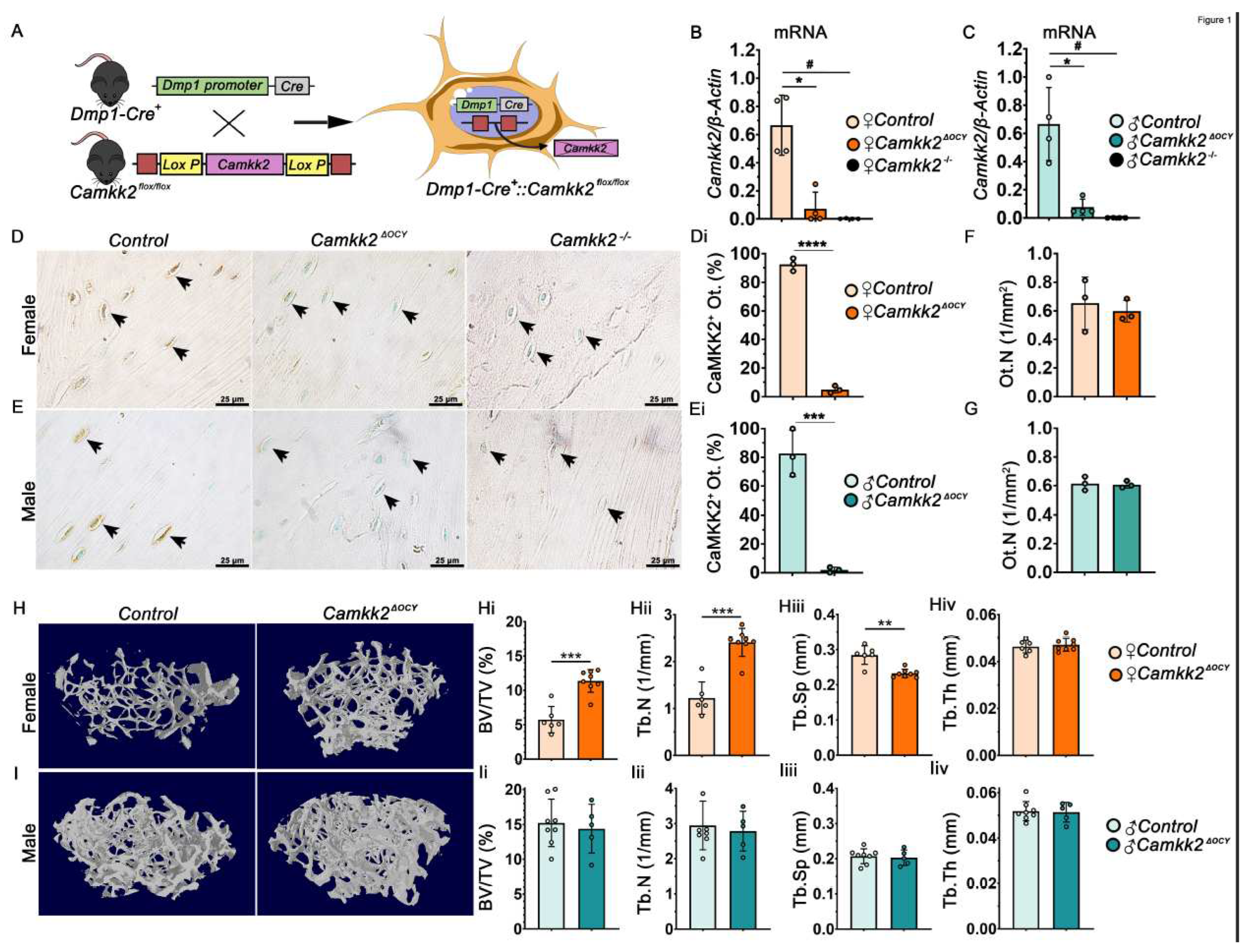
Figure 1A).
Dmp1-Cre+::Camkk2+/+ mice were used to control for potential non-specific effects of
Dmp1- regulated Cre recombinase. For all skeletal phenotyping and osteocyte isolation experiments, mice of either sex were used at 12 weeks (w) of age. Bone marrow isolations were performed using 6 w-old wild-type (WT) mice.
µCT Imaging: Long bones were excised at 12 w of age and fixed in 4% paraformaldehyde (PFA) for 48 h at 4
0C and transferred to 70% ethanol. Micro-computed tomography (μCT) was performed on femurs at a 5.87 µm image pixel size using a Bruker 1172 μCT system (59 kV, 167 µA, 0.7 rotation step, 0.5 aluminum filter). Reconstructed μCT images (NRecon software, Kontich, Belgium) were analyzed using CT Analyzer software (Skyscan, Kontich, Belgium). The trabecular bone compartment was analyzed within 1 mm proximal of the distal growth plate, while femoral midshaft architecture was measured using cross-sections approximately 3.5 mm proximal to the distal growth plate. Reconstructed 3D models were generated using CTVox software (Skyscan, Kontich, Belgium), from which trabecular bone volume per total volume (BV/TV) (%),trabecular number (Tb.N) (mm−1), trabecular thickness (Tb.Th) (mm), and trabecular separation (Tb.Sp) (mm) as well as cortical bone parameters were calculated using established guidelines [
20].
Histology: Bone histology was performed by the Indiana Center for Musculoskeletal Health Musculoskeletal Histology Core. Following μCT analysis, dynamic and static histomorphometry were performed on undecalcified femurs embedded in poly-methyl methacrylate (plastic). Longitudinal sections cut at 5 µm thickness were stained with Von Kossa and McNeal's (VKM) to assess OB numbers and osteoid parameters. Sections were also stained for tartrate-resistant acid phosphatase (TRAP) activity following with hematoxylin counterstain to measure OC parameters. Bone formation and mineralization parameters were measured based on incorporation of dual fluorochrome labels of calcein and alizarin, which mice received via I.P. injection approximately 7 and 2 days prior to euthanasia. Parameters measured included single-label perimeter (sL.Pm), double-label perimeter (dL.Pm), and interlabel width (Ir.L.Wi). From these primary measurements, the following outcome parameters were calculated: mineral apposition rate (MAR = Ir.L.Wi/7 days [µm/day]); mineralizing surface (MS/BS = (0.5*sL.Pm + dl.Pm)/B.Pm*100 [%]); and bone formation rate (BFR/BS = MAR*MS/BS*365 [µm3/µm2/year]). Static and dynamic measurements and calculations followed the guidelines of the American Society for Bone and Mineral Research Histomorphometry Nomenclature Committee [
21].
