1. Introduction
The standard of care for advanced urothelial carcinoma includes platinum-based chemotherapy and programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) inhibitors, administered respectively as frontline, second-line, or maintenance therapy [
1,
2,
3]. The EV-301 trial (NCT03474107) showed that enfortumab vedotin (EV) significantly prolonged survival when compared with standard chemotherapy in patients with locally advanced or metastatic urothelial carcinoma who had previously received platinum-based treatment and a PD-1 or PD-L1 inhibitor [
4]. EV received regulatory approval by the Food and Drug administration on July 9, 2021 for patients with locally advanced or metastatic urothelial cancer who have previously received a PD-1 or PD-L1 inhibitor and platinum chemotherapy, or have previously received one or more prior lines of therapy if cisplatin ineligible [
5]. The European Medicines Agency approved EV on April 13, 2022, for patients with advanced or metastatic urothelial cancer and who have already received platinum-based chemotherapy and immunotherapy [
6].
EV, an antibody-drug conjugate directed against nectin-4, is composed of a fully human monoclonal antibody specific for nectin-4 and monomethyl auristatin E (an agent that disrupts microtubule formation). Nectin-4 is a cell-adhesion molecule that is highly expressed in urothelial carcinoma and may contribute to tumor-cell growth and proliferation [
7]. Targeted delivery of monomethyl auristatin E results in cell-cycle arrest and apoptosis [
8].
In the registrational EV301 trial, the confirmed overall response was 40.6% [95% CI, 34.9 to 46.5] in the EV arm. The results of subgroup analyses were consistent with those of the primary analysis. A complete response was observed in 4.9% of the patients (14 of 288) in the EV group and disease control was observed in 71.9% (95% CI, 66.3 to 77.0) [
4]. In the EV301 trial, as in other similar trials with antibody-drug conjugates in urothelial cell cancer, patients were excluded from the trial if they had active central nervous system (CNS) metastases [
9]. Subjects who had received prior treatment for CNS metastases were permitted for inclusion in the study if all the following were true: 1) CNS metastases that have been clinically stable for at least six weeks prior to screening; 2) If requiring steroid treatment for CNS metastases, the subject is on a stable dose (≤ 20 mg/day) of prednisone or equivalent for at least two weeks; 3) Baseline scans show no evidence of new or enlarged brain metastases; 4) Subject does not have leptomeningeal disease [
10]. However, the trial results did not report subgroup analyses in patients with brain metastases, nor was it reported how many patients entered the trial with brain metastases.
