Introduction
Inflammation—one result of a host’s immune response to foreign objects or cellular damage—is meant to be an acute physiologic process that is upregulated to clear infections and downregulated when threats are neutralized (1). However, when the body is under consistent insult, the immune system reacts with a chronic inflammatory state that can cause damage at the cellular level leading to tissue dysfunction and eventually disease (2). Over 50% of Americans suffer from one or more chronic diseases that is either caused or prolonged by an unresolved inflammatory environment created by a misfiring immune response (3). Chronic inflammation is driven by a long-standing disruption in homeostasis due to an overabundance of unstable molecular oxidants that create pro-inflammatory compounds, which damage proteins, lipids, carbohydrates, and even DNA (4,5). Despite this, inflammation should not be limited to a bad-actor role in the body but rather should be seen as an imperative signal, which rallies innate and adaptive immune defenses against infection or tissue injury to instigate healing processes that attempt to restore homeostasis (6). In chronic disease, the body’s ability to achieve homeostasis is disrupted by an oxidant/antioxidant imbalance ultimately leading to a pro-oxidant state called oxidative stress (4). Mechanisms within the body emerge to compensate for or derail the inflammatory conditions; however, oxidative stress contributes to age-related diseases such as cognitive decline, atherosclerosis, arthritis, and sarcopenia among others (7–9). Thus, the identification of therapeutic mechanisms to resolve oxidative stress is crucial to support prevention of chronic disease and promote healthy aging. In response to this challenge, the nutrition community turns to our most potent remedies: whole foods and nutrients.
Clinicians are eagerly looking for specific guidance to support the use of antioxidants, whether dietary or as nutraceutical supplements, to achieve therapeutic outcomes and most importantly, avoid harm. At present, there is no consensus regarding the risk-benefit calculus for the clinical use of some exogenous antioxidants – some studies report harm, while others report benefits (10). On the other hand, there is substantial evidence showing that diets higher in minimally processed plant foods promote health and reduce disease (11). The antioxidants present within these foods may mediate the positive effects; however, this remains uncertain. Although healthy dietary patterns have significant barriers to adoption, it remains a crucial tool for fighting chronic inflammation as a means of addressing chronic disease in patients. Nevertheless, much confusion exists around the clinical use of food constituents (food as medicine) and the appropriate use of high doses of concentrated and refined nutrients in the form of nutraceuticals (supplementation) as anti-inflammatory agents. With almost 60% of Americans reporting the use of supplements, understanding the dose- response mechanism for specific antioxidants allows precision in clinical practice to fight the underlying cause of many chronic diseases (12). This leads us to the very heart of our research question: does a dose-response relationship exist with dietary antioxidants and our innate oxidant response system that would qualify as a hormetic response?
Antioxidants are complex compounds that have multi-factorial effects throughout the body. The categorization of plant-based antioxidants, known as phytochemicals or even phytonutrients, is based on the molecular structure that gives rise to the varied functions these compounds serve. Over 8,000 antioxidants are classified as phenols, with half coming from the flavonoid subclass (13). Two ployphenols in particular, curcumin and resveratrol, are derived from foods that have shown strong correlations in observational studies with reduced risk of chronic disease and improved longevity (14,15). The impact on human biochemistry of the isolation of these compounds from their health-promoting foods, turmeric and red wine respectively, is at the root of the clinical questions that gave rise to this review. If a phytochemical like curcumin is credited for antioxidant, anti-inflammatory, and anti-tumor activities, is there a point at which this highly concentrated compound whose health benefits were first recognized in a different format as part of a whole food, can create imbalance in the oxidative homeostasis and lead to negative outcomes? Not to mention the potentially important entourage or food matrix effect that may be lost in the isolation process. In short, are high doses of concentrated, isolated antioxidants harmful while low doses, as would mirror normal dietary intake, beneficial?
Born in the science of toxicology, hormesis has historically been defined as the difference in effect of a chemical messenger when experienced at a low dose, which increases resiliency, versus that at a high dose, which induces toxicity (16). The hormetic response is a foundational construct throughout the human body, as seen in the positive benefits from intermittent fasting (17), physical exercise (18), and mitochondrial replication or cognitive exercises among others (19), which suggests its likely presence in other key functions like oxidant homeostasis. These hormetic influences trigger biochemical processes that translate eustress into an activation of cellular defense signaling pathways that prove beneficial to the experiencer (20). But does this well-accepted phenomenon apply to dietary antioxidants as well?
Studies support the evolving theory that health benefits associated with high dietary intake of antioxidant-rich foods likely fight inflammation by triggering a protective cellular response either through reactive oxygen species (ROS) quenching (oxidant-scavenging) or through activation of key antioxidant pathways such as the NRF2-KEAP1 stress response network (hormetic response) (21). While the hormetic behavior of antioxidants is supported by the observation that the bioavailability of dietary polyphenols in the serum is very low while the subsequent polyphenol metabolites are more abundant (22), the research community has not yet supported this theory in human trials. This observation raises important considerations regarding the role that the Gastrointestinal Tract (GIT) may play in selective uptake of nutrients as well as transforming antioxidants prior to absorption. Various elements influence the capacity of the GIT to digest and absorb antioxidants such as microbial species, epigenetic factors, anatomy, age, and many more. Therefore, interindividual variation in GIT adsorption is almost guaranteed. This implies that every person will likely have a different dietary intake threshold to exhibit a hormetic response. A key consideration for this personalization of hormetic response in vivo is the important distinction between the dose of antioxidant given to an individual and the amount that becomes available to the individual’s antioxidant response system known as the bioavailability.
What is clear is that oxidant balance plays a critical role in the function of the human body and our ability to prevent and remedy chronic disease driven by unresolved inflammation. In this review, we seek to outline the current state of research around the theory of nutritional hormesis and how that theory accommodates the multiple roles antioxidants play in human biochemistry. In addition, we describe the challenges related to the translation of antioxidant research into clinical practice and provide our insights to bridge these barriers. We aim to support the field of antioxidant research to better guide clinical decisions by ensuring accurate translation of the epidemiological and laboratory-based findings of the last 10 years into the human patient population.
Objectives
Assess the strength of existing evidence for dietary antioxidants as hormetic agents in humans.
Describe the evidence for exogenous antioxidants to support anti-inflammatory clinical goals.
Clarify the various roles antioxidants play, i.e. hormetic agents and oxidant scavengers.
Identify research gaps and challenges for the next generation of antioxidant research.
Methods
Literature Search
In conjunction with librarians at our institution, PubMed and Scopus database searches were conducted in the Spring of 2022 to gather a subset of medical literature articles describing the relationship between dietary antioxidants, hormesis, and chronic disease. The search strategy (Table 1) was adapted for each individual database and incorporated both subject terms and free text terms, as applicable. Additionally, snowballing was used when articles contained relevant citations. Five batches of articles were generated using PubMed or Scopus search terms.
Table 1.
Search terms used for each batch.
Table 1.