Immunohistochemistry: Long bones were decalcified in 14% EDTA (pH 7.4) and dehydrated before paraffin embedding. Five µm thick serial sections were generated, deparaffinized and incubated with primary antibody against CaMKK2 (rabbit anti-CaMKK2-NT; cat# 033168, US Biological, Salem, MA; 1:100 dilution) and anti-rabbit secondary (Jackson Immunoresearch, West Grove, PA, USA) and counterstained with Gill No. 1 Hematoxylin or Methyl Green (Millipore Sigma, Burlington, MA). Images captured using a Leica DMi8 microscope were processed with Leica LAS-X software (Leica CM1950, Wetzlar, Germany), and quantified by counting immunopositive and total osteocytes within cortical bone from 4 regions of interest per sample using ImageJ (NIH, USA).
Osteocyte Culture and Media Collection: Osteocyte-enriched fractions derived from mouse long bones were isolated as previously reported by Stern et al [
22]. Cell suspensions from individual mice were cultured in twelve-well plates coated with type-I rat tail collagen (Millipore Sigma) at a seeding density of 1X10
5 cells per well. In α-minimal essential medium supplemented with 2.5% fetal bovine serum (FBS), 2.5% bovine calf serum (BCS) and 1% penicillin and streptomycin (PS) (Thermo Fisher, Hampton, NH). Cells were maintained at 37
0C and 5% CO
2 in a humidified incubator. Osteocytes were not disturbed for the first 7 days while they attached to the plate. Media changes were performed every 48 hours beginning on day 7, and conditioned media was collected from osteocyte cultures derived from individual mice every 48 hours on days 9, 11, 13, and 15 post isolation. Conditioned media (CM) was filtered using a 0.22 µm syringe filter and aliquoted before storing at -20
0C. Serum-free CM was collected for mass spectrometry and immunodepletion assays after a 4 h incubation on day 7 of cultures.
Osteoclast Cultures and Media Supplementation: Bone marrow cells (BMCs) were isolated from the long bones of 6-week-old WT mice and plated on 0.1% gelatin-coated dishes at a density of 1.5X105 cells/cm2 in osteoclast differentiation media (α-Minimum Essential Media (Invitrogen, Thermo Fisher) containing 10% FBS (R&D Systems, Minneapolis, MN, USA), P/S (Invitrogen), 30 ng/mL M-CSF (R&D Systems), and 50 ng/mL RANKL (Peprotech, Rockhill, NJ, USA)), which was supplemented with 50% osteocyte conditioned media. BMCs were plated on gelatin-coated wells or glass coverslips to observe OC differentiation and actin-ring formation, respectively, whereas 96-well Corning® Osteo Assay Surface strips (hydroxyapatite-coated, Corning, Thermo Fisher) were used to assess osteoclast resorption. Recombinant human calpastatin domain I (non-cell permeable) was purchased from Miilipore Sigma (catalogue #: 208900), reconstituted in sterile PBS and diluted before adding to OC cultures at 0.1 µM, 0.5 µM, 1.0 µM and 5.0 µM final concentrations. OC precursor migration was observed by performing a controlled scratch assay after 3 days of differentiation. A sterile 10 µl pipette tip was used to scrape across the middle of each well and cell migration was observed over the course of 24 hours. TRAP activity was assessed after 7 days of differentiation using the Acid Phosphatase Kit (Sigma). Actin ring formation was observed after 7 days by staining OCs mounted on glass coverslips with phalloidin-rhodamine (1:50; Thermo Fisher) diluted in PBS containing 1% BSA and mounted on slides using ProLong™ Gold Antifade mounting solution with DAPI (Invitrogen). To measure OC-mediated resorption after 8 days of differentiation, cells were removed from the 96-well Osteo Assay strip surface by incubating with 10% bleach solution for 5 minutes at room temperature, and resorption areas were visualized by Von Kossa staining. Resorbed areas appeared clear whereas the remaining hydroxyapatite-coated areas stained black with Von Kossa stain. Number of multinuclear (>3 nuclei) OCs as well as the number of resorption pits and resorption area were measured using Image J (NIH, USA).
qRT-PCR: Primary osteocyte RNA was isolated using the RNAqueous kit (Invitrogen, Thermo Fisher). Samples were treated with DNAse I for 20 min at 37°C to remove genomic DNA contamination and DNAse I was inactivated before synthesizing cDNA. RNA concentrations were determined using the BioPhotometer spectrophotometer (Eppendorf, Hauppauge, NY, USA). cDNA was synthesized from 1 μg RNA using a high-capacity reverse-transcriptase kit (Invitrogen, Thermo Fisher). QPCR reactions were performed using iTaq Universal SYBR Green Supermix and the CFX Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Relative expression to β-Actin was determined via the 2–ΔΔCt method included in the CFX Manager Software (Bio-Rad Laboratories). Primers used in qPCR reactions were purchased from IDT (Integrated DNA Technologies, Coralville, IA) and are as follows: Actin-F (5’GGGAAATCGTGCGTGACATC), Actin-R (5’CCAAGAAGGAAGGCTGGAAAAG), Rankl-F (5’ CATTTGCACACCTCACCATCAAT), Rankl-R (5’GTCTGTAGGTACGCTTCCCG), Opg-F (5’ TCCCGAGGACCACAATGAACAAGT), Opg-R (5’TTAGGTAGGTGCCAGGAGCACATT), Camkk2-F (5’CATGAATGGACGCTGC) and Camkk2-R (5’TGACAACGCCATAGGAGCC).
Mass Spectrometry: Serum-free osteocyte CM was concentrated 13-fold using centrifugal filter columns (Amicon® Ultra 3K; Millipore Sigma), and protein concentrate was exchanged into ammonium bicarbonate buffer (25 mM). Samples were then dried down and resuspended in 8 M urea with 100 mM ammonium bicarbonate, pH 7.8. Disulfide bonds were reduced by incubation for 45 min at 57 °C with a final concentration of 10 mM Tris (2-carboxyethyl) phosphine hydrochloride (Catalog no C4706, Sigma Aldrich). A final concentration of 20 mM iodoacetamide (Catalog no I6125, Sigma Aldrich) was then added to alkylate these side chains and the reaction was allowed to proceed for one hour in the dark at 21°C. Samples were diluted to 1 M urea using 100 mM ammonium bicarbonate, pH 7.8. One µg of trypsin (V5113, Promega) was added, and the samples were digested for 14 hours at 37 °C. The following day, samples were desalted using Omix tips (Agilent).