In this paper, we report the first three cases on activity of EV in urothelial cancer patients with brain metastases.
2. Case Presentations
2.1. Case 1
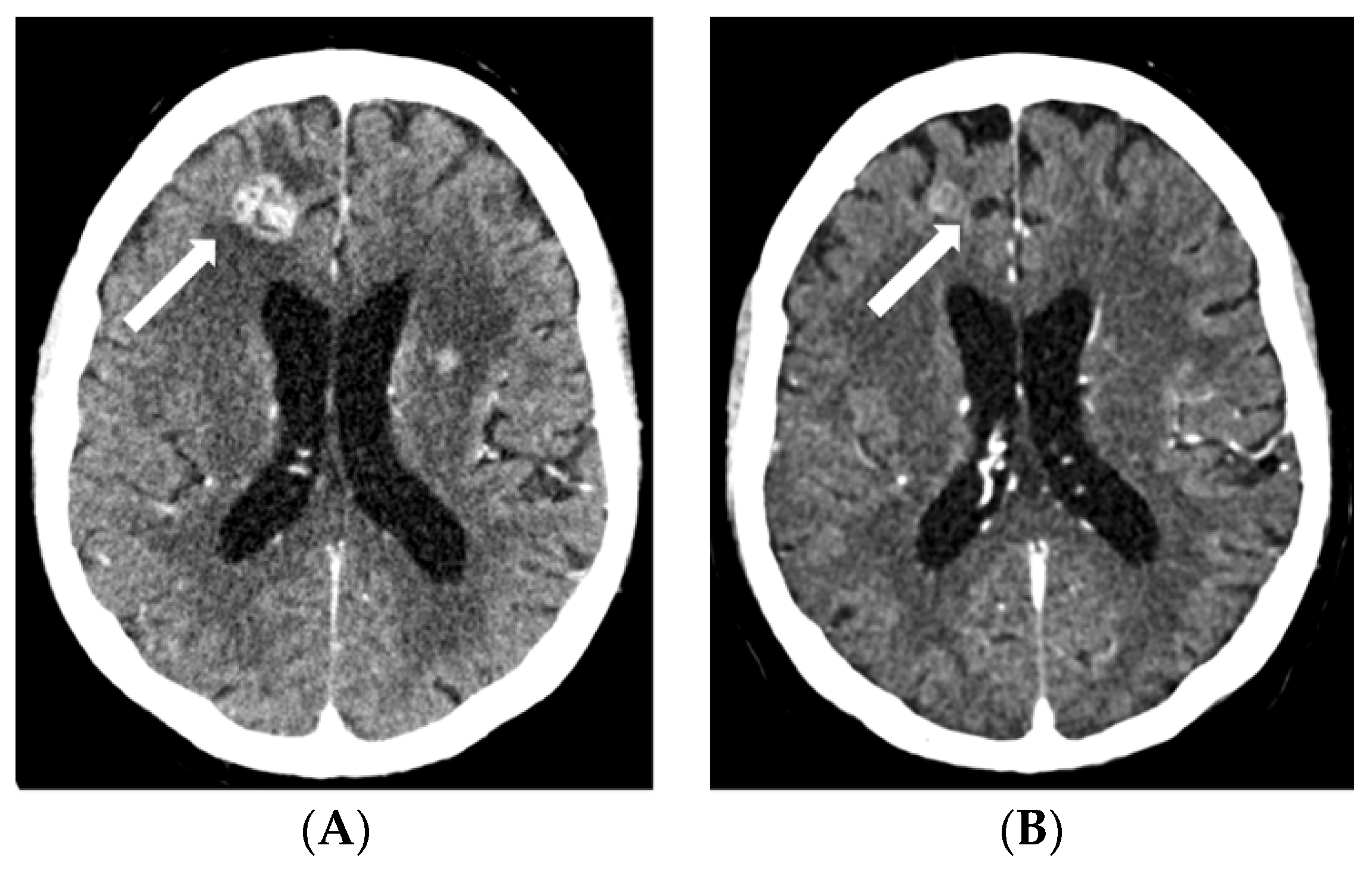
A 58-year-old male Caucasian patient underwent a nephroureterectomy and lymphadenectomy in April 2021. The pathology report showed a pathological T3N2 urothelial cell carcinoma of the upper tract. Cross-sectional imaging of the chest, abdomen and pelvis showed no evidence of metastases. He was subsequently treated with four cycles of adjuvant gemcitabine and cisplatin chemotherapy. The last cycle of chemotherapy was given on July 9, 2021. In September 2021, body computed tomography (CT) showed new nodules in both lungs and a solitary liver lesion. Subsequently, the patient received, in a platinum-refractory setting, pembrolizumab every three weeks. In January 2022, after six cycles of pembrolizumab, the patient had already progressed on CT scan. The patient still had a good performance status and chose for further treatment. He started on paclitaxel in February 2022. Following two cycles of paclitaxel, progressive disease was noted by RECIST v1.1 with growth of all lung and liver metastases and appearance of new lesions. Best supportive care was suggested, however he remained in close follow-up as he was still without disease-related complaints. In May 2022, the patient still had a good performance status and a rechallenge was proposed with platinum-based chemotherapy. After two cycles of carboplatin-gemcitabine, he had a stable disease of the lung and liver metastases. However, an additional CT was taken, due to the recent onset of facial numbness on the right side, which showed a nodular hypodense lesion measuring 15mm in the axial plane localized within the junction of the left thalamus and cerebral crus of the midbrain. Following multidisciplinary discussion, the patient was not deemed eligible for stereotactic radiotherapy because of the location of the lesion. Carboplatin-gemcitabine was administered for another two cycles to maintain systemic disease control. Re-evaluation in August 2022 showed further enlargement of the cystic brain metastasis to 17mm (
Figure 1). As EV became available in October 2022, the patient received EV at a dose of 1.25 mg/kg on days 1, 8, and 15 of a 28-day cycle. Prior to the second cycle, facial numbness had completely disappeared. The patient had tolerated EV quite well with the main side effects being anorexia grade 2, maculopapular rash grade 1, peripheral neuropathy grade 1, and diarrhea grade 1. After three cycles, a partial response of the visceral lesions with a near complete response of the thalamic brain metastasis was noted (RECIST v1.1) (
Figure 1,
Figure 2 and
Figure 3). However, the dose had to be reduced to 1mg/kg starting from cycle four due to anorexia grade 3. This patient is currently still on treatment.