Search terms used for each batch.
| Batch |
Search Terms |
# of Results |
Purpose |
1
PubMed Date: Through 6/2022 |
(Hormetic response*[tiab] OR hormesis[tiab] OR hormesis[MeSH]) AND (Plant-derived antioxidant*[tiab] OR Plant antioxidant*[tiab] OR Polyphenols[tiab] OR Polyphenols[Mesh] OR Phenolic acids[tiab] OR Flavonoids[tiab] OR Flavonoids[Mesh] OR Anthocyanins[tiab] OR Anthocyanins[Mesh] OR Lignans[tiab] OR Lignans[Mesh] OR Stilbenes[tiab] OR Stilbenes[Mesh] OR Carotenoids[tiab] OR Carotenoids[Mesh] OR Xanthophylls[tiab] OR Xanthophylls[Mesh] OR Carotenes[tiab] OR Vitamin E[tiab] OR Vitamin E[Mesh] OR Vitamin A[tiab] OR Vitamin A[Mesh] OR Vitamin C[tiab] OR Ascorbic Acid[Mesh]) |
143 |
Hormesis |
2
Scopus Date: Through 6/2022 |
((TITLE("Hormetic response*" OR hormesis) OR ABS("Hormetic response*" OR hormesis))) AND ((TITLE("Plant-derived antioxidant*" OR "Plant antioxidant*" OR Polyphenols OR "Phenolic acids" OR Flavonoids OR Anthocyanins OR Lignans OR Stilbenes OR Carotenoids OR Xanthophylls OR Carotenes OR "Vitamin E" OR "Vitamin A" OR "Vitamin C") OR ABS("Plant-derived antioxidant*" OR "Plant antioxidant*" OR Polyphenols OR "Phenolic acids" OR Flavonoids OR Anthocyanins OR Lignans OR Stilbenes OR Carotenoids OR Xanthophylls OR Carotenes OR "Vitamin E" OR "Vitamin A" OR "Vitamin C"))) |
107 |
Hormesis |
3
PubMed Date: Through 6/2022 |
(("Hormesis"[MeSH Terms] OR "hormetic response"[All Fields]) AND ("Vegetables"[MeSH Terms] OR "edible plants"[All Fields] OR "food"[All Fields] OR "antioxidants"[All Fields] OR "polyphenol"[All Fields] OR "resveratrol"[All Fields])) AND (humans[Filter]) |
93 |
Hormesis |
4
PubMed Date: Through 2021 |
(("antioxidants"[MeSH Terms] OR "polyphenols"[MeSH Terms] OR "lycopene"[MeSH Terms] OR "resveratrol"[MeSH Terms] OR "carotenoids"[MeSH Terms]) AND ("disease"[MeSH Terms] OR "chronic disease"[MeSH Terms] OR "alzheimer disease"[MeSH Terms] OR "neoplasms"[MeSH Terms] OR "heart diseases"[MeSH Terms] OR "mortality"[MeSH Terms] OR "diabetes mellitus"[MeSH Terms] OR "hypertension"[MeSH Terms] OR "stroke"[MeSH Terms] OR "obesity"[MeSH Terms] OR "arthritis"[MeSH Terms] OR "lung diseases"[MeSH Terms]) AND "diet"[MeSH Terms]) AND ((meta-analysis[Filter] OR randomizedcontrolledtrial[Filter]) AND (humans[Filter])) |
273 |
Correlation between antioxidants and chronic disease |
5
PubMed Date: Through 2021 |
(polyphenols[mesh] OR carotenoids[mesh] OR curcumin[mesh] OR resveratrol[mesh] OR ascorbic acid[mesh] OR flavonoids[mesh] ) AND (food[mesh] OR diet[mesh] OR dietary supplements[mesh]) AND biomarkers[mesh] AND ((y_10[Filter]) AND (randomizedcontrolledtrial[Filter]) AND (humans[Filter])) |
274 |
RCTs about antioxidants and biomarker endpoints |
Batch 1, 2, and 3 gathered articles discussing the relationship between hormesis, plant antioxidants, and human health. Batch 3 was a refined Batch 1 search to focus more on human studies. Batch 4 was a PubMed search created to examine the broader concept of whether antioxidant intake is linked to positive health outcomes in humans. Finally, Batch 5 was a PubMed search to identify randomized controlled trials using surrogate biomarker endpoints to identify the mechanistic role of antioxidants in humans.
Search Strategy
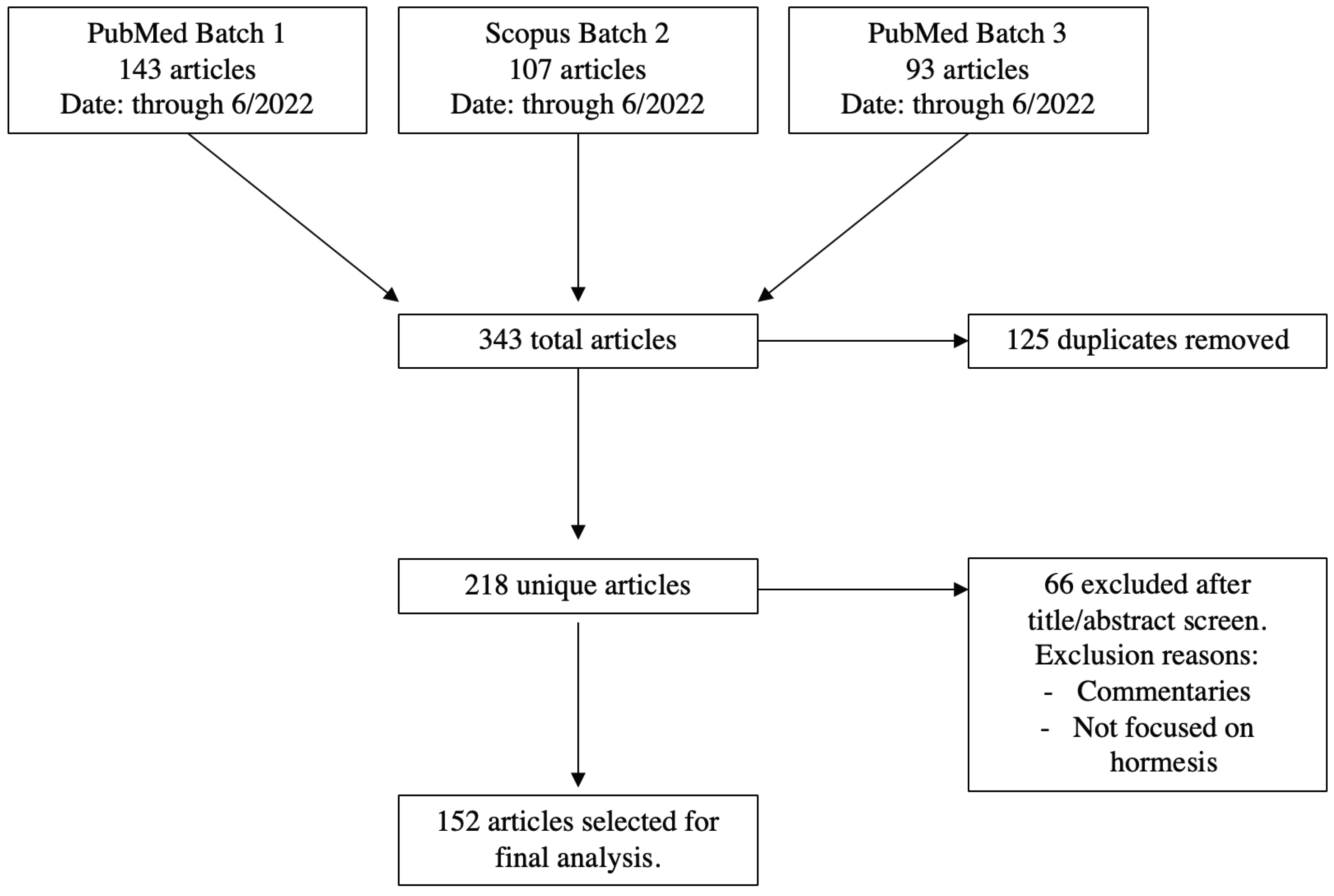
A total of 343 articles were collected from Batch 1, 2, and 3. After removing duplicates, 218 unique articles remained. All titles and abstracts were screened, and 66 articles were removed because they were commentaries or not focused on hormesis (Figure 1).
Figure 1.
PRISMA diagram for articles included in the analysis.
Figure 1.
PRISMA diagram for articles included in the analysis.
Data Abstraction
All articles were independently reviewed via title and abstract and classified as review or non-review articles. Review articles were then examined to determine whether results from human studies were discussed. If the review reported most findings from trials in humans, it was marked with a “review focused on human results” tag. The non-review articles were tagged either with a human, cell, animal, plant, or other organism label depending on the model used for the study. For example, if human liver cells were used, then the study was tagged with a “Cell” label. A third researcher served as a tiebreaker if necessary.
