Submitted:
06 February 2023
Posted:
07 February 2023
Read the latest preprint version here
Abstract
Keywords:
Introduction
Materials and methods
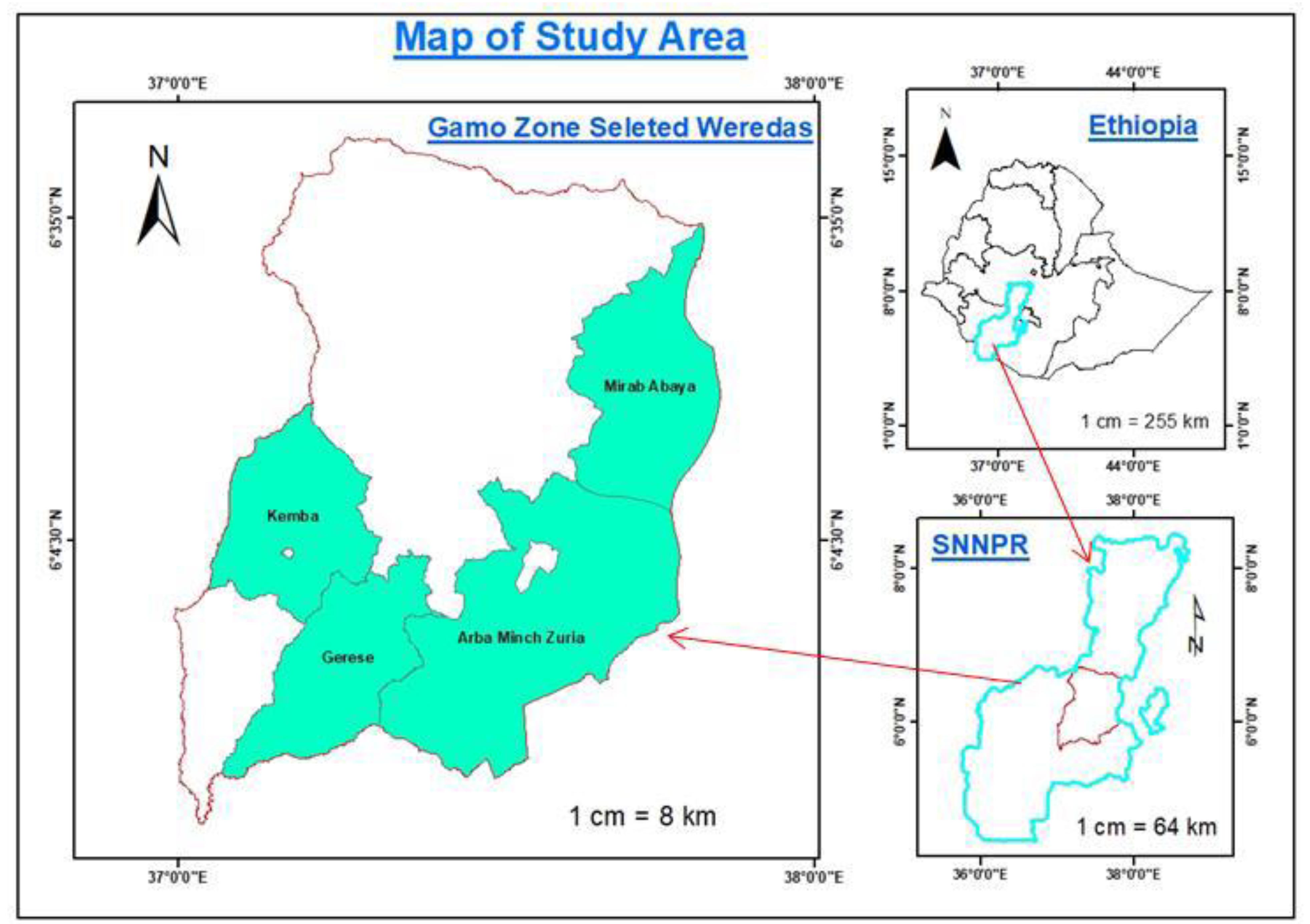
Description of the study area
Farmers’ preference scoring and sample collection of ILFTS
Evaluation of nutritional quality of ILFTS
Chemical analyses of ILFTS
In-vitro dry matter digestibility potential of ILFTS
Statistical analysis
Result
Socioeconomic characteristics’ of farmers
Farmers’ preference of ILFTS
Nutritional value parameters
The correlation among nutrients and farmers feed value scoress
Discussion
Farmers’ preference of ILFTS
Nutritional value parameters
The correlation between nutrients and farmers feed value score
Conclusion
References
- Abebe, A.; Tolera, A.; Holand, Ø.; Ådnøy, T.; Eik, L.O. Seasonal variation in nutritive value of some browse and grass species in Borana rangeland, southern Ethiopia. Trop. Subtrop. Agroecosystems 2012, 15, 261–271. [Google Scholar]
- Abraham, G.; Kechero, Y.; Andualem, D. Nutritional Quality of Indigenous Legume Browse in the Southern Ethiopia: Farmers’ Preference and Correlation of Local Valuation of Feed Value with Scientific Indicators. 2023. [CrossRef]
- Abraham, G.; Kechero, Y.; Andualem, D.; Dingamo, T. Indigenous legume fodder trees and shrubs with emphasis on land use and agroecological zones: Identification, diversity, and distribution in semi-humid condition of southern Ethiopia. Vet. Med. Sci. 2022, 8, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Adejoro, F.A. The use of condensed tannins and nitrate to reduce enteric methane emission and enhance utilization of high forage diets in sheep (Doctorial desertation). University of Pretoria, Pretoria, South Africa. 2019.
- Adjorlolo, L.K.; Adogla-Bessa, T.; Amaning-Kwarteng, K.; Ahunu, B.K. Effect of season on the quality of forages selected by sheep in citrus plantations in Ghana. Trop. Grassl.-Forrajes Trop. 2014, 2, 271–277. [Google Scholar] [CrossRef]
- Andualem, D.; Gelgele, M.; Bayssa, M. In vitro gas production kinetics of selected multipurpose tree browses in Gelana rangelands. Livest. Res. Rural Dev. 2021, 33, 18. [Google Scholar]
- AOAC, 2006. Official Methods of Analysis of AOAC International, 18th Edition. ed. AOAC International, USA.
- AOAC, 1990. Official method of analysis of the association of official analytical chemists, 15th edition. ed. Association of official analytical chemists,inc, USA.
