Submitted:
22 January 2023
Posted:
02 February 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. LC Model and Implications
Model
- Subsequently SARS CoV2 overwhelms and exhausts CD4+ and CD8+ T cells and natural killer cells (NKs)
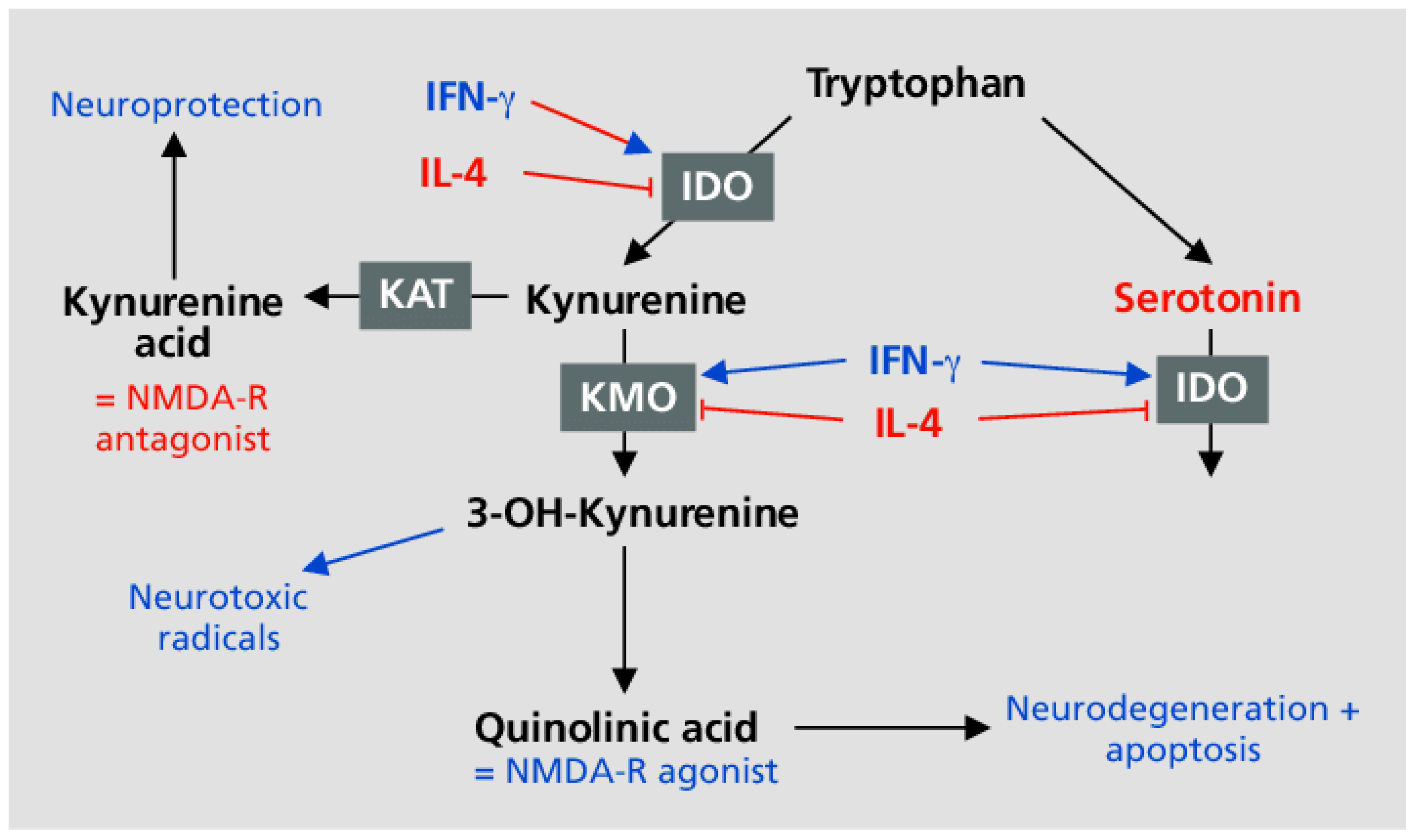
- Decreased secretion of IFN-γ lowers hepatic synthesis of complement component 1 inhibitor (C1-INH) [9]
- Uninhibited C1 triggers the Classic Complement Pathway (CCP) and crosstalk with the Kallikrein Kinin System (KKS) [10]
- The KKS triggers an increase in BKN, which is normally catabolized by angiotensin converting enzyme (ACE)
- The ACE DD genotype in African Americans, an evolutionary adaptation to falciparum malaria, downregulates this leakage (tighter endothelial junctions) and elevates the relative frequency of LC in Caucasian females
- The switch of pleiotropic TGF-β from anti-inflammatory to proinflammatory appears to be more organ specific, e.g., neurovascular pericytes [24].
Implications
- Late onset AD may appear earlier
- Cancer risk/progression and fibrosis may also increase in Caucasian females
- The presence of CD147 receptors (but not ACE2 receptors) on platelets (and erythrocytes) creates platelet aggregates, further complicating the microcirculation [32] (elevated mean platelet volume (MPV)).