Data Analysis
Statistical tests were tabulated in Excel.
Results
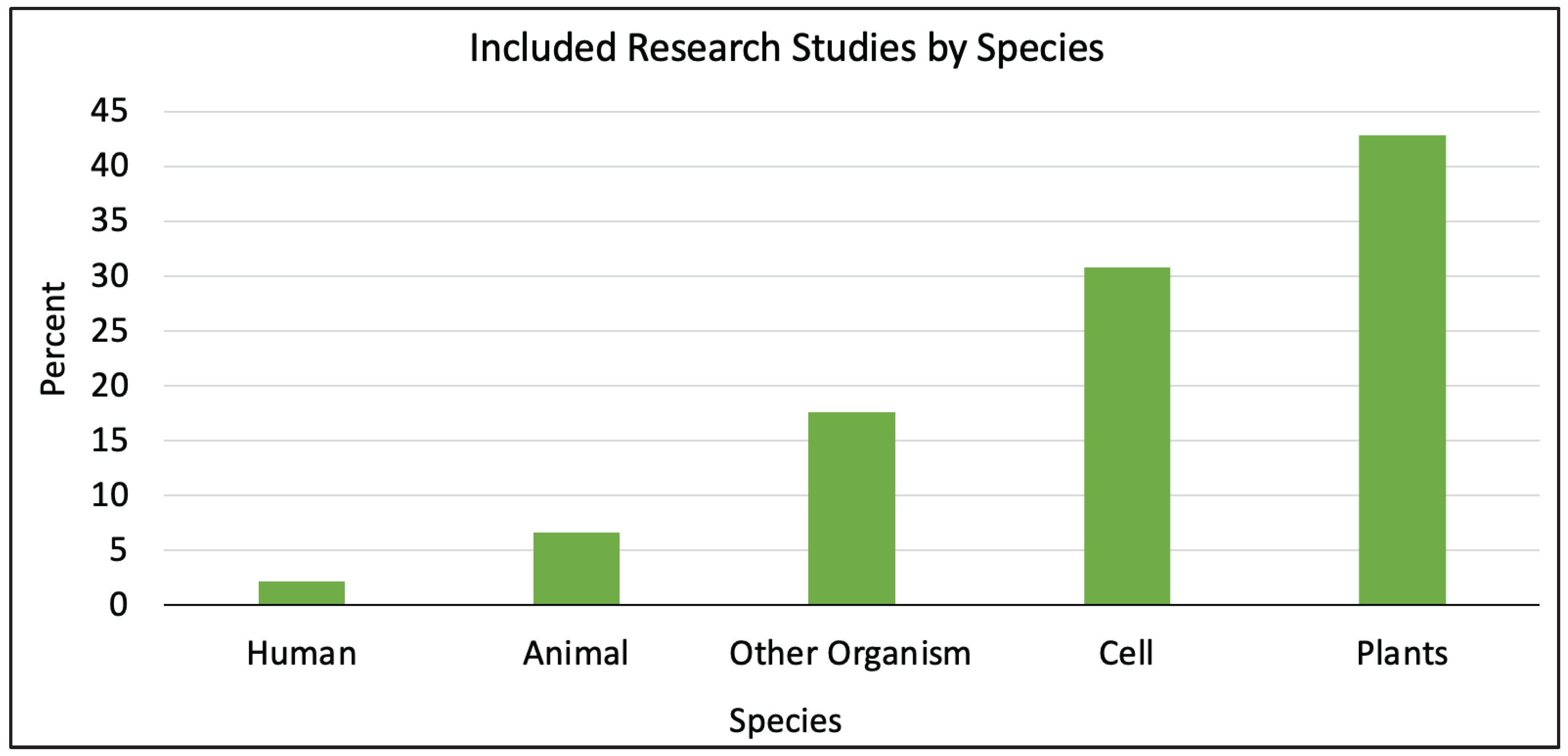
Of the 343 articles reviewed, 152 articles met the inclusion criteria, of which 40.1% (61/152) were review articles. Human results were the focus of 3 of the review articles (4.9%, 3/61). Most of the included studies were not reviews (59.9%, 91/152). Of the non-review articles, 2.2% (2/91) were performed in humans, 30.8% (28/91) in cell culture, 6.6% (6/91) in animals, 42.9% (39/91) in plants, and 17.6% (16/91) were in other organisms such as
Caenorhabditis elegans or
Drosophila melanogaster (
Figure 2). Most of the non-review articles were studying cell or plant models (73.6%, 67/91) versus human or animal models (8.8%, 8/91). Only two non-review studies were completed in humans (1.3%, 2/152).
Discussion
While numerous papers include antioxidants in their list of hormetic agents along with caloric restriction, physical exercise, and fasting, we found that most studies investigate hormesis in cell or plant models rather than in humans. This is consistent with a 2020 review describing the dose-response induced by phytochemicals that found, of included articles, 88% used cell culture models and only 2% used rodent models (23). We build off this review paper by identifying barriers that limit transition of research into humans and provide a discussion of ways to transcend these obstacles.
We identified only two studies examining hormesis in relation to humans. The first investigated the effect of different amounts and types of alcoholic beverages on in vivo plasma antioxidant activity. One drink of red wine, lager, or stout (alcoholic and alcohol free) increased plasma antioxidant activity while three drinks increased plasma pro-oxidant activity (24). The pro-oxidant effect observed after consumption of 3 drinks was thought to result from the production of free radicals from ethanol metabolism. Therefore, less alcohol consumption would lead to less ethanol metabolism likely producing fewer free radicals. The framework utilized in this study to measure serum antioxidant concentration may be valuable for future researchers to investigate antioxidant concentrations in humans after consumption of plant foods. Moreover, the second, a double-blind, randomized, placebo-controlled study, tested the effects high (25 mg/d) and low (5 mg/d) dose supplementation with hydroxytyrosol (a polyphenol) on the induction of phase 2 enzymes in humans (25). Results showed an insignificant difference of expression of most phase 2 enzymes in peripheral blood mononuclear cells between any group. Additionally, there was no significant difference between cardiovascular surrogate markers or inflammatory markers except for plasma hs-CRP and urinary isoprostanes. This study is likely underpowered due to a small sample size (n = 21) and the use of a Latin square design, which limited the window between the start and end of treatment to 7 days. The short treatment duration likely minimized the effect size on phase 2 enzymes and biomarkers, amplifying the lack of power from a small sample size.
Hormetic Responses in Cell Culture
Although cell culture models are incapable of capturing the complexity of mammalian physiology, they do provide a platform to conduct high throughput experiments capable of uncovering molecular mechanisms and other important concepts regarding phytochemical induced hormesis. A seminal 2010 review by Calabrese et al. described the dose-dependent effect of resveratrol on numerous cell lines, which included both healthy and cancerous tissues (26). Generally, in human tumor cell lines, low doses of resveratrol enhanced cell proliferation in the range of 30-60% while high doses suppressed cell proliferation (26). Furthermore, in non-tumor endothelial cell lines, low doses of resveratrol enhanced reendothelialization and improved cell migration whereas high doses were associated with a suppression of tissue repair and cell migration (26). In non-tumor immune cells, low doses of resveratrol enhanced the response of multiple immune cells (e.g. T-cells and spleen cells), while the response was suppressed at high doses (26). Whether the low-dose effect is harmful (cancer cell lines) or beneficial (non-cancer cell lines) to overall health, it is uncertain if the outcomes are clinically significant or negligible. Future research in animals or humans will help clarify this unknown. Additionally, this review concludes with the important observation that low doses of resveratrol exhibit either beneficial or harmful effects depending on the endpoint of interest. As discussed above, low doses increased tumor cell proliferation, which would negatively affect health. This contradicts one prevailing definition of hormesis, which states that low dose stimulation is beneficial to the organism. Future investigators must keep this in mind when defining hormesis and rationalizing results for low and high doses concentrations.