- Arigbede, O.M.; Anele, U.Y.; Südekum, K.-H.; Hummel, J.; Oni, A.O.; Olanite, J.A.; Isah, A.O. Effects of species and season on chemical composition and ruminal crude protein and organic matter degradability of some multi-purpose tree species by West African dwarf rams: Chemical composition and degradability of browse trees. J. Anim. Physiol. Anim. Nutr. 2012, 96, 250–259. [Google Scholar] [CrossRef]
- Assefa, A.; Kechero, Y.; Tolemariam, T.; Kebede, A.; Shumi, E. Anthelmintic effects of indigenous multipurpose fodder tree extracts against Haemonchus contortus. Trop. Anim. Health Prod. 2018, 50, 727–732. [Google Scholar] [CrossRef]
- Ayenew, A.; Tolera, A.; Nurfeta, A.; Assefa, G. Farmers’ preference and knowledge on indigenous multipurpose browse species towards their feed value in north western Ethiopia. Trop. Subtrop. Agroecosystems 2021, 24. [Google Scholar] [CrossRef]
- Balehegn, M. 2017. Silvopasture Using Indigenous Fodder Trees and Shrubs: The Underexploited Synergy Between Climate Change Adaptation and Mitigation in the Livestock Sector, in: Leal Filho, W., Belay, S., Kalangu, J., Menas, W., Munishi, P., Musiyiwa, K. (Eds.), Climate Change Adaptation in Africa, Climate Change Management. pp. 493–510. [CrossRef]
- Balehegn, M.; Hintsa, K. Effect of maturity on chemical composition of edible parts of Ficus thonningii Blume (Moraceae): An indigenous multipurpose fodder tree in Ethiopia. Livest. Res. Rural Dev. 2015, 27, 233. [Google Scholar]
- Birhan, M.; Gesses, T.; Kenubih, A.; Dejene, H.; Yayeh, M. Evaluation of Anthelminthic Activity of Tropical Taniferous Plant Extracts Against Haemonchus contortus. Vet. Med. Res. Rep. 2020, 11, 109–117. [Google Scholar] [CrossRef]
- Boogaard, B.K.; Oosting, S.J.; Bock, B.B. Elements of societal perception of farm animal welfare: A quantitative study in The Netherlands. Livest. Sci. 2006, 104, 13–22. [Google Scholar] [CrossRef]
- Bouazza, L.; Bodas, R.; Boufennara, S.; Bousseboua, H.; López, S. Nutritive evaluation of foliage from fodder trees and shrubs characteristic of Algerian arid and semi-arid areas. J. Anim. Feed Sci. 2012, 21, 521–536. [Google Scholar] [CrossRef]
- Brown, D.; Ng’ambi, J.W.; Norris, D. Effect of tanniniferous Acacia karroo leaf meal inclusion level on feed intake, digestibility and live weight gain of goats fed a Setaria verticillata grass hay-based diet. J. Appl. Anim. Res. 2018, 46, 248–253. [Google Scholar] [CrossRef]