- The presence of the CD147 epitope on the spike protein S poses significant risks involving microvascular thrombosis in the short term for all exposed to the spike protein S.
- The preference of SARS CoV2 for CD4+ T cells (laden with CD147 receptors) may cause growing cognitive impairment (without actual dementia) and reactivation of EBV (further exhausts CD4+ T cells) in LC and post vaccination.
- The incidence of PD in diabetics post Covid-19 should also increase due to a depressed TGF-β/IFN-γ in PD.
3. Discussion
3.1. To LC and Beyond
3.2. AD, PD, and LBD
3.3. TGF and IFN
3.4. ACE and BKN
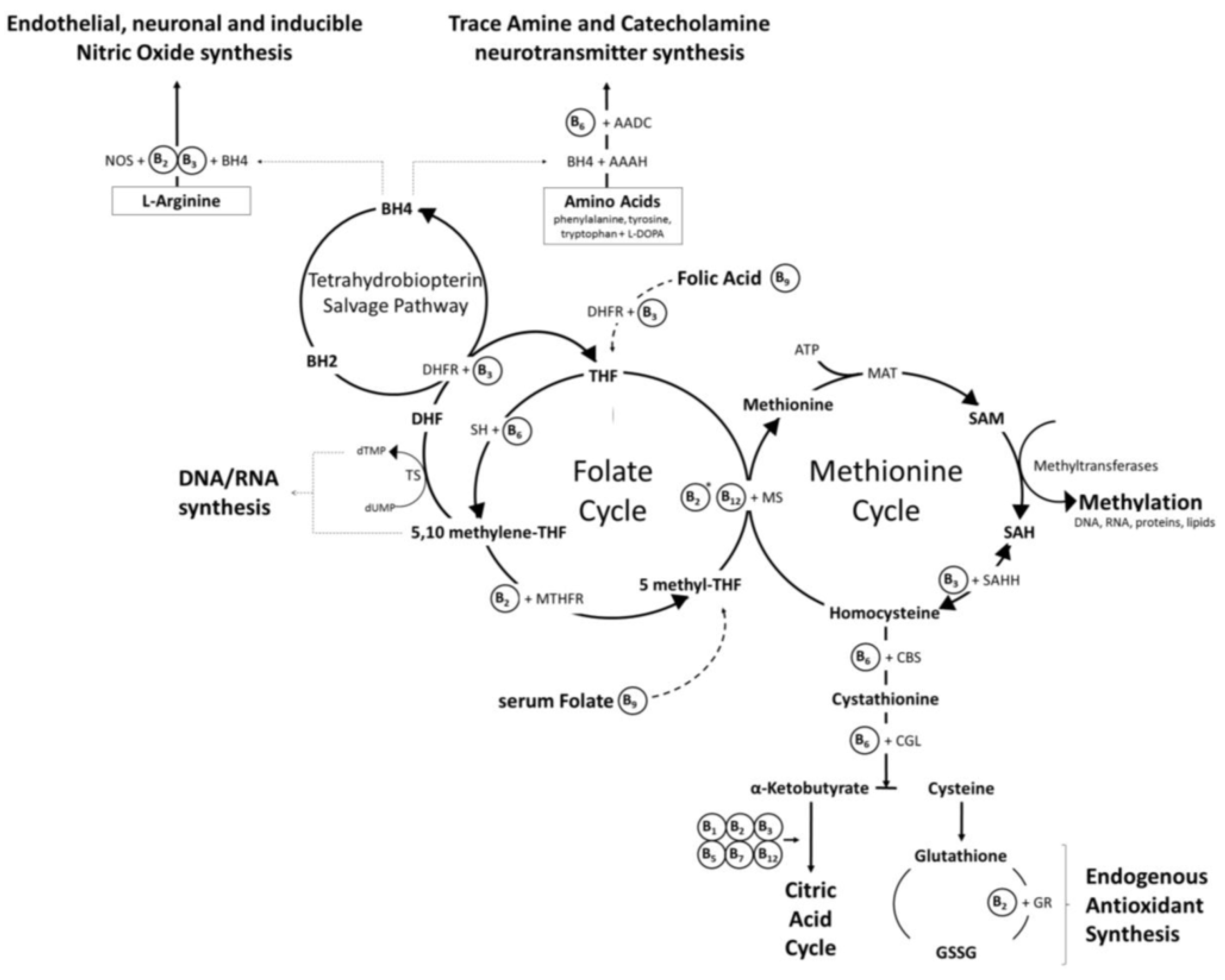
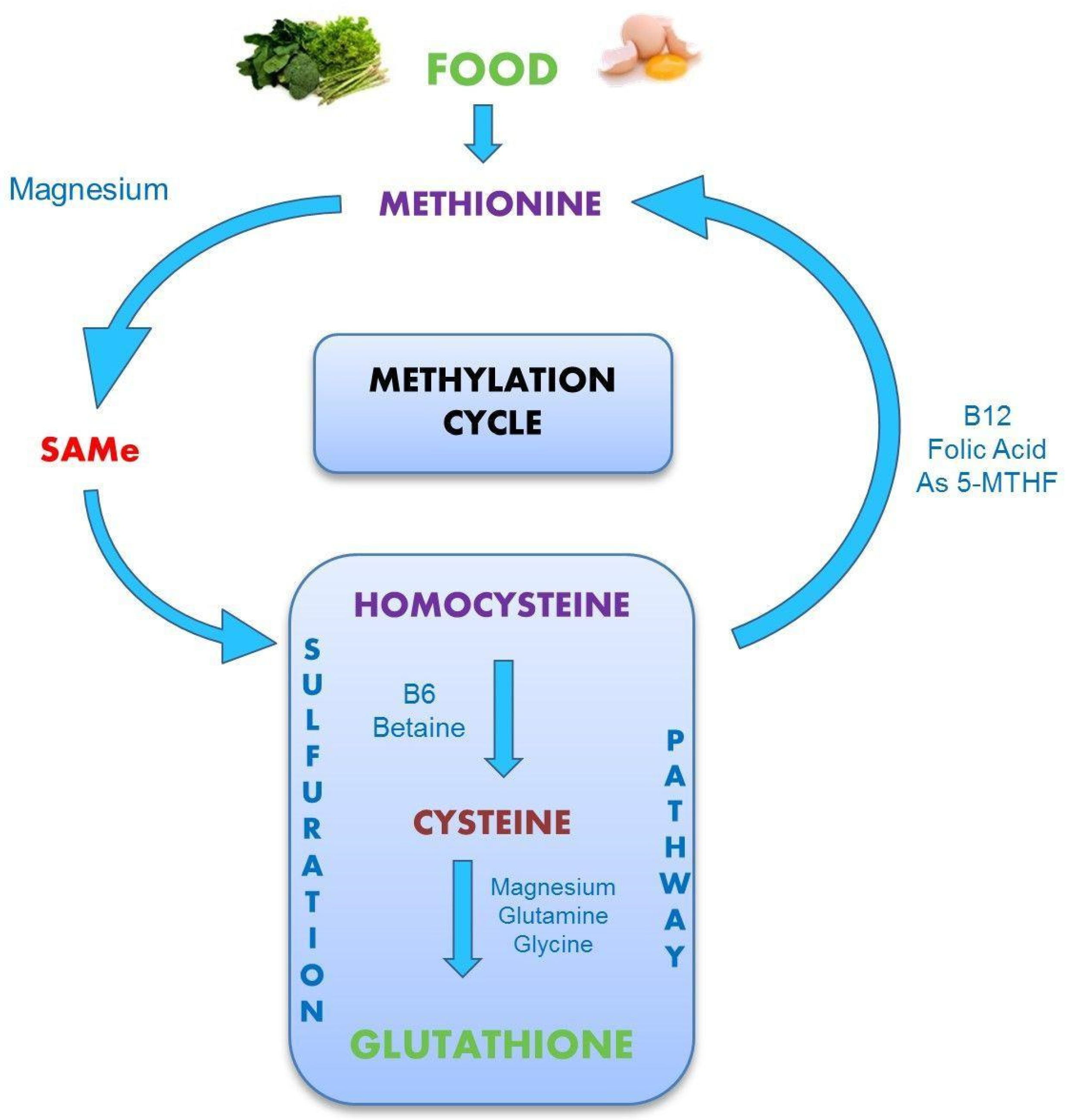
3.5. Homocysteine and B Vitamins
3.6. Vitamin D and Ca:Mg
3.7. APOE and Methylation
3.8. Treatment
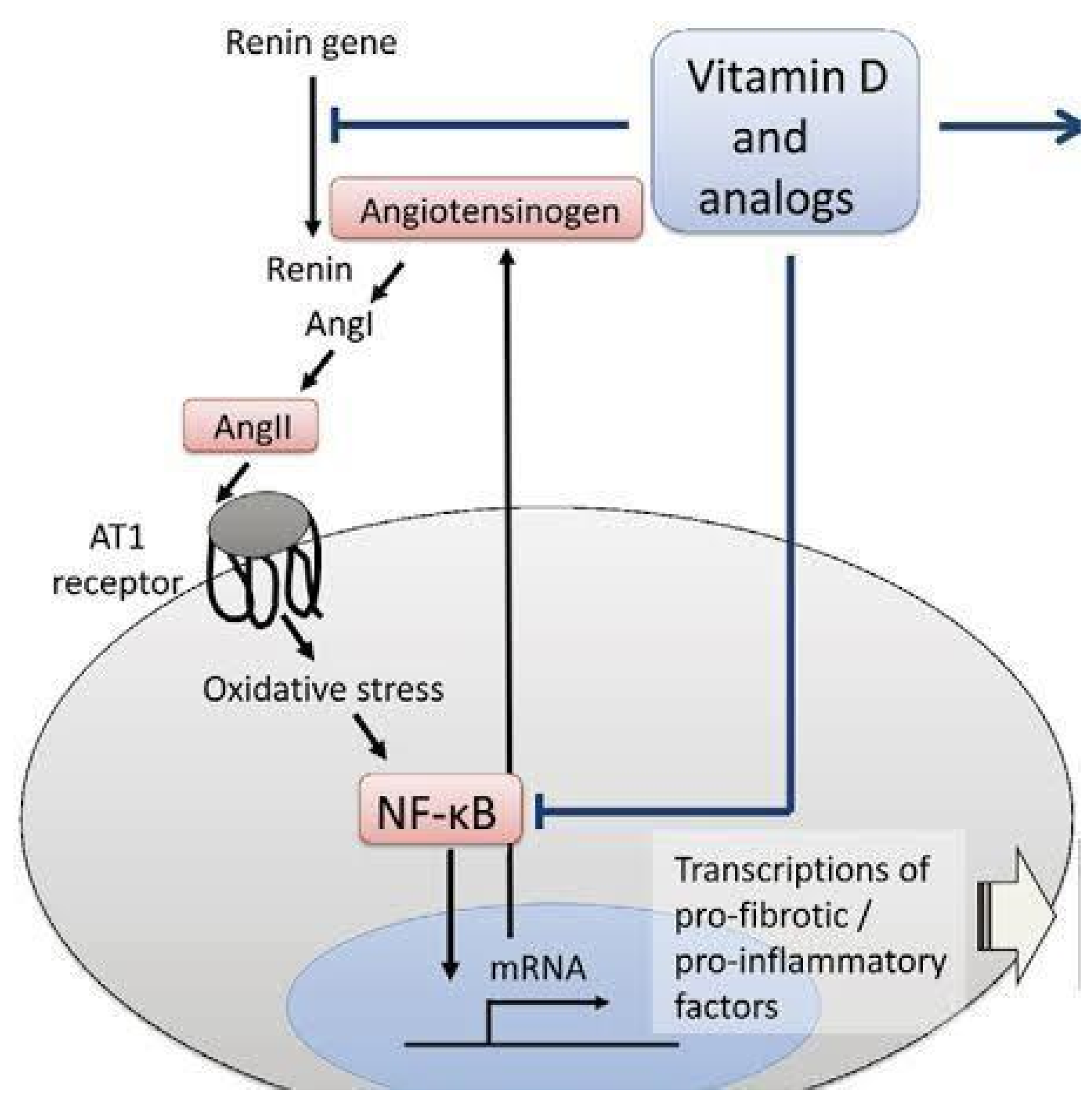
- Magnesium and vitamin D (50 ng/mL target) are at the top of the list for both prevention and treatment. Magnesium is a critical mineral in the human body and is involved in ~80% of known metabolic functions [142]. Vitamin D possesses invaluable antioxidant and anti-inflammatory properties (see Figure 9). Approximately 75% of human immune system functions depend on maintaining a healthy, physiological serum 25(OH)D concentration [143].
- 2.
- 3.