Peptide samples were analyzed by LC-MS on an Orbitrap Fusion Lumos (ThermoFisher) equipped with an Easy NanoLC1200 HPLC (ThermoFisher). Peptides were separated on a 75 µm × 15 cm Acclaim PepMap100 separating column (Thermo Scientific) downstream of a 2 cm guard column (Thermo Scientific). Buffer A was 0.1% formic acid in water. Buffer B was 0.1% formic acid in 80% acetonitrile. Peptides were separated on a 120 min gradient with the primary separation from 4% B to 33% B. Precursor ions were measured in the Orbitrap with a resolution of 120,000. Fragment ions were measured in the Orbitrap with a resolution of 15,000. The spray voltage was set at 1.8 kV. Orbitrap MS1 spectra (AGC 4×105) were acquired from 400-2000 m/z followed by data-dependent HCD MS/MS (collision energy 30%, isolation window of 2 Da) for a three second cycle time. Charge state screening was enabled to reject unassigned and singly charged ions. A dynamic exclusion time of 60 seconds was used to discriminate against previously selected ions.
Proteomics Data Analysis: Resulting RAW files were analyzed in Proteome Discover™ 2.4.1.15 (Thermo Fisher Scientific, RRID: SCR_014477) with a Mus musculus UniProt FASTA (both reviewed and unreviewed sequences) plus FBS and common laboratory contaminants. Default minora feature detector settings were used. SEQUEST HT searches were conducted with a maximum number of 2 missed cleavages; precursor mass tolerance of 10 ppm; and a fragment mass tolerance of 0.02 Da. Carbamidomethylation on cysteine (C) residues was included as a static modification. Dynamic modifications used for the search were oxidation of methionines, phosphorylation on S, T, and Y, Met-loss or Met-loss plus acetylation of protein of N-termini. Percolator False Discovery Rate was set to a strict setting of 0.01 and a relaxed setting of 0.05. Quantification methods utilized feature mapper chromatographic alignment tools of maximum shift 10 and min s/n threshold of 5. Precursor ion quantification used precursor intensities of unique and razor peptides without scaling and with normalization based on total peptide amount. Quantitative rollup of the summed abundances was done with the top 3 N unmodified peptides, no imputation with a hypothesis of background-based t-tests. Resulting normalized abundance values for each sample type, abundance ratio and log2 (abundance ratio) values; and respective p-values from Proteome Discover™ were exported to Microsoft Excel, Uniprot accession numbers were uploaded to the online Database for Annotation, Visualization and Integrated Discovery (DAVID) to perform a functional annotation analysis. All processed and raw data are available upon request.
Calpastatin Enzyme-Linked Immunosorbent Assay (ELISA): Quantification of calpastatin levels in osteocyte CM was performed using the Mouse CAST (Calpastatin) ELISA kit (NBP2-75048; Novus Biologicals, Centennial, CO, USA) according to manufacturer’s directions.
Immunoblotting: Primary osteocytes or WT OCs were harvested and placed into iced tween lysis buffer (25 mM Hepes (pH 7.5), 50 mM NaCl, 25 mM NaH2PO4, 0.5% Tween 20, 10% glycerol, 1 mM dTT, 10 mM β-glycerophosphate, 2 mM EGTA, 2 mM EDTA, 25 mM NaF, 1 mM sodium vanadate, 1 mM PMSF, 1 mg/ml aprotinin, 1 mg/ml leupeptin, 10 mg/ml pefabloc, and 100 nM okadaic acid), sonicated on ice, centrifuged 14000 rpm for 30 min at 40C. Equal amount of protein lysates (7.5 µg/lane) isolated from primary osteocytes or osteoclasts was fractionated under denaturing conditions on SDS-PAGE and transferred onto Immobilon-P membranes (Millipore Sigma). Blocking, primary and secondary antibody incubations were performed in Tris-buffered saline (TBS) containing 5% non-fat dry milk. Washes were performed in TBS with Tween-20 (0.1%, v/v, Millipore Sigma). Membranes were probed with primary antibodies for Cast (Cell Signaling Technology, Danvers, MA; 1:1000), CaMKK2 (BD Biosciences; 1:1000), Talin (C-9) (Santa Cruz Biotechnology, Dallas, TX; SC-365875 1:100), Rabbit p-PKA-C (T197), total PKA-C (D45D3) (both Cell Signaling 1:1000) or b-Actin (MilliporeSigma; 1:3000) followed by horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch; 1:5000). The target proteins were visualized with Clarity Enhanced Chemiluminescence substrate (Bio-Rad) using a ChemiDox MP Image System (Bio-Rad), and band densities quantified using ImageJ.
Immunoprecipitation of Calpastatin from Conditioned Media: Osteocyte CM used directly for western blot or media supplementation for osteoclast assays was precleared with Protein G Magnetic Sepharose Xtra Beads (GE Healthcare) and incubated with either rabbit anti-Cast antibody (1:1000, Cell Signaling #4416) or control IgG (1:1000, MilliporeSigma) overnight on a slow rotating mixer at 40C. Protein G Magnetic Sepharose Xtra beads were added and slurry allowed to incubate for 1 h with rotation at 40C, and centrifuged to separate immunoprecipitate containing beads from supernatant. For media supplementation, the supernatant was passed through a 0.22 µm syringe filter and aliquoted for storage at -200C. For immunoblots, beads containing the immunoprecipitates were washed three times with DPBS at room temperature and boiled in 1x protein loading dye prior to loading (30 µg/lane) on SDS-PAGE gel (Novex) for immunoblotting with Rabbit α-Calpastatin antibody (Cell signaling 1:1000).
Statistical Analysis: Statistical analyses were performed using the GraphPad Prism software (GraphPad Software, San Diego, CA). Normality assumptions were evaluated using histograms and QQ plots. Data sets that passed normality test were analyzed using unpaired, two-tailed Student’s
t-test when comparing Control and
Camkk2OCY or ordinary one-way ANOVA followed by Tukey’s post-hoc analysis when comparing >2 groups [
23]. When data sets did not pass normality test, non-parametric tests were used – Mann-Whitney test for 2 sample comparisons or Kruskal-Wallis test followed by Dunn’s post-hoc to compare >2 groups).
All values are represented as mean ± standard deviation (SD).
Results
Conditional Deletion of CaMKK2 in Osteocytes Enhances Trabecular Bone Mass Only in Female Mice
We crossbred
Camkk2flox/flox mice with transgenic mice expressing
Cre driven by a 8-kb dentin matrix protein 1 (
Dmp1) promoter to generate heterozygous
Dmp1-8kb-
Cre::
Camkk2flox/WT mice which were then mated to generate male and female
Dmp1-8kb-
Cre::
Camkk2WT/WT (Control) and
Dmp1-8kb-
Cre::
Camkk2flox/flox (
Camkk2ΔOCY) mice [
11,
18,
19] (
Figure 1A).