- Bueno, I.C.S.; Brandi, R.A.; Fagundes, G.M.; Benetel, G.; Muir, J.P. The Role of Condensed Tannins in the In Vitro Rumen Fermentation Kinetics in Ruminant Species: Feeding Type Involved? Anim. Open Access J. MDPI 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Gutierrez, E.; Aranda-Aguirre, E.; Robles-Jimenez, L.E.; Castelán-Ortega, O.A.; Chay-Canul, A.J.; Foggi, G.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; González-Ronquillo, M. Effect of tannins from tropical plants on methane production from ruminants: A systematic review. Vet. Anim. Sci. 2021, 14, 100214. [Google Scholar] [CrossRef]
- Chimphango, S.B.M.; Gallant, L.H.; Poulsen, Z.C.; Samuels, M.I.; Hattas, D.; Curtis, O.E.; Muasya, A.M.; Cupido, C.; Boatwright, J.S.; Howieson, J. Native legume species as potential fodder crops in the mediterranean renosterveld shrubland, South Africa. J. Arid Environ. 2020, 173, 104015. [Google Scholar] [CrossRef]
- Datt, C.; Datta, M.; Singh, N.P. Assessment of fodder quality of leaves of multipurpose trees in subtropical humid climate of India. J. For. Res. 2008, 19, 209–214. [Google Scholar] [CrossRef]
- Derero, A.; Kitaw, G. Nutritive values of seven high priority indigenous fodder tree species in pastoral and agro-pastoral areas in Eastern Ethiopia. Agric. Food Secur. 2018, 7, 68. [Google Scholar] [CrossRef]
- Dida, M.F.; Challi, D.G.; Gangasahay, K.Y. Effect of feeding different proportions of pigeon pea (Cajanus cajan) and neem (Azadirachta indica) leaves on feed intake, digestibility, body weight gain and carcass characteristics of goats. Vet. Anim. Sci. 2019, 8, 100079. [Google Scholar] [CrossRef] [PubMed]
- Dires, A.; Asefa, M.; Ashenafi, A.; Wasihun, W. 2021. Discover the land of paradize: Gamo zone travel guide, 2nd edition. ed. Gamo zone culture tourism and sport department, Arba-Minch, Ethiopia.
- Enri, S.R.; Probo, M.; Renna, M.; Caro, E.; Lussiana, C.; Battaglini, L.M.; Lombardi, G.; Lonati, M.; Enri, S.R.; Probo, M.; et al. Temporal variations in leaf traits, chemical composition and in vitro true digestibility of four temperate fodder tree species. Anim. Prod. Sci. 2020, 60, 643–658. [Google Scholar] [CrossRef]
- FAO, 2018. Ethiopia: Report on feed inventory and feed balance (2018). Food and Agriculture Organization of the United Nations, Rome, Italy.