A review focusing on neural stem cell models for curcumin-induced hormesis found a hormetic-like, biphasic dose-response relationship for cell proliferation across 4 studies that used different lineages of neural stem cells (27). Researchers hypothesized the increase in cell proliferation was due to curcumin induced activation of p38 MAP kinase and MEK/ERK. Curcumin has also displayed hormetic features across many different cell types and endpoints including supporting wound healing in human skin fibroblasts, suppressing inflammation in buffalo granulosa cells, stimulating heme oxygenase-1 (HO-1) activity in astrocytes, and many more (27). It is well established that, at least in cell culture models, curcumin induces dose-dependent, bi-phasic effects across many domains. The next step in characterizing the hormetic response of curcumin is moving research to animal and human models.
Hormetic Responses in Animals
Experimental animal models provide us an opportunity to evaluate the dose-dependent effects of phytochemicals in a robust, in vivo setting, which can be more persuasive than in vitro cell culture models. One study investigated the cardioprotective effects of resveratrol in mice hearts exposed to ischemia. Animals were first treated with resveratrol at doses of 2.5, 25, and 100 mg/kg daily for 21 days. Then, ex vivo heart ischemia was followed by 2 hours of reperfusion, during which left ventricle function was evaluated. At 60 and 120-minutes, hearts exposed to 2.5 and 25 mg/kg resveratrol had improved aortic flow and left ventricular developed pressure compared to controls while ventricular function was significantly reduced at doses of 100 mg/kg—perhaps indicating a U- or J-shaped curve to the response (28). The phenomenon observed here reflects low dose resveratrol stimulation (positive effect) and high dose resveratrol inhibition (negative effect), which is symbolic of the hormetic response. A similar trend was observed when researchers measured infarct size in rat hearts; intake of a higher dose of resveratrol (100 mg/kg) was associated with a larger infarct size whereas a lower dose (2.5 mg/kg) resulted in a smaller infarct size (28). A related study aimed to understand the cardioprotective effects of curcumin on myocardial damage in rat hearts and found low doses (100 and 200 mg/kg) prevented myocardial damage while a high dose (400 mg/kg) enhanced myocardial deterioration (29). Of note, the dosing for these studies varies greatly between antioxidants with the high dose of one (resveratrol) being the low dose of another (curcumin). There is no broadly applicable dosing of antioxidants.
Furthermore, a review describing the dose-dependent effects of green tea polyphenols (GTP) in rodents found medium dose and low dose GTP diets (0.01% - 0.1%) inhibited rat colon carcinogenesis while high dose GTP diets did not (30). Additionally, a similar result was also observed when looking at the effect of GTP on hepatic function in rodents exposed to dextran sulfate sodium (DSS). GTPs reduced DSS induced damage at doses of 0.01% and 0.1% (30). The review concluded that low and medium doses (0.01% - 0.1%) of GTPs are beneficial via mitigating intestinal inflammation and carcinogenesis (30). GTPs were hypothesized to minimize DSS induced hepatotoxicity and nephrotoxicity via modulation of self-protective enzymes.
Another example of hormetic response to antioxidants is the carcinogenic dose-response relationship exhibited by dietary caffeic acid in the forestomach and kidney of mice and rats. Researchers were interested in measuring how varying concentrations of caffeic acid affected cell proliferation in these model organisms. Rats were fed caffeic acid at concentrations of 0, 0.05, 0.14, 0.40, and 1.64% over the span of 4 weeks, and forestomach cell proliferation, a hallmark of carcinogenesis, was measured. The arm at 0.14% showed a 30% decrease in the number of cells/mm while the 0.40% group displayed a 2.5-fold increase in cells/mm (31). The authors concluded that the delayed cell division at low caffeic acid concentrations may reflect a protective cancer effect while the high dose accelerated cell growth may promote cancer. Moreover, as of June 2022, there were no clinical trials listed on ClinicalTrials.gov that were tagged with the term “hormesis;” therefore, that it is uncertain when human results will be available.
There are numerous examples of hormetic dose responses from the toxicology literature described in a hormesis database, which compiles over 5600 dose-response relationships across 900 agents (32). Although the database provides a comprehensive analysis of hormetic responses through the lens of toxicology, we were unable to use it to advance our knowledge of antioxidant induced hormesis in humans because the database contains results from human models that are “generally in vitro.” This agrees with our analysis of the literature that there are few to no studies utilizing in vivo human models.
Translation Challenges
The examples above in animal and cell culture models demonstrate that hormetic responses induced by antioxidants exist, which strengthens the likelihood that a similar result may be observed in humans. If true, this may have important implications for supplement use by the public as well as how clinicians utilize antioxidants in clinical practice. However, we must be careful when extrapolating results to humans because of the numerous complexities of the human body and human experience. For one, cell culture utilizes isolated cells stripped from their natural environment, which does not recapitulate the many interactions between cells in the ecosystem of the human body. Thus, many signals that occur in vivo are likely missing in cell culture experiments. In addition, when an agent is introduced into cell culture, it is typically placed in the media surrounding the cells. This is vastly different than the way cells in the human body would be exposed to a compound entering through the GIT, as it would for food or dietary supplements. While it is less intuitive, it is clear that even animal models may be a poor predictor of outcomes in humans. Each animal has its own set of underlying genetics, anatomy and physiology, pathological responses, habitat, microbiome, and much more that influence how it responds to certain stimuli (33). Thus, an outcome in one species may not translate to humans or even a different more similar species (34). Other characteristics that limit translation of some animal model findings to humans include inadequate study design (e.g. insufficient power, limited representation of interindividual variability), failure to measure outcomes over a long duration, insufficient description of statistical tests, and limited reproducibility of intra and inter experimental results (35). In summary, results from cell culture and animal models may guide our investigations in humans but should not be a stand in for human data, as they sometimes are when data in humans is lacking with the exception of when human data is unethical or unattainable (34).
Our review of the current state of the literature regarding the impact of antioxidants on chronic disease reveals limited discussion around a hormetic bi-phasic response and an inconsistent nomenclature that impacts the ability of the community to identify and locate the research. The last expert review on this topic was by compiled by Mark Birringer in 2011 (21), and the last literature review was done in 2005 by David Lindsay (36). A 2020 systemic review in the Journal of Clinical Medicine by Jodynis-Leibert and Kujawska reveals little progress in the application of the hormetic response within animal models, let alone the complexity of human biochemistry, based on their analysis of the literature from 1990-2019 (23). Thus, given the limitations of extrapolating the impact of antioxidants on plants or in vitro, the research community must shift the focus onto the role of antioxidants as hormetic agents within the complexity of human biochemistry in vivo.
Opportunities and Challenges to shift Antioxidant Research into Humans
Subsequently, we offer insight into some of the existing barriers within the literature discussing hormesis and ways the research community may proceed to study nutritional hormesis in humans.
Lack of precision in data collected from dietary sources of antioxidants and correlated absorption into the human body
The study of nutrition does not lend itself easily to many of the research approaches that have provided clarity in other areas of medicine such as medications. Many factors contribute to this difficulty including the complexity of food and the difficulty in creating blinding opportunities; how do you blind subjects to eating an apple or not? Several limitations in data collection were highlighted in our literature review related to the accuracy in measuring dietary intake of antioxidants and the ability to quantify the amount of antioxidant delivered to cellular targets (the bioavailable dose).
One common weakness of many studies is the use of inexact food measurement tools such as food frequency questionnaires, 24-hr recalls, or food records (37). One possible solution is to use a controlled diet environment that would allow complete control over intake for the duration of the study. This would ensure a higher level of accuracy in the amount and type of antioxidants that were consumed. However, the increased cost of such studies in terms of research dollars and participant burden would need to be weighed against the benefit from increasing data accuracy, which we feel is warranted to establish precise thresholds that will allow us to understand when a dose-response relationship goes from beneficial to harmful. Once this aspect of the data is more precise, the question of antioxidant bioavailability can be addressed.