Camkk2 mRNA levels were 13-fold lower in primary
Camkk2ΔOCY osteocytes compared to control osteocytes in both sexes (
Figure 1B-C). Deletion of CaMKK2 from osteocytes in female and male
Camkk2ΔOCY mice was also confirmed by immunohistochemistry (IHC;
Figure 1D-Di, 1E-Ei). Conditional deletion of CaMKK2 did not affect osteocyte numbers in either sex (
Figure 1F-G).
Examination of distal femora using micro-computed tomography (µCT) revealed a 2.2-fold increase in bone volume fraction (%BV/TV) in 12 week (w) old female
Camkk2ΔOCY mice compared to age-and sex-matched controls (
Figure 1H-Hi). Female
Camkk2ΔOCY long bones also possessed significantly higher trabecular number and lower trabecular separation compared to controls mice, whereas no differences were observed in trabecular thickness (
Figure 1Hii-Hiv). In contrast, BV/TV (%) and trabecular bone microarchitecture remained similar between 12 w old male
Camkk2ΔOCY and control mice (
Figure 1I-Iiv). Cortical bone area, cross-sectional thickness and polar moment of inertia were similar among cohorts of both sex (
Supplementary Figure S1A-B). These data indicate a sex divergent effect on trabecular bone mass by osteocyte-derived CaMKK2.
Ablation of Osteocyte-Derived CaMKK2 Diminishes OCs in Female Mice Without Altering OB Function
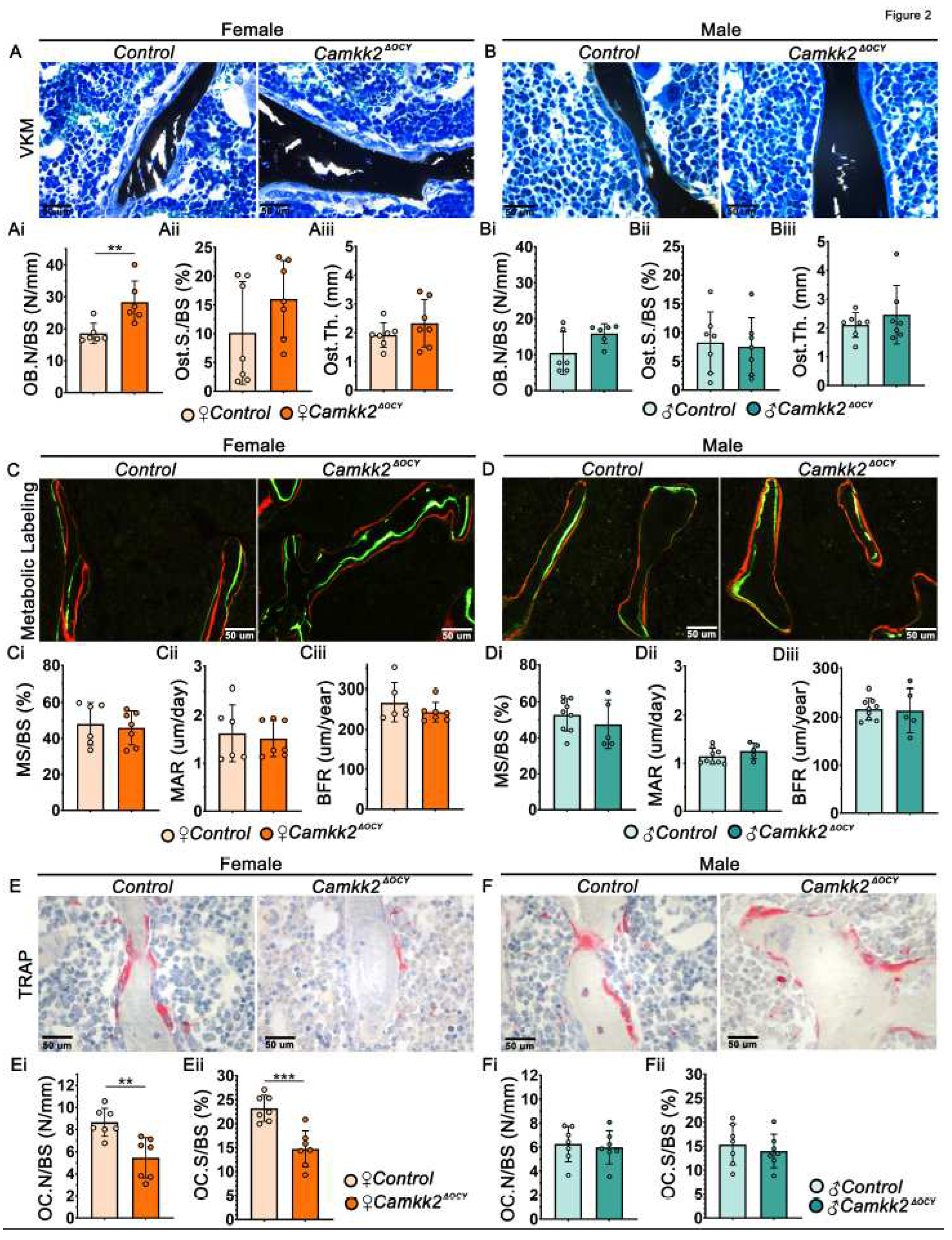
We next evaluated whether deletion of CaMKK2 in osteocytes affected bone remodeling. Examination of von-Kossa McNeil (VKM) stained femur sections revealed significantly higher numbers of OBs in female
Camkk2ΔOCY mice compared to female controls, whereas OB numbers were similar in male
Camkk2ΔOCY and control femurs (
Figure 2A-Ai; 2B-Bi). In contrast, osteoid surface and osteoid thickness remained similar between sex-matched cohorts (
Figure 2Aii-Aiii; 2Bii-Biii). Further, dynamic histomorphometry of double-fluorochrome labelled long bone sections revealed no differences in mineralizing surface, mineral apposition rate or bone formation rate between sex-matched
Camkk2ΔOCY and control mice (
Figure 2C-Ciii; 2D-Diii). Thus, changes in bone formation were not likely the cause of the female-specific increase in cancellous bone mass in female
Camkk2ΔOCY.
Static histomorphometry of tartrate-resistant acid phosphatase (TRAP)-stained bone sections revealed a 1.7-fold reduction in OC numbers and OC surface to bone surface in female
Camkk2ΔOCY mice compared to controls, whereas no such differences were observed in male mice (
Figure 2E-Eii; 2F-Fii). These histomorphometry data revealed a role for osteocytic CaMKK2 in the regulation of OCs in female mice.