- Franzel, S.; Carsan, S.; Lukuyu, B.; Sinja, J.; Wambugu, C. Fodder trees for improving livestock productivity and smallholder livelihoods in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 98–103. [Google Scholar] [CrossRef]
- Gebrehiwot, G.; Negesse, T.; Abebe, A. Effect of Feeding Leucaena leucocephala Leaves and Pods on Feed Intake, Digestibility, body Weight Change and Carcass Characteristic of Central-Highland Sheep Fed basal Diet Wheat bran and Natural Pasture Hay in tigray, Ethiopia. Int. J. Agric. Environ. Biotechnol. 2017, 10, 367. [Google Scholar] [CrossRef]
- Gebremedhin, A.T.; Gedo, A.H.; Edo, G.Y.; Haile, S.T. Evaluation of multi-functional fodder tree and shrub species in mid-altitudes of South Omo Zone, Southern Ethiopia. J. Hortic. For. 2020, 12, 27–34. [Google Scholar] [CrossRef]
- Grant, K.; Kreyling, J.; Dienstbach, L.F.H.; Beierkuhnlein, C.; Jentsch, A. Water stress due to increased intra-annual precipitation variability reduced forage yield but raised forage quality of a temperate grassland. Agric. Ecosyst. Amp Environ. 2014, 186, 11–22. [Google Scholar] [CrossRef]
- Hadgu, K.M.; Kooistra, L.; AH Rossing, W.; van Bruggen, A.H.C. Assessing the effect of Faidherbia albida based land use systems on barley yield at field and regional scale in the highlands of Tigray, Northern Ethiopia. Food Sec 2009, 1, 337–350. [Google Scholar] [CrossRef]
- Haugerud, A.; Collinson, M.P. Plants, genes and people: Improving the relevance of plant breeding in Africa. ExplAgric 1990, 26, 341–362. [Google Scholar] [CrossRef]
- Kuntashula, E.; Mafongoya, P.L. Farmer participatory evaluation of agroforestry trees in eastern Zambia. Agric. Syst. 2005, 85, 39–53. [Google Scholar] [CrossRef]
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Lelamo, L. A review on the indigenous multipurpose agroforestry tree species in Ethiopia: Management, their productive and service roles and constraints. Heliyon 2021, 7, e07874. [Google Scholar] [CrossRef]
- Li, X. Plant cell wall chemistry: Implications for ruminant utilisation. J. Appl. Anim. Nutr. 2021, 9, 31–56. [Google Scholar] [CrossRef]
- Lumu, R.; Katongole, C.B.; Nambi-Kasozi, J.; Bareeba, F.; Presto, M.; Ivarsson, E.; Lindberg, J.E. Indigenous knowledge on the nutritional quality of urban and peri-urban livestock feed resources in Kampala, Uganda. Trop. Anim. Health Prod. 2013, 45, 1571–1578. [Google Scholar] [CrossRef]
- Makau, D.N.; VanLeeuwen, J.A.; Gitau, G.K.; McKenna, S.L.; Walton, C.; Muraya, J.; Wichtel, J.J. Effects of Calliandra and Sesbania on Daily Milk Production in Dairy Cows on Commercial Smallholder Farms in Kenya. Vet. Med. Int. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. 2000. Quantification of Tannins in Tree Foliage.
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A. 2002. Animal Nutrition, 6th ed. Prentice Hall, Essex, UK.