The bioavailability of nutrients is the amount of the nutrient that makes its way through the digestion and absorption process such that it becomes available to support use or storage in the body (38). Nutrients, including antioxidants, enter the body through a selective process in the GIT, digestion and nutrient absorption. Setting a dose-response threshold requires increased specificity to acknowledge the factors involved in the digestion and absorption of dietary nutrients in the GIT. Thus, the use of blood biomarkers that represent antioxidant bioavailability rather than or in conjunction with dietary patterns may greatly improve the precision and accuracy of the research in this field.
Factors that impact digestion and absorption and ultimately bioavailability of antioxidants include (39):
Medications: The side effects from medications extend into the microbial composition of the intestines and alter the environment in which antioxidants interact with oxidants and the bioavailability. Antibiotics as well as many non-antibiotic drugs predictably alter the microbiome towards pro-inflammatory functionality (40). Thus, the current and former medical history of a patient may alter the behavior of the antioxidant within their specific microbial terrain, which would alter the hormetic threshold.
Age: Changes to the GIT with aging impact the ability to digest and absorb nutrients and antioxidants (41). Thus, the dose of antioxidants that a person is able to access from a dietary source (bioavailability) varies greatly depending on the health of their GIT. Age is an independent risk factor for impaired gut function. In addition, age-related changes to the immune system (immunosenescence) result in an increase in the amount of pro-inflammatory messengers produced, which leads to states of higher oxidative stress (42). Given that the majority of immune cells reside in and around the GIT, immunosenescence likely changes the reaction to oxidative stress due to age alone.
Food: Variability in nutrient concentrations within a food due to the health of the environment (soil/air/water), farming practices, time from harvest, and method of preparation changes the quantities of antioxidants within any given food (43,44). Crinnion’s study comparing food value between organically and non-organically grown produce confirms that food grown organically contains significantly more antioxidants than their non-organic counterparts at least in some cases (44).
GIT Function & the Microbiome: Digestion consists of bioaccessibility and absorption. Bioaccessibility is the liberation and solubilization of food components—getting the nutrients out of the food. Absorption is getting those now free nutrients transported across the gut barrier. The gut microbiota are bacteria, archaea, yeast, fungi, viruses, and phages that comprise the gut microbiome. The gut microbiome plays a very important role in bioaccessibility; for instance, up to 10% of energy requirements come from energy harvest by the gut microbiota (45–48). A specific instance of this has been elegantly shown through a resistant starch feeding study in which Ruminococcus bromii was required to be present in the gut microbiomes of participants for complete utilization of the resistant starch (100% with vs. 20-30% without R. bromii) (49). This alludes to the large interindividual variability of microbiomes and, thus, functional outcomes such as bioaccessibility, which has been shown throughout the literature (48,50–52). How does this role in bioaccessibility affect polyphenols? An estimated 90% of polyphenols in food make it to the gut microbiome unprocessed, where the microbiota processes them and improves bioavailability for the host (53–56). Therefore, the dose-response relationship of polyphenol intake is likely powerfully modified by the composition and/or function of the gut microbiome. This fact has all but been overlooked to date.
Consensus is lacking on how to assess antioxidant and oxidants in human models in order to understand the effect of antioxidants on oxidative stress
The methods used to assess antioxidant concentrations vary widely and prevent a more detailed understanding of dose response. It is unclear which biomarkers are the most reliable measure of antioxidant concentrations and which we should look to for an understanding of oxidative stress. Should we use plasma concentrations of antioxidants or oxidants? Should we look at the Total Antioxidant Capacity of a food or nutrient? Should we assess oxidative stress using C-reactive protein (CRP), homocysteine, or lipid peroxides? If we can develop standards of measurement for antioxidant activity and oxidant exposures, we can apply these standards to all antioxidants to elucidate the impact on oxidative stress. Perhaps standard methods could be developed by National Institute of Standards and Technology (NIST) in conjunction with leaders in the field, developing a standardized methodology as well as language. This would allow for easy data harmonization and comparison across multiple studies, which will be necessary to gain a complete understanding of this complex relationship.
Lack of standardization in research approach erodes the impact of cumulative research
Study Populations Unclear
When reviewing the literature, we sought to identify hormetic responses found in human subjects. In our literature search, 40.1% (61/152) of included reports were review papers. Review papers, by nature, combine results from many different studies; thus, data from multiple types of subjects (e.g. cell culture and animal models) is described together. In many cases, we found it challenging and cumbersome to pinpoint the subject of particular data points without examining the provided reference, meaning the science is not being communicated precisely enough as is. Some review papers did describe the subject of the research for all items of evidence; however, this was not universal and is an area for improvement.
Lack of Keyword Standardization
All 4 studies previously discussed in the animal section were not captured in our initial search (28–31). It is unclear exactly why these articles were excluded; however, 3 of 4 were tagged with the keyword, “Dose-Response Relationship, Drug” and not “hormesis” which was used in our search. The absence of universal keywords by all articles discussing hormesis is potentially limiting when attempting to identify relevant literature.
Throughout our review process we encountered keywords used inconsistently and interchangeably across research papers, which made it difficult to discern the precise meaning and scope of the research. Key terms that would benefit from standardization in the field are listed in Table 2. Our review process was bogged down by crucial key words such as antioxidant and polyphenol, being used with alternative meanings that were not applicable across research papers. For example, the key word ‘Antioxidant’ is defined by the National Center for Complimentary and Integrative Health as either man-made or natural substances that have a positive effect on the health of the cell. This definition is sufficiently broad that it could include nearly all vitamins, minerals, and fatty acids that are involved in the maintenance of the cell as well as non-nutritive plant phytochemicals. Thus, clarity would increase with the consistent use of specific qualifiers such as including the word ‘phytochemical’ before antioxidants to narrow the topic to exclude man-made substances. The group of researchers from IntechOpen in their chapter on Antioxidants Categories and Mode of Action further categorize the field by narrowing the topic to enzymatic versus non-enzymatic antioxidants, which distinguishes between antioxidants that catalyze reactions (SuperOxide Dismutase, Glutathione peroxidases, and Catalase) and those that contain hydroxyl groups that scavenge free radicals throughout the body (vitamin C, E, B-carotene, phenols, tannins, terpenes) (57). Research terms that can be specific and descriptive will help narrow the focus and allow more meaningful consolidation of research in the field. For example, rather than using the broad term 'antioxidant,’ employing qualifiers with this term such as nonenzymatic phytochemical antioxidants.
Table 2.
Key words found to be used inconsistently and interchangeably across the literature.
Table 2.
Key words found to be used inconsistently and interchangeably across the literature.
| Vocabulary Used |
Definition |
Types |
| Antioxidant |
Antioxidants are man-made or natural substances that may prevent or delay some types of cell damage (58). |
Vitamin C, vitamin E, plant polyphenol, carotenoids, and glutathione are nonenzymatic antioxidants (59). |
| Polyphenol |
Polyphenols are secondary metabolites of plants and are generally involved in defense against ultraviolet radiation or aggression by pathogens. More than 8,000 polyphenolic compounds have been identified in various plant species. All plant phenolic compounds arise from a common intermediate, phenylalanine, or a close precursor, shikimic acid (60). |
Phenolic acids
Flavonoids
Stilbenes
Lignans |
| Plant-based antioxidants |
Plant-derived antioxidants are a large group of natural products with reducing or radical-scavenging capacity (61). |
Non-plant-based antioxidants are synthetically derived for use in food preservation. |
| Phytonutrients |
Phytonutrients is a broad name for a wide variety of compounds produced by plants... Some researchers estimate there are up to 4,000 phytonutrients (62). |
Antioxidants, flavonoids, phytochemicals, flavones, isoflavones, catechins, anthocyanidins, isothiocyanates, carotenoids, allyl sulfides, polyphenols |
| Phytochemical |
Phytochemicals can be defined, in the strictest sense, as chemicals produced by plants. However, the term is generally used to describe chemicals from plants that may affect health but are not essential nutrients (63). |
From LinusPauling Institute (63): carotenoids, chlorophyll and chlorophyllin, curcumin, fiber, flavonoids, garlic, indole-3-carbinol, isothiocyanates, lignans, phytosterols, resveratrol, soy isoflavones
From Antioxidants, 2019 (59): flavonoids, catechins, carotenoids, carotene, lycopene, and herbs and spices such as diterpene, rosmariquinone, thyme, nutmeg, clove, black pepper, ginger, garlic, curcumin, and derivatives. |
Confusion about whether or not antioxidants can be toxic
Some observational studies challenge the notion that there is an upper limit for antioxidant utility by showing a positive correlation between increased antioxidant intake and better outcomes. Data from the PREDIMED trial showed reduced all-cause mortality for the highest polyphenol intake (self-reported) compared to lowest intake via multivariate analysis (64). Moreover, a clinical trial investigating the impact of carotenoids on breast cancer recurrence reported that women with the highest plasma carotenoid concentration had a significantly reduced risk of a new breast cancer event (65). This trend is not isolated to these particular studies. Various meta-analyses of observational studies also found a reduction in adverse health events with increased antioxidant intake (66–69) or circulating concentrations of antioxidants (70).