- Antioxidants are vital in the defense of COVID-19 infection. However, if the onboard supply is suboptimal, the vast numbers of ROS generated may overwhelm mitochondria and markedly compromise ATP production. Most endogenously produced and some exogenous antioxidants require ATP (and magnesium) to attain activated status. Vitamin C (water-soluble), vitamins A, D3, E, K (all fat-soluble), Zn, D-ribose, selenium, and many others require no processing [112]. Furthermore, hydroxylation of C1 of 25(OH)D in the synthesis of active vitamin D occurs in the mitochondria and is suppressed by calcium [146]. Loss of mitochondria due to oxidative stress compromises vitamin D efficacy in addition to the elevated Ca:Mg.
- 4.
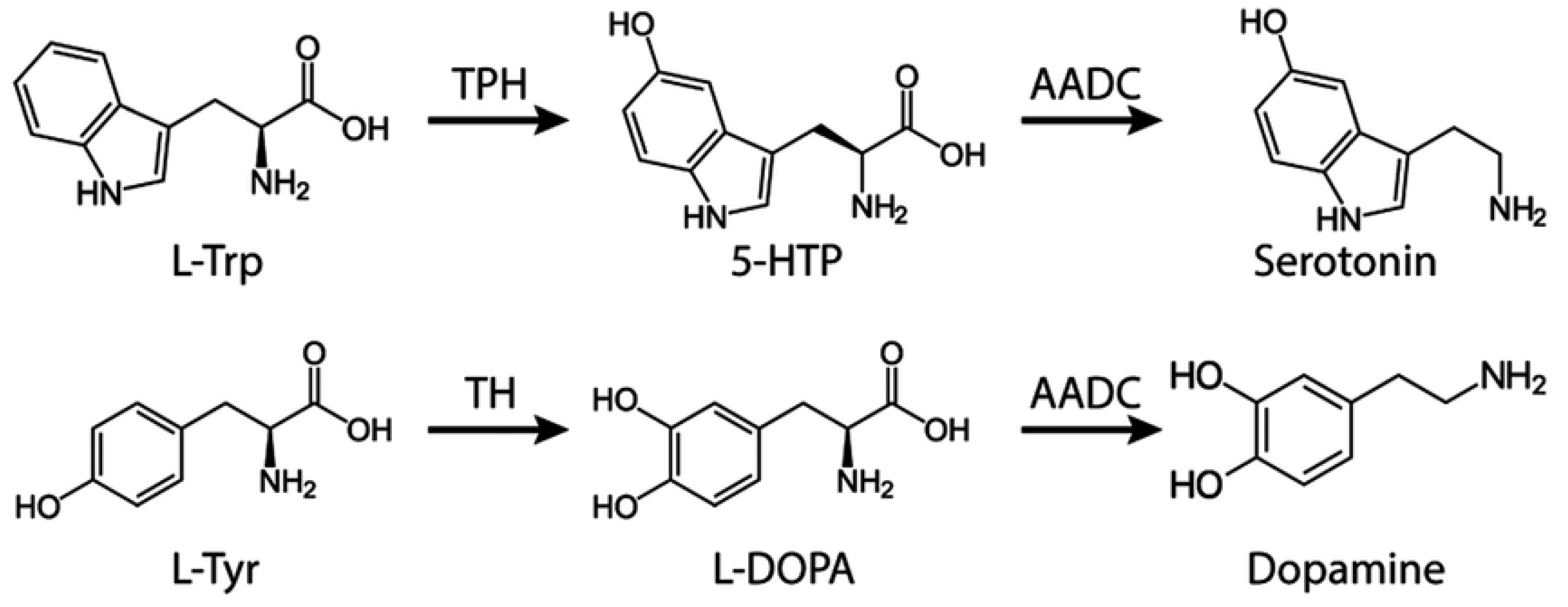
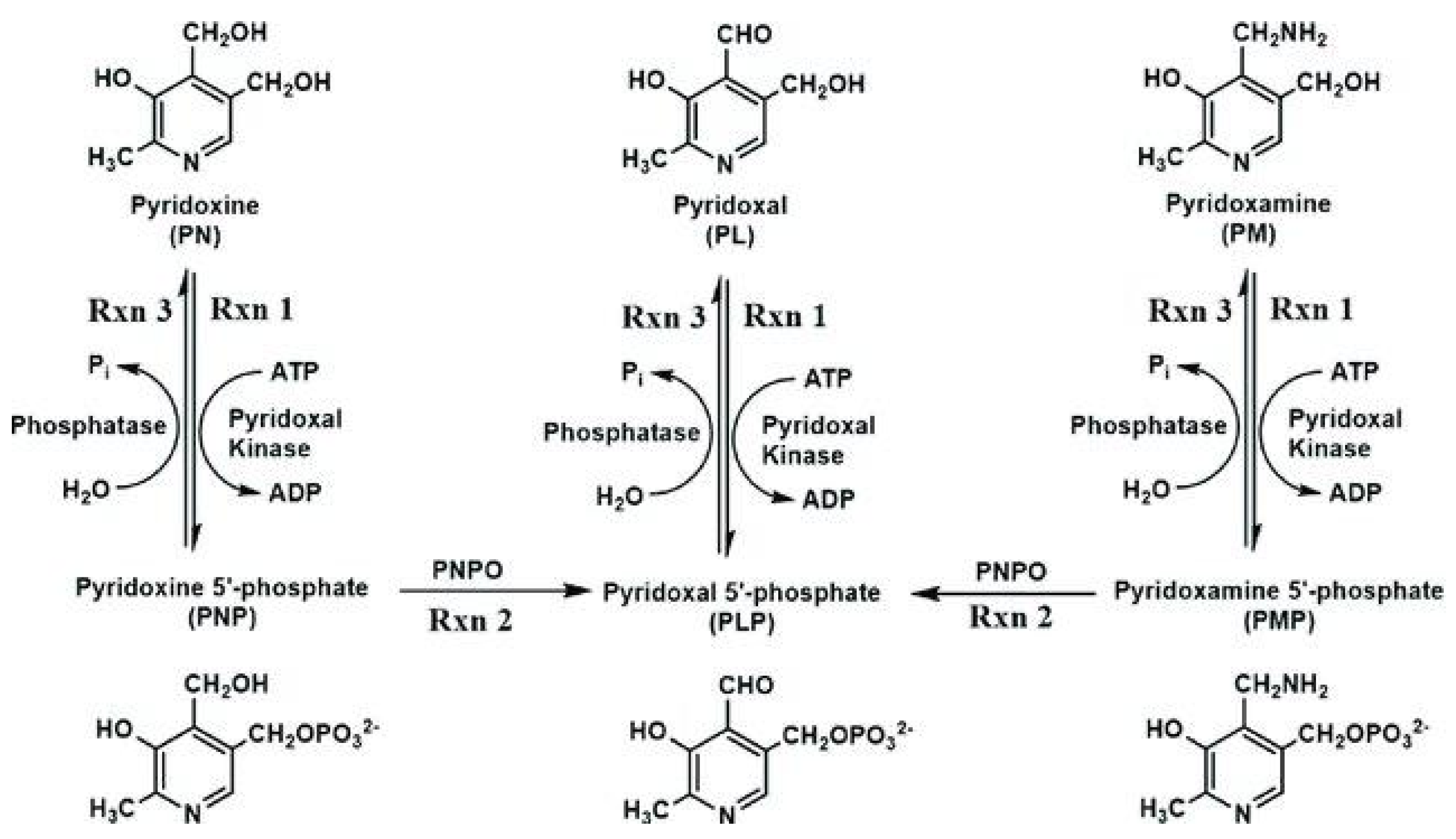
- P5P aka PLP (pyridoxal phosphate) is the active form of B6, which is required for activation of many of the B vitamins associated with homocysteine metabolism. P5P is the required cofactor for its own synthesis.
- 5.
- A sedentary lifestyle risks eventual obesity and diabetes. Exercise also facilitates a better IFN-γ:TGF-β balance by increasing IFN-γ levels [80].
- 6.
- 7.
- Dehydration triggers the renal resorption of Na+ and water. This also means loss of Mg++ (and K+) to maintain electroneutrality. Also, the thirst reflex diminishes with age. What good is increased dietary/supplemental Mg++ in the face of a magnesuric drain. Hydration maintenance, easily overlooked, potentially trumps all the other suggestions.