- Meijer, S.S.; Catacutan, D.; Ajayi, O.C.; Sileshi, G.W.; Nieuwenhuis, M. The role of knowledge, attitudes and perceptions in the uptake of agricultural and agroforestry innovations among smallholder farmers in sub-Saharan Africa. Int. J. Agric. Sustain. 2015, 13, 40–54. [Google Scholar] [CrossRef]
- Mekonnen, K.; Glatzel, G.; Sieghardt, M. Assessments of Fodder Values of 3 Indigenous and 1 Exotic Woody Plant Species in the Highlands of Central Ethiopia. Mt. Res. Dev. 2009, 29, 135–142. [Google Scholar] [CrossRef]
- Mekoya, A.; Oosting, S.J.; Fernandez-Rivera, S.; Van der Zijpp, A.J. Multipurpose fodder trees in the Ethiopian highlands: Farmers’ preference and relationship of indigenous knowledge of feed value with laboratory indicators. Agric. Syst. 2008, 96, 184–194. [Google Scholar] [CrossRef]
- Mitiku, B. Evaluation and Demonstration of Indigenous Fodder Trees and Shrubs in Alicho-wriro District, Siltie Zone. J. Nat. Sci. Res. 2018, 8. [Google Scholar]
- Mohameed, A.; Kassahun, G.; Ayantu, M. Identification and Nutritional Evaluation of Potential Indigenous Browse Species in Guba Lafto District, North Wollo, Ethiopia. J. Anim. Sci. Res. 2020, 4. [Google Scholar] [CrossRef]
- Moore, K.J.; Jung, H.-J.G. Lignin and fiber digestion. J. RANGE Manag. 2001, 54, 420–430. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef]
- Rubanza, C.D.K.; Shem, M.N.; Otsyina, R.; Ichinohe, T.; Fujihara, T. Nutritive Evaluation of Some Browse Tree Legume Foliages Native to Semi-arid Areas in Western Tanzania. Asian-Australas. J. Anim. Sci. 2003, 16, 1429–1437. [Google Scholar] [CrossRef]
- Shenkute, B.; Hassen, A.; Assafa, T.; Amen, N.; Ebro, A. Identification and nutritive value of potential fodder trees and shrubs in the mid rift valley of Ethiopia. J. Anim. Plant Sci. 2012, 22, 1126–1132. [Google Scholar]
- Tilley, J.M.A.; Terry, R.A. A Two-Stage Technique for the in Vitro Digestion of Forage Crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B. 1985. Analysis of Forages and Fibrous Foods: A Laboratory Manual for Animal Science. Cornell University, Ithaca, NY (USA).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Yayneshet, T.; Eik, L.O.; Moe, S.R. Seasonal variations in the chemical composition and dry matter degradability of exclosure forages in the semi-arid region of northern Ethiopia. Anim. Feed Sci. Technol. 2009, 148, 12–33. [Google Scholar] [CrossRef]
- Yisehak, K.; Janssens, G.P.J. Evaluation of nutritive value of leaves of tropical tanniferous trees and shrubs. Livest. Res. Rural Dev. 2013, 25, 28. [Google Scholar]
- Yu, P.; McKinnon, J.J.; Christensen, D.A. Hydroxycinnamic acids and ferulic acid esterase in relation to biodegradation of complex plant cell walls. Can. J. Anim. Sci. 2005, 85, 255–267. [Google Scholar] [CrossRef]