Although the general trend of higher intake and favorable outcomes is described for various types of antioxidants in meta-analyses, conflicting results exist that impart reasonable doubt into the assumption that greater consumption of antioxidants is always beneficial. For example, in a fertility study by Dias et al., the theory that more antioxidants will lead to positive health outcomes is challenged by results that showed a decline in sperm viability (a distinct U-shaped curve) after an initial positive impact at lower doses of antioxidant intake likely due to the inhibition of essential signaling pathways that ROS trigger, which creates homeostatic balance (71). Furthermore, a meta-analysis found a U-shaped, dose-response relationship between dietary vitamin C and all-cause mortality (67). Although unclear why, it is possible that high doses of vitamin C in the presence of transition metals serve as pro-oxidants. The same meta-analysis found a U-shaped association between circulating lycopene and all-cause mortality. The authors suggest that circulating lycopene may be a surrogate for intake of highly processed tomato products such as sugar-sweetened ketchup or pizza sauce, which contain high amounts of lycopene. If true, the negative results found for high levels of circulating lycopene may be due to harmful effects of ultra-processed foods rather than lycopene itself.
In summary, there is uncertainty about the optimal dose of antioxidant intake. Clear toxicity limits to antioxidants have not been well established and are likely dependent on the particular antioxidant in question. Outside of supplements, antioxidants are almost universally found in foods, creating unavoidable confounding. Food exerts its effects through multiple interactions between the phytochemicals it contains and biochemical processes in the human body—and the gut microbiome. This factor further complicates the equation because we may not be able to tease apart which items within a food are causing harm and which are promoting health easily or, perhaps, even at all. The entourage or food matrix effect is elucidating that foods are not simply the sum of their parts, meaning that anyone component in isolation may not exhibit the same effect as it would in the whole food.
Studies do not consider the importance of the duration of exposure to a dose of antioxidants
Hormesis is typically studied by introducing a pre-set dose or series of doses of a compound to either a cell or animal model, followed by observing a response, often immediately or shortly after. This design allows researchers to determine the dose-response relationship of the system and compose curves to illustrate the correlation. Although this results in valuable data, it may be equally important to appreciate the temporal trends of a dose-response curve. For example, a stimulatory, low dose may only be beneficial if applied for a short amount of time. A longer duration could potentially elicit harmful effects on the system. In contrast, a high dose seen for a short period of time immediately after a meal may not be detrimental due to its transitory nature. Even though this is a theoretical situation, it provides a framework for thinking how time may affect a hormetic response. This is especially important to consider when investigating hormetic phenomena in humans because of the complex interplay between compounds we ingest and the cells within the body. Therefore, we propose use of area under the curve (AUC) instead of simply dose. Time in range could also be potentially beneficial in the future once hormetic relationships have been established.
Bioindividuality has not been fully acknowledged from a genetic and epigenetic perspective
The genetic blueprint that an individual patient overlays onto the bioavailability of antioxidants will create variation in the dosing of antioxidants from dietary and supplemental sources. Smoliga, Baur, and Hausenblas make note of the “inter-individual difference in bioavailability” in their 2011 review of clinical trials investigating resveratrol (72). Single Nucleotide Polymorphisms (SNPs) such as SEPP1 and GPX, impact antioxidant bioavailability by changing the transport, receptor levels, and enzymatic activity (73). The clinical implication of nutrigenomics in response to antioxidants requires additional insight into how an individual’s genetic and epigenic profile will require personalization of any therapeutic recommendations. While the promise of personalized and precision nutrition, including nutrigenomics, are great we are not there yet. To build the evidence base, the National Institutes of Health is launching the Nutrition for Precision Health study, powered by the All of Us Research Program (74). While antioxidant hormesis is not a stated objective of this research program, it may lead to a large human data set in which this relationship can be explored.
Changing definition of hormesis makes it difficult to compare research results
We found that the definition of hormesis varies depending on the area of study, the timeframe of study completion, and the preference of the study authors (Table 3). Further, the cultural understanding of hormesis adds to the confusion around what exactly a hormetic response looks like and whether or not a given compound or lifestyle behavior exhibits this pattern. In summary, the field of nutrition lacks a cohesive and agreed-upon definition of hormesis that would provide a clear standard by which antioxidant behavior patterns may be judged.
Table 3.
Studies using different definitions of hormesis.
Table 3.
Studies using different definitions of hormesis.
| Author |
Date |
Definition |
Subject Area |
| DP Hayes (75) |
2007 |
The biphasic nature of the U-shaped response can... be subdivided into low and high-dose regions where the toxicity response differentially occurs (the arms of the U), plus a region of no toxic effect (the trough of the U). |
Nutrition |
| Calabrese (26) |
2010 |
Hormesis is a biphasic dose response phenomenon that is characterized by a low-dose stimulation and a high-dose inhibition. |
Toxicology |
| Chirumbolo (16) |
2011 |
A biphasic dose–response relationship for which low doses display stimulation and high doses inhibition |
Toxicology |
| Jodynis-Liebert and Kujawska (23) |
2020 |
The phenomenon in which a chemical is able to induce biologically opposite effects at different doses |
Nutrition |
Improved study design can bring consistency with cumulative effects across studies
We observed a lack of consistency in study design and application across different types of antioxidants, making it difficult to aggregate findings across research papers. As a foundation for employing cumulative research, attention needs to be applied to systematically studying each antioxidant to define the oxidant-resolving patterns as oxidant scavenging (linear curve), hormetic (J- or U-shaped curve), or possibly a hybrid of the two.
Limitations
This study has 2 major strengths and 3 major limitations. First, the strengths. We searched two large databases and found articles discussing the relationship between plant antioxidants and hormesis. Our results were consistent with the general consensus of the literature, which is that hormesis has been rarely studied in human populations. In addition, we offered actionable advice for researchers and the field for future experiments exploring the hormetic responses in humans. Yet, this study has limitations. Our initial search did not include the terms “dose-response,” which we found later was a keyword tagged by many of the articles discussing hormesis. We may have missed articles that found a hormetic response but did not tag their report with the keyword, “hormesis.” Furthermore, our initial aim was to summarize our current knowledge of the hormetic dose-response relationship induced by plant-based antioxidants in humans; however, our literature search revealed very limited data on this subject. Thus, we adapted our study and moved in the direction of offering advice to researchers on aspects we believe are important when studying hormesis in humans. Finally, we did not evaluate clinical trials that used antioxidants either in the form of foods or supplements in humans. It is possible that some trials did find a hormetic dose-response relationship but did not tag their findings with hormesis and therefore would have been excluded from our analysis.