4. Conclusion
References
- Wang K, Chen W, Zhang Z, Deng Y, Lian J-Q, Du P. . CD147-Spike Protein Is a Novel Route for SARS-CoV-2 Infection to Host Cells Signal Transduction and Targeted Therapy 2020, 5, 283. [CrossRef] [PubMed]
- Chambers P, Basigin Binds Spike S on SARS-CoV2. Open Access Library Journal 2021, 9, 1–19. [CrossRef]
- Behl T, Kaur I, Aleya L, Sehgal A, Singh S, Sharma N. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Science of The Total Environment 2022, 808, 152072. [Google Scholar] [CrossRef]
- Fenizia C, Galbiati S, Vanetti C, Vago R, Clerici M, Tacchetti C, Daniele T. SARS-CoV-2 Entry: At the Crossroads of CD147 and ACE2. Cells 2021, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Song X, Hu W, Yu H, Zhao L, Zhao Y, Zhao X. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytometry, 2020; 1–10. [CrossRef]
- Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C. Platelet gene expressions and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef]
- Varghese J, Sandmann S, Schrempf I-M, Frömmel C, Dugas M. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19 (2021) Sci Rep 11, 12775. [CrossRef]
- Kim M-O, Salloum S, Wang JY, Wong LP, Regan J. Type I, II, and III Interferon Signatures Correspond to Coronavirus Disease 2019 Severity. The Journal of Infectious Diseases 2021, 224, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Zahedi K, Prada AE, Davis AE 3rd. Transcriptional regulation of the C1 inhibitor gene by gamma-interferon. J Biol Chem 1994, 269, 9669–74 https://pubmedncbinlmnihgov/8144555/. [Google Scholar] [CrossRef]
- Bossi, F. , Peerschke, E.I., Ghebrehiwet, B. and Tedesco, F. Cross-Talk between the Complement and the Kinin System in Vascular Permeability. Immunology Letters 2011, 140, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.E. , Li, P., Lenhart, J.R., Chappell, M.C. and Brosnihan, K.B. Estrogen Regulation of Angiotensin-Converting Enzyme mRNA. Hypertension 1999, 33, 323–328. [Google Scholar] [CrossRef]
- Wang W, Zhou Y, Wei R, Jiang G, Li F, Chen X. Bradykinin promotes proliferation, migration, and invasion of cervical cancer cells through STAT3 signaling pathways. Oncol Rep 2019, 42, 2521–2527. [Google Scholar] [CrossRef]
- Greco S, Elia MG, Muscella A, Romano S, Storelli C, Marsigliante S. Bradykinin stimulates cell proliferation through an extracellular-regulated kinase 1 and 2-dependent mechanism in breast cancer cells in primary culture, Journal of Endocrinology 2023, 186, 291–301. [CrossRef] [PubMed]
- Sun D-P, Lee Y-W, Chen J-T, Lin Y-W, Chen R-M. The Bradykinin-BDKRB1 Axis Regulates Aquaporin 4 Gene Expression and Consequential Migration and Invasion of Malignant Glioblastoma Cells via a Ca2+-MEK1-ERK1/2-NF-κB Mechanism. Cancers 2020, 12, 667 . [Google Scholar] [CrossRef]
- Ichinose M, Barnes PJ. Bradykinin-induced airway microvascular leakage and bronchoconstriction are mediated via a bradykinin B2 receptor. Am Rev Respir Dis 1990, 142, 1104–7. [Google Scholar] [CrossRef] [PubMed]
- Sarker MH, Hu DE, Fraser PA. Acute effects of bradykinin on cerebral microvascular permeability in the anaesthetized rat. The Journal of Physiology 2000, 528, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Schultheiß C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes SS. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022, 3, 100663. [Google Scholar] [CrossRef] [PubMed]
- Freitas F, Tibiriçá E, Singh M, Fraser PA, Mann GE. Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin. Antioxidants 2020, 9, 1269. [Google Scholar] [CrossRef] [PubMed]
- Delvig AA, Lee JJ, Chrzanowska-Lightowlers ZM, Robinson JH. TGF-beta1 and IFN-gamma cross-regulate antigen presentation to CD4 T cells by macrophages. J Leukoc Biol. 2002, 72, 163–6 https://pubmedncbinlmnihgov/12101276/. [Google Scholar] [CrossRef]
- Sobral LM, Montan PF, Martelli-Junior H, Graner E, Coletta RD. Opposite effects of TGF-β1 and IFN-γ on transdifferentiation of myofibroblast in human gingival cell cultures. Journal of Clinical Periodontology 2007, 34, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu Y, Kimuro S, Pauty J, Takagaki K, Nomiyama S, Inagawa A. TGF-beta and TNF-alpha cooperatively induce mesenchymal transition of lymphatic endothelial cells via activation of Activin signals. PLoS ONE 2020, 15, e0232356. [Google Scholar] [CrossRef]
- Masola V, Carraro A, Granata S, Signorini L, Bellin G. In vitro effects of interleukin (IL)-1 beta inhibition on the epithelial-to-mesenchymal transition (EMT) of renal tubular and hepatic stellate cells J Transl Med 2019, 17, 12. [CrossRef]
- Liu ZW, Zhang YM, Zhang LY, Zhou T, Li YY, Zhou GC. Duality of Interactions Between TGF-β and TNF-α During Tumor Formation. Front Immunol 2022, 12, 810286. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven J, Aalderink M, Scotter EL, Oldfield RL, Bergen PS. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation 2016, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Rota E, Bellone G, Rocca P. Increased intrathecal TGF-β1, but not IL-12, IFN-γ and IL-10 levels in Alzheimer’s disease patients. Neurol Sci 2006, 27, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Taipan R, das Neves SP, Sousa AL, Fernandez J Pinto, Correia AP. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer's disease and their correlation with cognitive decline Neurobiology of Aging 2019, 76, 125–132. [CrossRef]
- Zhang X, Huang WJ, Chen WW. TGF-β1 factor in the cerebrovascular diseases of Alzheimer's disease. Eur Rev Med Pharmacol Sci. 2016, 20, 5178–5185 https://pubmedncbinlmnihgov/28051272/. [Google Scholar]
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition BMJ 2019, 364, l877. [CrossRef] [PubMed]