| Parameter | Category | Agroecological zone | Total | ||
|---|---|---|---|---|---|
| Lowland | Midland | Highland | |||
| Sex of the respondents’ | Male | 18 | 17 | 20 | 55 (91.7%) |
| Female | 2 | 3 | 0 | 5 (8.3 %) | |
| Age category of the respondents’ | 21 – 30 years | 1 | 0 | 0 | 1 (1.67%) |
| 31 – 40 years | 6 | 7 | 6 | 19 (31.67%) | |
| 41 – 50 years | 7 | 8 | 10 | 25 (41.57%) | |
| Above 51 years | 6 | 5 | 4 | 15 (25%) | |
| Marital status of the respondents’ | Married | 19 | 18 | 20 | 57 (95%) |
| Widowed | 1 | 2 | 0 | 3 (5%) | |
| Educational level of the respondents’ | Illiterate | 1 | 4 | 8 | 13 (21.67%) |
| Basic education | 5 | 8 | 2 | 15 (25%) | |
| Grade 1 - 4 | 3 | 2 | 3 | 8 (13.33%) | |
| Grade 5 – 8 | 7 | 6 | 4 | 17 (28.33%) | |
| Grade 9 – 12 | 3 | 0 | 2 | 5 (8.3%) | |
| Above 12 | 1 | 0 | 1 | 2 (3.33%) | |
| Position of the respondent in the community | Locality admin | 4 | 1 | 1 | 6 (10%) |
| Spiritual leader | 1 | 2 | 2 | 5 (8.3%) | |
| Elder | 6 | 0 | 3 | 9 (15%) | |
| Ordinary farmer | 9 | 17 | 14 | 40 (66.7%) | |
| Land holdings | <0.25 ha | 0 | 3 | 3 | 6 (10%) |
| 0.26 – 0.5 ha | 4 | 9 | 5 | 18 (30%) | |
| 0.51 – 1 ha | 9 | 6 | 7 | 22 (36.67%) | |
| >1ha | 7 | 2 | 5 | 14 (23.3%) | |
| Total | 20 | 20 | 20 | 60 (100%) | |
| Species/ agro-ecology | Feed value | Growth rate | Biomass yield | Compatibility | Multifunctionality | Overall mean |
|---|---|---|---|---|---|---|
| Lowland | ||||||
| Acacia tortilis | 3.53ab | 2.1ef | 2.73cd | 2.60f | 3.23bcd | 2.84bc |
| Acacia seyal | 3.63a | 2.00f | 2.78c | 2.53f | 3.17bcd | 2.82bcd |
| Acacia albida | 3.57a | 2.00f | 2.7cd | 3.92a | 3.28bc | 3.09a |
| Tamarindus Indica | 3.07de | 2.48c | 3.55a | 3.17bc | 3.22bcd | 3.1a |
| Aeschynomene elaphroxylon | 3.25cd | 2.9a | 2.9c | 2.97cde | 1.93f | 2.79bcd |
| Acacia polyacantha | 3.22cde | 2.9a | 2.95c | 3.25b | 3.08d | 3.08a |
| Acacia Senegal | 3.68a | 2.65b | 2.88c | 2.87de | 3.29bc | 3.07a |
| Acacia hockii | 2.98e | 2.28d | 2.7cd | 2.75ef | 3.19bcd | 2.78cd |
| dichrostachys cinerea | 2.72f | 2.23de | 2.5d | 3.05bcd | 3.09cd | 2.72d |
| Acacia mellifera | 3.68a | 2.35cd | 2.95c | 3.23b | 3.25abc | 3.1a |
| Acacia nilotica | 2.6f | 2.63b | 2.78c | 2.97cde | 3.4a | 2.87bc |
| Acacia brevispica | 3.61a | 2.38cd | 2.5d | 3.05bcd | 2.68e | 2.84bc |
| Acacia sieberiana | 3.32bc | 2.05f | 3.25b | 3.23b | 2.59e | 2.89b |
| Mean + SD | 3.29+0.4 | 2.38+0.38 | 2.86+0.4 | 3.04+0.39 | 3.03+0.4 | 2.92+0.19 |
| Significance level | *** | *** | *** | *** | *** | *** |
| Midland | ||||||
| Acacia lahai | 2.98d | 2.8bc | 2.7d | 2.95b | 3.38a | 2.97d |
| Acacia abyssinica | 3.70ab | 2.8bc | 3.00c | 3.05b | 3.38a | 3.2ab |
| Piliostigma thonningii | 3.52bc | 2.73c | 3.25b | 3.00b | 3.40a | 3.18ab |
| Millettia ferruginea | 2.88d | 2.83bc | 3.23bc | 3.08b | 3.50a | 3.1c |