Conditioned Media from Female Osteocytes Lacking CaMKK2 Inhibits OC Differentiation and Function
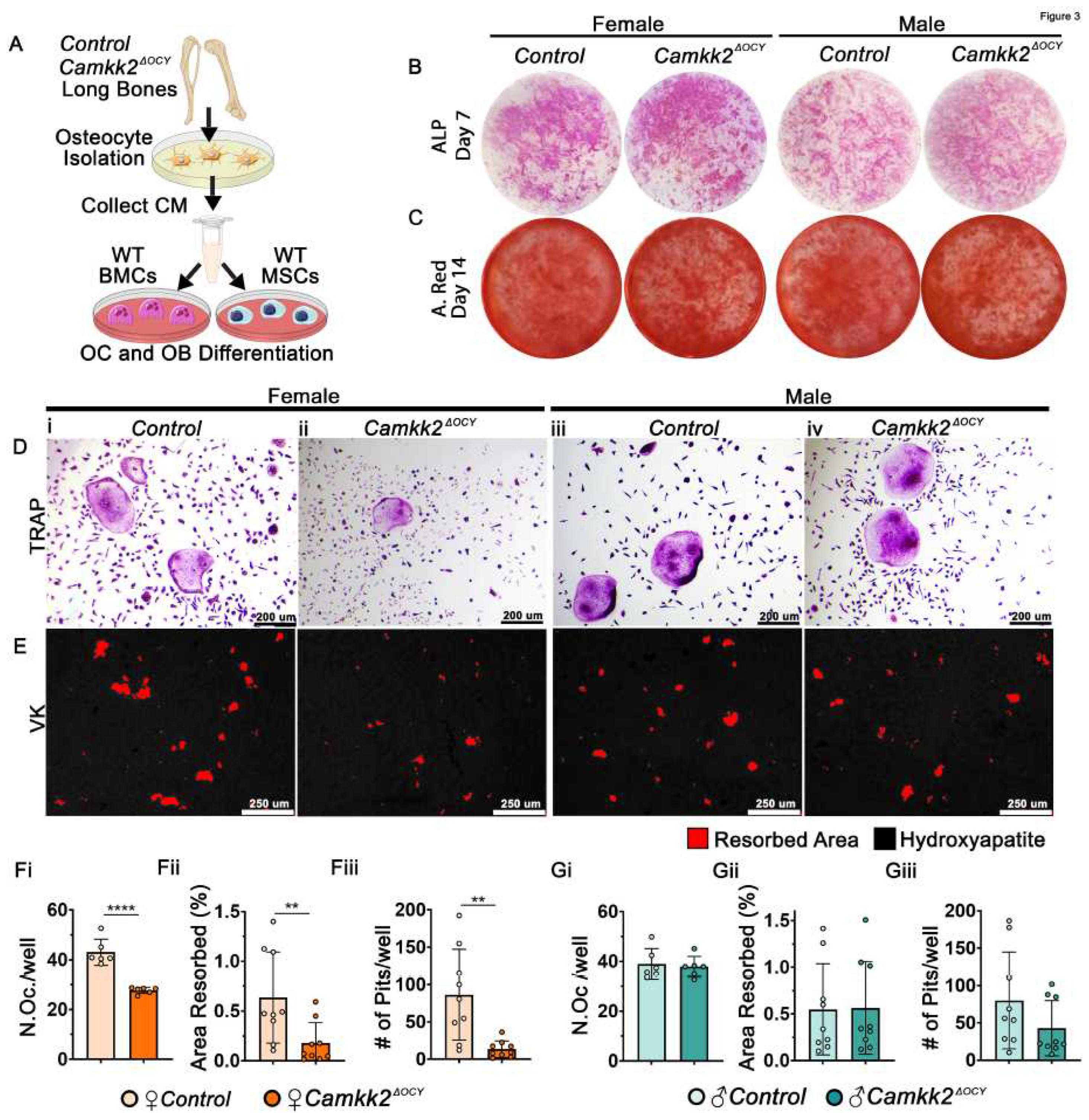
We surmised that the differential regulation of OCs by female
Camkk2ΔOCY osteocytes occurs through secreted factors. To test this, we isolated primary osteocytes from male and female control and
Camkk2ΔOCY long bones and evaluated the ability of the respective conditioned media (CM) to support OB and OC differentiation by primary wildtype (WT) bone marrow (BM)-derived cells (
Figure 3A). We observed no differences in the ability of male or female, control or
Camkk2ΔOCY osteocyte CM to support OB differentiation by WT BM-derived mesenchymal stem cells (MSCs)
in vitro as evidenced by alkaline phosphatase or alizarin red staining intensities (
Figure 3B-C). In contrast, WT BM cells exposed to CM from female
Camkk2ΔOCY osteocytes yielded 1.7-fold fewer multinuclear TRAP-positive OCs than cells receiving female control CM (
Figure 3D, 3Fi). On the other hand, WT BM cells yielded similar numbers of TRAP-positive multinuclear OCs when treated with male control or
Camkk2ΔOCY osteocyte CM (
Figure 3D, 3Gi). Next, to assess the effects of osteocyte CM on OC function, we plated WT BM cells on hydroxyapatite-coated wells in the presence or absence of osteocyte CM and assessed resorption. BM cells that received female
Camkk2ΔOCY osteocyte CM formed 3-fold fewer resorption pits and resorbed 3-fold lower hydroxyapatite area than cells exposed to control CM (
Figure 3E, 3Fii-Fiii), whereas no such differences were observed when BM cells were treated with male
Camkk2ΔOCY or control CM (
Figure 3E, 3Gii-Giii).