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, Vattulainen P, Gissler M, Ylikorkala O. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study BMJ 2019, 364, l665. [CrossRef] [PubMed]
- Chen PY, Qin L, Li G. Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis. Nat Metab 2019, 1, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Wang C, Jin R, Zhu X. Function of CD147 in Atherosclerosis and Atherothrombosis. J. of Cardiovasc Trans. Res. 2015, 8, 59–66. [Google Scholar] [CrossRef]
- Nishikawa M, Kanno H, Zhou Y. Massive image-based single-cell profiling reveals high levels of circulating platelet aggregates in patients with COVID-19. Nat Commun 2021, 12, 7135. [Google Scholar] [CrossRef]
- Villalba MCM, Ramírez OV, Jiménez MM, Garcia AA, Alfonso JM, Baéz GG. Interferon gamma, TGF-β1 and RANTES expression in upper airway samples from SARS-CoV-2 infected patients. Clinical Immunology 2020, 220, 108576. [Google Scholar] [CrossRef]
- Tesseur I, Nguyen A, Chang B, Li L, Woodling NS, Wyss-Coray T. Deficiency in Neuronal TGF-β Signaling Leads to Nigrostriatal Degeneration and Activation of TGF-β Signaling Protects against MPTP Neurotoxicity in Mice. J Neurosci. 2017, 37, 4584–92. [Google Scholar] [CrossRef] [PubMed]
- Mohamed A, Maha A, Hanan H, Samaa T. Association of Serum Interferon Gamma Level with Parkinson's Disease in Egyptian Patients EJMM-Egyptian Journal of Medical Microbiology [The] 2014, 23, 1–5. [CrossRef]
- Hu ZJ, Xu J, Yin JM, Li L, Hou W, Zhang LL. Lower Circulating Interferon-Gamma Is a Risk Factor for Lung Fibrosis in COVID-19 Patients. Front Immunol. 2020, 11, 585647. [Google Scholar] [CrossRef]
- Weng H, Mertens PR, Gressner AM, Dooley S. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J Hepatol 2007, 46, 295–303. [Google Scholar] [CrossRef] [PubMed]
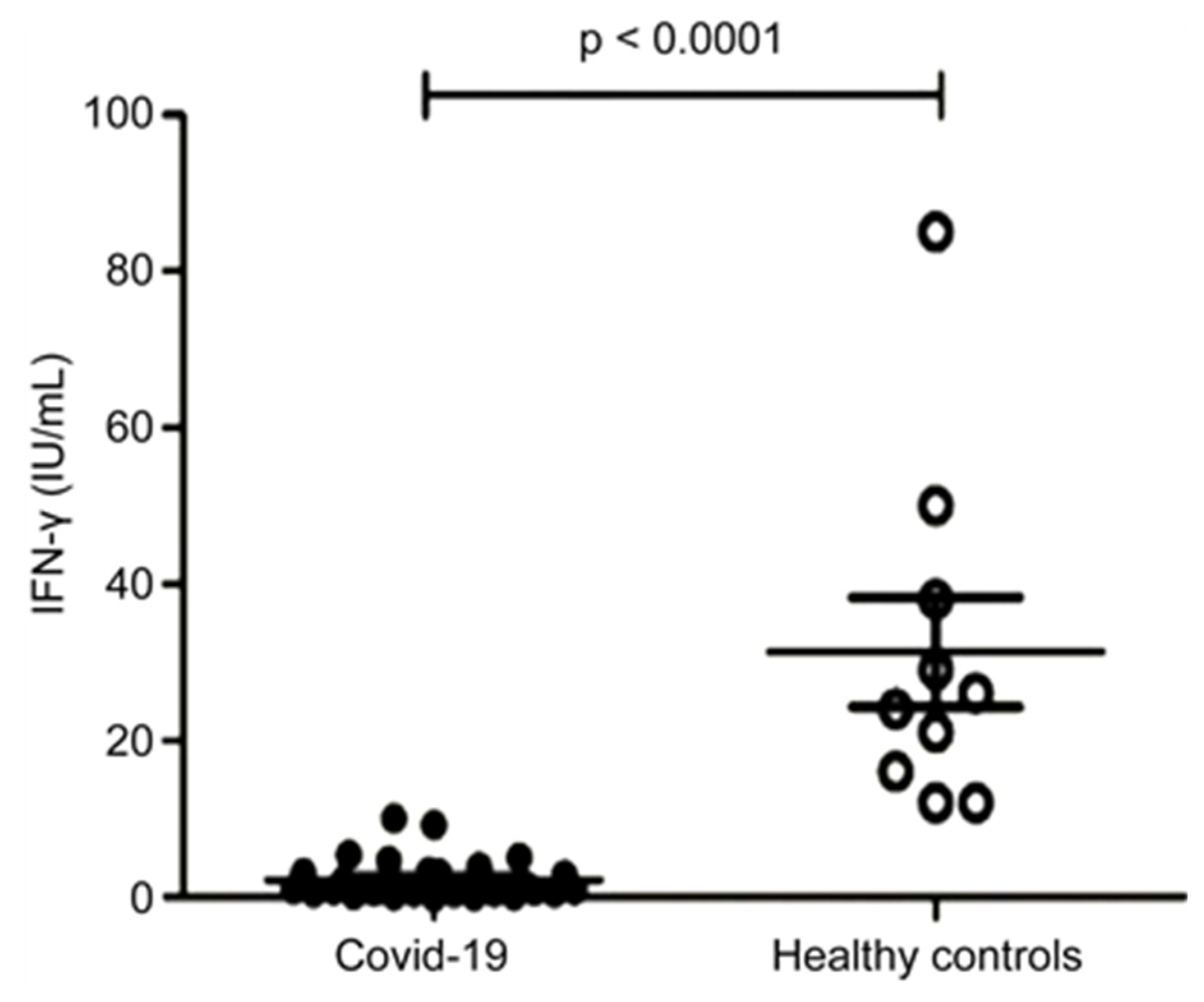
- Dhanda AD, Felmlee D, Boeira P, Moodley P, Tan H, Scalioni LDP. Patients with moderate to severe COVID-19 have an impaired cytokine response with an exhausted and senescent immune phenotype. Immunobiology 2022, 227, 152185. [Google Scholar] [CrossRef] [PubMed]
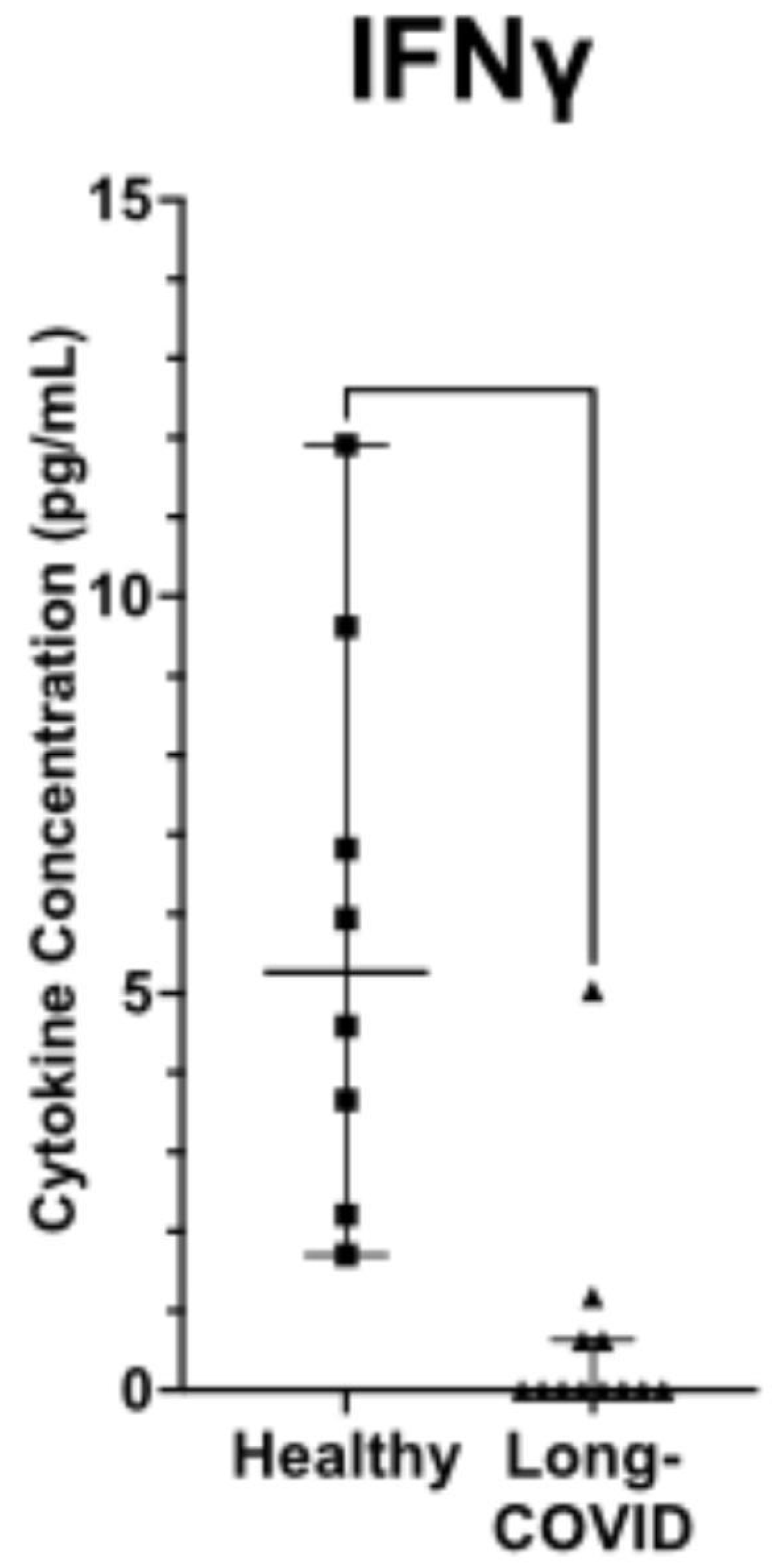
- Elizabeth SC, Williams P, Martins TB, Hill HR, Coiras M, Shah KS. Plasma cytokine levels reveal deficiencies in IL-8 and gamma interferon in Long-COVID. medRxiv, 2022. [CrossRef]
- Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ, Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion Frontiers in Immunology 2018, 9. [CrossRef]
- Yoshimatsu Y, Wakabayashi I, Kimuro S. TNF-α enhances TGF-β-induced endothelial-to-mesenchymal transition via TGF-β signal augmentation. Cancer Sci. 2020, 111, 2385–99. [Google Scholar] [CrossRef]
- Borthwick LA, Gardner A, De Soyza A. Transforming Growth Factor-β1 (TGF-β1) Driven Epithelial to Mesenchymal Transition (EMT) is Accentuated by Tumour Necrosis Factor α (TNFα) via Crosstalk Between the SMAD and NF-κB Pathways. Cancer Microenvironment 2012, 5, 45–57. [Google Scholar] [CrossRef]
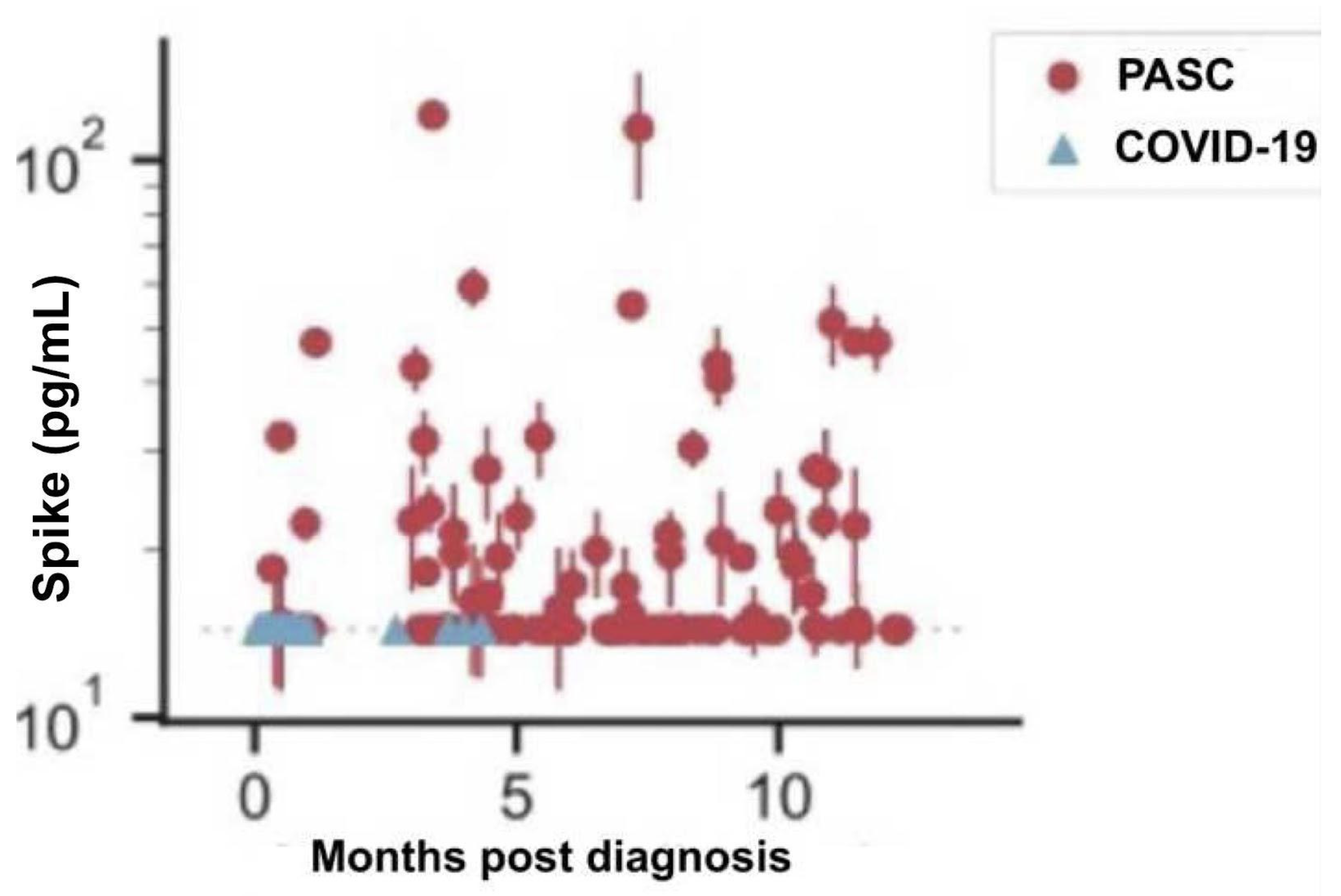
- Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ, Alter G. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated with Post-acute Coronavirus Disease 2019 Sequelae. Clinical Infectious Diseases, 2022; ciac722. [CrossRef]
- Mouton A, Blanc F, Gros A. Sex ratio in dementia with Lewy bodies balanced between Alzheimer’s disease and Parkinson’s disease dementia: a cross-sectional study. Alz Res Therapy 2018, 10, 92. [Google Scholar] [CrossRef]
- Reekes TH, Higginson CI, Ledbetter CR. Sex specific cognitive differences in Parkinson disease. npj Parkinsons Dis. 2020, 6, 7. [Google Scholar] [CrossRef]
- 46. de Souza HDN, Oliveira A, Ulrich H. Bradykinin reverts contralateral rotation and restores dopamine levels in an animal model of Parkinson disease. 11th World Congress on Neurology and Therapeutics, March 27-29, 2017 Madrid, Spain; Spain. Available online: https://www.iomcworld.org/conference-abstracts-files/2155-9562-C1-047-007.pdf.
- Manson JM, Sammel MD, Freeman EW, Grisso JA. Racial differences in sex hormone levels in women approaching the transition to menopause. Fertil Steril. 2001, 75, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Pepino MY, Finkbeiner S, Beauchamp GK, MMennella JA. , Obese Women Have Lower Monosodium Glutamate Taste Sensitivity and Prefer Higher Concentrations Than Do Normal-weight Women. Obesity 2010, 18, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Bautista RJH, Mahmoud AM, Königsberg M, López NE, Guerrero D. Obesity: Pathophysiology, monosodium glutamate-induced model and anti-obesity medicinal plants, Biomedicine & Pharmacotherapy 2019, 111, 503–516. [CrossRef] [PubMed]
- Bahadoran Z, Mirmiran P, Ghasemi A. Monosodium Glutamate (MSG)-Induced Animal Model of Type 2 Diabetes. In: Guest, P. (eds) Pre-Clinical Models. Methods in Molecular Biology, 2019; 1916, Humana Press: New York, NY. [CrossRef]
- Tann C, Chong H, Tan E. Getting ‘Smad’ about obesity and diabetes. Nutr & Diabetes, 2012; e29. [CrossRef]
- He Z, Yang Y, Xing Z. Intraperitoneal injection of IFN-γ restores microglial autophagy, promotes amyloid-β clearance and improves cognition in APP/PS1 mice. Cell Death Dis 2020, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Zheng C, Zhou XW, Wang JZ. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl Neurodegener, 2016; 5, 7. [CrossRef]
- Shen, L. Associations between B Vitamins and Parkinson’s Disease. Nutrients 2015, 7, 7197–7208. [Google Scholar] [CrossRef] [PubMed]
- Ma H, Yan J, Sun W, Jiang M, Zhang Y. Melatonin Treatment for Sleep Disorders in Parkinson's Disease: A Meta-Analysis and Systematic Review. Front Aging Neurosci 2022, 14, 784314 https://wwwfrontiersinorg/articles/103389/fnagi2022784314/full. [Google Scholar]