Conclusions
Given the limited robust evidence, it is difficult for clinicians to ascertain the appropriate therapeutic dose of dietary antioxidants or, even more concerning, antioxidant supplements for patients. It is currently unclear how the two functional mechanisms of antioxidants (free radical scavenging and hormesis) cooperate in human biochemistry. The role of antioxidants as hormetic agents has been overwhelmingly studied in plants and cell culture, leaving clinicians blinded to the effects of these chemical messengers in their patients. Meta-analyses based on observational research establish an association between diets that are high in plant antioxidants with a reduction in chronic disease and improved well-being (67,76,77), which may run contrary to the hormesis theory of antioxidants or be in keeping with the theory based on lower absorption and bioavailability. A knowledge gap exists between the observational human studies and the cell culture and animal model research, which shows a biphasic, hormetic quality to the role of antioxidants. Cell culture and animal model research cannot accurately be translated directly into clinical care; therefore, antioxidant therapy cannot be employed in the clinic at this time. The question remains, at what dose and/or blood concentration do polyphenols promote an anti-inflammatory response versus overwhelming the biological infrastructure and become pro-inflammatory? Our concern is that well-meaning clinicians as well as the public apply the thinking, more is better, when discussing the role of dietary antioxidants. In fact, some of the literature may be the rationale for this thinking; however, it may turn out to be harmful due to a hormetic response. In summary, we have described why it is currently difficult to translate our understanding of hormesis to therapeutic antioxidant intake in humans and have proposed steps for the research community to address the knowledge gap between cells/animals and humans. A stronger understanding of the functions of antioxidants in humans will surely help tackle the chronic disease epidemic and improve the lives of patients.
Data Sharing
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
Author Contributions
JW and LAF designed research; LAF, JW, and BK conducted research; JW and BK analyzed data; JW, BK, and LAF wrote the paper. JW and LAF had primary responsibility for final content. All authors have read and approved the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge Elaine Sullo, MLS, MAEd, Associate Director for Reference and Instruction at the George Washington University’s Himmelfarb Health Sciences Library, for her guidance and support in developing the search strategies for this review and Linda Cotton, MA, Senior Instructional Multimedia Producer at the George Washington School of Medicine and Health Sciences, for her design of the graphical abstract.
Conflicts of Interest
The authors declare no conflict of interest.
Running Title
Plant-based antioxidant activity in the human body.
Abbreviations
AUC: area under the curve
CRP: C-reactive protein
GIT: gastrointestinal tract
GTP: green tea polyphenols
NRF2-KEAP1: NF-E2 p45-related factor 2 - Kelch-like ECH-associated protein 1
ROS: reactive oxygen species
SNPs: single nucleotide polymorphisms
References
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Chronic Inflammation - StatPearls - NCBI Bookshelf [Internet]. [cited 2022 Jul 16]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493173/.
- Boersma, P.; Black, L.I.; Ward, B.W. Prevalence of Multiple Chronic Conditions Among US Adults, 2018. Preventing Chronic Disease 2020, 17, 200130. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 2010. p. 118–26.
- Hunter, P. The inflammation theory of disease. EMBO Rep 2012, 13, 968–970. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature. Nature Publishing Group; 2008. p. 428–35.
- Pawelec, G.; Goldeck, D.; Derhovanessian, E. Inflammation, ageing and chronic disease. Current Opinion in Immunology 2014, 29, 23–28. [Google Scholar] [CrossRef]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin Chim Acta 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. International Journal of Food Properties 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, Davis EM, Donahue KE, Doubeni CA, Jaén CR, et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer. JAMA 2022, 327, 2326.
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr 2017, 57, 3640–3649. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Data Brief 399: Dietary Supplement Use Among Adults: United States, 2017–2018. Hyattsville, MD; 2021 Feb.
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef]
- Tagde P, Tagde P, Islam F, Tagde S, Shah M, Hussain ZD, Rahman MdH, Najda A, Alanazi IS, Germoush MO, et al. The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders. Molecules 2021, 26, 7109.
- Chirumbolo, S. Hormesis, resveratrol and plant-derived polyphenols: some comments. Human & Experimental Toxicology 2011, 30, 2027–2030. [Google Scholar]
- Raefsky, S.M.; Mattson, M.P. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radical Biology and Medicine 2017, 102, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, T.; Genovesi, F.; Nemmer, M.; Carling, C.; Alberti, G.; Howatson, G. Nutritional interventions for reducing the signs and symptoms of exercise-induced muscle damage and accelerate recovery in athletes: current knowledge, practical application and future perspectives. Eur J Appl Physiol [Internet] Eur J Appl Physiol; 2020 [cited 2022 Jul 30], 120, 1965–1996. Available from: https://pubmed.ncbi.nlm.nih.gov/32661771/.
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Ke, P.-Y.; Wu, C.-Y.; Peng, H.-H.; Young, J.D. Hormetic Effects of Phytochemicals on Health and Longevity. Trends in Endocrinology & Metabolism 2019, 30, 335–346. [Google Scholar]
- Fedullo, A.L.; Ciccotti, M.; Giannotta, P.; Alviti, F.; Bernardi, M.; Raguzzini, A.; Toti, E.; Sciarra, T.; Peluso, I. Hormetic Effects of Bioactive Compounds from Foods, Beverages, and Food Dressing: The Potential Role in Spinal Cord Injury. Oxidative Medicine and Cellular Longevity 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Birringer, M. Hormetics: Dietary Triggers of an Adaptive Stress Response. Pharmaceutical Research 2011, 28, 2680–2694. [Google Scholar] [CrossRef]
- Murakami, A. Non-specific protein modifications may be novel mechanism underlying bioactive phytochemicals. Journal of Clinical Biochemistry and Nutrition 2018, 62, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence. Journal of Clinical Medicine 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Prickett, C.D.; Lister, E.; Collins, M.; Trevithick-Sutton, C.C.; Hirst, M.; Vinson, J.A.; Noble, E.; Trevithick, J.R. Alcohol: Friend or FOE? Alcoholic Beverage Hormesis for Cataract and Atherosclerosis is Related to Plasma Antioxidant Activity. Nonlinearity in Biology, Toxicology, Medicine 2004, 2 154014204909002.
- Crespo, M.C.; Tomé-Carneiro, J.; Burgos-Ramos, E.; Loria Kohen, V.; Espinosa, M.I.; Herranz, J.; Visioli, F. One-week administration of hydroxytyrosol to humans does not activate Phase II enzymes. Pharmacological Research 2015, 95–96, 132–137.
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Human & Experimental Toxicology 2010, 29, 980–1015. [Google Scholar]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Mattson, M.P.; Rattan, S.I.S. Curcumin and hormesis with particular emphasis on neural cells. Food and Chemical Toxicology 2019, 129, 399–404. [Google Scholar] [CrossRef]
- Juhasz, B.; Mukherjee, S.; Das, D.K. Hormetic response of resveratrol against cardioprotection. Exp Clin Cardiol 2010, 15, e134-8. [Google Scholar] [PubMed]
- Tanwar, V.; Sachdeva, J.; Kishore, K.; Mittal, R.; Nag, T.C.; Ray, R.; Kumari, S.; Arya, D.S. Dose-dependent actions of curcumin in experimentally induced myocardial necrosis: a biochemical, histopathological, and electron microscopic evidence. Cell Biochemistry and Function 2010, 28, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch Biochem Biophys 2014, 557, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lutz, U.; Lugli, S.; Bitsch, A.; Schlatter, J.; Lutz, W.K. Dose Response for the Stimulation of Cell Division by Caffeic Acid in Forestomach and Kidney of the Male F344 Rat. Toxicological Sciences 1997, 39, 131–137. [Google Scholar] [CrossRef]
- CALABRESEE; BLAINR The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicology and Applied Pharmacology 2005, 202, 289–301. [CrossRef]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochemical Pharmacology 2014, 87, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Extrapolating from Animals to Humans. Science Translational Medicine 2012;4.