Extracellular Calpastatin is Enriched in Female CaMKK2-Deficient Osteocyte Conditioned Media Compared to Control and its Sequestration Relieves OC Inhibition
It is well-documented that osteocytes regulate OC differentiation through the altered expression of receptor activator of nuclear factor κ-B ligand (Rankl) and Opg [
24,
25]. However, we found no significant differences in
Rankl or Opg mRNA levels or their ratio in male and female
Camkk2ΔOCY osteocytes compared to controls (
Supplementary Figure S2A-B), indicating the presence of another mechanism. To identify OC-inhibitory factors secreted by CaMKK2-deficient female osteocytes, we concentrated serum-free CM harvested from male and female control and
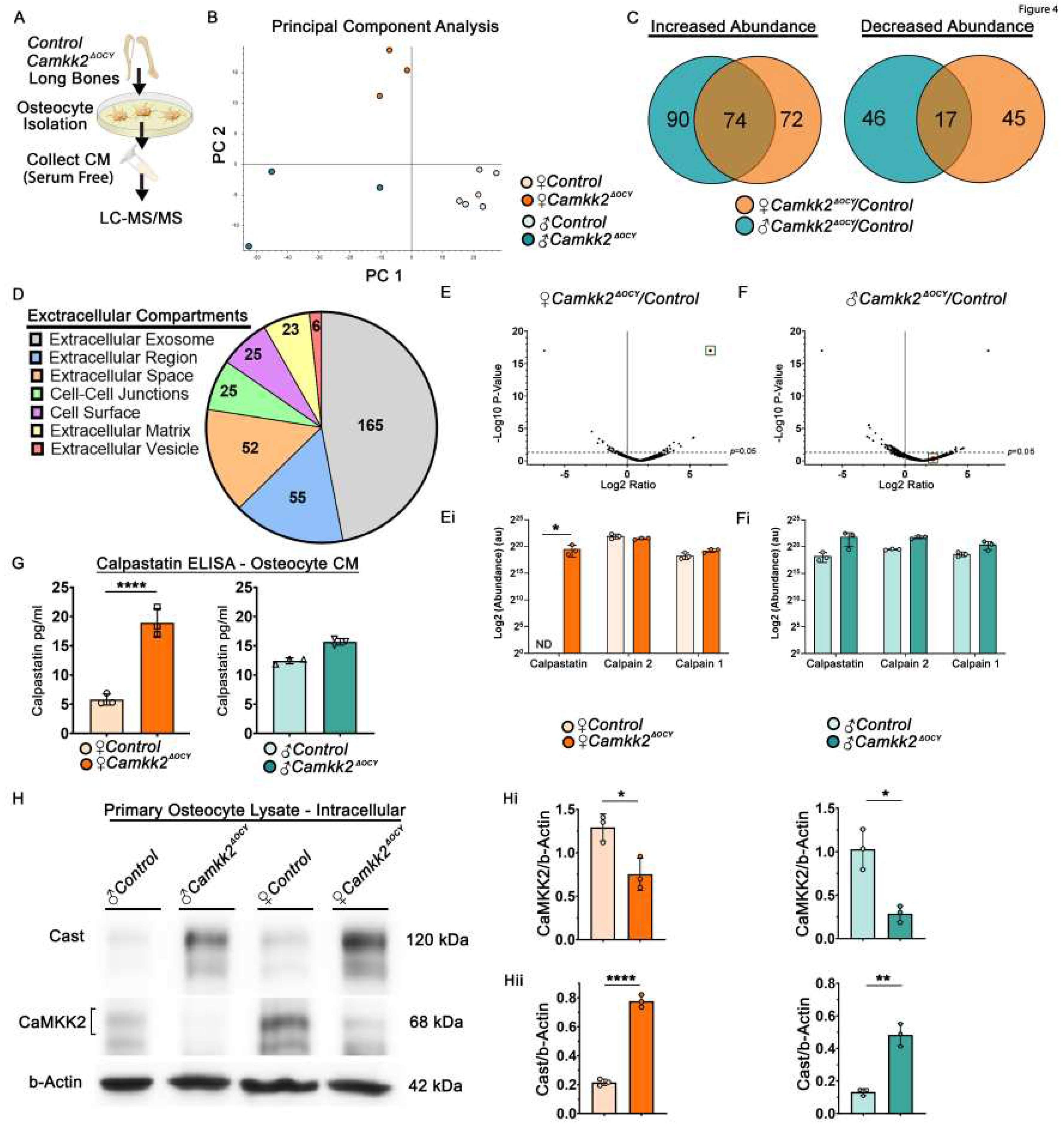
Camkk2ΔOCY osteocytes and performed proteomic analysis of the secretome using mass spectrometry (LC-MS/MS) (
Figure 4A). The experiments were conducted in triplicate and to initially assess the underlying reproducibility and accuracy of the data, we conducted principal component analysis. This revealed grouping of experimental replicates from the same cohort, and divergence of male and female osteocyte CM samples (
Figure 4B), consistent with reproducible proteomic data. Although
Camkk2ΔOCY male CM samples displayed more overall variability than other groups within the principal component analysis, they still clustered with each other and apart from the other groups.
Mass spectrometry detected a total of 1,182 proteins, including 9,293 unique peptides, in male and female osteocyte CM. Of these, 236 proteins were upregulated, 108 proteins were downregulated in male and female
Camkk2ΔOCY osteocyte CM compared to sex-matched controls, and 117 proteins were significantly altered only in female
Camkk2ΔOCY secretome (
Figure 4C). Functional annotation analysis confirmed association of the majority of detected proteins with extracellular compartments, including exosomes, vesicles, and extracellular matrix (ECM;
Figure 4D), confirming that our CM primarily consisted of secreted and cell surface associated proteins. Reactome pathway analysis identified several pathways associated with degradation of ECM, protein metabolism, and Golgi-associated vesicle biosynthesis and anterograde transport to be significantly altered selectively in female
Camkk2ΔOCY osteocyte CM (
Table 1).
Of the proteins associated with ECM degradation, calpastatin, the specific inhibitor of Ca
2+-dependent cysteine proteases called calpains, was of particular interest as its activity is regulated by Ca
2+; it possesses CaM-binding domains; and its target calpain has crucial roles in OCs [
26,
27,
28,
29]. Though predominantly intracellular, calpastatin and calpains are also secreted molecules [
30,
31]. Calpastatin was among a group of 62 proteins that were highly enriched in CM from female
Camkk2ΔOCY osteocytes, but not detected in CM from female control osteocytes. On the other hand, levels of calpastatin in male
Camkk2ΔOCY osteocyte CM were not significantly different from those in male control CM (
Figure 4E-Ei; 4F-Fi). Enzyme-linked immunosorbent assay (ELISA) of CM indicated a 3-fold increase in secreted calpastatin in female
Camkk2ΔOCY CM compared to female control CM (
Figure 4G). On the other hand, extracellular calpastatin levels in male
Camkk2ΔOCY osteocyte CM were only slightly higher than those in control male CM, and the differences were not statistically significant (
Figure 4G). Of note, the female control CM possessed the least amount of extracellular calpastatin of all four cohorts. We next examined intracellular levels of calpastatin in these osteocytes and found that both male and female
Camkk2ΔOCY osteocytes possessed 3.7–3.9-fold higher intracellular calpastatin compared to sex-matched controls (
Figure 4H-Hii). Thus, whereas intracellular calpastatin is elevated in CaMKK2-deficient osteocytes of both sexes, the differential increase in extracellular calpastatin was observed only in CM from CaMKK2-deficient female osteocytes.