- Müller N, Myint A-M, Schwarz MJ. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders - relation to drug treatment. Dialogues in Clinical Neuroscience 2009, 11, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Ngai P, McCormick S, Small C, Zhang X, Zganiacz A, Aoki N, Gamma interferon responses of CD4 and CD8 T-cell subsets are quantitatively different and independent of each other during pulmonary Mycobacterium bovis BCG infection. Infect Immun 2007, 75, 2244–52. [CrossRef] [PubMed]
- Williams GP, Schonhoff AM, Jurkuvenaite A, Gallups NJ, Standaert DG, Harms AS, CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson's disease. Brain 2021, 144, 2047–2059. [CrossRef]
- Shen, XR. , Geng, R., Li, Q. ACE2-independent infection of T lymphocytes by SARS-CoV-2. Sig Transduct Target Ther 2022, 7, 83. [Google Scholar] [CrossRef]
- Contaldi E, Magistrelli L, Comi C. T Lymphocytes in Parkinson’s Disease. Journal of Parkinson's Disease 2022, 12, S65–S74. [Google Scholar] [CrossRef] [PubMed]
- Ferrari DP, Bortolanza NM, Del Bel E. Interferon-γ Involvement in the Neuroinflammation Associated with Parkinson’s Disease and L-DOPA-Induced Dyskinesia Neurotox Res 2022, 39, 705–719. [CrossRef] [PubMed]
- Gulzar N, Copeland KF. CD8+ T-cells: function and response to HIV infection. Curr HIV Res 2004, 2, 23–37. [Google Scholar] [CrossRef]
- Roff SR, Noon-Song EN, Yamamoto JK. The Significance of Interferon-γ in HIV-1 Pathogenesis, Therapy, and Prophylaxis. Front Immunol 2014, 4, 498. [Google Scholar] [CrossRef] [PubMed]
- Koutsilieri, E. , Sopper, S., Scheller, C. et al. Parkinsonism in HIV dementia. J Neural Transm 2002, 109, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Lees, JR. Interferon gamma in autoimmunity: A complicated player on a complex stage. Cytokine 2015, 74, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chohan H, Senkevich K, Patel RK, Bestwick JP, Jacobs BM. Type 2 Diabetes as a Determinant of Parkinson's Disease Risk and Progression. Mov Disord 2021, 36, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Fernández E, Warner T. Association between diabetes and subsequent Parkinson disease: A record-linkage cohort study. Neurology 2019, 92, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Kalra S, Kalra B, Agrawal N. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr 2011, 3, 2. [Google Scholar] [CrossRef]
- Swaroop J, Rajarajeswari D, Naidu J. Association of TNF-α with insulin resistance in Type 2 diabetes mellitus. The Indian journal of medical research 2012, 135, 127–30. [Google Scholar] [CrossRef]
- Sudar-Milovanovic E, Gluvic Z, Obradovic M, Zaric B, Isenovic ER. Tryptophan Metabolism in Atherosclerosis and Diabetes. Curr Med Chem 2022, 29, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Murray, MF. Tryptophan depletion and HIV infection: a metabolic link to pathogenesis. Lancet Infect Dis 2003, 3, 644–52. [Google Scholar] [CrossRef] [PubMed]
- Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson's disease. J Neural Transm Suppl. 2006; 373–381. [CrossRef]
- Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Town T, Laouarn Y, Pittenger C. Blocking TGF-β–Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med, 2008; 681–687. [CrossRef]
- Yang C, Xu P. The role of transforming growth factor β1 /Smad pathway in Alzheimer’s disease inflammation pathology. Mol Biol Rep 2023, 50, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Xu L, Pan CL, Wu XH, Song JJ, Meng P, Li L. Inhibition of Smad3 in macrophages promotes Aβ efflux from the brain and thereby ameliorates Alzheimer's pathology. Brain Behav Immun 2021, 95, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Tee JK, Peng F, Tan YL, Yu B, Ho HK. Magnesium Isoglycyrrhizinate Ameliorates Fibrosis and Disrupts TGF-β-Mediated SMAD Pathway in Activated Hepatic Stellate Cell Line LX2. Front Pharmacol 2018, 9, 1018. [Google Scholar] [CrossRef] [PubMed]
- Luo X, Deng Q, Xue Y, Zhang T, Wu Z, Peng H. Anti-Fibrosis Effects of Magnesium Lithospermate B in Experimental Pulmonary Fibrosis: By Inhibiting TGF-βRI/Smad Signaling. Molecules 2021, 26, 1715. [Google Scholar] [CrossRef] [PubMed]
- Ulloa L, Doody J, Massagué J.Inhibition of transforming growth factor-β/SMAD signalling by the interferon-γ/STAT pathway. Nature, 1999; 397, 710–713. [CrossRef]
- Vijayaraghavaavaava AKR. Alteration of Interferon Gamma (IFN-?) in Human Plasma with Graded Physical Activity. J Clin of Diagn Res 2014, 8, BC05–BC07. [Google Scholar] [CrossRef] [PubMed]
- Li X, Wang K. Effects of moderate-intensity endurance exercise on angiotensin II and angiotensin II type I receptors in the rat heart. Mol Med Rep 2017, 16, 2439–2444. [Google Scholar] [CrossRef]
- Fernandez-Castelo S, Arzt ES, Pesce A, Criscuolo ME, Diaz A, Finkielman S. Angiotensin II regulates interferon-gamma production. J Interferon Res 1987, 7, 261–8. [Google Scholar] [CrossRef]
- Gebreyesus K, Kilbourne EJ, Sabban EL. Bradykinin elevates tyrosine hydroxylase and dopamine beta-hydroxylase mRNA levels in PC12 cells. Brain Research 1993, 608, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Hu J, Igarashi A, Kamata M, Nakagawa H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem 2001, 276, 47863–8. [Google Scholar] [CrossRef] [PubMed]
- Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 1990, 86, 1343–6. [Google Scholar] [CrossRef] [PubMed]
- Narain Y, Yip A, Murphy T, Brayne C, Easton D, Evans JG. The ACE gene and Alzheimer's disease susceptibility. J Med Genet 2000, 37, 695–7 https://jmgbmjcom/content/37/9/695. [Google Scholar] [CrossRef] [PubMed]