2.2. Case 2
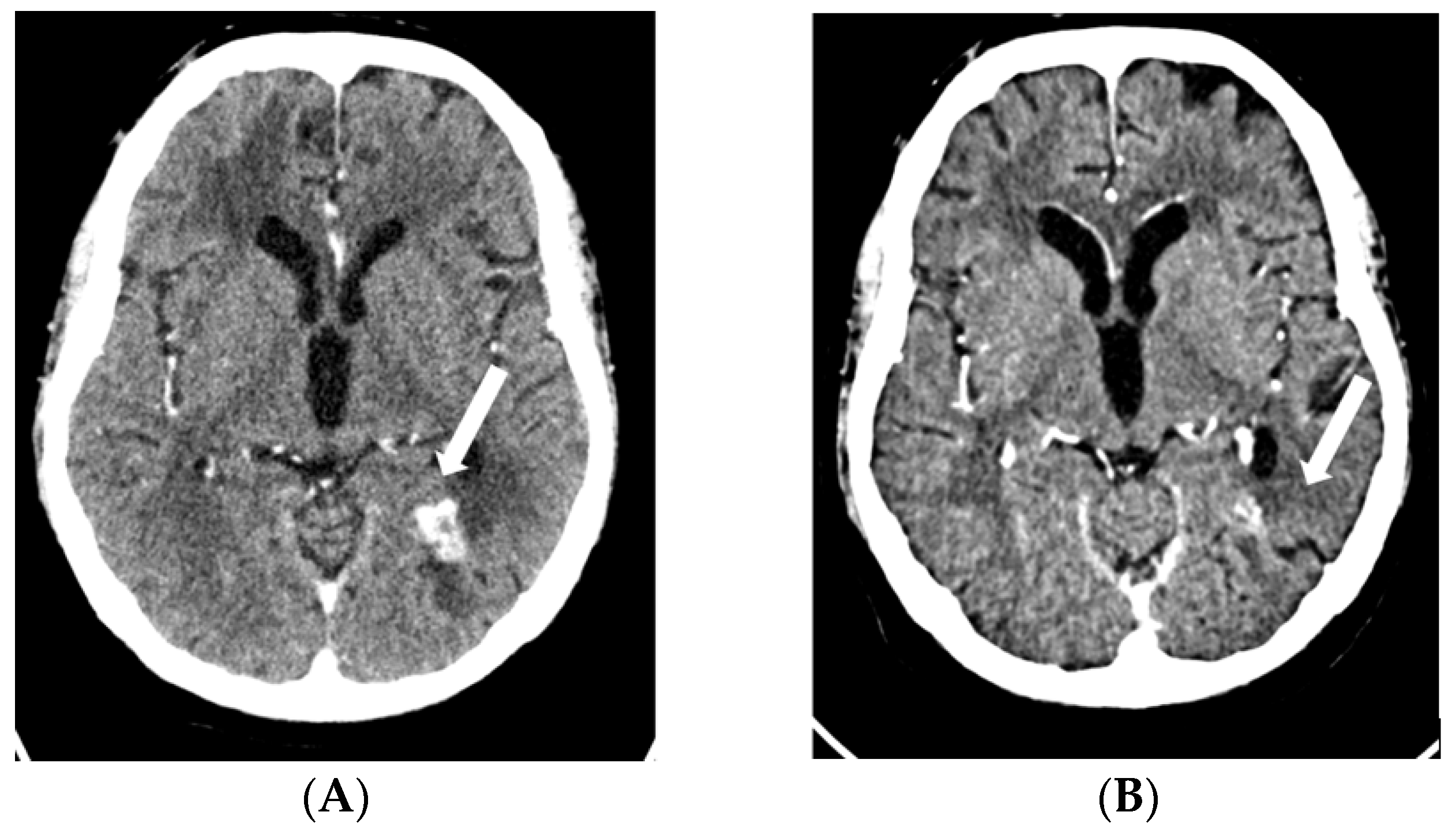
A 74-year-old male patient presented with unexplained bowel obstruction in November 2020. A CT scan showed a primary bladder tumor, multiple pelvic and retroperitoneal lymph node metastases as well as infiltration of the duodenum. A diagnostic sampling of the bladder tumor confirmed urothelial carcinoma. The patient started on front-line chemotherapy with gemcitabine and carboplatin and achieved partial response at the end of chemotherapy. Subsequently, the patient received avelumab in maintenance every two weeks. In February 2022, the patient showed new left pleural metastasis and was started on EV. Despite good response to therapy, the patient decided to hold therapy after five months due to significant side effects (grade 2 fatigue, grade 1 skin toxicity). In October 2022, the patient experienced new neurological symptoms and an MRI brain confirmed new diffuse meningeal carcinomatous infiltration of the posterior fossa and the base of the skull. The patient was rechallenged with EV and showed a significant reduction of the meningeal infiltration two months later (
Figure 4).
2.3. Case 3
A 50-year-old male Caucasian patient, who had a smoking history of 27 pack years, was diagnosed with muscle invasive bladder cancer in August 2019. Staging tests demonstrated a cT2N0M0 urothelial carcinoma. However, the patient refused neoadjuvant chemotherapy followed by cystectomy, as well as bladder preservation with trimodal treatment. After a second opinion, he underwent a repeat transurethral resection of the bladder and received intravesical instillations with Bacillus Calmette-Guérin at another center and was lost to follow-up.
In March 2021, he presented with a 2-week history of progressive dyspnoea. Chest X-ray revealed a left-sided pleural effusion. Chest CT showed lung and nodal metastases as well as a solitary left ischial bone lesion, which was subsequently confirmed on bone scan. A transbronchial biopsy was performed and confirmed urothelial carcinoma. As his kidney function was impaired, he received six cycles of split dose cisplatin-gemcitabine in combination with atezolizumab as part of a clinical trial between May 2021 and September 2021, achieving a partial response. Atezolizumab was continued in maintenance until May 2022. The patient then presented with a mild headache, responsive to step one analgesia. A brain CT showed multiple brain metastases, while body CT showed increasing lung metastases and a new implant in his left psoas muscle. The patient was treated with palliative whole brain radiotherapy (20 Gy in 5 fractions) and vinflunine up to two cycles between June 2022 and August 2022. Treatment was discontinued due to symptomatic progression (ataxia). CT scan showed no CNS response, accompanied by increasing lung nodules and worsening of the psoas metastases.