Osteoclast Inhibitory Effects of Recombinant Non-Cell Permeable Calpastatin is Sex-Dependent
To understand whether extracellular calpastatin inhibited OC differentiation and function, we treated male and female WT BM cells undergoing RANKL-mediated OC differentiation with varying doses of recombinant human calpastatin domain I, which is non-cell-permeable (NCP) [
32,
33]. Treatment of female WT BM cells with 0.5, 1.0 and 5.0 µM NCP-calpastatin elicited a 1.7, 1.9 and 3.7-fold decrease in resorption pit number and area resorbed, compared to untreated cells (
Supplementary Figure S3A, S3B-3D). In contrast, only 5.0 µM NCP-calpastatin caused a significant 2-fold reduction in resorption pit number and area resorbed by WT BM cells undergoing RANKL-mediated OC differentiation, compared to untreated cells (
Supplementary Figure S3A, S3E-3F). On the other hand, NCP-calpastatin elicited a dose-dependent reduction in the number of multinuclear OCs produced by male and female WT BM cells (
Supplementary Figure 3D and 3G). Of note, treatment with 5.0 µM NCP-calpastatin resulted in smaller and fewer OCs and resorption pits in cells from either sex. Taken together, our results indicate that whereas extracellular NCP-calpastatin inhibited OC differentiation in both sexes, it inhibited OC function, only in females.
Extracellular Calpastatin in Female Camkk2ΔOCY CM Regulates Bone Resorption by Osteoclasts
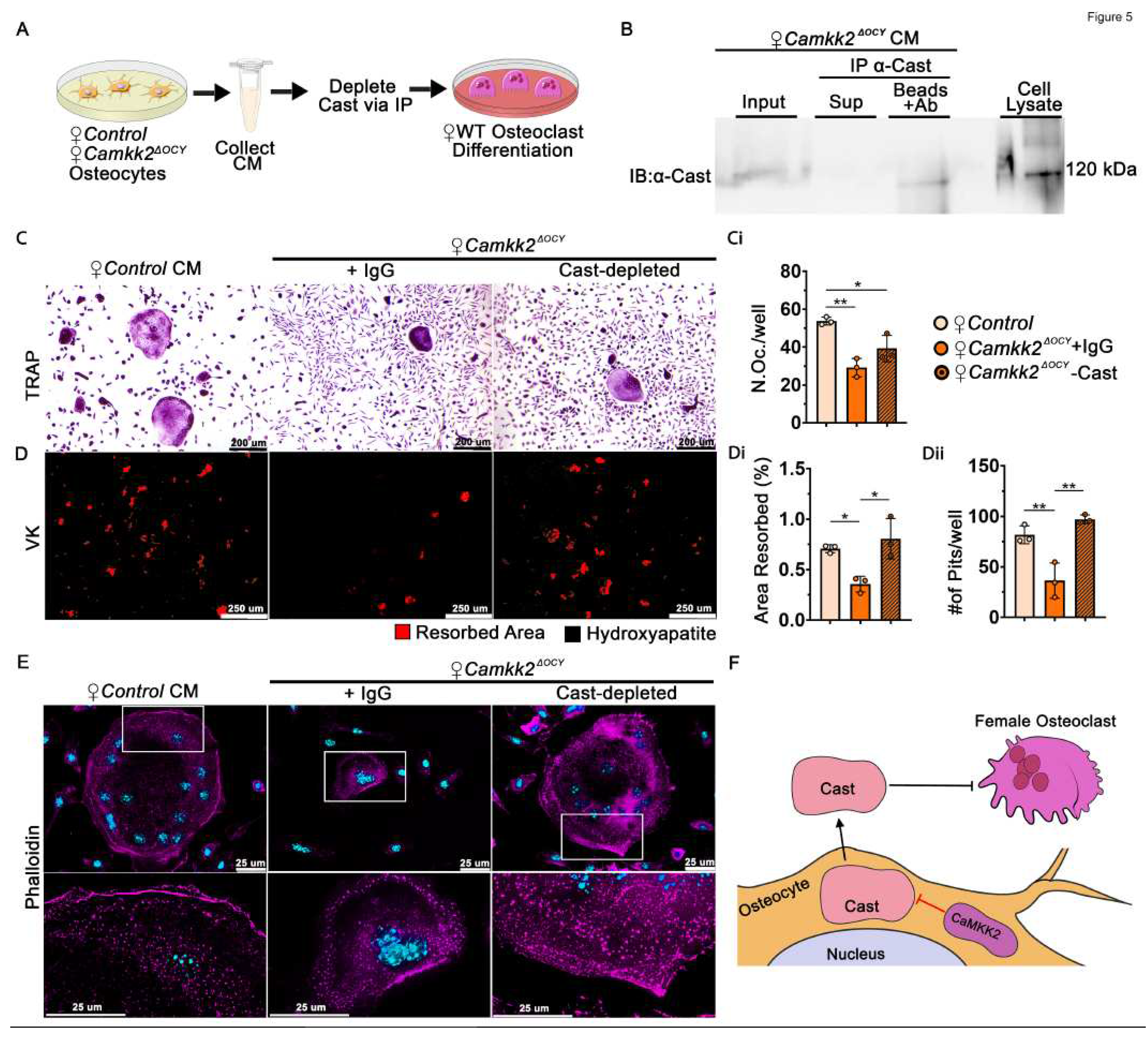
To further investigate the OC-inhibitory role of secreted calpastatin, we incubated female
Camkk2ΔOCY osteocyte CM with anti-calpastatin antibody or control IgG and assessed the ability of calpastatin-depleted or IgG-incubated CM to support RANKL-mediated OC differentiation by WT BM cells (
Figure 5A-C). Consistent with our previous results (
Figure 3D, 3F), we observed 1.8-fold fewer OCs and a 2.8-fold reduction in the number of resorption sites and area resorbed when WT BM cells were treated with IgG-incubated female
Camkk2ΔOCY osteocyte CM (
Figure 5C-Ci; 5D-Dii). Depletion of calpastatin fully reversed the inhibition of OC resorption by female
Camkk2ΔOCY CM (
Figure 5D-Dii).
The main function of calpastatin is calpain inhibition. Secreted calpains cleave ECM components to enable cell spreading, ECM attachment and migration in other cell types [
32,
33]. Since female
Camkk2ΔOCY osteocyte CM inhibits OC differentiation, we surmised that extracellular calpastatin present in the CM blocks migration of osteoclast progenitors towards each other. We performed
in vitro monolayer scratch assays to test this and found WT BM cells treated with control CM as well as IgG- or calpastatin-depleted
Camkk2ΔOCY CM to display similar rates of migration (
Supplementary Figure S4A-C), indicating that extracellular calpastatin does not inhibit cell migration.
We next assessed whether calpastatin in the CM affects formation of actin ring-mediated sealing zone that is required for attachment of OCs to bone matrix. WT-BM-derived OCs treated with female control CM, IgG-exposed or calpastatin-depleted
Camkk2ΔOCY CM were stained with rhodamine-phalloidin to detect filamentous (F) actin. OC precursors treated with female control osteocyte CM formed multinuclear OCs with large distinct F-actin rings on glass coverslips (
Figure 5E). In contrast, OCs treated with IgG-incubated female
Camkk2ΔOCY CM were smaller with poorly defined F-actin rings, whereas depletion of calpastatin resulted in larger OCs with more distinct F-actin rings (
Figure 5E). Taken together, our data indicate a novel paracrine role for osteocyte-derived CaMKK2 in the regulation of OC differentiation and function through a secreted calpastatin-mediated mechanism.