| Albizia Schimperiana | 3.75a | 3.00b | 3.15bc | 3.74a | 3.41a | 3.4a |
| Erythrina brucei | 3.71a | 3.78a | 3.63a | 3.12b | 2.69b | 3.38a |
| Erythrina abyssinica | 3.34c | 3.63a | 3.50a | 3.10b | 2.68b | 3.23b |
| Mean + SD | 3.41+0.39 | 3.08+0.48 | 3.21+0.37 | 3.14+0.4 | 3.21+0.34 | 3.21+0.17 |
| Significance level | *** | *** | *** | *** | *** | *** |
| Highland | ||||||
| Millettia ferruginea | 3.55b | 3.33b | 3.0a | 3.19 | 3.54a | 3.44 |
| Erythrina brucei | 3.81a | 3.95a | 3.5ab | 3.28 | 2.68c | 3.44 |
| Albizia Schimperiana | 3.83a | 3.88a | 3.4b | 3.33 | 2.79b | 3.45 |
| Mean + SD | 3.73+0.24 | 3.72+0.38 | 3.5+0.27 | 3.27+0.2 | 3.0+0.37 | 3.44+0.1 |
| Significance level | *** | *** | *** | NS | *** | NS |
| ILFTS | DM | Ash | CP | NDF | ADF | HC | ADL | IVDMD | ME (KJ/DM) |
CT (mg/g) |
| Lowland | ||||||||||
| Acacia tortilis lf | 902 | 98.7 | 202.0 | 329.0 | 123.0 | 206.0 | 95.1 | 591.0 | 8.87 | 5.17 |
| Acacia seyal lf | 906 | 63.1 | 220.4 | 282.2 | 158.1 | 124.1 | 68.6 | 575.1 | 6.63 | 1.65 |
| Acacia albida lf | 919.2 | 40.7 | 202.8 | 272.4 | 130.5 | 141.9 | 82.4 | 520.6 | 7.81 | 7.09 |
| Tamarindus indica lf | 905.7 | 81.8 | 158.7 | 447.2 | 189.5 | 257.7 | 72.3 | 673.5 | 10.1 | 1.94 |
| Aeschynomeneelaphroxylon lf | 895.7 | 58.9 | 170.4 | 414.2 | 146.6 | 267.6 | 57.8 | 699.3 | 10.5 | 2.2 |
| Acacia polyacantha lf | 913.7 | 88.5 | 195.8 | 334.6 | 133.0 | 201.6 | 108.2 | 490.5 | 7.35 | 4.48 |
| Acacia Senegal lf | 924.5 | 55.5 | 259.7 | 271.7 | 111.7 | 160.0 | 55.4 | 683.8 | 10.3 | 2.21 |
| Acacia hockii lf | 838.3 | 75.2 | 131.3 | 441.3 | 246.4 | 194.9 | 204.7 | 394.1 | 5.9 | 6.03 |
| DichrostachysCinerea lf | 877.2 | 44.8 | 101.1 | 446.5 | 188.4 | 258.1 | 155.8 | 470.4 | 7.1 | 6.78 |
| Acacia mellifera lf | 923.6 | 51.5 | 240.9 | 305.2 | 126.6 | 178.6 | 53.4 | 669.5 | 10.0 | 2.67 |
| Acacia nilotica lf | 839.6 | 77.8 | 100.4 | 501.7 | 257.1 | 244.6 | 220.1 | 303.6 | 4.55 | 5.27 |
| Acacia brevispica lf | 948.4 | 67.2 | 271.6 | 406.5 | 161.3 | 245.2 | 88.8 | 601.3 | 9.02 | 3.87 |
| Acacia sieberiana lf | 919.9 | 25.9 | 204.4 | 211.7 | 164.5 | 47.2 | 89.6 | 538.9 | 8.08 | 3.57 |
| Acacia albida fruit | 924.7 | 110.8 | 81.8 | 551.9 | 224.9 | 327.0 | 93.3 | 656.7 | 9.85 | 7.08 |
| Acacia tortilis pod | 912.6 | 37.2 | 118.6 | 421.0 | 260.3 | 160.7 | 100.6 | 529.7 | 7.95 | 6.79 |
| PiliostigmaThonningii lf | 888.8 | 134.2 | 169.9 | 618.1 | 259.3 | 358.8 | 85.9 | 740.5 | 11.1 | 2.54 |
| Midland | ||||||||||
| Acacia lahai lf | 871.1 | 60.7 | 137.0 | 406.9 | 167.8 | 239.1 | 136.8 | 469.7 | 7.04 | 3.78 |
| Acacia abyssinica lf | 919.5 | 53.9 | 229.5 | 224.6 | 139.6 | 85.0 | 87.5 | 543.2 | 8.15 | 1.95 |
| Millettia ferruginea lf (M) | 936.8 | 60.1 | 206.3 | 437.0 | 238.7 | 198.3 | 100.3 | 526.0 | 7.89 | 2.91 |