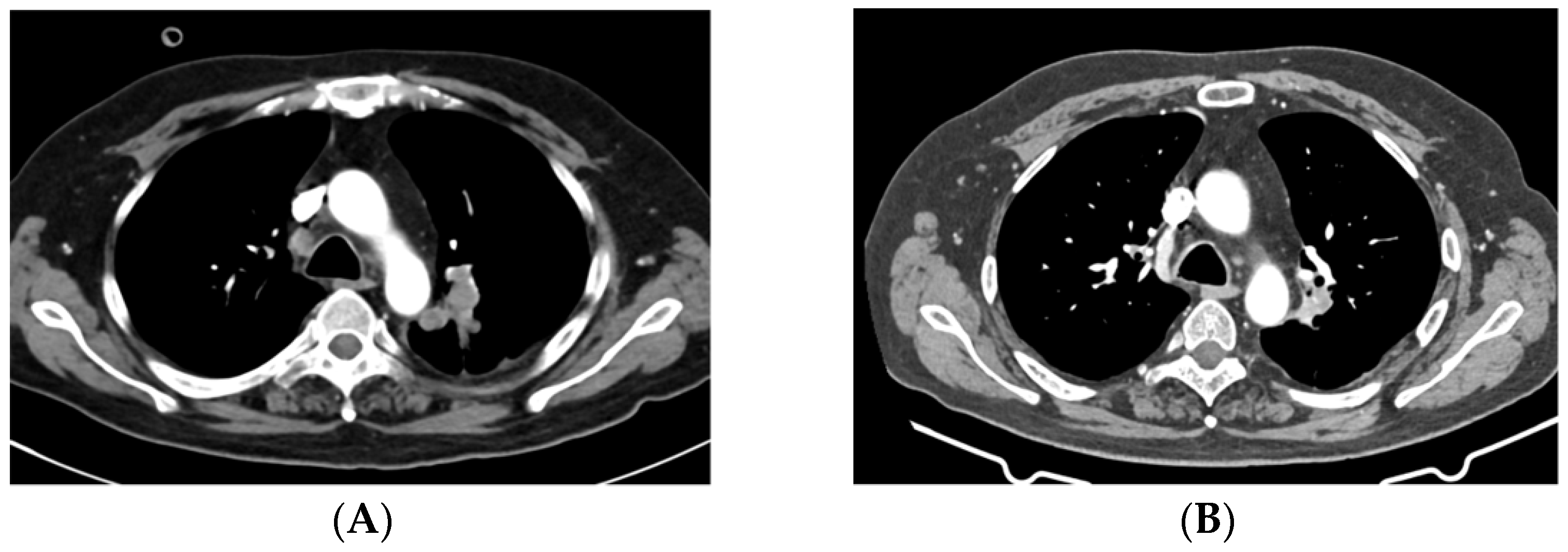
In September 2022, EV was started at a dose of 1.25 mg/kg on days 1, 8, and 15 of a 28-day cycle. After two cycles, the patient developed a grade 2 erythematous rash with associated skin sloughing. EV was withheld and a skin biopsy was taken. Topical steroids were prescribed. One week later, the rash had completely disappeared. The biopsy results ruled out Steven-Johnson Syndrome syndrome and toxic epidermal necrolysis. Thus, EV was rechallenged at a reduced dose (1mg/kg), with no new episodes of skin toxicity. In December 2022, following three cycles of EV, CT showed partial response to therapy in both brain and non-CNS metastases (
Figure 5,
Figure 6 and
Figure 7). This patient is currently still on treatment.
3. Discussion
The most common sites of distant metastases of urothelial carcinoma are the lymph nodes, liver, peritoneum, lungs and bones. Metastases to the CNS are rare and reported in around 1-8% of patients [
11].
The primary approaches to the treatment of brain metastases include surgery, stereotactic radiosurgery (SRS), or whole brain radiation. However, if surgery or SRS is not feasible, there are no data available to guide the systemic treatment approaches in pretreated brain metastatic urothelial cell cancer patients. The registrational EV301 trial did not include patients with active CNS metastases [
4]. Subjects with treated CNS metastases were permitted given the conditions specified above. No data were released up until now of the activity of EV in patients with brain metastases.
Sacituzumab govitecan (SG), is another antibody-drug conjugate that is currently being investigated in a phase 3 trial in a similar population as the EV301 trial [
4,
9]. SG is composed of an anti-trophoblast cell-surface antigen 2 (Trop-2) IgG1 kappa antibody coupled to SN-38, the active metabolite of irinotecan, a topoisomerase I inhibitor. This drug is already approved for triple negative breast cancer based on the randomized phase 3 ASCENT trial. A subgroup of this trial were patients with asymptomatic brain metastases. In an exploratory analysis of these patients , SG was numerically better than treatment physician choice (TPC) for tumor response and progression free survival but no overall survival. However, this benefit was clinically marginal with an overall response rate of 3% for the SG group (1/32) vs 0% for TPC. Moreover, no patients with active brain metastases were included [
12,
13].
The three cases, who had heavily pretreated urothelial cancer, demonstrated a profound response to the brain metastases following EV.
The cases we present here are the first reports of activity of EV in patients with active brain metastases therefore offering a new therapeutic option in this patient population.