- Brown NJ! Blais Jr C, Gandhi SK, Albert A. ACE Insertion/Deletion Genotype Affects Bradykinin Metabolism. Journal of Cardiovascular Pharmacology 1998, 32, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Singh PK, Chen ZL, Ghosh D, Strickland S, Norris EH. Increased plasma bradykinin level is associated with cognitive impairment in Alzheimer's patients. Neurobiol Dis 2020, 139, 104833. [Google Scholar] [CrossRef]
- Douillet CD, Velarde V, Christopher JT, Mayfield RK, Trojanowska ME, Jaffa AA. Mechanisms by which bradykinin promotes fibrosis in vascular smooth muscle cells: role of TGF-beta and MAPK. Am J Physiol Heart Circ Physiol 2000, 279, H2829–37. [Google Scholar] [CrossRef] [PubMed]
- Bandera EV, Qin B, Lin Y, Zeinomar N, Xu B, Chanumolu D. Association of Body Mass Index, Central Obesity, and Body Composition With Mortality Among Black Breast Cancer Survivors. JAMA Oncol 2021, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/Black People 2022. CA Cancer J Clin 2022, 72, 202–229. [Google Scholar] [CrossRef]
- Jorgovanovic, D. , Song, M., Wang, L. et al. Roles of IFN-γ in tumor progression and regression: a review. Biomark Res 2020, 8, 49. [Google Scholar] [CrossRef]
- Liang J, Shang Y. Estrogen and cancer. Annu Rev Physiol. 2013, 75, 225–40. [Google Scholar] [CrossRef]
- Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018, 363, k4209. [Google Scholar] [CrossRef] [PubMed]
- Yu HS, Lin TH, Tang CH. Bradykinin enhances cell migration in human prostate cancer cells through B2 receptor/PKCδ/c-Src dependent signaling pathway. Prostate 2013, 73, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Smith AD, Refsum H, Bottiglieri T, Fenech M, Hooshmand B, McCaddon A. Homocysteine and Dementia: An International Consensus Statement. J Alzheimers Dis 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Jang Y, Yoon H, Park NS, Rhee MK, Chiriboga DA. Asian Americans' concerns and plans about Alzheimer's disease: The role of exposure, literacy and cultural beliefs. Health Soc Care Community 2018, 26, 199–206. [Google Scholar] [CrossRef]
- Rojo-Sebastián A, González-Robles C, García de Yébenes J. Vitamin B6 Deficiency in Patients With Parkinson Disease Treated With Levodopa/Carbidopa. Clin Neuropharmacol. 2020, 43, 151–157. [Google Scholar] [CrossRef]
- Politis M, Loane C. Serotonergic dysfunction in Parkinson's disease and its relevance to disability. ScientificWorldJournal. 2011, 11, 1726–34. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, DO. B Vitamins and the Brain: Mechanisms, Dose and Efficacy--A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Tyagi N, Sedoris KC, Steed M. Mechanisms of homocysteine-induced oxidative stress. American Journal of physiology. Heart and Circulatory Physiology 2005, 289, H2649–56. [Google Scholar] [CrossRef] [PubMed]
- Keskin A, U Ustun G, Aci R, Duran U. Homocysteine as a marker for predicting disease severity in patients with COVID-19. Biomark Med 2022, 16, 559–568. [Google Scholar] [CrossRef]
- McCaddon A, Regland B. COVID-19: A methyl-group assault? Med Hypotheses 2021, 149, 110543. [Google Scholar] [CrossRef] [PubMed]
- Fan X, Zhang L, Li H, Chen G, Qi G, Ma X. Role of homocysteine in the development and progression of Parkinson's disease. Ann Clin Transl Neurol 2020, 7, 2332–2338. [Google Scholar] [CrossRef]
- Farkas M, Keskitalo S, Smith DE, Bain N, Semmler A, Ineichen B. Hyperhomocysteinemia in Alzheimer's disease: the hen and the egg? J Alzheimers Dis 2013, 33, 1097–104. [Google Scholar] [CrossRef] [PubMed]
- Gilfix, BM. Vitamin B12 and homocysteine. CMAJ 2005, 173, 1360. [Google Scholar] [CrossRef]
- Morris MC, Schneider JA, Tangney CC. Thoughts on B-vitamins and dementia. J Alzheimers 2006, 9, 429–33. [Google Scholar] [CrossRef]
- Łoboś P, Regulska-Ilow B. Link between methyl nutrients and the DNA methylation process in the course of selected diseases in adults. Rocz Panstw Zakl Hig 2021, 72, 123–136 https://pubmedncbinlmnihgov/34114759/. [Google Scholar]
- Hadtstein F, Vrolijk M. Vitamin B-6-Induced Neuropathy: Exploring the Mechanisms of Pyridoxine Toxicity. Adv Nutr 2021, 12, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Noah L, Dye L, Bois De Fer B, Mazur A, Pickering G, Pouteau E. Effect of magnesium and vitamin B6 supplementation on mental health and quality of life in stressed healthy adults: Post-hoc analysis of a randomised controlled trial. Stress Health 2021, 37, 1000–1009. [Google Scholar] [CrossRef]
- Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003; CD004393. [CrossRef]
- Chambers P, Antioxidants and Long Covid. Open Access Library Journal 2022, 9, e9414. Available online: https://www.scirp.org/journal/paperinformation.aspx?paperid=120821.
- Xu J, Patassini S, Begley P, Church S, Waldvogel HJ, Faull RLM. Cerebral deficiency of vitamin B5 (d-pantothenic acid; pantothenate) as a potentially-reversible cause of neurodegeneration and dementia in sporadic Alzheimer's disease. Biochem Biophys Res Commun 2020, 527, 676–681. [Google Scholar] [CrossRef]
- Scholefield M, Church SJ, Xu J, Patassini S, Hooper NM, Unwin RD. Substantively Lowered Levels of Pantothenic Acid (Vitamin B5) in Several Regions of the Human Brain in Parkinson’s Disease Dementia. Metabolites 2021, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Leonardi R, Jackowski S. Biosynthesis of Pantothenic Acid and Coenzyme A. EcoSal Plus 2007, 2, 101128/ecosalplus3634. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer's Association Calcium Hypothesis Workgroup. Calcium Hypothesis of Alzheimer's disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement 2017, 13, 178–182e17. [Google Scholar] [CrossRef] [PubMed]
- Magnesium in Alzheimer’s disease Section 3 of the book Magnesium in the Central Nervous System (2011). Vink R, Nechifor M, editors. Adelaide (AU): University of Adelaide Press. https://www.ncbi.nlm.nih.gov/books/NBK507256/.