- Beck, A.P.; Meyerholz, D.K. Evolving challenges to model human diseases for translational research. Cell and Tissue Research 2020, 380, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.G. Nutrition, hormetic stress and health. Nutrition Research Reviews 2005, 18, 249–258. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Augustin, J.L.; Schaefer, M.M.; Rasmussen, H.; Ordovas, J.M.; Dallal, G.E.; Dwyer, J.T. Lack of efficacy of a food-frequency questionnaire in assessing dietary macronutrient intakes in subjects consuming diets of known composition. The American Journal of Clinical Nutrition 2000, 71, 746–751. [Google Scholar] [CrossRef]
- Melse-Boonstra, A. Bioavailability of Micronutrients from Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Frontiers in Nutrition 2020;7.
- Combs Jr, G.F.; McClung, J.P. The Vitamins (Fifth Edition). 2017.
- le Bastard Q, Al-Ghalith GA, Grégoire M, Chapelet G, Javaudin F, Dailly E, Batard E, Knights D, Montassier E. Systematic review: human gut dysbiosis induced by non-antibiotic prescription medications. Alimentary Pharmacology & Therapeutics 2018, 47, 332–345.
- Soenen, S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. The ageing gastrointestinal tract. Current Opinion in Clinical Nutrition and Metabolic Care 2016, 19, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Myers SS, Zanobetti A, Kloog I, Huybers P, Leakey ADB, Bloom AJ, Carlisle E, Dietterich LH, Fitzgerald G, Hasegawa T, et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142.
- Crinnion, W.J. Organic foods contain higher levels of certain nutrients, lower levels of pesticides, and may provide health benefits for the consumer. Altern Med Rev 2010, 15, 4–12. [Google Scholar] [PubMed]
- McNeil, N.I. The contribution of the large intestine to energy supplies in man. The American Journal of Clinical Nutrition 1984, 39 338–42.
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiological Reviews 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Jeffery, I.; O’Toole, P. Diet-Microbiota Interactions and Their Implications for Healthy Living. Nutrients 2013, 5, 234–252. [Google Scholar] [CrossRef]
- Duncan, S.H.; Flint, H.J. Probiotics and prebiotics and health in ageing populations. Maturitas 2013, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. British Journal of Nutrition 2009, 101, 541–550. [Google Scholar] [CrossRef]
- Davis, L.M.G.; Martínez, I.; Walter, J.; Goin, C.; Hutkins, R.W. Barcoded Pyrosequencing Reveals That Consumption of Galactooligosaccharides Results in a Highly Specific Bifidogenic Response in Humans. PLoS ONE 2011, 6, e25200. [Google Scholar] [CrossRef]
- Thompson S v, Hannon BA, An R, Holscher HD. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition 2017, 106, 1514–1528.
- Roca-Saavedra, P.; Mendez-Vilabrille, V.; Miranda, J.M.; Nebot, C.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Food additives, contaminants and other minor components: effects on human gut microbiota—a review. Journal of Physiology and Biochemistry 2018, 74, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.; Bird, A. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Romo-Hualde, A.; Macarulla, M.T.; Portillo, M.P.; Milagro, F.I.; Martínez, J.A. Metabolic faecal fingerprinting of trans-resveratrol and quercetin following a high-fat sucrose dietary model using liquid chromatography coupled to high-resolution mass spectrometry. Food & Function 2015, 6, 2758–2767. [Google Scholar]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Azat Aziz, M.; Shehab Diab, A.; Abdulrazak Mohammed, A. Antioxidant Categories and Mode of Action. Antioxidants. IntechOpen; 2019.
- Chun, O.; Frei, B.; Gardner, C.; Alekel, L.; Killen Jr, J. Antioxidants: In Depth. National Center for Complementary and Integrative Health. 2013.
- Shalaby E, editor. Antioxidants. IntechOpen; 2019.
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Medicine and Cellular Longevity 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, R.; Pospíšil, P.; Kruk, J. Plant-Derived Antioxidants in Disease Prevention 2018. Oxidative Medicine and Cellular Longevity 2018, 2018, 1–2. [Google Scholar] [CrossRef]
- What Are Phytonutrients? [Internet]. [cited 2022 Jul 21]. Available from: https://fruitsandveggies.org/stories/what-are-phytochemicals/.
- Phytochemcials [Internet]. [cited 2022 Jul 21]. Available from: https://lpi.oregonstate.edu/mic/dietaryfactors/phytochemicals.
- Tresserra-Rimbau A, Rimm EB, Medina-Remón A, Martínez-González MA, López-Sabater MC, Covas MI, Corella D, Salas-Salvadó J, Gómez-Gracia E, Lapetra J, et al. Polyphenol intake and mortality risk: a re-analysis of the PREDIMED trial. BMC Medicine 2014, 12, 77.
- Rock, C.L.; Flatt, S.W.; Natarajan, L.; Thomson, C.A.; Bardwell, W.A.; Newman, V.A.; Hollenbach, K.A.; Jones, L.; Caan, B.J.; Pierce, J.P. Plasma Carotenoids and Recurrence-Free Survival in Women with a History of Breast Cancer. Journal of Clinical Oncology 2005, 23, 6631–6638. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. The American Journal of Clinical Nutrition 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Parohan, M.; Zargar, M.S.; Shab-Bidar, S. Dietary Antioxidants, Circulating Antioxidant Concentrations, Total Antioxidant Capacity, and Risk of All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Observational Studies. Advances in Nutrition 2018, 9, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Orsini, N.; Wolk, A. Vitamin C and survival among women with breast cancer: A Meta-analysis. European Journal of Cancer 2014, 50, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, E.; Liu, L.; Zhang, W.; Wei, X.; Gao, X.; Song, N.; Fu, C. Dietary intake of vitamins A, C, and E and the risk of colorectal adenoma. European Journal of Cancer Prevention 2013, 22, 529–539. [Google Scholar] [CrossRef] [PubMed]
- LIX; XUJ Dietary and circulating lycopene and stroke risk: a meta-analysis of prospective studies. Scientific Reports 2014, 4, 5031. [CrossRef] [PubMed]
- RDias, T.; Martin-Hidalgo, D.; M Silva B, F. Oliveira P, G. Alves M. Endogenous and Exogenous Antioxidants As a Tool to Ameliorate Male Infertility Induced by Reactive Oxygen Species. Antioxidants & Redox Signaling 2020, 33, 767–785. [Google Scholar]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and health - A comprehensive review of human clinical trials. Molecular Nutrition & Food Research 2011, 55, 1129–1141. [Google Scholar]
- Pascual-Geler, M.; Robles-Fernandez, I.; Monteagudo, C.; Lopez-Guarnido, O.; Rodrigo, L.; Gálvez-Ontiveros, Y.; Cozar, J.M.; Rivas, A.; Alvarez-Cubero, M.J. Impact of oxidative stress SNPs and dietary antioxidant quality score on prostate cancer. International Journal of Food Sciences and Nutrition 2020, 71, 500–508. [Google Scholar] [CrossRef]
- Nutrition for Precision Health, powered by the All of Us Research Program [Internet]. [cited 2022 Jul 21]. Available from: https://commonfund.nih.gov/nutritionforprecisionhealth.
- Hayes, D.P. Nutritional hormesis. European Journal of Clinical Nutrition 2007, 61, 147–159. [Google Scholar] [CrossRef]
- Parohan, M.; Anjom-Shoae, J.; Nasiri, M.; Khodadost, M.; Khatibi, S.R.; Sadeghi, O. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: a systematic review and dose–response meta-analysis of prospective cohort studies. European Journal of Nutrition 2019, 58, 2175–2189. [Google Scholar] [CrossRef]
- Abbasalizad Farhangi, M.; Vajdi, M. Dietary Total Antioxidant Capacity (TAC) Significantly Reduces the Risk of Site-Specific Cancers: An Updated Systematic Review and Meta-Analysis. Nutrition and Cancer 2021, 73, 721–739. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).