Author Contributions
C. Vulsteke: Conceptualization, data curation, investigation, writing-original draft; L. De Cocker: Visualization, writing-review and editing; A. De Meulenaere: writing-review and editing; L. Croes: writing-review and editing; D. Delombaerde: writing-review and editing; B. Szabados: Data curation, writing-review and editing; T. Powles: Data curation, investigation, writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bellmunt J, Orsola A, Leow JJ, Wiegel T, De Santis M, Horwich A. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014, 25, iii40–8. [CrossRef]
- Warren M, Kolinsky M, Canil CM, Czaykowski P, Sridhar SS, Black PC, et al. Canadian Urological Association/Genitourinary Medical Oncologists of Canada consensus statement: Management of unresectable locally advanced and metastatic urothelial carcinoma. Can Urol Assoc J 2019, 13, 318–327. [CrossRef]
- Kamat AM, Bellmunt J, Galsky MD, Konety BR, Lamm DL, Langham D, et al. Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of bladder carcinoma n.d. [CrossRef]
- Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Durán I, Lee J-L, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021, 384, 1125–1135. [CrossRef]
- FDA grants regular approval to enfortumab vedotin-ejfv for locally advanced or metastatic urothelial cancer | FDA n.d. hhttps://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer (accessed January 30, 2023).
- Padcev | European Medicines Agency n.d. https://www.ema.europa.eu/en/medicines/human/EPAR/padcev (accessed January 30, 2023).
- Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, et al. Therapeutics, Targets, and Chemical Biology Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models n.d. [CrossRef]
- Rosenberg J, Sridhar SS, Zhang J, Smith D, Ruether D, Flaig TW, et al. EV-101: A phase I study of single-agent enfortumab vedotin in patients with nectin-4–positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol 2020, 38, 1041–1049. [CrossRef]
- Vulsteke C, Grivas P, Tagawa ST, Bellmunt J, Santis M De, Duran I, et al. TROPiCS-04: Study of sacituzumab govitecan (SG) in patients (pts) with locally advanced (LA) unresectable or metastatic urothelial cancer (mUC) that has progressed after prior platinum (PLT) and checkpoint inhibitor (CPI) therapy. Https://DoiOrg/101200/JCO2022406_supplTPS582 2022, 40, TPS582–TPS582. [CrossRef]
- A Study to Evaluate Enfortumab Vedotin Versus (vs) Chemotherapy in Subjects With Previously Treated Locally Advanced or Metastatic Urothelial Cancer (EV-301) - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT03474107 (accessed January 30, 2023).
- Boyle HJ, Lavergne E, Droz JP, Bonnin N, Flechon A. Brain metastases in patients with urothelial carcinoma. Https://DoiOrg/101200/Jco2013316_suppl282 2013, 31, 282–282. [CrossRef]
- Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med 2021, 384, 1529–1541. [CrossRef]
- Diéras V, Weaver R, Tolaney SM, Bardia A, Punie K, Brufsky A, et al. Abstract PD13-07: Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Res 2021, 81, PD13–07. [CrossRef]
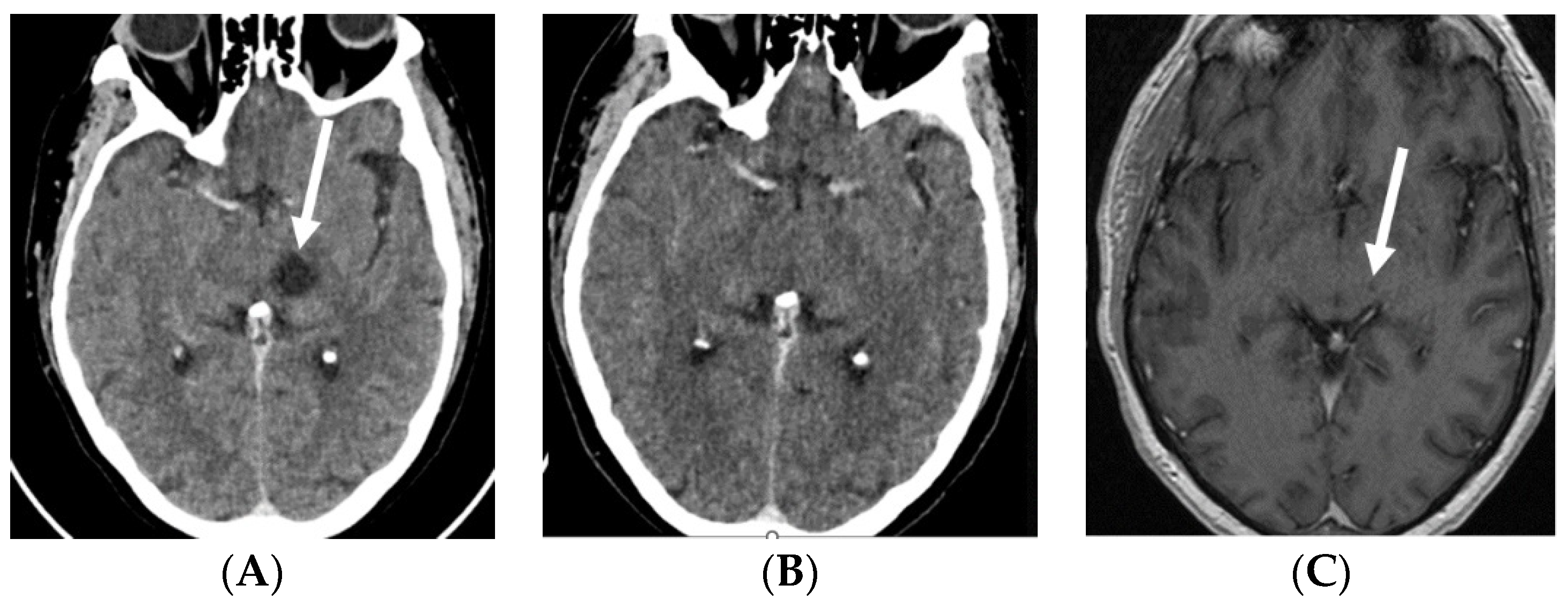
Figure 1.