- Surmeier DJ, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E. Calcium and Parkinson's disease. Biochem Biophys Res Commun 2017, 483, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Miyake Y, Tanaka K, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y. Fukuoka Kinki Parkinson's Disease Study Group. Dietary intake of metals and risk of Parkinson's disease: a case-control study in Japan. J Neurol Sci. 2011, 306, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Wang L, Zhou C, Yu H, Hao L, Ju M, Feng W. Vitamin D, Folic Acid and Vitamin B12 Can Reverse Vitamin D Deficiency-Induced Learning and Memory Impairment by Altering 27-Hydroxycholesterol and S-Adenosylmethionine. Nutrients 2023, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Chambers, P. Ca:Mg + D, the Shield that Interdicts the Crown Viruses and Vaccines. Open Access Library Journal 2022, 9, 1–24. Available online: https://www.scirp.org/journal/paperinformation.aspx?paperid=119926. [CrossRef]
- Fonseca SC, Rivas I, Romaguera D, et al. Association between consumption of fermented vegetables and COVID-19 mortality at a country level in Europe. medRxiv, 2020. [CrossRef]
- Kiana TV, Nikan Z, Armin E, Mohammad MS, Parnia M, Pari M. Association of fruits, vegetables, and fiber intake with COVID-19 severity and symptoms in hospitalized patients: A cross-sectional study Frontiers in Nutrition 2022, 9, 2022. [CrossRef] [PubMed]
- Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153e14. [Google Scholar] [CrossRef]
- Kim YH, Jung KI. Song CH. Effects of Serum Calcium and Magnesium on Heart Rate Variability in Adult females. Biological Trace Element Research 2012, 150, 116–122. [Google Scholar] [CrossRef]
- Haensel, A. , Mills, P.J., Nelesen, R.A., Ziegler, M.J. and Dimsdale, J.E. The Relationship between Heart Rate Variability and Inflammatory Markers in Cardiovascular Diseases. Psychoneuroendocrinology 2008, 33, 1305–1312. [Google Scholar] [CrossRef]
- Schiopu C, Ștefănescu G, Diaconescu S, Bălan GG, Gimiga N, Rusu E. Magnesium Orotate and the Microbiome–Gut–Brain Axis Modulation: New Approaches in Psychological Comorbidities of Gastrointestinal Functional Disorders. Nutrients 2022, 14, 1567. [Google Scholar] [CrossRef] [PubMed]
- Winther G, Pyndt Jørgensen B, ElfvingmB, Nielsen D, Kihl P, Lund S. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatrica 2015, 27, 168–176. [Google Scholar] [CrossRef]
- Luo J, Zhang C, Zhao Q. Dietary calcium and magnesium intake and risk for incident dementia: The Shanghai Aging Study. Alzheimer's & Dementia 2022, 8, e12362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. Ca:Mg Ratio, APOE Cytosine Modifications, and Cognitive Function: Results from a Randomized Trial. Journal of Alzheimer's Disease 2020, 75, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Kong LM, Liao CG, Chen L, Yang HS, Zhang SH, Zhang Z. Promoter hypomethylation up-regulates CD147 expression through increasing Sp1 binding and associates with poor prognosis in human hepatocellular carcinoma. J Cell Mol Med 2011, 15, 1415–28. [Google Scholar] [CrossRef] [PubMed]
- Liao CG, Liang XH, Ke Y. Active demethylation upregulates CD147 expression promoting non-small cell lung cancer invasion and metastasis. Oncogene 2022, 41, 1780–1794. [Google Scholar] [CrossRef]
- Ehrlich, M. DNA hypomethylation in cancer cells. Epigenomics 2009, 1, 239–59. [Google Scholar] [CrossRef] [PubMed]
- Kulis M, Esteller M. DNA methylation and cancer. Adv Genet 2010, 70, 27–56. [Google Scholar] [CrossRef]
- Johnson AA, Akman K, Calimport SR, Wuttke D, Stolzing A, de Magalhães JP. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res 2012, 15, 483–94. [Google Scholar] [CrossRef]
- Song H, Yang J, Yu W. Promoter Hypomethylation of TGFBR3 as a Risk Factor of Alzheimer's Disease: An Integrated Epigenomic-Transcriptomic Analysis. Front Cell Dev Biol 2022, 9, 825729. [Google Scholar] [CrossRef] [PubMed]
- Chouliaras L, Kumar GS, Thomas AJ, Lunnon K, Chinnery PF, O'Brien JT. Epigenetic regulation in the pathophysiology of Lewy body dementia. Prog Neurobiol 2020, 192, 101822. [Google Scholar] [CrossRef]
- Tulloch J, Leong L, Chen S, Keene CD, Millard SP, Shutes-David A. APOE DNA methylation is altered in Lewy body dementia. Alzheimers Dement 2018, 14, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Harhangi BS, de Rijk MC, van Duijn CM, Van Broeckhoven C, Hofman A, Breteler MM. APOE and the risk of PD with or without dementia in a population-based study. Neurology 2000, 54, 1272–6. [Google Scholar] [CrossRef]
- Foraker J, Millard SP, Leong L, Thomson Z, Chen S, Keene CD, Bekris LM, Yu CE. The APOE Gene is Differentially Methylated in Alzheimer's Disease. J Alzheimers Dis 2015, 48, 745–55. [Google Scholar] [CrossRef] [PubMed]
- Suchy-Dicey A, Howard B, Longstreth WT Jr, Reiman EM, Buchwald D. APOE genotype, hippocampus, and cognitive markers of Alzheimer's disease in American Indians: Data from the Strong Heart Study. Alzheimers Dement 2022, 18, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Workinger, J.L. , Doyle, R.P. and Bortz, J. Challenges in the Diagnosis of Magnesium Status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Nakayama Y, Ueda S, Okuda S. Molecular mechanism underlying the renoprotective action of vitamin D. Circ J 2014, 78, 599–600. [Google Scholar] [CrossRef]
- Wimalawansa, SJ. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections—Sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- Xian X, Wang Y, Zheng J. COVID-19 and Alzheimer’s disease: how one crisis worsens the other. Transl Neurodegener 2021, 10, 15. [Google Scholar] [CrossRef]
- Bikle, DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014, 21, 319–29. [Google Scholar] [CrossRef] [PubMed]
- Thye A Y-Y, Tan L T-H, Law J W-F, Pusparajah P, Letchumanan V. Long COVID-19: psychological symptoms in COVID-19 and probiotics as an adjunct therapy. Progress In Microbes & Molecular Biology, 2022. [CrossRef]
- Gutiérrez-Castrellón P, Gandara-Martí T, Abreu Y Abreu AT, Nieto-Rufino CD, López-Orduña E. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef]
- Yong SJ, Tong T, Chew J, Lim WL. Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential. Front Neurosci 2020, 13, 1361. [Google Scholar] [CrossRef] [PubMed]
- Shrestha NK, Burke PC, Nowacki AS, Simon JF, Hagen A, Gordon SM. Effectiveness of the Coronavirus Disease 2019 (COVID-19) Bivalent Vaccine medRxiv 2023, preprint. https://www.medrxiv.org/content/10.1101/2022.12.17. 22283625v1.
- McCracken IR, Saginc G, He L, Huseynov A, Daniels A, Fletcher S. Lack of Evidence of Angiotensin-Converting Enzyme 2 Expression and Replicative Infection by SARS-CoV-2 in Human Endothelial Cells. Circulation 2021, 143, 865–868. [Google Scholar] [CrossRef]
- Ganier C, Du-Harpur X, Harun N, Wan B, Arthurs C, Luscombe NM. CD147 (BSG) but not ACE2 expression is detectable in vascular endothelial cells within single cell RNA sequencing datasets derived from multiple tissues in healthy individuals biorxiv 2020, preprint. [CrossRef]
- Hsu CC, Cheng CH, Hsu CL, Lee WJ, Huang SC, Huang YC. Role of vitamin B6 status on antioxidant defenses, glutathione, and related enzyme activities in mice with homocysteine-induced oxidative stress. Food Nutr Res 2015, 59, 25702. [Google Scholar] [CrossRef]
- Gibson CC, Davis CT, Zhu W, Bowman-Kirigin JA, Walker AE, Tai Z. Dietary Vitamin D and Its Metabolites Non-Genomically Stabilize the Endothelium. PLoS One 2015, 10, e0140370. [Google Scholar] [CrossRef] [PubMed]
- Tsubokawa M, Nishimura M, Mikami T, Ishida M, Hisada T, Tamada Y. Association of Gut Microbial Genera with Heart Rate Variability in the General Japanese Population: The Iwaki Cross-Sectional Research Study. Metabolites 2022, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Brilla L, Teichler L, Hahn T, Freeman J, LiNY. Effect of Magnesium on Heart Rate Variability in Healthy Subjects. FASEB J 2010, 24, 917.3. [Google Scholar] [CrossRef]
- Wyller VB, Nguyen CB, Ludviksen JA, Mollnes TE. Transforming growth factor beta (TGF-β) in adolescent chronic fatigue syndrome. J Transl Med 2017, 15, 245. [Google Scholar] [CrossRef]
- Thanou A, Stavrakis S, Dyer JW, Munroe ME, James JA, Merrill JT. Impact of heart rate variability, a marker for cardiac health, on lupus disease activity. Arthritis Res Ther 2016, 18, 197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