| Albizia schimperiana lf (M) | 877.7 | 50.1 | 152.9 | 520.7 | 210.8 | 309.9 | 115.7 | 540.0 | 8.1 | 2.36 |
| Erythrina brucei lf (M) | 942.4 | 106.5 | 314.0 | 439.0 | 208.1 | 230.9 | 70.0 | 609.9 | 9.15 | 1.45 |
| Erythrina abyssinica lf (M) | 941.1 | 71.7 | 155.9 | 427.2 | 317.5 | 109.7 | 83.1 | 486.1 | 7.29 | 2.65 |
| Highland | ||||||||||
| Albizia schimperiana lf(H) | 939.3 | 40.9 | 247.9 | 300.6 | 152.6 | 148.0 | 90.5 | 580.2 | 8.7 | 1.7 |
| Erythrina brucei lf(H) | 939.9 | 82.8 | 276.3 | 453.0 | 225.4 | 227.6 | 80.1 | 601.0 | 9.01 | 1.91 |
| Millettia ferruginea lf (H) | 941.6 | 89.0 | 272.9 | 444.9 | 228.7 | 216.2 | 83.8 | 589.6 | 8.84 | 1.8 |
| Nutritive value of ILFTS (g/kg DM) | Agroecological zones (Mean+SD) |
|||
|---|---|---|---|---|
| Lowland | Midland | Highland | Mean | |
| DM | 903+30.6 | 911+31.2 | 940+119.3 | 909.9+30.5 |
| OM | 838+41.2 | 834+41.2 | 869+25.3 | 840.9+40.2 |
| Ash | 65.2+23.8 | 76.7+23.8 | 70.9+26.2 | 69.1+25.8 |
| CP | 177.3+60.2b | 195.1+61.6ab | 265.7+15.5a | 192.9+62.4 |
| NDF | 375.8+97.0 | 439.1+119.6 | 399.5+85.7 | 396.3+102.4 |
| ADF | 174.8+50.8 | 220.3+58.9 | 202.2+43.0 | 190.8+54.4 |
| HC | 201+69.5 | 218.8+98.9 | 197.3+43.0 | 205.5+74.1 |
| ADL | 103.1+51.2 | 97+22.7 | 84.8+5.3 | 99.2+41.2 |
| IVDMD | 559.9+113.7 | 559.3+91.8 | 590.3+10.4 | 563.4+98.8 |
| IVOMD | 603.6+124.3 | 614.7+127.5 | 638.9+28.3 | 610.9+115.3 |
| DOMD | 524.9+106.6 | 524.4+86.0 | 553.4+9.8 | 528.2+92.6 |
| ME(MJ/KG) | 8.4+1.7 | 8.39+1.7 | 8.85+1.55 | 8.45+1.48 |
| CT(mg/g) | 4.45+2.02a | 2.52+0.74ab | 1.80+0.11b | 3.59+1.93 |
| Ash | OM | CP | NDF | ADF | HC | ADL | IVDMD | IVOMD | DOMD | ME (MJ/kg) |
CT | Feed value |
|
| DM | -0.015 | 0.768*** | 0.685*** | -0.296 | -0.139 | -0.306 | -0.781*** | 0.509** | 0.455* | 0.509** | 0.509** | -0.385 | 0.615** |
| Ash | -0.652*** | 0.019 | 0.612** | 0.318 | 0.612** | -0.005 | 0.286 | 0.447* | 0.286 | 0.285 | -0.087 | 0.028 | |
| OM | 0.507** | -0.616** | -0.309 | -0.624** | -0.589** | 0.203 | 0.059 | 0.202 | 0.203 | -0.236 | 0.458* | ||
| CP | -0.434 | -0.388 | -0.315 | -0.594** | 0.403* | 0.364 | 0.403* | 0.402* | -0.648*** | 0.768*** | |||
| NDF | 0.714*** | 0.858*** | 0.323 | 0.031 | 0.149 | 0.031 | 0.031 | 0.126 | -0.314 | ||||
| ADF | 0.252 | 0.372 | -0.297 | -0.212 | -0.297 | -0.297 | 0.095 | -0.332 | |||||
| HC | 0.174 | 0.261 | 0.361 | 0.261 | 0.261 | 0.105 | -0.190 | ||||||
| ADL | -0.838*** | -0.774*** | -0.838*** | -0.838*** | 0.526** | -0.702** | |||||||
| IVDMD | 0.984*** | 1.000*** | 1.000*** | -0.445* | 0.600** | ||||||||
| IVOMD | 0.984*** | 0.984*** | -0.422* | 0.565** | |||||||||
| DOMD | 1.000*** | -0.4458 | 0.600** | ||||||||||
| ME(MJ/Kg) | -0.444* | 0.600** | |||||||||||
| CT | -0.543** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