Contrast-enhanced CT scans: baseline, prior to EV treatment (A) and after three cycles of EV (B), and contrast-enhanced T1-weighted MRI after three cycles of EV (C). Baseline CT shows a ring-enhancing cystic/necrotic tumor in the left thalamus, whereas follow-up CT fails to show minute residual tumor. However, this is still slightly visible on the MRI scan (arrows). Abbreviations: CT, computed tomography; EV, enfortumab vedotin; MRI, magnetic resonance imaging.
Figure 1.
Contrast-enhanced CT scans: baseline, prior to EV treatment (A) and after three cycles of EV (B), and contrast-enhanced T1-weighted MRI after three cycles of EV (C). Baseline CT shows a ring-enhancing cystic/necrotic tumor in the left thalamus, whereas follow-up CT fails to show minute residual tumor. However, this is still slightly visible on the MRI scan (arrows). Abbreviations: CT, computed tomography; EV, enfortumab vedotin; MRI, magnetic resonance imaging.
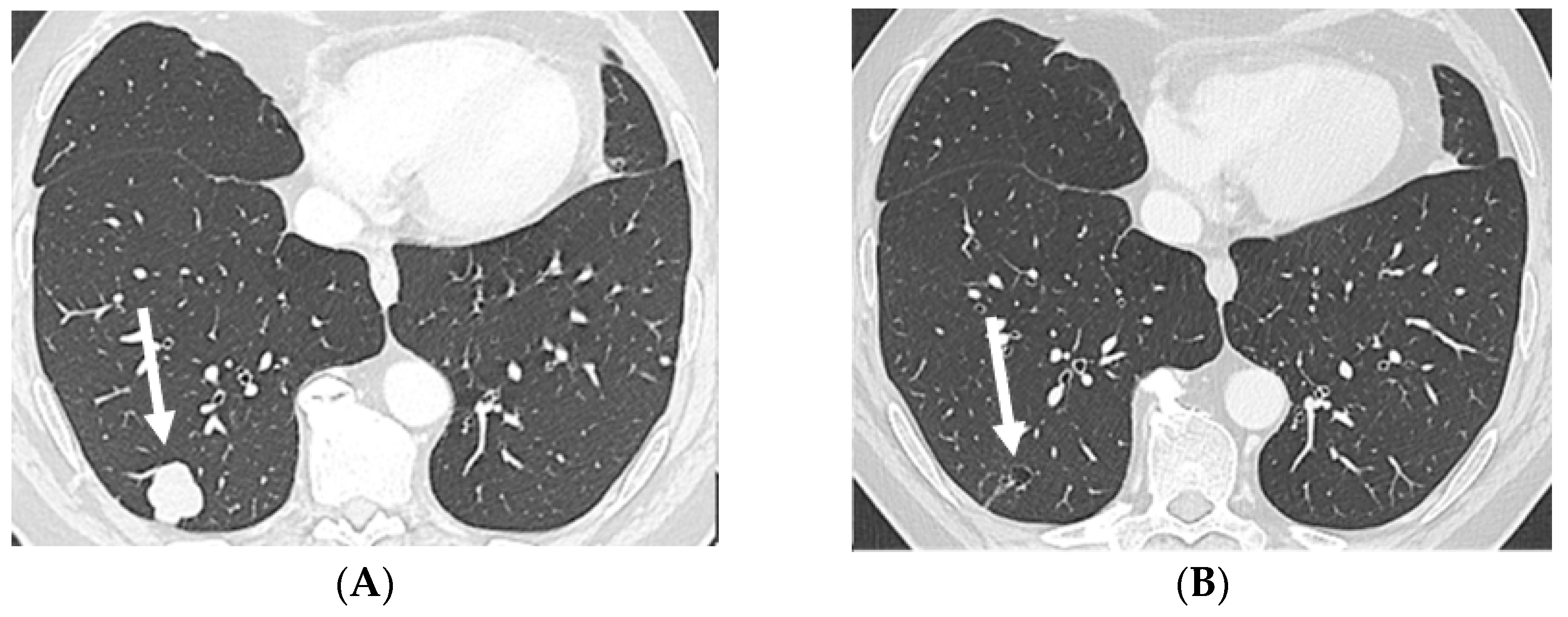
Figure 2.
Axial CT in lung window: baseline, prior to EV treatment (A) and after three cycles of EV (B) shows a large solid metastasis in the subpleural region of the right lower lobe regressing into a small, excavated and thin-walled air-containing cystic remnant after treatment (arrows). Abbreviations: CT, computed tomography; EV, enfortumab vedotin.