Discussion
The objective of the current study was to investigate a cell-intrinsic role of CaMKK2 in osteocytes, the master regulators of skeletal homeostasis [
6]. Conditional deletion of CaMKK2 from osteocytes elicits a sex-dependent effect on the skeleton. Specifically, we observed enhanced bone mass coupled with fewer OCs in female but not male
Camkk2ΔOCY mice. Further, CM isolated from female
Camkk2ΔOCY osteocytes suppressed OC formation and function. Calpastatin, a specific inhibitor of Ca
2+-activated calpains, was highly enriched in female
Camkk2ΔOCY CM, its intracellular levels were significantly elevated in male and female
Camkk2ΔOCY osteocytes whereas its extracellular levels were differentially altered only in female
Camkk2ΔOCY CM. Levels of extracellular calpastatin were significantly lower in female control osteocyte CM compared to males, and the reason for this suppression is not clear. Immunodepletion of calpastatin attenuated the inhibition of OC function by female
Camkk2ΔOCY CM. Moreover, treatment of WT BM-derived myeloid cells with exogenous non-cell permeable calpastatin caused a more pronounced inhibitory effect on OC resorption in female-derived cells than male. Based on these cumulative data, we propose that CaMKK2 is an inhibitor of calpastatin expression in osteocytes and that osteocyte-secreted calpastatin blocks OC function in a sex-specific manner (
Figure 5F). Thus, our studies identify a novel sex-specific paracrine role for osteocyte-derived extracellular calpastatin in the regulation of OCs.
Calpastatin is the chief inhibitor of Ca
2+-dependent cysteine proteases called calpains [
26]. Calpains proteolyze several intracellular substrates to critically regulate a multitude of cellular processes such as cell motility, spreading, and adhesion to ECM in multiple cell types including OBs and OCs [
28,
29,
34,
35,
36,
37]. In OCs, cleavage by intracellular calpains of their substrates enriched in actin ring such as talin, filamin A and Pyk2, is crucial for OC motility, spreading, sealing zone formation and bone resorption [
36,
38]. Though predominantly intracellular, extracellular calpains that cleave ECM components have been reported in several systems including OBs, hypertrophic chondrocytes, healing bone fracture calluses, and synovial fluid from rheumatoid arthritis (RA) and osteoarthritis (OA) patients [
39,
40,
41,
42,
43,
44,
45]. Calpastatin is also a secreted molecule found in the synovial fluid of RA and OA patients, plasma of patients with pulmonary arterial hypertension, and exosomes from luminal fluid of ovine uterus [
32,
46,
47]. In this study, we demonstrate for the first time that calpastatin is secreted by osteocytes and that extracellular calpastatin acts to regulate the formation and function of female OCs.
Mammalian calpastatin is encoded by a single gene (
Cast), but the use of multiple promoters and alternative splicing mechanisms leads to the generation of many calpastatin isoforms that vary in molecular mass from 17.5 kDa to 85 kDa [
48,
49,
50]. Multiple calpastatin isoforms are often present in the same tissue and even cell type. Calpastatin protein consists of an N-terminal L domain devoid of inhibitory activity and four repetitive inhibitory domains (I–IV) that bind to and inhibit calpain in a Ca
2+-dependent manner [
48,
49,
50]. We observed two intracellular calpastatin isoforms, a predominant 120 kDa species and a minor 80 kDa species in murine osteocytes through immunoblotting, and both isoforms were enhanced in CaMKK2-deficient osteocytes, regardless of biological sex (
Figure 4H). Though the size of the osteocyte-secreted calpastatin is unknown, we surmise that it is the 80 kDa isoform since the lacunar-canalicular transport has a molecular cut off limit of 70-80 kDa [
51,
52]. Further, phosphorylation of calpastatin by Ser/Thr kinases protein kinase A or protein kinase C decreases its efficiency of calpain inhibition [
48]. Being a Ser/Thr kinase, CaMKK2 potentially regulates calpastatin levels and/or activity via phosphorylation, and its absence could enhance the levels of both intracellular and extracellular calpastatin in both sexes.
Extracellular calpastatin is non-cell permeable [
32]. Accordingly, exposure of OCs to female osteocyte CM did not alter intracellular levels of calpastatin, calpain substrate talin or calpain activator PKA (
Supplementary Figure 4D). How might osteocyte-secreted calpastatin regulate OCs? The only known function of calpastatin is calpain inhibition, and others have postulated that the regulatory effects of secreted calpastatin mainly involve the inhibition of extracellular calpain [
32,
53]. Secreted calpains cleave ECM components to facilitate the disengagement of α
Vβ
3 integrins with ECM and enable cell migration [
32,
33]. However, migration of OC precursors is not influenced by extracellular calpastatin (
Supplementary Figure S4A-C). On the other hand, WT BM-derived myeloid progenitors treated with calpastatin-containing female
Camkk2ΔOCY osteocyte CM formed fewer functionally-deficient OCs that were smaller with indistinct F-actin rings, consistent with our
in vivo data from female
Camkk2ΔOCY long bones. Further, depletion of calpastatin from the CM completely reversed the inhibition of OC function by female
Camkk2ΔOCY osteocyte CM. Although the mechanism by which extracellular calpastatin regulates OCs is unclear, we hypothesize that osteocyte-secreted calpastatin inhibits OCs either by inhibiting secreted calpain or via an independent mechanism.
It is also intriguing why the OC-inhibitory effects of extracellular calpastatin, osteocyte-derived or recombinant, are more pronounced in females. Further, the in vivo phenotype of fewer OCs and enhanced bone mass was also only observed in female Camkk2ΔOCY mice. It is likely that sex-divergent signaling mechanisms downstream of secreted calpastatin in female and male OC precursors are responsible for this dichotomy. On the other hand, our proteomics analyses also identified 116 other proteins to be uniquely altered in the female Camkk2ΔOCY osteocyte secretome. Some of these proteins may also play OC-inhibitory roles, potentially collaborating with secreted calpastatin.
In conclusion, we identified a novel cell-intrinsic role for CaMKK2 in osteocytes that results in paracrine effects on OC formation and activity leading to enhanced bone mass only in female mice. This phenotype is in part due to a novel mechanism wherein extracellular calpastatin secreted by osteocytes inhibits bone resorption by female OCs. This novel osteocyte-secreted -calpastatin mechanism could be therapeutically leveraged to treat osteoporosis in women.