Figure 2.
Axial CT in lung window: baseline, prior to EV treatment (A) and after three cycles of EV (B) shows a large solid metastasis in the subpleural region of the right lower lobe regressing into a small, excavated and thin-walled air-containing cystic remnant after treatment (arrows). Abbreviations: CT, computed tomography; EV, enfortumab vedotin.
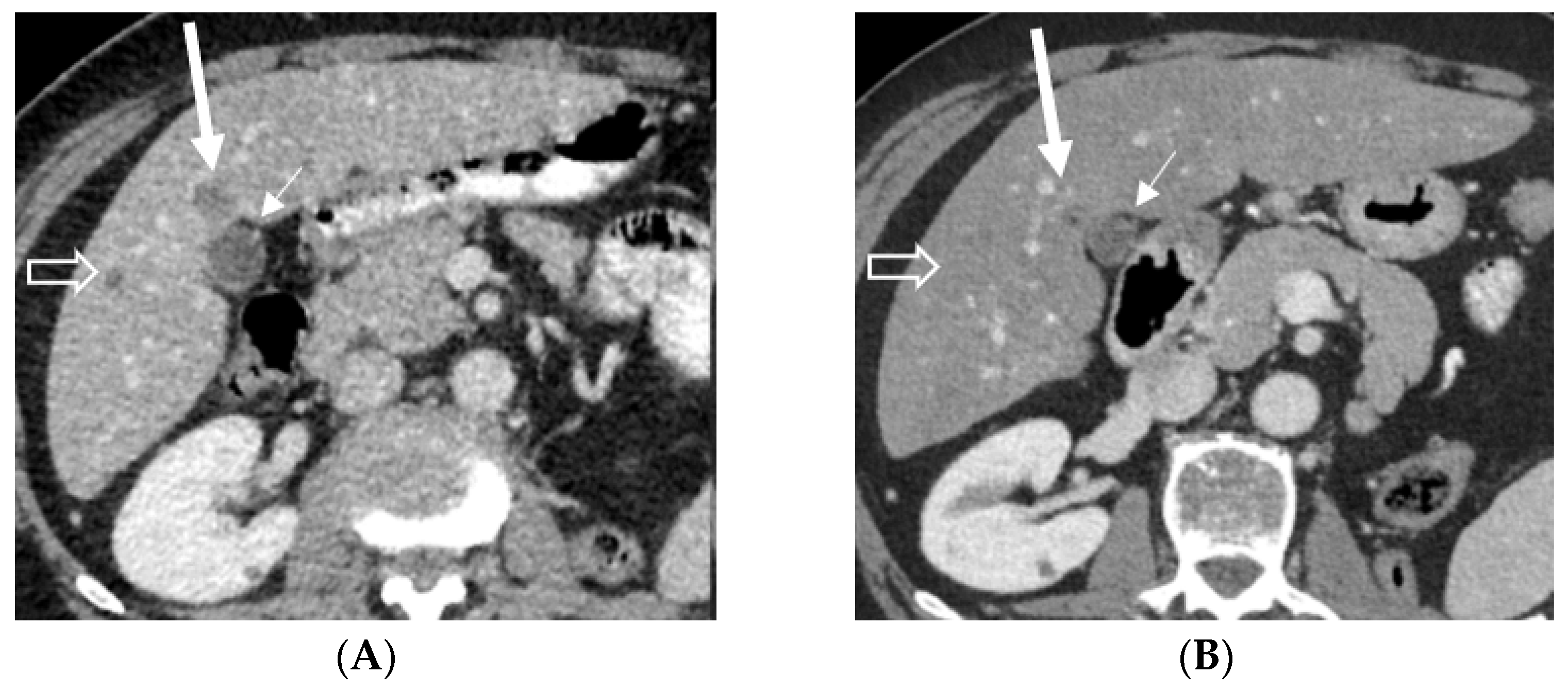
Figure 3.
Axial contrast-enhanced abdominal CT shows almost a complete disappearance of the hypodense liver metastasis (long arrow) in front of the gallbladder (short arrow) and disappearance of a smaller metastasis (open arrow) in the right lobe after treatment. Abbreviations: CT, computed tomography.
Figure 3.
Axial contrast-enhanced abdominal CT shows almost a complete disappearance of the hypodense liver metastasis (long arrow) in front of the gallbladder (short arrow) and disappearance of a smaller metastasis (open arrow) in the right lobe after treatment. Abbreviations: CT, computed tomography.
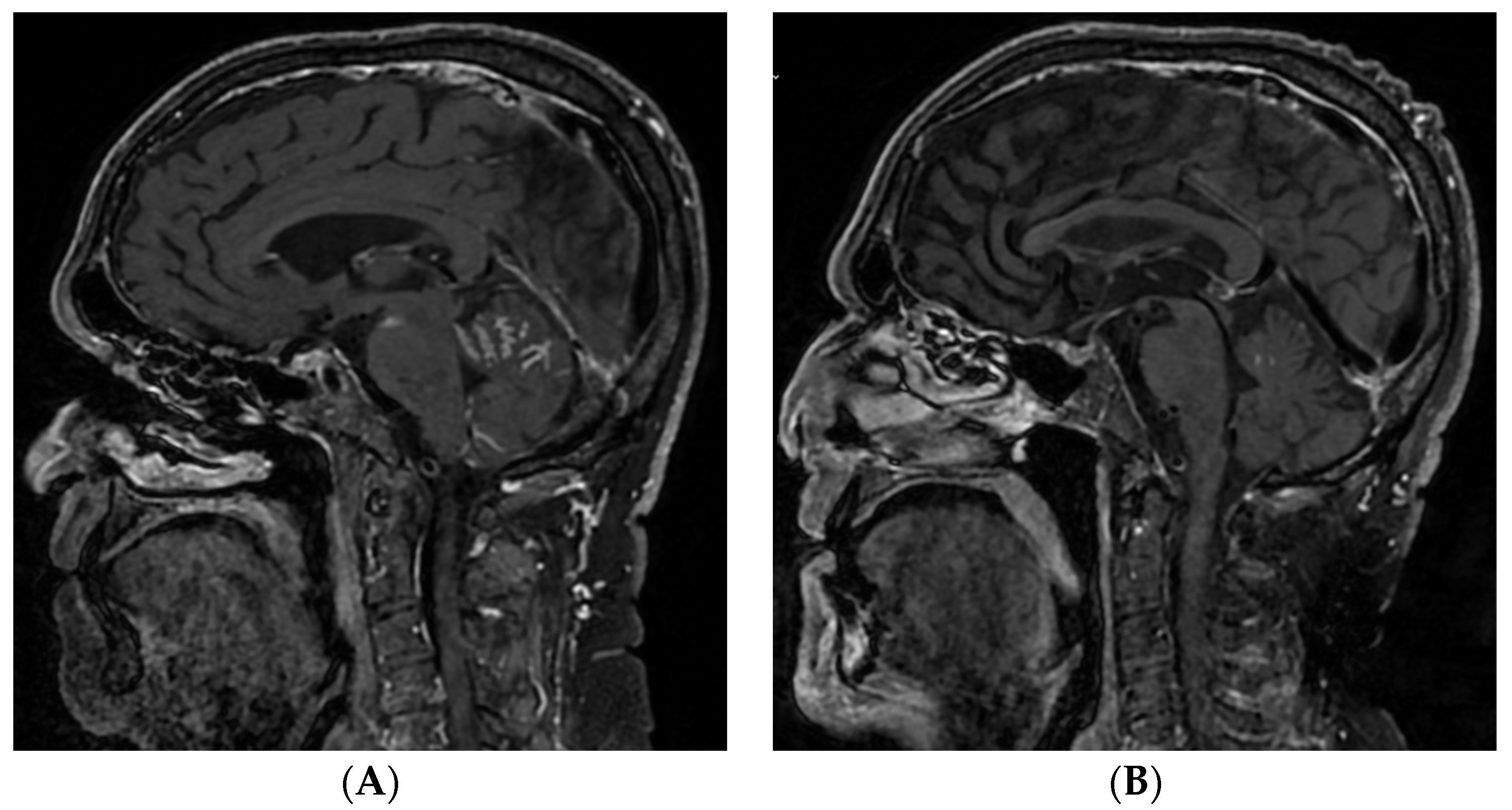
Figure 4.
MRI scan prior to rechallenge with EV (A) and after two months (B). A significant reduction can be seen (squared boxes) of the initial meningeal carcinomatous infiltration of the posterior fossa and base of the skull. Abbreviations: EV, enfortumab vedotin; MRI, magnetic resonance imaging
Figure 4.
MRI scan prior to rechallenge with EV (A) and after two months (B). A significant reduction can be seen (squared boxes) of the initial meningeal carcinomatous infiltration of the posterior fossa and base of the skull. Abbreviations: EV, enfortumab vedotin; MRI, magnetic resonance imaging
Figure 5.
CT scan showing right frontal metastases before ((A), 19mm) and after 3 cycles of EV ((B), 12mm). Abbreviations: CT, computed tomography; EV, enfortumab vedotin.
Figure 5.
CT scan showing right frontal metastases before ((A), 19mm) and after 3 cycles of EV ((B), 12mm). Abbreviations: CT, computed tomography; EV, enfortumab vedotin.
Figure 6.
CT scan showing left parieto-occipital metastases before ((A), 17mm) and after 3 cycles of EV ((B), 9mm). Abbreviations: CT, computed tomography; EV, enfortumab vedotin.
Figure 6.
CT scan showing left parieto-occipital metastases before ((A), 17mm) and after 3 cycles of EV ((B), 9mm). Abbreviations: CT, computed tomography; EV, enfortumab vedotin.
Figure 7.
CT scan shows a left hilar lung lesion before start of EV ((A), 43mm) and after 3 cycles of EV ((B), 23mm). Abbreviations: CT, computed tomography; EV, enfortumab vedotin.
Figure 7.
CT scan shows a left hilar lung lesion before start of EV ((A), 43mm) and after 3 cycles of EV ((B), 23mm). Abbreviations: CT, computed tomography; EV, enfortumab vedotin.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).